Abstract
Kaposi’s sarcoma (KS)-associated herpesvirus is associated with the proliferative/malignant disorders KS, primary effusion lymphoma (PEL), and multicentric Castleman’s disease (MCD) in patients with AIDS. In spite of recent advances in the treatment of KS, PEL and MCD represent therapeutic challenges. Recent advances in dissecting the pathogenesis of these diseases have indicated that the viral cytokine IL-6 and the cellular cytokines/growth factors IL-10, IL-6, stromal cell-derived factor 1, and vascular endothelial growth factor are important contributors to the growth, survival, and spread of PEL and MCD and are therefore potential targets for drug development.
Keywords: primary effusion lymphoma, multicentric Castleman’s disease, angiogenesis, cell migration
INTRODUCTION
The introduction of highly active antiretroviral treatment (HAART) has significantly extended the survival of patients with AIDS, but as more patients with AIDS succumb to cancer rather than from infectious complications, there is a need for innovative approaches in the treatment of AIDS-associated malignancies.
Primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD) are distinct B cell lineage malignancies associated with Kaposi’s sarcoma-associated herpesvirus (KSHV) infection in patients with AIDS. KSHV (also named human herpesvirus 8) is a γ-herpesvirus discovered in 1994 in association with KS tissues [1]. It has structural similarity to EBV and herpesvirus saimiri, and besides its association with KS, PEL and MCD are detected in a small proportion of normal individuals [2]. PEL is a large-cell immunoblastic lymphoma, which usually presents as an effusion malignancy in the body cavities [3,4,5]; MCD is a devastating lymphoproliferative disorder, which characteristically presents with severe general symptoms and lymphadenopathy [6, 7]. Typically, PEL is latently infected with KSHV [1] and is often coinfected with EBV, which contributes to KSHV persistence in PEL [8, 9]. Instead, most MCD lesions are EBV-negative, and there is evidence of KSHV reactivation and replication, particularly during clinical exacerbations [10, 11]. KSHV plays a pathogenetic role in PEL and MCD through expression of the latency-associated nuclear antigen (LANA), viral G-protein-coupled receptor, viral (v)FLICE-inhibitory protein, viral cyclin, and other gene products that variously perturb cell division and survival [12].
PEL cells are of B cell lineage but lack the phenotypic markers of mature B cells while expressing CD138 (syndecan-1) and multiple myeloma 1/IFN regulatory factor 4, which are typical of plasma cells [13,14]. Unlike PEL, KSHV-infected cells in MCD express a high level of IgMλ and CD20 but do not express CD138 [15, 16]. PEL cells have been successfully propagated in vitro and represent a unique source of KSHV-infected cells, whereas no KSHV-infected cell line has yet been derived from MCD tissues.
Despite treatment with high-dose chemotherapy and other drugs, PEL and MCD usually display aggressive clinical courses, with median survival times of approximately 6 months from diagnosis for PEL and 12 months for MCD [7, 17, 18]. In this review, we will summarize the current understanding of the role played by the viral cytokine IL-6 and the cellular chemokine receptor CXCR4 in the pathogenesis of the KSHV-associated malignancies PEL and MCD, with a particular focus on how this information could be translated into novel therapies for these lethal diseases.
STRUCTURAL AND FUNCTIONAL CHARACTERISTICS OF vIL-6
vIL-6, a cytokine product of KSHV that is encoded at open reading frame K2, displays ∼25% amino acid identity to cellular IL-6 [19, 20]. It was originally identified in KSHV-infected PEL cells, where it is constitutively expressed to a limited degree and to a much greater extent when viral replication is induced [19].
The IL-6 family of cytokines exerts its biological activities through a receptor complex composed by a nonsignaling receptor, which is cytokine-specific, and by the signal-transducing chain gp130 [21]. Use of the gp130 common transducer by all members of the IL-6 cytokine family explains the overlapping biological activities of these cytokines [21]. Cellular IL-6 binds to the specific receptor subunit, the IL-6R, and the complex of IL-6/IL-6R associates with gp130, promoting its homodimerization and signaling. Extensive structural studies have revealed that human (hu)IL-6 forms an antiparallel 4-helix bundle and have mapped the contact points of huIL-6 with IL-6R and gp130 [22, 23].
