Abstract
Tumor cell-associated chemokine receptors play distinct roles in cancer biology, including enhancement of lymph node (LN) metastasis. To determine if CCR7 influences tumor formation in skin, we inoculated B16 cells transduced with CCR7 and luciferase (CCR7-luc-B16) or with retroviral vector and luciferase (pLNCX2-luc-B16) into ear skin and footpads of wild-type (WT) mice. In contrast to pLNCX2-luc-B16 cells, 97% of CCR7-luc-B16 cell-inoculated mice formed skin tumors as well as cervical LN metastases by Day 21 following ear inoculation. CCR7-expressing and control B16 cells, however, formed tumors of similar size and with high-efficiency in SCID-beige mice. Cells from both lines accumulated in the skin of WT mice in similar numbers until Day 7. By Day 11, however, control cells decreased tenfold, whereas CCR7-luc-B16 cells formed small tumor nodules. Tumor cells were infrequently detected in draining cervical LNs up to 11 days after injection of both cell lines, but stable nodal metastases were only observed after CCR7-luc-B16 ear tumors had been established (∼Day 21). ELISPOT assays revealed that IFN-γ-producing cells in draining LNs from CCR7-luc-B16-injected ears were reduced through Day 7. After footpad injection, tumor formation by CCR7-expressing B16 cells was enhanced only with small, initial tumor cell inocula. With larger inocula, tumor formation was equivalent, but the numbers of tumor-infiltrating leukocytes were reduced by approximately sixfold in CCR7-B16 tumors compared with pLNCX2-B16 tumors of equal size. IFN-γ and CXCL10 were reduced 35- and sixfold, respectively, in CCR7-B16 cell tumors (vs. control tumors). Thus, CCR7 expression enhances tumorigenesis in addition to facilitating LN metastasis.
Keywords: chemokine, tumor immunology
INTRODUCTION
G-protein-coupled chemokine receptors are up-regulated in many human cancers and have been demonstrated to play nonredundant roles in metastasis by regulating cancer cell trafficking and survival [1]. Lymph nodes (LNs) are a common site of cancer metastasis, and several chemokine receptors, including CCR7, have been reported to influence the ability of cancer cells to metastasize to these secondary lymphoid organs [2,3,4]. Nodal metastasis is an important phenomenon in cancer biology, as it has been highly associated with poor prognosis in many common cancers, including melanoma [5,6,7].
CCR7 is a chemokine receptor notable for its role in influencing the migration of mature dendritic cells (DC) and specific subsets of T cells to LN [8, 9]. Clinical studies have indicated that multiple types of cancer cells, including those of the breast, stomach, lung, cervix, and melanoma, may express CCR7 and that expression is correlated with nodal metastasis and poor patient survival. In breast cancer, for example, LN-positive tumors expressed CCR7 nearly three times as frequently as LN-negative tumors [5]. In gastric cancer, CCR7-positive tumors were associated with frequent LN metastasis, greater lymphatic invasion, and poor surgical outcomes [10].
We have reported that CCR7-overexpressing B16 melanoma cells (CCR7-B16 cells) metastasized with much higher efficiency (compared with control B16 cells) to draining LN [3]. Moreover, inhibition of CCL21, a CCR7 ligand synthesized predominantly by lymphatic endothelial cells and stromal cells in LN with neutralizing antibodies, blocked LN metastasis following inoculation of CCR7-B16 cells in peripheral tissues [3]. These results suggested that the expression of CCR7 was sufficient to experimentally augment nodal metastasis by melanoma cells.
Although it is clear that expression of CCR7 enhances nodal metastasis, it is unclear whether it directly influences tumor formation in mice with normal immune systems. Because unmanipulated B16 cells are normally poorly immunogenic, we have augmented the immunogenicity of parental B16 cells in this and other studies [4, 11] by retroviral transduction of B16 cells with luciferase and other foreign genes present in commercial retroviral vectors. Our previous studies indicated that CCR7-B16 cells formed tumors of similar size compared with control B16 cells, when relatively large numbers of B16 cells were inoculated into the footpad [3], but the ability of CCR7 to regulate tumor formation was not tested with inoculation of suboptimal numbers of tumor cells. To address this question, we inoculated varying numbers of CCR7-B16 cells in ear skin or footpads of C57BL/6 wild-type (WT) mice and monitored tumor growth, nodal metastases, and the ability of LN cells to produce IFN-γ in response to tumor cells over time. Interestingly, under cell number-limiting conditions, CCR7 expression enhanced tumor formation in ear skin as well as in the footpad. Moreover, CCR7-expressing B16 tumors expressed significantly less IFN-γ as well as CXCL10, an IFN-γ-regulated chemokine. These studies indicate that CCR7 expression by tumor cells in vivo can influence tumorigenesis as well as the expression of cytokines and chemokines within the tumor microenvironment.
MATERIALS AND METHODS
Animals and cell lines
Female C57BL/6 mice and SCID-beige mice (8–12 weeks old) were purchased from the National Cancer Institute (NCI) Animal Production Colony (Frederick, MD, USA) and used in accordance with the guidelines of the NCI Animal Use and Care Committee. Syngeneic B16/F1 melanoma cells were grown in DMEM (Invitrogen, Carlsbad, CA, USA) with 10% heat-inactivated FBS and supplements as described [4].
