Abstract
Functional neuroimaging studies suggest that a lateral network in the brain is associated with the sensory aspects of pain perception while a medial network is associated with affective aspects. The highest concentration of opioid receptors is in the medial network. There is significant evidence that endogenous opioids are central to the experience of pain and analgesia. We applied an integrative multimodal imaging approach during acupuncture. We found functional magnetic resonance imaging signal changes in the orbitofrontal cortex, insula, and pons and [11C]diprenorphine positron emission tomography signal changes in the orbitofrontal cortex, medial prefrontal cortex, insula, thalamus, and anterior cingulate cortex. These findings include brain regions within both the lateral and medial pain networks.
Keywords: acupuncture, placebo, analgesia, opioid, PET, fMRI, 11C-diprenorhine
INTRODUCTION
The subjective experience of pain involves neuronal networks associated with both sensory discrimination of the stimulus as well as affect and cognitive responses to the stimulus [33]. The sensory discrimination of a stimulus arises from activation of peripheral receptors that transmit information via the spinal cord, brainstem and thalamus to S1 and S2 and possibly the insular cortex (the lateral network). The affective component of pain is associated with a neuronal network that includes amygdala, insula, anterior cingulate cortex (ACC) and medial regions of the frontal lobe (medial network) [34].
Functional magnetic resonance imaging (fMRI) and Positron Emission Tomography (PET) studies of acupuncture needle manipulation [3,11,20–22,24,25,29,31,42–44] and acupuncture analgesia [5,45,46] demonstrate involvement of these neuronal networks during acupuncture. Additionally, there is strong evidence that acupuncture analgesia is mediated at least in part by opioid systems [4,6,26,32].
The current study assessed the effect of manual acupuncture on pain perception in healthy subjects, employing a multimodal approach, using both fMRI and endogenous opioid peptide release as measured by PET. [11C]diprenorphine is a relatively nonspecific partial opioid agonist that is used for in vivo characterization of mu, delta, and kappa opioid receptors in conjunction with positron emission tomography (PET). We hypothesized that brain regions with fMRI signal changes and/or changes in PET measures of opioid receptor binding associated with acupuncture would include the brainstem, thalamus, insula, amygdala, ACC, and medial territories of the prefrontal cortex (PFC).
MATERIALS AND METHODS
Human Subjects
Twenty-two acupuncture-naïve, right-handed subjects (12 males) consented to and participated in this study, as approved by the Human Research Committee at Massachusetts General Hospital. Subjects participated in four sessions. In all sessions heat pain was administered using a TSA-2001 Thermal Sensory Analyzer with a 3 × 3 cm probe (Medoc Advanced Medical Systems, Rimat Yishai, Israel).
Recognizing the large individual variability in response to acupuncture treatment [17], we chose to pre-screen subjects entering the fMRI and PET scanning sessions (Sessions 3 and 4). Prior to Sessions 3 and 4, all subjects participated in a separate behavioral study (Session 2) to evaluate their response to acupuncture treatment. Subjects received identical experimental heat pain before and after treatment, and only those whose pain ratings were reduced were allowed to proceed to the following brain imaging sessions.
Experimental Procedures
Session 1
We used the first behavioral session to familiarize subjects with the rating scales and determine appropriate stimulus intensities using methods employed in our previous studies [19,23]. In this session, subjects were also trained to rate heat pain stimuli applied to the right forearm using the 0–20 Gracely Box Scales [7,8].
In summary, temperatures eliciting subjective intensity ratings in the LOW-level pain range (~ 8; weak on the 0–20 Sensory Scale) and HIGH-level pain range (~ 15; strong) were selected for each individual using an ascending stimuli sequence in which the stimuli temperature increased in one degree intervals. We then applied series of 8 noxious stimuli (4 HIGH and 4 LOW in random order) to the right arm. Temperatures were adjusted when necessary to ensure that each subject’s subjective ratings of HIGH and LOW remained in the desired range, as these would be used in the following sessions.
Session 2
In Session 2, subjects received heat pain stimuli (4 pain stimuli sequences applied to different areas of the right forearm, each stimuli sequence consisting of 6 HIGH and 6 LOW level noxious stimuli) before and after a 29-minute application of verum acupuncture at LI 4, described below. Only subjects who had decreased pain ratings after treatment for both pain levels (average pain ratings for both HIGH and LOW levels of noxious stimuli reduced by at least 0.5 on the Gracely 0–20 Sensory Scale) were then randomized into two groups (verum acupuncture and placebo acupuncture) and studied using fMRI and PET imaging (Sessions 3 and 4) performed in a counterbalanced manner.
