Abstract
Many fundamental cellular processes depend on enzymes that utilize chemical energy to catalyze unfavourable reactions. Certain classes of ATPases provide a particularly vivid example of the process of energy conversion, employing cycles of nucleotide turnover to move and/or rearrange biological polymers such as proteins and nucleic acids. Four well characterized classes of ATP-dependent protein/nucleic acid translocases and remodelling factors are found in all three domains of life (bacteria, archaea, and eukarya): ASCE P-loop NTPases, GHL proteins, actin-fold enzymes, and chaperonins. These unrelated protein superfamilies have each evolved the ability to couple ATP binding and hydrolysis to the generation of motion and force along or within their substrates. The past several years have witnessed the emergence of a wealth of structural data that help explain how such molecular engines couple nucleotide turnover to conformational change. In this review, we highlight several recent advances to illustrate some of the mechanisms by which each family of ATP-dependent motors facilitates the rearrangement and movement of proteins, protein complexes and nucleic acids.
Introduction
Numerous protein families have evolved the ability to catalyze molecular transactions using ATP, the universal energy currency of the cell. ATPases can function as nucleotide-dependent switches, ion pumps, and polymer-dependent molecular motors, to name a few (Vetter and Wittinghofer, 1999). While many ATPases undergo significant conformational changes as they hydrolyze nucleotide, the polymer-dependent motors further couple these conformational changes to the often-dramatic rearrangement and mechanical manipulation of polypeptides, protein complexes and nucleic acids. The vast majority of these motors are built from one of four basic folds – ASCE P-loop, GHL, actin, and chaperonin – that have evolved the ability to push, pull and wind or unwind target substrates. The cellular roles of these motors beautifully illustrate the adaptive powers of evolution, both through the divergence of function for closely related motors, and the convergence of function between unrelated ATPase superfamilies.
The microbial ATPase folds covered here each illustrate a unique mechanism by which ATPase activity is coupled to motion or force generation. However, four main generalizations can be drawn about the requirements for an ATP dependent motor, summarized here and in Table 1. First, to generate the large conformational changes required for motor function, ATP and a divalent cation are generally bound at an interdomain or intersubunit interface, with conserved amino acids being contributed by both ATP binding subunits or subdomains. At a minimum, this configuration leads to instances in which two adjacent domains within a single polypeptide create a monomeric motor; however, this arrangement often leads to large multimeric motor assemblies that respond to complex allosteric interactions between multiple ATP and target-substrate binding sites. Second, binding of adenosine nucleotides often relies on a flexible glycine rich loop (generally termed a P-loop), in which backbone amides help hydrogen bond to the phosphate groups of ATP. In most motor ATPases, the P-loop is accompanied by adenine binding residues, which in some protein classes are highly conserved. Third, motors typically utilize a conserved acidic residue to position and activate a water molecule for attack on the γ-phosphate of ATP. Finally, in order to physically respond to different nucleotide states, the motor must be able to sense the difference between ATP and its hydrolysis products. This action is accomplished either by direct interactions between a positively-charged γ-phosphate sensor and ATP, or indirectly via a negatively-charged residue that binds a divalent cation (typically Mg2+) in contact with the γ-phosphate. Although these conserved features are present in all four microbial motor ATPase families, it is their unique positioning within distinct folds that is primarily responsible for generating the remarkable diversity of ATP dependent motor mechanisms.
Table 1.
Functional roles of conserved catalytic motifs among motor ATPase families. Abbreviations: W-A = Walker A, W-B = Walker B, RF = arginine finger, CE = catalytic glutamate, H = H loop, D = D loop, Q = Q loop, C = C region or ABC signature motif, SI = sensor I, SII = sensor II. All other motifs are listed as roman numerals.
| P-loop | Metal binding | γ-phosphate sensor | Hydrolysis | Adenine binding | |
|---|---|---|---|---|---|
| RecA | W-A | W-A | RF | CE | - |
| W-B | |||||
| ABC | W-A | W-A | C | CE | - |
| W-B | D | ||||
| H | |||||
| Q | |||||
| AAA+ | W-A | W-A | RF | CE | - |
| W-B | SII | SI | |||
| GHL | III | I | V | I | II |
| IV | |||||
| Actin | III | I | - | VI | IV |
| II | |||||
| III | |||||
| Chaperonin | III | I | - | II | V |
| II | |||||
| III | |||||
| IV |
Given the broad nature of the topic, this review will focus primarily on structural studies that have begun to reveal the nature and scope of ATP-dependent conformational changes in microbial protein/nucleic-acid motor ATPases, and how these rearrangements drive function. We will first discuss the conserved modules for ATP binding and hydrolysis and how these elements differ among families, and then describe the ATP-dependent conformational changes in the context of quaternary motor assemblies. GTP-hydrolyzing tubulin family members, the Walker-A Cytoskeletal ATPases (WACA) (which are phylogenetically classified as P-loop GTPase family members) (Leipe et al., 2002), and the nucleotide-dependent RNA and DNA polymerizing machineries, though motor proteins in their own right, are too broad to be contained in this survey; the reader is directed to a number of recent reviews for coverage of these NTPase families, (Michie and Lowe, 2006; Rothwell and Waksman, 2005; Steitz, 2006; Thanbichler and Shapiro, 2008). We will focus instead on those motor proteins whose members almost exclusively use ATP to translocate or remodel their target substrates.
ASCE P-loop Motors
The Additional Strand Catalytic “E” (ASCE) P-loop family (Leipe et al., 2003) is one of the largest and most functionally diverse classes of ATP-dependent motors. Although ASCE P-loop members are sometimes referred to as “RecA-like” proteins, due to the similarity of their fold to the RecA recombination factor (Story et al., 1992), there are critical differences between this subgroup and other ASCE proteins. For example, the core nucleotide-binding folds of RecA-like and AAA+ (ATPases Associated with various cellular Activities) proteins are related, but differ in the details of their topology, conserved ATP-binding residues, and the orientation of individual motor domains in higher-order quaternary states (Caruthers and McKay, 2002; Erzberger and Berger, 2006; Iyer et al., 2004b; Wang, 2004). Despite these differences, RecA and AAA+ proteins both rely on similar mechanisms for the generation of movement, in which ATP binding induces internal conformational changes within a single motor subunit, which are then amplified by the remodelling of inter-subunit interfaces via trans-acting γ-phosphate sensor motifs.
RecA-like Motors
The RecA-like family can be divided into four major evolutionarily related groups: the ABC ATPases, superfamily 1/superfamily 2 (SF1/SF2) helicases, the PilT/FtsK secretory ATPases, and the classic RecA/F1 family (Iyer et al., 2004b). RecA-like ATPases are defined by a six- to seven-strand β-sheet sandwiched between a series of α-helices (Fig. 1A) (Story and Steitz, 1992; Story et al., 1992). Residues responsible for ATPase function are located almost exclusively on loops connecting the C-termini of central β-strands to downstream helical elements. In almost all cases, non-conserved accessory domains are attached to or inserted within the RecA-like fold, such as the C-terminal helical bundles commonly found in RecA/F1 family members, or domains Ib and IIb found in SF1/SF2 helicases.
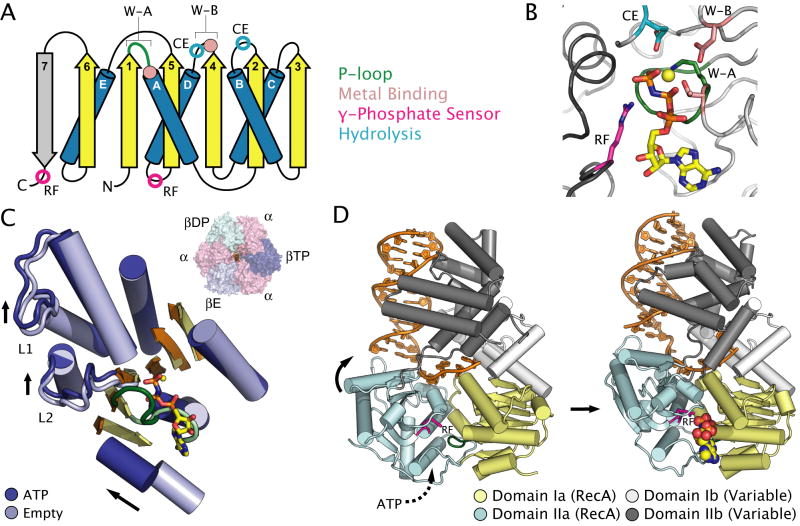
Fig. 1. Nucleotide binding and conformational changes in RecA-like ATPases.
A. Topology diagram of the RecA fold. Conserved motifs and catalytic residues are shown as solid coloured circles (see key). Motifs and catalytic residues conserved spatially in the structure, but not in primary amino acid sequence are shown as open circles. β-sheets and α-helices are coloured yellow and blue respectively. Secondary structure elements shown in grey are not conserved in all members of the family. Abbreviations: RF = arginine finger, CE = catalytic glutamate, W-A = Walker A, W-B = Walker B.
B. The active site of the Saccharomyces cerevisiae F1-ATPase β-subunit (PDB entry 2HLD) (Kabaleeswaran et al., 2006), highlighting the location of catalytic residues common to all RecA-like ATPases. The adjacent α-subunit, which contributes the arginine finger, is shown in dark grey. Magnesium is shown as a yellow sphere. Residues are coloured as in A.
C. Structural superposition of the conserved RecA-fold of the ATP bound (βTP) subunit (dark blue) and the empty (βE) subunit (light blue) of S. cerevisiae F1-ATPase (PDB entry 2HLD) (Kabaleeswaran et al., 2006), with most loops removed for clarity. Arrows highlight ATP-dependent conformational changes discussed in the text. Inset: the location of the superposed subunits with respect to the quaternary structure of F1-ATPase.
D. ATP-dependent conformational changes between two RecA-like folds illustrated in the structure of E. coli UvrD bound to DNA (PDB entry 2IS1 and 2IS4) (Lee and Yang, 2006). ATP is shown as coloured spheres. Domains 1A and 2A comprising the RecA folds are shown in yellow and cyan respectively, while domains 1B and 2B are shown in light and dark grey. DNA illustrated in stick representation is coloured orange.
Conserved elements known as Walker-A and Walker-B motifs, so termed for their identification in the sequence of the bovine mitochondrial F1-ATPase and other ATP binding enzymes (Walker et al., 1982), contain the only catalytic residues that are strictly conserved among all RecA family members. The Walker-A motif (W-A), also referred to as the phosphate-binding, or P-loop, is located between strand β-1 and helix α-A, and generally bears the consensus sequence [G/A]xxxxGK[T/S] (Fig. 1A) (Walker et al., 1982). The backbone amide groups of the glycines coordinate the phosphate groups of ATP, the conserved lysine forms ion pairs with the β- and γ-phosphates, and the threonine/serine residue ligands a critical Mg2+ ion (Fig. 1B). The Walker-B motif (W-B), located between strand β-4 and helix α-D, contains the consensus sequence φφφφ[D/E] (in which φ is a hydrophobic residue) (Walker et al., 1982), where the conserved acidic group further coordinates the Mg2+ ion associated with the β- and γ-phosphates of ATP (Abrahams et al., 1994; Story and Steitz, 1992). The spatial position of the catalytic glutamate that gives its name to the ASCE superfamily is conserved in the active site of different RecA-like family members, but is located at different points in the primary amino acid sequence. For example, in the F1/RecA and PilT/FtsK families, the catalytic residue is located on a loop between strand β-2 and helix α-B (Fig. 1A) (Story and Steitz, 1992; Yeo et al., 2000). By contrast, in SF1/SF2 and the ABC ATPase families, the catalytic glutamate is located immediately after the aspartate of the W-B motif (Fig. 1A) (Geourjon et al., 2001; Hung et al., 1998; Subramanya et al., 1996). In both instances, this residue is postulated to activate a water molecule for nucleophilic attack on the γ-phosphate (Geourjon et al., 2001). Another component of the active site conserved in many RecA-like ATPases is a polar residue at the C-terminal tip of strand β-5 that might function as a γ-phosphate sensor in order to transmit conformational changes to other parts of the protein upon ATP binding (Iyer et al., 2004b; Story and Steitz, 1992; Yoshida and Amano, 1995). Such a function might parallel the analogous switch-II region of G-proteins (Milburn et al., 1990), which are distantly related to ASCE P-loop ATPases within the broader P-loop NTPase superfamily (Leipe et al., 2002). Finally, nearly all RecA-like ATPases, apart from the ABC class (see below and Fig. 1 and S1), contain an arginine (or lysine) finger similar to those first observed in GTPase activating proteins (Wittinghofer et al., 1997). Since this amino acid typically contributes to the active site of a partner subunit in-trans, the arginine finger helps couple ATP binding and hydrolysis to large-scale conformational changes between adjacent RecA folds. The positive charge on the guanidinium group of the arginine is critical for ATPase activity, and helps to stabilize the pentavalent phosphoanhydride transition state that forms during hydrolysis (Braig et al., 2000; Nadanaciva et al., 1999; Ogura et al., 2004).