Retroviral transduction of B16/F1 melanoma cells
B16/F1 melanoma cells were first retrovirally transduced with cDNA for murine (m)CCR7 (CCR7-B16 cells) or with empty vector (pLNCX2-B16 cells) alone using a pLNCX2 vector (Clontech, Mountain View, CA, USA) as described previously [3]. In some experiments, pLNCX2- and CCR7-B16 cell lines were then transduced with firefly luciferase cDNA using an alternative, retroviral plasmid murine stem cell virus vector (Clontech) [4]. These B16 cell lines are hereby referred to as CCR7-luc-B16 and pLNCX2-luc-B16 cell lines, respectively. Examination of proliferation rates (by serial cell counting over several days) and flow cytometry-based cell-cycle kinetics (BD Biosciences, San Jose, CA, USA) revealed no significant differences between the CCR7- and CCR7-luc-B16 cell lines and their respective control B16 cells lines.
s.c. inoculation of transduced cell lines
The cell lines described above were harvested in exponential growth phase by trypsinization and washed in PBS before injection. For footpad injections, cells [4×105 in 20 μl PBS (or as indicated in figures)] were injected into the left hind footpads. For ear injections, cells (1×105 in 20 μl PBS) were injected into the s.c. space under the central dorsal surface of the ears immediately above the cartilage. Tumor growth was measured with a caliper. Tumor thickness in the footpad was evaluated by measuring the maximal depth of the tumor within the footpad (mm) and then subtracting the baseline foot thickness of the contralateral, noninjected footpad of the same mouse. Tumor size was then approximated by multiplying tumor depth, maximal tumor width, and maximal perpendicular length. For LN metastasis analysis, draining cervical LNs (for ear injection) or draining popliteal LNs (for footpad injection) were harvested, scored by inspection, photographed, and in some cases, assayed for luciferase activity in vitro.
In vitro luciferase activity assay
pLNCX2-luc-B16 cells or CCR7-luc-B16 cells (1×105 per injection) were inoculated into ear skin of C57BL/6 WT mice, which were euthanized at different time-points, and the injected ears were excised and minced in 500 μl Glo lysis buffer (Promega, Madison, WI, USA) using scissors and the back of a syringe. Draining cervical LNs were collected and minced in 400 μl Glo lysis buffer. Luciferase activity was determined using a microplate luminometer (Turner BioSystems, Sunnyvale, CA, USA) and converted into total cell numbers based on the corresponding standard curves.
ELISPOT assay for IFN-γ-producing cells in draining cervical LNs
ELISPOT reagents for detecting mIFN-γ were purchased from BD Biosciences. For ELISPOT assays, pLNCX2-luc-B16 or CCR7-luc-B16 cells (1×105 per injection) were inoculated into ear skin of C57BL/6 WT mice. At different time-points, draining cervical LNs from injected ears and contralateral cervical LNs from uninjected ears were harvested. Two or three LNs from the same group were randomly pooled together and considered as one sample. LN cells (1×106) were stimulated with 104 corresponding B16 cells in a precoated ELISPOT plate (Millipore, Bedford, MA, USA) for 24 h. Spots were counted using an ELISPOT analyzer (CTL, Cleveland, OH, USA), and the mean spot size was calculated by the same software.
Analysis of tumor-infiltrating leukocytes within footpad tumors
pLNCX2-B16 cells or CCR7-B16 cells (4×105) were inoculated into the left hind footpad of C57BL/6 WT mice. Three weeks later, tumors were excised, minced, and incubated in B16 culture medium containing 1 mg/ml collagenase D (Roche, Indianapolis, IN, USA) and 0.5 mg/ml DNase I (Sigma Chemical Co., St. Louis, MO, USA) at 37°C for 45 min. Tumor pieces were then gently separated using the back of the syringe and filtered through a 100-μm spleen filter. Single cell suspensions were washed twice with FACS buffer (PBS+1% BSA+2.5 mM EDTA) before use. Tumor cells were stained with FITC-CD45 (BD Biosciences) to distinguish tumor-infiltrating leukocytes from tumor cells or stromal cells. FITC-CD45 staining was combined with PE-CD4, PE-CD8, PE-GR1, or PE-B220 (BD Biosciences) to define different cell populations. Samples were collected with FACSCalibur (BD Biosciences) and analyzed using CellQuest software (BD Biosciences).
Quantitative (q)RT-PCR SuperArray analysis
Murine inflammatory cytokine and receptor PCR array plates were purchased from SuperArray Bioscience (Frederick, MD, USA). This plate includes primer sets for 84 key genes involved in the inflammatory response, including chemokines, cytokines, and their cognate receptors. Single cell suspensions of pLNCX2-B16 or CCR7-B16 footpad tumors were prepared as mentioned above. Total RNA was extracted from tumor cells using the RNeasy kit (Qiagen, Valencia, CA, USA), and 5 μg total RNA was converted into cDNA using the SuperScript II First-Strand synthesis kit (Invitrogen). RT-PCR was performed on array plates according to the manufacturer’s protocol (SuperArray Bioscience). This experiment was repeated three times.
