Abstract
Dengue virus (DENV), a re-emerging arbovirus, readily infects dendritic cells (DC) in culture and in vivo. However, there have been contradictory reports regarding the effect of DENV infection on DC activation and maturation. DC undergo a series of functional changes following exposure to infectious agents, including cytokine production and costimulatory and MHC molecule induction, culminating in stimulation of adaptive immune responses. Immunological memory to primary DENV infection critically influences disease severity during subsequent infections with heterologous serotypes. To explore these phenomena, we examined DENV infection-dependent and -independent effects on DC secretory, phenotypic, and allostimulatory functions. DENV infection of DC resulted in the secretion of a broad array of cytokines and chemokines. Type I IFN produced by DC inhibited propagation of infection and induced the chemokine IFN-γ-inducible protein 10 (IP-10; CXCL10). Based on intracellular cytokine staining, infected DC produced less IP-10 but more TNF-α than uninfected bystander cells in the same culture. DENV exposure activated surface molecule expression on infected and bystander cells; infected DC had enhanced programmed death ligand 2 (PD-L2) and MHC II expression but reduced levels of PD-L1, CD80, CD86, and MHC I relative to bystander DC. Dengue-infected DC cultures stimulated resting allogeneic CD4 T cell proliferation, although an increasing multiplicity of infection was associated with decreasing stimulatory capacity of DC. These data demonstrate that functional maturation of DC in response to dengue infection is modified by the presence of virus through IFN-dependent and -independent mechanisms with consequences for the development of adaptive immunity.
Keywords: flavivirus, IFN-α, IP-10, PD-L1, T-lymphocyte
INTRODUCTION
Dendritic cells (DC) are innate immune cells critical in the detection of and response to microbial challenge. Bone marrow-derived myeloid DC are targets for a variety of RNA viruses, including measles [1, 2], HIV [3, 4], hantaan [5], yellow fever [6], and influenza viruses [7], and can be instrumental in viral dissemination [8, 9]. Myeloid DC precursors migrate via the blood to temporary sites of residence in a wide range of tissues, where they maintain an “immature”, preactivated state. Immature DC (iDC) constitutively sample their environment, where binding of pathogen-derived products to specific cellular receptors potentiates transcriptional, morphological, and functional changes. A microbial “danger signal” [10] thus initiates a program of DC “maturation” [11].
DC maturation includes a transient burst of antigen uptake followed by a switch to enhanced antigen processing and presentation, the secretion of cytokines and chemokines, and homing to secondary lymphoid tissues to stimulate T cell responses [11, 12]. The maturation process evolves as the DC encounters additional signals such as intracellular viral dsRNA [7] or CD40-CD40 ligand interactions with T cells [2]. Plasticity in DC activity ostensibly allows for priming of the most appropriate adaptive response for pathogen clearance, and pathogens develop evasion strategies to modify DC functions.
Dengue is a disease of the tropics and subtropics that affects more than 50 million individuals each year with significant morbidity and mortality. Dengue illness is the product of infection with one of four dengue viruses (DENV). These are antigenically related, mosquito-borne, positive-sense RNA viruses of the family Flaviviridae, genus Flavivirus. The broadening range of DENV and its major vector Aedes aegypti increases the likelihood of hyperendemicity, cocirculation of multiple serotypes within a geographical region (reviewed in ref. [13]). This is a critical fact, as DENV infection results in lasting, serotype-specific immunity but does not provide protection against other “heterologous” serotypes. On the contrary, prior heterotypic DENV exposure is a major risk factor for developing severe disease, dengue hemorrhagic fever (DHF) [14, 15]. Increased vascular permeability is a cardinal feature of DHF, and one hypothesized mechanism is excessive cytokine production as a result of reactivation of serotype cross-reactive T cells [16], such as CD4 and CD8, which are induced by primary infection [17, 18] and are preferentially expanded during secondary DENV infection [19]. However, the mechanisms of priming and reactivation of DENV-specific T cells are not well characterized.
The principal cellular targets of DENV in vivo remain poorly defined. Monocytes and macrophages have been considered major targets of DENV infection [20,21,22]. Recently, attention has shifted to DC, originally shown to be infected by DENV ex vivo and in vitro by Wu et al. [23]. Subsequent work demonstrated that DENV induces DC activation with surface phenotype changes and secretion of inflammatory mediators [24,25,26,27]. Reports regarding the effects that active viral replication has on DC functions are varied, and the effects on adaptive immunity [28] are not well defined. Virus-infected DC may have functional responses distinct from bystander DC, potentially altering T cell priming. As functional maturation of DC is critical in regulating adaptive immunity, we investigated the direct and indirect roles of DENV in regulating DC production of inflammatory mediators, surface molecule regulation, and the priming of allogeneic CD4 T cells.
MATERIALS AND METHODS
Monocyte isolation and culture
We generated monocyte-derived DC following methods described previously [29] with minor modifications. Briefly, PBMC were isolated from the heparinized blood of healthy adult volunteers in a dengue-nonendemic region using Ficoll-hypaque (Amersham/GE Healthcare Bio-Sciences AB, Uppsala Sweden). Subjects were enrolled under a clinical study protocol approved by the University of Massachusetts Medical School Institutional Review Board (Worcester, MA, USA). We selected U.S.-born subjects without a history of receipt of flavivirus vaccines and without a history of international travel. Monocytes were isolated using MACS™ (Miltenyi Biotec, Germany) CD14-positive selection, according to the manufacturer’s protocol. The CD14− fraction was collected and subsequently used for T cell isolation. CD14+ cells were washed and resuspended at 1.4–1.8 × 106 cells/mL in 24-well plates in RPMI 1640 containing penicillin/streptomycin (1 mg/mL), 10% heat-inactivated FCS (Hyclone, Logan, UT, USA), 800 U/mL recombinant human (rh)GM-CSF, and 500 U/mL rhIL-4 (Peprotech, Rocky Hill, NJ, USA). At Days 3–4, 0.5 mL medium was removed and replaced with fresh medium containing 1600 U/mL rhGM-CSF and 1000 U/mL rhIL-4. On Days 6–7, DC were collected by vigorous pipetting and gentle scraping. These cells were CD3−, CD8−, CD14−, CD19−, and CD56− but were MHC class II+ and MHC class Ilow and expressed the myeloid DC marker CD1a+ (data not shown). Cells had an iDC (CD83–/low) phenotype.
