Abstract
Endogenous polymorphonuclear leukocyte (PMN)-associated sialidase activity enhances PMN adhesion to and migration across the endothelium through the removal of sialylated cell-surface residues. We tested the hypothesis that PMNs also express sialyltransferase (ST) activity that restores sialyl residues to the PMN surface. We developed a highly sensitive fluorometric assay to demonstrate that intact human PMNs can mediate and accept sialyl residue transfer. This ST activity is inhibited by a ST inhibitor, CMP, which also inhibits the transendothelial migration of PMNs in response to IL-8 in vitro and in vivo. We conclude that intact PMNs express sialidase and ST activities that permit rapid modulation of their surface sialylation and their ability to adhere to and migrate across the endothelium.
Keywords: sialic acid cell trafficking, inflammation
INTRODUCTION
Circulating neutrophils or polymorphonuclear leukocytes (PMNs) traffic to sites of inflammation, where they exit the intravascular space through a multistep process called diapedesis [1]. This process initially requires a carbohydrate-dependent step by which nonactivated PMNs loosely adhere to adhesion molecules expressed on activated endothelial cells (ECs). Following this loose tethering, PMNs are activated by EC-derived mediators, resulting in the tight adherence of PMNs to the endothelium, primarily via the binding of PMN-expressed integrins to ICAMs on the EC surface. This step precedes the migration of PMNs to and through the interendothelial cell junction. During diapedesis, interactions between multiple glycosylated surface molecules and their counter-ligands regulate PMN adhesion to and migration across the endothelium; these interactions are required for full expression of the acute inflammatory phenotype [1].
Sialic acids or N-acetylneuraminic acids are a family of amino sugars that are coupled to the outermost ends of glyconjugates on the surface of eukaryotic cells and impart a negative charge to the molecule and the cell. Sialic acid residues on the cell surface reduce cell-to-cell adhesion, prevent the deposition of complement on the cell surface, permit evasion of immune recognition, and regulate the binding of ligands to their cell surface receptors [2, 3].
Enzymatic modification of the sialylation status of these cell-surface glycoconjugates may provide an additional layer of regulation of PMN interactions with other PMNs, other cells, or informational molecules (e.g., cytokines, hormones). Microbial and cellular sialidases, by removing sialyl residues from these surface glycoconjugates, can rapidly modify their affinity for binding partners [3, 4]. Upon activation, human PMNs mobilize endogenous sialidase activity from intracellular compartments to the plasma membrane, where it cleaves sialyl residues from cell-surface glycoconjugates [4]. Such desialylation results in marked PMN functional changes, including increased adhesiveness to and migration across the endothelium.
Our laboratory has focused on host sialic acid modulation and its regulation of PMN and monocyte function [4,5,6,7]. We found that stimulation of PMNs in vitro and in vivo is associated with a loss of cell-surface sialic acid, enhanced homotypic and heterotypic intercellular adhesion, and priming of the response to agonistic stimuli [4, 6, 7]. We hypothesize that during a tightly regulated inflammatory response, resialylation of PMN surface molecules through sialyltransferase (ST) activity is required to return the cell to its resting state (i.e., resialylate). To date, PMN ST activity and its potential roles in the inflammatory process have not been studied. We now demonstrate that intact human PMNs contain endogenous ST activity. Further, prior ST inhibition with CMP impairs transendothelial migration (TEM) of PMNs in response to IL-8 in vitro and in vivo. Such ST activity may insure the availability of cell-surface sialyl residues on resting PMNs for their initial carbohydrate-dependent tethering to EC adhesion molecules. Alternatively, resialylation of the PMN surface may promote the “de-adherence” or release of PMNs from the abluminal endothelial surface during TEM, so they might continue their movement into inflamed tissues. Thus, a highly orchestrated system of sialidases and STs may permit PMNs to respond dynamically to a rapidly changing environment.
MATERIALS AND METHODS
Cell preparation
Human RBCs were obtained from the peripheral blood of healthy volunteers by venipuncture under a protocol approved by the Institutional Review Board (IRB). Sterile and defibrinated SRBCs were purchased from Waltz Farm (Smithsburg, MD, USA) and stored at 4ºC. PMNs were processed from the peripheral blood of healthy human donors as described previously [4] under the same IRB-approved protocol.
Reagents
Type 5 sialidase (Clostridium perfringens), 2,3 deoxy-N-acetylneuraminic acid (2-DN), CMP, and GMP were purchased from Sigma Chemical Co. (St. Louis, MO, USA). CMP was also obtained from Tokyo Kasei (Japan). CMP-5-FITC-neuraminic acid (CMP-5-FITC-NEU) was purchased from Calbiochem (San Diego, CA, USA) and later prepared by one of the coauthors (R. Brossmer). α2,6-ST was purchased from Genzyme (Cambridge, MA, USA), and protease inhibitor cocktail tablets were purchased from Roche (Indianapolis, IN, USA). Recombinant human (rhu)IL-8 was obtained from R&D Systems (Minneapolis, MN, USA), and Dulbecco’s PBS (DPBS) was purchased from Invitrogen (Carlsbad, CA, USA).