Depletion of CD45+-infiltrating leukocytes from footpad tumors and qPCR methods
Single cell suspensions of pLNCX2-B16 or CCR7-B16 footpad tumors were treated with anti-CD45 magnetic beads (Miltenyi Biotec, Auburn, CA, USA), washed, and then depleted of CD45+ cells by placement through a magnetic separation column. Complete depletion of CD45 (>99%) was confirmed by FACS staining with FITC-CD45 antibody. Total RNA was extracted from tumor cells collected before and after CD45 depletion, and cDNA was synthesized as described above. RT-PCR was performed in a Chromo4 RT-PCR machine (Bio-Rad, Hercules, CA, USA). The following primer pairs were used: mGAPDH, forward primer 5′ CGT GTT CCT ACC CCC AAT GT 3′, reverse primer 5′ TGT CAT CAT ACT TGG CAG GTT TCT 3′; mIFN-γ, forward primer 5′ CAG CAA CAG CAA GGC GAA A 3′, reverse primer 5′ CTG GAC CTG TGG GTT GTT GAC 3′; mCXCL9, forward primer 5′ AAG GAA TGC ACG ATG CTC CT 3′, reverse primer 5′ TGA GGT CTT TGA GGG ATT TGT AGT G 3′; mCXCL10, forward primer 5′ CCA GTG AGA ATG AGG GCC ATA G 3′, reverse primer 5′ CTC AAC ACG TGG GCA GGA T 3′. This experiment was repeated three times.
In vitro stimulation of B16 cell lines with IFN-γ and detection of CXCL10 by ELISA
CCR7-B16 or pLNCX2-B16 cells were stimulated with or without plate-bound mCCL21 (R & D Systems, Minneapolis, MN, USA) at 1 μg/ml for 3 h. mIFN-γ at 10 ng/ml or 1 ng/ml (PeproTech, Rocky Hill, NJ, USA) was then added into the cell culture for 24 h. Cell culture supernatants were collected to analyze mCXCL10 secretion by ELISA according to the manufacturer’s recommendations (R & D Systems).
Statistical calculations
Statistical calculations were performed with Student’s t-test using the analysis software Prizm (GraphPad, San Diego, CA, USA). P values less than 0.05 were considered statistically significant.
RESULTS
CCR7-B16 tumor progression in the ear skin
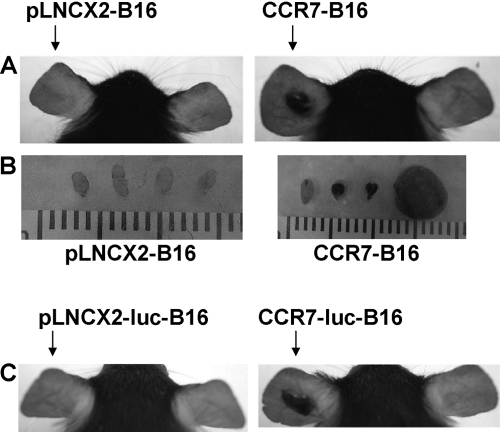
To determine if CCR7 directly influences tumor formation in the skin, we inoculated B16 cells transduced with CCR7 (CCR7-B16) or with an empty retroviral vector (pLNCX2-B16) in ear skin of WT mice. A small minority of WT mice inoculated with pLNCX2-B16 cells formed ear tumors (Fig. 1A and Table 1), which was consistent with our prior findings [4]. Furthermore, the draining cervical LNs were free of visible metastasis (Fig. 1B). By contrast, 97% of the WT mice inoculated with CCR7-B16 cells developed ear tumors within 3 weeks, which were accompanied by cervical LN metastasis in 90% of mice (Fig. 1, A and B, and Table 1). To minimize the possibility that the results above were a result of clonal selection, we repeated these experiments with B16 cells that had been independently transduced with luciferase and CCR7 or appropriate control vector (designated as CCR7-luc-B16 and pLNCX2-luc-B16 cells). Using these independently derived cell lines, we demonstrated that CCR7 expression in these B16 cells resulted in 100% tumor formation compared with 15% tumor formation using pLNCX2-luc-B16 cells (Fig. 1C and Table 1). These outcomes were essentially the same as those described for CCR7- and pLNCX2-B16 cells. Combined with our prior data indicating that parental (untransduced) B16 tumors grew progressively in the ear skin [4], our results suggest that retrovirally transduced B16 cells (regardless of expression of luciferase) do not have the same tumorigenic potential as parental B16 cells. As the CCR7-B16 cells were transduced with the same retroviral vector as the control cells, however, we conclude that the additional expression of CCR7 not only increases LN metastasis but also directly (or indirectly) enhances tumorigenesis in B16 cells.
Fig. 1.
B16 cell tumor formation and LN metastasis following ear skin inoculation. (A) Representative photos of mouse ears from pLNCX2-B16- or CCR7-B16-injected ears (1×105/ear) at Day 20. (B) Representative photos of draining cervical LNs from pLNCX2-B16- or CCR7-B16-injected mice at Day 20 are shown. (C) Representative photos of mouse ears from pLNCX2-luc-B16- or CCR7-luc-B16-injected ears (1×105/ear) at Day 18. Tumor and LN metastasis incidence is reported in Table 1.
TABLE 1.
Incidence of Tumor Formation and LN Metastasis in C57BL/6 WT Mice or SCID-Beige Mice Inoculated with Different Tumor Cell Lines
| Tumor cells | Mice | Tumor incidence (%) | LN metastasis (%) |
|---|---|---|---|
| pLNCX2-B16 | C57BL/6 | 4/30 (13) | 0 |
| CCR7-B16 | C57BL/6 | 29/30 (97) | 90 |
| pLNCX2-luc-B16 | C57BL/6 | 3/20 (15) | |
| CCR7-luc-B16 | C57BL/6 | 20/20 (100) | |
| pLNCX2-B16 | SCID-beige | 10/10 (100) | |
| CCR7-B16 | SCID-beige | 10/10 (100) |
Tumor and/or LN metastasis incidence (pooled from several experiments) was evaluated by visually examining ear and cervical LN (after dissection) from injected mice at Day 18 or 20. LN metastasis was not assessed in WT mice injected with luciferase-expressing B16 cells or in SCID-beige mice.
