Abstract
Purpose
Anemia is an important comorbidity in heart failure, and has been associated with increased mortality. The goals of this study were to define the prevalence of anemia in a community heart failure population, examine trends in prevalence over time, and evaluate the role of anemia in heart failure patients with preserved and reduced ejection fraction.
Methods
Two cohorts of Olmsted County residents with heart failure were examined. The retrospective cohort included incident heart failure cases from 1979–2002 (n=1063). The prospective cohort included active heart failure cases from 2003–2006 (n=677). Clinical characteristics were collected. Anemia was defined by WHO criteria.
Results
The prevalence of anemia was 40% in the retrospective and 53% in the prospective cohorts. Anemia prevalence increased by an estimated 16% between 1979 and 2002 (p=0.008), and was higher in those with preserved (≥50%), vs. reduced (<50%) ejection fraction (58% vs. 48%, respectively, p<0.001) from 2003–2006. Anemia was associated with a large increase in the risk of death (p<0.001 both cohorts). The relationship between mortality and hemoglobin followed a J-shaped curve, with increased mortality with hemoglobin below 14mg/dL and above 16mg/dL. In the prospective cohort, after adjustment for clinical characteristics, the HR(95%CI) for death were 3.07(1.26–6.82) in those with hemoglobin ≥16mg/dL and 2.39(1.37–4.27) in those with hemoglobin<10mg/dL using hemoglobin 14–16mg/dL as the referent.
Conclusions
In the community half of heart failure patients are anemic, and the prevalence of anemia increased over time. Anemia is more prevalent in heart failure with preserved ejection fraction and is associated with a large increase in mortality.
Keywords: anemia, hemoglobin, heart failure, epidemiology, prevalence, mortality
Introduction
More than 5 million Americans are currently living with heart failure, constituting a major public health problem1. As heart failure treatments advance, there has been associated improvement in survival, but overall prognosis remains poor. Recently, the role of anemia in heart failure has become a focus of interest as a possible target for therapeutic intervention2.
Anemia is seemingly common among U.S. patients with heart failure, with estimated prevalence ranging from 23–48%3, 4. However, previous studies cannot be readily generalized as they pertain mostly to patients with systolic dysfunction4–8, had incomplete ejection fraction ascertainment9, 10, or were performed using clinical trial populations4, 7, 8. In addition, varied definitions for anemia have been used including physician’s diagnosis11, hematocrit<30–37%3, 7, 9, hemoglobin <12.0mg/dL12, and the World Health Organization (WHO) definition (hemoglobin <12 mg/dL in women, <13 mg/dL in men)4, 10, 13. Whether the prevalence of anemia has changed over time is unknown.
Previous studies have demonstrated that anemia is associated with increased morbidity and mortality. However, a recent study10 noted that patients with elevated hemoglobin levels (≥17mg/dL) were at increased mortality risk compared to those with hemoglobin 13.0–13.9mg/dL, and at a risk similar to those with hemoglobin levels 11.0–11.9 mg/dL. These findings have not been replicated, and require further examination.
The present study was designed to address these gaps in knowledge by ascertaining the prevalence of anemia in community heart failure patients. We aimed at assessing whether there has been a change in prevalence over time and determining whether anemia and hemoglobin level are associated with mortality in community heart failure patients with a wide range of ejection fraction and heart failure severity.
Methods
Study design and setting
This is a population-based study conducted in Olmsted County, Minnesota using the resources of the Rochester Epidemiology Project. According to the U.S. Census Bureau (www.census.gov) the 2005 Olmsted County population is estimated at 135,189, the majority of whom are white (90.2%), including 50.8% women.
We employed two complementary study designs in this analysis: a retrospective cohort including incident heart failure patients diagnosed from 1979–2002 and a prospective cohort enrolling patients with active heart failure from 2003–2006. The retrospective cohort allows examination of trends in prevalence of anemia over more than two decades14, 15, and captures heart failure patients at the time of diagnosis. The prospective cohort represents a contemporary heart failure population, contains complete echocardiographic data, and provides information on heart failure severity including biomarker data and New York Heart Association (NYHA) functional class, lacking from prior studies. Investigations were approved by the appropriate institutional review board.