Session 3 and 4
Session 3 and Session 4 were the fMRI and PET imaging scan sessions, conducted in a randomized order with about 1–2 week interval between sessions.
fMRI session
All fMRI brain imaging was performed with a 3-axis gradient head coil in a 3 Tesla head only Siemens MRI System equipped for echo planar imaging. Thirty axial slices (4 mm thick with 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with 4 s TR, 40 ms TE, 435 time points, 90° flip angle and 3.13 × 3.13 mm in-plane spatial resolution. A high-resolution 3D MPRAGE sequence was also collected for anatomic localization.
The procedures for the fMRI session were similar to those for Session 2. Subjects received heat pain stimuli (4 pain stimuli sequences applied to different areas of the right forearm, each stimuli sequence consisting of 6 HIGH and 6 LOW noxious stimuli) before and after a 29-minute application of verum or sham acupuncture at LI 4 depending on their group randomization.
PET session
The PET session lasted approximately 5.5 hours. Subjects were outfitted with physiological monitoring, visual presentation, and response indicator equipment. An initial assessment of pain responses was performed right before the subject entered the PET camera. Two 90-minute PET scans measuring opioid receptor binding were collected, one at baseline rest beginning at 9:30 AM and the other during acupuncture administration beginning at 1:30 PM. At the time of imaging, subjects were positioned in the gantry of a PET camera. Head alignments were made, relative to the canthomeatal line, using projected laser lines whose positions were known with respect to the slice positions of the scanner. An individually molded thermoplastic mask was used to minimize head motion. A peripheral venous catheter was inserted for radiopharmaceutical injection.
[11C]diprenorphine was injected manually as an intravenous bolus of 15 mCi for both acquisitions. PET data acquisition began at the moment that [11C]diprenorphine injection began and continued for 90 minutes. After the first PET imaging period, the subject left the PET camera gantry during the hour required for residual radioactivity decay. Subjects were then repositioned in the PET gantry as described above. During the second PET imaging period, acupuncture (active or sham) began immediately following [11C]diprenorphine injection and continued during PET data acquisition. The only difference between the two PET imaging periods was that acupuncture (via methods described below) was performed during the second PET imaging period.
Images were acquired using a PC-4096 PET camera (Scanditronix AB, Uppsala, Sweden). The primary imaging parameters of the PC-4096 camera are in-plane and axial resolution of 6.0 mm FWHM, 15 contiguous slices of 6.5 mm. PC4096 camera data was acquired in 2D mode and reconstructed using a conventional filtered back projection algorithm with an in-plane resolution of 6.0 mm FWHM. Photon attenuation measurements were made with a rotating pin source containing 68Ge.
Acupuncture administration
This study used acupoint Large Intestine 4 (LI 4) on the right hand, which has well-documented analgesic effects [38]. The acupuncturist practiced sterile technique. Subjects were blinded subjects to the nature (verum or sham) of acupuncture by covering the point of insertion with tape. Treatment lasted about 30 minutes. Immediately following acupuncture treatment, subjects used the Subjective Acupuncture Sensation Scale (SASS) to quantify their sensations of stabbing, throbbing, tingling, burning, heaviness, fullness/distention, numbness, soreness, and aching, and other feelings about the stimulated acupoint [17,20]. This set of deqi descriptors is based on sensations described in the more recent Traditional Chinese Medicine literature [18].
Verum acupuncture
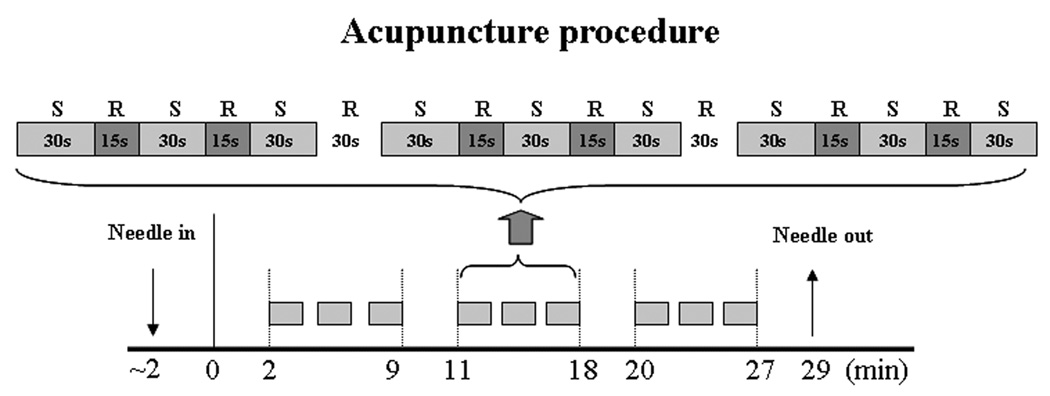
Manual acupuncture needle manipulation was performed using a balanced tonifying and reducing technique at the acupoint on the right side of the body in three, 7-minutes on, 2-minutes off blocks (Figure 1). The depth of acupuncture needle insertion was about 1 cm. During the 7-minute stimulation period, the acupuncturist manually stimulated acupoint (LI 4) for 30 seconds followed by a 15-second break before stimulation started again. Rotation frequency was approximately 180 rotations per minute at an angle of 45° from the perpendicular to the skin surface.
