Abstract
The liver contains a unique repertoire of immune cells and a particular abundance of NK cells. We have found that CD11c defines a distinct subset of NK cells (NK1.1+CD3−) in the murine liver whose function was currently unknown. In naïve animals, CD11c+ liver NK cells displayed an activated phenotype and possessed enhanced effector functions when compared with CD11c− liver NK cells. During the innate response to adenovirus infection, CD11c+ NK cells were the more common IFN-γ-producing NK cells in the liver, demonstrated enhanced lytic capability, and gained a modest degree of APC function. The mechanism of IFN-γ production in vivo depended on TLR9 ligation as well as IL-12 and -18. Taken together, our findings demonstrate that CD11c+ NK cells are a unique subset of NK cells in the murine liver that contribute to the defense against adenoviral hepatitis.
Keywords: IFN-γ, adenovirus, dendritic cells, Toll-like receptor 9, hepatic
INTRODUCTION
Adenovirus is a nonenveloped dsDNA virus that is responsible for a wide range of human illnesses most commonly affecting the respiratory and gastrointestinal systems. Systemic exposure to adenovirus results in viral hepatitis marked by an intense mononuclear cell infiltrate and an increase in serum liver enzymes [1]. Clearance of adenovirus occurs primarily through a CD8 T cell response, the magnitude of which depends on the efficiency of the preceding innate immune response [2].
IFN-γ possesses potent antiviral properties, as it amplifies the innate response of NK cells and macrophages, increases MHC class II expression on macrophages, and activates CD8 T cells [3, 4]. IFN-γ is required for effective elimination of some but not all viral infections, and the specific antiviral effects of IFN-γ vary within different organs. For example, infection with DNA viruses such as the murine cytomegalovirus (MCMV) and the herpes simplex virus (HSV) is exacerbated in mice deficient in IFN-γ or its receptor [5], whereas IFN-γ is not detected during infection with lymphocytic choriomeningitis virus [6]. Depletion of IFN-γ with a mAb results in increased MCMV viremia in the liver and a concomitant increase in liver injury, but the absence of IFN-γ does not affect viral burden in the spleen [7, 8]. Moreover, IFN-γ produced in the liver is more important than perforin-based cytotoxicity for hepatic clearance of MCMV, and the opposite is true of the spleen [8]. During infection with adenovirus, IFN-γ is required for optimal clearance of infected hepatocytes as it up-regulates target cell expression of MHC class I [2] and induces an adenovirus-specific T cell response [1, 9]. Furthermore, IFN-γ may have additional T cell-independent roles in the resolution of liver damage and clearance of a low burden of adenovirus [10].
NK cells are abundant in the liver and relatively rare in peripheral lymphoid organs [11] suggesting that they are important for certain immune responses in the liver. Within hours of infection with adenovirus, liver NK cells become activated and produce IFN-γ, which is required for an effective adenovirus-specific T cell response [1]. Depletion of NK cells prior to infection with adenovirus results in decreased recruitment of virus-specific CD8 T cells to the liver and reduced intrahepatic CD8 T cell cytotoxicity of infected targets, whereas these responses are unaffected in the spleen [9]. Additionally, during infection with adenovirus, liver NK cells can cause apoptosis of infected hepatocytes in a manner independent of T cells [1].
NK cell production of IFN-γ often results from the monokines IL-12 and IL-18, which are generated in response to viral infection [3]. However, the requirement of these monokines for efficient IFN-γ responses depends on the type of virus and anatomic compartment. For example, hepatic IFN-γ production during MCMV infection depends on IL-12 but not IL-18 [12] and during respiratory infection with adenovirus, IFN-γ production in the lung depends on IL-18 but not IL-12 [13]. In mice infected with adenovirus, correlative evidence links IFN-γ production by intrahepatic lymphocytes with IL-12 [9] and whether hepatic NK cells depend on IL-18 is unknown. In addition, adenovirus is sensed through TLR-dependent and TLR-independent mechanisms [14, 15] but which pathway is necessary for liver IFN-γ production during infection with adenovirus is uncertain.
In this report, we have found that NK cells (NK1.1+CD3−) were the most common IFN-γ-producing cells in the liver during the peak innate response to adenovirus infection and that the majority of IFN-γ-producing liver NK cells expressed CD11c. These CD11c+ NK cells accounted for a large proportion of NK cells in the resting liver and could be distinguished morphologically, phenotypically, and functionally from CD11c− liver NK cells. In response to adenovirus infection in vivo, CD11c− and CD11c+ liver NK cells increased in number and demonstrated increased lytic function in vitro. However, only CD11c+ liver NK cells up-regulated expression of MHC class II and costimulatory molecules and gained some degree of APC function. Furthermore, we provide evidence that intact TLR9 signaling in addition to IL-12 and IL-18 is crucial for a productive in vivo IFN-γ response to adenovirus.
