Abstract
IL-2 is essential for CD4+CD25+forkhead box P3+ (FoxP3+) naturally occurring regulatory T cell (Treg) homeostasis and activation. Binding of IL-2 to its receptor leads to phosphorylation of STAT5, and binding of phosphorylated STAT5 to the foxp3 promoter increases foxp3 transcription, resulting in elevated levels of FoxP3 protein in Tregs. Transcriptional regulation by the elevated levels of FoxP3 is thought to be essential for the strong suppressor function seen in activated Tregs. IL-2 belongs to a family cytokines, which all depend on the common γ-receptor chain (γc). Given the well-documented effects of IL-2 on Treg function, the effect of other IL-2 family cytokines (IL-7, -15, and -21) on Tregs was examined. We observed that IL-7 and IL-15 induce STAT5 phosphorylation and up-regulation of FoxP3 in Tregs. STAT5 activation correlated with enhanced viability. However, only in the presence of IL-2 did Tregs acquire potent suppressor function. This finding is surprising, as IL-15 as well as IL-2 use the same IL-2R βc and γc for signaling. In contrast, IL-21 activated STAT3 but did not activate STAT5 and had no effect on Treg viability, activation, or function. We therefore conclude that phosphorylation of STAT5, mediated through the IL-2Rγ, promotes Treg survival in a resting and activated state. However, activation of STAT5 alone in conjunction with TCR signaling is not sufficient for the induction of potent suppressor function in Tregs, as IL-7 and IL-15 are not capable of inducing potent Treg suppressor function.
Keywords: FoxP3, IL-7, IL-15, CD25, IL-21, γc cytokines
INTRODUCTION
Thymus-derived CD4+CD25+forkhead box P3+ (FoxP3+) naturally occurring T regulatory cells (Tregs) are CD4+ T cells that have the capacity to suppress T cell responses [1, 2]. Tregs are refractory to TCR-induced proliferation [2, 3]. They depend on IL-2 for survival [4, 5], and signaling via the high-affinity IL-2R complex, in combination with TCR engagement, is essential for Treg proliferation as well as the acquisition of their potent suppressive function [6]. Treg-mediated inhibition of T cell proliferation and activation is cell contact-dependent [7], but soluble factors such as TGF-β [8] and IL-10 [9, 10] contribute to their suppressive activity as well. Tregs are crucial for maintaining peripheral tolerance by suppressing self-antigen-specific T cell responses in peripheral tissues (reviewed in refs. [11, 12]).
The transcription factor FoxP3 was described as a marker distinguishing Tregs from CD4 Th cells [13,14,15], and transduction of naïve Th cells with FoxP3 is sufficient to confer regulatory function [13]. Stimulation of Tregs with IL-2 leads to phosphorylation and activation of STAT5 and consequently, binding to the FoxP3 promotor, resulting in enhanced FoxP3 expression [16]. The transcriptional regulation induced by increased levels of FoxP3 is proposed to induce the strong, suppressive activity in naïve Tregs. However, the mechanisms by which Tregs suppress T cells remain incompletely understood.
Interestingly, some cytokines of the IL-2 family, although dependent on the same common receptor γ-chain (γc) for signaling, have different effects on Tregs. IL-2 and to a lesser-extent, IL-4 can induce proliferation and activation of Tregs in vitro [17], and IL-7 [18] and IL-15 [19, 20] may increase Treg numbers in vivo.
To understand better the effect of IL-2 family cytokines on Tregs and the effect of STAT5 activation, we analyzed the effect of IL-2 family cytokines on STAT5 phosphorylation, FoxP3 expression, Treg-mediated suppression, and Treg survival. We found that cytokine receptor expression in CD4 Th and Tregs is similar, with the exception of the constitutively high levels of CD25 (IL-2Rα) expression in Tregs: IL-7R is down-regulated upon TCR activation, and CD25 and IL-15R are up-regulated. All three cytokines, IL-2, -7, -15, can induce phosphorylation of STAT5 if the receptor is expressed and can induce FoxP3 up-regulation. However, only IL-2 is capable of inducing strong suppressor function in Tregs, indicating that FoxP3 up-regulation alone is not sufficient to promote the suppressive effects of Tregs. Interestingly, IL-21 activates STAT3 in Tregs but does not induce suppressor function either.
MATERIALS AND METHODS
Mice
Four- to 5-week-old C57BL/6×C3H/HeN F1 (B6C3F1) mice were purchased from the National Cancer Institute (NCI) Animal Production Area Facility (Frederick, MD, USA). TCR II mice bear a TCR transgene specific for SV40 TAg362–384 restricted by I-Ak [21] and were a kind gift of Douglas Hanahan (University of California San Francisco, CA, USA). These mice were bred onto a RAG−/− background. All mice were maintained in a specific pathogen-free facility and used for experiments at the ages of 7–9 weeks. Mice were treated in accordance with the National Institutes of Health Guidelines under protocols approved by the NCI-Frederick Institutional Animal Care and Use Committees.