To determine if a functional immune system regulates B16 tumor growth in the ear, we inoculated CCR7-B16 and pLNCX2-B16 cell lines into ears of SCID-beige mice, which lack T, B, and NK cells. Both B16 cell lines developed ear tumors equally well within 3 weeks (Table 1). Thus, a T, B, or NK cell-dependent mechanism suppressed tumor formation of pLNCX2-B16 cells in WT mice, but this suppression could be overcome by overexpression of CCR7 in tumor cells.
B16 tumor formation in footpads under specific inoculation conditions
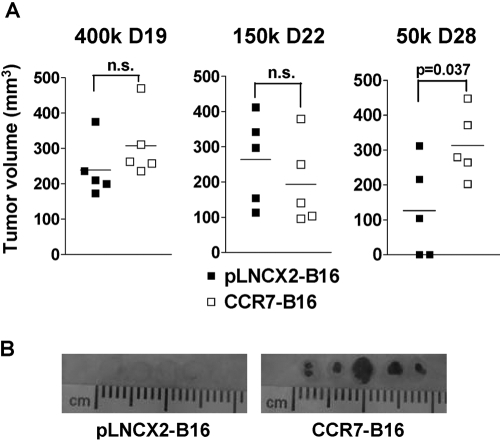
A previous study in our lab showed that CCR7-B16 and pLNCX2-B16 cells formed footpad tumors with similar sizes when mice were inoculated with 4 × 105 cells per footpad [3]. To determine if this result was also true if small tumor cell inocula were used, we injected WT mice with 4 × 105, 1.5 × 105, or 5 × 104 cells per footpad. Although both B16 cell lines formed tumors of similar sizes at the first two inocula, two out of five pLNCX2-B16-injected mice did not form tumors when mice were injected with the lowest inoculum of cells. The remaining three mice developed smaller tumors (on average) than those observed in CCR7-B16-injected mice (Fig. 2A). Yet, at all inoculation doses, only CCR7-B16 tumors metastasized efficiently to draining popliteal LNs (Fig. 2B, and data not shown). Of note, equivalent amounts of CCL21 protein, the ligand for CCR7, were detected by ELISA assays in tumor lysates from similarly sized pLNCX2-B16 and CCR7-B16 footpad tumors (data not shown), indicating that CCL21 was likely to be present in the tumor microenvironment. These results indicated that CCR7 overexpression increases B16 cell tumor formation when small tumor inocula are used.
Fig. 2.
B16 cell tumor formation and LN metastasis following footpad inoculation. (A) Footpad tumor volumes (mm3) were measured by a caliper at the indicated dose and time-point. n.s., Not significant. (B) Representative photos (21 days postinoculation) of draining popliteal LNs from pLNCX2-B16 (left)- or CCR7-B16 (right)-injected footpads (4×105 cells/footpad) are shown. One of four experiments with similar results is shown.
Kinetics of B16 tumor growth in ears and draining cervical LNs
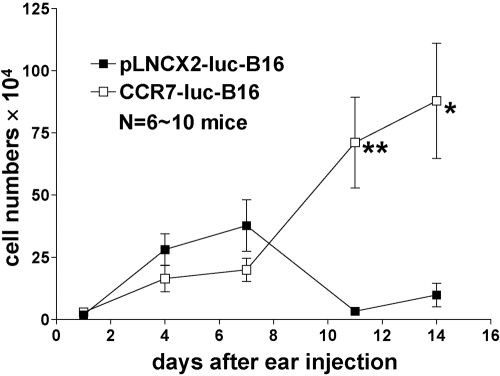
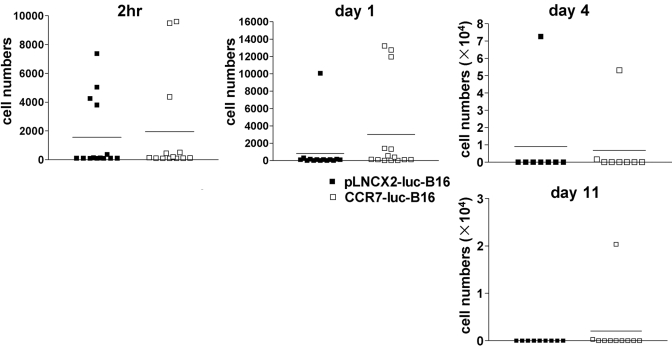
To examine the kinetics of B16 tumor growth in vivo, we inoculated WT mice with pLNCX2-luc-B16 or CCR7-luc-B16 cells, which enabled us to quantify small numbers of tumor cells at the primary inoculation site as well as in draining LNs. At different time-points, we determined luciferase activity in injected ears and draining cervical LNs and then converted luciferase activity into cell numbers based on a standard curve. pLNCX2-luc-B16 and CCR7-luc-B16 cells accumulated in ears in similar numbers up to Day 7, suggesting that overexpression of CCR7 by B16 cells did not convey obvious growth advantages in the first few days after tumor cell implantation. By Day 11, however, the numbers of pLNCX2-luc-B16 cells decreased by tenfold. CCR7-luc-B16 cell numbers, however, continued to increase (Fig. 3) and formed small tumor nodules (data not shown). As early as 2 h after ear inoculation, tumor cells were detected in one-third of cervical LNs draining the ears of mice injected with pLNCX2-luc-B16 or CCR7-luc-B16 cells. Tumor cells, however, were only infrequently detected through Day 11 (Fig. 4). Stable nodal metastases were only observed after CCR7-B16 ear tumors had been established for approximately 3 weeks (Fig. 1B), suggesting that CCR7 does not contribute to early nodal metastasis.
