Abstract
Nucleotide receptors serve as sensors of extracellular ATP and are important for immune function. The nucleotide receptor P2RX7 is a cell-surface, ligand-gated cation channel that has been implicated in many diseases, including arthritis, granuloma formation, sepsis, and tuberculosis. These disorders are often exacerbated by excessive mediator release from activated macrophages in the inflammatory microenvironment. Although P2RX7 activation can modulate monocyte/macrophage-induced inflammatory events, the relevant molecular mechanisms are poorly understood. Previous studies suggest that MAPK cascades and transcriptional control via CREB-linked pathways regulate the inflammatory capacity of monocytic cells. As P2RX7 promotes MAPK activation and inflammatory mediator production, we examined the involvement MAPK-induced CREB activation in P2RX7 action. Our data reveal that stimulation of multiple monocytic cell lines with P2RX7 agonists induces rapid CREB phosphorylation. In addition, we observed a lack of nucleotide-induced CREB phosphorylation in RAW 264.7 cells expressing nonfunctional P2RX7 and a gain of nucleotide-induced CREB phosphorylation in human embryonic kidney-293 cells that heterologously express human P2RX7. Furthermore, our results indicate that P2RX7 agonist-induced CREB phosphorylation is partly mediated via Ca2+ fluxes and the MEK/ERK system. Mechanistic analyses revealed that macrophage stimulation with a P2RX7 agonist induces CREB/CREB-binding protein complex formation, which is necessary for CREB transcriptional activation. Also, we demonstrate that P2RX7 activation induces a known CREB-dependent gene (c-fos) and that dominant-negative CREB constructs attenuate this response. These studies support the idea that P2RX7 stimulation can directly regulate protein expression that is not dependent on costimulation with other immune modulators such as LPS.
Keywords: CBP, Ca2+, ERK1/2, macrophages, c-FOS
INTRODUCTION
The regulation of leukocyte activation and mediator release is critical for immune responses such as inflammation and its associated symptoms. Over the last decade, extracellular ATP has received increasing recognition as an immunomodulatory agent and has been proposed to serve as a danger signal upon its release at the onset of inflammation [1, 2]. In accordance with this idea, extracellular ATP can bind to P2RX receptor family members that are ligand-gated cation channels expressed on the surface of leukocytes and that appear to function as extracellular ATP sensors that influence the progression of inflammatory diseases [3].
Early evidence for an immunological role of extracellular nucleotides came from our observations that certain ATP analogs could rescue mice from gram-negative bacterial LPS-induced death, thereby implicating nucleotide receptors in the modulation of bacterial sepsis [4]. In this regard, one member of the P2RX family that is highly expressed in activated leukocytes is P2RX7, and many subsequent studies have revealed a role for P2RX7 in modulating LPS-induced signaling events. For example, it has been observed that LPS-induced IL-1β release from peritoneal macrophages isolated from P2RX7−/− mice is compromised when compared with that observed with wild-type control littermates [5]. Moreover, murine RAW 264.7 macrophages that express a nonactive form of P2RX7 have reduced LPS-induced NO release as compared with macrophages with functional P2RX7 [6]. In terms of other diseases, P2RX7−/− mice also exhibit a reduced incidence and severity of anti-collagen-induced arthritis symptoms when compared with their littermate (P2RX7+/+) controls [7]. Interestingly, mutations in P2RX7 have been linked to increased susceptibility to extrapulmonary tuberculosis [8], and P2RX7 activation potentiates multinucleated giant cell (MGC) formation, which commonly occurs at sites of chronic inflammation [9,10,11,12,13]. Thus, P2RX7 activity affects a wide range of inflammatory events, and yet, the molecular mechanisms by which these events occur are not well-defined.