MATERIALS AND METHODS
Animals
Adult 6- to 8-week-old male C57BL/6 (B6, H-2Kb) and Balb/c (H-2Kd) mice were purchased from Taconic Farms (Germantown, NY, USA). IL-12−/− and IL-18−/− mice (both H2-Kb) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). TLR9−/− mice (H2-Kb) were originally generated in the laboratory of Akira and co-workers [16]. Animals were maintained in pathogen-free conditions in the animal housing facility at Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY, USA), and the Institutional Animal Care and Use Committee approved all animal procedures. Mice were infected with 5 × 1010 particles of wild-type human adenovirus type 5 [American Type Culture Collection (ATCC), Manassas, VA, USA] via lateral tail vein injection.
Cell isolation
Liver dendritic cells (DC; NK1.1−CD11c+CD3−), CD11c+ NK cells (NK1.1+CD11c+CD3−), and CD11c− NK cells (NK1.1+CD11c−CD3−) were isolated as described previously [17] with minor modifications. Animals were killed by CO2 inhalation, and the portal vein was injected in situ with 2 ml 1% type IV collagenase (Sigma Chemical Co., St. Louis, MO, USA) in PBS. The livers were then removed from the animals and mechanically disrupted prior to 20 min incubation in 1% collagenase at 37°C. The resulting tissue slurry was passed through a sterile, 100-μm cell strainer (Falcon, BD Biosciences, San Jose, CA, USA) and centrifuged three times at 30 + g for 5 min to remove hepatocytes. Erythrocytes were then lysed with a hypotonic solution of ammonium chloride. The resulting nonparenchymal cells (NPC) were resuspended in complete RPMI-1640 medium containing 10% FCS and enriched by a 40% Optiprep (Sigma Chemical Co.) density gradient. The low-density, buoyant cells were separated into CD45+ and CD45− fractions with immunomagnetic beads, per the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA, USA), and purified further by passage through a second positive selection column. Prior to immunomagnetic bead incubation, FcγRIII/II were blocked with the mAb 2.4G2 (Fc block; 1 μg/million cells; Monoclonal Antibody Core Facility, MSKCC). CD45+ cells were stained with fluorescently conjugated antibodies to CD11c, NK1.1, and CD3ε (BD PharMingen, San Diego, CA, USA) for separation into DC and NK subsets by FACS using a MoFlo cell sorter (DakoCytomation, Fort Collins, CO, USA). Dead cells were excluded with 4′,6-diamidino-2-phenylindole, dilactate (Molecular Probes, Eugene, OR, USA) and care was taken to exclude doublets and highly autofluorescent cells. Post-sort purities were routinely greater than 97%. Spleens were mechanically disrupted and incubated in 1% collagenase for 20 min at 37°C and then passed through a 70-μm filter. Splenocytes were obtained following lysis of RBC with a hypotonic solution and two washes in media.
Flow cytometry
Four-color analyses were performed on CD45+ liver cells and splenocytes (1 million) using a FACSCalibur flow cytometer (BD Biosciences). FcRs were blocked prior to staining, and dead cells were excluded with 7-amino-actinomycin D (BD Biosciences) and forward-scatter gating. FITC, PE, PerCP, and APC-conjugated antibodies were obtained from BD PharMingen unless otherwise specified and included CD11c (clone HL-3), NK1.1 (PK136), CD3ε (145-2C11), MHCII (I-Ab, AF6-120.1), CD40 (3/23), CD80 (16-10A1), CD86 (GL-1), CD45R/B220 (RA3-6B2), DEC 205 (NLDC-145, Serotec, Raleigh, NC, USA) and CD49b (DX5), NKG2D (CX5), Ly49A (A1), Ly49D(4E5), CD244.2 (2B4), CD69 (H1.2F3), CD62 ligand (CD62L; Mel-14), CD27 (LG.3A10), CD90.2 (30-H12), and CD8α (OX-8). Ig isotype controls were used where appropriate. Flow cytometry data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Cytokine analysis
In vitro cytokine production was determined by culturing 3 × 104 freshly sorted cells in 100 μl media for 72 h. CpG ODN 1826 (10 μg/ml, Oligos Ect., Wilsonville, OR, USA), IL-12 (2 ng/ml, R&D Systems, Minneapolis, MN, USA), or IL-18 (50 ng/ml, R&D Systems) was added to some wells. Supernatant IFN-γ was determined with a cytometric bead array (BD Biosciences). For intracellular IFN-γ determination, CD45+ liver cells and splenocytes were isolated from infected and noninfected animals and cultured for 6 h in the presence of Brefeldin A (Golgi plug, BD PharMingen) without restimulation. After washing, 1 million cells were stained with surface antibodies, fixed and permeabilized, and stained for intracellular IFN-γ per the manufacturer’s protocol (BD PharMingen).
Microscopy
FACS-purified liver DC and NK cells were centrifuged at 600 rpm onto glass slides and fixed in 100% methanol before staining with H&E. Typically, greater than 200 cells were examined and photographed at 100× magnification using a Zeiss Axiophot 2 microscope (Carl Zeiss, Thornwood, NY, USA).
