Abstract
Detecting the formation of mineralized nodules in osteogenic cell culture provides a means of assessing mature osteoblast cell function and the status of culture. In the present study, to continuously monitor the formation of mineralized nodules during the entire culture period, different concentrations of two fluorescent dyes (xylenol orange and calcein blue) were evaluated for their ability to specifically label calcified areas and their toxicity to cells in osteogenic cultures. Results showed that 20 μM xylenol orange and 30 μM calcein blue gave rise to distinct fluorescent staining for mineralized nodules, which were correlated exactly with von Kossa and alizarin red S staining at the same locations in cultures. In the assessment of toxicity, both dyes at the aforementioned concentration did not alter cell viability or change the total DNA content in cultures. To demonstrate the advantage of using these fluorochromes to monitor mineralized nodules formation, consecutive fluorescent images of each staining were recorded at the same location of individual culture over the entire duration. Result indicates that both xylenol orange and calcein blue can provide contrasting fluorescent staining to continuously monitor mineralized nodules formation in living osteogenic cell cultures without deleterious effects.
Introduction
Osteogenic cell cultures established from calvaria, marrow stroma, or immortalized cell lines have been widely used to study many aspects of bone biology (1). The formation of mineralized nodules in these osteoblastic or osteoblast-like cell cultures provides an informative index to assess the function of cells or the status of cultures (2–6). Currently, the two common methods to visualize mineralized nodules in histology and cell culture are the von Kossa method using silver nitrate and alizarin red S staining (7–9). The von Kossa method is based on the binding of silver ions to the anions (phosphates, sulfates, or carbonates) of calcium salts and the reduction of silver salts to form dark brown or black metallic silver staining. Unlike the non-specificity of von Kossa for calcium, alizarin red S reacts with calcium cation to form a chelate. Because of the required cell fixation prior to staining, both methods terminate the culture and preclude further examination or manipulation of the culture. Previously, during the observation of osteoblastic lineage progression in osteogenic cell cultures, in vitro staining of ongoing cultures with xylenol orange was initially examined in order to continuously monitor the status of mineralized nodules formation over the entire duration of the same culture (10, 11). The purpose of the present study was to further assess the use of xylenol orange and another reagent, calcein blue, in the labeling of mineralized nodules in osteogenic cell culture.
Xylenol orange (C31H28N2O13SNa4) and calcein blue (C15H15NO7), both calcium-chelating fluorochrome, have been administered by intraperitoneal injection to label newly calcified tissues in vivo (12, 13). The appropriate in vivo dose for each fluorochrome has been determined to avoid the toxic acute hypocalcemia resulting from overdose. Each fluorochrome has its own specific excitation and emission wavelength [xylenol orange: 440/570 and 610 (nm), calcein blue: 375 and 435 (nm)] (14) which yields contrasting fluorescent color (red for xylenol orange and blue for calcein blue). In the present study, to investigate the usefulness of these fluorochromes in labeling mineralized nodules in vitro, we have tested the effects of different concentrations on labeling and evaluated their toxic effects on cells in osteogenic cell cultures.
Materials and Methods
Primary Calvarial Osteoblast Cultures
Mouse osteoblastic cell cultures were established from calvarial cells isolated from 6- to 8-day-old neonatal CD-1 mice. Calvarial cells were prepared using a modified sequential digestion described by Wong and Cohn (15). Briefly, after surgical isolation from skull and removal of sutures and adherent mesenchymal tissues, calvaria were subjected to four sequential 15-min enzyme digestions at 37C in solution containing 0.05% trypsin-EDTA and 0.1% collagenase P (Roche Diagnostics, Indianapolis, IN). Cells released from the 2nd to 4th digestions were collected, centrifuged, resuspended, and plated at 1.5 × 104 cells/cm2 (1.5 × 105 cells/well) in 35 mm 6-well culture plates in DMEM (Invitrogen, Carlsbad, CA) containing 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and non-essential amino acids (100 μM). The day of plating was designed day 0. Plated cells became confluent around day 5–6; then the culture medium was changed to differentiation medium, which consisted of α-MEM (Invitrogen) containing 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), ascorbic acid (50 μg/ml), and β-glycerophosphate (4 mM). Medium was changed every other day for the entire duration of culture.
