Abstract
Chitin synthase III (CSIII), an enzyme required to form a chitin ring in the nascent division septum of Saccharomyces cerevisiae, may be transported to the cell surface in a regulated manner. Chs3p, the catalytic subunit of CSIII, requires the product of CHS6 to be transported to or activated at the cell surface. We find that chs6Δ strains have morphological abnormalities similar to those of chs3 mutants. Subcellular fractionation and indirect immunofluorescence indicate that Chs3p distribution is altered in chs6 mutant cells. Order-of-function experiments using end4–1 (endocytosis-defective) and chs6 mutants indicate that Chs6p is required for anterograde transport of Chs3p from an internal endosome-like membrane compartment, the chitosome, to the plasma membrane. As a result, chs6 strains accumulate Chs3p in chitosomes. Chs1p, a distinct chitin synthase that acts during or after cell separation, is transported normally in chs6 mutants, suggesting that Chs1p and Chs3p are independently packaged during protein transport through the late secretory pathway.
INTRODUCTION
Three temporally and spatially regulated chitin synthesis events occur during the Saccharomyces cerevisiae cell cycle (Shaw et al., 1991; Bulawa, 1993; Cid et al., 1995). Chitin synthase III activity (CSIII) is required early in the cell cycle for chitin ring formation and later in the cell cycle for lateral cell wall chitin synthesis (Shaw et al., 1991). Late in mitosis, chitin synthase II activity (CSII) is required for primary septum formation (Sburlati and Cabib, 1986; Silverman et al., 1988). After cell separation, chitin synthase I (CSI) activity is required for cell wall repair in the birth scar (Cabib et al., 1989). The catalytic subunits of CSI, CSII, and CSIII are Chs1p, Chs2p, and Chs3p, respectively. These proteins are integral membrane proteins that are transported to the plasma membrane via the secretory pathway.
Chs2p is synthesized late in the cell cycle, delivered apparently directly to the septum plasma membrane, and rapidly internalized into the vacuole where it is degraded (Choi et al., 1994; Chuang and Schekman, 1996). In contrast, Chs3p and Chs1p are synthesized continuously and remain metabolically stable (Choi et al., 1994; Chuang and Schekman, 1996; Ziman et al., 1996). Unlike constitutively secreted proteins in yeast, Chs1p and Chs3p are distributed between an intracellular membrane compartment, called the chitosome, and the plasma membrane (Flores Martinez and Schwencke, 1988; Leal-Morales et al., 1988; Chuang and Schekman, 1996; Ziman et al., 1996). The chitosome may serve as a cell cycle-regulated reservoir of these enzymes, ready to be mobilized when their function is required. Transport of Chs3p to the site of incipient chitin ring formation may be mediated by a branch of the secretory pathway involving vesicular traffic from the endosome to the plasma membrane (Chuang and Schekman, 1996; Ziman et al., 1996).
Several genes are required for CSIII activity or localization (Bulawa, 1993). CHS4 encodes a posttranslational activator of CSIII (Trilla et al., 1997). CHS5 is required for the localization of Chs3p to polarized rings on the cell surface (Santos and Snyder, 1997). CHS4, CHS5, and CHS6 are each required for CSIII activity in vivo; however, extracts of chs6 mutant cells display CSIII activity, whereas membranes isolated from chs4 or chs5 cells do not (Bulawa, 1992). This discrepancy suggested that Chs6p might be required to transport CSIII to a site on the cell surface where its activity could be optimally expressed. We report here that chs6 mutants are specifically defective in transport of Chs3p, but not of Chs1p, from the chitosome to the plasma membrane.
MATERIALS AND METHODS
Strains and Plasmids
CHS6 was amplified by PCR of genomic DNA from strain YPH499 (Sikorski and Hieter, 1989) using oligonucleotide primers homologous to the upstream (5′-cgcggatccgcgggacaaggtgccaggtaatag) and downstream (5′-gtccgctcgagcgggcgtacctttcccaaatacac) untranslated regions of the gene. The amplified DNA was cloned into pBluescript II SK+ (Stratagene, La Jolla, CA) using the BamHI and XhoI sites incorporated into the ends of the upstream and downstream primers, respectively, and in the polylinker. The 360-base pair (bp) EcoRI fragment that is internal to the coding region of the gene was replaced with a 950-bp EcoRI fragment containing the TRP1 gene that was isolated from plasmid pJJ246 (Jones and Prakash, 1990). Strain Z128 (Z106–2B × Z107–11A; see Table 1) was transformed with a linear DNA fragment containing the chs6::TRP1 gene, and a transformant was sporulated for tetrad analysis. Tryptophan prototrophy segregated 2:2 and cosegregated with resistance to 100 μg/ml Calcofluor (Sigma Chemical, St. Louis, MO), yielding strains Z130–1A and Z130–1D. DNA-DNA blot hybridization experiments confirmed homologous recombination only in the two Trp+ meiotic products (our unpublished observations).
Table 1.
Strains used in this study
| Name | Genotype | Reference |
|---|---|---|
| KFC1-4C | MATa chs6::TRP1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| Y1307 | MATα CHS3-3XHA ade2-101 his3Δ-200 lys2-801 trp1-901 ura3-52 | Santos et al. (1997) |
| JCY478 | MATα chs6::TRP1 ade2 his3 leu2 lys2 trp1 ura3 | This study |
| JCY479 | MATa CHS3-3XHA chs6::TRP1 ade2 his3 leu2 lys2 trp1 ura3 | This study |
| JCY480 | MATα CHS6 ade2 his3 leu2 lys2 trp1 ura3 | This study |
| JCY481 | MATa CHS3-3XHA CHS6 ade2 his3 leu2 lys2 trp1 ura3 | This study |
| Z130-1Aa | MATa MYC-CHS1 HA-PMA1 ade2-101 his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| Z130-1D | MATa chs6::TRP1 MYC-CHS1 HA-PMA1 ade2-101 his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| YPH499 | MATa ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | Sikorski and Hieter (1989) |
| YPH500 | MATα ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | Sikorski and Hieter (1989) |
| Z128 | MATa MYC-CHS1 HA-PMA1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| MATα MYC-CHS1 HA-PMA1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | ||
| Z125-6Cb | MATa end4-1 MYC-CHS1 HA-PMA1 ade2 leu2 lys2-801 trp1-Δ63 ura3 | This study |
| Z106-2B | MATa MYC-CHS1 HA-PMA1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | Ziman et al. (1996) |
| Z131b | MATa end4-1 chs6::TRP1 MYC-CHS1 HA-PMA1 ade2 leu2 lys2-801am trp1-Δ63 ura3 | This study |
| Z132-2Ab | MATa end4-1 CHS6 ade2 MYC-CHS1 HA-PMA1 leu2 lys2-801am trp1-Δ63 ura3 | This study |
| Z132-2Bb | MATa END4 CHS6 ade2 MYC-CHS1 HA-PMA1 leu2 lys2-801am trp1-Δ63 ura3 | This study |
| Z132-2Cb | MATa end4-1 chs6::TRP1 ade2 MYC-CHS1 HA-PMA1 leu2 lys2-801am trp1-Δ63 ura3 | This study |
| Z132-2Db | MATa END4 chs6::TRP1 ade2 MYC-CHS1 HA-PMA1 leu2 lys2-801am trp1-Δ63 ura3 | This study |
| Z134-5A | MATa chs6::TRP1 HA-PMA1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| Z106-2C | MATa HA-PMA1 ade2-101oc his3-Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 | This study |
| Z136 | Z106-2C [pRS306(7MYC-CHS6)] | This study |
MYC-CHS1 is marked by URA3 and is a next-door insertion to chs1::HIS3. HA-PMA1 is marked by LEU2.