Functional assays
FACS-purified hepatic DC and NK cells were cultured in triplicate with 2 × 103 (51Cr) sodium chromate-labeled (100 μCi Na51CrO4 per 2×106 targets at 37ºC for 90 min; PerkinElmer Life and Analytical Sciences, Inc., Boston, MA, USA) yeast artifical chromosome-1 (YAC-1) lymphoma cells (ATCC) for 6 h. Select effectors were treated with concanamycin-A (CMA; 100 ng/ml, ICN Biomedical, Costa Mesa, CA, USA) for 2 h at 37ºC before the addition of targets. Supernatant chromium release was measured with a TopCount NXT microplate scintillation and luminescence counter (PerkinElmer Life and Analytical Sciences, Inc.). Percent-specific lysis was calculated as (cpm experimental–cpm spontaneous release) × 100/(cpm maximum release–cpm spontaneous release). MLRs were performed by culturing γ-irradiated (3000 rad), FACS-purified DC and NK cells with 1 × 105 Balb/c T lymphocytes in triplicate wells. CpG ODN 1826 (10 μg/ml) was added to some wells, and APC and T cell-alone controls were included in all experiments. On Day 3, 3H-thymidine (1 μCi/well, PerkinElmer Life and Analytical Sciences, Inc.) was added to the cultures, and radioactive uptake was measured 20 h later (TopCount NXT, PerkinElmer Life and Analytical Sciences, Inc.). Antigen-specific CD4 T cell activation was assayed in a similar manner with OT-II transgenic T cells specific for the KISQAVHAAHAEINEAG peptide (OVA323–399). γ-Irradiated stimulators were pulsed for 1 h at 37°C with peptide (1 μg/ml, Peptide Synthesis Core, MSKCC) and plated at various concentrations with OT-II T cells in triplicate wells. Thymidine was added on Day 3, and radioactive uptake was measured 20 h later.
Statistics
Statistical significance was determined by Student’s t-test or one-way ANOVA where appropriate, using Prism 4.0 statistical software (Graphpad Software, San Diego, CA, USA). A P value of less than 0.05 was considered significant.
RESULTS
CD11c+ NK cells contribute to early hepatic IFN-γ production during adenovirus infection
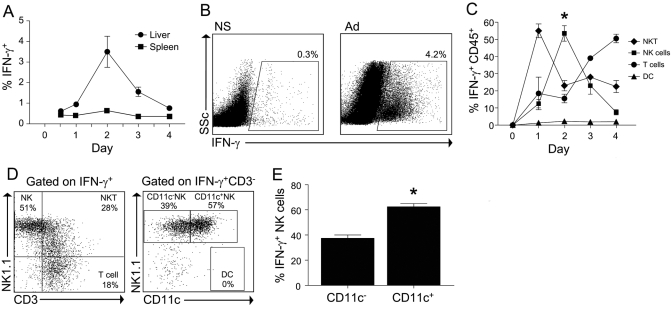
Using intracellular cytokine analysis of liver NPC, we determined that hepatic IFN-γ production peaked at 2 days following i.v. infection with adenovirus. The IFN-γ response to viral challenge was substantially greater in the liver compared with the spleen and consistent with the hepatotropic properties of the adenovirus (Fig. 1, A and B). NKT cells (NK1.1+CD3+) accounted for the majority of IFN-γ+ cells in the liver on Day 1; however, total liver IFN-γ production was low at this time. NK cells became the dominant source of IFN-γ in the liver by Day 2 during the peak IFN-γ response (Fig. 1, C and D). Further phenotyping of the IFN-γ+ liver NK cells revealed that greater than 60% expressed CD11c (Fig. 1E). Notably, liver DC did not produce IFN-γ.
Fig. 1.
CD11c+ NK cells contribute to early hepatic IFN-γ production during adenovirus infection. Following infection with adenovirus, liver CD45+ cells and splenocytes were isolated at the indicated time-points and cultured for 6 h in the presence of Brefeldin-A without restimulation. (A) The percentage of liver leukocytes and splenocytes that were IFN-γ+ is shown. (B) Representative FACS plots of liver CD45+ cells isolated from animals treated 48 h previously with normal saline (NS) or adenovirus (Ad). SSc, Side-scatter. (C) The composition of IFN-γ+ cells at serial time intervals during adenovirus infection was determined by intracellular staining. The number of NKT cells (NK1.1+CD3+), NK cells (NK1.1+CD3−), DC (NK1.1−CD11c+CD3−), and conventional T cells (NK1.1−CD3+) is expressed as a percentage of total IFN-γ+ cells. (D) Representative FACS plots to demonstrate the gating strategy used to determine the composition of the various IFN-γ+ cell types. (E) The expression of CD11c on IFN-γ+ liver NK cells is shown at 48 h after adenovirus infection. Data represent the mean (±sem) of two separate experiments in which the hepatic leukocytes of three to four mice per group were pooled. *, P < 0.05.