P2RX7 is activated by high concentrations of extracellular ATP or the potent agonist 2′(3′)-O-(4-benzoylbenzoyl)-ATP (BzATP) [14] and mediates a rapid exchange of ions (i.e., K+ and Ca2+) across the plasma membrane. In addition, P2RX7 has been associated with the passage of molecules <900 Da in size via the formation of a nonspecific pore on the cell surface upon prolonged ligand exposure [15]. Numerous studies have implicated P2RX7 activity in monocyte and macrophage responses, including the processing and release of IL-1β, IL-6, and IL-18, the expression of inducible NO synthase (iNOS) and the production of NO, the activation of NF-κB, and the generation of reactive oxygen species [5, 6, 16,17,18,19,20,21,22].
Although the mechanisms by which P2RX7 regulates gene expression are unclear, the promoter region of many P2RX7-modulated proinflammatory genes, such as IL-1β, TNF-α [23], iNOS [6], IL-6 [24], and cyclooxygenase-2 (COX-2) [16], contains consensus binding sites for the CREB [25], which is a transcription factor that is ubiquitously expressed and functions in many cellular processes such as glucose homeostasis, growth factor-dependent cell survival, memory, and inflammatory mediator production [26]. CREB mediates the activation of cAMP-responsive genes by binding as a dimer to conserved CREs (5′-TGACGTCA-3′) in the promoter regions of target genes. CREB activation requires phosphorylation at serine-133 (S133), which promotes recruitment of the transcriptional coactivator CREB-binding protein (CBP) and p300, thereby enabling gene transcription [25, 26]. Phosphorylation of CREB at S133 (pCREB) is mediated by multiple kinases, including those downstream of the ERK1/2. Of note, MAPKs such as ERK1/2 are activated in monocytic cells upon stimulation with P2RX7 agonists [17], providing a potential link between P2RX7 stimulation and CREB activation.
Although CREB regulation is critical for inflammatory mediator release by monocytic cells in response to other stimuli [27,28,29,30], little is known about the role of nucleotide receptors in CREB activation. Because of the role of P2RX7 in augmenting macrophage-induced inflammatory events, together with the observation that the production of many inflammatory mediators known to be augmented by P2RX7 has been linked to CREB activation in response to other stimuli, the hypothesis that P2RX7 stimulation promotes CREB activation in monocytic cells was tested. The present study demonstrates that P2RX7 agonists alone promote CREB phosphorylation in multiple monocytic cell types, including murine RAW 264.7 macrophages, human THP-1 monocytes, and primary human peripheral blood-derived monocytes. Our results also indicate that P2RX7 function/expression is required for nucleotide-induced CREB phosphorylation. Furthermore, our data support a role for Ca2+ fluxes as well as the MEK/ERK/p90 ribosomal S6 kinase (p90Rsk) pathway in P2RX7 agonist-induced CREB phosphorylation. Analysis of the mechanism of CREB activation revealed that stimulation of RAW 264.7 macrophages with a P2RX7 agonist induces CREB/CBP complex formation, which is necessary for CREB transcriptional activation. To address the significance of P2RX7 agonist-induced CREB phosphorylation, we assessed the role of P2RX7 activation in the production of c-Fos protein, a known CREB-dependent gene [26] and modulator of macrophage and osteoclast function [31, 32]. In accordance with this, expression of c-Fos protein is induced in a P2RX7-dependent manner and blockade of CREB activity using two separate dominant-negative (dn)CREB vectors attenuates P2RX7 agonist-induced c-Fos expression. Taken together, the data support the idea that P2RX7 stimulation alone can lead to the induction of protein expression and is not dependent on costimulation with other immune modulators such as LPS.
