Abstract
The calcium activated K+ channel KCa3.1 plays an important role in T lymphocyte Ca2+ signaling by helping to maintain a negative membrane potential, which provides an electrochemical gradient to drive Ca2+ influx. We previously showed that nucleoside diphosphate kinase beta (NDPK-B), a mammalian histidine kinase, is required for KCa3.1 channel activation in human CD4 T lymphocytes. We now show that the mammalian protein histidine phosphatase (PHPT-1) directly binds and inhibits KCa3.1 by dephosphorylating histidine 358 on KCa3.1. Overexpression of wild-type, but not a phosphatase dead, PHPT-1 inhibited KCa3.1 channel activity. Decreased expression of PHPT-1 by siRNA in human CD4 T cells resulted in an increase in KCa3.1 channel activity and increased Ca2+ influx and proliferation after T cell receptor (TCR) activation, indicating that endogenous PHPT-1 functions to negatively regulate CD4 T cells. Our findings provide a previously unrecognized example of a mammalian histidine phosphatase negatively regulating TCR signaling and are one of the few examples of histidine phosphorylation/dephosphorylation influencing a biological process in mammals.
Keywords: nucleoside diphosphate kinase, NM23-h2, PHPT-1, CRAC channel, histidine kinase
The influx of Ca2+ into T cells via Ca2+ release-activated channels (CRAC) located in the plasma membrane plays a central role in T cell activation. The increase in cytosolic Ca2+ activates the phosphatase calcineurin, which results in the assembly of Nuclear factor of activated T-cells transcriptional complexes and the subsequent transcription of a number of genes required for T cell activation. Sufficient influx of Ca2+ via CRAC channels to activate a T cell also requires the activation of one of two K+ channels, the calcium-activated K+ channel, KCa3.1 [also known as IK Ca2+, small conductance calcium-activated potassium channels (SK)4, or KCNN4], or the voltage-dependent channel Kv1.3 (1, 2). By mediating the efflux of K+, both of these channels function to maintain a negative membrane potential, which is required to maintain a favorable electrochemical gradient for Ca2+ influx.
KCa3.1 channels are expressed at low levels in resting naïve T cells, are rapidly up-regulated after T cell activation, and are required for maximal Ca2+ influx and proliferation during the reactivation of naïve T cells (1, 2). We have previously shown that nucleoside diphosphate kinase beta (NDPK-B, also known as Nm23h2), a mammalian histidine kinase, activates KCa3.1 by phosphorylating histidine (H) 358 in the carboxyl terminus (CT) of KCa3.1 and is required for KCa3.1 channel activation, Ca2+ influx, and proliferation of human CD4 T cells (3). Histidine phosphorylation is a reversible process and therefore histidine phosphatases that dephosphorylate KCa3.1 or NDPK-B should function as a negative regulator of CD4 T cells by dephosphorylating and inhibiting KCa3.1 channel activity. Thus far, only a couple of histidine phosphatases (PT) have been identified in mammalian cells and include mammalian phosphohistidine phosphatase 1 (PHPT-1) and phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) (4–6). PHPT-1 is an evolutionarily conserved 14-kDa protein that is widely expressed in mammalian cells and tissues including immune cells and has been shown to dephosphorylate Gβ and ATP citrate lyase, although the physiologic relevance of this process is currently unknown (7). In contrast to PHPT-1, LHPP exhibits a much more restricted expression pattern and is not expressed in peripheral blood lymphocytes (6). We now provide evidence that PHPT-1 functions as a negative regulator of CD4 T lymphocytes by dephosphorylating and inhibiting KCa3.1.
Results
PHPT-1 Inhibits KCa3.1 Channel Activity in Whole-Cell Patch Clamp Experiments.