Antibodies and reagents
Anti-CD3 (500A2), anti-CD3-FITC (145-2C11), anti-CD4-PerCP (RM4-5), anti-CD25-biotin (7D4), anti-CD25-PE (PC61), anti-phospho (p)-Stat3 (Y705)-Alexa488, and anti-p-STAT5 (Y694)-PE antibodies were purchased from BD PharMingen (San Diego, CA, USA); anti-FoxP3-allophycocyanin (FJK-16 s) and anti-IL-7Rα-PE-Cy5 antibodies were purchased from eBioscience (San Diego, CA, USA); anti IL-15R-biotin antibody was purchased from R&D Systems (Minneapolis, MN, USA); anti-CD16/32 hybridoma (2.4G2; ATCC HB-217) was used as a cell supernatant; and anti-IL-21R-biotin antibody was a kind gift from Dr. Thomas Malek (University of Miami School of Medicine, FL, USA).
TAg362–384 (TNRFNDLLDRMDIMFGSTGSADI) peptide was purchased from New England Peptide (Gardner, MA, USA), and the purity was >90% based on HPLC assay. Human (h)IL-2 was donated by Cetus (Emeryville, CA, USA); murine recombinant (mr)IL-7, mrIL-15, and mrIL-21 were purchased from R&D Systems. For all assays, T cell media were used: RPMI, supplemented with 10% heat-inactivated FCS, 2 μM L-glutamine, 1 mM sodium pyruvate, 1 U/ml penicillin, and 1 μg/ml streptomycin. Cells were cultured at 37°C in a humidified incubator with 5% CO2.
Treg purification
Single-cell suspensions of spleen and lymph nodes were prepared by grinding tissues between the frosted end of microscope slides and passed through a 50-μm nylon filter (Falcon Labware). CD4+ T cells were enriched from lymphocyte suspension by negative selection with magnetic beads (Dynal Biotech, Carlsbad, CA, USA). The CD4-enriched cells were blocked with anti-CD16/32 (2.4G2) and labeled with 0.5 μg/ml PE-coupled anti-CD25 antibody (BD PharMingen) followed by anti-PE magnetic beads (Miltenyi Biotec, Auburn, CA, USA) and selected on a MS column (Miltenyi Biotec). The purity of the CD25-positive fraction (Treg) was determined by flow cytometry and was always >93% and >85% FoxP3+. The CD25-negative fraction was used as Th responder cells.
APC
Thy1.2-positive cells were depleted from spleen cells by magnetic labeling with anti-CD90.2 (Thy1.2) particles (BD PharMingen) following the manufacturer’s instructions, irradiated with 3000 Rad using a 137-Cs source and washed before being used as APC.
Flow cytometry
Cells were blocked with anti-CD16/32 antibodies and incubated with the indicated antibodies on ice in the dark for 20 min, washed once, and analyzed on an LSR 2 cytometer (Becton Dickinson, San Jose, CA, USA).
Treg activation and antigen-specific proliferation assay
To preactivate Tregs, 2–4 × 105 purified CD4+25+ cells were incubated with the indicated cytokines for 3 days in anti-CD3 antibody-coated (5 μg/ml) 24-well tissue-culture plates. Cells were then washed and counted. For the inhibition assay, 20,000 lymph node cells from TCR-II transgenic mice were used with the indicated ratio of preactivated Tregs, 100,000 APC, and 100 ng/ml TAg362–384 peptide in a 96-well round-bottom plate. Proliferation was measured 72 h later by pulsing each well with 1 μCi 3H-thymidine overnight.
Treg inhibition assay
Inhibition of Th cell proliferation was measured by incubating 20,000 purified CD4+25–Th cells together with the indicated ratio of freshly isolated CD4+25+ Tregs, 0.5 μg/ml anti-CD3, 100,000 APC, and the indicated cytokines. Proliferation was measured 72 later by pulsing each well with 1 μCi 3H-thymidine overnight.
STAT phosphorylation assay
Tregs were purified as described above using biotin-labeled anti-CD25. For Treg activation, freshly isolated Tregs were activated for 2 days with plate-coated anti-CD3 antibody (5 μg/ml, clone 500A2) and 100 U/ml hIL-2. Cells were then harvested, washed three times in RPMI + 0.5% BSA, and incubated for 6 h at 37°C and 5% CO2. For the STAT phosphorylation assay, freshly isolated or activated Tregs were washed once, and 5 × 105 cells were resuspended in 100 μl RPMI + 0.5% BSA at 37°C for 20 min and then stimulated with 50 ng/ml of the indicated cytokine or 100 U/ml for IL-2 and incubated for 20 min at 37°C. The cells were fixed immediately on ice and then stained with APC-conjugated anti-FoxP3 antibody according to the manufacturer’s instructions. After FoxP3 staining, the cells were fixed with 2% paraformaldehyde on ice for 20 min, then treated with 90% methanol for 30 min on ice, and then stained for CD4, p-STAT3, and p-STAT5 and analyzed by flow cytometry using FoxP3 expression to gate on Tregs.