ST assay
For the study of PMN ST activity, we adapted a ST assay described previously in which CMP-5-FITC-NEU served as the labeled sialyl residue donor and sialidase-treated RBCs as the sialyl residue acceptor [8]. Based on preliminary studies in which we optimized the concentrations of each component of the assay and the duration of treatment, a standard reaction mixture contained a sialyl residue acceptor (RBCs or PMNs), CMP-5-FITC-NEU, and a ST source (Fig. 1). CMP-5-FITC-NEU was added over a dosage range of 12.5–50 μM. As the signal decreased at the 12.5-μM concentration, we routinely used the CMP-5-FITC-NEU at 25 μM. Purified α2,6-ST at a final concentration of 0.5 nU/ml or PMNs as an endogenous source of ST activity were used in a final volume of 100 μl cell incubation buffer (CIB) containing PBS plus 5 mg/ml BSA, pH 6.8. Maximal ST activity was observed with α2,6-ST at a final concentration of 8 × 10–10 U/ml, with decreasing activity observed down to 0.5 × 10–10 U/ml (40% maximal). We therefore chose 0.5 nU/ml, which was 95% maximal activity. Sialyl residue acceptor SRBCs and PMNs were desialylated with 25 mU/ml sialidase/1–2 × 108/cells in 1 ml CIB for 1 h at 37ºC, washed once in CIB, and used at 4 × 106 cells or at 8 × 106 cells, respectively, in a volume of 40 μl. Examination of sialidase doses from 0.025 to 100 mU/ml demonstrated that maximal desialylation was achieved at 25 mU/ml. Although the amount of desialylation increased linearly from the earliest time-point, 30 (57% maximal ST activity)–120 min, for convenience, we chose a treatment time of 60 min (75% maximal activity). When used as an endogenous source of ST activity, human PMNs were adjusted to a stock concentration of 1 × 108 PMN/ml, lysed by freeze/thawing, and centrifuged, and the protein concentration of the supernatant was determined (Bio-Rad Laboratories, Hercules, CA, USA). Intact human PMNs at 8 × 106/0.1 ml assay volume were also used as a source of ST activity as well as an acceptor of sialyl residues.
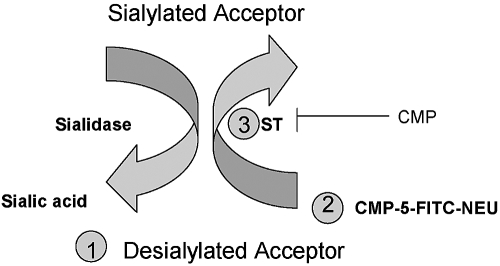
Fig. 1.
ST assay. The components of the ST assay include (1) a molecule or cell capable of accepting sialyl residues (sialyl acceptor). Although previous ST assays used molecules such as asialofetuin, in our assay, we used sialidase-treated RBCs or PMNs. (2) A sialic acid (or N-acetyl neuraminic acid) donor. In our assay, we use a fluorophore, CMP-5-FITC-NEU. (3) A source of ST activity. In our assay, we used exogenous ST or PMNs. Addition of CMP-5-FITC-NEU and a ST source to a sialyl acceptor mediates the transfer of sialic acid onto the acceptor (Sialylated Acceptor), which is detected by flow cytometry. This reaction is competitively blocked by CMP. Sialidase removes the sialyl residues from the acceptor cells (Desialylated Acceptor).
After gentle mixing, all tubes were incubated for 2 h at 37ºC in a humidified atmosphere containing 5% CO2. There was a progressive increase in resialylation of erythrocytes from 30 to 120 min. To optimize the ST signal, we therefore selected the 2-h assay time. The cells were washed, pelleted, and resuspended in CIB and analyzed fluorocytometrically on a FACScan II (Becton Dickinson, Mountain View, CA, USA). Based on forward- and right-angle, light-scatter properties, an electronic gate was placed around the RBCs or PMNs, which were excited at 488 nM, and emission was measured at 530 nM. At least 10,000 events were recorded per condition. Data were analyzed with a CellQuest analysis program.
For each experiment, controls included omission of each of the components of the assay mixture (i.e., ST source, CMP-5-FITC-NEU, or sialyl residue acceptor) as well as boiled, exogenous α2,6-ST. When indicated, 2-DN (0.04 mM, final concentration), an inhibitor of sialidase activity [9], was introduced to prevent the possible removal of the transferred sialyl residues. ST activity was measured after subtraction of background fluorescence [i.e., complete assay mixture minus the exogenous α2,6-ST or for endogenous ST, in the presence and absence of the ST inhibitor CMP (0.3 mM final concentration)] and was expressed as percent of maximal activity. We observed similar inhibitory effects of CMP on ST activity over a dosage range of 0.125–0.450 mM. For experiments in which we estimated the endogenous ST activity, we constructed a standard curve using serial twofold dilutions of purified α2,6-ST enzyme, from which tissue ST activity was interpolated and expressed as nU/μg protein.
Confocal microscopy
In addition to measuring the ST activity by flow cytometry, we visualized ST activity by confocal microscopy. PMNs were treated with 100 mU/ml bacterial sialidase for 30 min at 37ºC, and then the CMP-5-FITC-NEU was added in the absence or presence of CMP (0.3 mM) or a similar concentration of GMP for 37ºC for 2 h. PMNs were fixed with 4% paraformaldehyde, and cell nuclei were stained with the nuclear dye 4′,6-diamidino-2-phenylindole (DAPI; 20 nM for 30 min at 22ºC) and cover-slipped with a DABCO-based antifade mounting media. Digital microscopy images were captured by means of a FluoView 500 confocal microscope (Olympus Instruments, San Jose, CA, USA), fitted with standard filters for the visualization of DAPI and CMP-5-FITC-NEU. All images of substrates and controls were captured using identical acquisition parameters (FluoView500 software) and assembled into panels (CorelDraw 12).