CD11c+ NK cells are a discrete subset of resting liver NK cells
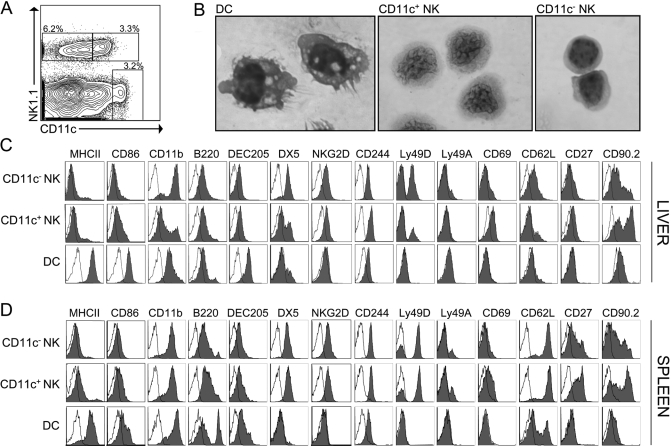
NK1.1+CD11c+ cells in the spleen have been demonstrated to have dual NK cell and APC function [18]. To determine the properties of steady-state CD11c+ liver NK cells, we studied uninfected C57BL/6 mice. CD11c+ NK cells comprised a substantial percentage of the liver NK cell compartment (37±5%; Fig. 2A) and could be distinguished morphologically from CD11c− liver NK cells and liver DC. Liver DC were large cells with a low nuclear-to-cytoplasmic (N:C) ratio, vacuolated cytoplasm, cytoplasmic projections, and dense hyperchromatic nuclei. CD11c− liver NK cells were small lymphocytes with a high N:C ratio. CD11c+ liver NK cells had distinctive features with intermediate size, intermediate N:C ratio, and a peculiar dense, folded-appearing nuclear chromatin pattern (Fig. 2B).
Fig. 2.
CD11c+ NK cells comprise a substantial portion of NK1.1+CD3− cells in the uninfected liver. (A) Liver NPC from unmanipulated mice were enriched with anti-CD45 immunomagnetic beads and analyzed by flow cytometry. The control-plot is gated on CD3− hepatic leukocytes to highlight conventional liver DC (NK1.1−CD11chiCD3−), CD11c+ NK cells (NK1.1+CD11c+CD3−), and CD11c− NK cells (NK1.1+CD11c−CD3−). The percentage indicates the fraction of CD3− cells. (B) FACS-purified DC, CD11c+ NK cells, and CD11c− NK cells were stained with H&E after cytospin and imaged at 100× original magnification. Similar results were obtained from two separate experiments, and the microscopic appearances of each cell population were greater than 90% consistent. (C and D) CD45+ liver cells and splenocytes from uninfected mice were analyzed for the indicated surface markers by flow cytometry after gating on DC, CD11c+ NK cells, and CD11c− NK cells. Closed histograms represent staining of the indicated surface markers, and open histograms represent isotype controls. Data are representative of a minimum of three separate experiments.
Consistent with their microscopic differences, CD11c+ liver NK cells had a different cell-surface phenotype than liver DC and CD11c− liver NK cells (Fig. 2C). Resting CD11c+ liver NK cells displayed low MHC class II and intermediate CD86 expression (Fig. 2C), and they lacked the costimulatory molecules CD40 and CD80 and negative immunoregulatory molecules programmed death 1 ligand (PD-L1) and PD-L2 (not shown). When compared with CD11c− liver NK cells, CD11c+ liver NK cells expressed similar levels of the NK receptors NKG2D and CD244 but had lower levels of CD11b and DX5, indicating a relatively immature NK cell phenotype [19]. A higher activation state of CD11c+ liver NK cells was suggested by greater CD69 and lower CD62L when compared with CD11c− liver NK cells. Additionally, a large fraction of CD11c+ liver NK cells expressed CD90.2. This molecule, expressed on T cells and some NK cells, has been shown previously to be expressed as a subset of cytotoxic rat spleen DC [20]. Taken together, steady-state CD11c+ liver NK cells shared more phenotypic similarities with NK cells than with DC. Furthermore, when compared with CD11c+ spleen NK cells, CD11c+ liver NK cells showed lower expression of B220, Ly49D, the NK cell differentiation markers CD11b, DX5, and CD27, and the lymphoid homing molecule CD62L (Fig. 2, C and D).