FoxP3 expression
Lymph node cells and splenocytes from FoxP3-gfp knock-in mice [22] were prepared in a single cell suspension as described above and sorted by FACS based on gfp expression. The resulting populations were placed into culture dishes with plate-bound anti-CD3 and the indicated cytokines as described above. Forty-eight hours later, cells were harvested and analyzed for FoxP3 expression by flow cytometric analysis. Similar results were obtained using Tregs isolated based on CD25 expression.
RESULTS
Cytokine receptor expression by Th and Tregs
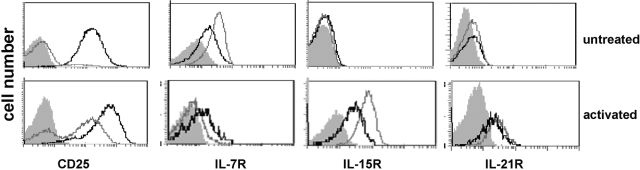
TCR engagement in the presence of moderate concentrations of IL-2 or IL-4 has been reported to induce full inhibitory capacity in Tregs [17, 23]. To determine which additional IL-2 family cytokines may influence naturally occurring Treg function and survival, we first tested cytokine receptor expression on Tregs. We isolated Tregs from naïve mice based on CD4 and CD25 expression and analyzed receptor expression of freshly isolated and activated Tregs by flow cytometry (Fig. 1). Freshly isolated Tregs (consistently greater than 95% CD4+CD25+ and at least 85% FoxP3+) expressed low but detectable levels of IL-7R and IL-21R but no detectable IL-15R. The level of expression was comparable with resting CD4+ Th cells (Fig. 1). Upon activation for 2 days with anti-CD3 and IL-2, expression of CD25 was elevated in Treg and Th cells. Similarly, IL-15R and IL-21R expression was up-regulated, and IL-7R was down-regulated on both cell types after activation, although the decrease in IL-7R expression was more pronounced in Th cells. The expression of these cytokine receptors suggested that Tregs have the potential to respond to all four IL-2 family cytokines, supporting further studies about their role in Treg function.
Fig. 1.
Treg expression of receptors for IL-2 family cytokines. Tregs and Th cells were prepared as described and stained for receptor expression. Alternatively, isolated cells were preactivated for 48 h with immobilized anti-CD3 antibody and IL-2 prior to staining for receptor expression. Gray filled, Isotype control; gray line, Th cells; black line, Tregs. Cytofluorimetric analysis for each receptor was performed at least three times.
Costimulation of Treg proliferation by IL-2 family cytokines
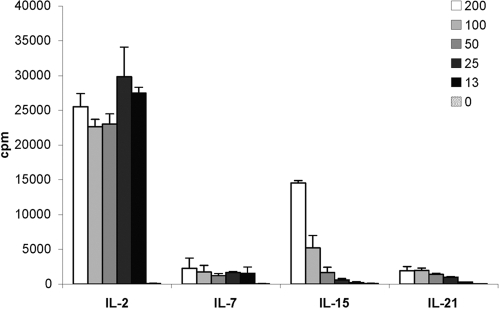
It has been well-described that in combination with TCR engagement, IL-2 provides a sufficient costimulatory signal to induce a proliferative response of Tregs in vitro [23, 24] and in vivo [25]. To determine whether other IL-2 family cytokines can promote Treg expansion, we stimulated Tregs with plate-bound anti-CD3 antibody in the presence of IL-2 family cytokines (Fig. 2). As reported previously, IL-2 induced strong proliferation of Tregs, even at levels as low as 5 U/ml; a nearly 500-fold increase in 3H-thymidine incorporation was observed when Tregs were stimulated in the presence of IL-2 when compared with anti-CD3 antibody alone. Despite the expression of IL-7R on naïve Tregs, proliferation in the presence of IL-7 was minimal. A similar observation was noted for IL-21 as well. Surprisingly, IL-15 induced dose-dependent proliferation. At the maximal dose tested (200 ng/ml), Treg proliferation was about half of the maximal response induced with IL-2 but was nearly 300-fold greater than anti-CD3 stimulation of Tregs alone. The proliferating cells were identified as Tregs by flow cytometric analysis of CFSE dilution within the CD4+CD25+FoxP3+ population of cells (data not shown).
Fig. 2.
Influence of IL-2 family cytokines on Treg proliferation. Purified Tregs were incubated with IL-2 family cytokines and immobilized anti-CD3 for 72 h, and proliferation was determined by pulsing the cultures with 3H-thymidine for the final 18 h. The mean ± sd of triplicates of one of three similar experiments is shown.