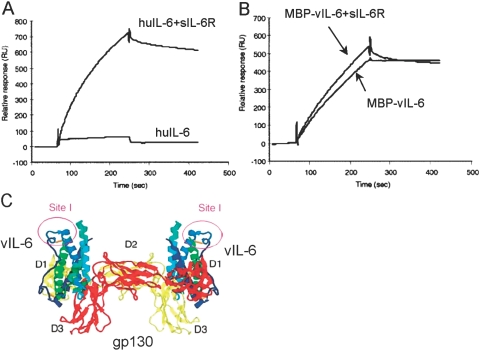
vIL-6 differs from other IL-6 family members in that it can directly bind to gp130 and promote intracellular STAT/MAPK signaling without a requirement for IL-6R or another nonsignaling subunit [24, 25]. By ELISA, we found that vIL-6 can bind to gp130 directly, and by plasmon surface resonance, we measured that vIL-6 binding to gp130 displays an affinity that is ∼1000-fold lower than that of the huIL-6/IL-6R complex [24] (Fig. 1). Certain studies, including our own [24, 26], have provided evidence that IL-6R plays no role in vIL-6 signaling, but others have shown that vIL-6 can signal through gp130, with or without IL-6R [27]. Studies in vitro have demonstrated that vIL-6 displays similar biological activities to those of huIL-6, including its ability to promote the proliferation of the murine hybridoma B9 and human myeloma INA-6 cell lines and to induce IgM secretion from the SKW6.4 human B cell line, but is ∼1000-fold less potent than huIL-6. In this regard, recent studies have provided evidence that vIL-6 is largely an intracellular cytokine, which is inefficiently secreted but can be biologically active intracellularly [28].
Fig. 1.
Properties of vIL-6 binding to gp130. (A) Binding of huIL-6 (50 mg/ml) to immobilized, soluble gp130, with or without soluble IL-6R (sIL-6R), analyzed by plasmon resonance (BIAcore 2000 system). (B) Binding of recombinant (Escherichia coli-derived), purified maltose-binding protein (MBP)-vIL-6 to gp130. (C) Schematic representation of the vIL-6/gp130 complex based on crystallographic analyses. gp130 (red and yellow) binds to vIL-6 helices in a tetrameric complex. Site I denotes an epitope recognized by a vIL-6-neutralizing mAb that we have generated.
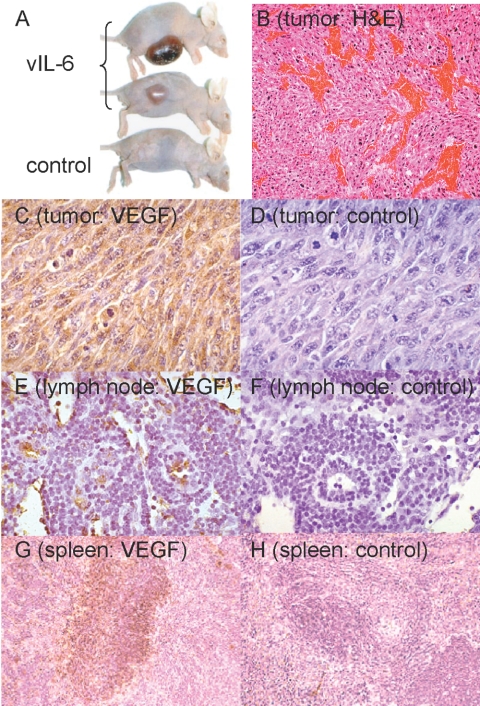
To better assess the biological activities of vIL-6 as a viral cytokine produced by KSHV-infected cells in the context of PEL and MCD, we injected NIH3T3 cells stably expressing vIL-6 in athymic mice [29]. Expression of vIL-6 promoted tumor growth and mouse cachexia, and a direct relationship was found between the levels of vIL-6 expression by the NIH3T3 cells and their growth as s.c. tumors in athymic mice. Histologically, vIL-6-producing tumors were significantly more vascular than controls, and this coincided with a significantly greater expression of vascular endothelial growth factor (VEGF) in the tumor tissues (Fig. 2). Not only did vIL-6-expressing tumor tissues express higher levels of VEGF, but also, lymph nodes and spleen of the tumor-bearing mice contained higher levels of VEGF, which we detected by immunocytochemistry [29]. This result is consistent with a systemic effect of vIL-6, and indeed, we detected circulating vIL-6 in mice bearing vIL-6-expressing tumors. Additional effects attributable to circulating vIL-6 included the occurrence of plasmacytosis in the spleen and lymph nodes and polyclonal hyper-γ-globulinemia. These results indicated that vIL-6 is a multifunctional cytokine that promotes tumor angiogenesis and tumor growth, at least in part by inducing VEGF expression, and induces plasmacytosis in lymphoid organs. Recent experiments have confirmed that vIL-6 can promote VEGF expression and additionally described that vIL-6 induces the VEGFRs-1 and -2 and angiopoietin-2 [30]. Cellular IL-6 has similar biological activities, a result that is consistent with vIL-6 and cellular IL-6 signaling through the common gp130 signal transducer [21, 31, 32].