This type of research is feasible in Olmsted County because medical care is provided by few providers including Mayo Clinic, Olmsted Medical Center, and a few private practitioners. Each institution’s records are easily retrievable because Mayo Clinic maintains extensive indices which, through the Rochester Epidemiology Project, are extended to the records of other care providers to county residents. The result is the linkage of medical records from all sources of care through a centralized system16.
Heart Failure Retrospective Cohort (1979–2002): Patient Identification
Olmsted County residents with a possible heart failure diagnosis were identified by International Classification of Diseases, Ninth Revision (ICD9) code 428 (heart failure). Codes are primarily assigned based on physician diagnoses during outpatient visits or at hospital discharge. From all patients with ICD9 code 428, a subset was randomly selected for case validation and data abstraction. Validation occurred using methods previously described14, and described here in brief. Experienced nurse abstractors blinded to study hypotheses reviewed records to ensure each met Framingham criteria17 and had a physician’s diagnosis of heart failure. When this method was utilized previously14, missing data were minimal and Framingham criteria could be applied in 98% of cases. The inter-abstractor agreement was 100%, indicating these methods of classification are highly reproducible.
Heart Failure Prospective Cohort (2003–2006): Patient Identification and Recruitment
Following a clinical visit, details are transcribed and appear in the electronic medical record within 24 hours. Natural language processing of the electronic text is utilized to identify potential heart failure cases. Nurse abstractors then review the cases to confirm heart failure diagnosis using Framingham criteria17 and collect additional data. All patients with active heart failure were identified from 2003–2006, including both incident and prevalent heart failure cases. Patients were prospectively recruited into the study, which includes a blood draw and echocardiogram. Hospitalized patients were contacted during hospitalization, and outpatients were contacted at their next clinic appointment.
Data Collection: Retrospective and Prospective Cohorts
Laboratory Data
Hemoglobin closest to heart failure diagnosis was utilized. Hemoglobin values within one year of heart failure diagnosis were available for 97% and >99% of patients in the retrospective and prospective cohorts, respectively. Anemia was defined by WHO criteria (hemoglobin <13mg/dl in men, <12mg/dl in women)18. Creatinine clearance was calculated based on the last creatinine value (within 30 days of heart failure diagnosis) using the Cockroft Gault equation19. Brain natriuretic peptide (BNP) was measured upon enrollment in the prospective cohort by immunoradiometric assay (nonextracted) with antibody to human BNP using the Shionoria assay (Shionogi, Osaka, Japan) in the Immunochemical Core Laboratory of Mayo Clinic, Rochester, Minnesota.
Echocardiography
Echocardiograms were obtained and analyzed at Mayo Clinic Echocardiography laboratory according to the American Society of Echocardiography guidelines20. Left ventricular ejection fraction was measured using M-mode, quantitative, and semi-quantitative methods as previously described and validated21 with excellent correlation between the methods. Ejection fraction values were averaged when multiple measurements were performed. Patients with an ejection fraction ≥50% were classified as having preserved ejection fraction, while those with ejection fraction <50% were classified as having reduced ejection fraction15. All patients in the prospective cohort had complete echocardiographic data. Echocardiograms were not consistently performed in the retrospective cohort, and data were not included in analysis.
Additional patient data
Research nurses collected baseline characteristics from the medical record. Physician’s diagnosis defined prior coronary artery disease, chronic obstructive pulmonary disease (COPD), and malignancy. Hypertension was defined as systolic blood pressure >140mmHg, diastolic blood pressure >90mmHg, or use of anti-hypertensive medications22. Smoking status was classified as ‘ever’ or ‘never’. Diabetes mellitus was defined using the American Diabetes Association criteria23. Body mass index (BMI) was calculated using patient weight at the time of heart failure diagnosis and earliest adult height. NYHA functional class was assessed using standard definitions.