T cell isolation
CD45RA+ CD45RO− CD4 T cells were isolated from CD14− PBMC by magnetic-negative selection using a naïve T cell isolation kit (StemSep™, StemCell Technologies, Canada) according to the manufacturer’s protocol. Cell separation was performed using LS columns (Miltenyi Biotec). Negatively selected T cells were washed, counted, and resuspended in RPMI 1640 supplemented with penicillin/streptomycin and 10% heat-inactivated human AB serum (Gemini Bio-Products, West Sacramento, CA, USA; RPMI AB10) at 1.0 × 106 cells/mL. Isolated T cells were typically 90–95% CD3+ and 95% CD4+. The CD3+CD4+ cells were routinely >98% CD45RA+CD45RO− (data not shown). T cells were added to wells in a 96-well plate at 50,000 T cells in 50 μL RPMI AB10 for coculture experiments.
Dengue infection of iDC
DENV2 strain New Guinea C was propagated in the mosquito cell line C6/36. Virus titers were determined by plaque assay on Vero or LLC-MK2 cells. In control experiments, virus was rendered nonreplicative by placing an aliquot 5–6 cm under a germicidal lamp (2300 μW/cm2 UVA irradiation at 254 nm) and incubating for 30 min on ice.
For infection, DC pellets in 15 mL polypropylene tubes were incubated at 37°C 5% CO2 for 90 min with C6/36 medium (mock infection), UV-inactivated virus, or live virus. Except where indicated, DC were infected at a multiplicity of infection (MOI) of 2 pfu/cell. Following adsorption, the cells were washed and plated in 24-well plates (1 mL/well) or 48-well plates (0.5 mL/well) at 1.0 × 106 cells/mL and maintained at 37°C, 5% CO2. TNF-α at 50 ng/mL (R&D Systems, Minneapolis, MN, USA; Peprotech), IFN-α at 1000 U/mL (5 ng/mL, Peprotech), and/or LPS at 50 ng/mL (from Escherichia coli, Sigma Chemical Co., St. Louis, MO, USA) were added where noted. For DC infection using a range of MOIs, virus stock was serially diluted fivefold in medium, and DC were infected in a fixed volume as described above.
Multiplex analysis of cytokines and chemokines
Cell culture supernatants were analyzed for levels of 23 cytokines and chemokines using the BeadLyte cytokine assay kit (Upstate Biotechnology, Lake Placid, NY, USA) as per the manufacturer’s protocol. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a 5-parameter curve-fitting algorithm applied for standard curve calculations. The lower limit of detection threshold was 10 pg/mL for all analytes, with the exception of IL-12 p40 (40 pg/mL), IFN-γ (13 pg/mL), eotaxin (69 pg/mL), and MIP-1α (86 pg/mL). Upper limits of detection were greater than 10,000 pg/mL with the exception of RANTES (8750 pg/mL). For the purposes of graphical analysis, samples outside the limits of detection were plotted at the limit.
Antibodies for flow cytometric analysis
The following mAb to human targets were purchased from BD/PharMingen (San Diego, CA, USA): FITC anti-CD3, FITC anti-CD45RA, FITC IgG2a, FITC IgG1k, PE anti-HLA-A, -B, and -C, PE anti-CD40, PE anti-IFN-γ-inducible protein 10 (IP-10), PE anti-CD45RO, PE anti-CD56, PE anti-CD1a, PE-anti-programmed death ligand 1 (PD-L1), PE anti-CD80, PE IgG2a, PE IgG1k, PerCP anti-CD4, PerCP IgG1k, allophycocyanin (APC) anti-CD3, APC anti-CD19, APC anti-CD56, APC anti-CD83, APC anti-CD1a, APC anti-HLA-DR, APC anti-PD-L2, APC anti-CD86, APC anti-TNF-α, and APC IgG1k. Purified mouse IgG1k was purchased from Becton Dickinson (San Jose, CA, USA) and used as a blocking antibody in some experiments. Cross-reactive, anti-dengue complex mAb (clone M8051125, IgG2a) was purchased from Fitzgerald Industries (Concord, MA, USA) and was custom-conjugated to FITC or labeled with Zenon™ Pacific Blue (Molecular Probes/Invitrogen, Eugene, OR, USA) reagent, according to the manufacturer’s protocol. In some experiments, DENV envelope staining was performed using FITC goat anti-mouse IgG (Sigma Chemical Co.) as a secondary antibody.
IFN-blocking experiments
DC were harvested and washed as described previously. The cells were then incubated with medium alone or with medium plus IgG2a or anti-CD118 [IFN-αR subunit 2 (IFNAR2)] antibody (PBL Laboratories, Piscataway, NJ, USA) at 50 μg/mL for 30 min. The addition of virus resulted in dilution of antibody to 10 μg/mL. Viral adsorption, washing, and DC culture were carried out as described above in the presence of antibody where specified. Upon resuspension, the appropriate antibody (IgG2a or anti-IFNAR2) was added at 10 μg/mL for the duration of culture as required.
Immunocytochemistry and flow cytometric analysis
For staining of cell-surface markers, DC were washed twice with Dulbecco’s PBS (Gibco, Grand Island, NY, USA), 2% FCS, and 0.5% sodium azide [PBS-azide wash buffer (PAWB)]. In some experiments, DC were incubated for 15 min with 500 ng mouse IgG1k to reduce nonspecific staining. Cell marker-specific antibodies were added at 50 ng per antibody for 30 min at 4°C. Cells were washed three times and fixed using Cytofix (Becton Dickinson) or Cytofix/Cytoperm (Becton Dickinson), according to the manufacturer’s protocol. Cells were assayed for DENV E protein expression by permeabilization and staining for 30 min with anti-DENV (1 μg) antibody. For intracellular cytokine staining, 1 μg brefeldin A (Golgiplug, Becton Dickinson) per million cells was added 6–8 h prior to harvesting and fixation. DC were washed two times with Perm/Wash (Becton Dickinson), and then anti-IP-10 (0.5 μg) and/or anti-TNF-α (0.5 μg) and anti-DENV (1 μg) antibodies were added for 30 min, after which, the cells were washed three times with Perm/Wash buffer and resuspended in PAWB for analysis. Flow cytometry was performed on FACSCalibur, FACSAria, or LSRII flow cytometers (BD Immunocytometry Systems, San Jose, CA, USA) with data collection using CellQuest 3.1.3 or FACSDiva 5.x software. Data were analyzed using FlowJo™ software version 6 or higher (Treestar, Ashland, OR, USA).