Figure 1.
Acupuncture administration procedure. S indicates verum (placebo) acupuncture stimulation at LI4; R indicates rest without stimulation with acupuncture needle still in place. During the fMRI scanning session, imaging data was collected from t = 0 to t =29 minutes. During the PET scanning session, tracer injection and imaging acquisition began at t = 2.
Sham / placebo acupuncture administration
Placebo treatment used a validated sham acupuncture needle (Streitberger placebo) [16,17,19,28,31,37,40] and was identical to active manual acupuncture except that the needle was not inserted into the skin and the acupuncturist held and rotated the needle more gently. The sham needles differed from regular needles by possessing blunt and retractable tips. Instead of penetrating the skin, the point of the Streitberger needle retracts up the handle shaft when the acupuncturist presses it into the skin.
Data analysis
Behavioral data analysis
Verum and placebo acupuncture analgesia were evaluated using a t-test comparing pre-and post-treatment differences in subjective ratings of identical noxious stimuli.
fMRI data analysis
Pre-processing and statistical analysis of fMRI data were performed using SPM2 software (Wellcome Department of Cognitive Neurology). Pre-processing included motion correction, normalization to MNI stereotactic space, and spatial smoothing with an 8 mm Gaussian kernel. Additionally, fMRI signal change associated with needle manipulation during real and placebo acupuncture and those associated with baseline (rest with no needle manipulation) were calculated with a general linear model. Global signal scaling was not applied. Low-frequency noise was removed with a high-pass filter applied with default values to the fMRI time series at each voxel. Group analysis was performed using a random-effects model. A two sample t-test was used to compare fMRI signal change differences evoked by verum and placebo acupuncture stimulation. The threshold was set P<0.005 with 5 contiguous voxels.
PET data analysis
All brain data were corrected for inter-scan movement. The PET images were used to generate functional images of the binding potential (BP) of [11C]diprenorphine using the simplified reference tissue model [9,47] with the occipital cortex used as the reference tissue. Data were then normalized and smoothed. Statistical analysis of the PET data was conducted following the theory of statistical parametric mapping. Data were analyzed using the SPM2 software package (Welcome Department of Cognitive Neurology, London, UK). The analysis of variance considered the scan condition (baseline or during treatment) as the main effect and subjects as a block effect (verum or sham acupuncture). In other words, the multi-group, multi-subject function in SPM was used to compare binding decreases during treatment (as compared to baseline) between groups (verum vs sham acupuncture). Planned contrasts at each voxel were conducted; this method fits a linear statistical model, voxel-by-voxel to the data. These hypotheses were tested as contrasts in which linear compounds of the model parameters were evaluated using t statistics. Data from all conditions were used to compute the contrast error term. Threshold was set at P < 0.05 with 5 continuous voxels.
RESULTS
Twelve of 22 consenting volunteers completed the study (6 verum acupuncture and 6 placebo acupuncture subjects). One subject was dropped after Session 1 because he could not reliably perform the rating task (High pain stimuli were not consistently rated higher than Low pain stimuli); eight subjects were dropped on account of their poor acupuncture response after Session 2; and one dropped out during a scanning session, citing discomfort with the procedures of scanning. Of the remaining 12 subjects, there were three male and three females in each group with the age (mean ± SD) of 28±7 years for the acupuncture group and 36±10 years for the placebo acupuncture group. There was no significant difference between the two groups.
The behavioral results from Session 2 showed comparable pain rating difference (pre-treatment pain rating minus post-treatment pain rating) during verum acupuncture between the groups that later underwent verum or placebo acupuncture in Sessions 3 and 4. In the fMRI session, subjects in the acupuncture group reported pre- and post-treatment pain ratings of 8.7±1.3 and 7.6±1.6, respectively, for LOW pain and 13.9±1.3 and 12.8±2.1 for HIGH pain. Subjects in the control group reported pre and post ratings of 8.4±2.3 and 9.0±2.0, respectively, for LOW pain and 13.5±2.6 and 14.0±2.9 for HIGH pain. Statistical analyses with paired t-tests showed that the reduction in pre- and post-treatment pain ratings was significantly greater in the acupuncture group when compared to the placebo group (p<0.05).
Average SASS ratings for each acupoint fell between 0.0 and 2.7 on the 10.0-point scale (Table I). The maximum individual scale registered at 8.2, but most ratings fell in the mild to moderate range (<5.0). As expected, we found significantly lower SASS ratings for the sham acupuncture treatment than for the verum acupuncture treatment in both fMRI and PET sessions. The subjective anxiety rating during verum acupuncture and placebo acupuncture ranged from 0 to 3.3 with an average score of 0.3. There was no significant difference between acupunture and placebo treatment in the fMRI or PET session.