his3 genotype obscured by HIS3 integrated with epitope tag, not determined.
To create an end4–1 chs6::TRP1 double mutant strain, Z125 was sporulated and an end4–1 trp1 segregant, Z125–6C, was transformed with the linear DNA fragment containing chs6::TRP1, yielding strain Z131. Homologous recombination in this strain was confirmed by DNA-DNA blot hybridization experiments (our unpublished data). Z131 was crossed to Z106–2B yielding Z132, which was sporulated yielding strains Z132–2A, 2B, 2C, and 2D (a tetratype with respect to the end4–1 and chs6::TRP1 alleles).
Z130–1D MATa chs6::TRP1 was crossed to YPH500, yielding strain Z134–5A. The full-length CHS6 gene on a BamHI–XhoI fragment was subcloned into pRS316 (Sikorski and Hieter, 1989), resulting in plasmid pRS316(CHS6), and introduced into Z134–5A. Analysis of transformants indicated the full-length CHS6 gene complemented the Calcofluor-resistant phenotype of the chs6::TRP1 mutant.
MYC-CHS6 was created by adding the myc epitope (Kolodziej and Young, 1991) to pRS316(CHS6) using the primer 5′-gcaaaaaaggattacatctattgcccttatggaagaacaaaagcttatttctgaagaagatttgttgagaaagggatccttttggccatcggaaaccaaaaagcaaaatgaaatacc and an oligonucleotide-directed mutagenesis protocol (Kunkel, 1985) yielding plasmid pRS316(MYC-CHS6). A SpeI–EcoRI fragment containing the myc epitope-tagged CHS6 gene was inserted into pRS306 (Sikorski and Hieter, 1989) yielding plasmid pRS306(MYC-CHS6). MYC-CHS6 on a BglII fragment was isolated from pRS316(CHS6) and inserted into the BamHI site of plasmid pRS313 (Sikorski and Hieter, 1989) resulting in pRS313(MYC-CHS6). This CHS6 DNA had less 5′-untranslated sequence but retained complementing activity. To improve the detection of Myc-Chs6p, 6 myc epitope containing DNA was amplified by PCR from plasmid pJR1265 (Ziman et al., 1996) using the primers 5′-cgggatccccccctcgaggtcgacggtatcg and 5′-cgggatccccgctctagaactagtggttcccccgggctgcagga. This PCR product has BamHI sites at its termini allowing insertion into pRS313(MYC-CHS6), yielding pRS313(7MYC-CHS6). A HindIII fragment containing the N-terminal portion of 7MYC-CHS6 was used to replace the HindIII fragment of pRS306(MYC-CHS6) resulting in pRS306(7MYC-CHS6). This integration plasmid was linearized with NruI and used to transform Z106–2C to uracil prototrophy resulting in strain Z136, which has 7MYC-CHS6 as the sole copy of CHS6 in the genome.
JCY478, JCY479, JCY480, and JCY481 were spores from a tetratype tetrad with respect to CHS3–3XHA and chs6::TRP1 derived from a cross between KFC1–4C and Y1307.
Cell Fractionation and Gradient Analysis
Methods for converting cells to spheroplasts, Con A-coating, and lysis were essentially as described previously (Con A fractionation; Chuang and Schekman, 1996; Ziman et al., 1996). Briefly, cell growth was terminated with 10 mM NaN3 and 10 mM KF on ice, treated with 100 mM Tris, pH 9.4, 10 mM DTT at room temperature for 10 min, and converted to spheroplasts using 60 U lyticase per OD600 cell equivalents (Shen et al., 1991) for 15–40 min at 30°C. For Con A fractionation experiments, cells were incubated in 1 mg/ml Con A for 10 min at room temperature. Cells were osmotically lysed using 20 mM triethanolamine, pH 7.2, 1 mM EDTA, 5 or 12.5% (wt/wt) sucrose (lysis buffer), and protease inhibitors as described (Ziman et al., 1996). For the experiments in Figures 2 and 3, a low-speed (500 × g) supernatant fraction was applied to either a linear sucrose equilibrium or a linear Ficoll velocity gradient (Rexach et al., 1994) in the same buffer. Gradients (5.2 ml) were constructed in 13 × 51 mm Beckman Ultraclear tubes (Beckman, Fullerton, CA) and centrifuged in a Beckman SW55 rotor. Equilibrium gradients were centrifuged for 20 h at 250,000 × g, and velocity gradients were centrifuged for 2 h at 100,000 × g. Fractions (0.2 ml, Figure 2; 0.4 ml, Figure 3) were collected from the top using an Isco gradient fractionator (Isco, Lincoln, NE). Samples were evaluated by SDS-PAGE and immunoblot using ECL (Amersham, Arlington Heights, IL). Fluorograms were scanned using a Microtek ScanMaker III flatbed scanner (Microtek Lab, Redondo Beach, CA), and pixel density was determined using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). For the experiment in Figure 8, 100 OD600 cell equivalents were lysed in 1 ml lysis buffer. An aliquot (750 μl) of the 10,000 × g supernatant fraction was adjusted to 40% (wt/vol) Nycodenz using an equal volume of 80% (wt/vol) Nycodenz. This mixture was transfered to the bottom of a Beckman Ultraclear tube, overlaid with 700 μl of 35% (wt/vol) Nycodenz, followed by 200 μl of lysis buffer. Gradients were centrifuged at 55,000 rpm in a TLS55 rotor for 3 h. Four 0.1-ml fractions were collected from the top by hand.
