Abstract
BACKGROUND
The matrix metalloproteinase (MMP) family is known to play a key role in tissue remodelling during embryonic development and in pathological conditions, such as cardiovascular disease, arthritis and cancer metastasis. It has been shown previously that p53 regulates positively or negatively the expression of different MMPs. Because of p53 overexpression in trophoblastic cells, and its potential role in regulating MMP-2 and MMP-9 expression in different cell lines, we hypothesized that the expression of MMP-9 could also be regulated by p53 in first trimester cytotrophoblasts (CTB).
METHODS and RESULTS
Transfection experiments in CTB demonstrated that wild-type p53 down-regulates the −670 (P < 0.001) but not the −531 and −90 human MMP-9 promoter/CAT reporter plasmid activity, whereas p53 mutants partially lost this repressive activity. However, endogenous p53 is not able to regulate MMP-9 expression in CTB. The presence of high molecular weight complexes of p53 in CTB suggests a potential mechanism of inactivation of p53 transcriptional activity towards MMPs in these cells.
CONCLUSIONS
Although p53 is mutated in trophoblast, it is functionally incompetent towards MMPs in these cells.
Keywords: matrix metalloproteinase-2, matrix metalloproteinase-9, p53, first trimester cytotrophoblast, gelatinase
Introduction
Cytotrophoblastic cells (CTB) of the human placenta proliferate, migrate and invade the uterus during pregnancy to allow implantation and placentation (Red-Horse et al., 2004). The invasive property of CTB is limited in time and in space and depends to their ability to secrete proteases. Matrix metalloproteinases (MMPs) are involved in this invasive process since phenanthroline, a non-specific inhibitor of MMPs, inhibits invasion of matrigel by CTB (Bischof et al., 1995). The MMP family is known to be essential for degradation of nearly all components of the extracellular matrix (ECM). This confers an important role to these enzymes in various physiological and pathological processes such as embryonic development (Behrendsten and Werb, 1997), inflammation (Cowland and Borregaard, 1999), infection (Giraudon et al., 1998), vascular (Jones et al., 2003; Kuzuya and Iguchi, 2003) and degenerative diseases (Backstrom et al., 1996), tumour invasion (Polette et al., 2004) and metastasis (Nagase and Woessner, 1999; Vu and Werb, 2000; Chang and Werb, 2001). MMP-9 represents the largest member and one of the most studied for its involvement in the invasiveness of trophoblasts (Librach et al., 1991; Polette et al., 1994; Shimonovitz et al., 1994; Hurskainen et al., 1996; Xu et al., 2000). During early stages of pregnancy, the level of expression of MMP-9 (gelatinase B) is very low in trophoblasts and increases gradually after the 8th week. Thus, expression of MMP-9 coincides with the maximal invasive potential of CTB and suggests that MMP-9 is implicated in the invasiveness of these cells (Cohen et al., 2006).
MMP-9 is encoded by a 7.7 kb gene comprising 13 exons and 12 introns (Van den Steen et al., 2002). The 2.2 kb promoter sequence of the human (h)MMP-9 contains several consensus motifs for regulatory elements as shown in Fig. 1. Several cis-regulatory regions appear to act synergistically in basal and induced MMP-9 gene expression. For example, the AP-1 element at position −79 bp plays a dominant role in transcriptional activation of MMP-9; it is necessary but not sufficient for basal or induced MMP-9 gene transcription and requires specific interactions with nuclear factor κB and stimulating protein-1 elements at positions −600 and −563, respectively (Sato and Seiki, 1993; Bischof et al., 2003).
Figure 1:
MMP-9 promoter.
Starting from the transcription initiation site and moving upstream towards the 5′ end of the promoter, one observes a TATA motif like sequence at position −29 bp, correlated with the transcriptional start site, at positions −79, −209, −533 and −1652 bp four 12-O-tetradecanoyl-phorbol-13-acetate or activator protein-1 (AP-1) binding sites, at positions −328 and −600 bp two nuclear factor κB (NFκB) motifs, at position −474 bp a consensus sequence for a transforming growth factor-β inhibitory element (TIE), at positions −541 and −554 two polyoma enhancer A binding protein-3 (PEA3) elements, and at position −563 bp a consensus sequence for the binding of nuclear stimulating protein-1 (Sp1).
The tumour suppressor p53 is a transcription factor that activates transcription of its target genes by binding to a specific consensus DNA sequence consisting of two copies of a 10 bp DNA motif 5′-PuPuPuC(A/T)(T/A)GPyPyPy-3′ separated by 0–13 bp (El-Deiry et al., 1992). Wild-type (wt) p53, but not mutants of p53, can efficiently bind the p53-binding element on target genes. The promoter of MMP-2 contains a p53-binding element that mediates activation of MMP-2 gene transcription (Bian and Sun, 1997). Furthermore, p53 can also repress expression of genes lacking the p53-binding site in their promoter by interacting with other transcription factors (Subler et al., 1992; Donehower and Bradley, 1993; Werner et al., 1996; Sun et al., 1999; Ala-aho et al., 2002). Most mutants of p53 have lost this wt-p53 repressive ability. It has been shown that wt-p53 is able to potently inhibit expression of MMP-13 (Sun et al., 2000; Ala-aho et al., 2002), MMP-1 (Sun et al., 1999; Sun et al., 2004) and MMP-9 (Liu et al., 2006; Meyer et al., 2005). Immunohistochemical studies of first trimester trophoblast have shown that p53 is detectable in CTB and in syncytiotrophoblast (Haidacher et al., 1995; Marzusch et al., 1995; Cohen et al., 2007). Since wt-p53 is generally not detectable by immunohistochemistry due to its short half-life, Marzusch et al. (1995) suggested that p53 might be overexpressed in these cells and contribute to excessive trophoblastic proliferation in normal placentation. We recently demonstrated the presence of high molecular complexes of p53 (HMWC) in both cytoplasmic and nuclear fractions of CTB which could be involved in p53 stabilization in these cells (Cohen et al., 2007). The aim of this study was to determine the potential role of p53 in regulating MMP-9 expression in CTB.