TEM of PMNs in vitro
The ability of human PMNs to migrate across human pulmonary artery EC (HPAEC) monolayers in response to a chemotactic stimulus (rhuIL-8) was measured as described previously [7]. Only EC monolayers retaining >97% of a 14C-BSA tracer as a measure of barrier integrity were studied. After treatment of HPAECs for 4 h with 25 U/ml TNF-α, calcein-alveolar macrophage (calcein-AM; Molecular Probes, Eugene, OR, USA)-labeled human PMNs (5×105 cells/well) were introduced into the upper compartment of assay chambers, incubated for 2 h at 37ºC, after which time, each lower compartment was sampled and fluorometrically assayed. A standard curve was established for each experiment from which PMN numbers could be interpolated from fluorescence units, and PMN TEM was expressed as percent migration.
Recruitment of murine PMNs in vivo
Crl:CD-1 (ICR) BR mice (Jackson Laboratories, Bar Harbor, ME, USA) were used for i.p. recruitment of PMNs. Mice were injected i.p. with 0.2 ml sterile, nonpyrogenic, normal saline (0.9% sodium chloride, Baxter Healthcare Corp., Deerfield, IL, USA) or rhuIL-8 (750 ng/mouse). Mice were i.v.-infused with CMP or GMP (30 μg/g body weight in 0.1 ml) at 5 min before and 1 h after normal saline or rhuIL-8. Cells were harvested by peritoneal lavage with cold DPBS at 4 h, and total cells and PMNs were counted directly in a hemocytometer. In control animals, equivalent volumes of saline were administered at the same time-points in lieu of CMP or GMP.
Statistical analysis
Experimental data were analyzed using the GraphPad Prism statistical analysis package (GraphPad Software, Inc., San Diego, CA, USA). Statistical differences were analyzed by one-way ANOVA with comparison among groups analyzed by Tukey’s multiple comparison test or by a two-tailed Mann-Whitney test. A P value <0.05 was considered statistically significant.
RESULTS
Exogenous α2,6-ST transfers sialyl residues onto SRBCs
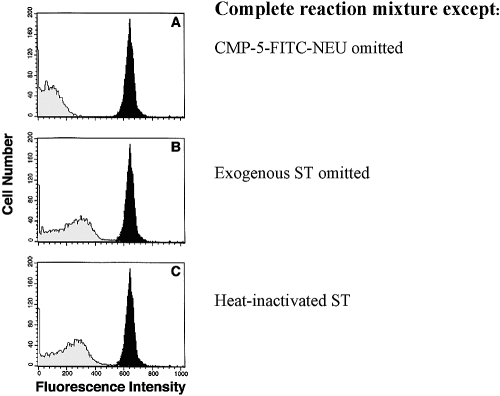
In preliminary experiments, we established that sialidase treatment prepared the surface of human RBCs and SRBCs for the comparable acceptance of ST-mediated transfer of CMP-5-FITC-NEU (data not shown). Aliquots of SRBCs kept at 4ºC for up to 3 weeks had no decrement in acceptor ability. The complete mixture of SRBCs, CMP-5-FITC-NEU, and exogenous α2,6-ST resulted in a strong signal on flow cytometry (Fig. 2A–C, dark peaks). Omission of CMP-5-FITC-NEU (i.e., autofluorescence; A, light peak) or exogenous ST (B, light peak) from the reaction mixture or the use of heat-inactivated ST (C, light peak) markedly reduced the signal. CMP-5-FITC-NEU did not bind nonspecifically to cells after washing (data not shown).
Fig. 2.
ST assay using sheep erythrocytes as sialyl residue acceptors. A complete reaction mixture consisting of sialidase-treated SRBCs, purified α2,6-ST, and CMP-5FITC-NEU was added at the concentrations described in Materials and Methods and incubated for 2 h at 37ºC. The complete mixture gave a strong signal [solid peaks, A–C; mean fluorescence intensity (MFI)=637]. Omission of the CMP-5-FITC-NEU (light peak, A; MFI=72) or ST (light peak, B; MFI=219) or heat inactivation (100ºC for 30 min) of ST (light peak, C; MFI=205) reduced the fluorescence of the SRBCs. No signal was evident when the SRBC acceptor was omitted.
Sialidase pretreatment is required for SRBCs but not for PMNs to accept sialyl residues
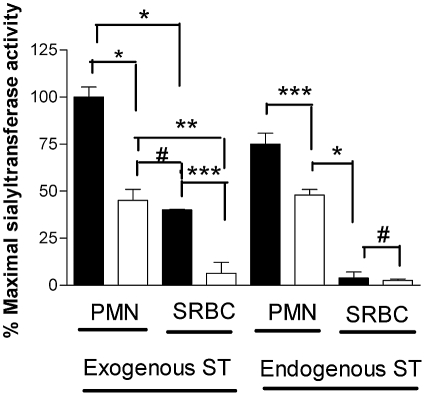
As RBCs were capable of accepting sialyl residues, we asked whether intact human PMNs might also function as sialyl residue acceptors in the presence of exogenous (α2,6-ST) ST. It was reported previously that in the absence of sialidase treatment, SRBCs were unable to serve as an acceptor for the labeled CMP-5-FITC-NEU. We therefore examined the necessity for sialidase pretreatment of SRBCs and PMNs to serve a similar preparative function in our assay [8]. When exogenous α2,6-ST was added to the reaction mixture, sialidase-treated PMNs and SRBCs were able to serve as a sialyl residue receptor, although SRBCs had <50% of the ST activity compared with similarly treated PMNs (Fig. 3). There was an increase in the ability of PMNs to accept sialyl residues with increasing doses of sialidase pretreatment until a plateau was attained at 30 mU sialidase/ml (data not shown). In the absence of sialidase pretreatment, SRBCs displayed no fluorescent signal, indicating that the “unmasking” of acceptor sites on SRBCs by sialidase is necessary for ST activity. Exogenous α2,6-ST also mediated an easily detectable but diminished CMP-5-FITC-NEU transfer to PMNs without prior sialidase treatment, which suggests that unlike SRBCs, PMNs were able to unmask sialyl acceptor sites.