Fig. 3.
Kinetics of B16 tumor growth in ears. Following injection of pLNCX2-luc-B16 or CCR7-luc-B16 cells (1×105 per mouse) into ear skin of WT mice, mice were euthanized, and homogenized ear skin lysates were assessed for luciferase activity, which was converted to total tumor cell numbers using a standard curve. One of three experiments with similar results is shown. In this experiment, the following numbers of mice for each group at each time-point were used: Day 1 (six mice), Day 4 (eight mice), Day 7 (10 mice), Day 11 (10 mice), Day 14 (nine mice). **, P < 0.01; *, P< 0.05.
Fig. 4.
Kinetics of B16 tumor metastasis to draining cervical LNs. pLNCX2-luc-B16 or CCR7-luc-B16 cells (1×105 per mouse) were injected into ear skin of WT mice. At different time-points, the draining cervical LNs were processed to determine the luciferase activity, which was converted to total tumor cell numbers using a standard curve. One of three experiments with similar results is shown.
Lack of early host anti-tumor immune responses in mice inoculated with CCR7-B16 cells
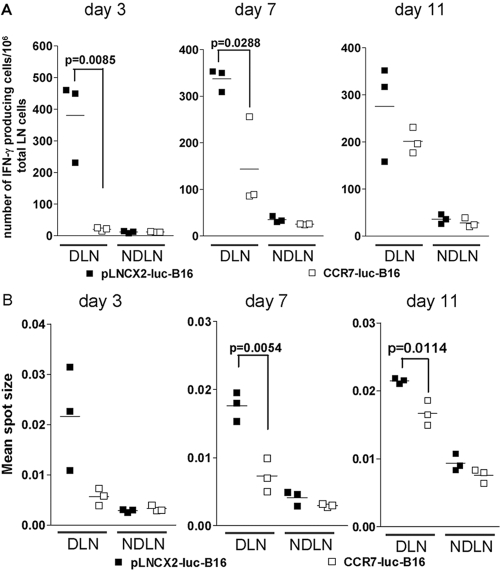
We hypothesized that host anti-tumor immune responses might suppress tumor formation of pLNCX2-B16 but not CCR7-B16 cells. We first focused on draining cervical LNs, where host anti-tumor immune response would be generated after ear injection. As the distributions of CD4, CD8, and NK cells in draining cervical LNs from pLNCX2-luc-B16- or CCR7-luc-B16-injected ears were not significantly different (data not shown), we measured numbers of IFN-γ-producing cells and average IFN-γ production by ELISPOT assay to determine if functional changes were present. Three days after ear inoculation, a robust host anti-tumor immune response was measured in draining cervical LNs from pLNCX2-luc-B16-injected ears. By contrast, the numbers of IFN-γ-producing cells in draining cervical LNs from CCR7-luc-B16-injected ears remained at baseline levels, suggesting an early inhibition of host anti-tumor immune response by CCR7-luc-B16 cells (Fig. 5A).
Fig. 5.
ELISPOT analysis of IFN-γ expression in LN cells from tumor-bearing mice. Number of IFN-γ-producing cells (A) and the average IFN-γ amount secreted by individual cells (B) in draining cervical LNs were determined by ELISPOT assay. Draining cervical LNs (DLN) from injected ears or contralateral cervical LNs (NDLN) from uninjected ears were collected at the indicated time-points after ear inoculation. Two to three LNs from the same group were randomly pooled together and considered as one sample. One million total LN cells were stimulated for 24 h with 104 corresponding B16 cells used for initial injection. Positive spots were analyzed using an ELISPOT analyzer, which also calculated the mean spot size. One of two experiments with similar results is shown.
The average IFN-γ secretion by individual cells, which was reflected by the mean spot size, showed a similar trend (Fig. 5B). By Day 7, the number of IFN-γ-producing cells and mean spot size from draining cervical LNs from CCR7-luc-B16-injected ears were significantly lower than from draining cervical LNs from pLNCX2-luc-B16-injected ears (Fig. 5, A and B). Although the difference in number of IFN-γ-producing cells between the two groups was not significantly different by Day 11, the average IFN-γ produced by individual cells in draining cervical LNs from CCR7-luc-B16-injected ears was still significantly lower compared with draining cervical LNs from pLNCX2-luc-B16-injected ears (Fig. 5, A and B). Over the subsequent 2 weeks, the overall level of host IFN-γ response was decreased in mice injected with either cell line, and no differences in IFN-γ production were observed (data not shown). Thus, early host responses are not observed in mice inoculated with CCR7-expressing B16 cells, raising the possibility that CCR7-B16 cells may be able to suppress initial anti-tumor responses.
Reduction in tumor-infiltrating leukocytes in CCR7-B16 footpad tumors
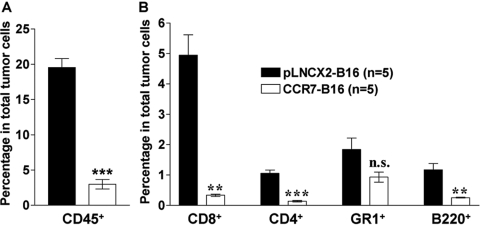
In the footpad injection model, both B16 cell lines developed tumors with similar sizes in footpads when injected at 4 × 105 cells/footpad. However, when we analyzed tumor-infiltrating leukocytes by staining single cell suspension of footpad tumors with anti-CD45 antibody, we found a striking, approximately sixfold reduction in the percentage of CD45+ cells in CCR7-B16 tumors compared with pLNCX2-B16 tumors of equal size (Fig. 6A). Moreover, after gating on the CD45+ population, flow cytometric analysis of tumor-infiltrating leukocytes further revealed striking 15-, seven-, and fourfold reductions in CD8, CD4, and B cells, respectively, within the tumors arising from injection of CCR7-B16 cells (vs. pLNCX2-B16 cells; Fig. 6B), suggesting that there is a global reduction of tumor-infiltrating leukocytes within CCR7-B16 footpad tumors.