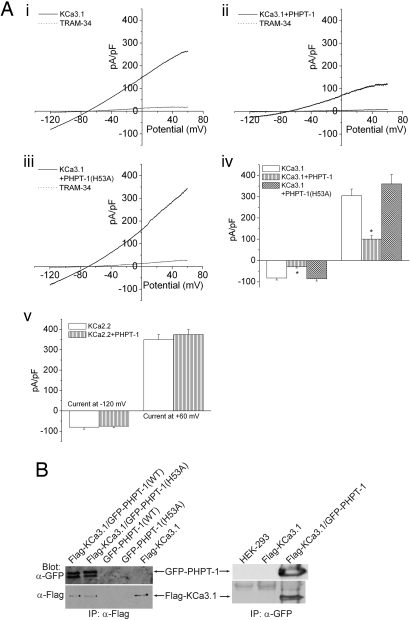
To determine whether PHPT-1 could dephosphorylate KCa3.1 and thereby function to inhibit KCa3.1 channel activity in CD4+ T lymphocytes, we tested whether overexpression of GFP-PHPT-1 inhibited KCa3.1 channel activity in CHO cells overexpressing KCa3.1 (CHO-KCa3.1). We found that overexpression of GFP-PHPT-1 inhibited KCa3.1 channel activity by >60–70% (Fig. 1A). Moreover, the inhibitory effect of PHPT-1 required its phosphatase activity because overexpression of a mutant PHPT-1 containing the substitution of histidine 53 for alanine (H53A), which has previously been shown to inhibit PHPT-1 phosphatase activity (8, 9), did not inhibit KCa3.1 channel activity (Fig. 1Aiii). The inhibition of KCa3.1 channel activity was specific because PHPT-1 did not inhibit the related calcium-activated K+ channel KCa2.2 (Fig. 1Av), which we have previously shown is not activated by NDPK-B (3).
Fig. 1.
Overexpression of PHPT-1 inhibits KCa3.1 channel activity in whole-cell patch-clamp experiments. (A) CHO cells overexpressing KCa3.1 were transfected with GFP, GFP-PHPT-1(WT), or GFP-PHPT-1(H53A), and KCa3.1 channel activity was determined by whole-cell patch-clamp experiments on GFP-positive cells. Shown are current–voltage (i–v) plots of CHO-KCa3.1 cells: control (i), overexpressing GFP-PHPT1(WT) (ii), and overexpressing GFP-PHPT-1(H53A) (iii). Cells in i–iii were inhibited by 1 μM of the selective KCa3.1 blocker TRAM-34 (16). (iv) Bar graph summary of TRAM-34-inhibited currents plotted at −120 and +60 mV (n = 8). (v) Bar graph summary as described in iv showing that overexpression of GFP-PHPT-1(WT) does not inhibit the related calcium-activated potassium channel KCa2.2. (B) PHPT-1 and KCa3.1 coimmunoprecipitate in cells. Flag-KCa3.1 and GFP-PHPT-1(WT) or GFP-PHPT-1(H53A) were transfected into HEK293 cells either alone or together, and cell lysates were then immunoprecipitated (IP) with anti-Flag or anti-GFP antibodies as described (15). The immunoprecipitated proteins were then Western blotted with anti-GFP or anti-Flag antibodies as indicated. Current is represented as pico Amp/pico farad (pA/pF). *, P < 0.05 as compared with control KCa3.1 current. Data displayed as mean ± SEM.
PHPT-1 and KCa3.1 Coimmunoprecipitate in Cells.
Direct binding of phosphatases (PT) to their target is one mechanism that sometimes determines PT specificity (10). To determine whether PHPT-1 physically associates with KCa3.1, we expressed Flag-tagged KCa3.1 with GFP-tagged PHPT-1 in HEK 293 cells and determined whether the two proteins coimmunoprecipitate (3). These studies demonstrated that GFP-PHPT-1(WT) and PHPT-1(H53A) coimmunoprecipitated with anti-Flag antibodies when coexpressed with Flag-KCa3.1 (Fig. 1B). The ability of the two proteins to coimmunoprecipitate was specific because GFP-PHPT-1 was not immunoprecipitated by anti-Flag antibodies in the absence of expression of Flag-KCa3.1 (Fig. 1B Left, lanes 3 and 4). Moreover, the two proteins also coimmunoprecipitated in a reciprocal immunoprecipitation using anti-GFP antibodies (Fig. 1B Right).