MATERIALS AND METHODS
Materials
Unless otherwise specified, reagents for cell culture were purchased from Mediatech (Herndon, VA, USA). The nucleotides ATP and BzATP were obtained from Sigma Chemical Co. (St. Louis, MO, USA). LPS (Escherichia coli, serotype 0111:B4), PMA, and anisomycin were also purchased from Sigma Chemical Co. The active MEK1/2 inhibitor UO126 was purchased from Promega (Madison, WI, USA), whereas the inactive analog of this inhibitor (UO124) was obtained from Calbiochem (San Diego, CA, USA). The intracellular calcium chelator BAPTA-AM was purchased from Invitrogen (Carlsbad, CA, USA). Antibodies used for immunoblotting β-tubulin, c-Fos, CREB, antiphospho (S133)-CREB [which also labels phosphorylation of the related protein activating factor-1 (ATF-1)], and antiphospho (Thr359/Ser363)-p90Rsk were purchased from Cell Signaling Technology (Danvers, MA, USA); antiactive ERK1/2 (which recognizes the dually phosphorylated TXY motif) was purchased from Promega; pan-reactive ERK1/2 was purchased from Upstate Biotechnology (Waltham, MA, USA); and anti-growth factor receptor-bound protein 2 (Grb2) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). GST coupled to kinase-inducible domain-interacting domain of CBP (GST–KIX) was a gift from Jennifer Nyborg (Colorado State University, Fort Collins, CO, USA) and was expressed and purified as described [33] by the laboratory of Dr. Randal Tibbetts (University of Wisconsin, Madison, WI, USA). dnCREB vectors were purchased from Clontech (Mountain View, CA, USA), and the empty vector control plasmid, designated as MT, was made by cutting out the CREB sequence from the porcine CMV (pCMV)-CREB133 (S133A) dnCREB plasmid.
Cell culture
Human THP-1 monocytes were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in RPMI supplemented with 10% FBS (HyClone Laboratories, Logan, UT, USA), 2 mM sodium pyruvate, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin. Murine RAW 264.7 macrophages were obtained from ATCC, whereas P2RX7-defective RAW cells were generated by treating RAW 264.7 cells with a 99% lethal dose (1 mM) of BzATP, and the remaining cells were cloned by limiting dilution. One isolated clone was sequenced and found to have a S342F mutation in the second transmembrane region of the P2RX7 gene (cells generated by Phil Fisette, University of Wisconsin, Madison). Both RAW 264.7 cell lines were maintained in RPMI supplemented with 5% cosmic calf serum, 2 mM sodium pyruvate, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin. Human embryonic kidney (HEK)-293 cells were obtained from ATCC, whereas HEK-293 cells transfected with the pIREShyg (empty vector control) and pIRES/hP2RX7 expression vectors were generated as described previously [34]. All HEK-293 cell lines were maintained in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 2 mM sodium pyruvate, and 100 U/ml penicillin/streptomycin. All cells were grown in 10 cm tissue-culture dishes at 37°C in a humidified atmosphere with 5% CO2.
Isolation of human blood-derived monocytes
Human blood-derived monocytes were purified as described previously [35]. Briefly, heparinized blood was drawn from healthy adult volunteers at the University of Wisconsin Hospitals and Clinics (Madison, WI, USA) in compliance with the requirements of the University of Wisconsin Health Sciences, Human Subjects Committee protocol. The blood was separated using a Percoll density gradient, and the PBMC layer was removed along with 1 mL RBC suspension for monocyte purification. Platelets were removed by three washes in HBSS with 2% heat-inactivated bovine serum. Monocytes were enriched with RosetteSep monocyte enrichment cocktail and a standard Ficoll centrifugation. The monocytes were collected at the Ficoll:plasma interface. CD14+ cells were determined by flow cytometry and comprised of 90–95% of the cell preparations.
Immunoblotting
After isolation, human blood-derived monocytes were plated at a density of 2 × 106 cells/well in 12-well CoStar (Corning Inc., Corning, NY, USA) plates, washed after 2 h with HBSS, and incubated at 37°C for 16–24 h. THP-1 monocytes, RAW 264.7 macrophages, and HEK-293 cells were plated at a density of 3 × 105–5 × 105 cells/well in 24-well tissue-culture plates and incubated at 37°C, 16–24 h. Following each experiment and subsequent cell lysis with SDS sample buffer (20 mM Tris, 2 mM EDTA, 1 mM Na3VO4, 2 mM DTT, 2% SDS, and 20% glycerol), the proteins were resolved on SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA), which were blocked in 5% nonfat milk in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20) or 5% BSA/TBST for antiphospho-CREB, antiphospho-p90Rsk, or anti-β-tubulin antibodies. Immunoblotting was performed by incubating the membranes with the primary antibody according to the manufacturer’s protocols. The membranes were then washed with TBST and incubated with secondary antibodies conjugated to HRP (Santa Cruz Biotechnology), and the immunoreactive bands were visualized using SuperSignal West chemiluminescent substrates (Pierce, Rockford, IL, USA). The PVDF membranes were visualized using the Epichemi II darkroom (UVP, Upland, CA, USA) equipped with a 12-bit, cooled charged-coupled device camera. Image processing and analysis were performed using ImageJ 1.33u (National Institutes of Health, Bethesda, MD, USA). To evaluate equal protein loading, membranes were reprobed with antibodies that react with Grb2 (1:2500 in 5% milk-TBST), β-tubulin (1:1000 in 5% BSA-TBST), or pan-reactive ERK1/2 (1:2000 in 5% milk-TBST).