Examination of Mineralization Using Fluorescent Dyes
Xylenol orange powder (Sigma, St. Louis, MO) was dissolved in distilled water and filtered to make the 20 mM stock solution. Calcein blue powder (Sigma) was dissolved in 100 mM KOH (diluted in distilled water) and filtered to make the 30 mM stock solution. Xylenol orange and calcein blue was added to medium overnight in a series of 10-fold dilutions ranging from 2 mM ~ 2 μM and 3 mM ~ 3 μM (the final concentration in culture medium) respectively. Prior to microscopic examination and photography, cultures received fresh medium without fluorochrome to avoid non-specific fluorescent background. Xylenol orange expresses a red color under the fluorescent microscope using a TRITC Red filter (Chroma Technology, Rockingham, VT). In contrast, calcein blue emits blue fluorescence using a Sapphire GFP filter (Chroma Technology).
Confirmation of mineralization using von Kossa and alizarin red S staining
The presence of mineralized nodules observed by xylenol orange or calcein blue staining was further confirmed using either von Kossa or alizarin red S staining. For the von Kossa silver nitrate staining method, cultures were fixed in cold methanol for 15–20 min. After rinsing, the fixed plates were incubated with 5% silver nitrate solution under UV light using 2 cycles of auto-crosslink (1200 mjoules × 100) in a UV Stratalinker (Strategene, La Jolla, CA). Mineralized nodules were seen as dark brown to black spots. On the other hand, for alizarin red S (sodium alizarin sulphonate) staining, 2% alizarin red S (Sigma) was prepared in distilled water and the pH was adjusted to 4.1–4.3 using 0.5% ammonium hydroxide. Cultures were fixed with 10% formalin (15 min), washed, and stained with alizarin red S for 10–15 min. After removal of unincorporated excess dye with distilled water, the mineralized nodules were labeled as red spots.
Observation and Photography of Fluorescent Expression
Cell cultures were examined and photographed with a Zeiss Axiovert 200 (Carl Zeiss, Thornwood, NY) microscope using Openlab software (Improvision, Lexington, MA). The Zeiss Axiovert 200 microscope is equipped with the motorized X-Y-Z platform, motorized fluorescent cube, and AxioCam color digital camera that is controlled by a user defined computation program (10). The microscope workstation allows the user to reproducibly record images of cultures at the same location at defined time points.
Examination of Cell Viability
Cell viability was examined with a red-fluorescent dead-cell indicator called ethidium homodimer-1 (EthD-1) (excitation/emission: 495 nm/515 nm) (Molecular Probes, Eugene, OR), which has high affinity for DNA and low membrane permeability. The high affinity of EthD-1 permits the use of very low concentrations to stain dead and dying cells that have damaged cell membranes. Cultures were incubated with EthD-1 at a final concentration of 2 μM for 30 minutes, then washed and examined under the fluorescent microscope with a TRITC filter. Dead cells were labeled as red fluorescent dots. Fluorescent images of EthD-1 staining were photographed and then quantified by counting the number of red dots using a computation program developed from Openlab software.
Quantification of DNA Content and Calcium Content
Total DNA content was quantified using the DNA-binding fluorochrome 4′,6-diamimidino-2-phenylindole (DAPI) (Sigma) method. Scraped or trypsin-released cells were lysed in 0.5 M NaOH, and neutralized with 0.5 M acetic acid. After the addition of DAPI reagent, the amount of DNA was determined by the binding of DAPI to DNA (excitation 344 nm and emission 466 nm) using a Fluoro Count fluorimeter (Packard Instrument, Meriden, CT). Calcium content was determined from cultures grown in 12 well dishes using the Calcium Reagent Set (TECO Diagnostics, Anaheim, CA) according to manufacturers’ recommendations. Briefly, cultures harvested at different time points were washed with PBS (calcium and magnesium free) followed by addition of 6N HCL to release calcium from culture matrix. After reaction with calcium color reagent, calcium content in each sample was measured on an EL800 plate reader (Bio-Tek Instruments, Winooski, VT).
Statistical Analysis
Statistical analysis was carried out using SPSS-11.0 software (SPSS, Chicago, IL). All experiments were repeated three times and data were analyzed with one-way ANOVA followed by the Scheffe post hoc test. All values were expressed as the mean±SE and p<0.05 was considered statistically significant.