PHPT-1 Directly Inhibits KCa3.1 Channel Activity in Inside/Out (I/O) Patches.
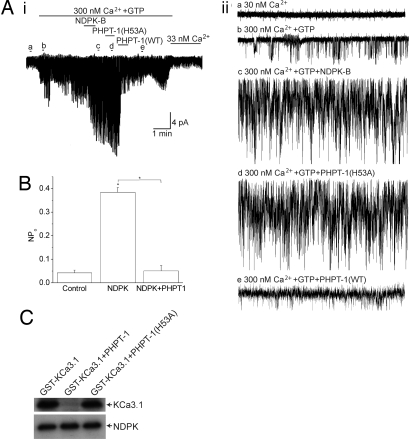
The above studies suggest that PHPT-1 inhibits KCa3.1 by directly binding and dephosphorylating H358 on KCa3.1. Thus, if PHPT-1 directly dephosphorylates H358 on KCa3.1, PHPT-1 should inhibit KCa3.1 activity in isolated membrane patches. As previously shown (3), addition of GST-NDPK-B to I/O patches isolated from CHO-KCa3.1 cells led to a marked increase in KCa3.1 channel activity due to the phosphorylation of H358 in KCa3.1 (Fig. 2 Ai and Aii, compare trace c with trace b). Whereas KCa3.1 channel activity was unchanged after the addition of recombinant His-PHPT-1(H53A) to the same membrane patch (Fig. 2 Ai and Aii, trace d), addition of His-PHPT-1(WT) led to a marked inhibition of KCa3.1 channel activity (Fig. 2 Ai and Aii, trace e).
Fig. 2.
PHPT-1 directly inhibits KCa3.1 by dephosphorylating H358 in the CT of KCa3.1. (A) I/O patches were isolated from CHO-KCa3.1 cells. Baseline channel activity was first recorded in I/O patches in the absence (i and ii, trace a) or presence of 300 nM Ca2+ and GTP (i and ii, trace b) as described (3). KCa3.1 channels were then activated by the addition of GST-NDPK-B (10 μg/ml) (i and ii, trace c). To determine whether PHPT-1 inhibits KCa3.1 channel activity, His-PHPT-1(H53A) (10 μg/ml) was first added to the same patch (i and ii, trace d), followed by the addition His-PHPT-1(WT) (i and ii, trace e). Aii traces a–e are I/O recordings over 5 sec as indicated. (B) Effect of PHPT-1 on the open channel probability (NPo). Bar graph summary of KCa3.1 NPo from control (trace b), NDPK-B (trace c), and PHPT-1(WT) (trace e); n = 3 patches, P < 0.001. All recordings were at +100 mV. His-PHPT-1(WT), but not His-PHPT-1(H53A), inhibits KCa3.1 channel activity. (C) PHPT-1 dephosphorylates H358 in KCa3.1. To phosphorylate H358 on KCa3.1, Flag-tagged-NDPK-B was immunoprecipitated from transfected HEK293 cell lysates and then incubated with 2.5 μg of GST-KCa3.1(CT) in kinase buffer containing [γ-32P]GTP as described (3). The reaction products were then incubated with 2.5 μg of His-PHPT-1(WT) or His-PHPT-1(H53A) for 30 min at 37°C. Reaction products were then separated by SDS/PAGE and visualized by autoradiography. GST-KCa3.1(CT) and Flag-NDPK-B are indicated. Current represented as pico Amps. *, P < 0.05 as compared with control and as indicated in the figure. Data displayed as mean ± SEM.
PHPT-1 Inhibits KCa3.1 by Dephosphorylating H358 in the Carboxyl Terminus (CT) of KCa3.1.