RecA-like ATPases typically exhibit nucleotide-dependent movement of substrate-binding loops that are adjacent to the ATP binding site. In some cases, this movement appears to be coupled to twisting of the central β-sheet of the RecA-fold. This β-sheet deformation has been proposed to store energy elastically (Sun et al., 2003) and can lead to large motions in accessory domains. The F1-ATPase, which has served as a model system for studies of oligomeric RecA-type proteins, illustrates both of these effects clearly (Fig. 1C) (Abrahams et al., 1994; Kabaleeswaran et al., 2006). F1 is a hetero-hexamer containing three active β-subunits and three inactive α-subunits in an alternating arrangement (Abrahams et al., 1994). Two loops, designated here as L1 and L2 in accordance with the nomenclature for RecA (Story et al., 1992), contact the internal γ-shaft subunit and undergo small but distinct conformational changes between the ATP-bound and empty subunits. Similar ATP-dependent loop movements have also been observed in other well-studied hexameric or filamentous RecA-like motors whose nucleic acid binding sites reside in the central channel formed by the L1/L2 loops. These proteins include RecA itself (Krishna et al., 2007b), the phage T7-gp4 helicase (Singleton et al., 2000), the bacteriophage φ12 P4 RNA packaging motor (Mancini et al., 2004), and the E. coli Rho transcription termination factor (Skordalakes and Berger, 2006). Interestingly, in the SF1 helicases PcrA and UvrD that bind target nucleic acid substrates in a very different fashion, loops topologically equivalent to L1/L2 also undergo ATP-dependent conformational shifts and contain residues that base stack with bound nucleic acid (Fig. 1D) (Lee and Yang, 2006; Velankar et al., 1999). No other RecA-like proteins to date have illustrated the drastic flexing of the β-sheet observed in F1, which might reflect a unique characteristic of this motor but could also be due to the fact that structures of RecA-like hexameric translocases bound to both ATP and their protein or nucleic acid substrates have not been obtained.
Although the intra-domain conformational changes due to ATP binding are relatively small, the oligomeric nature of RecA-like motors allows these transitions to be amplified through inter-subunit or inter-domain (in the case of SF1/SF2 family members) interfaces. Structural analyses of the SF1 helicases PcrA and UvrD have illustrated this principle particularly well, since they remain the only RecA motors imaged in the presence of nucleic-acid substrates in multiple nucleotide-bound states (Fig. 1D) (Lee and Yang, 2006; Velankar et al., 1999). In the inactive or empty conformation, the two RecA folds of the helicases splay apart, removing the arginine finger in domain IIa from the ATP-binding site in domain Ia. Upon binding ATP, domain IIa rotates inward to insert its arginine finger into the catalytic pocket of domain Ia, thereby promoting ATP hydrolysis. This movement is thought to be coupled to the translocation of single-stranded nucleic acid across the tandem RecA domain surface, using base stacking and nucleic acid backbone-binding residues located on substrate-binding loops to grip and pull DNA (Velankar et al., 1999). Unwinding of duplex DNA occurs concomitantly with this motion by splitting the duplex across a wedge element in domain IIa (Lee and Yang, 2006; Velankar et al., 1999). Significant inter-domain movements have been observed in the conserved RecA domains of other nucleic-acid bound SF1 helicases such as E. coli Rep (Korolev et al., 1997), as well as in SF2 helicases such as HCV-NS3 (Kim et al., 1998), archaeal Rad54 (Durr et al., 2005), and Hel308 (Buttner et al., 2007). It is notable that only nucleic-acid bound SF1/SF2 helicases have illustrated both the open and closed states clearly. A possible explanation was provided by recent single-molecule fluorescence resonance energy transfer (FRET) studies of substrate dependent domain movements in the SF2 RNA helicase YxiN from Bacillus subtilis (Theissen et al., 2008). This study revealed that cooperative binding of both ATP and nucleic acid substrate were required for the helicase to adopt the closed conformation. Although the substrate binding modes and quaternary organization of the hexameric motors are quite different, the movements observed in SF1/SF2 helicases currently serve as the most complete model of inter-domain communication in the RecA-like polymer-dependent ATPases.
The catalytic motifs and inter-subunit/domain conformational changes described thus far can be generalized to most RecA-like proteins with the exception of the ABC ATPases. Although the small molecule transporters of the ABC class are outside the scope of this review, a number of ABC proteins have been identified as DNA-dependent remodelling or repair factors (Hopfner and Tainer, 2003). Structures of these ATPases, including structural maintenance of chromosomes (SMC) proteins (Lammens et al., 2004), the mismatch repair enzyme MutS (Obmolova et al., 2000), and the double-strand break repair enzyme Rad50 (Hopfner et al., 2000), have all revealed that ABC ATPases form head-to-head dimers with conserved ABC motifs forming two bipartite ATP binding sites along a dyad symmetry axis (Fig. S1) (Hopfner et al., 2000). A notable exception to this organization is illustrated by UvrA, a protein involved in nucleotide excision repair, in which the ATP binding sites are formed at the interface between tandem ABC folds contained in a single polypeptide chain (Pakotiprapha et al., 2008). Both arrangements differ markedly from the head-to-tail dimers that generate a single ATP binding site in classical RecA-like proteins. As a result, the unique ABC dimers lack the arginine fingers found in other RecA family members, but have gained several new motifs (Table I and Fig. S1). Another distinction is that ABC ATPase multimers do not form the ring-shaped assemblies characteristic of many RecA ATPases, but rather use cycles of ATP-dependent dimerization events to capture and/or bind to DNA (Lamers et al., 2000; Obmolova et al., 2000). In this way, ABC transporters share some functional parallels with GHL enzymes (see below), highlighting an intriguing functional convergence between two unrelated ATPase folds.
While SF1/SF2 and DNA-dependent ABC ATPases typically couple the ATP-dependent rearrangement of two RecA folds to movement along or association with nucleic acid substrates (Hopfner and Tainer, 2003; Singleton et al., 2007), how nucleotide turnover is linked among the multiple active sites of ring-shaped hexameric RecA-like motors is less well understood (Singleton et al., 2007). In this regard, the F1-ATPase has served as a valuable reference system for ring shaped RecA-like motors. This heterohexameric assembly, which participates in ATP synthesis in-vivo, displays structural and catalytic asymmetry among its three active β-subunits, which alternate between high, medium and low affinity for ATP+Mg2+ (Abrahams et al., 1994). The asymmetry of F1 is directly coupled to the position of the γ-shaft subunit in the centre of the ring, and ensures that only the high affinity site can hydrolyze ATP at any one time. ATP turnover proceeds around the F1 ring, directly driving rotation of the internal γ-shaft (Mao and Weber, 2007; Noji et al., 1997; Weber and Senior, 2003). In contrast to F1, most toroidal RecA-type ATPases assemble and function as homohexamers, and retain the potential to accommodate six catalytically competent ATP binding sites. To date, structures of these motors have revealed a variety of ring symmetries. These structural states are often observed to have rotational three-fold, or “trimer of dimers” symmetry, as has been observed in an archaeal secretion ATPase of the GspE family (Yamagata and Tainer, 2007), the Rho transcription termination factor (Skordalakes and Berger, 2006), and the bacterial replicative helicases RepA of plasmid RSF1010 (Ziegelin et al., 2003) and DnaB (Bailey et al., 2007). By contrast, other oligomeric RecA motors have been imaged as dimers of trimers (Satyshur et al., 2007; Singleton et al., 2000; Xu et al., 2003), as six-fold symmetric particles (Gomis-Ruth et al., 2001; Mancini et al., 2004; Niedenzu et al., 2001; Yeo et al., 2000), or even as heptamers (Toth et al., 2003). Based on these structures and on F1, multiple mechanisms have been proposed for how inter-subunit conformational changes drive substrate through the motor ring, the most predominant of which rely on a concerted wave of L1/L2 loop movements that progress around the helicase in a rotary fashion. Nonetheless, given that a ring shaped RecA-type ATPase has not yet been visualized in the presence of both nucleotide and a nucleic acid or protein substrate, the detailed mechanism by which this family of motors uses ATP turnover to power the movement of target molecules remains a point of vigorous debate (Singleton et al., 2007).
AAA+ Motors
The AAA+ family of ASCE P-loop ATPases represents a second way in which nature has utilized conserved Walker-A and Walker-B motifs, and a mixed α/β fold, to perform mechanical work. The structures of domain II of eukaryotic N-ethylmaleimide Sensitive Factor (NSF) (Lenzen et al., 1998; Yu et al., 1998) and the δ′ subunit of the E. coli DNA polymerase III clamp-loader complex (Guenther et al., 1997), along with subsequent phylogenetic analyses, have formed the basis for classifying this family separately from other ASCE P-loop ATPases (Iyer et al., 2004a; Neuwald et al., 1999). The AAA+ family has been divided further into seven evolutionarily-related subgroups, or clades: clamp loaders, initiators/helicase loaders, classic AAAs, superfamily 3 (SF3) helicases, HCLR (HslU, ClpABC-CTD, LonAB, RuvB), helix 2 insert (H2I), and pre-sensor 2 insert (PS2I) (Iyer et al., 2004a). The structural core of the AAA+ ATPases consists of a five-stranded, parallel β-sheet sandwiched between α-helices that is similar to, but slightly more compact than, the RecA-like fold (Fig. 2A). This core is typically appended with a three-helix bundle at its C-terminus, sometimes referred to as the “lid”, which is conserved in six of the seven AAA+ clades (Erzberger and Berger, 2006; Iyer et al., 2004a).
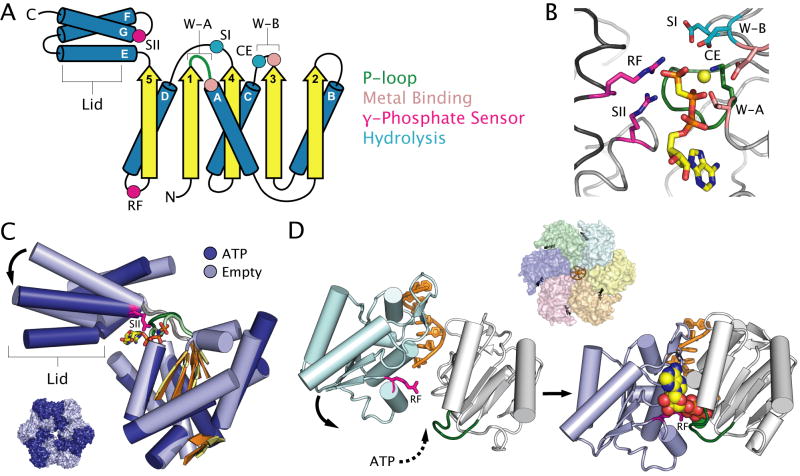
Fig. 2. Nucleotide binding and conformational changes in AAA+ ATPases.
A. Topology diagram of the typical two-domain AAA+ fold. Conserved motifs, catalytic residues and secondary structural elements are coloured as in Figure 1. Abbreviations: RF = arginine finger, CE = catalytic glutamate, W-A = Walker A, W-B = Walker B, SI = sensor I, SII = sensor II.
B. The active site of Aquifex aeolicus DnaA (PDB entry 2HCB) (Erzberger et al., 2006) highlighting the location of catalytic residues common to all AAA+ ATPases. Active site elements are coloured as in Figure 1.