Fig. 3.
Unlike PMNs, SRBCs require sialidase pretreatment to accept the ST-mediated transfer of sialyl residues. CMP-5-FITC-NEU (25 μM) was added to PMNs and SRBCs, which were treated (closed bars) or not treated (open bars) with sialidase (25 mU/ml for 1 h at 37ºC). Following the addition of an exogenous source of ST (α2,6-ST, 0.5 nU/ml), the cells were incubated at 37ºC for 2 h and analyzed by flow cytometry. Intact PMNs were used as a source of ST with intact PMNs as acceptor cells, and a similar number of SRBCs were added to SRBCs to assess whether these cells provided endogenous ST activity. The data represent the mean ± sem of four wells per variable. This experiment is representative of an experiment repeated three times. The data were analyzed by one-way ANOVA with comparison among groups analyzed by Tukey’s multiple comparison test. *, P < 0.001; **, P < 0.01; ***, P < 0.05; #, P> 0.05.
Intact PMNs are a ST source and sialyl residue acceptors
In assaying endogenous sources for ST activity, we routinely used a specific ST inhibitor [10, 11], CMP, to verify that the assay system was in fact detecting genuine ST activity. As preliminary tests demonstrated that 0.3 mM CMP fully inhibited ST activity in vitro and was not toxic to the PMNs, each assay for endogenous ST activity was performed in the presence and absence of 0.3 mM CMP. The difference in corrected values generated in the presence and absence of CMP was taken as the true ST activity (i.e., CMP-inhibitable activity).
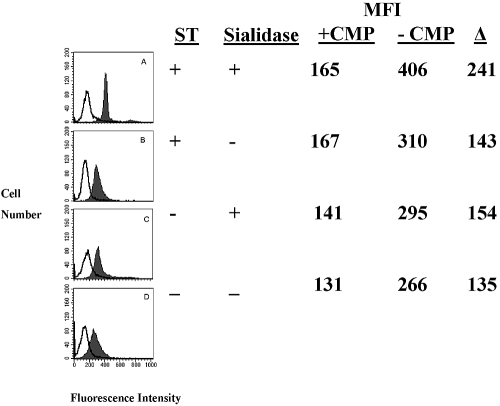
Since in preliminary experiments, PMN lysates contained ST activity, we asked whether intact PMNs or SRBCs also could serve as an endogenous source for ST. To optimally expose more sialyl acceptor sites, aliquots of PMNs or SRBCs were pretreated with sialidase and added to the reaction mixture as sialyl residue acceptors, and a second aliquot of untreated PMNs or SRBCs was added in place of the purified α2,6-ST enzyme as a source of ST activity (Fig. 3). In the absence of an exogenous source of ST, α2,6-ST, we observed no ST activity with SRBC, even with sialidase pretreatment (Fig. 3). Thus, SRBCs had no endogenous ST activity. In contrast, even in the absence of an exogenous source of ST, PMNs exhibited ST activity (Fig. 3). CMP inhibited the ability of sialidase-treated PMNs to serve as sialyl residue acceptors when α2,6-ST was added to the reaction (Fig. 4A). Although PMNs not treated with sialidase had a decreased ability to accept the α2,6-ST-mediated transfer of sialyl residues, it, too, was inhibited by CMP (Fig. 4B). The ability of CMP to inhibit exogenously added ST activity led us to ask whether there might be endogenous ST activity in PMNs. When intact PMNs were added to a second aliquot of PMNs pretreated with sialidase to optimize the likelihood of observing ST activity, CMP-inhibitable ST activity was observed (Fig. 4C).
Fig. 4.
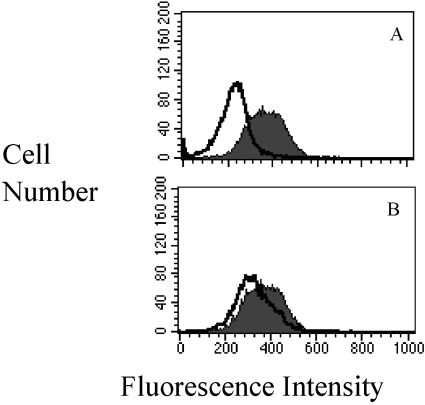
Human PMNs as a source of endogenous (i.e., CMP-inhibitable) ST activity. Although pretreatment of PMNs with sialidase and exogenous ST provided maximal ST activity (A, MFI=406 vs.165 with CMP), a strong signal was evident when exogenous ST was added to PMNs unexposed to sialidase pretreatment (B, MFI=310 vs. 167 with CMP). When PMNs were pretreated with sialidase, and intact, untreated PMNs were added as an endogenous source of ST, readily detectable ST activity was evident (C, MFI=295 vs. 141 with CMP). When an aliquot of intact, untreated PMNs was added to PMNs that were not exposed to sialidase, a lesser but distinct signal was apparent (D, MFI=266 vs. 131 with CMP). Transferase activity (shaded peaks) was compared in each case with PMNs, in which no exogenous ST was added and in the presence of the specific ST inhibitor, CMP (0.3 mM final concentration; open peaks).