Steady-state CD11c+ liver NK cells are potent killers with capacity for weak APC function
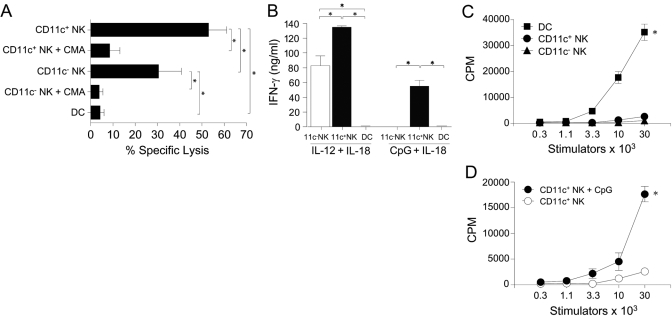
As CD11c+ NK cells appeared to be a phenotypically distinct subset of liver NK cells, we wanted to determine if they possessed different functions than CD11c− liver NK cells. CD11c+ liver NK cells sort-purified from uninfected animals consistently demonstrated greater lysis of YAC-1 lymphoma targets than CD11c− liver NK cells (Fig. 3A). Killing depended on perforin release, as it was abrogated by CMA, an inhibitor of the granule exocytosis pathway [21]. Based on known stimulators of spleen NK1.1+CD11c+ cells, we tested the ability of CD11c+ liver NK cells to produce IFN-γ. CD11c+ and CD11c− liver NK cells responded vigorously to the combination of IL-12 and IL-18; however, CD11c+ liver NK cells made considerably more IFN-γ (55.1±8.0 ng/ml) in response to the combination of CpG and IL-18 than did CD11c− liver NK cells (1.3±0.6 ng/ml; Fig. 3B). To determine the APC ability of resting CD11c+ liver NK cells, FACS-purified effectors were cultured with allogeneic T cells in a MLR. Although we have previously found that spleen NK1.1+CD11c+ cells were able cause the proliferation of allogeneic T cells [18], fresh CD11c+ liver NK cells were not capable of inducing alloproliferation (Fig. 3C). However, activation with the TLR9 ligand CpG enabled CD11c+ liver NK cells to become nearly half as potent at allogeneic T cell stimulation as unactivated DC (Fig. 3, C and D).
Fig. 3.
Steady-state CD11c+ liver NK cells are potent killers with the capacity for modest APC function. Liver DC, CD11c+ liver NK cells, and CD11c− liver NK cells were FACS-purified from noninfected mice. (A) Effector cells were cultured with chromium-labeled YAC-1 lymphoma cells in the presence or absence of CMA, an inhibitor of perforin release. A 30:1 E:T ratio is shown. Three separate chromium release experiments were performed with similar results, and the data represent the mean (±sem) percent-specific lysis of these three experiments. (B) FACS-purified effectors were cultured for 72 h with IL-12 (2 ng/ml) and IL-18 (50 ng/ml) or CpG (10 μg/ml) and IL-18 (50 ng/ml), and supernatants were analyzed for IFN-γ via cytometric bead analysis. Data are the mean (±sem) of duplicate wells and are representative of three separate experiments. (C) Indicated numbers of FACS-purified stimulator cells were incubated with 1 × 105 Balb/c T cells in a MLR in media alone or (D) CpG (10 μg/ml), and (3H)-thymidine incorporation was measured after 3 days. Mean (±sem) values are based on triplicate wells, and the data are representative of three individual experiments. *, P < 0.05.
CD11c+ liver NK cells are activated during viral infection
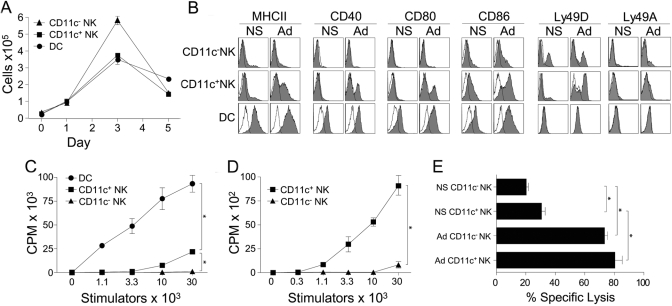
As CD11c+ liver NK cells demonstrated a robust IFN-γ response to adenovirus (Fig. 1), we sought to determine how they were otherwise altered during infection. Adenovirus infection resulted in an expansion of CD11c− NK cells (16-fold), CD11c+ NK cells (12-fold), and DC (16-fold), which peaked on the 3rd day following inoculation (Fig. 4A). To determine whether CD11c+ liver NK cells could respond to an infectious challenge like an APC, the expression of costimulatory molecules on CD11c+ liver NK cells was determined. Up-regulation of MHCII, CD40, CD80, and CD86 was seen on CD11c+ liver NK cells in infected mice. CD11c− liver NK cells retained their resting, immature state except for a slight increase in CD86 expression (Fig. 4B). Increased expression of costimulatory molecules peaked at 24 h following infection but only slightly decreased at serial time-points measured to 96 h (not shown). To determine if the increase in maturation had functional consequence, we purified CD11c+ liver NK cells from infected animals. CD11c+ liver NK cells activated in vivo by adenovirus acquired the ability to stimulate the proliferation of allogeneic T cells, albeit to a much lesser degree than classical liver DC (Fig. 4C). Consistent with their up-regulation of MHC class II, peptide-pulsed CD11c+ liver NK cells from infected mice were able to cause the proliferation of antigen-specific OT-II CD4 T cells (Fig. 4D). Notably, CD11c+ liver NK cells did not possess this ability when isolated from naïve animals (not shown), and CD11c–liver NK cells did not acquire these APC functions during infection. Maturation of CD11c+ liver NK cells was accompanied by a concomitant, marked increase in the expression of the activating C-type lectin NK cell receptor Ly49D with only a minor change in the inhibitory Ly49A receptor (Fig. 4B). Whether this NK cell receptor phenotype correlated with a change in NK cell function was investigated in lysis assays using liver NK cells isolated from adenovirus-infected mice. CD11c+ and CD11c− NK cells were both activated in vivo to become more efficient killers (Fig. 4E).