Results and Discussion
Optimization of Dye Concentration
To find the appropriate concentrations that produced distinct staining for mineralized nodules in calvarial osteoblast cultures, xylenol orange and calcein blue were tested in a series of 10-fold dilutions that yielded final concentrations in culture medium from 2 mM to 2 μM for xylenol orange and 3 mM to 3 μM for calcein blue. Multiple 18-day-old calvarial osteoblast cultures were incubated with different concentrations of xylenol orange (Figure 1A) and calcein blue (Figure 1B) overnight then subjected to von Kossa staining. Results showed that the lowest concentration (2 μM xylenol orange and 3 μM calcein blue) gave rise to a very weak fluorescent staining which was not comparable to the von Kossa staining (1st column in Figure 1A and 1B). Xylenol orange and calcein blue at a concentration of 20 μM and 30 μM, respectively, produced distinct fluorescent staining of mineralized nodules. Fluorescent stainings by xylenol orange and calcein blue were correlated exactly with the von Kossa staining at the same locations in cultures (2nd column in Figure 1A and 1B).
Figure 1.
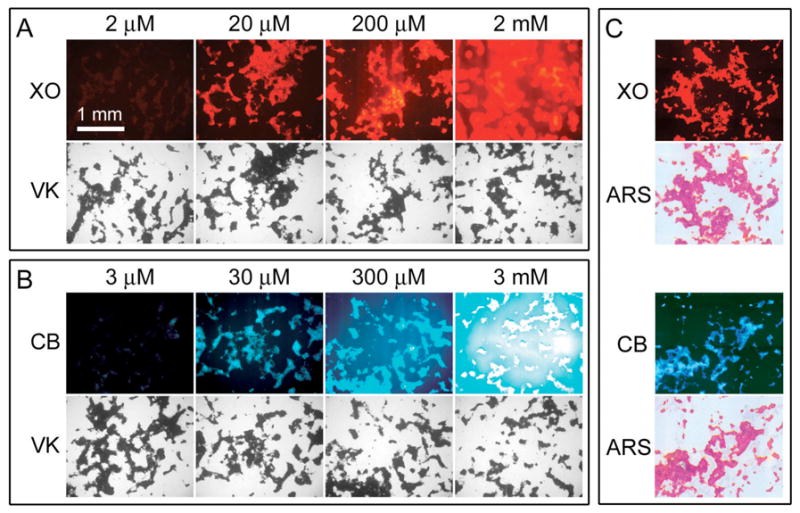
Optimization of dye concentration and confirmation of mineralization. (A, B) Xylenol orange (XO) and calcein blue (CB) were tested with a series of 10-fold dilutions overnight in day 18 cultures. Next day, after photography of fluorescent images, all cultures were fixed and subjected to von Kossa staining (VK). Contrasting fluorescent staining of mineralized nodules, which correlated exactly with the von Kossa pattern at the same locations, occurred with 20 μM XO and 30 μM CB. (C) In day 18 cultures stained with 20 μM XO or 30 μM CB, the presence of mineralized nodules was also confirmed by alizarin red S (ARS) staining. Scale bar (1 mm) applies to all pictures in panel A, B, and C.
However, at higher concentrations (200 μM and 2 mM for xylenol orange, 300 μM and 3 mM for calcein blue), the background fluorescence was greatly increased and the fluorescent staining of cultures became over-saturated (3rd and 4th columns in Figure 1A and 1B). In addition, the presence of mineralized nodules visualized with xylenol orange (20 μM) and calcein blue (30 μM) was further confirmed by the alizarin red S staining. As shown in Figure 1C, the alizarin red S staining correlated with those of xylenol orange and calcein blue in the same locations.
Assessment of Dye Toxicity
To avoid hypocalcemia resulting from the calcium-binding (chelating) activity of these dyes in vivo, the appropriate quantity of xylenol orange and calcein blue for injection has been well established. In the present study, the toxicity of these fluorescent dyes in calvarial osteoblast cultures was also assessed. Cell viability and DNA content was examined in 6-day-old calvarial osteoblast cultures that were treated with different concentrations of xylenol orange and calcein blue for 24 hours. In the examination of cell viability using EthD-1 (marker for dead cells), higher concentration of xylenol orange (2 mM) and calcein blue (3 mM) resulted in significantly more dead cells, indicating the toxicity of this concentration (Figure 2A). On the other hand, lower concentration of both dyes (20 μM xylenol orange and 30 μM calcein blue) did not alter cell viability when compared with controls (Figure 2A).
Figure 2.