Table 1.
real and placebo acupuncture subjective SASS ratings during fMRI and PET sessions (mean ± SD).
| soreness | heaviness | fullness | numbness | tingling | aching | burning | throbbing | stabbing | |
|---|---|---|---|---|---|---|---|---|---|
| fMRI-real | 1.6±1.6 | 1.4±2.2 | 1.6±2.4 | 3.0±3.5 | 3.6±3.4 | 1.9±2.0 | 0.7±1.3 | 0.9±1.3 | 1.0±1.5 |
| fMRI-placebo | 0.6±1.3 | 0.1±0.2 | 0.6±1.3 | 0.8±1.3 | 0.4±0.8 | 0.6±1.3 | 0.1±0.2 | 0.6±1.3 | 0.6±1.3 |
| PET-real | 1.4±1.6 | 1.4±2.9 | 1.5±3.2 | 2.2±3.5 | 2.1±3.0 | 1.4±2.6 | 0.6±0.2 | 1.3±2.2 | 1.1±2.6 |
| PET placebo | 0.1±0.2 | 0.1±0.2 | 0.1±0.2 | 0.1±0.2 | 0.7±1.3 | 0.1±0.1 | 0.1±0.1 | 0.1±0.1 | 0.1±0.1 |
SPM was used to analyze both the fMRI and [11C]diprenorphine PET data. The only a priori brain region demonstrating greater fMRI signal increase in the verum acupuncture versus placebo acupuncture group was the right medial orbitofrontal cortex (OFC) (see Table 2 & Figure 2). Further analysis showed that the fMRI contrast estimated β value (mean ± SD) at peak activation (10 54 −16) of right medial OFC was 0.32 ± 0.22 for the verum acupuncture group and −0.26 ± 0.16 for the placebo acupuncture group. A priori brain regions demonstrating greater fMRI signal increases during treatment in the placebo acupuncture group versus the verum acupuncture group included the left insula and bilateral brainstem (see Table II).
Table 2.
fMRI signal change differences evoked by real and placebo acupuncture treatment. Peak coordinates are in Montreal Neurological Institute (MNI) space. Threshold was set at P < 0.005 with 5 continuous voxels.
| Comparison | Area (Brodmann Area) | Z score | Number of voxels in cluster | Peak coordinate (x,y,z) |
|---|---|---|---|---|
| Real > Placebo | Right medial orbital prefrontal cortex (11) | 5.32 | 13 | 10 54 −16 |
| Right medial orbital prefrontal cortex (25) | 3.61 | 5 | 4 36 −16 | |
| Placebo >Real | Left inferior parietal lobule (40) | 4.04 | 23 | −62 −30 36 |
| Left superior temporal gyrus (22) | 3.52 | 157 | −44 −48 20 | |
| Right Pons | 3.12 | 18 | 12 −26 −24 | |
| Right inferior parietal lobule (40) | 3.11 | 29 | 44 −40 20 | |
| Right superior temporal gyrus (22) | 2.98 | 7 | 68 −34 18 | |
| Left operculum | 2.88 | 10 | −48 −4 14 | |
| Left anterior insula | 2.79 | 15 | −28 28 6 | |
| Left pons | 2.70 | 6 | −14 −28 −22 |
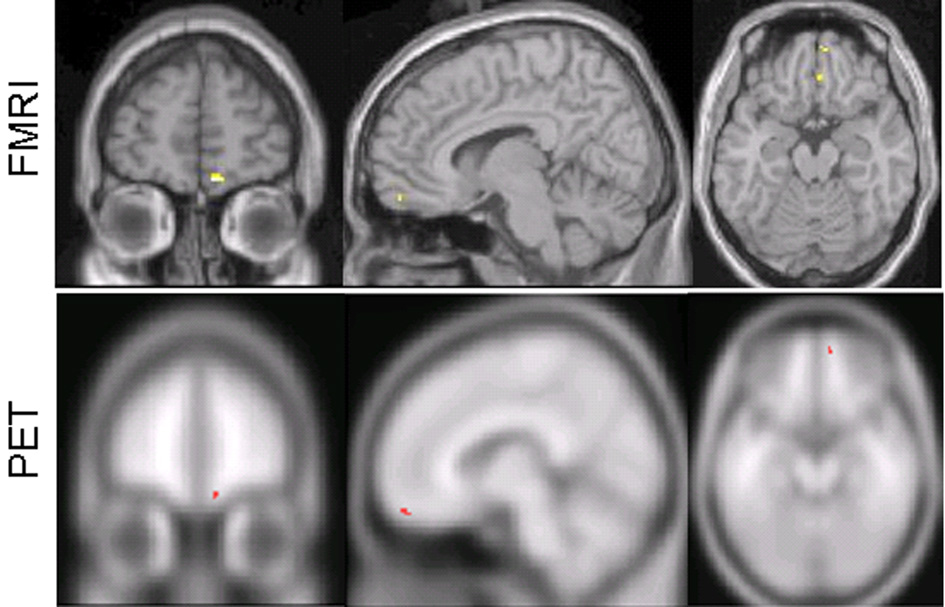
Figure 2.