Fig. 2.
Tumorigenicity of NIH3T3 cells overexpressing vIL-6 in immunodeficient mice. (A) Representative tumors in nude mice 4 weeks after they were injected s.c. with vIL-6-NIH3T3 cells. Control NIH3T3 and vIL-6-NIH3T3 cells were injected at 0.5 × 106 cells/mouse (BALBc nude). (B) Increased vascularization in vIL-6-NIH3T3 tumors. Representative image from tumor tissue stained with H&E (original magnification, ×100). (C, E, and G) VEGF visualized by immunohistochemical staining in tumor tissue (original, ×40), lymph node (original, ×200), and spleen (original, ×100), respectively. (D, F, and H) Control immunostaining of tumor tissue, lymph node, and spleen, respectively.
POTENTIAL CONTRIBUTIONS OF vIL-6 AND OTHER CYTOKINES TO MCD AND PEL PATHOGENESIS
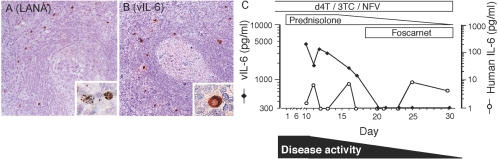
Immunohistochemical analysis of MCD lesions revealed that KSHV-infected cells, which we detected by their expression of the viral nuclear antigen LANA, also expressed vIL-6 [33]. Indeed, most cells that were positive for LANA were also positive for vIL-6 (Fig. 3). In addition, we found that most sera from AIDS patients with MCD contained measurable levels of vIL-6 [34].
Fig. 3.
vIL-6 detection in AIDS-associated MCD tissue and blood. Immunohistochemical detection of KSHV LANA (A) and vIL-6 (B) in the mantle zone of a lymph node affected with MCD (original magnification, ×200). The characteristic, nuclear-speckled staining of LANA and the cytoplasmic staining of vIL-6 are shown in the insets (original, ×1000). (C) Serum IL-6 and vIL-6 levels in the circulation of a patient with AIDS-MCD during an active phase of the disease and subsequent clinical remission. Antiretroviral therapy was with stavudine (d4T), lamivudine (3TC), and nelfinavir (NFV); prednisolone was added at the outset of general symptoms and tapered; the antiviral drug foscarnet was added as the clinical symptoms subsided.
To evaluate the potential contribution of vIL-6 to MCD exacerbations, we examined vIL-6 levels in sequential serum samples from a patient who presented with acute symptoms of the disease and went into clinical remission on high-dose prednisone treatment. We found that vIL-6 was first detected at 4756 pg/ml and subsequently declined and remained undetectable (less than 300 pg/ml), coincident with the decline of clinical symptoms and achievement of clinical remission. By contrast, levels of huIL-6 fluctuated in the patient’s serum without a temporal correlation with disease activity (Fig. 3). These observations indicated that MCD reactivation in an AIDS patient is associated with a selective increase in vIL-6 expression. Other studies have provided evidence that MCD exacerbations are associated with evidence of increased KSHV replication, which promotes vIL-6 expression [10, 35]. A prominent symptom of MCD activity is fever. Cellular IL-6 is a well-documented, pyrogenic cytokine [36], and vIL-6 is also expected to be pyrogenic. These results suggest that MCD reactivations are accompanied by increased KSHV replication and vIL-6 expression, and that increased vIL-6 contributes to the severe symptoms that characterize disease activity.