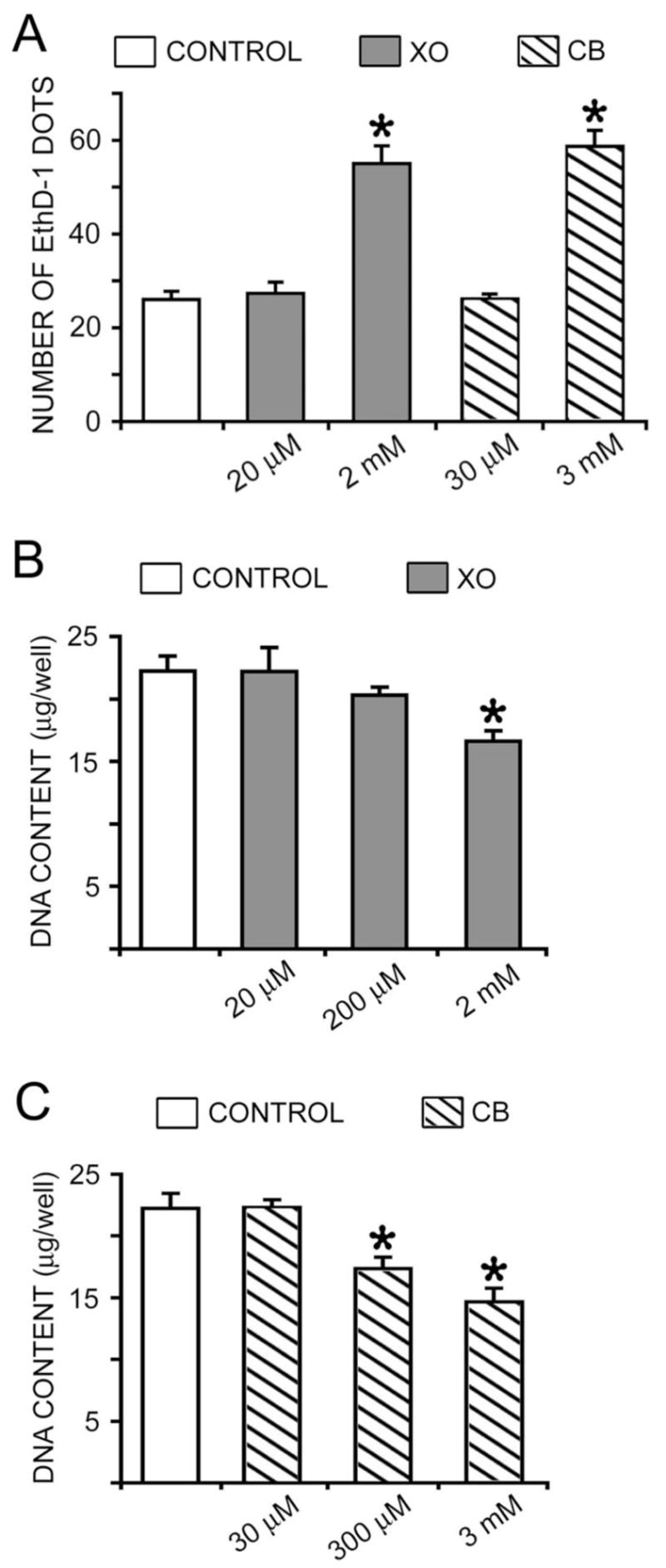
Effect of dye on cell viability and DNA content. Day 6 cultures were incubated overnight with different concentrations of xylenol orange (XO) or calcein blue (CB). (A) Cell viability was examined using the dead cell marker EthD-1. Higher concentration of XO (2 mM) and CB (3 mM) resulted in significantly more dead and/or dying cells marked with EthD-1. There was no difference between control cultures and those incubated with 20 μM XO or 30 μM CB. (B, C) Total DNA content was quantified with DAPI. Higher concentration of XO (2 mM) and CB (3 mM and 300 μM) significantly reduced the total amount of DNA in cultures. There was no difference between control cultures and those incubated with 20 μM XO, 200 μM XO, or 30 μM CB. (* p<0.05 in comparison with the control group)
The effect of different concentrations on cell viability was further examined using DNA content measurement (Figure 2B and 2C). Results showed that after 24 hours of incubation, xylenol orange at a concentration of 2 mM, but not 20 μM or 200 μM, significantly reduced the total DNA content in cultures (Figure 2B). Calcein blue at concentrations of 300 μM and 3 mM significantly decreased the total DNA content in cultures (Figure 2C). Similar to that of xylenol orange, 30 μM calcein blue did not change the total DNA content (Figure 2C). Taken together, the results of fluorescent staining and toxicity indicate that 20 μM xylenol orange and 30 μM calcein blue provide specific staining of mineralized nodules, but do not alter cell viability.