Materials and Methods
Dulbecco's modified Eagle's medium (DMEM) and antibiotics mixture (penicillin and streptomycin) were the products of Invitrogen (Basel, Switzerland). Fetal bovine serum (FBS) was from Biochrom AG (Oxoid AG, Basel, Switzerland). Etoposide and poly-l-ornithine (PLO) were from Sigma (Buchs, Switzerland). Pifithrin-alpha (PFT-α) was from Alexis Biochemicals (Lausanne, Switzerland). Lysis buffer 5X was from Promega (Catalys AG, Wallisellen, Switzerland). Bio-Rad protein assay, Trans-Blot transfer medium were from Bio-Rad (Munich, Germany). Hybond-N+membrane, Rainbow-stained protein molecular weight markers and enhanced chemiluminescence (ECL) western blotting detection system were from Amersham Biosciences (Buckinghamshire, UK). Sheep polyclonal MMP-9 antibody was from The Binding Site (Birmingham, UK). Goat polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) specific antibody and mouse monoclonal p53 specific antibody used for western blotting were from Santa Cruz (CA, USA). Mouse monoclonal wt-p53 specific antibody (clone Pab1620) and mouse monoclonal p53 antibody (clone Pab240) were from Oncogene (Stehelin, Basel, Switzerland). p53 small interfering RNA (siRNA) and Transpass R2 transfection reagent were from New England Biolab (Beverly, USA).
Cell culture
Placental tissue was obtained from patients undergoing a legal abortion during the first trimester (7–12 weeks of pregnancy). Informed written consent was obtained from all patients before their inclusion in the study, for which approval was obtained from the local ethics committee.
CTB were isolated from first trimester placentas as described (Bischof et al., 1995). In brief, fresh tissue specimens were isolated and washed several times in sterile hanks balanced salt solution (HBSS). Tissue was then enzymatically digested four times for 20 min at 37°C (0.25% trypsin, 0.25 mg/ml DNase I). Single cells were collected, trypsin cocktail was neutralized with FBS and cells are then resuspended in DMEM. This cell suspension was filtered through a 100 µm filter, then laid onto a Percoll gradient (70% to 5% Percoll diluted with HBSS) and centrifuged for 25 min at 1200g. The 30–45% bands containing CTB were collected, washed and suspended in DMEM. Cells were then immunopurified to eliminate mononuclear cells from the lymphomyloide lineage. Typically, our CTB preparations were 98% cytokeratin-7 positive with 2% contamination with vimentin positive cells (Bischof et al., 1995).
Treatment of CTB
Half of the cells were treated with a p53 inducer, etoposide (20 µM) or a p53 inhibitor, PFT-α (30 µM), in serum-free medium for 24 h. The other half of the cells were untreated and used as control in serum-free medium.
Plasmids
The −670, −531 and −90 hMMP-9 promoter/chloramphenicol acetyltransferase (CAT) reporter plasmid used in this study were a generous gift from Prof. Sato (Kanazawa University, Ishikawa, Japan) and have been described previously (Sato and Seiki, 1993). Wild-type and mutant p53 (p53-175H: Arg→His substitution at position 175 and p53-143A: Val→Ala substitution at position 143) expression plasmids were obtained from Prof. Vogelstein at Johns Hopkins Oncology Center and have been described previously (Baker et al., 1990).
Transfection
Cells were seeded into plates 24 h prior to transfection. PLO was used to transiently transfect CTB. The culture medium was replaced with 1 ml of transfection cocktail (2 µg PLO/μg plasmid in culture medium without FBS) and the cells incubated for 6 h at 37°C, in 5% CO2. The transfection cocktail was then replaced by 1 ml of 30% (v/v) dimethylsulphoxide (DMSO) in DMEM supplemented with 1% (v/v) FBS for 4 min at room temperature to shock the cells. The DMSO medium was aspirated and the wells were washed with medium before continuing incubation. Culture medium was changed after 24 h and cells harvested 24 h later by lysis with the reporter lysis buffer. CAT activity was run according to the Promega's protocol and radioactivity counted in Tri-Carb 1900TR (Packard, Zurich, Switzerland).
To study the effects of wt-p53 and some p53 mutants on −670 hMMP-9 promoter activity, 1.5 million CTB per well were seeded in 12-well plates and cotransfected with 3 µg of −670 hMMP-9 promoter/ CAT reporter plasmid and 0.5 µg of mutant or wt-p53 constructs.
For determination of the minimal promoter sequence required for p53 repression, 1.5 million CTB per well were plated in 12-well plates for 24 h and transfected with 3 µg of −670, −531 or –90 hMMP-9 promoter/CAT reporter plasmid and 0.5 µg wt-p53 or control vector.