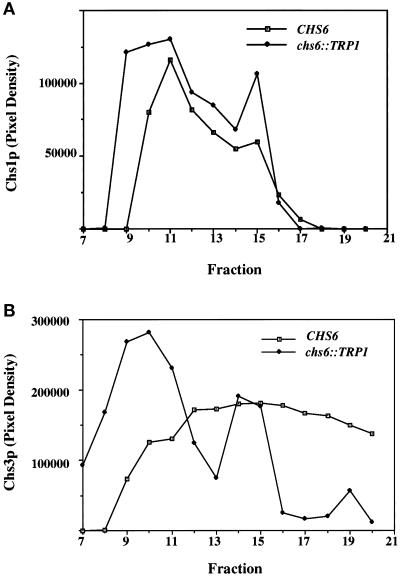
Figure 2.
Equilibrium density gradient analysis of membranes from chs6 cells. Z130–1D (chs6) cells were converted to spheroplasts and lysed, and a 500 × g supernatant fraction was sedimented to equilibrium in a linear sucrose density gradient. Gradient fractions were collected from the top and analyzed by SDS-PAGE and immunoblot. Films were quantified by densitometry. (A) Fractions were analyzed for Chs1p. (B) Fractions were analyzed for Chs3p.
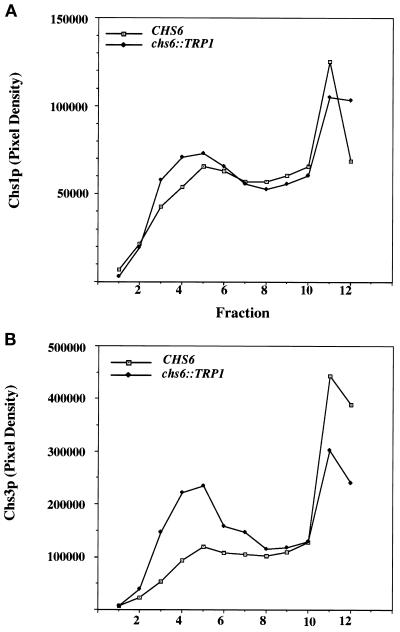
Figure 3.
Velocity gradient analysis of membranes from chs6 cells. Z130–1D (chs6) cells were converted to spheroplasts and lysed, and a 500 × g supernatant fraction was sedimented on a linear Ficoll gradient for 2 h. Gradient fractions were collected from the top and analyzed as in Figure 2. (A) Fractions were analyzed for Chs1p. (B) Fractions were analyzed for Chs3p.
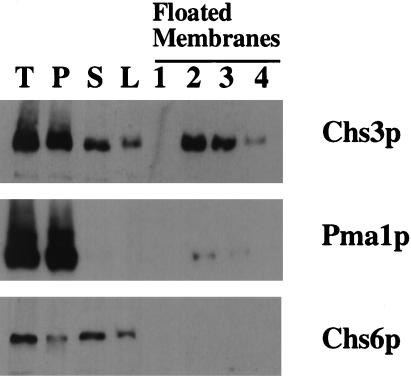
Figure 8.
Subcellular distribution of Chs6p. 7MYC-CHS6 (Z136) cells were grown to midlog phase and processed by Con A fractionation. The membranes in the 10,000 × g supernatant fraction were adjusted to 40% (wt/vol) Nycodenz and floated through a layer of 35% (wt/vol) Nycodenz. T, P, and S indicate the total, 10,000 × g pellet, and supernatant fractions, respectively. L indicates the load sample for the flotation gradient, and 1–4 represent 100 μl fractions from the top of the gradient.
Cell Surface Labeling with 2,4,6-Trinitrobenezenesulfonic Acid (TNBS)
Metabolic labeling with 35S-ProMix (Amersham) cell lysis using glass beads, and immunoprecipitation were performed essentially as described (Chuang and Schekman, 1996). Radiolabeled cells (2.5 to 5 OD600 units) were treated with 0.7 M sorbitol, 100 mM Tris, pH 9.4, 10 mM DTT, 10 mM NaN3 for 15 min at 25°C. All subsequent steps were performed on ice in siliconized Eppendorf tubes. Cells were washed with pre-TNBS buffer (0.7 M sorbitol, 0.3 M NaCl, 0.25 mM CaCl2, 50 mM sodium phosphate, pH 7.5), incubated in 0.7 M sorbitol, 10 mM TNBS (Sigma, St. Louis, MO), 0.25 mM CaCl2, 100 mM sodium bicarbonate, pH 9.2, for 30 min, washed with post-TNBS buffer (0.7 M sorbitol, 0.3 M NaCl, 20 mM sodium phosphate, pH 7.5), before lysis with glass beads (Chuang and Schekman, 1996). Antisera to Chs3p (Chuang and Schekman, 1996), Cell Wall Protein (CWP; Sanz et al., 1987), and Sec61p (Stirling et al., 1992) were used in the first immunoprecipitation. The resulting immunoprecipitates were heated at 65°C in 100 μl 2% SDS; one half was reprecipitated with the same antibody and the other half was precipitated with rabbit anti-DNP antiserum (Sigma). Immunoprecipitates were analyzed by SDS-PAGE followed by quantification on a PhosphorImager (Molecular Dynamics).
Electron Microscopy (EM)
Yeast cells were fixed for EM by rapid freezing with a Bal-Tec high pressure freezer (Kiss and Staehelin, 1995), followed by freeze substitution in anhydrous acetone containing 1% OsO4 and 0.1% uranyl acetate (McDonald, 1994). Samples were dehydrated and embedded in Epon-araldite. Thin sections were stained with 2% uranyl acetate and lead citrate and examined in a Jeol 1200 electron microscope (Jeol, Tokyo, Japan).
Indirect Immunofluorescence Microscopy
Cells were grown in YPD to midlog (OD600 = 0.5–1.0) and fixed by addition of 37% (vol/vol) formaldehyde to 5% (vol/vol). The cultures were gently shaken at 25°C for 15 min before centrifugation, cells were resuspended in 4% paraformaldehyde (dissolved in 0.045 N NaOH and then buffered with 100 mM KH2PO4, final pH is 6.5) and incubated at 25°C for 2 h, or overnight at 4°C. The fixed cells were washed twice with PBS, incubated in SKβME (1.2 M sorbitol, 100 mM potassium phospate, pH 7.5, 25 mM β-mercaptoethanol) for 5 min at 25°C, centrifuged, and resuspended to 5 OD600/ml in SKβME. Zymolyase-20T (ICN Pharmaceuticals, Costa Mesa, CA) was added at 100 μg/ml and spheroplasts were formed during an incubation at 30°C for 30 min. All subsequent centrifugations were done in a microcentrifuge at 7000 rpm. Spheroplasts were washed once in 1.2 M sorbitol and resuspended in 750 μl 1.2 M sorbitol. An aliquot (250 μl) of 8% SDS in 1.2 M sorbitol was added, and the tubes were inverted several times, immediately centrifuged, and washed three times with 1.2 M sorbitol.