Greater fMRI signal change increases were found during verum acupuncture when compared to sham acupuncture and greater [11C]diprenorphine PET binding potential decreases (suggesting increased endogenous opioid release) were found during verum acupuncture when compared to sham acupuncture.
A priori brain regions demonstrating greater [11C]diprenrophine binding decreases (associated with greater endogenous opioid release) in the verum group versus the placebo group were observed in the right medial OFC (see Table III & Figure 2), left medial PFC, right thalamus, and right insula. A greater binding increase in the verum group versus the placebo group included bilateral insula, right medial PFC/ACC, left OFC, and right brainstem. Common fMRI and PET changes (increased fMRI signal and decreased [11C]diprenorphine binding associated with greater endogenous opioid release) during verum acupuncture were seen only in the right medial OFC (see Figure 2). There were no common fMRI and PET changes during placebo acupuncture.
Table 3.
Greater [11C]diprenorphine PET binding potential decreases (corresponding with greater endogenous opioid release) during real and placebo acupuncture treatment. Peak coordinates are in Montreal Neurological Institute (MNI) space. Threshold was set at P < 0.05 with 5 continuous voxels. Italics represent a priori regions.
| Comparison | Area (Brodmann Area) | Z score | Number of voxels in cluster | Peak coordinate (x,y,z) |
|---|---|---|---|---|
| Real > Placebo | Cerebellum | 2.98 | 252 | −18, −76, −32 |
| Cerebellum | 2.83 | 5 | 30, −58, −26 | |
| DLPFC (9/44) | 2.85 | 176 | 48, 16, 30 | |
| DLPFC (9) | 2.14 | 27 | −28, 42, 34 | |
| Basal forebrain | 2.65 | 31 | 2, −6, −2 | |
| Precuneus (7) | 2.62 | 9 | −8, −64, 28 | |
| Medial PFC (18) | 2.56 | 11 | −4, 32, 36 | |
| Precentral gyrus (6) | 2.54 | 15 | −46, −2, 34 | |
| Superior frontal gyrus (10) | 2.41 | 26 | −18, 56, 22 | |
| Middle temporal gyrus (39) | 2.21 | 85 | −52, −66, 24 | |
| Superior temporal gyrus (22) | 2.16 | 30 | −62, −44, 14 | |
| Insula | 2.12 | 87 | 26, 12, −8 | |
| Inferior frontal gyrus (44) | 2.00 | 6 | −56, 8, 32 | |
| Thalamus | 1.97 | 19 | 8, −10, 12 | |
| Caudate | 1.96 | 6 | 16, 10, 12 | |
| Inferior temporal gyrus (20) | 1.88 | 12 | 62, −14, −20 | |
| Medial OFC (11) | 1.75 | 5 | 8, 62, −18 | |
| Placebo >Real | Insula | 2.92 | 148 | −38, −24, 16 |
| Insula | 2.40 | 122 | −46, 8, 0 | |
| Insula | 1.97 | 12 | 40, −8, 6 | |
| mPFC/dACC (9/32) | 2.77 | 95 | 8, 30, 34 | |
| Inferior temporal gyrus (37) | 2.47 | 25 | 62, −56, −4 | |
| OFC (10/46) | 2.42 | 173 | −34, 46, 4 | |
| Cerebellum | 2.32 | 5 | −4, −58, −42 | |
| Postcentral gyrus (40) | 2.28 | 145 | −64, −22, 16 | |
| GP/Putamen | 2.23 | 21 | −16, 6, −4 | |
| Superior temporal gyrus (22) | 2.13 | 15 | 58, 6, −4 | |
| mPFC (10) | 2.08 | 6 | 10, 54, −2 | |
| mPFC (10) | 1.96 | 7 | 14, 50, 14 | |
| Brainstem | 2.07 | 15 | −14, −22, −2 | |
| DLPFC (46) | 1.95 | 9 | 34, 40, 24 |
DISCUSSION
In this pilot study, we applied an integrative multimodal imaging approach (fMRI and [11C]diprenorphine PET) to investigate the brain mechanisms involved in verum acupuncture stimulation compared with placebo needle stimulation. Interestingly, the two imaging modalities revealed predominantly divergent findings. The fMRI study showed fMRI signal increases during verum acupuncture in the right orbitofrontal cortex and fMRI decreases during verum acupuncture in the left insula and bilateral brainstem. The PET study showed greater [11C]diprenorphine binding decreases during verum acupuncture in the right orbitofrontal cortex, left medial PFC, right thalamus, and right insula and greater [11C]diprenorphine increases during verum acupuncture in the bilateral insula, right medial PFC/ACC, left OFC, and right brainstem. Our results showed involvement of some brain regions only in fMRI results and the involvement of other brain regions only in [11C]diprenorphine PET results. For these two modalities, there was convergence only in the right medial OFC.