DC-T cell coculture
Infected, mock-infected, or cytokine-treated DC were harvested, washed, and resuspended in RPMI AB10 at 105 cells/mL and then serially diluted as needed. DC were added to T cells (5×104 cells in 50 μL) in 100 μL aliquots in quintuplicate wells for each condition to a final volume of 150 μL/well. In experiments using a range of MOIs, DC were washed and resuspended as described, with a final count of 5 × 104 cells/mL and added to 100 uL T cell suspension (at 5×105 T cells/mL) in aliquots of 50 μL (2500 DC; T:DC ratio of 20:1). T cells incubated with 1 μg/mL PMA plus 0.1 μg/mL ionomycin (Sigma Chemical Co.) served as a positive control. Negative control wells were T cells receiving medium alone.
Tritiated thymidine incorporation
Following 3–4 days of coculture, 1 μCi tritiated thymidine (Perkin-Elmer, Wellesley, MA, USA) was added to each well in 50 μL RPMI AB10, 18–20 h before harvesting. Cells were harvested onto glass fiber filter mats, and the incorporation of radioactive thymidine was measured using a Betaplate liquid scintillation counter. PMA/ionomycin-stimulated cells routinely produced counts in the range of 50,000 to greater than 100,000 cpm; negative controls were routinely less than 100 cpm.
Statistics
All statistical analyses used the Wilcoxon signed-rank test. For cell-surface phenotyping, the ratio of mean fluorescence intensities (MFI) was calculated for each comparison (MFI DENV+/MFI DENV−, MFI DENV+/MFI mock, or MFI DENV−/MFI mock) and compared with a hypothetical value of 1. Statistical analyses of coculture experiments were performed between groups on raw cpm values, and compared values were derived from the means of quintuplicate wells. For all analyses, P values of <0.05 were considered statistically significant.
RESULTS
Monocyte-derived iDC are highly susceptible to infection with DENV
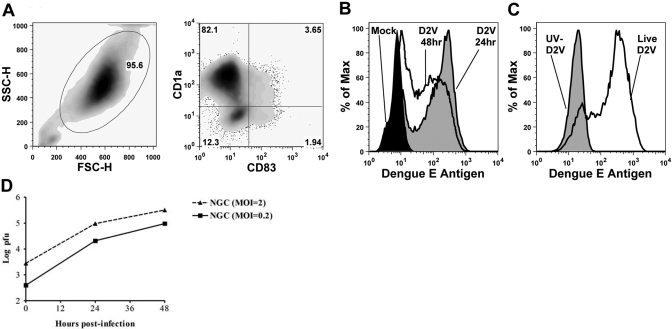
Monocyte-derived iDC are the in vitro counterparts of in vivo myeloid DC, including dermal interstitial DC [11]. Early studies demonstrated dengue infection of in vitro-cultured DC [23,24,25]. To study the effects of DENV on DC functions, we generated myeloid DC in culture and infected them with DENV2. Consistent with other studies, we observed highly efficient infection of DC by DENV (Fig. 1A). Maximal E protein production was found before 48 h (Fig. 1B). Only iDC treated with live DENV and not cells exposed to UV-irradiated virus expressed DENV E protein (Fig. 1C). Progeny virus was detected at 24 h postinfection and increased at 48 h postinfection, reaching titers of ∼105 pfu/ml (Fig. 1D). Cell numbers and viability were not significantly different in infected and uninfected DC cultures (data not shown). These data demonstrate that DENV readily infects iDC and that DENV E protein was synthesized de novo in infected cells.
Fig. 1.
iDC are highly susceptible to infection with DENV. (A) Flow cytometric analysis of iDC derived from CD14 positively selected monocytes. Results are representative of at least 10 experiments. iDC were CD14− HLA-A, -B, and -Cmid HLA-DR+ (not shown). SSC/FSC-H, Side-/forward-scatter-height. (B) iDC were mock-infected (black fill) or infected for 24 (gray fill) or 48 h (no fill) with live DENV2 (D2V) at a MOI of 2 and stained for intracellular dengue E protein using a FITC anti-dengue complex mAb. The data shown are representative of three similar experiments. (C) iDC were treated with live virus (no fill) as in B or with UV-inactivated virus (gray fill). The data shown are representative of more than five experiments. (D) iDC were infected with live DENV2 strain New Guinea C (NGC) at the indicated MOI. Culture supernatants were collected after virus adsorption and at 24 and 48 h postinfection, and titers of infectious virus were determined by plaque assay on LLC-MK2 cells.
DENV infection of DC induces secretion of a characteristic array of inflammatory cytokines without IL-12p70, TGF-β, or IL-10
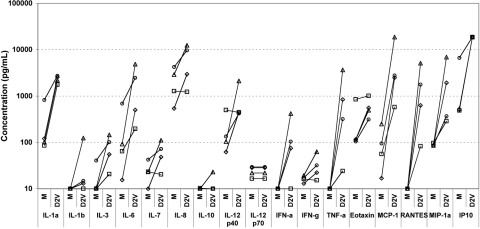
In vitro studies with other viruses have indicated that monocyte-derived DC are capable of production of a wide range of cytokines [30, 31]. DENV infection of iDC has been reported to induce the production of inflammatory cytokines such as IFN-α and TNF-α [24, 25] and chemokines including IL-8 and RANTES [26]. We hypothesized that DENV infection of iDC would induce a largely donor-independent, characteristic secretory response. Using Luminex technology, we compared the secretory responses in 24 h supernatants of mock-infected and DENV-infected DC. Several chemokines were secreted at high levels in response to DENV (Fig. 2), and IP-10 reached the highest concentrations (>18,750 pg/mL, the upper limit of quantitation, in four of four samples). RANTES, MIP-1α, and MCP-1 were also found in supernatants at substantially increased levels following DENV infection.
Fig. 2.
DENV2 infection of iDC cultures induces a broad inflammatory cytokine and chemokine secretory response. iDC were mock-infected (M) or infected with DENV2 (MOI=2). Supernatants were collected at 24 h and analyzed using a multiplex bead assay for a panel of immune mediators. Results from four independent experiments are shown.
Inflammatory cytokines TNF-α, IL-6, and IL-1α were increased in DC supernatants in response to DENV, as were the growth factors IL-7 and IL-3. IFN-α secretion was undetectable in mock-infected DC supernatants but increased in response to DENV2 in three of four experiments. DENV2 induced IL-12 p40 secretion in three of four experiments, yet IL-12 p70 heterodimer concentrations were one to two orders of magnitude lower with no DENV-specific increase. IL-10 production was detected slightly above the assay threshold in only one of four mock-infected samples (10 pg/mL) and one of four DENV-infected samples (23 pg/mL). In control experiments, LPS stimulation resulted in IL-10 secretion by 24 h (mean, 327 pg/mL; range, 288–433 pg/mL; n=3). We also noted no differences in TGF-β-1, -2, or -3 secretion between mock-infected and DENV-infected cultures (data not shown). These data demonstrate that DC secrete a characteristic array of inflammatory cytokines and chemokines in response to DENV2 infection, without inducing IL-10, TGF-β, or IL-12p70.