Fig. 4.
CD11c+ liver NK cells are expanded and activated during viral infection. (A) Mice were inoculated with 5 × 1010 particles of adenovirus via the lateral tail vein and killed at the indicated times. The absolute numbers of hepatic DC, CD11c+ NK cells, and CD11c− NK cells were enumerated by flow cytometry. (B) The surface expression of costimulatory molecules and NK cell receptors is shown for hepatic DC, CD11c+ NK cells, and CD11c− NK cells from animals infected with adenovirus 24 h prior. Each of the above is representative of a minimum of three separate experiments using pooled hepatic leukocytes of three mice per group. (C) A MLR was performed using stimulators isolated from the livers of mice infected with adenovirus 3 days earlier. These data are representative of two separate experiments. (D) CD11c+ and CD11c− liver NK cells were purified from infected animals, pulsed with the OVA323–399 peptide, and assayed for their ability to cause the proliferation of antigen-specific, transgenic OTII CD4 T cells. (E) The ability of CD11c+ and CD11c− liver NK cells, purified from mice, injected with normal saline (NS) or adenovirus 3 days prior was tested in a chromium release assay using YAC-1 lymphoma cells as targets. A 7.5:1 E:T ratio is shown. These data are representative of two separate experiments. *, P < 0.05.
IFN-γ produced by liver NK cells in response to adenoviral hepatitis depends on TLR9 ligation, IL-12, and IL-18
As CpG stimulated CD11c+ liver NK cells in vitro (Fig. 3, B and D) and adenovirus contains CpG motifs, we sought to determine whether TLR9 was necessary for CD11c+ liver NK cell activation. TLR9-deficient mice were infected with adenovirus, and intracellular IFN-γ was measured within isolated liver CD45+ cells. Strikingly, we found that in the absence of TLR9, IFN-γ production by CD11c+ as well as CD11c− liver NK cells was abrogated almost completely (Fig. 5). Notably, there was no significant difference in the percentages of CD11c+ or CD11c− NK cells in uninfected, TLR9-deficient animals when compared with wild-type controls (not shown). Likewise, from viral challenge of IL-12- and IL-18-deficient mice, we found that IL-12 and IL-18 were required in the liver for an optimal IFN-γ response to adenoviral hepatitis.
Fig. 5.
IFN-γ production by CD11c+ and CD11c− liver NK cells in response to adenovirus is dependent on TLR9 ligation, IL-12, and IL-18. Wild-type (WT), TLR9−/−, IL-12−/−, and IL-18−/− mice were inoculated wtih 5 × 1010 particles of adenovirus or normal saline and killed 48 h later. Intracellular cytokine analysis was performed on isolated, hepatic CD45+ cells. IFN-γ+ events within gated CD11c+ and CD11c–NK cells were determined based on isotype control staining. One of two experiments with similar results is shown.
DISCUSSION
The liver is home to a unique repertoire of immune cells that confers protection against a range of toxins, infectious agents, malignant cells, and unwarranted responses to dietary antigens. In particular, the liver is enriched in NK1.1+ lymphocytes that include NK cells and NK1.1+ T cells [11]. NK cells provide the first line of defense against microbial infection and malignant transformation by their ability to kill autologous cells without the necessity of priming [22]. NK cells in the liver demonstrate several curious properties specific to their location. The normal adult murine liver contains a considerable number of immature NK cells, suggesting that NK precursors originating in the bone marrow may complete their developmental program within the liver [19]. In contrast to NK cells resident in mouse spleen, bone marrow, and lymph node, liver NK cells contain a population of TRAIL-expressing cells [23, 24]. Furthermore, human liver NK cells display an activated phenotype compared with peripheral blood NK cells [25], and liver NK cells have been shown to mediate greater cytotoxic activity against tumor cells than peripheral NK cells in rodents and humans [26, 27].
In this report, we demonstrate that expression of the integrin CD11c defines a subset of liver NK cells that is particularly responsive to adenovirus infection and distinguishes them from steady-state CD11c− liver NK cells by morphologic, phenotypic, and functional attributes. Although we and others [17, 28, 29] have previously identified this subset of NK cells as a contaminant of liver DC preparations, their function in the normal and diseased liver was unknown. CD11c+ liver NK cells comprised approxiamtely one-third of liver NK cells and segregated into the immature liver NK cell compartment bearing low CD11b and low DX5 expression [19]. However, several additional features identify CD11c+ liver NK cells as an activated subset of liver NK cells. Higher expression of CD69 and lower expression of CD62L on CD11c+ compared with CD11c− NK cells are consistent with an activated phenotype. Functionally, resting CD11c+ liver NK cells demonstrated enhanced lysis (Fig. 3A) and a greater IFN-γ response to cytokine and TLR stimulation in vitro (Fig. 3B). Furthermore, CD11c+ liver NK cells possessed the capacity to cause T cell alloproliferation when stimulated by CpG motifs (Fig. 3D) or adenovirus (Fig. 4C).