Fig. 6.
Reduction in tumor-infiltrating leukocytes in footpad tumors arising from CCR7-B16-injected mice. Single cell suspension of footpad tumors was prepared as described in Materials and Methods. Indicated are percentage of CD45+ cells in total tumor cells and percentage of CD45-expressing CD4+, CD8+, GR1+, and B220+ cells among the total tumor cells. This figure depicts one of four experiments with similar results. **, P < 0.01; ***, P< 0.001.
Down-regulation of IFN-γ and CXCR3 ligands within CCR7-B16 tumors
Tumor-infiltrating immune cells play model-dependent roles during cancer development. Increased infiltration of innate immune cells, including macrophages, mast cells, and neutrophils, often promotes growth or facilitates survival of neoplastic cells and correlates with increased angiogenesis and poor prognosis. By contrast, an abundance of infiltrating lymphocytes, especially cytotoxic tumor-reactive CD8 T cells, commonly correlates with favorable prognosis [12, 13].
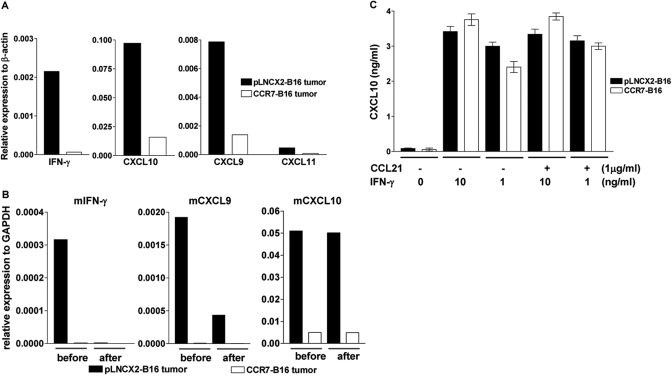
To investigate the underlying mechanisms that contributed to the global reduction of tumor-infiltrating leukocytes within CCR7-B16 tumors, we prepared single cell suspensions of footpad tumors from pLNCX2-B16- and CCR7-B16-injected mice and screened them for differential mRNA expression of a panel of cytokines, chemokines, and their cognate receptors. We performed qRT-PCR on SuperArray plates that include 84 key genes involved in the inflammatory response. Interestingly, we found that expression of several genes, including IFN-γ, CXCL9, CXCL10, and CXCL11, was reduced significantly in a CCR7-B16 tumor microenvironment compared with pLNCX2-B16 tumors (Fig. 7A and Table 2).
Fig. 7.
Down-regulation of IFN-γ and CXCR3 ligands in CCR7-B16 tumors. (A) Footpad tumor cells from pLNCX2-B16- and CCR7-B16-injected mice were screened for differential expression of IFN-γ and CXCL9, -10, and -11 by performing qRT-PCR on SuperArray plates. A more complete listing of up- and down-regulated mRNAs is found in Table 1. (B) qRT-PCR of the indicated cytokines and chemokines was performed on tumor cells (before and after depletion of CD45+ cells by affinity microbeads). (C) In vitro assessment of CXCL10 secretion by B16 cells in response to IFN-γ stimulation was performed by activating B16 cell lines with (or without) immobilized CCL21 (1 μg/ml) for 3 h prior to stimulating with IFN-γ at the indicated concentration. The cell culture supernatants were collected 24 h later to analyze CXCL10 production by ELISA. The figure depicts one of three independent experiments with similar results.
TABLE 2.
Fold Change in CCR7-B16 Versus pLNCX2-B16 Whole Tumor mRNA Expression of the Selected Cytokines, Chemokines, and Receptor Genes by qRT-PCR
| Gene name | Fold change | Gene name | Fold change |
|---|---|---|---|
| CCR7 | 23.6 | CCR2 | −3.2 |
| CCL6 | 4.1 | CCR3 | −6.9 |
| IFN-γ | −35.2 | CCR5 | −11.6 |
| CXCL9 | −5.7 | IL-1β | −4.4 |
| CXCL10 | −6.1 | TNF-α | −3.3 |
| CXCL11 | −6.5 | β 2 integrin | −6.9 |
| CCL3 | −3.6 | IL-2Rβ | −18.2 |
| CCL4 | −5.7 | IL-2Rγ | −13.3 |
| CCL5 | −8.5 | IL-10Rα | −8.1 |
| CCL8 | −9.7 |
CXCL9, CXCL10, and CXCL11 are IFN-γ-inducible chemokines that share CXCR3, a chemokine receptor that is primarily expressed on activated T cells (especially Th1 cells) and NK cells [14]. This finding is consistent with reduced numbers of CD8+ and CD4+ cells found within CCR7-B16 tumors. As tumor-infiltrating leukocytes are major sources of IFN-γ and T cell-trafficking chemokines, it was not clear whether reduced expression of these chemokines was the cause or the outcome of the striking reduction of tumor-infiltrating CD8+ and CD4+ T cells within CCR7-B16 tumors.