Endogenous DC type 1 IFN production inhibits propagation of DENV infection in vitro
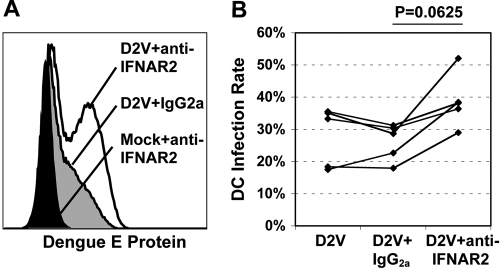
DENV susceptibility to the antiviral effects of IFNs has been described in immortalized cells but required pretreatment to be effective [32, 33]. Although monocyte-derived DC reportedly produce substantially less IFN-α than plasmacytoid DC in response to viruses [34, 35], we hypothesized that endogenous IFN-α/β induced following DENV exposure may inhibit active infection. We used an anti-CD118 (IFNAR2) mAb to block IFN-α/β signaling in DC during virus adsorption and culture. In five experiments, E protein staining at 24 h for DENV infection alone (mean±sd, 28.0±8.2%) and DENV plus IgG2a (26.2±5.1%) was similar but increased in the presence of blocking antibody (38.8±7.5%, P=0.06; Fig. 3). These results suggest that IFN-α/β produced by monocyte-derived DC inhibits ongoing DENV2 infection in vitro.
Fig. 3.
Endogenous IFN-α/β inhibits DENV infection of DC. iDC were preincubated for 30 min with medium alone or medium plus IgG2a or anti-IFNAR2 antibody at 10 μg/mL. Cells were then mock-infected or DENV2-infected (MOI=2). DC were harvested and stained for intracellular dengue E protein expression at 24 h. (A) Representative flow cytometry for dengue E protein expression in the specified cell populations. (B) Percent of DC staining positive for dengue E protein in experiments with five separate donors under the specified conditions. Exact P value by Wilcoxon signed rank test.
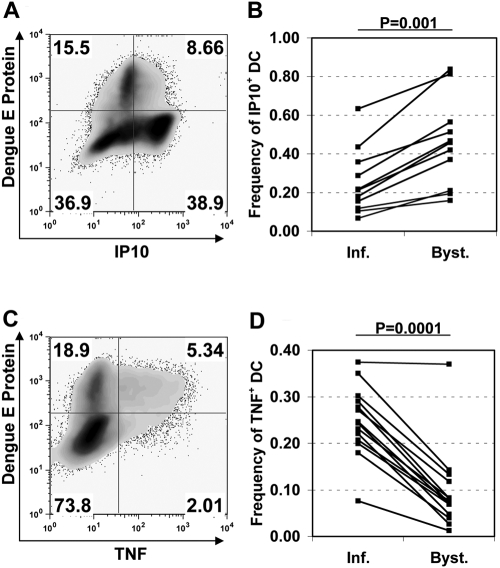
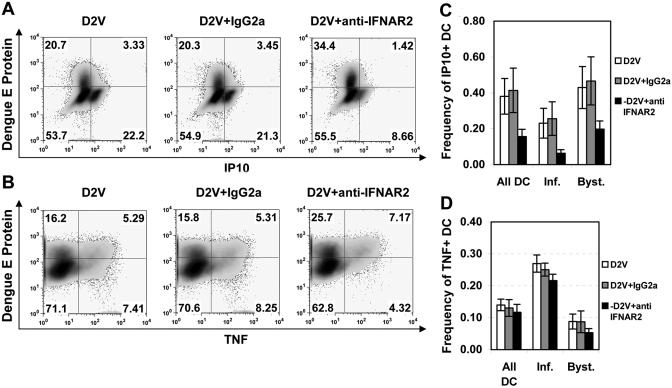
DENV-infected and bystander DC differentially produce TNF-α and IP-10
As DENV proteins are implicated in IFN inhibition [36, 37], we hypothesized that a consequence of differential IFN signaling would be distinct cytokine production profiles in actively infected versus bystander DC. Using intracellular cytokine staining for TNF-α and IP-10, we noted marked differences in the production of these two proteins depending on the presence of viral E protein. The frequency of cells staining positive for IP-10 was consistently lower in infected cells than in bystander cells (mean±sd, 0.252±0.160 vs. 0.455±0.216, P=0.001; Fig. 4, A and B). Bystander DC demonstrated a range of IP-10 staining, including IP-10 high populations, whereas infected DC had uniformly low-to-negative IP-10 staining. On the other hand, TNF-α-positive cells were 2.6 times more abundant in infected DC than in uninfected bystander cells (mean±sd frequency, 0.248±0.072 vs. 0.096±0.084, P=0.0001; Fig. 4, C and D). These findings demonstrate that DENV infection of DC differentially regulates IP-10 and TNF-α in virus-infected and bystander cells.
Fig. 4.
Infected DC are deficient in IP-10 production but more effectively induce TNF-α when compared with bystanders. iDC were infected with DENV2 (MOI=2), and brefeldin A was added 6–8 h prior to harvest and fixation at 24 h. Cells were stained using an anti-dengue E protein mAb and mAb directed against IP-10 and/or TNF-α. (A) Representative flow cytometry plot of DC showing dengue antigen staining versus intracellular IP-10. (B) Analysis of multiple experiments showing the frequency of IP-10-expressing cells in infected (Inf.) or bystander (Byst.) DC populations, as assessed by dengue E protein staining (exact P value by Wilcoxon signed rank test). (C) Flow cytometric plot from the same donor as shown in A, demonstrating dengue E protein staining compared with TNF-α production. (D) Analysis of multiple experiments, as described in B, for TNF-α production (Gaussian approximation of P value by Wilcoxon signed rank test).