The regulation of IFN-γ production during infection with wild-type adenovirus was similar for CD11c+ and CD11c− liver NK cells, which all required IL-12 to produce IFN-γ. In contrast to infection with MCMV [12], IL-18 was also required for NK cell IFN-γ production in the adenovirus-infected liver (Fig. 5). Furthermore, we determined that an intact TLR9 receptor is necessary for the downstream signaling required for type II IFN production during systemic adenovirus infection. TLR9 is a pathogen-associated molecular pattern receptor that recognizes unmethylated CpG DNA motifs present in bacterial DNA and in viruses such as murine MCMV and HSV [30]. The innate immune recognition of adenovirus has recently been determined to occur through TLR9-dependent as well as TLR-independent mechanisms that occur in a cell-type specific manner [14, 15]. Although liver (Fig. 3D) and spleen NK1.1+CD11c+CD3− cells can recognize TLR9 ligands directly [31, 32], purified liver NK cells did not secrete IFN-γ in response to live or heat-killed adenovirus in vitro (not shown). This suggests the presence of an in vivo cellular partner that recognizes adenovirus through TLR9 and can provide necessary signals to liver NK cells that may include IFN-α, IL-12, and/or IL-18.
Systemic infection with adenovirus caused up-regulation of MHC class II and costimulatory molecules on CD11c+ but not CD11c− NK cells and endowed them with modest APC function (Fig. 4, B and C). That an immune cell can acquire dual lytic and antigen-presenting function has been noted previously in humans. Human NK cells up-regulate surface expression of MHC class II molecules and gain the ability to present antigen and costimulate T cells after prolonged in vitro activation in IL-2 [33,34,35]. Conversely, circulating human myeloid and plasmacytoid DC can kill tumor cells after type I or II IFN stimulation [36, 37], TLR7/8 ligation [38], or influenza or HIV infection [39, 40], and unstimulated human peripheral DC may lyse select squamous cell tumor targets [41].
On T lymphocytes, up-regulation of CD11c has been shown to occur following activation in vivo [42], and in the setting of viral infection, this phenotype correlates with increased CTL activity [43]. The particular phenotype of NK1.1+CD11c+ has been correlated to murine spleen and lymph node NK cells possessing heightened innate functions (enhanced ability to lyse tumor cells and to secrete IFN-γ) and APC function [18, 44,45,46]. However, the APC function of mouse NK cells has become an area of recent debate. Where some laboratories have shown that some or all murine NK cells can function as APC once activated [47], others have challenged this notion [48]. This area has been confounded by differences in isolation procedures among different laboratories, varying definitions of NK cell subsets examined, and the use of different strains of mice. For example, as Balb/c mice do not express the NK1.1 antigen, the pan-NK cell marker DX5 is used to identify NK cells; however, this marker is promiscuous and is also expressed on a subset of DC [48]. In our models using C57BL/6 mice, we have not been able to demonstrate an APC contaminant within liver or spleen CD11c+NK1.1+ cells. In fact, here, we have found that CD11c+ NK cells in the liver do not exhibit APC function in their resting state. This appears to contrast with the APC ability of fresh, unactivated spleen NK1.1+ CD11c+ cells [18] and may reflect the unique, immunomodulatory microenvironment of the liver.
In summary, we have demonstrated that IFN-γ production by hepatic NK cells in response to adenovirus infection depends on TLR9 as well as the monokines IL-12 and IL-18. During the innate phase of the antiviral immune response to adenovirus and vaccinia virus (not shown), IFN-γ is produced preferentially by a subset of liver NK cells that expresses CD11c, suggesting that this subset of liver NK cells is specialized for responding to viral infection in the liver. CD11c+ liver NK cells are morphologically, phenotypically, and functionally distinct from CD11c− liver NK cells and behave differently than their splenic counterparts. CD11c+ liver NK cells are more potent cytolytic effectors, and like immature DC, they have the capacity to up-regulate costimulatory molecules and gain APC function in an inflammatory environment. In response to adenovirus infection, CD11c+ liver NK cells become activated immune responders with pleiotropic functions. The wide-reaching immune capabilities and immunoregulatory potential of this subset of NK cells likely have relevance, not only to hepatic infection but also to malignancy, tolerance, and autoimmunity within the liver.
Acknowledgments
This work was supported by National Institutes of Health grants DK068346 (to R. P. D) and CA123938-01 (to B. M. B.). The authors thank Jan Hendrikx, Vincent Sahi, Patrick Anderson, and Mark Kweens of the Memorial Sloan-Kettering Cancer Center flow cytometry core facility for their expertise in cell sorting.