As PMNs served as acceptor cells in the ST assay in the absence of sialidase pretreatment (Figs. 3and 4B), we asked whether intact PMNs that were not pretreated with sialidase could be used as the sialyl residue acceptor and endogenous source of ST activity. After incubation with CMP-5-FITC-NEU, CMP-inhibitable ST activity was evident in intact, untreated PMNs (Fig. 4D). These data suggest that during an inflammatory response, intact PMNs can mediate the autocrine/paracrine transfer of sialyl residues from PMN to PMN.
Direct visualization of ST activity on intact PMNs
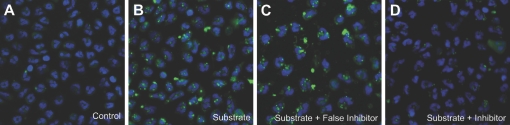
The use of a fluorescent instead of a radiolabeled substrate for the ST activity also permitted direct visualization of the ST activity on intact PMNs (Fig. 5). In the absence of the CMP-5-FITC-NEU, there was little background fluorescence, even with sialidase pretreatment of the PMNs (Fig. 5A); however, with the addition of the fluorescent substrate, there was cell-associated fluorescence evident (Fig. 5B). As CMP-5-FITC-NEU does not penetrate the cell [12], the signal most likely represents cell-surface-associated fluorescein. The fluorescence was not distributed uniformly throughout each cell, and although most cells had fluorescence, there were cell-to-cell differences observed. Addition of the ST inhibitor, CMP, to the reaction (Fig. 5D), but not a control molecule of similar molecular weight and charge, GMP (Fig. 5C) reversed the reaction
Fig. 5.
α2,6-ST mediates the transfer of sialyl residues onto intact PMNs. CMP-5-FITC-NEU was added to PMNs that were treated with 100 mU/ml bacterial sialidase for 30 min at 37ºC. The PMNs were then incubated in the presence and absence of the ST inhibitor, CMP (0.3 mM), or a noninhibitory molecule of similar molecular weight and charge, GMP (0.3 mM), for 2 h and then fixed in 4% paraformaldehyde. Cells were stained with DAPI (20 nM), coverslipped with mounting media, and examined by confocal microscopy as described in Materials and Methods. In the absence of CMP-5-FITC-NEU (A), no fluorescence was observed. When CMP-5-FITC-NEU was added to the reaction mixture, cell-associated fluorescence was observed, and the cells appeared intact (B). Although the addition of GMP did not reduce the fluorescent signal (C), the CMP greatly diminished the transfer of the substrate onto the cells (D).
Endogenous PMN sialidase activity unmasks sialyl residue acceptors
As RBCs not treated with sialidase cannot serve as sialyl residue acceptors, we reasoned that for PMNs to serve as sialyl residue acceptors in the absence of prior sialidase treatment, some previously unrecognized, constitutive level of endogenous sialidase activity may expose sites on the PMN surface to accept sialyl residues. To test this hypothesis, we first showed that unstimulated, intact PMNs not exposed to sialidase had CMP-inhibitable activity (Fig. 6A). The addition of CMP to the ST assay mixture reduced the MFI from 364 (solid peak) to 222 (open peak). We reported previously that 2-DN inhibits sialidase activity, and a molecule of similar weight and charge, ketodeoxyoctonate, does not [4,5,6,7]. Addition of 2-DN to those same PMNs not exposed to sialidase resulted in a small but consistently reproducible decrement in ST activity (decrease in MFI from 364 to 317; Fig. 4B, solid vs. open peak). Therefore, in nonstimulated PMNs, a basal level of sialidase activity appears necessary for the constitutive turnover of surface sialyl residues, perhaps as part of a cell-surface remodeling process. This activity was not detectable with the less-sensitive biochemical assays of sialidase activity used in our earlier reports [4,5,6,7] and was insufficient to cause PMN functional changes normally associated with desialylation [4, 7].
Fig. 6.
CMP inhibits endogenous ST activity. (A). PMNs (8×106) were added to 10 μM CMP-5-FITC-NEU in the absence (solid peak) or presence (open peak) of 0.3 mM CMP and analyzed by flow cytometry. CMP treatment reduced the MFI from 364 to 222. (B) To determine if intact, untreated PMNs exhibit some basal level of sialidase activity necessary to expose sialyl acceptor sites for the ST activity, PMNs were analyzed in the absence (solid peak) or presence (open peak) of 2-DN (0.04 mM), an inhibitor of sialidase activity. A small but highly reproducible reduction in ST activity was noted (reduction in MFI from 364 to 317). The data show a single experiment that was repeated independently three times.
ST inhibition reduces TEM of PMNs in vitro
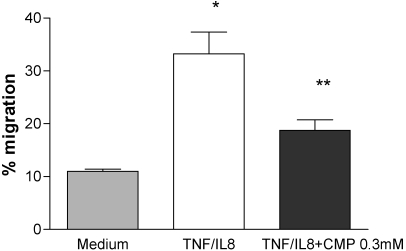
We hypothesized that once migrating PMNs reach the abluminal surface of endothelial cells, a restoration of sialyl residues to the PMN surface would be required to “deadhere” and to continue their migration. There was a robust (greater than threefold increase) migration of calcein-AM-labeled human PMNs across TNF-α-treated HPAEC monolayers in response to IL-8 (Fig. 7). Addition of the ST inhibitor, CMP, inhibited TEM >40% (P<0.03). This suggests that ST activity plays a critical role in TEM in a static, in vitro assay.