Mortality Follow-up
We relied on passive surveillance of the medical record via the Rochester Epidemiology Project. In addition to deaths noted in clinical care, death certificates for Olmsted County residents are obtained annually, the Mayo Clinic registration office records all obituaries and local death notices, and death data is obtained from the State of Minnesota Department of Vital and Health Statistics annually16.
Statistical Analysis
Baseline characteristics are represented as frequencies or mean values with standard deviations. Differences in baseline characteristics comparing the two cohorts and by anemia status were analyzed using a t-test for continuous variables and χ2 test for categorical variables. Year-specific prevalence rates of anemia, diabetes, hypertension, mean creatinine clearance, age, and BMI were calculated. Changes in prevalence over time were calculated using generalized linear models. Subjects were divided into groups based on hemoglobin level (<10, 10.0–11.9, 12.0–13.9, 14–15.9, ≥16.0mg/dL). Survival was assessed using Kaplan-Meier methods with censoring at the time of last follow-up. Cox proportional hazard regression analysis was performed to assess both the unadjusted and adjusted hazard ratios by hemoglobin group, using hemoglobin of 14.0–15.9mg/dL as the referent. The proportional hazard assumption using the Schoenfeld residuals was valid in both cohorts. Data was >99% complete with the exception of smoking status (n=13 missing retrospective), creatinine clearance (n= 58 missing retrospective, n=29 missing prospective), and BNP (n=51 missing prospective). A p value of <0.05 was used as the level of significance. Analyses were performed using SAS Version 8.02 (SAS Institute, Cary NC) and JMP Version 6.0 (SAS Institute, Cary NC).
Results
Patient Populations
There were 1063 heart failure patients in the retrospective and 677 in the prospective cohort. Age and sex distributions in both cohorts were similar. The prospective cohort had an increased proportion of patients with higher BMI, hypertension, diabetes, coronary artery disease, smoking, COPD, and malignancy (p<0.0001 for all).
Prevalence of Anemia and Associated Characteristics
Retrospective Cohort
The prevalence of anemia was 40% and increased over time (Figure 1), with an estimated increase of 0.67% annually. This equates to an estimated 16% increase from 1979–2002. During the same period, the mean age remained stable (p=0.13), while mean creatinine clearance increased (p<0.001). However, the frequency of other comorbidities including hypertension (p=0.0003), diabetes mellitus (p=0.026), and mean BMI (p=0.001) increased over time.
Figure 1. Time trends in the prevalence of anemia.
Trends in the prevalence of anemia by year of heart failure diagnosis were examined from 1979–2002.
Prospective Cohort
The prevalence of anemia in the prospective cohort was 53% from 2003–2006, which is consistent with the prevalence predicted by data from the retrospective cohort. Patients with preserved ejection fraction had an increased prevalence of anemia (58%) compared with patients with reduced ejection fraction (48%, p<0.001).
In both cohorts, anemia was associated with decreased creatinine clearance and coronary artery disease (Table 1). In the retrospective cohort, patients with anemia were older, and a similar trend was observed in the prospective cohort. Prospective cohort data indicated that anemia was associated with higher BNP.
Table 1.