To study the effects of etoposide on −670 hMMP-9 promoter activity, 3 million CTB per well were plated in six-well plates and then transfected with 5 µg of −670 hMMP-9 promoter/CAT reporter plasmid. After 24 h, the medium was replaced and half of the cells were treated with etoposide (20 µM) for 24 h. The other untreated cells were used as control.
To study the effects of exogenous p53 on HMWC formation in trophoblast, 20 million CTB per dish were plated in 100 mm dish and then transfected with 20 µg of wt-p53 or control vector.
p53 silencing RNA
To study the effect of endogenous p53 on MMP-9 expression, CTB or MCF-7 cells (human breast adenocarcinoma cell line) were plated in six-well plates and then transfected with p53 short-cut siRNA mix or control siRNA (25 nM). Transpass R2 transfection reagent was used to transfect cells, as described by the manufacturer.
Zymography
Proteolytic activity of culture supernatants were assayed using gelatin-substrate gel electrophoresis as described previously (Martelli et al., 1993). Zymograms were scanned with an Epson Perfection 1 200 Photo scanner and the surface of the digestion bands measured by the Kodak 1D Image analysis software (Kodak, Rochester, NY, USA).
ELISA for MMP-2 and MMP-9
MMP-2 (gelatinase A) and MMP-9 concentrations were measured in the cell supernatants using our own enzyme-linked immunosorbent assay (ELISA) as described and validated elsewhere (Meisser et al., 1999).
Subcellular fractionation
Plated CTB were rinsed in HBSS, trypsinized and collected by centrifugation (800g, 10 min). The pelleted cells were rinsed in ice-cold phosphate-buffered saline (PBS) buffer (0.01 M sodium phosphate, 138 mM NaCl and 2.7 mM KCl, pH 7.4) and collected by centrifugation. The pellets were resuspended in 20 volumes of 10 mM Hepes buffer containing 1.5 mM MgCl2, 10 mM NaCl and 0.5 mM dithiotreitol (DTT) and a Roche protease inhibitor cocktail tablet, pH 7.9. Cells suspension were incubated in ice for 30 min and collected by centrifugation. The pellet was resuspended in 10 volumes of 10 mM Hepes containing 1.5 mM MgCl2, 10 mM NaCl and 0.5 mM DTT, 0.5% nonidet P40 (NP40) and homogenized gently by passing the suspension at least five times through a 20 gauge needle fitted to a syringe. Nuclear fraction was obtained by centrifugation at 1000g, 10 min. The supernatant was collected for cytosolic analysis. Nuclei were resuspended by gentle homogenization in 0.88 M sucrose and 3 mM MgCl2 and centrifuged at 2500g for 20 min to remove cell debris. The pellet was resuspended in PBS buffer and stored at −80°C until use. Assay for the cytoplasmic marker enzyme lactate dehydrogenase (LDH) was performed on each nuclear fraction to determine cytoplasmic contamination. No LDH activity was detected in our fractions.
LDH assay
Nuclear fraction (4 µg of protein) was incubated in sodium phosphate buffer, 0.1 M, pH 7, with sodium pyruvate (0.125 mg) and NADH (0.125 mg) for 30 min. The assay measured the rate of NADH absorbance decrease at 340 nm.
Western blot
Proteins (40 µg) were not reduced but were denatured by boiling at 100°C for 10 min. Samples were then subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis using a 10% running gel. Rainbow-stained molecular weight markers were used as standards. Proteins were electro-transferred to nitrocellulose membranes. Non-specific binding was blocked for 30 min at 37°C with 5% powdered milk in 0.2% NP40 buffer. p53 specific antibodies (diluted 1/1000) were incubated overnight with the nitrocellulose membrane. After washing, the membranes were incubated with the appropriate horse-radish peroxidase-linked secondary antibody (2 h, room temperature). After washing, the bands were revealed by chemiluminescence (ECL detection kit). Films were scanned with an Epson Perfection 1 200 Photo scanner and the surface of the bands measured by the Kodak 1D Image analysis software (Kodak).
Statistics
Data were analysed using paired Student's t-test and a P-value of <0.05 was considered statistically significant.
Results
Regulation of hMMP-9 promoter by p53 in CTB
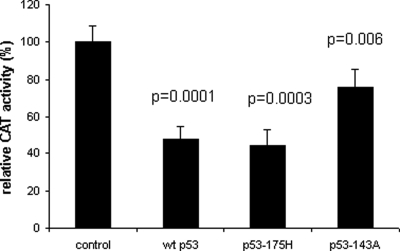
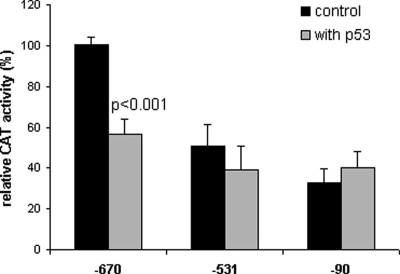
Transcription assays were performed with CAT reporter driven by −670 promoter of the hMMP-9 gene cotransfected with the expression vector of p53 in CTB. As shown in Fig. 2, wt-p53 and -p53 mutants commonly found in human cancer, p53-175H and p53-143A, are able to down-regulate −670 promoter activity. CAT activity is significantly reduced by wt-p53 and by p53-175H (∼50%), whereas p53-143A has partially lost the ability to repress the hMMP-9 promoter activity (∼25%). In order to map the site(s) responsible for p53-mediated repression, CTB have been cotransfected with wt-p53 expression plasmid or control vector and various 5′ deletions of hMMP-9/CAT promoter. As shown in Fig. 3, only −670 hMMP-9 CAT activity is significantly inhibited by wt-p53. So, cis-acting elements involved in the p53-induced down-regulation of hMMP-9 gene expression are localized between −670 and −531 bp of the 5′ flanking region of hMMP-9 gene.