Fixed spheroplasts were attached to slides that had been pretreated with 2% polyethyleneamine. Cells were blocked in BB (PBS with 10 mg/ml BSA and 0.05% Tx-100). Mouse HA.11 anti-HA mAb (BAbCo, Berkeley, CA) was used at a 1:1500 dilution (in BB) for 45 min at 25°C. Cy3 (indocarbocyanine)-conjugated donkey anti-mouse secondary Ab (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at a 1:500 dilution (in BB) for 30 min at 25°C. The slides were washed with PBS after each antibody incubation. Samples on slides were stained with 30 ng/ml DAPI (in water) for 5 min, dipped into water, mounted in Citifluor (Ted Pella, Redding, CA), and examined with a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Thornwood, NY). Images were captured with a 200-E CCD camera (Sony Electronics, San Jose, CA).
RESULTS
Morphological Abnormality of chs6 Null Strains
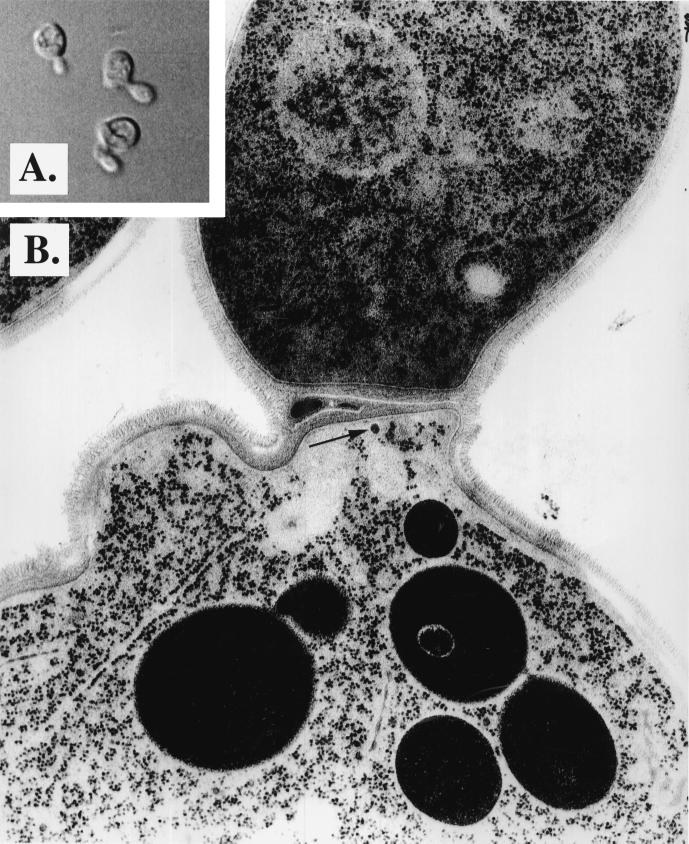
CSIII accounts for 90% of the cell wall chitin; thus, a defect in synthase activation or transport could have morphological consequences (Shaw et al., 1991). To examine the ultrastructural phenotype of a chs6 null strain, the CHS6 gene was replaced with the chs6::TRP1 gene (see MATERIALS AND METHODS). Meiotic segregants from a chs6::TRP1 heterozygote were analyzed by growth under several conditions. Trp+ prototrophy segregated 2:2 and cosegregated with slow growth at 37°C and resistance to the chitin-binding cellular poison, Calcofluor. Cells grown in complex liquid medium to midlog phase and viewed by light microscopy displayed substantial abnormalities, including 25% exhibiting cell surface bumps, 17% exhibiting bent necks, and 8% exhibiting other abnormal morphologies (Figure 1A). These phenotypes were not seen in the isogenic CHS strain (our unpublished data). Examination of mutant cells by electron microscopy revealed the cell surface bumps to be protrusions from the cell and not normal buds (Figure 1B). These protrusions may represent aborted buds, destabilized regions of the cell wall, or simply new buds on cells with incomplete cell separation. Septa appeared thicker than normal. Membrane transport defects, if any, were not apparent. Occasionally, some ∼50 nm vesicles were observed to accumulate near the septum (Figure 1B, arrow).
Figure 1.
Morphological characterization of chs6 strains. (A) chs6 cells (Z130–1D) were grown to midlog phase in YPD medium and examined by light microscopy. (B) The same cells were fixed and processed for electron microscopy (see MATERIALS AND METHODS).
Subcellular Distribution of Chs1p and Chs3p in chs6 Strains
On the basis of the observation that chs6 cells are defective for CSIII activity in vivo, Chs6p was suggested to function in the transport of Chs3p to its site of activation (Bulawa, 1993). To examine the distribution of Chs3p in these cells, we fractionated total membranes either by equilibrium bouyant density or by velocity sedimentation (see MATERIALS AND METHODS). In wild-type cells, both Chs3p (Figure 2A) and the plasma membrane ATPase, Pma1p (our unpublished data), were broadly distributed. In contrast, Chs3p shifted to a bimodal distribution in membranes from the chs6 strain, with most of the Chs3p fractionating at a lower density characteristic of chitosomes (fraction 10, Figure 2B). The high density peak of Chs3p (fraction 15) cofractionated with a peak of membranes containing Pma1p (our unpublished data: see DISCUSSION).
A velocity sedimentation comparison of chs6 and wild-type membranes revealed an increase in slowly sedimenting Chs3p (Figure 3B), characteristic either of chitosomes or mature secretory vesicles. Chitosomes rather than secretory vesicles are indicated because Chs3p accumulated in a late sec mutant (e.g., sec1) fractionates at a higher density than Chs3p detected in chitosomes (Chuang and Schekman, 1996).
Surprisingly, equilibrium and velocity sedimentation demonstrated little effect of chs6 on the distribution of Chs1p (Figures 2A and 3A). In equilibrium density gradients from wild-type cells, Chs1p and Chs3p exhibited distinct fractionation patterns. Therefore, we suggest that Chs6p is required selectively for the transport of Chs3p from the chitosome.