Despite acupuncture having been used for more than 2000 years to relieve pain, scientific validation is incomplete and the underlying mechanisms of the therapy are only partly understood. It is now widely held that a critical component of acupuncture analgesia is its mediation by endogenous opioids [4,6,26]. Yet, directly linking the specific brain regions with endogenous opioid release during acupuncture needle stimulation in human beings remains to be experimentally undertaken.
Recently, functional magnetic resonance imaging (fMRI) has been used to investigate the neurobiological mechanisms underlying acupuncture needle manipulation [11,20,22,24,25,29,42–45]. It is commonly reported that manual acupuncture needle manipulation induces fMRI signal change in widespread neuronal networks. Many of the brain regions affected by acupuncture needle manipulation are known to contain high concentrations of opioid peptides or opioid peptide receptors. However, the exact brain regions that are involved in the endogenous opioid release remain unclear. In the current study, as expected, some brain regions had significant fMRI signal changes without corresponding changes in [11C]diprenorphine BP and vice versa. While these findings are informative (and will be discussed below), the goal of the current multimodal study was to look for regions of overlap. We would posit that overlapping changes may represent a relationship between fMRI signal and [11C]diprenorphine BP changes in these brain regions. In the current study, the only brain region that exhibited such an overlap between modalities was the right medial OFC. These results suggest that right medial OFC may be an important brain region involved in endogenous opioid modulation during acupuncture analgesia. Other fMRI signal changes may be mediated by other neurotransmitters or, given the small sample size, there may not have been enough statistical power to detect an overlap. Conversely, there are also [11C]diprenorpine BP changes without a corresponding change in fMRI signal in some brain regions. Again, this may be attributable to the small sample size. While the common finding of changes in fMRI signal and [11C]diprenorphine BP in the right medial OFC are intriguing, further studies should be conducted.
Previous studies suggest that the orbital prefrontal cortex can be roughly divided into two interacting networks: the orbital prefrontal network and medial prefrontal network [30]. The first consists of the agranular insular areas and orbital areas 13b, 13l, 13m, 11l, 12r, 12m and 12l. This network receives signals related to sensory inputs and seems to play an important role in sensory integration. Another network, the medial prefrontal network, consists of all areas on the medial wall including 25, 32, 14r, 14c, 24a, 24b, 11m, 13a, Iai and 12o. Studies suggest these brain regions constitute the origin of the descending projections to the hypothalamus and periaqueductal gray (PAG). The medial orbital prefrontal cortex observed in our study is located near area 11m and thus would project into the medial PAG and hypothalamus.
It is well known that the PAG plays a crucial role in the descending pain inhibition system. For instance, studies suggest that stimulation of specific regions of the midbrain, PAG and surrounding areas could inhibit pain responses to noxious stimulation in both animal studies [27,35] and patients suffering from pain disorders [1,10]. It has been hypothesized that acupuncture may somehow trigger this descending inhibition system to produce an analgesic effect. This hypothesis has been supported by experiments performed on animals, where it has been found that PAG lesions abolish acupuncture analgesia [39]. Thus, we speculate that fMRI and PET activation in the medial OFC may indicate the activation of the descending pain inhibition system.
Previous studies in healthy control subjects have demonstrated high uptake of [11C]diprenorphine in diffuse cortical areas known from postmortem studies to have high concentrations of opioid receptors [36], particularly in subcortical (thalamus) and cortical (insula, prefrontal, and ACC) components of the affective pain network [12]. Chronic pain studies have demonstrated decreased [11C]diprenorphine binding potential (BP: the ratio of receptor occupancy [Bmax] to affinity [KD]) in brain regions associated with the affective pain network (insula, ACC and frontal lobe) during pain states when compared to non-pain states [13,15,41]. Decreased [11C]diprenorphine BP is hypothesized to represent increased binding of opioid receptors by endogenous opioids. Conversely, [11C]diprenorphine BP in components of the affective pain network was significantly increased in a cohort of patients after surgical relief of trigeminal neuralgia pain [14], consistent with the hypothesis that relief of pain is associated with decreased endogenous opioid release. Recent studies using a high affinity mu-selective opiate agonist, [11C]carfentanil, and PET have demonstrated decreased mu opioid receptor BP in the thalamus and amygdala following administration of acute experimental noxious stimuli consistent with the hypothesis of pain-induced release of endogenous opioid peptides [2,48].