The PEL cell lines BC-1, BCBL-1, and BCP-1 secrete vIL-6 (1680–19,200 pg/ml/48 h) when propagated in culture under standard culture conditions (1×106 cell/ml), and vIL-6 levels markedly increase after culture with the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (2630–118,100 pg/ml/48 h). In addition, most PEL body-cavity effusions contain high levels of vIL-6 (1390–66,630 pg/ml). They additionally contain variable amounts of huIL-6 (957–37,494 pg/ml), IL-10 (<8–1,342,318 pg/ml), and VEGF (1133–11,417 pg/ml) [37].
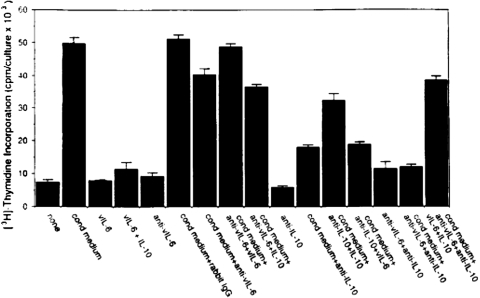
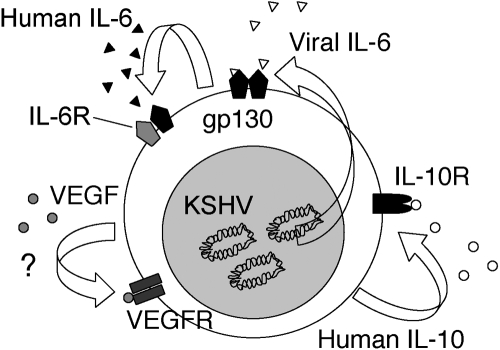
We examined the effects of vIL-6 on PEL cell growth. Using neutralizing antibodies to vIL-6, IL-6, and IL-10, we determined that vIL-6 and to a greater extent huIL-10 serve as autocrine growth factors for the PEL cell lines BCBL-1 and BC-1 but found no evidence that huIL-6 plays such role [38] (Fig. 4). The prominent role of IL-10 in sustaining PEL autocrine growth was confirmed by blocking experiments using a soluble form of the recombinant IL-10R [38]. Other investigators, however, have reported that huIL-6 can also stimulate the growth of selected PEL cells under certain culture conditions and have suggested that the in vitro, antiproliferative effects that the drug rapamycin exerts on PEL cells are largely attributable to its inhibition of autocrine growth factor activity by IL-6 and IL-10 [39, 40]. VEGF, secreted at biologically active concentrations by PEL cell lines, has no direct, biological activity on the PEL cells, which do not express the principal VEGF signaling receptor VEGFR2 and variably express VEGFR1. Thus, PEL cells generally secrete IL-10, IL-6, vIL-6, and VEGF; IL-10 and vIL-6 exert autocrine growth factor activity in PEL (Fig. 5).
Fig. 4.
Effects of vIL-6 and IL-10 neutralization on PEL cell proliferation. BCBL-1 PEL cells were cultured for 3 days, with or without 25% autologous conditioned medium alone or with anti-vIL-6 IgG, control IgG, mAb to IL-10, or a combination of anti-vIL-6 and anti-IL-10 antibodies. Cell proliferation was measured by radioactive thymidine uptake.
Fig. 5.
Schematic representation of autocrine growth factor activity in PEL. KSHV-encoded vIL-6, cellular IL-10, IL-6, and VEGF are constitutively expressed and secreted in the culture supernatants of PEL cells. Cellular IL-10 and vIL-6 bind to the IL-10R and gp130, respectively, activate STAT3, and promote PEL cell growth.
The biological activity of PEL-derived VEGF is quite evident when PEL cells are inoculated in the peritoneal cavity of recipient, immunodeficient mice. In this experimental model, the i.p. injection of the PEL cell line BCBL-1 (10×106 cells) into SCID/beige mice is rapidly followed (5–10 days) by the development of a lymphomatous effusion, which resembles human PEL. When we treated groups of mice with neutralizing antibodies specific for human VEGF, beginning 2 days after injection of the PEL cells and continuing twice/week for 4 weeks, we found that accumulation of ascites was prevented in all of the treated mice [41]. This experiment demonstrated that VEGF produced by PEL cells plays a critical role as an inducer of vascular permeability, resulting in the accumulation of body-cavity effusions, and is consistent with the detection of VEGF in human PEL effusions [34] and in effusions accompanying other malignancies [42, 43]. In conjunction with its well-documented activity as a principal promoter of neovascularization, VEGF is responsible for the increased permeability that accompanies many forms of angiogenesis, particularly tumor angiogenesis [44].