Chs3p Is Redistributed from Cell Surface Rings to Internal Patches in chs6 Mutants
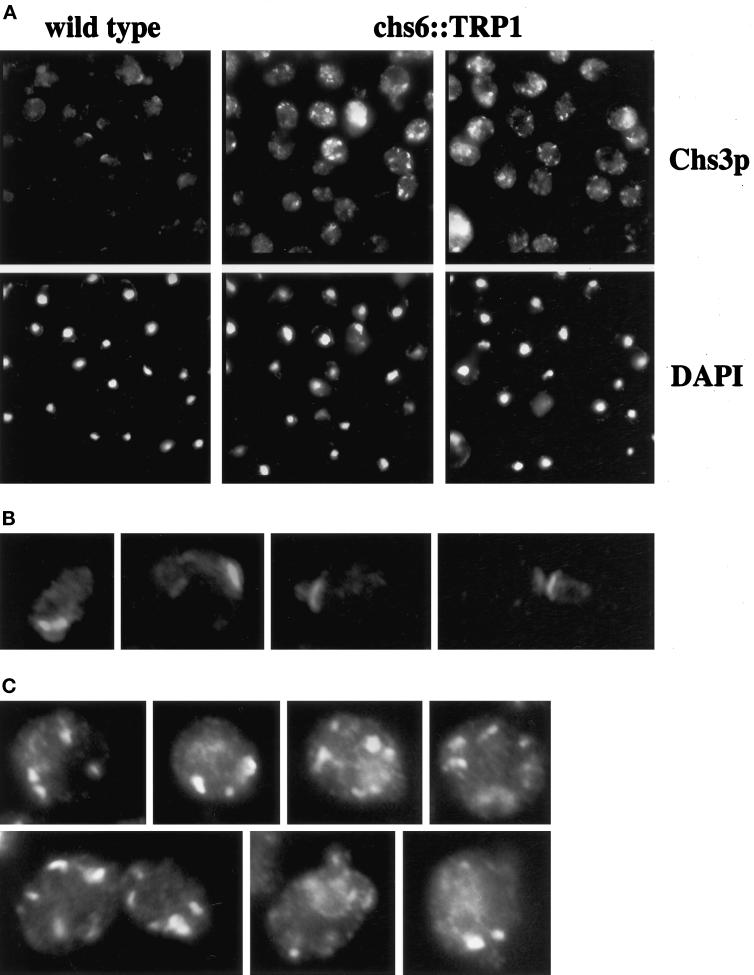
To corroborate our observation of an altered Chs3p distribution in subcellular fractionation experiments, we analyzed the localization of Chs3p in chs6 null mutant cells by indirect immunofluorescence. For these experiments, we used an epitope-tagged (HA) version of Chs3p in cells whose chromosomal locus was replaced with CHS3–3XHA (Santos and Snyder, 1997). The localization pattern of Chs3p determined with affinity-purified anti-Chs3p antibodies and of the epitope-tagged version determined with an anti-HA antibody were indistinguishable (Chuang and Schekman, 1996; Santos and Snyder, 1997).
CHS3–3XHA-tagged chs6 and wild-type strains were prepared and stained in parallel with untagged control strains. In wild-type cells, Chs3p-3XHA localized primarily to cell surface rings at the incipient bud site in large unbudded cells and at the mother-bud neck in small budded cells and some large budded cells (Figure 4, A and B). As previously noted (Chuang and Schekman, 1996; Santos and Snyder, 1997), Chs3p-3XHA did not display ring localization in medium budded cells. Many cells also had a few larger internal patches that were more easily observed in longer exposures.
Figure 4.
Indirect immunofluorescence localization of Chs3p in wild-type and chs6 cells. Wild-type (JCY481) (A and B) and chs6 (JCY479) (A and C) cells carrying the integrated CHS3–3XHA gene were grown at 25°C, fixed, and labeled with anti-HA mAbs in combination with a Cy3-conjugated secondary Ab followed by DAPI staining. (A) Lower magnification view of Chs3p and DAPI staining in wild-type (left) and chs6 (middle, right). (B) Close-up of four wild-type cells showing cell-surface staining of Chs3p at the incipient bud site of large unbudded cells and at the mother-bud neck of small-budded and large-budded cells. (C) Close-up of seven chs6 cells showing Chs3p localization in internal patches. The exposure time for all Chs3p panels was the same.
The localization of Chs3p-3XHA in the chs6 mutant was dramatically altered. Chs3p-3XHA was not found in the cell surface ring characteristic of wild-type at any cell cycle stage (Figure 4, A and C). Instead, Chs3p-3XHA was restricted to large internal patches, clearly more abundant than in wild-type cells (Figure 4). In addition, the patches in chs6 were more intensely stained; immunoblot analysis did not indicate differences in Chs3p levels (our unpublished data). Patches sometimes appeared to be peripheral to the plasma membrane (Figure 4C). For both strains, the staining observed was specific, because no labeling was detected in control strains lacking the epitope tag (our unpublished data). Thus, the loss of CHS6 results in a redistribution of Chs3p to internal patches, at the expense of localization to its normal position in cell-surface rings.
Chs6p Is Required for Transport of Newly Synthesized Chs3p to the Plasma Membrane
The altered subcellular distribution of Chs3p in chs6 mutants prompted us to investigate the transport dependence of newly synthesized Chs3p on Chs6p function. We adapted a procedure (Novick and Schekman, 1983) to tag cell surface-exposed proteins using the membrane-impermeant reagent TNBS. Reaction of TNBS with primary amines results in covalent addition of trinitrophenyl (TNP) groups, which can be detected using cross-reactive antibodies against dinitrophenyl (DNP). In a typical TNBS-tagging experiment (see MATERIALS AND METHODS), cells were radiolabeled briefly with 35S-Promix, chased for various times, treated with TNBS, and lysed. Extracts were subjected to immunoprecipitation using antibodies directed against a protein of interest. The resulting immunoprecipitate was split and either reprecipitated with the same antibody (representing a total fraction) or precipitated with anti-DNP antibodies (representing the cell surface-exposed fraction). The amount of the protein localized to the cell surface was measured by dividing the anti-DNP–reactive signal by the total signal after SDS-PAGE and quantification with a PhosphorImager.