Baseline Characteristics
| Retrospective Cohort (1979–2002) |
Prospective Cohort (2003–2006) |
||||||
|---|---|---|---|---|---|---|---|
| No anemia (n=622) |
Anemia (n=408) |
P value | No anemia (n=317) |
Anemia (n=359) |
P value | ||
| Age (years) | 75.1 (13.0) | 78.4 (11.5) | <0.001 | 75.5 (13.7) | 76.5 (12.9) | 0.32 | |
| Male | 281 (45.2) | 192 (47.1) | 0.55 | 156 (49.2) | 175 (48.7) | 0.90 | |
| Hypertension | 433 (69.6) | 269 (65.9) | 0.21 | 247 (77.9) | 276 (76.9) | 0.75 | |
| Ever smoker | 328 (53.1) | 210 (52.6) | 0.89 | 179 (56.6) | 227 (63.4) | 0.07 | |
| Diabetes | 115 (18.5) | 73 (17.9) | 0.81 | 95 (30.0) | 130 (36.2) | 0.09 | |
| BMI (kg/m2) | 26.9 (6.7) | 26.9 (6.8) | 0.99 | 29.5 (8.1) | 28.5 (6.9) | 0.08 | |
| Coronary artery | 200 (32.2) | 164 (40.2) | 0.008 | 170 (53.6) | 222 (61.8) | 0.03 | |
| disease | |||||||
| COPD | 141 (22.7) | 86 (21.1) | 0.55 | 93 (29.4) | 116 (32.3) | 0.42 | |
| Cancer | 85 (13.7) | 107 (26.2) | <0.001 | 101 (31.9) | 128 (35.7) | 0.30 | |
| Creatinine | |||||||
| clearance | 59.8 (37.0) | 48.3 (29.4) | <0.001 | 64.2 (35.6) | 53.5 (31.3) | <0.001 | |
| (mL/min) | |||||||
| NYHA class ¾ | -- | -- | 236 (74.5) | 271 (75.5) | 0.76 | ||
| BNP (mg/dL) | -- | -- | 270.0 (142.0, 529.0) | 321.5 (176.5, 625.3) | 0.002* | ||
| Ejection fraction ≥50% | -- | -- | 151 (47.6) | 208 (57.9) | 0.007 | ||
BNP was log transformed for analysis in determining p value.
Age, BMI, and creatinine clearance values are expressed as mean (standard deviation). BNP values are expressed as median (interquartile range). All other values are given as frequency (%).
BMI= body mass index, BNP= brain natriuretic peptide, COPD= chronic obstructive pulmonary disease, NYHA= New York Heart Association
Anemia, Hemoglobin, and Mortality
In the retrospective cohort, 917 of 1063 (86%) of heart failure patients died after a mean follow-up of 5.3 ±4.8 years. In the prospective cohort, after a mean follow-up of 21±13 months, 241 of 677 (36%) patients had died. Estimated two-year mortality (95% CI) was 30% (27%–33%) and 33% (29%–37%) in the retrospective and prospective cohorts, respectively. Mortality was higher in those with anemia in both cohorts (p<0.001, Figure 2A&B). In the prospective cohort two-year mortality (95% CI) was 41% (36–47%) in those with anemia compared to 24% (19–29%) in those without anemia. Results were similar in the retrospective cohort.
Figure 2. Mortality by Presence of Anemia and Hemoglobin Level.
Mortality during follow-up was analyzed by anemia status in the retrospective (A) and prospective (B) cohorts. Two-year mortality estimates by hemoglobin level are shown in the retrospective (C) and prospective (D) cohorts.
*Note- Kaplan Meier curves were truncated at three years follow-up in both cohorts to allow direct comparison.
Although anemic heart failure patients had higher mortality, the association between hemoglobin and mortality followed a J-shaped curve in both cohorts (Figure 2C&D). An increase in two-year mortality was noted with hemoglobin below 14.0mg/dL or above 16.0mg/dL. For example, in the prospective cohort estimated two-year mortality (95% CI) was 30%(6–48%) with hemoglobin ≥16 mg/dL, 19%(11–26%) with hemoglobin 14.0–15.9mg/dL, 28%(21–34%) with hemoglobin 12.0–13.9 mg/dL, 41%(34–48%) with hemoglobin 10.0–11.9mg/dL, and 49%(35–59%) with hemoglobin <10 mg/dL.