C. Structural superposition of the conserved AAA+ fold of ATP-bound (dark blue) and empty (light blue) subunits of E. coli HslU (PDB entry1DO2) (Bochtler et al., 2000), with most loops removed for clarity. Arrows highlight ATP-dependent conformational changes discussed in the text. Inset: the location of the superposed subunits with respect to the quaternary structure of HslU. Note that the β-sheet domain stays relatively immobile while the lid moves, compared to oligomeric RecA-type proteins where the β-sheet can flex considerably (Fig. 1C).
D. ATP-dependent conformational changes between two AAA+ folds illustrated in the structure of the human papillomavirus E1 helicase bound to DNA (PDB entry 2GXA) (Enmark and Joshua-Tor, 2006). ADP is shown as coloured spheres, and the bound chloride ion (which mimics the γ-phosphate) is shown as a green sphere. The cyan and purple subunits are coloured according to the inset. Inset: views of E1 from the bottom up relative to the main figure, illustrating the position of empty and ATP bound subunits within the quaternary structure.
The AAA+ family possesses a greater number of conserved catalytic residues than does the RecA-like family (Fig. 2B). The putative water-activating acidic residue is almost always located in the Walker-B motif (similar to SF1/SF2 helicases), while the arginine finger invariably lies on the C-terminus of helix α-D (Erzberger and Berger, 2006; Iyer et al., 2004a). The AAA+ family is also characterized by two additional, highly conserved catalytic residues, the sensor-I (SI) and sensor-II (SII) motifs first identified in the δ′ subunit of the E. coli clamp-loader complex (Guenther et al., 1997). The sensor-I motif is typified by a polar residue located at the C-terminus of strand β-4, a position structurally analogous to the switch-II or sensor residue found at the C-terminus of strand β-5 in many P-loop GTPases and RecA-like ATPases (Milburn et al., 1990; Story and Steitz, 1992). The sensor-I motif might play a role in orienting the catalytic water molecule for attack on the γ-phosphate (Guenther et al., 1997). The sensor-II motif is a positively-charged residue at the N-terminus of helix α-G of the AAA+ lid and is thought to couple ATP binding and hydrolysis to intra-subunit conformational changes between this region and the primary nucleotide-binding fold (Erzberger and Berger, 2006; Wang et al., 2001b). Due to the absence of a canonical AAA+ lid, SF3 helicases lack this element.
Among microbial AAA+ motor proteins, the ATPases of proteolytic degradosomes have been the most thoroughly characterized structurally. For example, the HslU engine of the HslU/V protease has been imaged at high resolution in numerous conformational states and bound to a variety of nucleotides (Bochtler et al., 2000; Song et al., 2000; Sousa et al., 2000; Wang et al., 2001a; Wang et al., 2001b). These different states have revealed intra-subunit conformational changes that are characteristic of most members of the AAA+ family (Fig. 2C). The most obvious conformational changes occur in the AAA+ lid domain that, in addition to the sensor-II arginine, contributes a number of residues directly to the active site. The lid can undergo a rigid body rotation of nearly 20° upon entering an ATP-bound state, allowing productive interactions to form between the sensor-II region and the γ-phosphate of ATP. Structures bound to SO4 (presumably mimicking a post hydrolysis PO4) and ADP populate conformations along this same rotation vector, suggesting that the observed configurations represent a functionally significant set of transitions (Wang et al., 2001b). Studies of numerous AAA+ motor proteins, including the σ-factor remodelling proteins NtrC (Lee et al., 2003b) and PspF (Rappas et al., 2005), and the chaperone ClpB (Lee et al., 2003a), have revealed marked variability in the positioning of the AAA+ lid domain. Distinct nucleotide-dependent conformational transitions have also been observed in the AAA+ domains of the DnaA initiator (Erzberger et al., 2002; Erzberger et al., 2006), and archaeal Replication Factor C (RFC) clamp loader (Seybert et al., 2006), as well as in the protein translocase motor of the protease FtsH (Niwa et al., 2002; Suno et al., 2006). Thus, movement of the AAA+ lid appears to be a general feature of most motor proteins of this family and is linked directly to the formation of a competent ATP-binding site.
The structure of the superfamily 3 (SF3) helicase E1 from papillomavirus bound to DNA represents one of the most complete structures of an AAA+ motor/substrate complex visualized to date. Although the complex is a homohexamer, E1 adopts a markedly asymmetric conformation in which the spatial displacement of each subunit varies sequentially around the ring and is coupled directly to the position of DNA-binding loops that protrude into the centre of the helicase (Enemark and Joshua-Tor, 2006). This configuration strongly suggests that the ATPase sites of E1 fire in a sequential manner similar to that seen for F1-ATPase, although six catalytic sites are utilized instead of three (Abrahams et al., 1994; Enemark and Joshua-Tor, 2006). Comparison of the differences between empty and nucleotide-bound active sites indicates that ATP-binding triggers inter-subunit clamping movements similar to those observed in RecA-type proteins (Fig. 2D). These movements, in turn, appear coupled to the shifting of substrate-binding loops emanating from the AAA+ fold and the translocation of single-stranded DNA through the central pore of the hexameric E1 motor (Enemark and Joshua-Tor, 2006). Structures of HslU (Bochtler et al., 2000; Song et al., 2000; Sousa et al., 2000; Wang et al., 2001a; Wang et al., 2001b), NtrC (Lee et al., 2003b), PspF (Rappas et al., 2005; Rappas et al., 2006), SV40 large T-antigen (Gai et al., 2004), and FtsH (Niwa et al., 2002; Suno et al., 2006) likewise exhibit ATP-dependent clamping movements and/or the displacement of internal substrate-binding loops, suggesting that a binding-ratchet motion might be a conserved feature of these oligomeric motors.
Despite relying on a common set of ATP-induced conformational changes, the relative relationship between inactive and active catalytic centres and their firing order might be variable. For example, the asymmetric structure of the E1 helicase bound to DNA strongly supports a rotary mechanism for ATP turnover (Enemark and Joshua-Tor, 2006) in which all six subunits sequentially take their turns binding and hydrolyzing nucleotide. By contrast, the SV40 large T-antigen has been imaged in a number of symmetric, nucleotide-dependent conformational states that have been interpreted as evidence for a concerted, all-or-none DNA translocation mechanism (Gai et al., 2004). Biochemical analysis of a covalently-linked ClpX hexamer, which allowed exquisite control over the number and placement of mutant subunits, has shown that activity is related to the number of active subunits within the hexamer, and that even a single catalytic subunit can support peptide translocation (Martin et al., 2005); these latter finding have been used to invoke a stochastic firing mechanism for the enzyme. As with the RecA-like ring-shaped motors, the coupling of ATP hydrolysis within the ring assembly to substrate movement through the motor might depend on whether the ATPase motor acts on protein or nucleic acid targets. In addition, it should be noted that E1 is the only substrate-bound hexameric AAA+ ATPase to be imaged at high resolution, raising the possibility that many of the symmetric structures observed in hexameric ATPases might not reflect fully functional states.
With respect to ASCE ATPases as a group, it is interesting that members of two different subgroups, E1 (AAA+) and F1 (RecA) have markedly different functions in the cell, yet both appear to use a sequential approach for coupling ATP turnover to the movement of target macromolecules. Despite this similarity, both proteins orient their ATPase folds in a distinct manner with respect to the central axis of the ring, and both use different secondary-structural elements to engage substrate (Wang, 2004). Given these parallels and differences, it will be interesting to see if other members of these two ASCE families have evolved similar motor mechanisms.
GHL Proteins
GHL proteins comprise a subset of molecular motors and switches within the GHKL ATPases, a diverse group of proteins named for the principal nucleotide-binding fold found in type II topoisomerases (DNA Gyrase), the chaperone Hsp90, the histidine Kinases and MutL repair factors (Dutta and Inouye, 2000), reviewed in (Corbett and Berger, 2004; Pearl and Prodromou, 2006; Wolanin et al., 2002; Yang, 2000). Like ASCE-class motor proteins, structural studies of GHL enzymes have revealed a common set of physical mechanisms that underlie a rich functional diversity. In particular, all microbial GHL proteins use ATP to trigger the dimerization of their ATPase domains and regulate the engagement of client macromolecular substrates.
GHL ATPases are composed of two structural domains, the ATP-binding GHKL core, and an α/β domain, sometimes referred to as the transducer element (Fig. 3A). The GHKL domain is a two layered α/β sandwich consisting of an eight-stranded, predominantly anti-parallel β-sheet with four to six α-helices situated on one face (Wigley et al., 1991). The transducer domain consists of a four- or five-stranded mixed β-sheet sandwiched between three to four interconnecting α-helices, and constitutes a fold found also in certain RNA binding proteins (Murzin, 1995). The GHL ATPase architecture was first observed in the structure of the E. coli gyrase B N-terminal domain (Wigley et al., 1991); however the family was defined only after comprehensive sequence analyses (Bergerat et al., 1997), along with structural studies of MutL (Ban and Yang, 1998) and Hsp90 (Meyer et al., 2003; Prodromou et al., 1997), revealed the conservation of the nucleotide-binding fold (Dutta and Inouye, 2000). Unlike ASCE P-loop motors, which situate the ATP-binding site on loops protruding from the C-terminal tips of a central β-sheet, the ATP-binding site of GHL proteins is located on the α-helical face of the GHKL domain within a large cleft partially bounded by the transducer domain (Wigley et al., 1991). Another distinct feature of GHL proteins is the presence of an N-terminal segment of polypeptide chain that is used by each subunit to engage the ATPase site of a partner protomer. This region, sometimes termed the “strap,” often contributes amino acids that directly coordinate bound nucleotide and help seal off the active site cleft (Ali et al., 2006; Ban and Yang, 1998; Brino et al., 2000; Wigley et al., 1991).
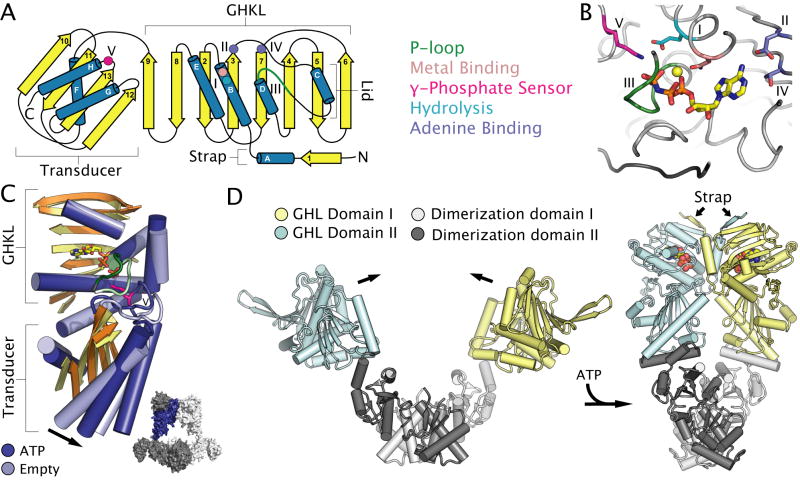
Fig. 3. Nucleotide binding and conformational changes in GHL ATPases.
A. Topology diagram of the two-domain GHL fold. The GHKL and transducer domain are labelled. Conserved motifs, catalytic residues and secondary structural elements are coloured as in Figure 1. Roman numerals define conserved motifs of GHL motors.
B. The active site of A. aeolicus topoisomerase VI (PDB entry 1MX0) (Corbett and Berger, 2003) highlighting the location of catalytic residues common to all GHL ATPases. Active site elements are coloured as in Figure 1.
C. Structural superposition of the GHL fold of ATP-bound (PDB entry 1MX0) (dark blue) and empty (PDB entry 1MU5) (light blue) B-subunits of Sulfolobus shibatae topoisomerase VI (Corbett and Berger, 2003), with most loops removed for clarity. Arrows highlight ATP-dependent conformational changes discussed in the text. Inset: the location of the ATP-bound subunit (shown in dark blue) with respect to the quaternary structure of the topoisomerase VI heterotetramer (with subunits shown in light and dark grey).
D. ATP-dependent conformational changes in a GHL homodimer illustrated by the structures of apo E. coli HtpG (PDB entry 2IOQ) (Shiau et al., 2006) and ATP-bound S. cerevisiae Hsp90 (PDB entry 2CGE) (Ali et al., 2006). ATP is shown as coloured spheres. Subunits of the homodimer are coloured cyan and dark grey, or yellow and light grey, with the cyan and yellow portion of each subunit corresponding to the conserved GHL fold.