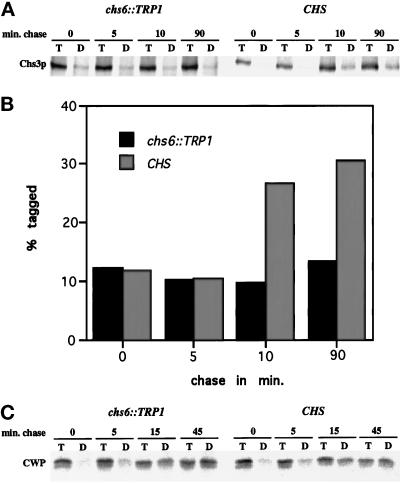
Initially, we analyzed the specificity of the TNBS-tagging procedure for cell surface-localized Chs3p. The ER integral membrane protein Sec61p (Stirling et al., 1992) and an abundant 33-kDa cell wall protein (CWP) (Sanz et al., 1987) were used as negative and positive controls, respectively. These experiments established that a fraction of Chs3p (varying from 20 to 40%) and a larger fraction of CWP (80–100%), could be precipitated with the anti-DNP antibody, but only if the cells had been treated with TNBS (our unpublished data). Importantly, if cells were harvested immediately after a radiolabeling pulse of 3 min (0 min chase), the level of TNBS tagging was significantly reduced when compared with chase points longer than 10 min (Figure 5). At the 0-min chase point, a majority of newly synthesized Chs3p and CWP is expected to be in transit, and thus inaccessible by TNBS. In all experiments, Sec61p was not tagged above background levels, since the amount of tagged Sec61p in the 0 min and in later chase points remained at a constant, low level (∼5%) (see Figure 7). Other experiments demonstrated the Sec61p could be tagged if the permeability of the cells was compromised (our unpublished data). We concluded that our adaptation of the TNBS procedure detected only cell surface-exposed proteins, consistent with the results of Novick and Schekman (1983).
Figure 5.
CHS6-dependent and time-dependent cell surface labeling of newly synthesized Chs3p. (A) Strains Z130–1D (chs6) and Z130–1A (CHS) were labeled with 35S-Promix for 3 min at 25°C and chased for the indicated times. After cell surface proteins were tagged with TNBS, cells were lysed and immunoprecipitated with anti-Chs3p Abs, and the resulting precipitate was divided in half and either reprecipitated with anti-Chs3p Abs (T) or anti-DNP Abs (D) and analyzed by SDS-PAGE. (B) Quantification of (A) after PhosphorImager analysis. (C) Strains Z130–1D (chs6) and Z130–1A (CHS) were analyzed as above, but metabolic labeling was for 4 min and anti-CWP Abs were used instead of anti-Chs3p Abs.
Figure 7.
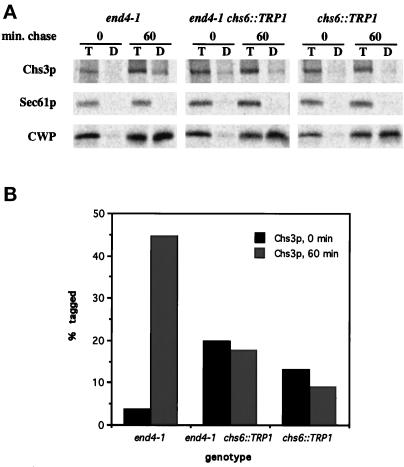
Comparison of cell surface labeling of newly synthesized Chs3p in end4–1, end4–1 chs6, and chs6 strains. (A) Strains Z132–1A (end4–1), Z132–1C (end4–1 chs6), and Z132–1D (chs6) were shifted to 37°C for 10 min, radiolabeled at 37°C for 2 min, and chased for 0 or 60 min. Chs3p, Sec61p, and CWP were analyzed by the TNBS-tagging procedure (see Figure 5). (B) Quantification of (A) after PhosphorImager analysis.
To determine whether loss of Chs6p affects the transport of newly synthesized Chs3p, we radiolabeled chs6 and wild-type cells at 25°C for 3 min, chased samples for various times, and subjected cells to TNBS tagging for analysis of Chs3p. Tagging of Chs3p in wild-type cells increased over time, with efficiencies of 20–30% after 90 min of chase (Figure 5, A and B). In contrast, tagging of Chs3p in chs6 was reduced (5–10%), and the lower level of tagging did not increase during the chase (Figure 5, A and B). Notably, tagging of CWP did not differ between chs6 and wild-type (Figure 5C), and a comparison of the most abundant anti-DNP precipitable proteins in TNBS-tagged chs6 versus wild-type cells showed no major differences. Additionally, the secretory pathway-dependent maturation of carboxypeptidase Y was not significantly altered in chs6 mutant cells (our unpublished data). These results suggest that Chs6p is specifically involved in the transport of newly synthesized Chs3p to the cell surface.
CHS6 Acts before END4 in the Transport of Newly Synthesized Chs3p
Accumulation of Chs3p in chitosomes of chs6 strains provided the opportunity to analyze the route of transport of this protein in the late secretory pathway. In previous experiments with end4–1, a mutant defective in the internalization step of endocytosis (Raths et al., 1993), Chs1p and Chs3p accumulated in the plasma membrane, suggesting that END4 function was required for Chs3p and Chs1p transport to or retention in chitosomes. These results were consistent with the vesicle-mediated transport of newly synthesized Chs3p either directly from the Golgi to the plasma membrane or indirectly via the chitosome (Chuang and Schekman, 1996; Ziman et al., 1996).
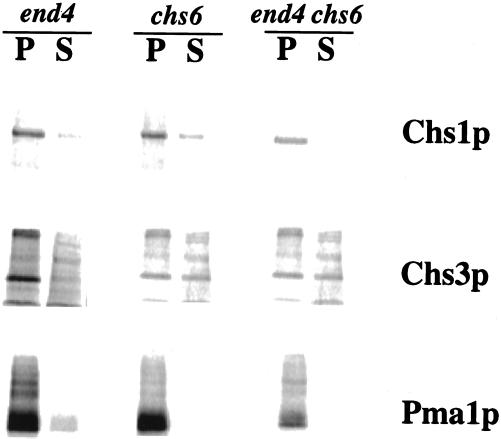
To distinguish between these possibilities, we used either differential centrifugation of lysates from metabolically labeled cells (Con A fractionation; Chuang and Schekman, 1996; Ziman et al., 1996) or cell-surface labeling (see MATERIALS AND METHODS) to analyze chs6 end4–1 double mutants and the respective single mutants. The Con A fractionation procedure generates a plasma membrane-free 10,000 × g supernatant fraction containing chitosomes (Chuang and Schekman, 1996; Ziman et al., 1996). Consistent with gradient fractionation data (Figures 2 and 3), we detected more Chs3p, but not Chs1p, in the 10,000 × g supernatant fraction from chs6 strains compared with wild-type (our unpublished data). As before (Chuang and Schekman, 1996; Ziman et al., 1996), end4–1 strains were impaired for transport of Chs1p and Chs3p to chitosomes recovered in the 10,000 × g supernatant fraction (Figure 6). A low level of Pma1p was found in the 10,000 × g supernatant fraction of the end4–1 strain, but not of the other two strains, indicating some plasma membrane contamination during the fractionation of this mutant. This may account for the small amount of contaminating Chs1p in the supernatant fractions of end4–1 that was not seen in previous experiments (Ziman et al., 1996). Cell lysis, as judged by the release of radiolabeled proteins into the 10,000 × g supernatant fraction, was similar for all strains (our unpublished data). A significant level of Chs3p, but not of Chs1p, was recovered in the supernatant fraction of the chs6 end4–1 double mutant strain (Figure 6). These data indicate that in regard to the recovery of Chs3p in chitosomes, the phenotype of chs6 is epistatic to that of end4 and specific to Chs3p, but not Chs1p.