To demonstrate biochemically that PHPT-1 can dephosphorylate H358 on KCa3.1, the CT of KCa3.1 was generated as a GST-fusion protein, and H358 was phosphorylated in vitro by using [γ-32P]GTP and NDPK-B as described (3). Addition of His-PHPT-1(WT), but not His-PHPT-1(H53A), led to dephosphorylation of H358 in KCa3.1 (Fig. 2C). NDPK-B undergoes autophosphorylation on H118, which is required for kinase activity. PHPT-1 did not dephosphorylate H118 on NDPK-B, indicating that PHPT-1 dephosphorylation of KCa3.1 is specific, and PHPT-1 does not inhibit KCa3.1 channel activity by dephosphorylating and inhibiting NDPK-B (Fig. 2C).
Silencing of PHPT-1 by siRNA in Primary Human CD4 T Cells Led to Increased KCa3.1 Channel Activity and TCR-Stimulated Calcium Influx and Proliferation.
We have previously shown that phosphorylation of KCa3.1 on H358 by NDPK-B is required for KCa3.1 channel activity and the reactivation of human CD4 T cells (3). Thus, by dephosphorylating and inhibiting KCa3.1, PHPT-1 is a candidate to function as a negative regulator of CD4+ T cells. Consistent with this idea, we found that down-regulation of PHPT-1 by siRNA led to a ≈1.5- to 2-fold increase in KCa3.1 channel activity (Fig. 3 Bi–Biii). These results were obtained with two independent sets of siRNAs to PHPT-1, indicating that the inhibitory effect was not because of an off target effect of the siRNA (Fig. 3Biv). The K+ current in activated CD4+ T cells is contributed by both KCa3.1 and Kv1.3, of which only KCa3.1 is regulated by NDPK-B. Unlike KCa3.1, Kv1.3 channel activity was similar between control and siRNA-treated cells, indicating that the increase in KCa3.1 channel activity in siRNA-treated cells is specific (Fig. 3Bv).
Fig. 3.
Silencing of PHPT-1 in CD4 T cells by siRNA leads to an increase in KCa3.1 channel activity. Purified CD4 T lymphocytes were transfected with a pool of siRNAs to PHPT-1 (Dharmacon) or a control siRNA by using AMAXA reagents and, after resting overnight, were stimulated with antibodies to CD3 and CD28. Whole-cell patch clamping was performed 48 h after stimulation as described (3). (A) Real-time PCR of PHPT-1 or interleukin 2 from control or CD4+ T cells stimulated with antibodies to CD3 or CD28 for 48 or 72 h (i) or from control or siRNA PHPT-1 transfected cells (ii). The relative amounts of PHPT-1 were standardized against GAPDH. In contrast to mRNA expression of IL-2, T cell stimulation did not lead to an increase in expression of PHPT-1 mRNA. (B) KCa3.1 and Kv1.3 current measured in siRNA control and siRNA PHPT-1 transfected CD4+ T cells. I–V trace of KCa3.1 current from siRNA control (i) and siRNA PHPT-1 transfected (ii) cells. Summary data of TRAM-34 inhibited current at +60 mV from CD4+ T cells transfected with siRNA (1–4) PHPT-1 (n = 8–12) (P < 0.001) (iii) or CD4+ T cells transfected with siRNA (1–2) or (3–4) PHPT-1 (iv). Data are displayed as ± SEM. (v) Kv1.3 current, which was not affected by silencing PHPT-1, was calculated as the remaining current after TRAM-34 treatment. Current is represented as pico Amp/pico farad (pA/pF). *, P < 0.05 as compared with control. Data are displayed as mean ± SEM.