IL-2 family cytokines affect survival of Tregs
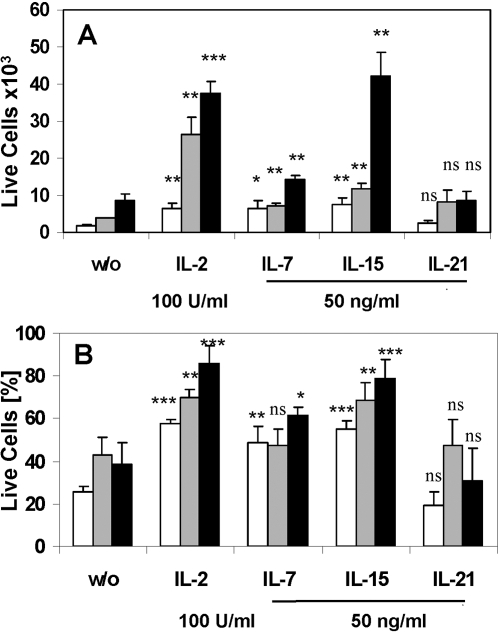
In addition to its effect on Treg proliferation, IL-2 has been shown by others to promote survival of Tregs in vitro and in vivo (reviewed in ref. [26]). To determine the effect of other IL-2 family cytokines on Treg survival, we tested their effect on naïve and preactivated Tregs using Trypan blue exclusion staining after a 3-day culture with the individual cytokines in combination with anti-CD3. We chose the optimal concentration of the cytokines based on our results for cytokine-induced proliferation (Fig. 2). Compared with Tregs cultured without cytokines, IL-2, IL-7, and IL-15 were able to increase the number (Fig. 3A, white bars) and sustain the viability (Fig. 3B, white bars) of naïve Tregs. However, consistent with proliferation data presented above, only IL-2 was able to expand naïve Tregs significantly when cultured in combination with TCR engagement (Fig. 3A, shaded bars). IL-2 and IL-15 were able to promote survival and sustain the expansion of preactivated Tregs as well (Fig. 3, A and B, black bars), but IL-2 had a much stronger effect than IL-15 on the naïve cells. In sharp contrast, IL-21 had no influence on Treg viability or expansion (Fig. 3, A and B), despite expression of its receptor (Fig. 1).
Fig. 3.
Influence of IL-2 family cytokines on Treg viability. Purified, naïve Tregs (2×104) were cultured with plate-bound anti-CD3 antibody and the indicated cytokines. After 72 h, the number of total live cells (A) and the viability of the cultures, as determined by Trypan blue staining (B), were enumerated. White bars, Freshly isolated Tregs; shaded bars, freshly isolated Tregs with immobilized anti-CD3 antibody; black bars, 3-day-preactivated Tregs. Significance was tested using a Student’s t-test by comparing the cytokine-treated culture with the same population of untreated cells [without cytokine (w/o)]: ***, P < 0.001; **, P < 0.01; *, P < 0.05. The mean ± sd of triplicates of one of two similar experiments is shown. ns, Not significant.
Induction of suppressor activity by IL-2 family cytokines
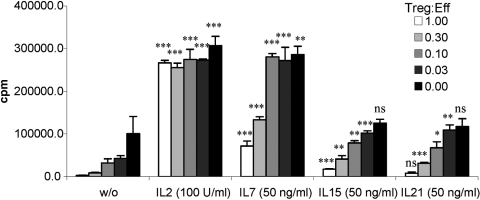
Murine Tregs isolated based on CD25 expression do not exhibit potent, spontaneous suppressor activity in vitro but rather require activation through TCR ligation and a second costimulatory signal. Although our findings suggest that IL-2 is the most potent at inducing Treg proliferation in combination with TCR engagement, induction of potent inhibitory function might be regulated independently. Therefore, we tested whether other cytokines can substitute for the essential costimulatory role of IL-2 by culturing Tregs with plate-bound anti-CD3 antibody in the presence of cytokines for 3 days and then measuring their suppressive activity on antigen-specific proliferation of TCR transgenic CD4+ T cells (Fig. 4). Not surprisingly, IL-2 had the strongest effect on inducing suppressor activity of naïve Tregs. In contrast, IL-7 showed a weak but measurable capacity of inducing Treg-mediated inhibition of proliferation (up to 37% at a 1:1 ratio). Interestingly, activation with IL-15, which induced Treg proliferation and survival, was profoundly weaker than IL-2, but comparable with IL-7. IL-21 had only a minimal effect on inducing Treg-suppressive activity.
Fig. 4.
Influence of IL-2 family cytokines on Treg suppressive function. Purified Tregs were activated with immobilized anti-CD3 and the indicated cytokines for 3 days, washed, and used in an inhibition assay together with TCR-II T cells at the indicated ratio. Cultures were supplemented with syngeneic splenocytes and antigen as described in Materials and Methods. Proliferation was determined after 72 h by pulsing the cultures with 3H-thymidine for the final 18 h. Statistical significance was tested using a Student’s t-test by comparing the cytokine-stimulated culture with the same population of untreated cells: ***, P < 0.001; **, P < 0.01; *, P < 0.05. The mean ± sd of triplicates of one of three similar experiments is shown. Treg:Eff, Treg:effector ratio.