Figure 6.
Pulse-chase fractionation of membranes from a end4–1 chs6 double mutant. Cells were incubated at 37°C for 10 min, pulse-labeled with 35S-Promix for 10 min, and chased for 30 min. Membranes were separated by differential centrifugation (Con A fractionation) and proteins were evaluated by immunoprecipitation, SDS-PAGE, and PhosphorImager analysis. P indicates the 10,000 × g pellet, and S indicates the 10,000 × g supernatant.
The end4–1, end4–1 chs6, and chs6 strains were also analyzed by the TNBS cell surface-labeling procedure. Cells were shifted to 37°C for 10 min, radiolabeled for 2 min, and chased for 0 or 60 min. Each sample was treated with TNBS, and the amount of anti-DNP-precipitable Chs3p, CWP, and Sec61p was determined by the double-immunoprecipitation method described earlier. Tagging of CWP indicated normal transport in all strains (Figure 7), and Sec61p remained untagged, as expected (Figure 7). Chs3p tagging was consistently higher in end4–1 than in wild-type (compare Figure 7 with Figure 5), and tagging in chs6 was impaired (Figure 7), consistent with results described earlier (Figure 5). In the chs6 end4–1 double mutant, we observed that the amount of “tagged” Chs3p in the 0-min chase point was consistently higher than in the other strains. We consider this to be background labeling, because it is unlikely that Chs3p transits the entire secretory pathway during a 2-min pulse labeling, and also because the amount of anti-DNP precipitable Chs3p and CWP was almost zero in end4–1 chs6 cells that had not been treated with TNBS. Importantly, the amount of tagged Chs3p in the end4–1 chs6 double mutant did not increase during the chase, just as was observed in the chs6 single mutant. These results provide independent verification of the epistasis observed by cell fractionation of lysed spheroplasts. We conclude that the absence of Chs6p results in a pre-endocytic defect in the transport of newly synthesized Chs3p.
Chs6p Is Mostly Soluble in Cell Extracts and Does Not Associate with Chitosomes
To determine the subcellular localization of Chs6p, we constructed myc-tagged CHS6 alleles (see MATERIALS AND METHODS). Both 1MYC-CHS6 and 7MYC-CHS6 were found to complement the Calcofluor-resistant phenotype of chs6 strains (our unpublished data). A 7MYC-CHS6 strain was lysed with glass beads and the lysate was subjected to high-speed centrifugation. Most of the Chs6p was in the soluble fraction (our unpublished data). This strain was also processed by Con A fractionation, and once again the majority (∼80–90%) of the Chs6p remained in the 10,000 × g supernatant (Figure 8). The membranes in the 10,000 × g supernatant fraction were subjected to bouyant density flotation on a Nycodenz step gradient. Whereas chitosome-associated Chs3p floated, Chs6p did not (Figure 8). These data indicate that most of the Chs6p is soluble and does not associate with membranes in cell extracts.
DISCUSSION
We have analyzed the effects of a null allele of chs6, a gene required for CSIII function. chs6 strains exhibit morphological phenotypes similar to that of chs3 strains, and like chs3 strains, are resistant to the chitin-binding cellular poison, Calcofluor. Additionally, both chs2 chs3 (Shaw et al., 1991) and chs2 chs6 (Baggott and Schekman, unpublished data) double-mutant combinations are inviable. These data suggest that Chs6p is required for CSIII function in vivo. However, unlike other mutants required for CSIII function, chs6 strains have normal levels of CSIII activity in vitro (Bulawa, 1992). It was previously suggested that Chs6p might be required for the transport of Chs3p (Bulawa, 1992, 1993).
Active CSIII in cell lysates of chs6 cells could represent an artificial situation in which a latent form of the enzyme in the chitosome becomes active because of an osmotic or chemical effect of the isolation medium or by an unregulated interaction with an activator in some other membrane, such as the plasma membrane. One attractive candidate for such an activator is Chs4p, which localizes to the nascent chitin ring similar to Chs3p (DeMarini et al., 1997). In addition, chs4 mutants are defective for CSIII in vivo and in vitro (Bulawa, 1992), resulting from a reduced Vmax of the enzyme (Trilla et al., 1997). Whatever the explanation, it is clear that CSIII has catalytic potential in the chs6 mutant but not in the chs5 mutant. Chs3p in chs5 mutants accumulates intracellularly as an inactive enzyme, possibly in the late-Golgi, based on equilbrium density gradient fractionation of Chs3p in wild-type and chs5 mutant membranes and the cofractionation of Chs5p with Golgi markers (Santos and Snyder, 1997). Chs3p may need to interact with a factor in the chitosome before transport to the plasma membrane. In chs5, Chs3p may not be exposed to this factor, whereas Chs3p accumulated in chitosomes of chs6 would. Thus, the distinct behavior of CSIII activity in chs5 and chs6 membrane preparations.
The altered distribution of Chs3p in chs6 mutants (Figures 2 and 3) suggests that transport of Chs3p to the plasma membrane requires Chs6p. Membrane fractionation revealed the majority of Chs3p in the low-density, slowly sedimenting chitosome membrane in lysates of chs6 mutant cells. A portion of Chs3p coincided with the denser plasma membrane fraction in chs6 (Figure 2B). However, this may represent a distinct membrane, or chitosomes trapped in plasma membrane vesicles, because cell surface tagging indicated little or no Chs3p exposed at the surface of chs6 or chs6 end4 mutant cells (Figures 5 and 7).
Chitosomes are distinct from mature secretory vesicles. Chs3p-containing membranes from wild-type and chs6 mutant cells fractionate at a lower bouyant density than secretory vesicles, and they lack other markers, such as Pma1p, which are transported to the cell surface (Chuang and Schekman, 1996). In addition, the chs6 mutant, unlike sec mutants that block late in the secretory pathway, has no effect on secretion of the periplasmic CWP (Figure 5). Furthermore, morphological inspection by thin-section EM (Figure 1) and by immunofluorescence localization of Chs3p (Figure 4) document quite distinct behaviors of chs6 and sec mutants (Novick et al., 1980).