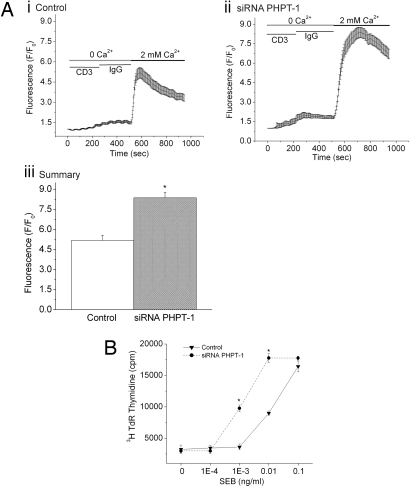
By mediating the efflux of K+, KCa3.1 functions to maintain a hyperpolarized membrane potential, which provides the electrochemical gradient that drives Ca2+ entry into reactivated CD4 T cells. As predicted, we found that down-regulation of PHPT-1 led not only to an increase in KCa3.1 channel activity, but also led to an increase in Ca2+ influx after cross-linking of the T cell receptor (TCR) (Fig. 4A). siRNA PHPT-1 transfected T cells were also more sensitive to antigen stimulation and were activated at 10-fold lower concentration by dendritic cells pulsed with staphylococcal enterotoxin B (SEB) when compared to control cells (Fig. 4B).
Fig. 4.
Silencing of PHPT-1 in CD4+ T cells by siRNA leads to an increase in Ca2+ influx and proliferation. Purified CD4 T cells were transfected with siRNA to PHPT-1 as described in Fig. 3. Cells were then stimulated for 48 h with antibodies to CD3 and CD28 and after resting overnight were loaded with Fluo-4 AM (10 μM). Ca2+ influx was determined by confocal microscopy at 488 nm with images taken every 5 sec after cross-linking with anti-CD3 antibodies (5 mg/ml) as described (17). Average values from 80–100 cells are shown for each series. Ca2+ influx was determined in control (Ai) and siRNA PHPT1 cells (Aii). (Aiii) Bar graph showing fluorescence values from Ai and Aii at peak with 2 mM Ca2+. (B) Purified CD4+ T cells were treated as described in A and, after resting overnight, were plated in 96-well plates with human DC that were activated for 24 h with lipopolysaccharide (100 ng/ml) in a ratio of 10:1 (30,000 CD4+ T cells:3,000 DC) in the presence of increasing concentrations of staphylococcal enterotoxin B (SEB) as described (18). Twenty-four hours after stimulation, cells were pulsed for 8 h with [3H]thymidine, and [3H]thymidine incorporation was assessed by scintillation counting (19). *, P < 0.05 as compared with control. Data are displayed as mean ± SEM.
Discussion
Although histidine phosphorylation has been proposed to play an important role in mammalian cells for more than 30 years, a critical role for reversible histidine phosphorylation in the regulation of specific biological processes are still lacking (11–13). The finding that NDPK-B activates KCa3.1 channels by phosphorylating H358 in the CT of KCa3.1 (3) and our findings reported here that PHPT-1 inhibits KCa3.1 by dephosphorylating H358 provides one of the best examples whereby reversible histidine phosphorylation regulates a biological function in mammalian cells. Moreover, the critical role for both NDPK-B and PHPT-1 in the regulation of KCa3.1 channel activity has uncovered an unexpected role for both of these molecules in the reactivation of human CD4 T cells and demonstrates that a histidine phosphatase functions as a negative regulator of T cells.
We still do not understand how PHPT-1 is regulated in T cells or how PHPT-1's target specificity is determined. Our finding that PHPT-1 dephosphorylates H358 on KCa3.1, but not H118 on NDPK-B, indicates that PHPT-1 specifically dephosphorylates only a subset of histidine phosphorylated proteins. One possibility is that binding a downstream target is required to localize PHPT-1 to its site of action. Consistent with this idea, we found that PHPT-1 coimmunoprecipitates with KCa3.1 but not NDPK-B. Another possible mechanism for PHPT-1 regulation could be at the level of PHPT-1 expression. For example, increased protein expression of PHPT-1 after T cell activation could lead to an increase in PHPT-1 activity, which in turn would mediate the dephosphorylation and inhibition of KCa3.1 channel activity resulting in T cell inhibition. Our inability to detect changes in PHPT-1 mRNA in activated T cells (Fig. 3Ai and data not shown) indicates that changes in PHPT-1 expression is unlikely to contribute to PHPT-1 regulation in T cells.