Monitoring the Formation of Mineralized Nodules
To demonstrate the advantage of xylenol orange and calcein blue in the observation of mineralized nodule formation, we have imaged both fluorescent staining at the same location over the entire duration of culture. Calvarial osteoblast cultures were periodically incubated with either 20 μM xylenol orange or 30 μM calcein blue and photographed at day 12, 15, 18, and 21. At day 21, all cultures were terminated and processed for von Kossa staining. Results showed that there was negligible fluorescent staining by both xylenol orange and calcein blue at day 12 (Figure 3A). Only a few spots of weak fluorescent staining were observed under higher magnification (data not shown). From day 15 to day 21, the continuous formation of mineralized nodules marked by xylenol orange and calcein blue was clearly demonstrated by an increase in both area and intensity (Figure 3A). At day 21, the fluorescent images of mineralized nodules from both dyes were exactly correlated with the pattern from von Kossa staining (Figure 3A).
Figure 3.
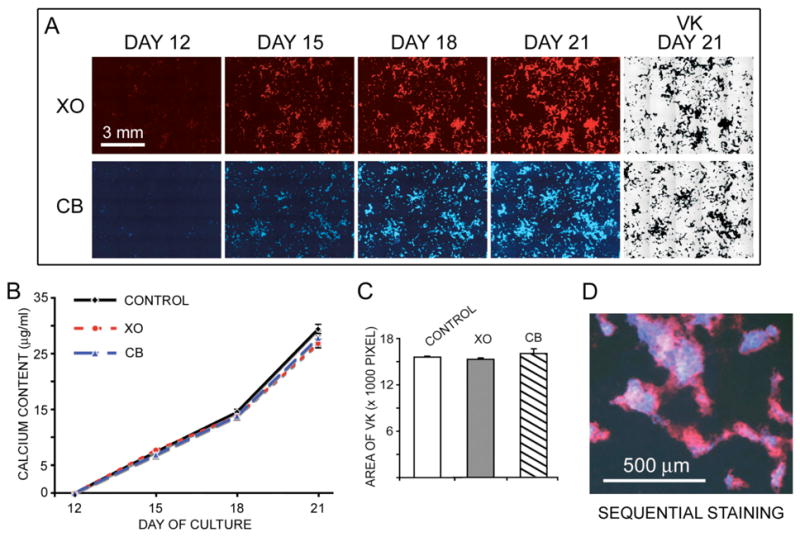
Continuous monitoring mineralized nodules formation. (A) A series of fluorescent images were obtained from the same culture incubated with either 20 μM xylenol orange (XO) or 30 μM calcein blue (CB) at different time point. At day 21, after photography of fluorescent image, both cultures were fixed and subjected to von Kossa staining. Scale bar (3 mm) applies to pictures in panel A. (B) Calcium content analysis showed no difference between control cultures and those stained with XO or CB at day 12, 15, 18, and 21. (C) Amount of mineralized nodules in day 21 cultures that had been exposed to either 20 μM XO or 30 μM CB at different time point were quantified with the areas of von Kossa staining. In comparison with the control cultures, no difference was found in cultures incubated with either XO or CB. (D) An overlapped image of sequential staining of CB (blue) at day 15 followed by XO (red) at day 18.
The possible effect of multiple doses of fluorescent dyes on the mineralization was also examined. Duplicate cultures from each time point (day 12, 15, 18, and 21) were harvested for calcium content analysis. The analysis showed that there was no significant difference in the calcium content between control cultures and those stained with xylenol orange or calcein blue at each time point (Figure 3B). From day 12 to day 21, the increased calcium content in cultures correlated with the accumulation of mineralized nodules stained by XO and CB. Similarly, comparison of total mineralized areas stained by von Kossa on day 21 also showed no difference between control cultures and those incubated with multiple doses of fluorescent dyes (Figure 3C). These results indicate that the presence of 20 μM xylenol orange or 30 μM calcein blue does not alter the rate of mineralization in cultures. Moreover, calcein blue and xylenol orange can also be used sequentially to distinguish old mineral deposition from new mineral formation. As shown in Figure 3D, the treatment of culture with calcein blue at day 15 followed by xylenol orange at day 18 revealed regions of old mineral formation (calcein blue) and new mineral formation (xylenol orange).