Influence of IL-2 family cytokines on Treg-mediated suppression
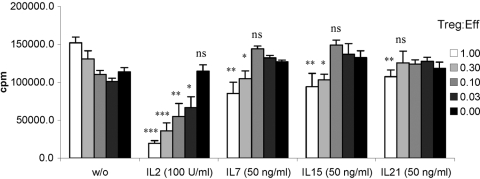
Previous reports have demonstrated that provision of exogenous IL-2 or IL-12 can reverse Treg-mediated suppression of proliferation when added to the in vitro suppression assay, and transcription of IL-2 mRNA is still inhibited [4, 24, 27, 28]. To test the ability of other IL-2 family cytokines to overcome the Treg-mediated suppression of Th cell proliferation (Fig. 5), we measured the proliferative response of Th cells cocultured with Tregs, anti-CD3 antibody, and cytokines. As described previously, IL-2 profoundly reversed Treg-mediated suppression of Th cell proliferation, IL-7 partially reversed Treg-mediated suppression, and IL-15 and IL-21 had only minimal effects on Treg-mediated suppression.
Fig. 5.
Inhibitory activity of Treg in the presence of IL-2 family cytokines. Purified Tregs were incubated with Th cells at the indicated ratios, together with anti-CD3, APC, and the indicated cytokines. Proliferation was determined by pulsing the cultures with 3H-thymidine for the final 18 h. Statistical significance was tested using a Student’s t-test by comparing the cytokine-stimulated culture with the same population of untreated cells: ***, P < 0.001; **, P < 0.01; *, P < 0.05. The mean ± sd of triplicates of one of three similar experiments is shown.
STAT3 and STAT5 activation in Tregs
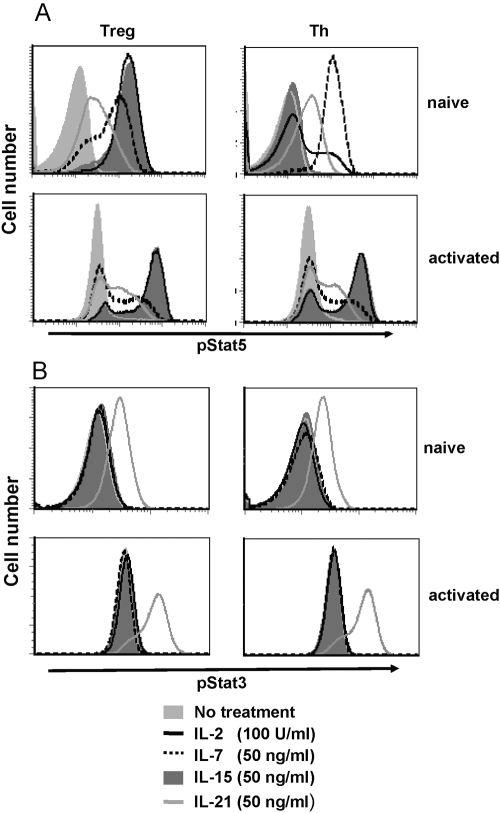
Binding of IL-2 family cytokines to their cognate receptor leads to phosphorylation and the activation of STATs, predominantly STAT1, STAT3, and STAT5, and subsequent translocation to the nucleus and regulation of target gene transcription [29]. To determine whether the signaling pathways of IL-2 family cytokines leading to STAT activation are functional in Tregs, we used intracellular flow cytometric analysis to assess phosphorylation of STAT3 and STAT5 in naïve and activated Tregs.
IL-2 induced STAT5 phosphorylation in naïve and activated Tregs (Fig. 6A). In contrast, IL-2 induced STAT5 phosphorylation in previously activated CD4+ Th cells but not in resting Th cells in which the high-affinity IL-2R is not expressed. The STAT5 phosphorylation pattern with IL-15 treatment was similar to the pattern observed with IL-2: strong phosphorylation was noted in naïve and activated Tregs as well as in activated Th cells but not in naïve Th cells. IL-7 also induced STAT5 phosphorylation in naïve Tregs, but this effect was strongly diminished in activated Tregs, consistent with down-regulation of the IL-7Rα upon activation. A similar pattern of STAT5 activation by IL-7 was seen in Th cells (Fig. 6A). IL-21 treatment led to low but detectable levels of phosphorylated STAT5 in all T cell populations. Taken together with our findings presented above, these results suggest that STAT5 activation in naïve Tregs may be necessary but is not sufficient to induce suppressor activity.
Fig. 6.
STAT3/STAT5 phosphorylation after stimulation with IL-2 family cytokines. Freshly purified and preactivated Treg and Th cells were stimulated with the indicated cytokines as described. STAT activation was determined by intracellular staining with p-STAT3- and p-STAT5-specific antibodies and analyzed by flow cytometry. Th cells were gated on CD4+FoxP3− and Treg on CD4+FoxP3−. The mean ± sd of triplicates of one of three similar experiments is shown.