Fig. 7.
Inhibition of TEM by a ST inhibitor, CMP. Calcein-AM-labeled human PMNs were added to HPAEC, pretreated for 4 h with rTNF-α (50 U/ml). TEM was measured in response to IL-8 (150 ng/ml) for over 2 h, as described previously [7], in the absence and presence of 0.3 mM CMP. We studied four chambers/variable. This figure is representative of three independent experiments. The groups were compared by a two-tailed Mann-Whitney test. *, P < 0.03, compared with medium; **, P < 0.03, compared with TNF/IL-8.
ST inhibition reduces PMN migration in vivo
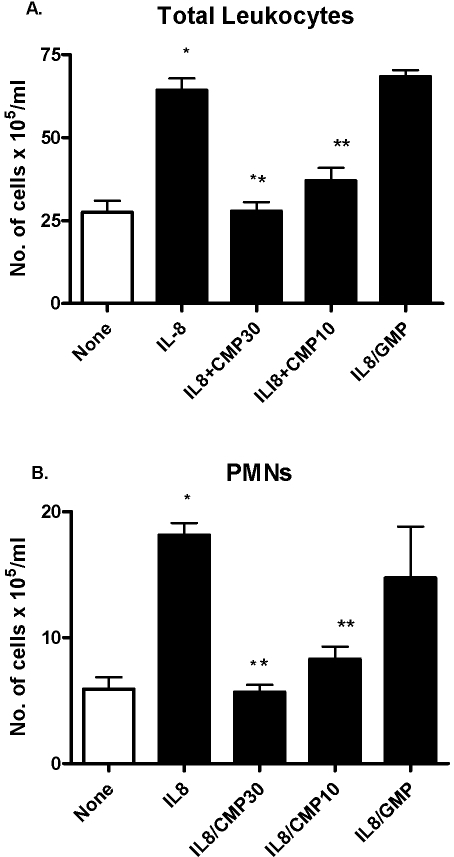
We asked whether the ability of ST inhibition to diminish TEM of PMNs in vitro could be extended to an in vivo experimental system. At 4 h after the i.p. administration of IL-8 or saline, we measured the appearance of total leukocytes and PMNs in peritoneal fluid (Fig. 8). In selected experiments, mice were i.v.-pretreated with CMP or a noninhibitory control molecule of similar molecular weight and charge, GMP. IL-8-treated mice displayed a two- to threefold increase in the i.p. recruitment of total leukocytes and PMNs compared with saline-treated controls. When mice were pretreated with the ST inhibitor, CMP, there was a marked reduction of total leukocytes and PMNs in the peritoneum. In contrast, when another purine nucleoside, GMP, was administered, no reduction in i.p. cellular migration was evident. Thus, ST activity is required for optimal PMN migration in response to an IL-8 stimulus in vivo.
Fig. 8.
Inhibition of leukocyte recruitment to IL-8-induced peritonitis by CMP. Outbred mice (6- to 8-week-old ICR) were administered 750 ng rIL-8 or PBS i.p. Four hours later, the mice were killed, the peritoneum washed out with PBS, and the cells collected by centrifugation. The number of total leukocytes (A) and PMNs (B) was enumerated by hemocytometry. Mice were given CMP, GMP, or PBS 1 h before IL-8 administration and 1 h afterwards. The results are the composite of four separate experiments with 10 mice in the “None” and “IL8” groups and four to eight mice in the “CMP” or “GMP” groups. The groups were compared by a two-tailed Mann-Whitney test.*, P < 0.0001, compared with “None”; **, P < 0.0002. There was no difference between the “IL8” and “IL8/GMP” groups.
DISCUSSION
Activated PMNs mobilize endogenous sialidase activity from an intracellular compartment to the plasma membrane [4]. Removal of as little as 20% of total cell-associated sialic acid residues profoundly increases PMN adherence to and migration across endothelial cell monolayers [7]. Inhibition of endogenous sialidase activity in vitro prevented these PMN-endothelial cell interactions and in vivo, reduced PMN recruitment to inflammatory sites [6, 7]. Moreover, we observed that fMLP-activated PMNs directly desialylate the surface of HPAECs, which in turn increases PMN migration across the EC monolayer [7].
As PMNs must respond dynamically to a range of dissimilar environmental stimuli, these cells require the capacity to restore sialyl residues to surface glycoconjugates. We now have demonstrated that intact human PMNs also have ST activity that catalyzes the transfer of sialyl residues onto host cells, including PMNs. Thus, PMNs have the capacity to rapidly alter their (and/or other) cell surface(s) through the removal (sialidase) or restoration (ST) of sialyl residues. We propose this modulation of sialylation as a novel mechanism for the regulation of PMN adhesion and motility.
STs are a family of enzymes that catalyzes the transfer of N-acetylneuraminic acid from CMP-β-N-acetylneuraminic acid onto carbohydrate groups of glycolipids and glycoproteins (i.e., glycoconjugates), usually at the terminal position. More than 10 distinct eukaryotic STs have been identified [13, 14], which differ in substrate specificity for acceptor sugars (e.g., galactose, N-acetylgalactosamine, or sialic acid) as well as the type of glycosidic linkages formed (e.g., α2,3, α2,6, α2,8).