Figure 2:
Repression of human (h)MMP-9 promoter activity by wild-type (wt)-p53 and mutants p53.
CTB were cotransfected with 6 µg of −670 hMMP-9 CAT reporter plasmid and 1 µg of mutant, wt-p53 constructs or control vector. After 48 h, cells were harvested and CAT activity was assayed and normalized to the amount of protein in the cellular lysates. Three independent transfections, each run in triplicate, were performed and the results expressed as mean ± SEM. Relative promoter activities were calculated by arbitrarily setting the activity of the control as 100. Paired Student's t-test was used to compare cells transfected with p53 constructs versus control vector.
Figure 3:
Determination of the minimal promoter sequence required for p53 repression.
CTB were transfected with 3 µg of −670, −531 or −90 hMMP-9 promoter/CAT reporter plasmid and 0.5 µg wt-p53 or control vector. After 48 h, cells were harvested and CAT activity was assayed and normalized to the amount of proteins in the cellular lysates. Three independent experiments, each run in triplicate, were performed and the results expressed as mean ± SEM. Paired Student's t-test was used to compare transfected cells with wt-p53 versus control plasmids.
Endogenous p53 regulation of MMP
We next examined the effect of endogenous p53 protein on MMP-9 expression. To this end, CTB were treated with an inducer of p53, etoposide, or with a specific inhibitor of p53, PFT-α (Komarov et al., 1999), or were transfected with p53siRNA. The effects on MMP-9 expression, secretion or promoter activity were then evaluated.
Effect of etoposide
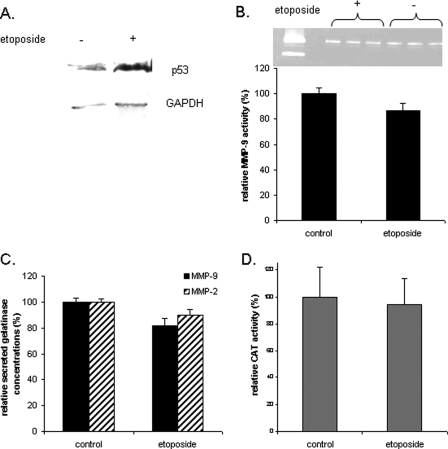
As shown in Fig. 4, whereas p53 expression was induced by etoposide (Fig. 4A), the levels of MMP-9 activity (Fig. 4B), the immunoreactivity of MMP-9 and MMP-2 (Fig. 4C), and the MMP-9 promoter activity (Fig. 4D) remained unchanged after etoposide treatment when compared with controls. Bian and Sun (1997) observed the same results concerning MMP-2 expression in the p53-negative Saos cell line.
Figure 4:
Effects of etoposide on p53 expression, MMP-9 activity, MMP-9 secretion and promoter activity in first trimester CTB.
Half of the CTB were treated with etoposide (a p53 inducer) for 48 h. The untreated cells were used as control. Cells were harvested and expression of p53 was evaluated by the western blot analysis under reducing conditions (A). Secreted MMP-9 activity and concentration measured by the gelatin zymography assay (B) and ELISA (C) in the culture medium. (B) CAT activity was also assayed and normalized to the amount of proteins in the cellular lysates (D). The representative zymogram (B) corresponds to one experiment run in triplicate. However, three independent experiments, each run in triplicate, were performed and the results expressed as mean ± SEM. Relative MMP-9 activity and concentration were calculated by arbitrarily setting the activity of the control as 100. Student's paired t-test was used to compare etoposide-treated and untreated cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of PFT-α
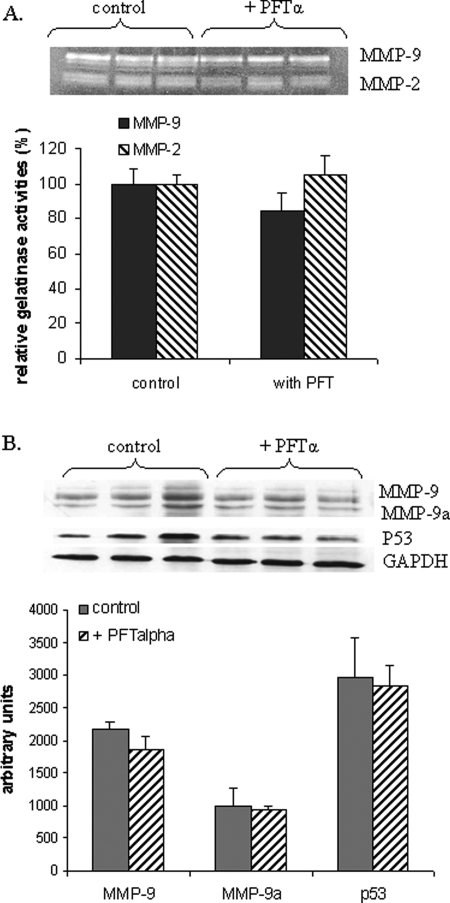
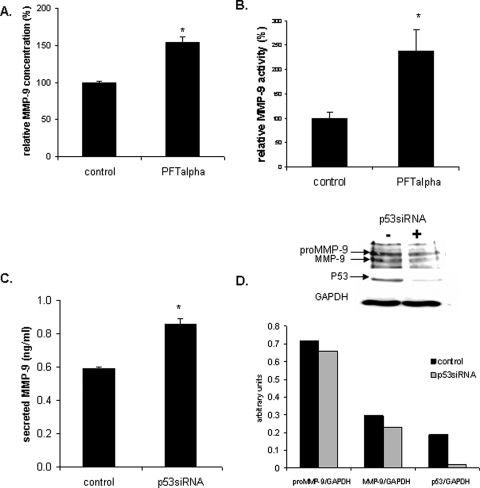
Because etoposide is not a specific inducer of p53, we also treated the CTB with PFT-α, a specific inhibitor of transcriptional activity of p53. As shown in Fig. 5, PFT-α did not change MMP-9 and MMP-2 activities (Fig. 5A) nor did it change MMP-9 expression (Fig. 5B) in contrast to the observations made in the same conditions in MCF-7 cells (Fig. 6A and B). As expected, since PFT-α inhibits only p53 transcriptional activity, p53 expression was not modified (Fig. 5B). These results suggest that in CTB endogenous p53 is not able to regulate MMP-2 and MMP-9 expression.