Advantage and Future Application
von Kossa staining using silver nitrate and alizarin red S staining have been widely used to detect calcification in skeletal tissues. Despite some pitfalls in von Kossa (16), it is still the standard method to visualize mineralization in osteogenic cell cultures. To examine the formation of mineralized nodules in cell culture, the methods of von Kossa and alizarin red S require the termination (fixation) of cultures. This step precludes the opportunity to continuously observe dynamic changes during the formation of mineralized nodules in living cell cultures. To overcome this limitation, the present study describes the development of a new protocol for the observation of mineralization in living cell cultures using different vital fluorescent dyes. In comparison to von Kossa and alizarin red S staining, 20 μM xylenol orange and 30 μM calcein blue has similar abilities to specifically label mineral deposition without the termination of cultures. This advantage provides the ability to continuously observe the extent of mineralized nodule formation in living cultures without deleterious effects.
Furthermore, the distinct colors of xylenol orange and calcein blue offer an alternative choice to visualize mineralization in the presence of other fluorescent substrate. In previous studies (10, 11), we have used xylenol orange to label mineralized nodules in cell cultures expressing different versions of GFP (green or blue fluorescence). The combination of blue fluorescence of calcein blue with red fluorescence of EthD-1 suggests another method to examine simultaneously the mineralization and cell viability in the same culture. Also, with the generation of red fluorescent proteins as reporter genes (such as DsRed), the contrasting blue fluorescent emission of calcein blue will be another choice for the visualization of mineralization.
Acknowledgments
The authors thank Dr. M. Kronenberg for providing a critical review of the manuscript. This study was supported by NIH grant R03-DE015224.
References
- 1.Majeska RJ, Gronowicz GA. Current methodologic issues in cell and tissue culture. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Vol. 2. Academic Press; San Diego: 2002. pp. 1529–1541. [Google Scholar]
- 2.Bellows CG, Aubin JE, Heersche JN, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 3.Bellows CG, Aubin JE, Heersche JN. Physiological concentrations of glucocorticoids stimulate formation of bone nodules from isolated rat calvaria cells in vitro. Endocrinology. 1987;121:1985–1992. doi: 10.1210/endo-121-6-1985. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987;122:49–60. doi: 10.1016/0012-1606(87)90331-9. [DOI] [PubMed] [Google Scholar]
- 5.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation, differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Igarashi C, Takeuchi Y, Harada S, Kikuchi T, Yamato H, Ogata E. Stimulation by 1,25-dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991;12:27–32. doi: 10.1016/8756-3282(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 7.Bills CE, Eisenberg H, Pallante SL. Complexes of organic acids with calcium phosphate: the von Kossa stain as a clue to the composition of bone mineral. Johns Hopkins Med J. 1971;128:194–207. [PubMed] [Google Scholar]
- 8.Kiernan JA. Histological and histochemical methods: theory and practice. Butterworth-Heinemann; Boston: 1999. Methods for inorganic ions; pp. 267–280. [Google Scholar]
- 9.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17:110–124. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- 10.Wang YH, Liu Y, Buhl K, Rowe DW. Comparison of the Action of Transient and Continuous PTH on Primary Osteoblast Cultures Expressing Differentiation Stage-Specific GFP. J Bone Miner Res. 2005;20:5–14. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- 11.Bilic-Curcic I, Kronenberg M, Jiang X, Bellizzi J, Mina M, Marijanovic I, Gardiner EM, Rowe DW. Visualizing levels of osteoblast differentiation by a two-color promoter-GFP strategy: Type I collagen-GFPcyan and osteocalcin-GFPtpz. Genesis. 2005;43:87–98. doi: 10.1002/gene.20156. [DOI] [PubMed] [Google Scholar]
- 12.Rahn BA, Perren SM. Xylenol orange, a fluorochrome useful in polychrome sequential labeling of calcifying tissues. Stain Technol. 1971;46:125–129. doi: 10.3109/10520297109067836. [DOI] [PubMed] [Google Scholar]
- 13.Rahn BA, Perren SM. Calcein blue as a fluorescent label in bone. Experientia. 1970;26:519–520. doi: 10.1007/BF01898484. [DOI] [PubMed] [Google Scholar]
- 14.Pautke C, Vogt S, Tischer T, Wexel G, Deppe H, Milz S, Schieker M, Kolk A. Polychrome labeling of bone with seven different fluorochromes: enhancing fluorochrome discrimination by spectral image analysis. Bone. 2005;37:441–445. doi: 10.1016/j.bone.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Wong GL, Cohn DV. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975;72:3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]