Our results, when taken together, are consistent with the idea that PHPT-1 inhibits KCa3.1 by dephosphorylating H358 in KCa3.1's carboxyl terminus. Based on these findings, we would predict that histidine phosphorylation of KCa3.1 should be increased in cells in which PHPT-1 expression is decreased by siRNA. However, we have thus far been unable to detect histidine phosphorylated KCa3.1 in vivo in cells labeled with orthophosphate. There are a number of reasons that may account for this. Histidine phosphorylation is very unstable and therefore probably turns over very quickly in a cell. There are also no known inhibitors of histidine phosphatases and therefore histidine-phosphorylated proteins may undergo dephosphorylation during cell lysis. To potentially inactivate histidine phosphatases, orthophosphate-labeled cells were lysed directly in 10% SDS. However, under these conditions, we were also unable to detect histidine phosphorylated KCa3.1. In addition, anti-phosphohistidine antibodies, which would be a valuable tool to detect in vivo histidine phosphorylated proteins, do not exist, and it is not possible to generate a phosphohistidine peptide to immunize rabbits using current technology.
Negative regulators of T and B cells are critical to both set a minimal threshold for T cell activation as well as to provide negative feedback to limit T cell activation. The important role for these molecules in attenuating T and B cell responses is evident by the finding that many negative regulators of T and B signaling are required to prevent the development of autoimmune diseases (14). Studies are currently underway to determine whether PHPT-1, like other negative regulators of TCR signaling (14), is required in vivo to prevent inappropriate or enhanced activation of T cells that may lead to autoimmunity.
Materials and Methods
Whole Cell and I/O Patch Clamping.
Protocols for whole cell and I/O patch clamping have been described (3).
Phosphorylation and Dephosphorylation Assay.
The GST-KCa3.1(CT) was phosphorylated by NDPK-B by using [γ-32P]GTP as described (3). His-PHPT-1(WT) or His-PHPT-1(H53A) were expressed in the expression vector pET-28 in Escherichia coli and purified as described (3, 8). Dephosphophorylation was assessed after the addition of His-PHPT-1(WT) or His-PHPT-1(H53A) (2.5 mg per reaction) to the same reaction for 30 min at 37°C. Reaction products were then separated by SDS/PAGE (12%) and visualized by autoradiography.
CD4 T Cell Isolation and Silencing of PHPT-1.
Human CD4+ were purified from adult blood buffy coats as described (3). To silence PHPT-1, purified human CD4+ T cells were electroporated with siRNAs described below by using AMAXA reagents, and after resting overnight, were stimulated for 48 h with anti-CD3 anti-CD28 antibodies. Whole-cell patch clamping was performed 72 h after transfection as described (3).
A SMART pool reagent (combination of four individual siRNAs 1–4) and a SMART pool upgrade (four individual siRNAs 1–4) to human PHPT-1 were purchased from Dharmacon. The sequence of the siRNAs used are as follows: siRNA 1, AGAUUCACGUGUACGGCUAUU (sense sequence) and 5′-PUAGCCGUACACGUGAA UCUUU (antisense sequence); siRNA 2, AUGCGGACAUCUACGACAAUU (sense sequence) and 5′-PUUGUCGUAGAUGUCCGCAUUU (antisense sequence); siRNA 3, GAAGCAAGGCUGCGACUGUUU (sense sequence) and 5′-PACAGUCGCAGCCUUGC UUCUU (antisense sequence); and siRNA 4, GGCUAACGACGGCUACUGAUU (sense sequence) and 5′-PUCAGUAGCCGUCGUUAGCCUU (antisense sequence). Experiments were performed by using SMART pool reagent (siRNA 1–4) and by combining siRNAs 1 and 2 and siRNAs 3 and 4.
Intracellular Ca2+ Activity.
Cells were loaded at 1 × 106 cells per ml with 10 μM Fluo-4 AM ester (Molecular Probes) and attached to poly(l-lysine)-coated coverslip for 20 min in an RC-20 bath flow chamber (Warner Instrument Corp.) and analyzed by laser confocal microscopy (Leica Microsystems) as described (15). Data are represented as F/F0, with F representing fluorescence values at different time points and F0 representing cellular fluorescence at time 0. Cells were perfused with the bath solution in the presence or absence of extracellular calcium and stimulated with 5 μg/ml of anti-CD3 cross-linked with 5 μg/ml of rat anti-mouse IgG.