Figure 5:
Effects of PFT-α on gelatinases activities and on MMP-9 expression.
Half CTB were treated with a known p53 inhibitor, 20 µM PFT-α, for 48 h. The untreated cells were used as control. Cells were harvested and secreted MMP-9 (gelatinase B) and MMP-2 (gelatinase A) activities measured by the gelatin zymography assay in the culture medium (A). Expression of MMP-9 was evaluated by the western blot analysis (B). The zymogram (A) and western blot (B) scan corresponds to one experiment run in triplicate. However, three independent experiments, each run in triplicate, were performed and the results expressed as mean ± SEM. Relative MMP-9 and MMP-2 activities were calculated by arbitrarily setting the activity of the control as 100. Student's paired t-test was used to compare PFT-α treated and untreated cells.
Figure 6:
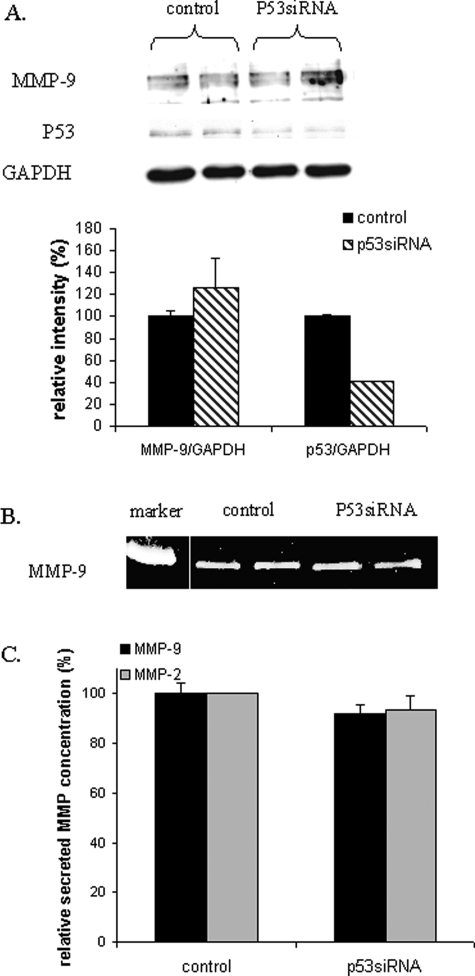
Effects of PFT-α and p53siRNA on gelatinase secretion and on MMP-9 and p53 expression in MCF-7 cells.
Effects of PFT-α on MMP-9 expression in MCF-7 were evaluated by ELISA (A) and zymography (B). To decrease the levels of p53 in MCF-7, cells were transfected with 25 nM p53siRNA or control siRNA. Secreted MMP-9 concentration was measured by ELISA in the culture medium (C). Cellular proteins (40 µg) were denatured and subjected to SDS–PAGE using a 10% running gel and blotted to PVDF. Western blot analysis was performed using a MMP-9 specific polyclonal antibody, a GAPDH specific antibody and a p53 specific antibody, followed by detection using ECL (D). Three independent experiments, each run in duplicate, were performed and the results expressed as mean ± SEM. Student's paired t-test was used to compare p53siRNA transfected cells and control siRNA transfected cells. *P < 0.05.
Effect of decreased expression of p53
In order to confirm the results obtained with PFT-α treatment of CTB, cells were transfected with p53siRNA. As shown in Fig. 6, although p53siRNA decreased the expression of p53 protein in CTB (Fig. 7A) and in MCF-7 (Fig. 6D), it did not change MMP-9 expression (Fig. 7A), activity (Fig. 7B) or immunoreactivity (Fig. 7C), whereas it increased MMP-9 secretion in MCF-7 (Fig. 6C). The secretion of the MMP-9, being the last step of its life, the lack of observation of reduced expression of MMP-9 in cellular extract could be due to the kinetics of experiment.