Overexpression of ST activity has been associated with oncogenic transformation and increased metastatic potential [11, 15, 16]. ST also plays a pivotal role in the maturation and function of myeloid cells and B and T lymphocytes [15, 17,18,19,20,21]. For example, CD22, a member of the Ig superfamily of adhesion molecules, is a B cell-restricted transmembrane protein required for lymphocyte activation and immune function [22]. ST-mediated sialylation of CD22 abrogates CD22-mediated B and T lymphocyte adhesion essential to lymphocyte function [22, 23]. In the case of CD8+ T lymphocytes, ST activity dictates whether a cell undergoes apoptosis or progresses to become a viable memory T cell [14]. ST activity regulates components of the innate immune system as well. For example, sialylation of the mannose receptor, a pattern recognition receptor, governs its ability to bind to its ligand [3].
STs are preferentially localized to the Golgi apparatus within the cell [13]; however, extensive work by Shur and colleagues [24, 25] has established that glycosyltransferases may be expressed on the cell surface, where they function as cell adhesion molecules, signal transducing receptors for extracellular oligosaccharide ligands, and regulators of cell growth. Ecto-STs have been described in platelets, lymphoblastoid cells, B lymphocytes, and neuronal cells and more recently, on the surface of early-activated CD8 T cells [13, 26, 27]. As there appears to be a connection between the Golgi and the cell surface [28], Golgi-associated STs might be exported to the plasma membrane.
Although it has been inferred that PMNs have ST activity within the Golgi complex that enables PMNs to restore sialyl residues during the recycling of surface receptors [29], to our knowledge, there have been no reports of ST activity on the surface of PMNs. Although we did not localize the ST activity of PMNs to the cell surface, we did find that intact, viable PMNs display ST activity. One could speculate that as PMNs migrate through the endothelial, paracellular pathway without disrupting barrier integrity, sialidase and ST activities might be expressed simultaneously but spatially segregated within distinct, subcellular compartments in polarized cells. Sialidase activity, concentrated on the leading front of a migrating PMN, may remove sialyl residues from glycoconjugates, such as CD31, at the interendothelial cell junction, thereby facilitating their adherence to as well as their ability to squeeze through this junction. In contrast, STs at the PMN “tail” may restore these residues to the cell surface, allowing for physical disengagement from substrates enabling PMNs to continue their migration through tissues. This might explain how PMNs can negotiate the paracellular pathway without compromising the barrier for the movement of macromolecules or fluid. It is also conceivable that PMN ST activity might resialylate endothelial cell surfaces, thereby “resealing” the paracellular pathway. Alternatively, the ability of CMP to decrease recruitment to an inflamed peritoneum in vivo might suggest that ST activity is required to insure the initial carbohydrate-dependent tethering to the activated EC through sialylation of the surface of nonactivated PMNs. Our data showing constitutive ST activity on PMNs is consistent with this hypothesis.
The sialidase/ST enzyme system, through its dynamic control of PMN surface sialylation, regulates the cell–cell and likely, the cell–matrix interactions, which are prerequisites to PMN motility. The constitutive ST activity of intact, untreated PMNs demonstrated in these studies may insure that PMNs do not become promiscuously adherent in response to every transient, minimal stimulus and/or that nonstimulated PMNs retain sialyl residues on their surface that facilitates their initial tethering. Finally, the coordinated activities of sialidase and ST activities in the PMN may allow a rapidly reversible, highly localized response to an inflammatory stimulus and may serve as a therapeutic target.
Acknowledgments
This work was supported by National Institutes of Health grant nos. AI42818 and HL086933. We greatly appreciate the technical assistance of Dayton Nance.
References
- Muller W A. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–533. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- Pilatte Y, Bignon J, Lambre C R. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993;3:201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- Su Y, Bakker T, Harris J, Tsang C, Brown G D, Wormald M R, Gordon S, Dwek R A, Rudd P M, Martinez-Pomares L. Glycosylation influences the lectin activities of the macrophage mannose receptor. J Biol Chem. 2005;280:32811–32820. doi: 10.1074/jbc.M503457200. [DOI] [PubMed] [Google Scholar]
- Cross A S, Wright D G. The mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest. 1991;88:2067–2076. doi: 10.1172/JCI115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N M, Gomatos P J, Cox J, Fowler A, Dow N, Wohlhieter J A, Cross A S. Desialylation of peripheral blood mononuclear cells promotes growth of HIV-1. Virology. 1997;228:123–131. doi: 10.1006/viro.1996.8373. [DOI] [PubMed] [Google Scholar]
- Cross A S, Sakarya S, Rifat S, Held T K, Drysdale B E, Grange P E, Cassels F J, Wang L-X, Stamatos N, Farese A, Casey D, Powell J, Bhattacharjee A K, Kleinberg M, Goldblum S E. Recruitment of murine neutrophils in vivo through endogenous sialidase activity. J Biol Chem. 2003;278:4112–4120. doi: 10.1074/jbc.M207591200. [DOI] [PubMed] [Google Scholar]