Four conserved sequence motifs, all of which contribute residues to the ATP-binding site, are found in GHKL family members (Dutta and Inouye, 2000), along with a fifth motif in the transducer that defines the GHL subclass (Fig. 3B). Motif I, located at the C-terminus of helix α-B contains a conserved glutamate that activates a water molecule for attack on the γ-phosphate (Ban and Yang, 1998; Jackson and Maxwell, 1993). Motif I also includes a conserved asparagine residue that ligands a Mg2+ ion required for nucleotide binding and hydrolysis (Wigley et al., 1991). Motif II contains an aspartate and glycine at the C-terminus of strand β-3 that make specific contacts to the adenine ring and strictly enforce the use of ATP over other nucleoside triphosphate cofactors (Dutta and Inouye, 2000; Wigley et al., 1991). Motif III, located between helix α-C and helix α-D, is composed of a glycine-rich, phosphate-binding region that is part of a large loop containing one or more α-helices known as the “ATP-lid” (Dutta and Inouye, 2000; Wigley et al., 1991). Motif IV, which lies adjacent to motif II at the N-terminus of strand β-7, contains a conserved threonine that makes further base-specific contacts with the adenine ring. Finally, motif V consists of a conserved basic residue located on a loop emanating from the transducer domain between strand β-13 and helix α-H. This positively-charged residue acts as a γ-phosphate sensor and is thought to be linked to the propagation of nucleotide-dependent conformational changes between the GHKL and transducer folds (Ali et al., 2006; Ban et al., 1999; Corbett and Berger, 2005; Wigley et al., 1991). In this regard, motif V is a functional analogue of the arginine finger found in ASCE P-loop motors.
Two distinct types of intra-subunit conformational changes occur during ATP turnover in GHL proteins. One is movement of the transducer domain with respect to the N-terminal GHKL region, while the other is the opening and closing of the ATP binding lid. The effects of nucleotide binding on these structural transitions have been seen in MutL (Ban et al., 1999), Hsp90 (Ali et al., 2006) and type II topoisomerases (Fig. 3C) (Bellon et al., 2004; Corbett and Berger, 2005). In the presence of ATP mimetics or transition-state analogues, the transducer domain rotates up and into the GHKL fold, inserting the motif V lysine into the active site where it contacts the γ-phosphate. In the absence of nucleotide, the transducer rotates away from the GHKL region, flipping the lysine into solution. Interestingly, in type II topoisomerases, ADP can support the adoption of both ATP-bound “restrained” and apo “relaxed” states, suggesting that nucleotide hydrolysis might not “power” conformational changes directly, but instead likely serves to facilitate the transition between the different structural states (Corbett and Berger, 2005; Wei et al., 2005). Multiple conformations of the transducer domain in relation to the GHKL domain also have been observed in the E. coli Hsp90 homologue HtpG in the presence of ADP, highlighting the importance of the γ-phosphate in stabilizing the restrained state (Huai et al., 2005; Shiau et al., 2006). The conformation of the lid region, which varies in size among different GHL family members, is also altered in an ATP-dependent manner. In type II topoisomerases and MutL, ATP binding orders the flexible lid, further stabilizing GHKL dimer contacts (Ban and Yang, 1998; Ban et al., 1999; Corbett and Berger, 2003; Lamour et al., 2002). A variety of conformations likewise have been observed for this region in different nucleotide-bound states of E. coli HtpG (Huai et al., 2005; Shiau et al., 2006) and yeast Hsp90 (Ali et al., 2006). The conformational variability in the particularly large Hsp90-family lids appears to alternatively expose or hide a number of hydrophobic residues, including some that are important for inter-subunit contacts, and others that might play a role in client protein binding (Shiau et al., 2006).
Structural studies of full-length Hsp90 chaperones (Ali et al., 2006; Shiau et al., 2006) and type IIB topoisomerases (Corbett et al., 2007; Graille et al., 2008) have at last begun to reveal the scope and nature of the large scale inter-subunit conformational changes that take place in intact GHL assemblies. Most notably, ATP-induced dimerization of the GHKL domains leads to a large, en-bloc movement akin to the closing of pincers (Fig. 3D). Although Hsp90 is thought to utilize this movement to pull on or remodel proteins and protein complexes, type II topoisomerases and MutL use it to trap DNA substrates in a central cavity between the GHL and C-terminal dimerization domains (Fig. 3C inset). It remains to be seen precisely how GHL proteins engage target protein/nucleic acid substrates and how these binding events are linked to the timing of ATP hydrolysis.
Actin fold
The actin-fold superfamily includes two types of motor/remodelling proteins in bacteria: the filament forming actin-related proteins and the Hsp70 class of ATP-dependent chaperones (Kabsch and Holmes, 1995). Both actin and Hsp70 function as ATP-dependent molecular switches that couple conformational changes to directed action upon a protein substrate (Genevaux et al., 2007; Goloubinoff and De Los Rios, 2007; Mogilner and Oster, 2003; Sousa and Lafer, 2006), however, the cellular functions of these two motors are very different. The Hsp70 monomer assists with protein folding, intracellular transport, and the remodelling of protein complexes by undergoing physical rearrangements that move a substrate-binding domain in relation to the actin fold (Sousa and Lafer, 2006). By contrast, filamentous actin polymerizes into extended multi-subunit assemblies that are able to exert force on objects at the filament ends via a Brownian ratchet mechanism (Mogilner and Oster, 2003). Although force generation for prokaryotic actins has yet to be measured directly, numerous lines of evidence suggest that these proteins play a motor function in the cell (Carballido-Lopez, 2006; Garner et al., 2004; Garner et al., 2007; Graumann and Defeu Soufo, 2004; Michie and Lowe, 2006). Despite their significant oligomeric and functional differences, actin-fold motors utilize a common nucleotide-dependent clamping mechanism, in which the differential movement of a pair of catalytic modules either directly generates movement of client-substrate binding domains (Hsp70), or generates binding interfaces that facilitate polymerization (actin).
The actin fold was first observed in the structure of hexokinase (Fletterick et al., 1975). It was not until 15 years later, however, that structures of eukaryotic actin and Hsp70 proteins became available to define the family (Bork et al., 1992; Flaherty et al., 1990; Flaherty et al., 1991; Kabsch et al., 1990); the discovery of prokaryotic actin filaments came later still (Jones et al., 2001; van den Ent et al., 2001). All structures of microbial actin homologues determined to date have revealed a two-domain architecture, with each domain divided into one large and one variable small subdomain. The two large subdomains (Ia and IIa) each consist of a repeat of a five-stranded, mixed β-sheet sandwiched between three α-helices. These two elements form the conserved core of the actin fold (Fig. 4A), with the base of the ATP binding cleft formed by helices α-C and α-F on the rear face of each β-sheet (Bork et al., 1992).
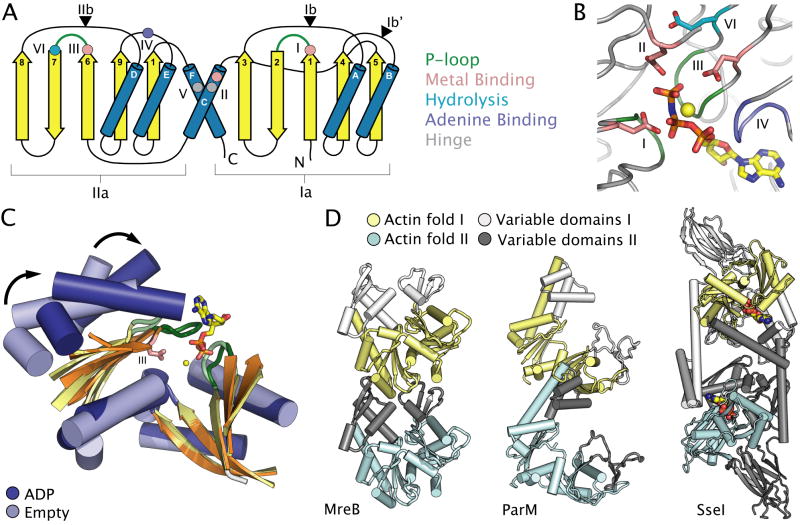
Fig. 4. Nucleotide binding and conformational changes in actin-fold ATPases.
A. Topology diagram of the two-domain actin fold. Conserved motifs, catalytic residues and secondary structural elements are coloured as in Figure 1. Roman numerals define conserved motifs of the actin fold. Triangles represent common insertion sites for the Ia and Ib subdomains.
B. The active site of Thermotoga maritima MreB (PDB entry 1JCG) (van den Ent et al., 2001) highlighting the location of catalytic residues common to all actin fold ATPases. Active site elements are coloured as in Figure 1.
C. Structural superposition of the actin fold of ADP-bound (PDB entry 1MWM) (dark blue) and apo (PDB entry 1MWK) (light blue) subunits of E. coli ParM (van den Ent et al., 2002), with most loops removed for clarity. Arrows highlight ATP-dependent conformational changes discussed in the text.
D. A variety of higher order oligomers observed in the actin-fold family. Structures correspond to T. maritima MreB (PDB entry 1JCE) (van den Ent et al., 2001), E. coli ParM (PDB entry 2QU4) (Orlova et al., 2007) and S. cerevisiae Sse1 (PDB entry 2QXL) (Liu et al., 2007). Subunits of the oligomers are coloured cyan and dark grey, or yellow and light grey, with the cyan and yellow portion of each subunit corresponding to the conserved actin fold. Bound nucleotide is shown as coloured spheres. The actin fold refers specifically to subdomains Ia and IIa, while subdomains Ib and IIb can be more variable. Regions coloured in grey correspond either to the variable Ib and IIb subdomains, or to the substrate binding domains found only in Hsp70-class chaperones.
Five sequence motifs, all lining the ATP-binding site, are highly conserved among the actin fold family, with a sixth motif found specifically in the actin and Hsp70 motors (Fig. 4B) (Bork et al., 1992). Motifs I and III form glycine-rich, phosphate-binding loops between strands β-1/β-2 and β-6/β-7, respectively. Both loops contribute to the binding of a single molecule of ATP (Flaherty et al., 1990; Kabsch et al., 1990). Each of these loops also contains a single acidic residue used in coordinating a divalent cation, typically Mg2+, which contacts the β- and γ-phosphates of bound ATP (Flaherty et al., 1990; Kabsch et al., 1990). Motif IV is rich in hydrophobic residues that stack against the hydrophobic face of the adenine ring, and contains an invariant glycine that is in close proximity to the α-phosphate (Flaherty et al., 1991). Motifs II and V lie on helices α-C and α-F, respectively, and define the closest contact points between the two large subdomains (Flaherty et al., 1990; Kabsch et al., 1990). The presence of a conserved alanine in motif II and a glycine in motif V is likely favoured by the fact that these amino acids define a major hinge point for ATP-dependent structural transitions in the actin fold (Bork et al., 1992). A negatively-charged or polar residue in motif II at the N-terminus of helix α-C additionally is involved in coordinating the divalent cation located in the active site (Flaherty et al., 1990; Kabsch et al., 1990). Motif VI consists of a single conserved acidic residue located in strand β-7 that is thought to activate a water molecule for in-line attack on the γ-phosphate of ATP (Flaherty et al., 1991). Finally, the two smaller subdomains (Ib and IIb) further contribute residues to the ATP binding centre. The structures and placement of these elements can vary among different actin fold members. For example, in the prokaryotic actin homologue FtsA, subdomain Ib is located between helix α-B and strand β-4, whereas in other actin and Hsp70 homologues, this module is positioned between helix α-A and strand β-3 (Fig. 4A) (van den Ent and Lowe, 2000).