In contrast to STAT5 responses, no STAT3 phosphorylation was detected in any of the T cell populations tested following stimulation with IL-2, IL-7, or IL-15 (Fig. 6B). However, IL-21 did induce STAT3 phosphorylation in all four cell populations tested (Fig. 6B). These findings suggest that STAT3 activation is not sufficient to activate Tregs or induce proliferation, even in the presence of partial STAT5 activation.
Cytokine-mediated up-regulation of FoxP3 in Tregs
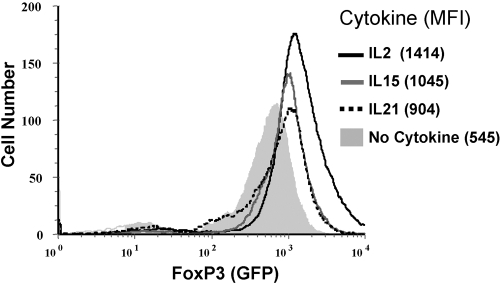
IL-2 and IL-15 are similarly potent in activating STAT5 (Fig. 6), and STAT5-mediated up-regulation of FoxP3 has been proposed as the mechanism inducing the potent suppressor function in Tregs. We therefore tested the expression levels of FoxP3 in Tregs after stimulation in the presence of IL-2, IL-15, or IL-21 for 48 h. As reported previously, stimulation with anti-CD3 antibody and IL-2 resulted in elevated levels of FoxP3, as measured by gfp expression by Tregs isolated from FoxP3-gfp knock-in mice [22] (Fig. 7). Similarly, stimulation with anti-CD3 antibody IL-15 or IL-21 also led to an increase in FoxP3 expression, albeit to a slightly lesser extent (Fig. 7). Interestingly, stimulation of Tregs with IL-2 or IL-15 without anti-CD3 antibody also resulted in an increased level of FoxP3 expression (data not shown), correlating with STAT5 phosphorylation (Fig. 6A).
Fig. 7.
FoxP3 expression after stimulation with IL-2 family cytokines. FACS-sorted Tregs from Foxp3-gfp knock-in mice were stimulated with anti-CD3 and the indicated cytokines. After 48 h cells, Tregs were analyzed for FoxP3 expression by determining GFP. Mean fluorescence intensity (MFI) is indicated in parentheses. Similar results were obtained using antibodies directed against FoxP3. Results presented are representative of five similar experiments.
DISCUSSION
IL-2 family cytokines are considered powerful tools in tumor immunotherapy as a result of their strong enhancement of T cell proliferation, viability, and effector function. Specifically, IL-15 and IL-21 show strong promise for enhancing CTL expansion and function [30,31,32,33]. As IL-2 signaling was described to be essential, not only for Treg development and homeostasis but also for the induction of potent suppressor function, it is important to elucidate the effects of other IL-2 family members on Treg function to understand better the influence of these cytokines on Treg homeostasis and function during immunotherapy.
Consistent with two recent studies [34, 35], we demonstrate that IL-7R expression by naïve Treg is low in comparison with naïve Th cells. Interestingly, similar to Th cells [36], Tregs down-regulate the IL-7R to essentially undetectable levels (Fig. 1). IL-7R expression correlates IL-7-induced STAT5 phosphorylation in naïve Tregs but diminished STAT5 activation in activated Tregs (Fig. 6). The observed reduction in STAT5 activation with IL-7 treatment of activated Tregs (presumably as a result of down-regulation of the receptor) is consistent with the more pronounced effect of IL-7 on the viability of naïve versus activated Tregs (Fig. 3).
Our findings are also consistent with others demonstrating a role for IL-7 in Treg homeostasis in vivo [18]. However, unlike IL-2, we demonstrate that IL-7 is not capable of inducing potent suppressor function or proliferation in Tregs, indicating that IL-7 may function as a homeostatic factor for naïve Tregs and has only limited effects on activated Tregs as a result of the diminished levels of IL-7R. Consistent with these findings, cancer patients treated with IL-7 for lymphopenia showed an increase in conventional T cells but not Tregs [37], and IL-2 increased the number of Tregs [25]. Thus, the role of IL-7 may be more complex than initially thought and requires further consideration.
As neither freshly isolated Tregs nor Th cells expressed detectable levels of IL-15Rα chain (Fig. 1), it was surprising that IL-15 activated STAT5 in Tregs but not in Th cells and promoted Treg survival (Fig. 3). A possible explanation might be that IL-15 can signal through its low-affinity receptor consisting of the β and γ subunits, which it shares with the IL-2R without the need for the unique IL-15Rα chain [38,39,40,41]. Although Tregs express high levels of IL-2Rβ, low levels are expressed by naïve Th cells [42]. Thus, expression of β/γc on Th cells may be too low to induce STAT5 activation by IL-15 but may be sufficient to activate STAT5 in Tregs. The low affinity of the β/γ receptor for IL-15 could also explain the high concentrations needed to induce proliferation in naïve Treg (Fig. 2). However, our observation of up-regulation of IL-15Rα expression upon TCR engagement in Tregs could also contribute to proliferation by naïve Tregs in response to IL-15.