References
- Liu Z X, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J Immunol. 2000;164:6480–6486. doi: 10.4049/jimmunol.164.12.6480. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C A, Nguyen K B, Pien G C, Cousens L P, Salazar-Mather T P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Heise M T, Virgin H W. The T-cell-independent role of γ interferon and tumor necrosis factor α in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli F, Casanova J L. The role of IL-12, IL-23 and IFN-γ in immunity to viruses. Cytokine Growth Factor Rev. 2004;15:367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay C H, Welsh R M. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Falck-Pedersen E, Elkon K B. Variation in adenovirus transgene expression between BALB/c and C57BL/6 mice is associated with differences in interleukin-12 and γ interferon production and NK cell activation. J Virol. 2001;75:4540–4550. doi: 10.1128/JVI.75.10.4540-4550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zajac A J, McPherson S A, Hsu H C, Yang P, Wu Q, Xu X, Wang X, Fujihashi K, Curiel D T, Mountz J D. Primary adenovirus-specific cytotoxic T lymphocyte response occurs after viral clearance and liver enzyme elevation. Gene Ther. 2005;12:1079–1088. doi: 10.1038/sj.gt.3302494. [DOI] [PubMed] [Google Scholar]
- Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien G C, Satoskar A R, Takeda K, Akira S, Biron C A. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-γ responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-γ release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]
- Nociari M, Ocheretina O, Schoggins J W, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Pillarisetty V G, Shah A B, Miller G, Bleier J I, DeMatteo R P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- Pillarisetty V G, Katz S C, Bleier J I, Shah A B, Dematteo R P. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-γ via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Kang H S, Dokun A, French A R, Greco S, Yokoyama W M. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- Trinite B, Voisine C, Yagita H, Josien R. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202–4208. doi: 10.4049/jimmunol.165.8.4202. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- Moretta A, Bottino C, Mingari M C, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, Yagita H, Kinoshita K, Okumura K, Smyth M J. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth M J, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- Tu Z, Bozorgzadeh A, Crispe I N, Orloff M S. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007;149:186–193. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D, Luo D, Froelich C J, Medema J P, Kummer J A, Willems E, Braet F, Wisse E. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol. 2002;72:668–676. [PubMed] [Google Scholar]
- Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- Lian Z X, Okada T, He X S, Kita H, Liu Y J, Ansari A A, Kikuchi K, Ikehara S, Gershwin M E. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- Hensley S E, Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol Ther. 2007;15:1417–1422. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- Chaudhry U I, Kingham T P, Plitas G, Katz S C, Raab J R, DeMatteo R P. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-γ and inhibit tumor growth. Cancer Res. 2006;66:10497–10504. doi: 10.1158/0008-5472.CAN-06-1908. [DOI] [PubMed] [Google Scholar]
- Welner R S, Pelayo R, Garrett K P, Chen X, Perry S S, Sun X H, Kee B L, Kincade P W. Interferon-producing killer dendritic cells (IKDC) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109:4825–4831. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M G, Bigler M, Haanen J B, Yssel H, Bacchetta R, de Vries J E, Spits H. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147:781–787. [PubMed] [Google Scholar]
- Hanna J, Gonen-Gross T, Fitchett J, Rowe T, Daniels M, Arnon T I, Gazit R, Joseph A, Schjetne K W, Steinle A, Porgador A, Mevorach D, Goldman-Wohl D, Yagel S, LaBarre M J, Buckner J H, Mandelboim O. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest. 2004;114:1612–1623. doi: 10.1172/JCI22787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zingoni A, Sornasse T, Cocks B G, Tanaka Y, Santoni A, Lanier L L. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- Fanger N A, Maliszewski C R, Schooley K, Griffith T S. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Zhao S, Deuse Y, Schakel K, Wehner R, Wohner H, Holig K, Wienforth F, Kiessling A, Bornhäuser M, Temme A, Rieger M A, Weigle B, Bachmann M, Rieber E P. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174:4127–4134. doi: 10.4049/jimmunol.174.7.4127. [DOI] [PubMed] [Google Scholar]
- Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens J P, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- Hardy A W, Graham D R, Shearer G M, Herbeuval J P. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-α. Proc Natl Acad Sci USA. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjic B M, Lu G, Pimenov A, Whiteside T L, Storkus W J, Vujanovic N L. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- Huleatt J W, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- Lin Y, Roberts T J, Sriram V, Cho S, Brutkiewicz R R. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol. 2003;33:2736–2743. doi: 10.1002/eji.200324087. [DOI] [PubMed] [Google Scholar]
- Chaudhry U I, Katz S C, Kingham T P, Pillarisetty V G, Raab J R, Shah A B, DeMatteo R P. In vivo overexpression of Flt3 ligand expands and activates murine spleen natural killer dendritic cells. FASEB J. 2006;20:982–984. doi: 10.1096/fj.05-5411fje. [DOI] [PubMed] [Google Scholar]
- Chan C W, Crafton E, Fan H N, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky T W, Stins M F, Lanier L L, Pardoll D M, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- Chen L, Calomeni E, Wen J, Ozato K, Shen R, Gao J X. Natural killer dendritic cells are an intermediate of developing dendritic cells. J Leukoc Biol. 2007;81:1422–1433. doi: 10.1189/jlb.1106674. [DOI] [PubMed] [Google Scholar]
- Vosshenrich C A, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo J P. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Ahmet F, Heger K, Brady J, Nutt S L, Vremec D, Pietersz S, Lahoud M H, Schofield L, Hansen D S, O'Keeffe M, Smyth M J, Bedoui S, Davey G M, Villadangos J A, Heath W R, Shortman K. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]