Early structures of hexokinase provided the first glimpse into the conformational plasticity of the actin fold (Steitz et al., 1981). A common inter-domain “propeller rotation” mechanism has since been suggested to unify the action of the family (Galkin et al., 2002). This movement is somewhat akin to the ATP-clamping action of SF1/SF2 helicases. However, the bipartite ATP-binding site in SF1/SF2 proteins is formed via a front-to-back arrangement of two homologous RecA domains, whereas the bipartite ATP-binding site of the actin fold is formed via a back-to-back arrangement of two homologous actin-fold subdomains (Kabsch et al., 1990; Subramanya et al., 1996). Structural studies of the bacterial actin homologue ParM, which is believed to use its polymerizing capabilities for segregating low copy number plasmids (Garner et al., 2007), clearly illustrate the nucleotide-dependent conformational changes that take place in a microbial actin (Fig. 4C) (van den Ent et al., 2002), revealing that the two large subdomains of the protein undergo a 25° rigid body rotation upon binding nucleotide. Interestingly, examination of the catalytic centre of actin-fold family members (Fig. 4B) shows that these proteins do not contain conserved arginine/lysine γ-phosphate sensors like GHL and ASCE P-loop motors. Instead, these proteins contain Mg2+-binding residues in the Ia and IIa subdomains that appear to undergo significant conformational changes upon adopting a nucleotide-bound conformation (Figs. 4A, 4C). Thus, these Mg2+ sensors might serve as signal relays for ATP-induced domain movements.
Hsp70-class chaperones differ from actin-based polymerization motors by virtue of an additional, C-terminal substrate-binding domain, the movement of which might be coupled to ATP-dependent conformational changes in the N-terminal actin fold. Most structures of Hsp70 homologues have exhibited an open or inactive orientation between the two large subdomains of the actin fold, but a recent structure of the yeast Hsp70 paralogue Sse1 has successfully captured an ATP-bound state for the first time (Liu and Hendrickson, 2007). Together with structurally-informed mutational analyses, comparison of this intermediate with other structures of prokaryotic and eukaryotic Hsp70 family members suggests that an actin-like inter-domain conformational rearrangement serves as the basis of function in this chaperone family (Liu and Hendrickson, 2007).
The relative orientation between subunits in different actin-fold families appears to vary significantly. The first high-resolution experimental structure of a filamentous actin (F-actin) was the bacterial actin homologue MreB bound to the ATP analogue AMP-PNP (van den Ent et al., 2001). This structure revealed a subunit interface similar to a theoretical model of eukaryotic F-actin obtained by fitting the crystal structure to X-ray fibre diffraction data (Fig. 4D) (Holmes et al., 1990; van den Ent et al., 2001). Remarkably, the subunit spacing captured in MreB crystals proved nearly identical to that seen in electron micrographs of MreB filaments formed in vitro (van den Ent et al., 2001), and is similar to the inter-subunit interactions within crystallographic filaments of the nucleotide-bound archaeal actin homologue Ta0583, from Thermoplasma acidophilum (Roeben et al., 2006). By contrast, ParM exhibits large structural differences in its inter-subunit interface compared to other prokaryotic and eukaryotic actins (Fig. 4D), primarily due to differences in the structure of its Ib and IIb subdomains (van den Ent et al., 2002). Two recent models of the ParM filament based on three-dimensional electron microscopic (EM) reconstructions reveal a unique set of subunit interfaces and an opposite helical handedness to that of F-actin, providing further evidence of the differences between ParM and other actin homologues. However, there is still some disagreement as to the relative orientations of actin monomers within the filament (Orlova et al., 2007; Popp et al., 2008). Based on the observation of both right and left handed filaments, it has been suggested that polymerization of actin proteins might have arisen more than once in evolution (Egelman, 2003; Orlova et al., 2007).
Hsp70 chaperones do not typically form higher-order oligomers, but are regulated by and require nucleotide exchange factors (NEFs) for function (Genevaux et al., 2007). Yeast Sse1 is thought to act as an NEF for cytosolic Hsp70s, and a recent Sse1 structure exhibits a dimeric arrangement of protomers that might recapitulate the Sse1-Hsp70 interface (Liu and Hendrickson, 2007). Interestingly, this dimer interface arises primarily from contacts between the small Ib and IIb domains as well as the Hsp70-specific substrate binding domain (SBDα) of both subunits (Liu and Hendrickson, 2007), forming a head-to-head orientation that differs from the head-to-tail configuration observed between protomers in actin filaments (Fig 4D). Thus, unlike other ATP-dependent molecular motors, members of the actin-fold family appear to have linked a common internal conformational change to unique forms of inter-subunit associations, giving rise to significantly different motor mechanisms.
Chaperonins
Chaperonins are among the most specialized and best-understood class of ATP-dependent molecular motors. Two families of chaperonins exist and, although their quaternary organizations are unique, both are based on a structurally homologous three-domain fold. The type I chaperonins, found in bacteria, mitochondria and chloroplasts, consist of 14 identical subunits arrayed into two heptameric rings stacked back-to-back against each other. By comparison, type II chaperonins are found in archaea and eukaryotes, and consist of two eight- or nine-subunit hetero-oligomeric rings stacked back-to-back (Horovitz and Willison, 2005). During the reaction cycle, the rings of type I chaperonins can be capped by a co-chaperonin lid (Horovitz and Willison, 2005), creating an interior cavity for protein substrate folding. Typically, only one of the rings is capped by the lid at any given time, leading to an enclosed “cis-ring” and an open “trans-ring” (Chen et al., 1994; Ishii et al., 1992; Langer et al., 1992; Saibil et al., 1993; Xu et al., 1997), although doubly capped complexes might also play a role in the chaperonin cycle (Azem et al., 1994; Beissinger et al., 1999; Inobe et al., 2008; Llorca et al., 1994, 1997a, b; Schmidt et al., 1994; Sparrer et al., 1997). The cis- and trans-rings are thought to alternate back and forth, utilizing positive cooperativity for ATP binding within a single ring, and negative cooperativity for ATP binding between rings to drive the process (Bochkareva et al., 1992; Bochkareva and Girshovich, 1994; Gray and Fersht, 1991; Horovitz and Willison, 2005; Rye et al., 1997; Rye et al., 1999). During this reaction, chaperonin subunits undergo dramatic conformational changes that are believed to promote active unfolding of mis-folded substrates (Krishna et al., 2007a; Lin and Rye, 2006; Motojima et al., 2004; Roseman et al., 1996; Shtilerman et al., 1999; Xu et al., 1997).
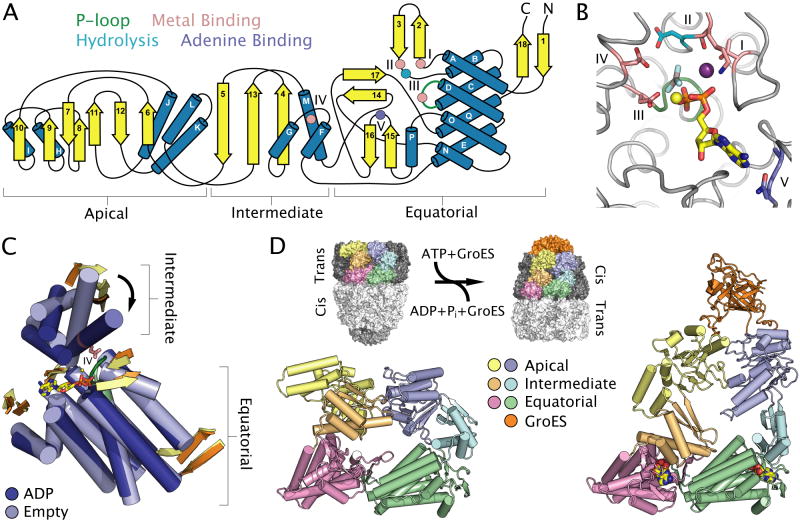
GroEL, a type I bacterial chaperonin, has the most complex topology of the microbial protein/nucleic-acid dependent motor ATPases. The chaperonin protomer consists of three distinct domains that are each composed of N- and C-terminal regions of the polypeptide chain (Fig. 5A) (Braig et al., 1994). The equatorial domain, which forms the inter-ring contacts and contains most of the ATP-binding residues, is composed of a two-layered α-helical sandwich and a series of small β-hairpins (Braig et al., 1994). Like other motor families, the phosphate groups of ATP are bound by a series of flexible loops, but the chaperonins represent the only class whose ATP-binding fold is not predicated on a central β-sheet element. The elongated intermediate domain, which contains other motifs involved in ATP binding, comprises a three-stranded β-sheet and a three-helix bundle, and serves to transmit allosteric signals between the equatorial and apical domains (Braig et al., 1994; Xu et al., 1997). The apical domain is composed of two stacked β-sheets and a series of five α-helices, and is involved in binding both substrate polypeptides and the GroES lid (Lin and Rye, 2006).
Fig. 5. Nucleotide binding and conformational changes in chaperonins.
A. Topology diagram of the three-domain chaperonin fold. Conserved motifs, catalytic residues and secondary structural elements are coloured as in Figure 1. Roman numerals define conserved motifs of the chaperonin fold.
B. The active site of E. coli GroEL (PDB entry 1PCQ) (Chaudhry et al., 2003) highlighting the location of catalytic residues common to type I and type II chaperonins. Active site elements are coloured as in Figure 1. The active-site potassium ion is shown as a purple sphere.
C. Structural superposition of the equatorial and intermediate domains of ADP- and GroES-bound (dark blue) and apo (light blue) subunits of E. coli GroEL (PDB entry 1AON) (Xu et al., 1997), with most loops removed for clarity. Arrows highlight ATP dependent conformational changes discussed in the text.
D. ATP-dependent conformational changes within the GroEL-GroES complex (PDB entry 1AON) (Xu et al., 1997). The equatorial (pink and green), intermediate (light orange and cyan) and apical (yellow and light blue) domains of two adjacent protomers are coloured to reveal the range of conformational changes and altered inter-subunit contacts. GroES is coloured orange. The left structure illustrates a dimer interface in the context of the apo cis-ring, while the right structure illustrates the dimer interface bound to ADP and GroES in the context of the trans-ring. ADP bound to the cis-ring is shown as coloured spheres. Inset: Effect of conformational changes on the GroEL14-GroES7 complex in which the two GroEL rings alternately bind and release GroES and substrate polypeptides. The two GroEL rings are illustrated in light and dark grey, while the subunits shown in the main panel are highlighted in colour.
The conserved ATP-binding residues, as observed in structures of GroEL bound to nucleotide, can be divided into five motifs (Fig. 5B). Motifs I and II lie at the base of the β-2/β-3 hairpin and contain residues involved in binding both the α-phosphate of ATP and a K+ ion that is critical for hydrolysis (Boisvert et al., 1996; Wang and Boisvert, 2003). Motif II further includes an aspartate that is thought to activate a water molecule for nucleophilic attack on the γ-phosphate (Chaudhry et al., 2003; Ditzel et al., 1998). Motif III resides on a loop between helices α-C and α-D and contains a highly conserved GDGTTT sequence that acts as the primary β- and γ-phosphate binding loop (Boisvert et al., 1996). The conserved aspartate in this region also helps to coordinate a Mg2+ ion, which contacts all three phosphates of ATP (Boisvert et al., 1996). Motif IV consists of a conserved aspartate on helix α-M of the intermediate domain and coordinates Mg2+ when GroES is bound (Chaudhry et al., 2003). Motif V is located on the hairpin loop between strands β-15 and β-16 of the equatorial domain and is primarily involved in making specific contacts to the adenine ring (Boisvert et al., 1996).
All structures of GroEL illustrate some degree of conformational variability within their 14 subunits; however, structures bound to ATP, protein substrate, and the co-chaperonin lid GroES (alone or in combination) have revealed the wide scope of conformational changes that take place within the chaperonin during catalysis. ATP binding causes three distinct but subtle conformational changes per protomer: movement of the β-2/β-3 hairpin, a lateral translation of helix α-C, and a subtle rigid-body rotation of the apical and intermediate domains with respect to the equatorial domain (Boisvert et al., 1996; Ranson et al., 2001; Wang and Boisvert, 2003). Substrate binds to the apical domain surface formed by helices H and I (Chen and Sigler, 1999), and induces modest conformational shifts in this domain with respect to the rest of the protein (Wang and Chen, 2003). In light of a recent apo structure of GroEL (Bartolucci et al., 2005), as well as a cryo-EM study of a GroEL substrate complex (Falke et al., 2005), both substrate and ATP binding appear to cause a counter clockwise rotation of the apical domains.