Role of endogenous IFN-α/β in DC IP-10 and TNF-α production
Potent IP-10 production in bystander cells suggested that a soluble factor was responsible, and IFN-α is capable of inducing IP-10 in monocyte-derived DC [38]. We therefore hypothesized that blocking IFN-α/β would suppress IP-10 but not TNF production. Anti-CD118 treatment inhibited IP-10 production. We noted a mean frequency ± sd of IP-10+ DC of 0.156 ± 0.041 following blocking antibody treatment, as opposed to 0.414 ± 0.124 with isotype control IgG2a treatment (Fig. 5A). In control experiments, the concentration of anti-CD118 used fully blocked DC IP-10 production in response to 1000 U/ml (>5 ng/mL) exogenous IFN-α (data not shown). Blocking endogenous IFN-α/β during infection had minimal effects on the frequency of TNF+ cells (Fig. 5B). We noted a mean frequency ± sd of TNF+ DC of 0.140 ± 0.018 in DENV-infected cultures versus 0.131 ± 0.026 with the addition of isotype control antibody and 0.117 ± 0.025 with blocking anti-CD118 antibody. These data demonstrate that endogenous IFN-α/β produced by DC in response to DENV infection acts in an autocrine/paracrine manner, contributing to DC production of IP-10 but not TNF-α. However, incomplete IP-10 block by anti-CD118 suggests that IP-10 production was not solely IFN-α/β-dependent.
Fig. 5.
Blocking endogenous IFN-α/β signaling inhibits IP-10 but not TNF-α production in DENV-infected DC cultures. (A) DC were infected with DENV2 (MOI=2) in the presence anti-CD118 IFNAR2-blocking antibody or an isotype control IgG2a antibody and stained for IP-10, TNF-α, and DENV2 E protein at 24 h postinfection. IP-10 production was analyzed by flow cytometry in infected (upper-right quadrant) and bystander (lower-right quadrant) DC. Representative flow cytometry from one of three experiments is shown. (B) Cells from A were also analyzed for TNF-α production. The data shown are representative of five experiments. (C and D) The frequency of IP-10-expressing cells (C) and TNF-α-expressing cells (D) was calculated for each cell population specified. Shown are the means ± sd for three experiments (C) and five experiments (D).
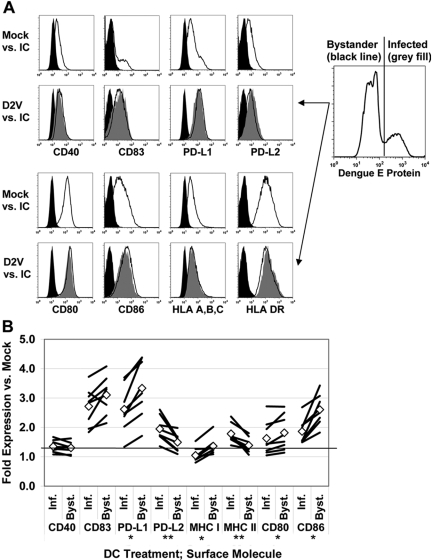
DENV infection induces differential expression of DC immunomodulatory surface molecules
Several of the secreted products we detected, including IFN-α and TNF-α, are implicated in the process of DC activation and maturation [29, 39, 40]. One aspect of DC maturation is the induction of surface molecules involved in T cell regulation. These molecules include B7 family costimulators CD80 and CD86 and cosuppressors PD-L1 and PD-L2, the TNF family costimulator CD40, the mature DC marker CD83, and MHC class I and II molecules HLA-A, -B, and -C and HLA-DR. In our analysis, mock-infected cells expressed low levels of CD40 and PD-L2 and intermediate amounts of MHC class I and PD-L1 (Fig. 6A). Approximately 50%, 90%, and 95% of mock-infected DC expressed CD86, CD80, and HLA-DR, respectively. CD83 expression was essentially absent. The ratio of MFI relative to mock-infected DC was used to quantify changes in protein expression levels in response to DENV infection and we used intracellular staining for DENV E protein to discriminate infected and bystander DC.
Fig. 6.
Infected DC activate surface molecule expression distinctly from uninfected bystander cells. DENV2-infected DC (MOI=2) were surface-stained at 24 h postinfection for the specified proteins and then fixed and intracellularly stained for dengue antigen. (A) Cells were gated on dengue antigen expression and analyzed by flow cytometry. Representative histograms are shown. (B) Expression of each surface molecule on dengue E protein-positive (Inf.) and E protein-negative (Byst.) DC, as quantitated by geometric MFI. Values shown represent fold-change in geometric MFI versus mock infection. Lines represent eight individual donors. ⋄, Mean values for the eight donors. *, P < 0.02, Inf. < Byst.; **, P < 0.02, Inf. > Byst., as determined by Wilcoxon signed rank test; IC, isotope control.
Infected and bystander cells increased surface expression of all molecules tested when compared with mock infection (P<0.02), with the exception of HLA-A, -B, and -C on infected DC (P=1.0). However, we noted reduced expression of the B-7 family molecules CD86 (geometric MFI ratio to mock-infected cells±sem for eight donors, 1.86±0.14 vs. 2.61±0.17), PD-L1 (2.62±0.26 vs. 3.33±0.32), and CD80 (1.63±0.19 vs. 1.82±0.20), as well as MHC class I molecules HLA-A, -B, and -C (1.05±0.08 vs. 1.36±0.10; P<0.02 for all comparisons) on infected DC in comparison with bystander cells. CD83 was also lower on infected DC (2.72±0.21 vs. 3.10±0.21) when compared with bystanders, although this difference was not statistically significant (P=0.055). Conversely, infected DC had significantly greater expression than did bystander cells of PD-L2 (geometric MFI relative to mock-infected cells±sem, 1.95±0.14 vs. 1.49±0.10) and HLA-DR (1.78±0.11 vs. 1.39±0.09; P<0.02 for both comparisons). CD40 (1.35±0.07 vs. 1.30±0.07) was unaffected by the presence of viral antigen (P=0.31). These data demonstrate that DENV2 exposure activates DC through direct and indirect mechanisms. The differences in protein expression could not be explained solely by viral suppression of IFN-α/β signaling, as relative differences were bidirectional. Active DENV replication positively and negatively modifies DC phenotype, depending on the surface molecule in question.