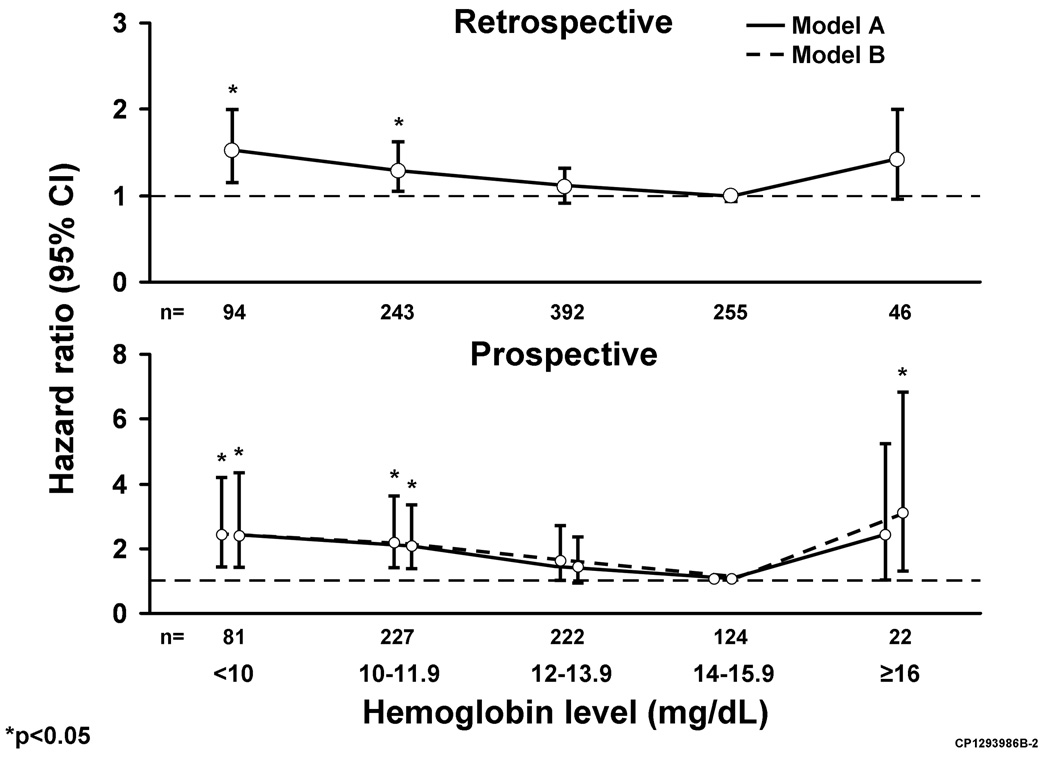
Cox proportional hazard regression further documented that mortality was higher with hemoglobin below 14mg/dL or above 16mg/dL in both cohorts. After adjusting for clinical characteristics in the prospective cohort the hazard ratios (95%CI) for death were 2.40 (0.99–5.23) with hemoglobin ≥16.0mg/dl, 1.41 (0.89–2.31) with hemoglobin 12.0–13.9 mg/dl, 1.99 (1.27–3.25) with hemoglobin 10.0–11.9mg/dL, and 2.37 (1.39–4.11) with hemoglobin <10.0mg/dL using hemoglobin 14.0–15.9mg/dL as the referent (Figure 3). A similar J-shaped mortality curve was demonstrated in the retrospective cohort using the same model (Figure 3). Adjustment for year of diagnosis did not significantly change results.
Figure 3. Adjusted Hazard Ratios for Mortality in Heart Failure Patients by Hemoglobin.
Hazard ratios for death are shown by hemoglobin level in the retrospective and prospective cohorts. Results are further adjusted for heart failure severity in the prospective cohort.
Model A adjusted for age, sex, coronary artery disease, diabetes mellitus, tobacco use, prior malignancy, body mass index, creatinine clearance.
Model B adjusted for above plus New York Heart Association functional class, ejection fraction, and brain natriuretic peptide (BNP).
Using the additional data available in the prospective cohort, we further adjusted for ejection fraction, NYHA functional class, and BNP. Hazard ratios were similar for those with hemoglobin <14.0mg/dL, while in those with hemoglobin ≥16mg/dL, the hazard ratio (95%CI) increased to 3.07 (1.26–6.82) (Figure 3). There was no interaction between hemoglobin and ejection fraction in the prospective cohort (p=0.76 interaction term).