Structural studies of GroEL-GroES bound to ADP have revealed that the binding of GroES creates much more drastic conformational changes in GroEL than nucleotide alone, giving rise to a downward motion of the intermediate domain to close off the nucleotide-binding site, and a dramatic upward movement and twist of the apical domain to engage the co-chaperonin lid (Fig. 5C and D) (Xu et al., 1997). This conformational change moves helices α-I and α-H from an exposed position in the centre of the ring to a buried position in the GroEL-GroES interface, creating a large, hydrophilic interior cavity. Since these two helices form the primary interaction between GroEL and hydrophobic peptides (Chen and Sigler, 1999; Wang and Chen, 2003), this GroES-induced rearrangement might constitute one mechanism by which substrate is released into the central cavity. Structures of the GroEL-GroES complex bound to the transition-state mimic ADP·AlF3, as well as a subsequent computational analysis of this and previous structures, have suggested that the γ-phosphate of ATP stabilizes the GroES-bound state by interacting with the motif IV aspartate through the active-site Mg2+ ion (Chaudhry et al., 2003; Chaudhry et al., 2004). A nearly identical interaction was observed in an ADP·AlF3 bound structure of the archaeal thermosome, a type II chaperonin (Ditzel et al., 1998). As with the actin fold family, chaperonins appear to lack a conserved arginine or lysine γ-phosphate sensor. The motif IV aspartate might serve instead as a Mg2+ sensor responsible for transmitting ATP binding signals between the equatorial and intermediate domains.
As might be expected from such large conformational transitions, the inter-subunit contacts among the 14 GroEL subunits also change significantly during the ATPase cycle. Consideration of the range and nature of observed conformations helps to explain the positive and negative cooperativity present in the allosteric transitions of this enzyme. Crystallographic models of the GroEL-GroES-ADP and GroEL-ATP complexes, as well as recent cryo EM studies of GroEL-GroES- and GroEL-nucleotide complexes, have all provided support for direct communication between the ATP binding sites of neighbouring subunits within a contiguous ring (Boisvert et al., 1996; Ranson et al., 2001; Ranson et al., 2006; Xu et al., 1997). Specifically, ATP-dependent movement of the β-2/β-3 hairpin disrupts a four-stranded β-sheet formed between this hairpin and the β-1/β-18 elements of an adjacent subunit. The large conformational change induced by GroES binding further alters many inter-subunit interactions (Fig. 5D). The most notable of these is a new contact formed between helix α-M of one subunit and helix α-C of an adjacent protomer. The fact that both of these helices are involved in intra- and inter-subunit conformational changes provides a possible explanation for the all-or-none cooperativity observed to arise from ATP-binding to a single GroEL ring (Bochkareva et al., 1992; Gray and Fersht, 1991). Finally, the inter-ring interface contains a series of structurally important salt bridges that provide direct communication between ATP-binding equatorial domains in either half of the particle (Bartolucci et al., 2005; Cabo-Bilbao et al., 2006; Xu et al., 1997). Cryo-EM studies of GroEL and GroEL-GroES complexes in the presence or absence of ATP have supported this idea by revealing conformational changes in the inter-ring interface (Ranson et al., 2001; Ranson et al., 2006). These findings provide a physical explanation for the negative cooperativity of ATP binding between the back-to-back GroEL heptamers (Bochkareva and Girshovich, 1994; Horovitz and Willison, 2005; Rye et al., 1997; Rye et al., 1999), and indicate that ATP binding to the trans-ring may provide the energy to eject Mg2+-ADP, GroES and substrate allosterically from the cis-ring to restart the cycle (Fig. 5D inset). Although the story is far from complete, these studies beautifully illustrate how a combination of low- and high-resolution structural analyses on distinct catalytic intermediates can reveal the dynamic architectural transitions in a complex molecular machine.
Conclusion
In microbes, ATP-dependent protein/nucleic-acid motors and remodelling factors range from the relatively specialized chaperonins to the remarkably diverse ASCE P-loop ATPases. Although each of these families utilizes a unique motor mechanism, certain common principles appear to underlie their functions. The phosphate groups of ATP are generally bound using flexible, often glycine-rich, loops that connect secondary structural elements. Acidic or polar residues are typically used to orient and/or activate a water molecule for nucleophilic attack on the γ-phosphate of ATP, promoting hydrolysis. Active sites are always located near an inter-domain or inter-subunit interface, allowing ATP binding to create or rearrange global protein-protein contacts. Finally, adjacent γ-phosphate (ASCE P-loop, GHL) or Mg2+(actin fold, chaperonin) sensors provided by neighbouring domains or subunits help propagate conformational changes within quaternary motor assemblies. These distinct sensor mechanisms constitute an intriguing difference between the four motor families. In all instances, when linked to disparate substrate binding sites or accessory domains, the conformational changes resulting from nucleotide turnover are capable of propelling a motor along a substrate or forcibly remodelling biological polymers.
Despite the large number of conformational states imaged by high-resolution structural studies over the past few years, much remains to be learned. For the majority of motors, it is still unclear how the conformational changes observed thus far are linked to the directed movement of protein and nucleic acid polymers. Moreover, there is often little direct evidence to explain how ATP binding and hydrolysis are coupled between different subunits in oligomeric motor complexes. These questions still exist, in part, because most structures imaged to date have been obtained in the absence of client substrates. Definitive insights into motor protein activity and the role of ATP await more high-resolution structures examining the effects of nucleotide turnover on protein- or nucleic-acid bound complexes. Such studies will help connect structural models to biochemical and single-molecule efforts to examine motor dynamics in solution. Given that most microbial motor ATPases and their eukaryotic homologues are critical for many fundamental cellular processes, mechanistic understanding of their function is likely to have important consequences for basic science and human health alike.
Supplementary Material
References
- Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azem A, Kessel M, Goloubinoff P. Characterization of a functional GroEL14(GroES7)2 chaperonin hetero-oligomer. Science. 1994;265:653–656. doi: 10.1126/science.7913553. [DOI] [PubMed] [Google Scholar]
- Bailey S, Eliason WK, Steitz TA. Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science. 2007;318:459–463. doi: 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- Ban C, Yang W. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell. 1998;95:541–552. doi: 10.1016/s0092-8674(00)81621-9. [DOI] [PubMed] [Google Scholar]
- Ban C, Junop M, Yang W. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 1999;97:85–97. doi: 10.1016/s0092-8674(00)80717-5. [DOI] [PubMed] [Google Scholar]
- Bartolucci C, Lamba D, Grazulis S, Manakova E, Heumann H. Crystal structure of wild-type chaperonin GroEL. J Mol Biol. 2005;354:940–951. doi: 10.1016/j.jmb.2005.09.096. [DOI] [PubMed] [Google Scholar]
- Beissinger M, Rutkat K, Buchner J. Catalysis, commitment and encapsulation during GroE-mediated folding. J Mol Biol. 1999;289:1075–1092. doi: 10.1006/jmbi.1999.2780. [DOI] [PubMed] [Google Scholar]
- Bellon S, Parsons JD, Wei Y, Hayakawa K, Swenson LL, Charifson PS, Lippke JA, Aldape R, Gross CH. Crystal structures of Escherichia coli topoisomerase IV ParE subunit (24 and 43 kilodaltons): a single residue dictates differences in novobiocin potency against topoisomerase IV and DNA gyrase. Antimicrob Agents Chemother. 2004;48:1856–1864. doi: 10.1128/AAC.48.5.1856-1864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- Bochkareva ES, Lissin NM, Flynn GC, Rothman JE, Girshovich AS. Positive cooperativity in the functioning of molecular chaperone GroEL. J Biol Chem. 1992;267:6796–6800. [PubMed] [Google Scholar]
- Bochkareva ES, Girshovich AS. ATP induces non-identity of two rings in chaperonin GroEL. J Biol Chem. 1994;269:23869–23871. [PubMed] [Google Scholar]
- Bochtler M, Hartmann C, Song HK, Bourenkov GP, Bartunik HD, Huber R. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–805. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- Boisvert DC, Wang J, Otwinowski Z, Horwich AL, Sigler PB. The 2.4 A crystal structure of the bacterial chaperonin GroEL complexed with ATP gamma S. Nat Struct Biol. 1996;3:170–177. doi: 10.1038/nsb0296-170. [DOI] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci U S A. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Braig K, Menz RI, Montgomery MG, Leslie AG, Walker JE. Structure of bovine mitochondrial F(1)-ATPase inhibited by Mg(2+) ADP and aluminium fluoride. Structure. 2000;8:567–573. doi: 10.1016/s0969-2126(00)00145-3. [DOI] [PubMed] [Google Scholar]
- Brino L, Urzhumtsev A, Mousli M, Bronner C, Mitschler A, Oudet P, Moras D. Dimerization of Escherichia coli DNA-gyrase B provides a structural mechanism for activating the ATPase catalytic center. J Biol Chem. 2000;275:9468–9475. doi: 10.1074/jbc.275.13.9468. [DOI] [PubMed] [Google Scholar]
- Buttner K, Nehring S, Hopfner KP. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat Struct Mol Biol. 2007;14:647–652. doi: 10.1038/nsmb1246. [DOI] [PubMed] [Google Scholar]
- Cabo-Bilbao A, Spinelli S, Sot B, Agirre J, Mechaly AE, Muga A, Guerin DM. Crystal structure of the temperature-sensitive and allosteric-defective chaperonin GroELE461K. J Struct Biol. 2006;155:482–492. doi: 10.1016/j.jsb.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R. The bacterial actin-like cytoskeleton. Microbiol Mol Biol Rev. 2006;70:888–909. doi: 10.1128/MMBR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruthers JM, McKay DB. Helicase structure and mechanism. Curr Opin Struct Biol. 2002;12:123–133. doi: 10.1016/s0959-440x(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Chaudhry C, Farr GW, Todd MJ, Rye HS, Brunger AT, Adams PD, Horwich AL, Sigler PB. Role of the gamma-phosphate of ATP in triggering protein folding by GroEL-GroES: function, structure and energetics. Embo J. 2003;22:4877–4887. doi: 10.1093/emboj/cdg477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry C, Horwich AL, Brunger AT, Adams PD. Exploring the structural dynamics of the E. coli chaperonin GroEL using translation-libration-screw crystallographic refinement of intermediate states. J Mol Biol. 2004;342:229–245. doi: 10.1016/j.jmb.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Chen L, Sigler PB. The crystal structure of a GroEL/peptide complex: plasticity as a basis for substrate diversity. Cell. 1999;99:757–768. doi: 10.1016/s0092-8674(00)81673-6. [DOI] [PubMed] [Google Scholar]
- Chen S, Roseman AM, Hunter AS, Wood SP, Burston SG, Ranson NA, Clarke AR, Saibil HR. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994;371:261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. Structure of the topoisomerase VI-B subunit: implications for type II topoisomerase mechanism and evolution. Embo J. 2003;22:151–163. doi: 10.1093/emboj/cdg008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu Rev Biophys Biomol Struct. 2004;33:95–118. doi: 10.1146/annurev.biophys.33.110502.140357. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. Structural dissection of ATP turnover in the prototypical GHL ATPase TopoVI. Structure. 2005;13:873–882. doi: 10.1016/j.str.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Benedetti P, Berger JM. Holoenzyme assembly and ATP-mediated conformational dynamics of topoisomerase VI. Nat Struct Mol Biol. 2007;14:611–619. doi: 10.1038/nsmb1264. [DOI] [PubMed] [Google Scholar]