To address this question, we depleted CD45+ cells from single cell suspensions of footpad tumors using anti-CD45 magnetic beads, which resulted in >99% depletion of CD45+ cells (data not shown). We then compared expression of IFN-γ, CXCL9, and CXCL10 mRNA from tumor cells prepared before and after CD45 depletion by qRT-PCR. As T cells and NK cells are the only known sources of IFN-γ, it was not surprising that expression of this cytokine was lost upon depletion of the CD45+ leukocyte population. The expression of CXCL9 mRNA was, however, only partially diminished in pLNCX2-B16 tumors, implying tumor-infiltrating leukocytes as well as tumor cells could express CXCL9 mRNA. Interestingly, after CD45+ cell depletion, the expression pattern of CXCL10 did not change compared with untreated samples, suggesting that tumor cells and/or stromal cells, but not tumor-infiltrating leukocytes, were the primary source of CXCL10 production (Fig. 7B).
We next asked if CCR7-B16 cells activated in vitro with CCL21 would down-regulate CXCL10 expression in response to IFN-γ stimulation. pLNCX2-B16 and CCR7-B16 cell lines increased protein expression of CXCL10 as detected by ELISA assay following stimulation with IFN-γ (Fig. 7C). The addition of CCL21 to CCR7-B16 cells did not result in loss of CXCL10 expression in CCR7-B16 cells compared with cells that had not been exposed to CCL21 (Fig. 7C). Similar results were obtained when we assessed mRNA expression of CXCL10 (in response to IFN-γ) by qRT-PCR methods (data not shown). Thus, CCR7 activation did not abrogate the up-regulation of CXCL10 in response to IFN-γ in vitro, suggesting that the down-regulation of CXCL10 in CCR7-B16 footpad tumors was more likely the result of the reduction of IFN-γ in the tumor microenvironment, although we cannot exclude the possibility that CCR7 activation may result in diminished expression of CXCL10 (in the presence of IFN-γ) under conditions present in tumors in vivo.
DISCUSSION
In observing the kinetics and establishment of CCR7-positive and -negative tumors and subsequent skin-draining metastases, we have made a number of findings that refine our view about the role of chemokine receptors in tumorigenesis and nodal metastasis. Consistent with our previous findings [3], CCR7 expression had little impact on the rate or frequency of tumor formation when large numbers of tumor cells were inoculated into mouse footpads. When smaller numbers of tumor cells were used, however, the expression of CCR7 by B16 cells increased tumor formation, particularly in our ear skin injection model. Although in gastric cancer, CCR7 expression has been clinically associated with greater tumor invasion [10], our data are the first to indicate that CCR7 directly influences tumor formation.
We observed that CCR7 expression does not markedly enhance the accumulation of B16 cells in the draining LN within the first few days following skin inoculation. Only several weeks after inoculation (when tumors were grossly visible) were LN metastases evident in CCR7-B16 cell-injected mice, suggesting that tumors must grow large enough to invade lymphatic vessels (or to secrete factors such as vascular endothelial growth factor-C to induce new lymphatic vessel formation [15, 16]) prior to effective dissemination to draining LN. These results are compatible with earlier results demonstrating a high degree of correlation (r=0.976) between B16 melanoma tumor size and regional LN metastasis [17]. Of note, lymphatic clearance of radiolabeled albumin as well as lymphatic vessel diameter also increased with progressive tumor growth [17], perhaps providing an increased likelihood of tumor cells escaping into lymphatic channels and passively flowing to regional LN.
The early accumulation of cancer cells in the LN has been reported to increase the ability of the host animal to mount effective anti-tumor responses [18]. Indeed, the low levels of B16 cell accumulation in cervical LN following ear skin injection that we have observed in other studies [4] appeared to be sufficient to render mice resistant to tumor formation at alternate inoculation sites, unless tumor cells expressed the chemokine receptor CCR10. In those studies, we had found that expression of CCR10 renders B16 cells resistant to killing by gp100 peptide-specific CD8 T cells through activation of PI-3K-dependent pathways [4]. The late LN metastasis that we observed in our current studies may suggest that gross infiltration of LN in human cancers is a relatively late event in cancer progression.
It was intriguing to discover that the number of tumor-infiltrating CD45+ leukocytes, especially CD8+ and CD4+ T cells, was reduced significantly in CCR7-B16 footpad tumors compared with pLNCX2-B16 footpad tumors of similar size. Large numbers of tumor-infiltrating CD8+ T cells are positively correlated with favorable clinical prognosis in cutaneous melanoma [19] and several other types of cancers [20,21,22,23]. Although the increased number of infiltrating leukocytes did not apparently slow tumor formation, the large inoculum of tumor cells used in comparison may have heavily favored tumor formation, despite the presence of infiltrating immune cells. When smaller numbers of tumor cells were inoculated in footpads, there was clear evidence that tumor development was hampered in control B16 cells.
In our attempt to understand the mechanism(s) by which CCR7 influences tumorigenesis, we assessed IFN-γ production by T cells recovered from the LNs draining the ear skin of tumor cell-injected mice. Interestingly, we observed striking, early suppression of host IFN-γ production following injection of CCR7-B16 cells but not control B16 cells. The development of a potent host anti-tumor response likely explains the profound decrease in control B16 cells between Days 7 and 11 following ear inoculation. It is likely that CCR7 activation by CCL21 present in the tumor microenvironment (see Results) resulted in increased resistance of B16 cells to immune cell-mediated killing, as we had previously demonstrated for CCR10 [4] and CXCR4 [24].