Discussion
In community heart failure patients, the prevalence of anemia is high and increasing over time. Currently, anemia is present in more than half of heart failure patients and is considerably more prevalent in those with preserved ejection fraction. Anemia is associated with a large increase in mortality independent of known clinical characteristics. The relationship between hemoglobin and mortality is not linear, as both reduced (<14mg/dL) and elevated (≥16mg/dL) hemoglobin are associated with increased mortality.
Prevalence of Anemia
Previous studies demonstrated an estimated prevalence of anemia in heart failure patients from 23–48%3, 4. In a general elderly population (NHANES) with similar age and sex distributions to our study, the prevalence of anemia was 10.6% in those age 65 or older (mean age 74.9 years, 56.6% female)24.
The present study extends previous reports by demonstrating that the burden of anemia in heart failure patients is substantial, with over half anemic by WHO criteria in recent years. This prevalence is higher than previously reported likely reflecting the unselected population represented in our community cohorts in contrast to the highly selective nature of trial participants and in studies limited to those with reduced ejection fraction. Further, the prevalence of anemia increased markedly over time and this steady increase cannot be readily explained by changes in age and renal function. As observed herein and consistent with prior studies4, 10, 11, the prevalence of anemia increases with age. However, no temporal change in mean age at heart failure diagnosis was detected. Additionally, despite the known correlation between anemia and chronic kidney disease in heart failure25, in this cohort the mean creatinine clearance increased over time.
One possible contributor could be the increase in heart failure patients with preserved ejection fraction. Prior data have been conflicting on whether the prevalence of anemia differs by ejection fraction, with studies demonstrating prevalence is higher3, lower13, and the same26 in patients with preserved vs. reduced ejection fraction. Our data from 2003–2006 performed in an unselected heart failure population with complete ejection fraction ascertainment demonstrate that the prevalence of anemia is higher in those with preserved vs. reduced ejection fraction. Owan et al15 recently reported that the proportion of heart failure patients with preserved ejection fraction is increasing over time. Given this proportionate increase in heart failure patients with preserved ejection fraction, and an increased prevalence of anemia in those with preserved ejection fraction it is plausible that this shift in case mix is contributing to the increased prevalence of anemia in community heart failure patients. As the pathogenesis of anemia in heart failure has not been fully elucidated27, 28, further work is needed to define the mechanisms of anemia in heart failure.
Anemia, Hemoglobin, and Association with Outcomes
Anemia and low hemoglobin have been associated with adverse outcomes in several clinical studies3–13. However, it becomes difficult to establish a causal relationship between anemia and mortality when studies include prevalent heart failure cases. By utilizing our retrospective cohort with only incident heart failure cases, we demonstrated that anemia and low hemoglobin at the time of heart failure diagnosis are associated with increased mortality. A previous study had demonstrated that patients with elevated hemoglobin levels are also at increased mortality risk10. Our study supports these findings, and extends them by demonstrating that adjusting for ejection fraction, NYHA functional class, and BNP did not attenuate this association.
Whether to treat anemia in heart failure patients remains controversial. Few studies to date have randomized patients to treatment with erythropoietin or darbepoetin with or without intravenous iron therapy vs. placebo and evaluated outcomes. Results have been promising, with trials demonstrating an improvement in peak oxygen consumption29, exercise duration29, NYHA functional class30, and health-related quality of life31 in patients treated with erythropoietin29, erythropoietin+iron30, or darbepoetin31 compared with placebo. Concerns have been raised about the safety of treating patients with these agents29 due to potential thrombotic complications. However, based on the promising results, a larger phase III trial evaluating treatment of anemic heart failure patients with darbopoeitin is underway. Given the high burden of anemia in heart failure, if treatment improves outcomes, the impact could be great.
Strengths and Limitations
Some limitations should be acknowledged to aid in data interpretation. First, despite its growing diversity, Olmsted County remains largely Caucasian, so generalization of these data to other racial and ethnic groups should be cautious. Second, information on anemia etiology including ferritin and transferrin levels were not available for analysis. Finally, the consent rate for the prospective cohort was 69% during the study period, which is similar to rates reported in other community studies of cardiovascular disease32. This study has several strengths. First, we examined the role of anemia in heart failure in a large unselected community population. Second, our retrospective cohort included only incident heart failure cases and spanned more than twenty years. Third, our prospective cohort had complete ejection fraction ascertainment and included data on heart failure severity.