Noteworthy, treatment with anti-human VEGF antibodies did not eradicate PEL from the mice injected i.p. with BCBL-1 cells, as a number of mice had evidence of PEL cell dissemination to the blood and distant sites [41]. This result is consistent with the experiments in vitro showing that PEL cells do not express VEGF receptors and fail to respond to VEGF [41] and provides evidence that the beneficial effects of VEGF neutralization derive from blocking host vascular hyperpermeability.
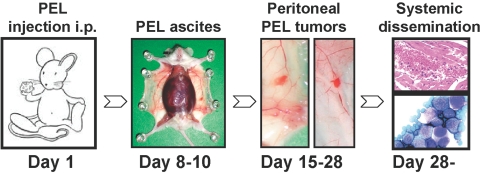
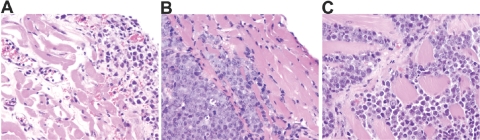
Recently, we have analyzed the pattern of PEL dissemination in a new mouse model of PEL (unpublished results). Immunodeficient NOD/SCID mice inoculated i.p. with BC-1 cells (20×106 cells/mouse) usually develop a progressive, lymphomatous ascite, which is visible on inspection 8–10 days after injection and is documented by recovery of PEL cells from the peritoneal fluid (Fig. 6). At autopsy, 3–4 weeks after PEL cell injection, 50–75% of the animals display a visible tumor mass growing from the parietal and/or visceral mesothelial layer of the peritoneal cavity, which is histologically consistent with PEL (Fig. 7A). This is accompanied by evidence of thickening of the diapham, which is infiltrated with PEL (Fig. 7B), PEL infiltration of the abdominal muscles (Fig. 7C), and colonization to distant sites (Fig. 6). We reasoned that PEL transition from a localized effusion malignancy to a disseminated lymphoma compromising essential body functions is critical to disease progression and patient demise.
Fig. 6.
Schematic representation of a PEL mouse model. Immunodeficient NOD/SCID mice are injected i.p. with PEL cells (BC-1 cells, 20×106/mouse) on Day 1. On Days 8–10, the mice develop a lymphomatous ascite; in the example shown, the peritoneal cavity is closed. On Days 15–18, 50–75% of the mice develop visible tumors attached to the peritoneal mesothelium. From Day 29 on, most mice have evidence of PEL dissemination outside the peritoneal cavity. In the example shown, PEL cells are identified microscopically under the renal capsule and in the blood.
Fig. 7.
Microscopic evidence of PEL dissemination in a murine model of the disease. Immunodeficient NOD/SCID mice were injected i.p. with PEL cells (BC-1 cells, 20×106/mouse) and were killed on Days 21–30. PEL infiltration of the peritoneal mesothelium (A), the diaphram (B), and the abdominal muscles (C) is visualized by microscopic morphology. Tissue sections were stained with H&E; original magnification, ×20.
The chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1 (SDF1) play important roles in mediating directed cell migration and transmigration and have been implicated in promoting tumor cell metastasis [45]. The peritoneal mesothelium is a rich source of SDF1 [46], and we found that PEL cells express the SDF1 receptor CXCR4 [47]. Therefore, we investigated whether SDF1/CXCR4 may contribute to PEL migration and attachment, which appear critical to PEL dissemination. Using monolayers of endothelial cells, which express cell surface SDF1, we have found that PEL cells can attach under conditions of sheer flow and that this attachment is mediated by the interaction between PEL-derived CXCR4 and endothelial cell surface-derived SDF1 [47]. Furthermore, we have found that endothelial cell surface SDF1 promotes PEL cell transmigration under static conditions [47]. These in vitro experiments suggested that PEL cells are capable of attaching to SDF1-expressing mesothelium by virtue of their surface expression of CXCR4, even in the course of transient contact with this surface expected from PEL cells floating in the ascites. As previous studies have shown that SDF1/CXCR4 signaling activates a cascade-type process leading to increased adhesiveness of integrins VLA-4 and LFA to their respective ligands VCAM and ICAM-1 [48, 49], these experiments further suggest that PEL cells could firmly attach to the mesothelium and then transmigrate and leave the peritoneal cavity.