IFN-γ was also strikingly reduced in the footpad tumors that followed the injection of CCR7-B16 cells (vs. pLNCX2-B16 cells), despite their similarity in sizes. Given the down-regulation of IFN-γ by tumor-infiltrating immune cells in CCR7-B16 tumors, we were not surprised to see that CXCL9 and CXCL10, both highly responsive to IFN-γ-mediated stimulation, were down-regulated as well. Our in vitro experiments (Fig. 7C) suggested that the CCR7-B16 and pLNCX2-B16 cell lines that we used were capable of producing CXCL10 with IFN-γ stimulation. As CCR7 activation did not block the ability of the B16 cells to produce CXCL10 after IFN-γ stimulation, we believe it is likely that a deficiency of IFN-γ is likely to be the major reason that CCR7-B16 cells showed diminished expression of CXCL10. This explanation does not preclude, however, the possibility that the resulting loss of CXCL10 might have resulted in even more ineffective trafficking of T cells into the tumor site. Indeed, several recent reports using melanoma as model tumors suggest that CXCR3 ligands, CXCL9 and CXCL10, can be produced by melanoma cells [25] or by macrophages infiltrating primary melanoma tumors [26]. Moreover, improved host responses to melanoma or reduced growth of melanoma xenografts have been linked to expression of CXCL10 [25, 27, 28]. For example, host T cell migratory and anti-tumor responses induced by IFN-α-transduced DC to melanoma cells were dependent on CXCL10 production by the melanoma cells in a murine brain metastasis model [25].
In summary, CCR7 plays key roles in tumorigenesis in skin and LN metastasis. Activation of CCR7 through ligands present in the tumor microevironment directly contributes to tumor formation through immunologically mediated mechanisms, possibly including early suppression of the host anti-tumor response. The loss of Th1 chemokines such as CXCL10 may also play a key role in limiting the influx of tumor-infiltrating leukocytes, including T cells, into the tumor microenvironment. These data provide additional impetus to test specific chemokine receptor antagonists (i.e., against CCR7) [24] for possible use as adjuncts for cancer therapy.
Acknowledgments
The authors thank Dr. Mark C. Udey (Dermatology Branch, NCI) and all of the members of the Hwang lab for their helpful comments.
References
- Kakinuma T, Hwang S T. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan M E, McClanahan T, Murphy E, Yuan W, Wagner S N, Barrera J L, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Wiley H E, Gonzalez E B, Maki W, Wu M T, Hwang S T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- Murakami T, Cardones A R, Finkelstein S E, Restifo N P, Klaunberg B A, Nestle F O, Castillo S S, Dennis P A, Hwang S T. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003;198:1337–1347. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabioglu N, Yazici M S, Arun B, Broglio K R, Hortobagyi G N, Price J E, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Forster R. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- Kodama J, Hasengaowa, Kusumoto T, Seki N, Matsuo T, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2007;18:70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- Gunn M D, Tangemann K, Tam C, Cyster J G, Rosen S D, Williams L T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H, Moore A M, Brown M J, Hwang S T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Ishigami S, Natsugoe S, Nakajo A, Tokuda K, Uenosono Y, Arigami T, Matsumoto M, Okumura H, Hokita S, Aikou T. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology. 2007;54:1025–1028. [PubMed] [Google Scholar]
- Kakinuma T, Nadiminti H, Lonsdorf A S, Murakami T, Perez B A, Kobayashi H, Finkelstein S E, Pothiawala G, Belkaid Y, Hwang S T. Small numbers of residual tumor cells at the site of primary inoculation are critical for anti-tumor immunity following challenge at a secondary location. Cancer Immunol Immunother. 2007;56:1119–1131. doi: 10.1007/s00262-006-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser K E, Eichten A, Coussens L M. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Lin W W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C H, Broxmeyer H E. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson D G, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg L M, Hawighorst T, Schirner M, Wolf G L, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson S D, Anaya P, Avery M, Hetzel F W, Sarantou T, Havstad S. Sentinel lymph node metastasis in experimental melanoma: relationships among primary tumor size, lymphatic vessel diameter and 99mTc-labeled human serum albumin clearance. Ann Surg Oncol. 1997;4:161–168. doi: 10.1007/BF02303800. [DOI] [PubMed] [Google Scholar]
- Ochsenbein A F, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel R M. Roles of tumor localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Timar J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia J R, Katsaros D, Gimotty P A, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman M N, Rubin S C, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc P H, Trajanoski Z, Fridman W H, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Piersma S J, Jordanova E S, van Poelgeest M I, Kwappenberg K M, van der Hulst J M, Drijfhout J W, Melief C J, Kenter G G, Fleuren G J, Offringa R, van der Burg S H. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- Sharma P, Shen Y, Wen S, Yamada S, Jungbluth A A, Gnjatic S, Bajorin D F, Reuter V E, Herr H, Old L J, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C H, Kakinuma T, Wang J, Zhang H, Palmer D C, Restifo N P, Hwang S T. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura F, Dusak J E, Eguchi J, Zhu X, Gambotto A, Storkus W J, Okada H. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Feldman A L, Friedl J, Lans T E, Libutti S K, Lorang D, Miller M S, Turner E M, Hewitt S M, Alexander H R. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer. 2002;99:149–153. doi: 10.1002/ijc.10292. [DOI] [PubMed] [Google Scholar]
- Kawamata A, Ito D, Odani T, Isobe T, Iwase M, Hatori M, Nagumo M. Thalidomide suppresses melanoma growth by activating natural killer cells in mice. Oncol Rep. 2006;16:1231–1236. [PubMed] [Google Scholar]