One limitation in our study is the small sample size. Although we found convergence with our two modalities of brain imaging tools only in the right medial OFC , there is no doubt that a whole network is involved in acupuncture analgesia. Thus, we speculate that if the sample size was dramatically increased, additional brain regions may show up as convergences. In any case, this pilot study suggests the potential power of using multiple imaging tools in acupuncture mechanism studies. It must additionally be noted that in order to maximize effective use of expensive neuroimaging resources, we only scanned subjects who responded to acupuncture in earlier sessions. This may limit how fully our data sample represents the general public. Further study is needed at this point.
In this experiment, given the unknown duration of acupuncture treatment effects, the first PET scan was always a baseline test and second PET scan was either a sham or active treatment, depending on a subject’s group randomization. Since our study only compared the difference between verum and placebo acupuncture, the order effect should not have significantly influenced our result.
In summary, common fMRI and PET signal changes may represent a specific marker for endogenous opioid driven changes in neural activity, a pharmacologically specific marker for fMRI signal change. Such studies may increase our understanding of which BOLD signal changes are associated with endogenous opioid release and which BOLD signal changes may be mediated by other neurotransmitter systems. Overall, these preliminary results suggest that integrative multimodal imaging studies have the potential to help elucidate the neural mechanisms of acupuncture.
Acknowledgements
Funding for this study came from: NIH (NCCAM) R21AT00949 and R21AT 001922 to Randy Gollub, PO1-AT002048 to Bruce Rosen/Randy Gollub, KO1AT003883 to Jian Kong, M01-RR-001066 for Mallinckrodt General Clinical Research Center Biomedical Imaging Core, P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR and the MIND Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baskin DS, Mehler WR, Hosobuchi Y, Richardson DE, Adams JE, Flitter MA. Autopsy analysis of the safety, efficacy and cartography of electrical stimulation of the central gray in humans. Brain Res. 1986;371:231–236. doi: 10.1016/0006-8993(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 2.Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99:589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- 3.Biella G, Sotgiu ML, Pellegata G, Paulesu E, Castiglioni I, Fazio F. Acupuncture produces central activations in pain regions. Neuroimage. 2001;14:60–66. doi: 10.1006/nimg.2001.0798. [DOI] [PubMed] [Google Scholar]
- 4.Cheng RS, Pomeranz BH. Electroacupuncture analgesia is mediated by stereospecific opiate receptors and is reversed by antagonists of type I receptors. Life Science. 1980;26:631–638. doi: 10.1016/0024-3205(80)90239-8. [DOI] [PubMed] [Google Scholar]
- 5.Cho ZH, Hwang SC, Wong EK, Son YD, Kang CK, Park TS, Bai SJ, Kim YB, Lee YB, Sung KK, Lee BH, Shepp LA, Min KT. Neural substrates, experimental evidences and functional hypothesis of acupuncture mechanisms. Acta Neurol Scand. 2006;113:370–377. doi: 10.1111/j.1600-0404.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- 6.Chou J, Tang J, Del Rio J, Yang H, Costa E. Action of peptidase inhibitors on methionine5-enkephalin-arginine6-phenylalanine7 (YGGFMRF) and methionine5-enkephalin (YGGFM) metabolism and on electroacupuncture antinociception. Journal of Pharmacology and Experimental Therapeutics. 1984;230:349–352. [PubMed] [Google Scholar]
- 7.Gracely RH, McGrath PA, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 8.Gracely RH, McGrath PA, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 9.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 10.Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 11.Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones A. The pain matrix and neuropathic pain. Brain. 1998;121:783–784. doi: 10.1093/brain/121.5.783. [DOI] [PubMed] [Google Scholar]
- 13.Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, Derbyshire S, Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. British Journal of Rheumatology. 1994;33:909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- 14.Jones AK, Kitchen ND, Watabe H, Cunningham VJ, Jones T, Luthra SK, Thomas DG. Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C] diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1999;19:803–808. doi: 10.1097/00004647-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Jones AK, Liyi Q, Cunningham VV, Brown DW, Ha-Kawa S, Fujiwara T, Friston KF, Silva S, Luthra SJ, ones T. Endogenous opiate response to pain in rheumatoid arthritis and cortical and subcortical response to pain in normal volunteers using positron emission tomography. International Journal of Clinical Pharmacology Research. 1991;11:261–266. [PubMed] [Google Scholar]