TARGETING vIL-6 AND CELLULAR CYTOKINES FOR THE TREATMENT OF MCD AND PEL
We have developed a series of mAb against vIL-6 [24]. Six of these were characterized in detail. All of these antibodies recognized specifically vIL-6 but not cellular IL-6 in Western blotting and ELISA. In functional studies, we determined that four of the monoclonal did neutralize vIL-6 activity measured in the B9 proliferation assay and the SKW6.4 Ig secretion assay, but they did not neutralize cellular IL-6 activity, also measured in these assays. One of these antibodies (v6m31.2.4) had the highest neutralizing capacity for vIL-6 [24]. Mapping studies using recombinant vIL-6 mutants that we produced indicated that all of the neutralizing antibodies recognize a 13-residue epitope in vIL-6, which includes Asp81 to Cys93 [24]. Structural analyses of vIL-6 binding to gp130 have indicated that this vIL-6 region is not found in the binding interface, suggesting that the neutralizing antibodies may alter the conformation of vIL-6 at the critical binding interface with gp130 [50]. Based on the recent, successful application of monoclonal antibodies (mAb) to the treatment of various human diseases, we believe that vIL-6 neutralization by monoclonal could be tested for the treatment of KSHV diseases, particularly MCD, where the cytokine appears to contribute significantly to disease morbidity.
The viral cytokine vIL-6 and the cellular cytokines/growth factors IL-10, IL-6, and VEGF all induce phosphorylation of STAT3 for intracellular signaling [51,52,53]. PEL cells consistently express a constitutively active STAT3, which is attributable, at least in part, to the autocrine growth factor activities of vIL-6 and IL-10. To target activated STAT3 in PEL cells, we used a dominant-negative form of STAT3, which contains a mutated DNA-binding motif and acts as a competitive inhibitor of wild-type STAT3 [54]. When PEL cells were transfected with this construct, significant apoptosis rapidly ensued. At 3 days after transduction, ∼90% of the PEL cells were dead, and control cells that do not express active STAT3 displayed minimal cell death. Similar PEL cell apoptosis was induced by tyrphostin AG490, a JAK2 inhibitor that was shown to inhibit STAT3 phosphorylation. Levels of AG490-induced apoptosis in PEL cells were markedly reduced by the broad-spectrum caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone, providing evidence for caspase-dependent cell death. Interestingly, we found that inhibition of STAT3 activity by AG490 was associated with decreased levels of the antiapoptotic protein survivin, which inhibits the activity of caspase proteases [55]. By contrast, levels of the other antiapoptotic proteins Mcl-1, Bcl-2, and Bcl-XL were unaffected in PEL treated with AG490. Furthermore, the overexpression of survivin in PEL cells rendered them resistant to apoptosis by AG490. Together, these results provide evidence that STAT3 is a desirable, therapeutic target for PEL [54]. There is much interest in the development of STAT3 inhibitors [56, 57]. Our results indicate that such drugs could be extremely useful in the treatment of PEL.
CONCLUDING REMARKS
The use of HAART aimed at controlling HIV replication and promoting immune reconstitution has been successful at prolonging the survival of patients with AIDS. Treatment of KSHV-induced malignancies is now a challenge, as more patients survive longer. Intense investigations of KSHV-induced malignant transformation and the pathogenesis of KSHV malignancies provided important clues for the design of rational therapies. Viral proteins, such as vIL-6, and cellular cytokines and their receptors have emerged as promising targets for treatment as they contribute to the pathogenesis of PEL and MCD.