Proliferation Assay.
Human dendritic cells (DC) were purified and cultured in the presence of granulocyte–macrophage colony-stimulating factor. For proliferation assays, DC were plated together with CD4 T cells in U-bottom 96-well plates at a ratio of 10:1 (T cells:DC) in the presence of various concentrations of staphylococcal enterotoxin B (SEB). Forty-eight hours after stimulation, cells were pulsed with [3H]thymidine, and [3H]thymidine incorporation was assessed by scintillation counting.
Acknowledgments.
We thank S. Hubbard and William A. Coetzee (New York University Medical Center) for helpful discussions and B. M. Hallberg (Karolinska Institute, Stockholm, Sweden) for the PHPT-1 expression vectors. E.Y.S. is supported by grants GM084195 and AI052459.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.K.T. is a guest editor invited by the Editorial Board.
References
- 1.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel. 2003;6:640–647. [PubMed] [Google Scholar]
- 2.Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell. 2006;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Ek P, et al. Identification and characterization of a mammalian 14-kDa phosphohistidine phosphatase. Eur J Biochem. 2002;269:5016–5023. doi: 10.1046/j.1432-1033.2002.03206.x. [DOI] [PubMed] [Google Scholar]
- 5.Klumpp S, et al. Protein histidine phosphatase: A novel enzyme with potency for neuronal signaling. J Cereb Blood Flow Metab. 2002;22:1420–1424. doi: 10.1097/01.wcb.0000045041.03034.99. [DOI] [PubMed] [Google Scholar]
- 6.Yokoi F, Hiraishi H, Izuhara K. Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase. J Biochem (Tokyo) 2003;133:607–614. doi: 10.1093/jb/mvg078. [DOI] [PubMed] [Google Scholar]
- 7.Klumpp S, Krieglstein J. Reversible phosphorylation of histidine residues in vertebrate proteins. Biochim Biophys Acta. 2005;1754:291–295. doi: 10.1016/j.bbapap.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Busam RD, et al. First structure of a eukaryotic phosphohistidine phosphatase. J Biol Chem. 2006;281:33830–33834. doi: 10.1074/jbc.C600231200. [DOI] [PubMed] [Google Scholar]
- 9.Ma R, et al. Mutational study of human phosphohistidine phosphatase: Effect on enzymatic activity. Biochem Biophys Res Commun. 2005;337:887–891. doi: 10.1016/j.bbrc.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 10.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: The substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besant PG, Attwood PV. Mammalian histidine kinases. Biochim Biophys Acta. 2005;1754:281–290. doi: 10.1016/j.bbapap.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Steeg PS, Palmieri D, Ouatas T, Salerno M. Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Lett. 2003;190:1–12. doi: 10.1016/s0304-3835(02)00499-8. [DOI] [PubMed] [Google Scholar]
- 13.Tan E, Besant PG, Attwood PV. Mammalian histidine kinases: Do they REALLY exist? Biochemistry. 2002;41:3843–3851. doi: 10.1021/bi012021r. [DOI] [PubMed] [Google Scholar]
- 14.Rangachari M, Penninger JM. Negative regulation of T cell receptor signals. Curr Opin Pharmacol. 2004;4:415–422. doi: 10.1016/j.coph.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava S, et al. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol Cell Biol. 2006;26:5595–5602. doi: 10.1128/MCB.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wulff H, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 18.Motsinger A, et al. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J Virol. 2003;77:8153–8158. doi: 10.1128/JVI.77.14.8153-8158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier E, et al. The B cell SH2/PH domain-containing adaptor Bam32/DAPP1 is required for T cell-independent II antigen responses. Curr Biol. 2003;13:1858–1866. doi: 10.1016/j.cub.2003.09.034. [DOI] [PubMed] [Google Scholar]