Figure 7:
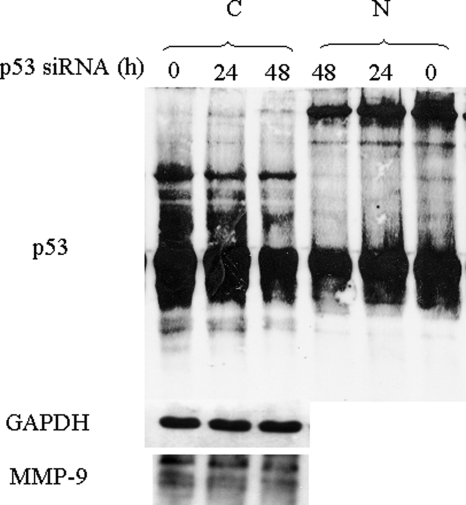
Effects of p53siRNA on MMP-9 and p53 expression and on gelatinases secretion of CTB.
CTB were transfected with 25 nM p53siRNA or control siRNA. Cellular proteins (20 µg) were denatured and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) using a 10% running gel and blotted to PVDF. Western blot analysis was performed using an MMP-9 specific polyclonal antibody, GAPDH specific antibody and a p53-specific antibody, followed by detection using ECL (A). Secreted MMP-9 activity (B) and concentration (C) was measured by zymography and ELISA, respectively, in the culture medium (C). Three independent experiments, each run in duplicate, were performed and the results expressed as mean ± SEM. Student's paired t-test was used to compare p53siRNA transfected cells and control siRNA transfected cells.
Status of p53
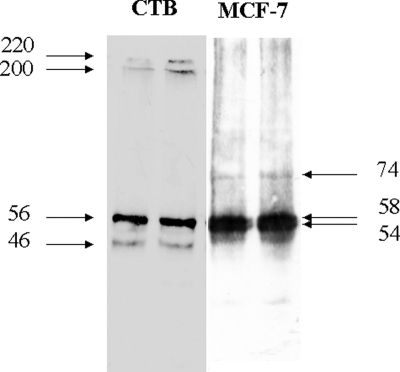
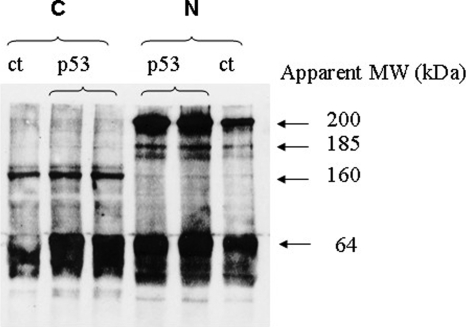
Whereas transfection studies showed that exogenous p53 inhibits MMP-9 promoter activity in CTB, endogenous p53 does not seem to inhibit endogenous MMP-9 expression. This led us to compare the status of p53 in CTB and in MCF-7 cells. MCF7 and CTB express a p53 protein estimated at 56–58 kDa on western blot, but in contrast to MCF-7, CTB express two HMWC of p53 (with apparent molecular weight of 220 and 195 kDa) as assessed by western blots of whole cell lysates under non-reducing conditions (Fig. 8). We then studied the effect of p53 expression level on HMWC in CTB. As shown in Fig. 9, transfection of exogenous wt-p53 in CTB increased both p53 monomer and nuclear HMWC of apparent MW of 220, 195 and 165 kDa, whereas it did not increase formation of HMWC in cytoplasm. In contrast, siRNA-induced decreased expression of endogenous p53 reduced the level of HMWC but not of p53 monomers in both cytoplasmic and nuclear fractions but remained without effect on MMP-9 expression (Fig. 10).
Figure 8:
Western blot of whole cell lysate of CTB and MCF-7 cells performed under non-reducing conditions and probed with p53 specific antibody, DO-1.
Two independent experiments, each run in duplicate, were performed. Markers: apparent molecular weight in kDa.
Figure 9:
Effect of exogenous p53 on HMWC level in trophoblast.
CTB were transfected with 5 µg of wt-p53 construct (p53) or control vector (ct). Western blot of cellular fractionations of CTB was performed under non-reducing conditions and probed with DO-1. C, cytoplasmic fraction; N, nuclear fraction; two independent experiments were performed.
Figure 10:
Effect of decreased level of endogenous p53 on HMWC level in trophoblast.
CTB were transfected with 25 nM p53siRNA or control siRNA. Western blot of cellular fractionations of CTB was performed under non-reducing conditions and probed with MMP-9 specific polyclonal antibody, GAPDH specific antibody and p53 specific antibody. C, cytoplasmic fraction; N, nuclear fraction; ct, control; two independent experiments were performed.
Discussion
The process of trophoblastic cell invasion involves degradation and remodelling of ECM mainly due to the action of MMPs. The gelatinases (gelatinase A: MMP-2; gelatinase B: MMP-9) that mainly degrade collagen IV and a number of other ECM proteins, such as collagen I, V, VII, IX, fibronectin, laminin, elastin and vitronectin, are the most studied MMPs in placental invasion (Librach et al., 1991; Bischof et al., 1995; Isaka et al., 2003; Staun-Ram et al., 2004). During the first trimester, MMP-2 is expressed in extravillous trophoblast, whereas MMP-9 is mainly expressed in villous CTB (Isaka et al., 2003), and in vitro, human CTB cells secrete MMP-2 and MMP-9 (Bischof et al., 1991, 1995).
p53 is a potent transcriptional regulator of MMPs (among other genes) in different cells (Bian and Sun, 1997; Sun et al., 1999, 2000, 2004; Meyer et al., 2005; Liu et al., 2006) either by direct interaction with a cis-element regulator, as described for MMP-2 (Bian and Sun, 1997), or by cis-element dependent, but binding independent mechanism as observed for MMP-9 in HT1080 cells (unpublished results).