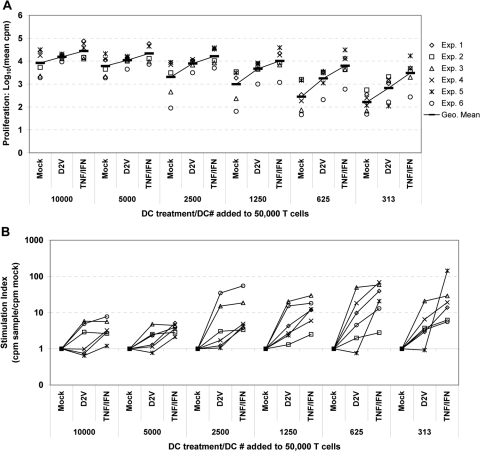
DENV infection activates DC priming of resting CD45RO− CD4 T cells
One major function of DC is the stimulation of adaptive immune responses through cell-surface-bound and soluble mediators. To investigate whether iDC gain T cell priming function upon exposure to DENV, we compared DC for their ability to stimulate proliferative responses of allogeneic, resting CD45RA+ CD45RO− CD4 T cells following mock infection, DENV infection, or cytokine-mediated maturation. As expected, we noted a dose-dependent increase in T cell proliferation with addition of increasing numbers of DC, regardless of treatment (Fig. 7A). In comparison with mock infection, DENV increased the T cell priming capacity of DC. This effect was associated with the highest values for SI [calculated as (cpm DENV-infected)/(cpm mock-infected)] with lower numbers of DC added (Fig. 7B). Increased T cell proliferation was observed even with >90% of DC staining positive for DENV antigen. DC matured with the addition of exogenous TNF-α/IFN-α were likewise superior to mock-infected DC as stimulator cells at all T:DC ratios (P<0.032), and SI values were highest when DC alloantigen was limiting. T cell proliferation induced by cytokine-matured DC was also statistically significantly higher than that observed with DENV-infected DC with addition of 2500 or fewer DC. These results demonstrate that DENV infection of DC enhances their ability to stimulate CD4 T cell alloresponses, although less effectively than the addition of exogenous cytokines.
Fig. 7.
DENV infection of DC cultures facilitates priming of allogeneic, quiescent CD4T+ cells. iDC were mock-infected (Mock) or infected with DENV2 (MOI=2) as described previously. Cells were washed and returned to culture for 24 h. Some mock-infected cells were stimulated by the addition of exogenous rhTNF-α (50 ng/mL) plus rhIFN-α (1000 U/mL=5 ng/mL; TNF/IFN). Following 24 h in culture, DC were harvested, washed, and counted, and twofold serial dilutions were performed. DC were added to 50,000 allogeneic CD45RA+CD45RO− CD4 T cells, and proliferation was quantitated by tritiated thymidine incorporation on Day 4 of coculture. Data points represent means from quintuplicate wells. (A) Proliferation responses from six independent experiments at a range of T:DC ratios from 5:1 to 160:1. Data are presented as log10 (mean cpm from quintuplicate wells) for clarity. Black lines represent the geometric mean values for all six experiments. (B) Stimulation indices were calculated from the data in A by normalizing to mock infection {Stimulation index (SI)=[cpm (treatment)/cpm(Mock)]}.
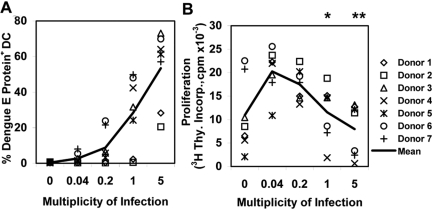
Increasing MOI of DC reduces T cell priming
In previous experiments, we noted differences between infected and bystander DC in cytokine/chemokine production and surface molecule expression. If infected and bystander DC also differed in T cell stimulatory capacity, we postulated that the efficiency of T cell priming would also be related to the multiplicity of DENV infection of DC cultures. We infected DC using serial dilutions of DENV from MOI 0.04 to 5 and cocultured them with T cells using a T:DC ratio of 20:1. DC infection was dose-dependent, albeit with considerable variability between DC donors (Fig. 8A). In coculture with T cells, DC infected with lower MOIs induced the greatest stimulation of T cell proliferation, whereas proliferative responses declined at higher MOIs (Fig. 8B). To determine the relative contributions of DC and T cells to interexperimental variation, DC from two donors were each tested against two different allogeneic T cell preparations. Both T cell response curves to a given DC donor were highly concordant; for those experiments, we plotted the mean value from the two cocultures from each DC donor in Figure 8B. These results suggest that bystander DC contribute more to stimulation of allogeneic, naïve T cells than DENV-infected DC in this in vitro system.
Fig. 8.
Higher DC infection rates are associated with decreased, quiescent CD4 T cell priming. iDC were mock-infected or infected at varying MOIs by using serial fivefold dilutions of virus stock in a fixed volume. Following 24 h of culture, DC were harvested, washed, counted, and added to 50,000 allogeneic CD45RO− CD4 T cells at a T:DC ratio of 20:1, and proliferation was determined as described previously. Data points represent the mean cpm for quintuplicate wells. Data points from DC Donors 4 and 5 are mean values from simultaneous testing against T cells from two donors. *, P < 0.02, versus MOI = 0.04, 0.2; **, P < 0.01, versus MOI = 0.04, 0.2, and 1, as determined by Wilcoxon signed rank test performed between groups.
DISCUSSION
We sought to identify the phenotypic and functional properties of DC following exposure to DENV and to elucidate the role that DENV plays in determining the characteristics of infected and bystander cells. We observed that DC exposed to DENV underwent cellular activation, as measured by the secretion of inflammatory mediators, enhanced expression of cell-surface molecules, and increased capacity for allospecific CD4 T cell priming. DC IFN-α/β production inhibited propagation of infection and induced IP-10 production. However, actively infected DC appeared to have blunted IFN-α/β responsiveness, as evidenced by low IP-10 production but enhanced TNF-α expression. Infected DC also showed an altered surface phenotype when compared with bystander cells and were less efficient at stimulating proliferation of allogeneic CD4 T cells.
The cytokine response of DC to DENV was broad, and the most striking changes were in chemokine production. DENV induction of chemokine expression in DC has been reported, specifically MIP-1α, MIP-1β, IL-8, RANTES, and MCP-1 [26]. Here, we report that DENV also induces high-level secretion of IP-10 from DC. IP-10 but not the other CXCR3 ligands, monokine induced by IFN-γ (CXCL9) and IFN-inducible T cell α chemoattractant (CXCL11), was reported to be critical for survival in a murine DENV challenge model [41]. Similar to previous reports [24, 25, 28], we found that DENV was also a potent inducer of inflammatory cytokines IFN-α and TNF-α but not IL-12p70. We did find increased IL-12p40 production in three of four experiments, suggesting that failure to produce IL-12p70 was related to a failure to produce the IL-12p35 subunit. One potential, alternative pathway for IL12p40 is IL-23, which uses the p40 subunit along with IL-23-specific p19 [42]. IL-23 has been reported to induce IL-17 secretion in CD4 T cells [43]; however, we did not note any increase in IL-17 levels in cocultures of T cells with DENV-infected DC (data not shown). Our findings also support previous work indicating that DENV does not stimulate IL-10 production by DC [25], although others [28] have reported contrasting findings. Although we measured IL-10 levels at 24 h postinfection as compared with 48 h used by Palmer et al. [28], we do not think this explains the difference in results. We were able to detect IL-10 in response to LPS stimulation at 24 h, and DENV antigen staining was high (33–90%) at 24 h, demonstrating productive infection. We also failed to detect IL-10 in supernatants from T-DC cocultures, suggesting that IL-10 was not secreted at later time-points. Further studies regarding the role of DC in production of this immunosuppressive molecule in response to DENV are needed.