- 16.Kleinhenz J, Streitberger K, Windeler J, Gussbacher A, Mavridis G, Martin E. Randomised clinical trial comparing the effects of acupuncture and a newly designed placebo needle in rotator cuff tendinitis. Pain. 1999;83:235–241. doi: 10.1016/s0304-3959(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 17.Kong J, Fufa DT, Gerber AJ, Rosman IS, Vangel MG, Gracely RH, Gollub RL. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain. 2005;6:55–64. doi: 10.1016/j.jpain.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13:1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- 19.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong J, Gollub RL, Webb JM, Kong JT, Vangel MG, Kwong K. Test-retest study of fMRI signal change evoked by electro-acupuncture stimulation. Neuroimage. 2007:1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong J, Kaptchuk TJ, Webb JM, Kong JT, Sasaki Y, Polich GR, Vangel MG, Kwong K, Rosen B, Gollub RL. Functional neuroanatomical investigation of vision-related acupuncture point specificity-A multisession fMRI study. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20481. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong J, Ma L, Gollub RL, Wei J, Yang X, Li D, Weng X, Jia F, Wang C, Li F, Li R, Zhuang D. A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J Altern Complement Med. 2002;8:411–419. doi: 10.1089/107555302760253603. [DOI] [PubMed] [Google Scholar]
- 23.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Liu HL, Cheung RT, Hung YC, Wong KK, Shen GG, Ma QY, Yang ES. An fMRI study comparing brain activation between word generation and electrical stimulation of language-implicated acupoints. Hum Brain Mapp. 2003;18:233–238. doi: 10.1002/hbm.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu WC, Feldman SC, Cook DB, Hung DL, Xu T, Kalnin AJ, Komisaruk BR. fMRI study of acupuncture-induced periaqueductal gray activity in humans. Neuroreport. 2004;15:1937–1940. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- 26.Mayer DJ, Prince DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Research. 1977;121:368–372. doi: 10.1016/0006-8993(77)90161-5. [DOI] [PubMed] [Google Scholar]
- 27.Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- 28.McManus CA, Schnyer RN, Kong J, Nguyen LT, Hyun Nam B, Goldman R, Stason WB, Kaptchuk TJ. Sham acupuncture devices - practical advice for researchers. Acupunct Med. 2007;25:36–40. doi: 10.1136/aim.25.1-2.36. [DOI] [PubMed] [Google Scholar]
- 29.Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2004;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 31.Pariente J, White P, Frackowiak RS, Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage. 2005;25:1161–1167. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Peets JM, Pomeranz B. CXBK mice deficient in opiate receptors show poor electroacupuncture analgesia. Nature. 1978;273:675–676. doi: 10.1038/273675a0. [DOI] [PubMed] [Google Scholar]
- 33.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 34.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 36.Sadzot B, Price JC, Mayberg HS, Douglass KH, Dannals RF, Lever JR, Ravert HT, Wilson AA, Wagner HNJ, Feldman MA, et al. Quantification of human opiate receptor concentration and affinity using high and low specific activity [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1991;11:204–219. doi: 10.1038/jcbfm.1991.52. [DOI] [PubMed] [Google Scholar]
- 37.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364–365. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 38.Stux G. Channels, Organs, and Points. In: Stux G, Pomeranz B, editors. Basics of Acupuncture. Berlin: Springer-Verlag; 1997. pp. 84–194. [Google Scholar]
- 39.Wang QA, Mao LM, Han JS. The role of periaqueductal gray in mediation of analgesia produced by different frequencies electroacupuncture stimulation in rats. Int J Neurosci. 1990;53:167–172. doi: 10.3109/00207459008986598. [DOI] [PubMed] [Google Scholar]
- 40.White P, Lewith G, Hopwood V, Prescott P. The placebo needle, is it a valid and convincing placebo for use in acupuncture trials? A randomised, single-blind, cross-over pilot trial. Pain. 2003;106:401–409. doi: 10.1016/j.pain.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Willoch F, Tolle TR, Wester HJ, Munz F, Petzold A, Schwaiger M, Conrad B, Bartenstein P. Central pain after pontine infarction is associated with changes in opioid receptor binding: a PET study with 11C-diprenorphine. AJNR American Journal of Neuroradiology. 1999;20:686–690. [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M-T, Hsieh J-C, Xiong J, Yang P-C, Pan H-B, Chen Y-CI, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain-preliminary experience. Radiology. 1999;212:133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- 43.Wu MT, Sheen JM, Chuang KH, Yang P, Chin SL, Tsai CY, Chen CJ, Liao JR, Lai PH, Chu KA, Pan HB, Yang CF. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. Neuroimage. 2002;16:1028–1037. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- 44.Yoo SS, Teh EK, Blinder RA, Jolesz FA. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. Neuroimage. 2004;22:932–940. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, Zeng YW, Luo F, Chen AC, Han JS. Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res. 2003;982:168–178. doi: 10.1016/s0006-8993(03)02983-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang WT, Jin Z, Huang J, Zhang L, Zeng YW, Luo F, Chen AC, Han JS. Modulation of cold pain in human brain by electric acupoint stimulation: evidence from fMRI. Neuroreport. 2003;14:1591–1596. doi: 10.1097/00001756-200308260-00010. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage. 2003;18:975–989. doi: 10.1016/s1053-8119(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 48.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]