Conclusions
Over half of community heart failure patients are currently anemic and the prevalence is increasing over time. Heart failure patients with preserved ejection fraction have increased prevalence of anemia compared with reduced ejection fraction. Anemia is associated with increased mortality, but hemoglobin follows a J-shaped curve, with increased mortality at both low and very high hemoglobin. Further work is needed to investigate the increasing prevalence of anemia in heart failure, and to determine whether treatment improves outcomes.
Acknowledgements
We thank Ellen Koepsell, RN and Kay Traverse, RN. for their study support:
Funding Sources- This study was supported by grants from the National Institute of Health (RO1 HL 59205, RO1 HL 72435) and by an American Heart Association Postdoctoral Greater Midwest Fellowship Award to Dr. Dunlay. Dr. Roger is an Established Investigator of the American Heart Association.
Footnotes
Disclosures- none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006 May 23;113(20):2454–2461. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 3.Kosiborod M, Smith GL, Radford MJ, et al. The prognostic importance of anemia in patients with heart failure. Am J Med. 2003 Feb 1;114(2):112–119. doi: 10.1016/s0002-9343(02)01498-5. [DOI] [PubMed] [Google Scholar]
- 4.Anand IS, Kuskowski MA, Rector TS, et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation. 2005 Aug 23;112(8):1121–1127. doi: 10.1161/CIRCULATIONAHA.104.512988. [DOI] [PubMed] [Google Scholar]
- 5.Sharma R, Francis DP, Pitt B, et al. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur Heart J. 2004 Jun;25(12):1021–1028. doi: 10.1016/j.ehj.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002 Jun 5;39(11):1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001 Oct;38(4):955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE) J Am Coll Cardiol. 2003 Jun 4;41(11):1933–1939. doi: 10.1016/s0735-1097(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 9.McClellan WM, Flanders WD, Langston RD, et al. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002 Jul;13(7):1928–1936. doi: 10.1097/01.asn.0000018409.45834.fa. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006 Jun 13;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003 Jan 21;107(2):223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 12.Szachniewicz J, Petruk-Kowalczyk J, Majda J, et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003 Aug;90(2–3):303–308. doi: 10.1016/s0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 13.Felker GM, Shaw LK, Stough WG, O'Connor CM. Anemia in patients with heart failure and preserved systolic function. Am Heart J. 2006 Feb;151(2):457–462. doi: 10.1016/j.ahj.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. Jama. 2004 Jul 21;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 15.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006 Jul 20;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. Mar. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993 Jul;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Nutritional Anemias: Report of a WHO Scientific Group. WHO Technical Report Series. 1968;405:1. [PubMed]
- 19.Cockroft DG. MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Amico AF, Lichtenberg GS, Reisner SA, et al. RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989 Dec;118(6):1259–1265. doi: 10.1016/0002-8703(89)90018-5. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004 Oct 15;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 25.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004 Mar 2;109(8):1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 26.Brucks S, Little WC, Chao T, et al. Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol. 2004 Apr 15;93(8):1055–1057. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 27.Francis GS, Kanderian A. Anemia and heart failure a new pathway? J Am Coll Cardiol. 2007 oct 23;50(17):1666–1667. doi: 10.1016/j.jacc.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 28.Okonko DO, Anker SD. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. 2004 Feb;10(1 Suppl):S5–S9. doi: 10.1016/j.cardfail.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Mancini DM, Katz SD, Lang CC, et al. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003 Jan 21;107(2):294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001 Jun 1;37(7):1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 31.Ponikowski P, Anker SD, Szachniewicz J, et al. Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2007 Feb 20;49(7):753–762. doi: 10.1016/j.jacc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families The Framingham offspring study. Am J Epidemiol. 1979 Sep;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]