IFN-α/β was reported to inhibit DENV infection of human cells in vitro but required pretreatment to be effective [33]. Monocyte-derived DC have been described as weak IFN-α producers when compared with plasmacytoid DC [34, 35], and IFN-α levels detected in supernatants from our experiments were low compared with a number of other virus-induced cytokines and chemokines. Nevertheless, autocrine and/or paracrine signaling by endogenous IFN-α/β was active in controlling ongoing DENV infection in DC cultures.
Although we found a wide range of secreted products in response to DENV infection, intracellular staining revealed distinct cytokine production phenotypes for infected and bystander DC. IP-10 production was potently induced in bystander cells, whereas DC with detectable DENV E protein demonstrated uniformly low levels of staining for IP-10. On the other hand, TNF-α production was induced more prominently in infected DC, similar to the findings of Sanchez et al. [27] using an attenuated DENV2 strain. Blocking experiments revealed that IP-10 production in response to DENV was largely IFN-α/β-dependent. Expression of the DENV nonstructural proteins has been shown to inhibit IFN-α signaling but not IFN-γ signaling [36, 37, 44, 45]. These findings suggest that decreased responsiveness to IFN-α/β may be the cause of weak IP-10 induction in DENV-infected DC. In control experiments, anti-IFNAR2 antibody blocked IP-10 induction in response to 50-fold higher levels of IFN-α than we measured in supernatants (data not shown), suggesting the production of additional regulators of IP-10, such as IFN-γ or IFN-λ [46]. Unlike IP-10, TNF-α production was not affected by IFN-α/β blockade and appeared to represent a direct response to ongoing viral replication in infected DC. The mechanism for this observation will require further dissection but could involve intracellular signaling mechanisms such as the retinoic-acid-inducible protein I-mitochondrial antiviral signaling pathway [47]. We previously reported direct induction of IL-8 secretion by the DENV NS5 protein [26].
We found that DENV exposure enhanced the expression of immunoregulatory surface proteins in infected and bystander DC. Our data include the novel finding that DENV induces expression of PD-L1 and PD-L2, the known ligands for PD-1, on infected and bystander cells. PD-1 is expressed on activated T cells, and binding to either of its ligands results in cosuppressive signals, which inhibit T cell proliferation and cytokine production [48, 49]. PD-L1 was shown to effectively inhibit Th1 and Th2 responses in antigen-specific T cells, and PD-L2 preferentially inhibited Th1 responses alone [50]. We also found enhanced expression of CD40, CD83, CD80, CD86, and MHC class II on infected and bystander cells, and only bystander cells up-regulated MHC class I. PD-L1, CD80, CD86, and MHC class I expression was lower in infected cells than bystander cells, whereas PD-L2 and HLA-DR expression showed the reverse pattern. PD-L1 has been reported to be induced by IFN-α in cultured DC and renal tubular cells [51, 52], and we observed up-regulation of PD-L1 and PD-L2 in response to IFN-α treatment of DC (data not shown). However, as infected DC had suppressed PD-L1 and enhanced PD-L2 expression relative to bystander cells, the differential expression of PD-L2 was not a result of viral inhibition of IFN-α/β. There is general agreement in the literature that bystander DC have enhanced expression of costimulatory and MHC molecules following DENV exposure [25, 27, 28]. However, infected cells have been reported to have greater [27] or lesser [25] expression relative to bystander cells, and in one study, infected DC showed no induction of surface molecules when compared with mock infection [28]. The differences in findings between studies may reflect differences in virus strains or genetic polymorphisms between DC donors.
In accordance with the activation of DC surface molecule expression, DENV-infected cultures also stimulated allogeneic, resting CD4 T cell proliferation. These results indicate that DENV is not immunosuppressive, as has been reported for measles virus [1, 53, 54] and respiratory syncytial virus [55, 56], among others. However, at the MOI used in most experiments, 2 pfu/cell, less than half of the cells in the culture was productively infected. At higher MOI, we observed reduced proliferation, suggesting that infected DC were less able to prime T cells than bystander DC and in some donors, inhibited bystander DC function. We did observe decreased T cell proliferation in cocultures with DENV-infected DC for a minority of donors, similar to a previous report [28]. Proliferation responses to mock-infected DC were generally high in these experiments, but the results suggest that donor-dependent effects may have a strong influence on the experimental results. Testing of DC and T cells in a 2 × 2 factorial design suggested that the DC source had a greater influence on the pattern of proliferation responses. However, the characteristics of the T cells are likely also important. We used negatively selected, “naïve” CD4+ T cells. In contrast, Palmer et al. [28] used positively selected CD3+ T cells, which included naïve and memory CD4+ and CD8+ T cells. These cells showed higher proliferation responses to mock-infected DC and may be more readily activated to express PD-1, thus becoming susceptible to inhibition by PD-L1/PD-L2 expression on the DC [50, 57].
These data support a model in which DENV infection of DC modulates DC function by direct (IFN-α/β-independent) and indirect (IFN-α/β-dependent) mechanisms. Differential DC functions determined by infection at the level of individual cells may have implications for disease pathogenesis. Infected DC would potentially be less effective than bystander DC at recruiting Th1 cells, which preferentially express CXCR3 (the receptor for IP-10) [58], resulting in inefficient control of virus replication. In addition, infected DC may subject antigen-specific, responding T cells to high levels of TNF-α, causing T cell death [59] and impairment of the adaptive response.
Acknowledgments
This work was supported by grants P01 AI34533 and U19 A157319 from the National Institutes of Health (NIH). The opinions expressed herein are those of the authors and should not be construed as the official policy of the NIH. We thank Jurand Janus and Marcia Woda for technical assistance and Daniel Libraty and Anuja Mathew for comments and suggestions about the experiments and manuscript.