We considered the possibility that the elevated level of Chs3p in chitosomes from the chs6 mutant resulted from enhanced internalization from the cell surface rather than defective transport to the cell surface. Previously, we showed that Chs3p is retained in the plasma membrane when an endocytosis-defective mutant, end4, is incubated at a restrictive temperature (Chuang and Schekman, 1996). Were enhanced internalization the explanation for the chs6 phenotype, a double mutant, end4 chs6, would be expected to block accumulation of Chs3p in the chitosome. However, cell fractionation and surface-tagging experiments show that the chs6 mutation overrides the effect of end4 and Chs3p continues to accumulate in the chitosome at the expense of cell surface expression (Figures 6 and 7).
The effect of chs6 is quite selective for Chs3p; Chs1p distributes between the chitosome and plasma membrane similarly in wild-type and mutant cells. Although Chs3p and Chs1p cofractionate in chitosomes isolated from wild-type cells (Ziman et al., 1996), they could be packaged independently into separate transport vesicles that target to different locations on the septal region of the plasma membrane. Alternatively, Chs6p may be required to recruit Chs3p into a common tranport vesicle that buds from the chitosome to deliver both Chs1p and Chs3p to the septum. The cytosolic localization of Chs6p (Figure 8) is consistent with a mechanism in which the protein binds to and dissociates from a membrane transport intermediate serving, for example, as a coat protein subunit or a coat adaptor molecule.
Our current hypothesis regarding the regulation of chitin synthesis is that both newly synthesized and endocytically derived Chs1p and Chs3p undergo a vesicle-mediated transport event that targets these proteins to a spatially restricted location of the plasma membrane. Chs3p is delivered to the incipient bud site in a Chs6p-dependent manner; transport of Chs1p to the birth scar in new daughters may depend on Chs6p homologs present in the yeast genome. Sorting of Chs3p and Chs1p to a chitosome compartment may have similarities with the sorting of the facilitative glucose transporter, GLUT4, into an endocytically derived intracellular storage pool in muscle cells and adipocytes (reviewed by James et al., 1994). Whereas the GLUT4 compartment confers insulin regulation to vesicle fusion, chitosomes may confer temporal or spatial regulation to vesicle fusion, at a time in the yeast-budding cycle when general secretion is not yet polarized.
ACKNOWLEDGMENTS
We thank Edina Harsay, Anne Spang, and Daniel Baggott for comments on the manuscript, and members of the Schekman laboratory for helpful discussion. Rapid freezing with the Bal-Tec high-pressure freezer and subsequent processing were performed in the Electron Microscope Laboratory at the University of California (Berkeley, CA). J.S.C. was supported by a fellowship from the Howard Hughes Medical Institute, and M.Z. was supported by a fellowship from the National Institutes of Health. Work in the laboratory is supported by a grant from the National Institutes of Health (GM-26755) and the Howard Hughes Medical Institute.
REFERENCES
- Bulawa CE. CSD2, CSD3, and CSD4, genes required for chitin synthesis in Saccharomyces cerevisiae: the CSD2 gene product is related to chitin synthases and to developmentally regulated proteins in Rhizobium species and Xenopus laevis. Mol Cell Biol. 1992;12:1764–1776. doi: 10.1128/mcb.12.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa CE. Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- Cabib E, Sburlati A, Bowers B, Silverman SJ. Chitin synthase 1, an auxiliary enzyme for chitin synthesis in Saccharomyces cerevisiae. J Cell Biol. 1989;108:1665–1672. doi: 10.1083/jcb.108.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WJ, Santos B, Duran A, Cabib E. Are yeast chitin synthases regulated at the transcriptional or the posttranslational level? Mol Cell Biol. 1994;14:7685–7694. doi: 10.1128/mcb.14.12.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid VJ, Duran A, del Rey F, Snyder MP, Nombela C, Sanchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AE, M, Fares H, Virgilio CD, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores Martinez A, Schwencke J. Chitin synthetase activity is bound to chitosomes and to the plasma membrane in protoplasts of Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;946:328–336. doi: 10.1016/0005-2736(88)90408-7. [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC, Slot JW. Insulin stimulation of GLUT-4 translocation: a model for regulated recycling. Trends Cell Biol. 1994;4:120–126. doi: 10.1016/0962-8924(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Jones JS, Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990;6:363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Staehelin LA. Rapid Freezing. Freeze Fracture and Deep Etching. New York: Wiley-Liss; 1995. High pressure freezing; pp. 88–104. [Google Scholar]
- Kolodziej PA, Young RA. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–92. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Morales CA, Bracker CE, Bartnicki-Garcia S. Localization of chitin synthetase in cell-free homogenates of Saccharomyces cerevisiae: chitosomes and plasma membrane. Proc Natl Acad Sci USA. 1988;85:8516–8520. doi: 10.1073/pnas.85.22.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KL. Methods in Cell Biology. New York: Academic Press; 1994. Electron microscopy and EM immunocytochemistry; pp. 411–444. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983;96:541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach MF, Latterich M, Schekman RW. Characteristics of endoplasmic reticulum-derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B, Snyder M. Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J Cell Biol. 1997;136:95–110. doi: 10.1083/jcb.136.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz P, Herrero E, Sentandreu R. Secretory pattern of a major integral mannoprotein of the yeast cell wall. Biochim Biophys Acta. 1987;924:193–203. [Google Scholar]
- Sburlati A, Cabib E. Chitin synthetase 2, a presumptive participant in septum formation in Saccharomyces cerevisiae. J Biol Chem. 1986;261:15147–15152. [PubMed] [Google Scholar]
- Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991;114:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SH, Chretien P, Bastien L, Slilaty SN. Primary sequence of the glucanase gene from Oerskovia xanthineolytica. Expression and purification of the enzyme from Escherichia coli. J Biol Chem. 1991;266:1058–1063. [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman SJ, Sburlati A, Slater ML, Cabib E. Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1988;85:4735–4739. doi: 10.1073/pnas.85.13.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trilla JA, Cos T, Duran A, Roncero C. Characterization of CHS4 (CAL2), a gene of Saccharomyces cerevisiae involved in chitin biosynthesis and allelic to SKT5 and CSD4. Yeast. 1997;13:795–807. doi: 10.1002/(SICI)1097-0061(199707)13:9<795::AID-YEA139>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Schekman RW. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol Biol Cell. 1996;7:1909–1919. doi: 10.1091/mbc.7.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]