Stimulation of Tregs with IL-15 activated STAT5 and enhanced Treg viability at levels comparable with IL-2. However, when provided in combination with TCR ligation, unlike IL-2, IL-15 did not induce suppressor activity in naïve Tregs, despite increased expression levels of FoxP3. Therefore, STAT5 activation seems to be sufficient to induce Treg survival and FoxP3 up-regulation but is not sufficient to induce potent suppressor function in naïve Tregs. The equivalent activation of STAT5 in naïve Tregs by IL-2 and IL-15 suggests that binding of IL-2 to its heterotrimeric receptor complex modulates or makes use of an additional signaling pathway, resulting in the induction of potent suppressor function. The hypothesis is also supported by studies that show that maintenance of suppressor function is regulated by a PI-3K-dependent mechanism independently of FoxP3 expression [20, 43]. Although some differences exist between human and murine Tregs, taken together, these reports and our current findings suggest that STAT5 activation induces Treg survival and FoxP3 up-regulation but is not sufficient to promote potent suppressor function in Tregs. As IL-2Rα has only a short cytoplasmic domain with no known ligand binding or modification motifs, the stronger binding of IL-2 to the heterotrimeric receptor complex [44] or recruitment of the dimeric βγ receptor complex to the lipid rafts following binding of IL-2 to the α chain [45, 46] could lead to receptor cross-talk with other lipid raft-resident receptor complexes.
Although engagement of the costimulatory receptor CD28 has been shown to be essential for Treg homeostasis and proliferation in vivo [47, 48], the role of CD28 in the induction of suppressor function seems to be less critical than the provision of IL-2 [17], and the provision of paracrine factors (e.g., IL-2 and its family members) can bypass the need for costimulation. Interestingly, a recent report has demonstrated that CD28-mediated costimulation is critical for TGF-β-induced Tregs [49]. However, in our studies, unlike IL-2, activation of natural Treg by TCR stimulation and CD28 ligation in the presence of IL-15 did not enhance the induction of suppressor function (data not shown). Although IL-15 did not induce suppressor function in naïve Tregs, IL-15 enhanced Treg viability and sustained the proliferation of activated Tregs without rendering effector cells resistant to Treg-mediated suppression. Therefore, immunotherapy with IL-15 has the potential to increase Treg accumulation in the tumor and should be considered carefully.
In contrast to the other cytokines, IL-21 only induced weak STAT5 phosphorylation and had no influence on Treg survival or induction of suppressor function. However, IL-21 slightly reduced Treg-mediated suppression of Th cell proliferation and induced a small increase in FoxP3 expression. Rather than acting on the Tregs, IL-21 presumably confers partial resistance of Th cells to Treg-mediated suppression of proliferation. This is consistent with a recent report demonstrating selective enrichment of CD8 effector cells using in vitro cultures supplemented with IL-21 [50]. Our results demonstrate that strong phosphorylation of STAT3 induced by IL-21 in Tregs cannot compensate for the lack of STAT5 phosphorylation, further suggesting that STAT5 phosphorylation is essential for Treg viability, which cannot be compensated for by STAT3 activation. The poor STAT5 activation by IL-21 in Tregs might partially explain the higher potency of IL-21 in tumor immunotherapy models when compared with other IL-2 family cytokines [30,31,32,33]. Although immunotherapy with IL-2 can increase Treg numbers in patients [25], IL-21 is unlikely to do so because of its inability to activate STAT5 in Tregs. This could reduce the potential for tumor relapse and increase the long-term efficacy of IL-21 tumor immunotherapy in comparison with IL-2.
Our findings suggest that IL-2 family cytokines and potentially other cytokines using the STAT5 signaling pathway can strongly increase Treg viability and up-regulation of FoxP3. However only IL-2 induced strong suppressor function in Tregs, indicating that STAT5-mediated up-regulation of FoxP3 expression alone is not sufficient to induce suppressor function. Nevertheless, cytokines such as IL-21, which do not strongly activate STAT5, should be considered for tumor immunotherapy, so as to prevent detrimental effects on long-term efficacy as a result of increased Treg function and accumulation. These cytokines may not provide efficient costimulation for Tregs and still promote enhancement of effector functions of conventional T cells in the context of a cancer vaccine. Further studies about how IL-15 signaling via the same β/γc as IL-2 can deliver STAT5-mediated signals without inducing potent suppressor function may provide valuable information for enhancing T cell effector function while concurrently dampening or preventing Treg suppressor function and may thus generate more powerful approaches for the immunotherapy of cancer.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the Department of Defense Prostate Cancer Research Program. We thank Drs. Joost Oppenheim and Xin Chen for their advice. We also appreciate the generous gift of anti-IL-21Rα antibody from Dr. Tom Malek and FoxP3-gfp knock-in mice from Drs. Vijay Kuchroo and Yasmine Belkaid.