References
- Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain P O, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol. 2000;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- Canque B, Rosenzwajg M, Camus S, Yagello M, Bonnet M L, Guigon M, Gluckman J C. The effect of in vitro human immunodeficiency virus infection on dendritic-cell differentiation and function. Blood. 1996;88:4215–4228. [PubMed] [Google Scholar]
- Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M J, Kraus A A, Ulrich R, Kruger D H, Schonrich G. Hantavirus infection of dendritic cells. J Virol. 2002;76:10724–10733. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Longman R S, Albert M L, Rice C M. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med. 2005;202:1179–1184. doi: 10.1084/jem.20051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G H, Johnston R E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J H, Janas A M, Olson W J, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M G, Kouri G P, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–184. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- Halstead S B, Nimmannitya S, Cohen S N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- Rothman A L, Ennis F A. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- Bashyam H S, Green S, Rothman A L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol. 2006;176:2817–2824. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- Mangada M M, Rothman A L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Dejnirattisai W, Xu X N, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus P T, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Chen Y C, Wang S Y. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol. 2002;76:9877–9887. doi: 10.1128/JVI.76.19.9877-9887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianneau P, Steffan A M, Royer C, Drouet M T, Jaeck D, Kirn A, Deubel V. Infection of primary cultures of human Kupffer cells by dengue virus: no viral progeny synthesis, but cytokine production is evident. J Virol. 1999;73:5201–5206. doi: 10.1128/jvi.73.6.5201-5206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D M, Halstead S B. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol. 1990;71:2909–2914. doi: 10.1099/0022-1317-71-12-2909. [DOI] [PubMed] [Google Scholar]
- Wu S J, Grouard-Vogel G, Sun W, Mascola J R, Brachtel E, Putvatana R, Louder M K, Filgueira L, Marovich M A, Wong H K, Blauvelt A, Murphy G S, Robb M L, Innes B L, Birx D L, Hayes C G, Frankel S S. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Ho L J, Wang J J, Shaio M F, Kao C L, Chang D M, Han S W, Lai J H. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol. 2001;166:1499–1506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- Libraty D H, Pichyangkul S, Ajariyakhajorn C, Endy T P, Ennis F A. Human dendritic cells are activated by dengue virus infection: enhancement by γ interferon and implications for disease pathogenesis. J Virol. 2001;75:3501–3508. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin C L, Fitzgerald K A, Rothman A L. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J Virol. 2005;79:11053–11061. doi: 10.1128/JVI.79.17.11053-11061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Hessler C, DeMonfort A, Lang J, Guy B. Comparison by flow cytometry of immune changes induced in human monocyte-derived dendritic cells upon infection with dengue 2 live-attenuated vaccine or 16681 parental strain. FEMS Immunol Med Microbiol. 2006;46:113–123. doi: 10.1111/j.1574-695X.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- Palmer D R, Sun P, Celluzzi C, Bisbing J, Pang S, Sun W, Marovich M A, Burgess T. Differential effects of dengue virus on infected and bystander dendritic cells. J Virol. 2005;79:2432–2439. doi: 10.1128/JVI.79.4.2432-2439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras B, Connolly J, Freitas H, Palucka A K, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilliox M J, Parmigiani G, Griffin D E. Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens. Proc Natl Acad Sci USA. 2006;103:3363–3368. doi: 10.1073/pnas.0511345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M S, Harris E. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology. 2001;289:297–311. doi: 10.1006/viro.2001.1114. [DOI] [PubMed] [Google Scholar]
- Diamond M S, Roberts T G, Edgil D, Lu B, Ernst J, Harris E. Modulation of dengue virus infection in human cells by α, β, and γ interferons. J Virol. 2000;74:4957–4966. doi: 10.1128/jvi.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- Izaguirre A, Barnes B J, Amrute S, Yeow W S, Megjugorac N, Dai J, Feng D, Chung E, Pitha P M, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-α expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan J L, Sanchez-Burgos G G, Laurent-Rolle M, Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci USA. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Jordan J L, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin W I, Garcia-Sastre A. Inhibition of α/β interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan E, Spagnoli G C, Ferrantini M, Heberer M. IFN-α2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71:669–676. [PubMed] [Google Scholar]
- Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Pang K C, Thomas E, Hertzog P, Hart D N, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Hsieh M F, Lai S L, Chen J P, Sung J M, Lin Y L, Wu-Hsieh B A, Gerard C, Luster A, Liao F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans J C, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams J S, Moore K W, Rennick D, de Waal-Malefyt R, Hannum C, Bazan J F, Kastelein R A. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie M H, de Sauvage F J, Gurney A L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Ho L J, Hung L F, Weng C Y, Wu W L, Chou P, Lin Y L, Chang D M, Tai T Y, Lai J H. Dengue virus type 2 antagonizes IFN-α but not IFN-γ antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol. 2005;174:8163–8172. doi: 10.4049/jimmunol.174.12.8163. [DOI] [PubMed] [Google Scholar]
- Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster G R, Jacobs M. Dengue virus inhibits α interferon signaling by reducing STAT2 expression. J Virol. 2005;79:5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon λ-1 (IFN-λ1/IL-29) induces ELR(–) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-γ-independent manner. Genes Immun. 2007;8:177–180. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Lin R, Nakhaei P, Paz S. MasterCARD: a priceless link to innate immunity. Trends Mol Med. 2006;12:53–56. doi: 10.1016/j.molmed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Brown J A, Dorfman D M, Ma F R, Sullivan E L, Munoz O, Wood C R, Greenfield E A, Freeman G J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- Cai G, Karni A, Oliveira E M, Weiner H L, Hafler D A, Freeman G J. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol. 2004;230:89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood C R, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long A J, Brown J A, Nunes R, Greenfield E A, Bourque K, Boussiotis V A, Carter L L, Carreno B M, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe A H, Freeman G J. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Schreiner B, Mitsdoerffer M, Kieseier B C, Chen L, Hartung H P, Weller M, Wiendl H. Interferon-β enhances monocyte and dendritic cell expression of B7–H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Waeckerle-Men Y, Starke A, Wuthrich R P. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+ cytotoxic T cells. Nephrol Dial Transplant. 2007;22:1527–1536. doi: 10.1093/ndt/gfl818. [DOI] [PubMed] [Google Scholar]
- Dubois B, Lamy P J, Chemin K, Lachaux A, Kaiserlian D. Measles virus exploits dendritic cells to suppress CD4+ T-cell proliferation via expression of surface viral glycoproteins independently of T-cell trans-infection. Cell Immunol. 2001;214:173–183. doi: 10.1006/cimm.2001.1898. [DOI] [PubMed] [Google Scholar]
- Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-γ in naive T cells. Immunology. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaff P M, de Jong E C, van Capel T M, van Dijk M E, Roholl P J, Boes J, Luytjes W, Kimpen J L, van Bleek G M. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175:5904–5911. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger L R. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res. 1997;232:25–28. doi: 10.1006/excr.1997.3493. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon P P, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]