- Ditzel L, Lowe J, Stock D, Stetter KO, Huber H, Huber R, Steinbacher S. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell. 1998;93:125–138. doi: 10.1016/s0092-8674(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Durr H, Korner C, Muller M, Hickmann V, Hopfner KP. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121:363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- Egelman EH. Actin's prokaryotic homologs. Curr Opin Struct Biol. 2003;13:244–248. doi: 10.1016/s0959-440x(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Enemark EJ, Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442:270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- Erzberger JP, Pirruccello MM, Berger JM. The structure of bacterial DnaA: implications for general mechanisms underlying DNA replication initiation. Embo J. 2002;21:4763–4773. doi: 10.1093/emboj/cdf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- Erzberger JP, Mott ML, Berger JM. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol. 2006;13:676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- Falke S, Tama F, Brooks CL, 3rd, Gogol EP, Fisher MT. The 13 angstroms structure of a chaperonin GroEL-protein substrate complex by cryo-electron microscopy. J Mol Biol. 2005;348:219–230. doi: 10.1016/j.jmb.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flaherty KM, McKay DB, Kabsch W, Holmes KC. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991;88:5041–5045. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletterick RJ, Bates DJ, Steitz TA. The structure of a yeast hexokinase monomer and its complexes with substrates at 2.7-A resolution. Proc Natl Acad Sci U S A. 1975;72:38–42. doi: 10.1073/pnas.72.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai D, Zhao R, Li D, Finkielstein CV, Chen XS. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell. 2004;119:47–60. doi: 10.1016/j.cell.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Galkin VE, VanLoock MS, Orlova A, Egelman EH. A new internal mode in F-actin helps explain the remarkable evolutionary conservation of actin's sequence and structure. Curr Biol. 2002;12:570–575. doi: 10.1016/s0960-9822(02)00742-x. [DOI] [PubMed] [Google Scholar]
- Garner EC, Campbell CS, Mullins RD. Dynamic instability in a DNA-segregating prokaryotic actin homolog. Science. 2004;306:1021–1025. doi: 10.1126/science.1101313. [DOI] [PubMed] [Google Scholar]
- Garner EC, Campbell CS, Weibel DB, Mullins RD. Reconstitution of DNA segregation driven by assembly of a prokaryotic actin homolog. Science. 2007;315:1270–1274. doi: 10.1126/science.1138527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevaux P, Georgopoulos C, Kelley WL. The Hsp70 chaperone machines of Escherichia coli: a paradigm for the repartition of chaperone functions. Mol Microbiol. 2007;66:840–857. doi: 10.1111/j.1365-2958.2007.05961.x. [DOI] [PubMed] [Google Scholar]
- Geourjon C, Orelle C, Steinfels E, Blanchet C, Deleage G, Di Pietro A, Jault JM. A common mechanism for ATP hydrolysis in ABC transporter and helicase superfamilies. Trends Biochem Sci. 2001;26:539–544. doi: 10.1016/s0968-0004(01)01907-7. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, De Los Rios P. The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem Sci. 2007;32:372–380. doi: 10.1016/j.tibs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth FX, Moncalian G, Perez-Luque R, Gonzalez A, Cabezon E, de la Cruz F, Coll M. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature. 2001;409:637–641. doi: 10.1038/35054586. [DOI] [PubMed] [Google Scholar]
- Graille M, Cladiere L, Durand D, Lecointe F, Gadelle D, Quevillon-Cheruel S, Vachette P, Forterre P, van Tilbeurgh H. Crystal Structure of an Intact Type II DNA Topoisomerase: Insights into DNA Transfer Mechanisms. Structure. 2008;16:360–370. doi: 10.1016/j.str.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Graumann PL, Defeu Soufo HJ. An intracellular actin motor in bacteria. Bioessays. 2004;26:1209–1216. doi: 10.1002/bies.20126. [DOI] [PubMed] [Google Scholar]
- Gray TE, Fersht AR. Cooperativity in ATP hydrolysis by GroEL is increased by GroES. FEBS Lett. 1991;292:254–258. doi: 10.1016/0014-5793(91)80878-7. [DOI] [PubMed] [Google Scholar]
- Guenther B, Onrust R, Sali A, O'Donnell M, Kuriyan J. Crystal structure of the delta' subunit of the clamp-loader complex of E. coli DNA polymerase III. Cell. 1997;91:335–345. doi: 10.1016/s0092-8674(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Tainer JA. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr Opin Struct Biol. 2003;13:249–255. doi: 10.1016/s0959-440x(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Horovitz A, Willison KR. Allosteric regulation of chaperonins. Curr Opin Struct Biol. 2005;15:646–651. doi: 10.1016/j.sbi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Huai Q, Wang H, Liu Y, Kim HY, Toft D, Ke H. Structures of the N-terminal and middle domains of E. coli Hsp90 and conformation changes upon ADP binding. Structure. 2005;13:579–590. doi: 10.1016/j.str.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Hung LW, Wang IX, Nikaido K, Liu PQ, Ames GF, Kim SH. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- Inobe T, Takahashi K, Maki K, Enoki S, Kamagata K, Kadooka A, Arai M, Kuwajima K. Asymmetry of the GroEL-GroES complex under physiological conditions as revealed by small-angle x-ray scattering. Biophys J. 2008;94:1392–1402. doi: 10.1529/biophysj.107.114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Taguchi H, Sumi M, Yoshida M. Structure of holo-chaperonin studied with electron microscopy. Oligomeric cpn10 on top of two layers of cpn60 rings with two stripes each. FEBS Lett. 1992;299:169–174. doi: 10.1016/0014-5793(92)80240-h. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004a;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Makarova KS, Koonin EV, Aravind L. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 2004b;32:5260–5279. doi: 10.1093/nar/gkh828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AP, Maxwell A. Identifying the catalytic residue of the ATPase reaction of DNA gyrase. Proc Natl Acad Sci U S A. 1993;90:11232–11236. doi: 10.1073/pnas.90.23.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Kabaleeswaran V, Puri N, Walker JE, Leslie AG, Mueller DM. Novel features of the rotary catalytic mechanism revealed in the structure of yeast F1 ATPase. Embo J. 2006;25:5433–5442. doi: 10.1038/sj.emboj.7601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Holmes KC. The actin fold. Faseb J. 1995;9:167–174. doi: 10.1096/fasebj.9.2.7781919. [DOI] [PubMed] [Google Scholar]
- Kim JL, Morgenstern KA, Griffith JP, Dwyer MD, Thomson JA, Murcko MA, Lin C, Caron PR. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Korolev S, Hsieh J, Gauss GH, Lohman TM, Waksman G. Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP. Cell. 1997;90:635–647. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Krishna KA, Rao GV, Rao KR. Chaperonin GroEL: structure and reaction cycle. Curr Protein Pept Sci. 2007a;8:418–425. doi: 10.2174/138920307782411455. [DOI] [PubMed] [Google Scholar]
- Krishna R, Prabu JR, Manjunath GP, Datta S, Chandra NR, Muniyappa K, Vijayan M. Snapshots of RecA protein involving movement of the C-domain and different conformations of the DNA-binding loops: crystallographic and comparative analysis of 11 structures of Mycobacterium smegmatis RecA. J Mol Biol. 2007b;367:1130–1144. doi: 10.1016/j.jmb.2007.01.058. [DOI] [PubMed] [Google Scholar]
- Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK. The crystal structure of DNA mismatch repair protein MutS binding to a G × T mismatch. Nature. 2000;407:711–717. doi: 10.1038/35037523. [DOI] [PubMed] [Google Scholar]
- Lammens A, Schele A, Hopfner KP. Structural biochemistry of ATP-driven dimerization and DNA-stimulated activation of SMC ATPases. Curr Biol. 2004;14:1778–1782. doi: 10.1016/j.cub.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Lamour V, Hoermann L, Jeltsch JM, Oudet P, Moras D. An open conformation of the Thermus thermophilus gyrase B ATP-binding domain. J Biol Chem. 2002;277:18947–18953. doi: 10.1074/jbc.M111740200. [DOI] [PubMed] [Google Scholar]
- Langer T, Pfeifer G, Martin J, Baumeister W, Hartl FU. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. Embo J. 1992;11:4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yang W. UvrD helicase unwinds DNA one base pair at a time by a two-part power stroke. Cell. 2006;127:1349–1360. doi: 10.1016/j.cell.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W, Yoshida M, Tsai FT. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003a;115:229–240. doi: 10.1016/s0092-8674(03)00807-9. [DOI] [PubMed] [Google Scholar]
- Lee SY, De La Torre A, Yan D, Kustu S, Nixon BT, Wemmer DE. Regulation of the transcriptional activator NtrC1: structural studies of the regulatory and AAA+ ATPase domains. Genes Dev. 2003b;17:2552–2563. doi: 10.1101/gad.1125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- Leipe DD, Koonin EV, Aravind L. Evolution and classification of P-loop kinases and related proteins. J Mol Biol. 2003;333:781–815. doi: 10.1016/j.jmb.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Lenzen CU, Steinmann D, Whiteheart SW, Weis WI. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell. 1998;94:525–536. doi: 10.1016/s0092-8674(00)81593-7. [DOI] [PubMed] [Google Scholar]
- Lin Z, Rye HS. GroEL-mediated protein folding: making the impossible, possible. Crit Rev Biochem Mol Biol. 2006;41:211–239. doi: 10.1080/10409230600760382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hendrickson WA. Insights into hsp70 chaperone activity from a crystal structure of the yeast Hsp110 Sse1. Cell. 2007;131:106–120. doi: 10.1016/j.cell.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, Marco S, Carrascosa JL, Valpuesta JM. The formation of symmetrical GroEL-GroES complexes in the presence of ATP. FEBS Lett. 1994;345:181–186. doi: 10.1016/0014-5793(94)00432-3. [DOI] [PubMed] [Google Scholar]
- Llorca O, Marco S, Carrascosa JL, Valpuesta JM. Symmetric GroEL-GroES complexes can contain substrate simultaneously in both GroEL rings. FEBS Lett. 1997a;405:195–199. doi: 10.1016/s0014-5793(97)00186-5. [DOI] [PubMed] [Google Scholar]
- Llorca O, Marco S, Carrascosa JL, Valpuesta JM. Conformational changes in the GroEL oligomer during the functional cycle. J Struct Biol. 1997b;118:31–42. doi: 10.1006/jsbi.1996.3832. [DOI] [PubMed] [Google Scholar]
- Mancini EJ, Kainov DE, Grimes JM, Tuma R, Bamford DH, Stuart DI. Atomic snapshots of an RNA packaging motor reveal conformational changes linking ATP hydrolysis to RNA translocation. Cell. 2004;118:743–755. doi: 10.1016/j.cell.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Mao HZ, Weber J. Identification of the betaTP site in the x-ray structure of F1-ATPase as the high-affinity catalytic site. Proc Natl Acad Sci U S A. 2007;104:18478–18483. doi: 10.1073/pnas.0709322104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Baker TA, Sauer RT. Rebuilt AAA + motors reveal operating principles for ATP-fuelled machines. Nature. 2005;437:1115–1120. doi: 10.1038/nature04031. [DOI] [PubMed] [Google Scholar]
- Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–658. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Michie KA, Lowe J. Dynamic filaments of the bacterial cytoskeleton. Annu Rev Biochem. 2006;75:467–492. doi: 10.1146/annurev.biochem.75.103004.142452. [DOI] [PubMed] [Google Scholar]
- Milburn MV, Tong L, deVos AM, Brunger A, Yamaizumi Z, Nishimura S, Kim SH. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990;247:939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–733. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Motojima F, Chaudhry C, Fenton WA, Farr GW, Horwich AL. Substrate polypeptide presents a load on the apical domains of the chaperonin GroEL. Proc Natl Acad Sci U S A. 2004;101:15005–15012. doi: 10.1073/pnas.0406132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin AG. A ribosomal protein module in EF-G and DNA gyrase. Nat Struct Biol. 1995;2:25–26. doi: 10.1038/nsb0195-25. [DOI] [PubMed] [Google Scholar]
- Nadanaciva S, Weber J, Wilke-Mounts S, Senior AE. Importance of F1-ATPase residue alpha-Arg-376 for catalytic transition state stabilization. Biochemistry. 1999;38:15493–15499. doi: 10.1021/bi9917683. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Niedenzu T, Roleke D, Bains G, Scherzinger E, Saenger W. Crystal structure of the hexameric replicative helicase RepA of plasmid RSF1010. J Mol Biol. 2001;306:479–487. doi: 10.1006/jmbi.2000.4398. [DOI] [PubMed] [Google Scholar]
- Niwa H, Tsuchiya D, Makyio H, Yoshida M, Morikawa K. Hexameric ring structure of the ATPase domain of the membrane-integrated metalloprotease FtsH from Thermus thermophilus HB8. Structure. 2002;10:1415–1423. doi: 10.1016/s0969-2126(02)00855-9. [DOI] [PubMed] [Google Scholar]
- Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407:703–710. doi: 10.1038/35037509. [DOI] [PubMed] [Google Scholar]
- Ogura T, Whiteheart SW, Wilkinson AJ. Conserved arginine residues implicated in ATP hydrolysis, nucleotide-sensing, and inter-subunit interactions in AAA and AAA+ ATPases. J Struct Biol. 2004;146:106–112. doi: 10.1016/j.jsb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Orlova A, Garner EC, Galkin VE, Heuser J, Mullins RD, Egelman EH. The structure of bacterial ParM filaments. Nat Struct Mol Biol. 2007;14:921–926. doi: 10.1038/nsmb1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakotiprapha D, Inuzuka Y, Bowman BR, Moolenaar GF, Goosen N, Jeruzalmi D, Verdine GL. Crystal Structure of Bacillus stearothermophilus UvrA Provides Insight into ATP-Modulated Dimerization, UvrB Interaction, and DNA Binding. Mol Cell. 2008;29:122–133. doi: 10.1016/j.molcel.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Popp D, Narita A, Oda T, Fujisawa T, Matsuo H, Nitanai Y, Iwasa M, Maeda K, Onishi H, Maeda Y. Molecular structure of the ParM polymer and the mechanism leading to its nucleotide-driven dynamic instability. Embo J. 2008;27:570–579. doi: 10.1038/sj.emboj.7601978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, Piper PW, Pearl LH. A molecular clamp in the crystal structure of the N-terminal domain of the yeast Hsp90 chaperone. Nat Struct Biol. 1997;4:477–482. doi: 10.1038/nsb0697-477. [DOI] [PubMed] [Google Scholar]
- Ranson NA, Farr GW, Roseman AM, Gowen B, Fenton WA, Horwich AL, Saibil HR. ATP-bound states of GroEL captured by cryo-electron microscopy. Cell. 2001;107:869–879. doi: 10.1016/s0092-8674(01)00617-1. [DOI] [PubMed] [Google Scholar]
- Ranson NA, Clare DK, Farr GW, Houldershaw D, Horwich AL, Saibil HR. Allosteric signaling of ATP hydrolysis in GroEL-GroES complexes. Nat Struct Mol Biol. 2006;13:147–152. doi: 10.1038/nsmb1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappas M, Schumacher J, Beuron F, Niwa H, Bordes P, Wigneshweraraj S, Keetch CA, Robinson CV, Buck M, Zhang X. Structural insights into the activity of enhancer-binding proteins. Science. 2005;307:1972–1975. doi: 10.1126/science.1105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappas M, Schumacher J, Niwa H, Buck M, Zhang X. Structural basis of the nucleotide driven conformational changes in the AAA+ domain of transcription activator PspF. J Mol Biol. 2006;357:481–492. doi: 10.1016/j.jmb.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Roeben A, Kofler C, Nagy I, Nickell S, Hartl FU, Bracher A. Crystal structure of an archaeal actin homolog. J Mol Biol. 2006;358:145–156. doi: 10.1016/j.jmb.2006.01.096. [DOI] [PubMed] [Google Scholar]
- Roseman AM, Chen S, White H, Braig K, Saibil HR. The chaperonin ATPase cycle: mechanism of allosteric switching and movements of substrate-binding domains in GroEL. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- Rothwell PJ, Waksman G. Structure and mechanism of DNA polymerases. Adv Protein Chem. 2005;71:401–440. doi: 10.1016/S0065-3233(04)71011-6. [DOI] [PubMed] [Google Scholar]
- Rye HS, Burston SG, Fenton WA, Beechem JM, Xu Z, Sigler PB, Horwich AL. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature. 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- Rye HS, Roseman AM, Chen S, Furtak K, Fenton WA, Saibil HR, Horwich AL. GroEL-GroES cycling: ATP and nonnative polypeptide direct alternation of folding-active rings. Cell. 1999;97:325–338. doi: 10.1016/s0092-8674(00)80742-4. [DOI] [PubMed] [Google Scholar]
- Saibil HR, Zheng D, Roseman AM, Hunter AS, Watson GM, Chen S, Auf Der Mauer A, O'Hara BP, Wood SP, Mann NH, Barnett LK, Ellis RJ. ATP induces large quaternary rearrangements in a cage-like chaperonin structure. Curr Biol. 1993;3:265–273. doi: 10.1016/0960-9822(93)90176-o. [DOI] [PubMed] [Google Scholar]
- Satyshur KA, Worzalla GA, Meyer LS, Heiniger EK, Aukema KG, Misic AM, Forest KT. Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure. 2007;15:363–376. doi: 10.1016/j.str.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Rutkat K, Rachel R, Pfeifer G, Jaenicke R, Viitanen P, Lorimer G, Buchner J. Symmetric complexes of GroE chaperonins as part of the functional cycle. Science. 1994;265:656–659. doi: 10.1126/science.7913554. [DOI] [PubMed] [Google Scholar]
- Seybert A, Singleton MR, Cook N, Hall DR, Wigley DB. Communication between subunits within an archaeal clamp-loader complex. Embo J. 2006;25:2209–2218. doi: 10.1038/sj.emboj.7601093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau AK, Harris SF, Southworth DR, Agard DA. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127:329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Shtilerman M, Lorimer GH, Englander SW. Chaperonin function: folding by forced unfolding. Science. 1999;284:822–825. doi: 10.1126/science.284.5415.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton MR, Sawaya MR, Ellenberger T, Wigley DB. Crystal structure of T7 gene 4 ring helicase indicates a mechanism for sequential hydrolysis of nucleotides. Cell. 2000;101:589–600. doi: 10.1016/s0092-8674(00)80871-5. [DOI] [PubMed] [Google Scholar]
- Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- Skordalakes E, Berger JM. Structural insights into RNA-dependent ring closure and ATPase activation by the Rho termination factor. Cell. 2006;127:553–564. doi: 10.1016/j.cell.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Song HK, Hartmann C, Ramachandran R, Bochtler M, Behrendt R, Moroder L, Huber R. Mutational studies on HslU and its docking mode with HslV. Proc Natl Acad Sci U S A. 2000;97:14103–14108. doi: 10.1073/pnas.250491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MC, Trame CB, Tsuruta H, Wilbanks SM, Reddy VS, McKay DB. Crystal and solution structures of an HslUV protease-chaperone complex. Cell. 2000;103:633–643. doi: 10.1016/s0092-8674(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Sousa R, Lafer EM. Keep the traffic moving: mechanism of the Hsp70 motor. Traffic. 2006;7:1596–1603. doi: 10.1111/j.1600-0854.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrer H, Rutkat K, Buchner J. Catalysis of protein folding by symmetric chaperone complexes. Proc Natl Acad Sci U S A. 1997;94:1096–1100. doi: 10.1073/pnas.94.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz TA, Shoham M, Bennett WS., Jr Structural dynamics of yeast hexokinase during catalysis. Philos Trans R Soc Lond B Biol Sci. 1981;293:43–52. doi: 10.1098/rstb.1981.0058. [DOI] [PubMed] [Google Scholar]
- Steitz TA. Visualizing polynucleotide polymerase machines at work. Embo J. 2006;25:3458–3468. doi: 10.1038/sj.emboj.7601211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story RM, Steitz TA. Structure of the recA protein-ADP complex. Nature. 1992;355:374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- Story RM, Weber IT, Steitz TA. The structure of the E. coli recA protein monomer and polymer. Nature. 1992;355:318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- Subramanya HS, Bird LE, Brannigan JA, Wigley DB. Crystal structure of a DExx box DNA helicase. Nature. 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- Sun S, Chandler D, Dinner AR, Oster G. Elastic energy storage in beta-sheets with application to F1-ATPase. Eur Biophys J. 2003;32:676–683. doi: 10.1007/s00249-003-0335-6. [DOI] [PubMed] [Google Scholar]
- Suno R, Niwa H, Tsuchiya D, Zhang X, Yoshida M, Morikawa K. Structure of the whole cytosolic region of ATP-dependent protease FtsH. Mol Cell. 2006;22:575–585. doi: 10.1016/j.molcel.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Thanbichler M, Shapiro L. Getting organized--how bacterial cells move proteins and DNA. Nat Rev Microbiol. 2008;6:28–40. doi: 10.1038/nrmicro1795. [DOI] [PubMed] [Google Scholar]
- Theissen B, Karow AR, Kohler J, Gubaev A, Klostermeier D. Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proc Natl Acad Sci U S A. 2008;105:548–553. doi: 10.1073/pnas.0705488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth EA, Li Y, Sawaya MR, Cheng Y, Ellenberger T. The crystal structure of the bifunctional primase-helicase of bacteriophage T7. Mol Cell. 2003;12:1113–1123. doi: 10.1016/s1097-2765(03)00442-8. [DOI] [PubMed] [Google Scholar]
- van den Ent F, Lowe J. Crystal structure of the cell division protein FtsA from Thermotoga maritima. Embo J. 2000;19:5300–5307. doi: 10.1093/emboj/19.20.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- van den Ent F, Moller-Jensen J, Amos LA, Gerdes K, Lowe J. F-actin-like filaments formed by plasmid segregation protein ParM. Embo J. 2002;21:6935–6943. doi: 10.1093/emboj/cdf672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velankar SS, Soultanas P, Dillingham MS, Subramanya HS, Wigley DB. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. Nucleoside triphosphate-binding proteins: different scaffolds to achieve phosphoryl transfer. Q Rev Biophys. 1999;32:1–56. doi: 10.1017/s0033583599003480. [DOI] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Song JJ, Franklin MC, Kamtekar S, Im YJ, Rho SH, Seong IS, Lee CS, Chung CH, Eom SH. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure. 2001a;9:177–184. doi: 10.1016/s0969-2126(01)00570-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Song JJ, Seong IS, Franklin MC, Kamtekar S, Eom SH, Chung CH. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure. 2001b;9:1107–1116. doi: 10.1016/s0969-2126(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Boisvert DC. Structural basis for GroEL-assisted protein folding from the crystal structure of (GroEL-KMgATP)14 at 2.0A resolution. J Mol Biol. 2003;327:843–855. doi: 10.1016/s0022-2836(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen L. Domain motions in GroEL upon binding of an oligopeptide. J Mol Biol. 2003;334:489–499. doi: 10.1016/j.jmb.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Wang J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J Struct Biol. 2004;148:259–267. doi: 10.1016/j.jsb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Weber J, Senior AE. ATP synthesis driven by proton transport in F1F0-ATP synthase. FEBS Lett. 2003;545:61–70. doi: 10.1016/s0014-5793(03)00394-6. [DOI] [PubMed] [Google Scholar]
- Wei H, Ruthenburg AJ, Bechis SK, Verdine GL. Nucleotide-dependent domain movement in the ATPase domain of a human type IIA DNA topoisomerase. J Biol Chem. 2005;280:37041–37047. doi: 10.1074/jbc.M506520200. [DOI] [PubMed] [Google Scholar]
- Wigley DB, Davies GJ, Dodson EJ, Maxwell A, Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991;351:624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A, Scheffzek K, Ahmadian MR. The interaction of Ras with GTPase-activating proteins. FEBS Lett. 1997;410:63–67. doi: 10.1016/s0014-5793(97)00321-9. [DOI] [PubMed] [Google Scholar]
- Wolanin PM, Thomason PA, Stock JB. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-10-reviews3013. REVIEWS3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Strater N, Schroder W, Bottcher C, Ludwig K, Saenger W. Structure of DNA helicase RepA in complex with sulfate at 1.95 A resolution implicates structural changes to an “open” form. Acta Crystallogr D Biol Crystallogr. 2003;59:815–822. doi: 10.1107/s0907444903004025. [DOI] [PubMed] [Google Scholar]
- Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- Yamagata A, Tainer JA. Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism. Embo J. 2007;26:878–890. doi: 10.1038/sj.emboj.7601544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. Structure and function of mismatch repair proteins. Mutat Res. 2000;460:245–256. doi: 10.1016/s0921-8777(00)00030-6. [DOI] [PubMed] [Google Scholar]
- Yeo HJ, Savvides SN, Herr AB, Lanka E, Waksman G. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol Cell. 2000;6:1461–1472. doi: 10.1016/s1097-2765(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Amano T. A common topology of proteins catalyzing ATP-triggered reactions. FEBS Lett. 1995;359:1–5. doi: 10.1016/0014-5793(94)01438-7. [DOI] [PubMed] [Google Scholar]
- Yu RC, Hanson PI, Jahn R, Brunger AT. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nat Struct Biol. 1998;5:803–811. doi: 10.1038/1843. [DOI] [PubMed] [Google Scholar]
- Ziegelin G, Niedenzu T, Lurz R, Saenger W, Lanka E. Hexameric RSF1010 helicase RepA: the structural and functional importance of single amino acid residues. Nucleic Acids Res. 2003;31:5917–5929. doi: 10.1093/nar/gkg790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.