References
- Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Lonard B M, Sester M, Sester U, Pees H W, Mueller-Lantzsch N, Kohler H, Gartner B C. Estimation of human herpesvirus 8 prevalence in high-risk patients by analysis of humoral and cellular immunity. Transplantation. 2007;84:40–45. doi: 10.1097/01.tp.0000267158.23795.11. [DOI] [PubMed] [Google Scholar]
- Knowles D M, Inghirami G, Ubriaco A, Dalla-Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–799. [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Nador R G, Cesarman E, Chadburn A, Dawson D B, Ansari M Q, Sald J, Knowles D M. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- Peterson B A, Frizzera G. Multicentric Castleman’s disease. Semin Oncol. 1993;20:636–647. [PubMed] [Google Scholar]
- Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, Cazals-Hatem D, Autran B, Clauvel J P, Raphael M. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10:61–67. [PubMed] [Google Scholar]
- Fan W, Bubman D, Chadburn A, Harrington W J, Jr, Cesarman E, Knowles D M. Distinct subsets of primary effusion lymphoma can be identified based on their cellular gene expression profile and viral association. J Virol. 2005;79:1244–1251. doi: 10.1128/JVI.79.2.1244-1251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Coleman T, Zhang J, Fagot A, Kotalik C, Zhao L, Trivedi P, Jones C, Zhang L. Epstein-Barr virus inhibits Kaposi’s sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J Virol. 2007;81:6068–6078. doi: 10.1128/JVI.02743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandadam M, Dupin N, Calvez V, Gorin I, Blum L, Kernbaum S, Sicard D, Buisson Y, Agut H, Escande J P, Huraux J M. Exacerbations of clinical symptoms in human immunodeficiency virus type 1-infected patients with multicentric Castleman’s disease are associated with a high increase in Kaposi’s sarcoma herpesvirus DNA load in peripheral blood mononuclear cells. J Infect Dis. 1997;175:1198–1201. doi: 10.1086/593567. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- Jarviluoma A, Ojala P M. Cell signaling pathways engaged by KSHV. Biochim Biophys Acta. 2006;1766:140–158. doi: 10.1016/j.bbcan.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Gloghini A, Gattei V, Rossi M F, Cilia A M, Godeas C, Degan M, Perin T, Canzonieri V, Aldinucci D, Saglio G, Carbone A, Pinto A. Association of Kaposi’s sarcoma-associated herpesvirus-positive primary effusion lymphoma with expression of the CD138/syndecan-1 antigen. Blood. 1997;90:4894–4900. [PubMed] [Google Scholar]
- Carbone A, Gaidano G, Gloghini A, Larocca L M, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1998;91:747–755. [PubMed] [Google Scholar]
- Goedert J J. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin Oncol. 2000;27:390–401. [PubMed] [Google Scholar]
- Corbellino M, Bestetti G, Scalamogna C, Calattini S, Galazzi M, Meroni L, Manganaro D, Fasan M, Moroni M, Galli M, Parravicini C. Long-term remission of Kaposi sarcoma-associated herpesvirus-related multicentric Castleman disease with anti-CD20 monoclonal antibody therapy. Blood. 2001;98:3473–3475. doi: 10.1182/blood.v98.12.3473. [DOI] [PubMed] [Google Scholar]
- Simonelli C, Spina M, Cinelli R, Talamini R, Tedeschi R, Gloghini A, Vaccher E, Carbone A, Tirelli U. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol. 2003;21:3948–3954. doi: 10.1200/JCO.2003.06.013. [DOI] [PubMed] [Google Scholar]
- Waterston A, Bower M. Fifty years of multicentric Castleman’s disease. Acta Oncol. 2004;43:698–704. doi: 10.1080/02841860410002752. [DOI] [PubMed] [Google Scholar]
- Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- Heinrich P C, Behrmann I, Haan S, Hermanns H M, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger M J, Chow D C, Brevnova E E, Garcia K C. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Narazaki M, Kishimoto T, Tosato G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpesvirus. Blood. 2001;98:3042–3049. doi: 10.1182/blood.v98.10.3042. [DOI] [PubMed] [Google Scholar]
- Chen D, Nicholas J. Structural requirements for gp80 independence of human herpesvirus 8 interleukin-6 (vIL-6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL-6. J Virol. 2006;80:9811–9821. doi: 10.1128/JVI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molden J, Chang Y, You Y, Moore P S, Goldsmith M A. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL- 6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272:19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- Hu F, Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J Virol. 2006;80:10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads M B, Medveczky P G. Kaposi’s sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: evidence for a unique autocrine signaling mechanism. J Biol Chem. 2004;279:51793–51803. doi: 10.1074/jbc.M407382200. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Jaffe E S, Chang Y, Jones K, Teruya-Feldstein J, Moore P S, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–4043. [PubMed] [Google Scholar]