- Sakarya S, Rifat S, Zhou J, Bannerman D D, Stamatos N M, Cross A S, Goldblum S E. Mobilization of neutrophil sialidase activity desialylates the pulmonary vascular endothelial surface and increases resting neutrophil adhesion to and migration across the endothelium. Glycobiology. 2004;14:481–494. doi: 10.1093/glycob/cwh065. [DOI] [PubMed] [Google Scholar]
- Sticher U, Brossmer R. Highly sensitive fluorometric assay for transferase activity using fluoresceinyl-NeuAc as donor. Anal Biochem. 1990;186:127–134. doi: 10.1016/0003-2697(90)90585-w. [DOI] [PubMed] [Google Scholar]
- Mendla K, Cantz M. Specificity studies on the oligosaccharide sialidase of human fibroblasts. Biochem J. 1984;218:625–628. doi: 10.1042/bj2180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E L, Bighouse K J. Cytidine 5′-monophosphosialic acid hydrolase. Subcellular location and properties. Biol Chem. 1974;249:7813–7823. [PubMed] [Google Scholar]
- Kleineidam R G, Schmelter T, Schwarz R T, Schauer R. Studies on the inhibition of sialyl- and galactosyltransferases. Glycoconj J. 1997;14:57–66. doi: 10.1023/a:1018560931389. [DOI] [PubMed] [Google Scholar]
- Gross H J. Fluorescent CMP-sialic acids as a tool to study the specificity of the CMP-sialic acid carrier and the glycoconjugate sialylation in permeabilized cells. Eur J Biochem. 1992;203:269–275. doi: 10.1111/j.1432-1033.1992.tb19856.x. [DOI] [PubMed] [Google Scholar]
- Broquet P, Baubichon-Cortay H, George P, Louisot P. Glycoprotein sialyltransferases in eucaryotic cells. Int J Biochem. 1991;23:385–389. doi: 10.1016/0020-711x(91)90164-i. [DOI] [PubMed] [Google Scholar]
- Priatel J J, Chui D, Hiraoka N, Simmons C J, Richardson K B, Page D M, Fukuda M, Varki N M, Marth J D. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–283. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- Cyopick P, Culliton R, Brockhausen I, Sutherland D R, Mills G B, Baker M. Role of aberrant sialylation of chronic myeloid leukemia granulocytes on binding and signal transduction by chemotactic peptides and colony stimulating factors. Leuk Lymphoma. 1993;11:79–90. doi: 10.3109/10428199309054733. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Srivantana U, Ullah A, Gagneja H, Berenson C S, Lance P. Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochim Biophys Acta. 2001;1536:148–160. doi: 10.1016/s0925-4439(01)00044-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Hasegawa Y, Higai K, Matsumoto K. Transcriptional regulation of human β-galoctoside α2,6-sialyltransferase (hST6Gal I) gene during differentiation of the HL-60 cell line. Glycobiology. 2000;10:623–628. doi: 10.1093/glycob/10.6.623. [DOI] [PubMed] [Google Scholar]
- Skacel P O, Edwards A J, Harrison C T, Watkins W M. Enzymic control of the expression of the X determinant (CD15) in human myeloid cells during maturation: the regulatory role of 6-sialyltransferase. Blood. 1991;78:1452–1460. [PubMed] [Google Scholar]
- Moody A M, Chui D, Reche P A, Priatel J J, Marth J D, Reinherz E L. Developmentally regulated glycosylation of the CD8αβ coreceptor stalk modulates ligand binding. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- Baum L G, Derbin K, Perillo N L, Wu T, Pang M, Uittenbogaart C. Characterization of terminal sialic acid linkages on human thymocytes. Correlation between lectin-binding phenotype and sialyltransferase expression. J Biol Chem. 1996;271:10793–10799. doi: 10.1074/jbc.271.18.10793. [DOI] [PubMed] [Google Scholar]
- Hennet T, Chui D, Paulson J C, Marth J D. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi D, Varki A, Braesch A S, Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J Biol Chem. 1993;268:7011–7018. [PubMed] [Google Scholar]
- Braesch-Andersen S, Stamenkovic I. Sialylation of the B lymphocyte molecule CD22 by α 2,6-sialyltransferase is implicated in the regulation of CD22-mediated adhesion. J Biol Chem. 1994;269:11783–11786. [PubMed] [Google Scholar]
- Appeddu P A, Shur B D. Molecular analysis of cell surface β-1, 4-galactosyltransferase function during cell migration. Proc Natl Acad Sci USA. 1994;91:2095–2099. doi: 10.1073/pnas.91.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Dubois D H, Miller D J, Shur B D. Activation of a G protein complex by aggregation of β-1,4 galactosyltransferase on the surface of sperm. Science. 1995;269:1718–1721. doi: 10.1126/science.7569899. [DOI] [PubMed] [Google Scholar]
- Kaufmann M, Blaser C, Takashima S, Schwartz-Albiez R, Tsuji S, Pircher H. Identification of an α2, 6-sialyltransferase induced early after lymphocyte activation. Int Immunol. 1999;11:731–738. doi: 10.1093/intimm/11.5.731. [DOI] [PubMed] [Google Scholar]
- Gross H J, Merling A, Moldenhauer G, Schwartz-Albiez R. Ectosialyltransferase of human B lymphocytes reconstitutes differentiation markers in the presence of exogenous CMP-N-acetyl neuraminic acid. Blood. 1996;87:5113–5126. [PubMed] [Google Scholar]
- Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron P H, Steele-Mortimer O, Paiement J, Bergeron J J, Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- Perez H D, Elfman F, Lobo E. Removal of human polymorphonuclear leukocyte surface sialic acid inhibits reexpression (or recycling) of formyl peptide receptors. A possible explanation for its effect on formyl peptide-induced polymorphonuclear leukocyte chemotaxis. J Immunol. 1987;139:1978–1984. [PubMed] [Google Scholar]