Transfection experiments in CTB demonstrated the inhibitory effect of exogenous p53 on MMP-9 promoter activity confirming unpublished results obtained in HT1080 cells, and the study of Liu et al. (2006) in human leiomyosarcoma cell line. However, here, we observed that in spite of an apparent p53 overexpression in trophoblastic cells, endogenous p53 is unable to regulate MMP-9 and MMP-2 expression or activity in these cells. Furthermore, in contrast to MCF-7 cells, invalidation of p53 gene in CTB remained without effect on MMP-9. This apparent paradox between the effects of the endogenous and exogenous (transfected) p53 and the high expression of p53 could be explained by several mechanisms: by the presence of mutant p53 (Finlay et al., 1988), by the presence of dominant negative spliced variants (Bourdon et al., 2005), by a resistance to proteolytic degradation of p53 (Zaika et al., 1999), by an accelerated nuclear export or an inhibition of nuclear import (O'Brate et Giannakakou, 2003) and/or by sequestration of p53 in a complex through binding to other proteins (Nikolaev et al., 2003; O'Brate et Giannakakou, 2003). Since cloning of trophoblastic p53 did not identify mutations or spliced variants (Cohen et al., 2007), these two possible explanations seem to be excluded. Incubating CTB in the presence of MG132 (a proteasome inhibitor) increases the amount of p53 in CTB as measured by western blots (Cohen et al., 2007) indicating that the normal proteolytic p53 pathway is active in CTB. Although modifications of the nuclear import/export of p53 have not been investigated in CTB, the presence of HMWC of p53 in CTB as described here and reported previously (Cohen et al., 2007) could suggest that, in trophoblast, overexpression and functional inactivation of p53 towards its target genes MMP-2 and MMP-9 could be due to ‘sequestration’ of p53 in HMWC. This of course does not exclude an altered import/export of p53. However, western blot experiments showed that p53 monomers are also present in trophoblast. Despite an apparent excess of p53 monomers when compared with HMWC, it is unfortunately impossible to compare absolute quantities of p53 monomers to HMWC on western blot due to the difference of transfer capacity of proteins with low and high molecular weight. Transfection experiments leading to increased levels of p53 in trophoblast showed that cytoplasmic HMWC were saturated at physiological level, whereas reduced p53 expression decreased both p53 monomer and HMWC in similar proportions. These results suggest that inactivation of p53 by HMWC formation cannot be the only explanation to p53 inactivation and that one has to understand also why p53 is inactive in trophoblast.
In conclusion, whereas exogenous p53 is able to down-regulate MMP-9 promoter activity in CTB, endogenous p53 is not able to regulate MMP-9 expression in first trimester CTB cells. The presence of HMWC of p53 in CTB suggests a potential mechanism of p53 inactivation but cannot account for a complete inactivation. Inactivation of p53 through mutation is the most common trait in cancer. By loosing its oncosuppressive activity, p53 becomes oncogenic in almost all malignant tumours (Soussi and Lozano, 2005). Although p53 is not mutated in the human placenta, it has become functionally incompetent. If these in vitro results are also true in the in vivo situation, it would explain how invasion of CTB could be regulated. Understanding why and how p53 is functionally incompetent in CTB might well be the key to understanding trophoblast invasion.
Funding
This work was supported by Novartis foundation.
Acknowledgements
The authors wish to express their gratitude to Prof. Sato for the donation of MMP-9 promoter constructs and to Prof. Vogelstein for the p53 constructs.
References
- Ala-aho R, Grénman R, Seth P, Kähäri VM. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene. 2002;21:1187–1195. doi: 10.1038/sj.onc.1205198. [DOI] [PubMed] [Google Scholar]
- Backstrom JR, Lim GP, Cullen MJ, Tökés ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SJ, Markowitz S, Fearon ER, Willson JKV, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Behrendsten O, Werb Z. Metalloproteinases regulate parietal endoderm differentiating and migrating in cultured mouse embryos. Dev Dyn. 1997;208:255–265. doi: 10.1002/(SICI)1097-0177(199702)208:2<255::AID-AJA12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bian J, Sun Y. Transcriptional activation by p53 of the human type IV collagenase (gelatinase A or matrix metalloproteinase 2) promoter. Mol Cell Biol. 1997;17:6330–6338. doi: 10.1128/mcb.17.11.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof P, Friedli E, Martelli M, Campana A. Expression of extracellular matrix-degrading metalloproteinases by cultured human cytotrophoblast cells: effects of cell adhesion and immunopurification. Am J Obstet Gynecol. 1991;165:1791–1801. doi: 10.1016/0002-9378(91)90034-o. [DOI] [PubMed] [Google Scholar]
- Bischof P, Martelli M, Campana A, Itoh Y, Ogata Y, Nagase H. Importance of matrix metalloproteinases in human trophoblast invasion. Early Pregnancy. 1995;1:263–269. [PubMed] [Google Scholar]
- Bischof P, Truong K, Campana A. Regulation of trophoblastic gelatinases by proto-oncogenes. Placenta. 2003;24:155–163. doi: 10.1053/plac.2002.0890. [DOI] [PubMed] [Google Scholar]
- Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Bischof P. Metalloproteinases and Human Placental Invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Haenggeli L, Irminger-Finger I, Bischof P. Status of p53 in cytotrophoblastic cells. Mol Hum Reprod. 2007;13:111–116. doi: 10.1093/molehr/gal105. [DOI] [PubMed] [Google Scholar]
- Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999;66:989–995. doi: 10.1002/jlb.66.6.989. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Bradley H. The tumor suppressor p53. Biochim Biophys Acta. 1993;1155:181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Kern S, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudon P, Vernant JC, Confavreux C, Belin MF, Desgranges C. Matrix metalloproteinase 9 (gelatinase B) in cerebrospinal fluid of HTLV-1 infected patients with tropical spastic paraparesis. Neurology. 1998;50:1920. doi: 10.1212/wnl.50.6.1920. [DOI] [PubMed] [Google Scholar]
- Haidacher S, Blaschitz A, Desoye G, Dohr G. Immunohistochemical evidence of p53 protein in human placenta and choriocarcinoma cell lines. Hum Reprod. 1995;10:983–988. doi: 10.1093/oxfordjournals.humrep.a136082. [DOI] [PubMed] [Google Scholar]
- Hurskainen T, Hoyhtya M, Tuttila A, Oikarinen A, Autio-Harmainen H. mRNA expressions of TIMP-1, -2, and -3 and 92-KD type IV collagenase in early human placenta and decidual membrane as studied by in situ hybridisation. J Histochem Cytochem. 1996;44:1379–1388. doi: 10.1177/44.12.8985130. [DOI] [PubMed] [Google Scholar]
- Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24:53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Iguchi A. Role of matrix metalloproteinases in vascular remodelling. J Atheroscler Thromb. 2003;10:275–282. doi: 10.5551/jat.10.275. [DOI] [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhan M, Hannay JAF, Das R, Bolshakov SV, Kotilingam D, Yu D, Lazar AF, Pollock RE, Lev D. Wild-type p53 inhibits nuclear factor-κB–induced matrix metalloproteinase-9 promotor activation: Implications of soft tissue sarcoma growth and metastasis. Mol Cancer Res. 2006;4:803–810. doi: 10.1158/1541-7786.MCR-06-0201. [DOI] [PubMed] [Google Scholar]
- Martelli M, Campana A, Bischof P. Secretion of matrix metalloproteinases by human endometrial cells in vitro. J Reprod Fertil. 1993;98:67–76. doi: 10.1530/jrf.0.0980067. [DOI] [PubMed] [Google Scholar]
- Marzusch K, Ruck P, Horny HP, Dietl J, Kaiserling E. Expression of the p53 tumour suppressor gene in human placenta: an immunohistochemical study. Placenta. 1995;16:101–104. doi: 10.1016/0143-4004(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Meisser A, Chardonnens D, Campana A, Bischof P. Effects of tumour necrosis factor-alpha, interleukin-1 alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Mol Hum Reprod. 1999;5:252–260. doi: 10.1093/molehr/5.3.252. [DOI] [PubMed] [Google Scholar]
- Meyer E, Vollmer JY, Bovey R, Stamenkovic I. Matrix metalloproteinases 9 and 10 inhibit protein kinase C-potentiated, p53-mediated apoptosis. Cancer Res. 2005;65:4261–4272. doi: 10.1158/0008-5472.CAN-04-2908. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nikolaev M, Li N, Puskas J, Gu QW. Parc A cytoplasmic anchor for p53. Cell. 2003;112:29–40A. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- O'Brate A, Giannakakou P. The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist Updat. 2003;6:313–322. doi: 10.1016/j.drup.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Polette M, Nawrocki B, Pintiaux A, Massenat C, Maquoi E, Volders L, Schaaps JP, Birembaut P, Foidart JM. Expression of gelatinases A and B and their tissue inhibitors by cells of early and term human placenta and gestational endometrium. Lab Invest. 1994;71:838–846. [PubMed] [Google Scholar]
- Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49:179–186. doi: 10.1016/j.critrevonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kD type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am J Obstet Gynecol. 1994;171:832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Comm. 2005;331:834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subler MA, Martin DW, Deb S. Inhibition of viral and cellular promoters by human wild-type p53. J Virol. 1992;66:4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sun Y, Wenger L, Rutter JL, Brinckerhoff CE, Cheung HS. p53 down-regulates human matrix metalloproteinase-1 (Collagenase-1) gene expression. J Biol Chem. 1999;274:11535–11540. doi: 10.1074/jbc.274.17.11535. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheung JM, Martel-Pelletier J, Pelletier JP, Wenger L, Altman RD, Howell DS, Cheung HS. Wild type and mutant p53 differentially regulate the gene expression of human collagenase-3 (hMMP-13) J Biol Chem. 2000;275:11327–11332. doi: 10.1074/jbc.275.15.11327. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zeng XR, Wenger L, Firestein GS, Cheung HS. P53 down-regulates matrix metalloproteinase-1 by targeting the communications between AP-1 and the basal transcription complex. J Cell Biochem. 2004;92:258–269. doi: 10.1002/jcb.20044. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Werner H, Karnieli E, Rauscher F, Leroith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci USA. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase-2, -9, and -14, tissue inhibitors of metalloproteinase-1, and matrix proteins in human placenta during the first trimester. Biol Reprod. 2000;62:988–994. doi: 10.1095/biolreprod62.4.988. [DOI] [PubMed] [Google Scholar]
- Zaika A, Marchenko N, Moll UM. Cytoplasmically “sequestered” wild type p53 protein is resistant to Mdm2-mediated degradation. J Biol Chem. 1999;274:27474–27480. doi: 10.1074/jbc.274.39.27474. [DOI] [PubMed] [Google Scholar]