- Vart R J, Nikitenko L L, Lagos D, Trotter M W, Cannon M, Bourboulia D, Gratrix F, Takeuchi Y, Boshoff C. Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 2007;67:4042–4051. doi: 10.1158/0008-5472.CAN-06-3321. [DOI] [PubMed] [Google Scholar]
- Tosato G, Seamon K B, Goldman N D, Sehgal P B, May L T, Washington G C, Jones K D, Pike S E. Monocyte-derived human B-cell growth factor identified as interferon- β 2 (BSF-2, IL-6) Science. 1988;239:502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem L W, Neufeld G, Levi B Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Tosato G, Fonville T W, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman’s disease. Blood. 2001;97:2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Tosato G, Nambu Y, Iwamoto A, Yarchoan R. Detection of vascular endothelial growth factor in AIDS-related primary effusion lymphomas. Blood. 2000;95:1109–1110. [PubMed] [Google Scholar]
- Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel J P, Agbalika F. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood. 2000;96:2069–2073. [PubMed] [Google Scholar]
- Kluger M J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Yarchoan R, Braun J, Iwamoto A, Tosato G. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood. 2000;96:1599–1601. [PubMed] [Google Scholar]
- Jones K D, Aoki Y, Chang Y, Moore P S, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- Asou H, Said J W, Yang R, Munker R, Park D J, Kamada N, Koeffler H P. Mechanisms of growth control of Kaposi’s sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- Sin S H, Roy D, Wang L, Staudt M R, Fakhari F D, Patel D D, Henry D, Harrington W J, Jr, Damania B A, Dittmer D P. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109:2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94:4247–4254. [PubMed] [Google Scholar]
- Dvorak H F, Sioussat T M, Brown L F, Berse B, Nagy J A, Sotrel A, Manseau E J, Van de Water L, Senger D R. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Ferrara N, Jaffe R B. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153:1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H F, Detmar M, Claffey K P, Nagy J A, van de Water L, Senger D R. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107:233–235. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan M E, McClanahan T, Murphy E, Yuan W, Wagner S N, Barrera J L, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Foussat A, Balabanian K, Amara A, Bouchet-Delbos L, Durand-Gasselin I, Baleux F, Couderc J, Galanaud P, Emilie D. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur J Immunol. 2001;31:350–359. doi: 10.1002/1521-4141(200102)31:2<350::aid-immu350>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yao L, Salvucci O, Cardones A R, Hwang S T, Aoki Y, De La Luz Sierra M, Sajewicz A, Pittaluga S, Yarchoan R, Tosato G. Selective expression of stromal-derived factor-1 in the capillary vascular endothelium plays a role in Kaposi sarcoma pathogenesis. Blood. 2003;102:3900–3905. doi: 10.1182/blood-2003-02-0641. [DOI] [PubMed] [Google Scholar]
- Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabovsky V, Feigelson S, Chen C, Bleijs D A, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, Lobb R R, Alon R. Subsecond induction of α4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D, He X, Snow A L, Rose-John S, Garcia K C. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291:2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Gu X, Tsai N T, Venema R C, Brooks S E, Marrero M B, Caldwell R B. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem. 2000;275:33189–33192. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- Mullberg J, Geib T, Jostock T, Hoischen S H, Vollmer P, Voltz N, Heinz D, Galle P R, Klouche M, Rose-John S. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol. 2000;164:4672–4677. doi: 10.4049/jimmunol.164.9.4672. [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Feldman G M, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- Reed J C. The survivin saga goes in vivo. J Clin Invest. 2001;108:965–969. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D R, IV, Ren Z, Mandal P K, Cameron A G, Dyer G A, Muranjan S, Campbell M, Chen X, McMurray J S. Investigation of the binding determinants of phosphopeptides targeted to the SRC homology 2 domain of the signal transducer and activator of transcription 3. Development of a high-affinity peptide inhibitor. J Med Chem. 2005;48:6661–6670. doi: 10.1021/jm050513m. [DOI] [PubMed] [Google Scholar]