References
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- Furtado G C, Curotto de Lafaille M A, Kutchukhidze N, Lafaille J J. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A M, Shevach E M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Shevach E M, McHugh R S, Piccirillo C A, Thornton A M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak T W, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa T C, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- Piccirillo C A, Shevach E M. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot J D, Gavin M A, Rudensky A Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko S A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford W T, Laurence A, Robinson G W, Shevach E M, Moriggl R, Hennighausen L, Wu C, O'Shea J J. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A M, Piccirillo C A, Shevach E M. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- Harnaha J, Machen J, Wright M, Lakomy R, Styche A, Trucco M, Makaroun S, Giannoukakis N. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–170. [PubMed] [Google Scholar]
- Koenen H J, Fasse E, Joosten I. IL-15 and cognate antigen successfully expand de novo-induced human antigen-specific regulatory CD4+ T cells that require antigen-specific activation for suppression. J Immunol. 2003;171:6431–6441. doi: 10.4049/jimmunol.171.12.6431. [DOI] [PubMed] [Google Scholar]
- Yates J, Rovis F, Mitchell P, Afzali B, Tsang J Y, Garin M, Lechler R I, Lombardi G, Garden O A. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- Forster I, Hirose R, Arbeit J M, Clausen B E, Hanahan D. Limited capacity for tolerization of CD4+ T cells specific for a pancreatic β cell neo-antigen. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom T B, Oukka M, Weiner H L, Kuchroo V K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Shevach E M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Thornton A M, Donovan E E, Piccirillo C A, Shevach E M. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M, Rosenberg S A. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T R, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- Sojka D K, Hughson A, Sukiennicki T L, Fowell D J. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- King I L, Segal B M. Cutting edge: IL-12 induces CD4+CD25– T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–645. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- Imada K, Leonard W J. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein S E, Oh S, Kovanen P E, Hinrichs C S, Pise-Masison C A, Radonovich M F, Brady J N, Restifo N P, Berzofsky J A, Leonard W J. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Wisner P, Yang G, Hu H M, Haley D, Miller W, O'Hara A, Alvord W G, Clegg C H, Fox B A, Urba W J, Walker E B. Combined IL-21 and low-dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med. 2006;4:24. doi: 10.1186/1479-5876-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti B D. Immunomodulatory and antitumor effects of interleukin-21 in patients with renal cell carcinoma. Expert Rev Anticancer Ther. 2006;6:905–909. doi: 10.1586/14737140.6.6.905. [DOI] [PubMed] [Google Scholar]
- Moroz A, Eppolito C, Li Q, Tao J, Clegg C H, Shrikant P A. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam A L, Xu-Yu Z, Szot G L, Lee M R, Zhu S, Gottlieb P A, Kapranov P, Gingeras T R, Fazekas de St Groth B, Clayberger C, Soper D M, Ziegler S F, Bluestone J A. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander S I, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Vacchio M S, Fan S, Visconti R, Frucht D M, Geenen V, Chrousos G P, Ashwell J D, O'Shea J J. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor α. J Immunol. 2002;168:2212–2218. doi: 10.4049/jimmunol.168.5.2212. [DOI] [PubMed] [Google Scholar]
- Rosenberg S A, Sportes C, Ahmadzadeh M, Fry T J, Ngo L T, Schwarz S L, Stetler-Stevenson M, Morton K E, Mavroukakis S A, Morre M, Buffet R, Mackall C L, Gress R E. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J G, Anderson D M, Kumaki S, Park L S, Grabstein K H, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce J P, Burkett P R, Boone D L, Chien M, Ma A. T cell-independent interleukin 15Rα signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor α (IL-15R α)-sushi as a selective and potent agonist of IL-15 action through IL-15R β/γ. Hyperagonist IL-15 × IL-15R α fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon B L. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D T, Garden O A, Pearce W P, Clough L E, Monk C R, Leung E, Rowan W C, Sancho S, Walker L S, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110 δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- De Jong J L, Farner N L, Widmer M B, Giri J G, Sondel P M. Interaction of IL-15 with the shared IL-2 receptor β and γ c subunits. The IL-15/β/γ c receptor-ligand complex is less stable than the IL-2/β/γ c receptor-ligand complex. J Immunol. 1996;156:1339–1348. [PubMed] [Google Scholar]
- Ellery J M, Nicholls P J. Possible mechanism for the α subunit of the interleukin-2 receptor (CD25) to influence interleukin-2 receptor signal transduction. Immunol Cell Biol. 2002;80:351–357. doi: 10.1046/j.1440-1711.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- Vamosi G, Bodnar A, Vereb G, Jenei A, Goldman C K, Langowski J, Toth K, Matyus L, Szollosi J, Waldmann T A, Damjanovich S. IL-2 and IL-15 receptor α-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc Natl Acad Sci USA. 2004;101:11082–11087. doi: 10.1073/pnas.0403916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C H, Hunig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–638. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman R M. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huddleston S J, Fraser J M, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]