BAPTA-AM treatment
BAPTA loading was done in a similar manner as described previously [36]. RAW cells plated in 24-well plates (3×105 cells/well) the day prior to the experiment were washed with RPMI with no phenol red or supplements and loaded with BAPTA-AM in RPMI with no phenol red or supplements for 30 min at 37°C. Following this incubation, the cells were washed two times with cell culture medium and treated as described in the figure legends.
GST-KIX pulldown
Murine RAW 264.7 macrophages were stimulated as described for various time-points and then lysed with 3× GST lysis buffer (30% glycerol, 150 mM Tris-Cl, pH 7.4, 600 mM NaCl, 3% Nonidet P-40, 6 mM MgCl2) containing inhibitors (100 μM sodium vanadate, 100 μM PMSF, 100 μM aprotinin, 100 μM leupeptin). Protein concentrations were determined through a bicinchoninic acid protein assay, and 100 μg cell lysate from each treatment was incubated with 20 μg recombinant GST-KIX protein immobilized on glutathione agarose beads in 1× GST lysis buffer at 4°C for 18 h. The beads were washed three times with 1× GST lysis buffer, and the bound proteins were eluted by boiling the beads in 2× SDS loading buffer.
Transfection of dnCREB constructs
HEK cells heterologously expressing human P2RX7 (HEK/P2RX7) were plated at a density of 1 × 105 cells/well on collagen-I (Sigma Chemical Co.)-coated 24-well plates the day before transfection. Cells were subsequently transiently transfected with pCMV empty vector (designated as MT), S133A, or pCMV-KCREB (KCREB) dn plasmids using FuGENE HD (Roche, Nutley, NJ, USA) at an 8:2 transfection reagent:DNA ratio according to the manufacturer’s specifications. Twenty-four hours after transfection, cells were treated and lysed in SDS sample buffer.
Statistical analysis
The Student’s two-tailed paired t-test was used to calculate the statistical differences between samples. Significance levels were set at P values <0.05.
RESULTS
P2RX7 agonists induce pCREB in monocytic cells
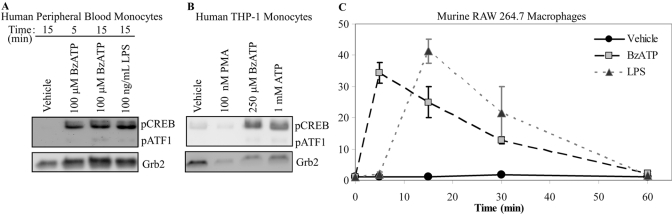
Previous studies examining CREB transactivation have indicated that the phosphorylation of S133 is critical for the activation of gene transcription [25], thus the effect of P2RX7 agonists on the pCREB was first examined. As shown in Figure 1, treatment of human blood-derived monocytes or human THP-1 monocytic cells promoted robust pCREB, as determined by immunoblotting analysis. In human blood-derived monocytes, CREB was detectably phosphorylated after 5 min of BzATP (100 μM) stimulation (Fig. 1A). Stimulation of monocytes with 100 ng/mL LPS was used as a positive control for CREB phosphorylation [37]. Furthermore, in response to ATP and BzATP stimulation, pCREB was also observed in a human monocyte cell line, THP-1 (Fig. 1B). Phosphorylation of CREB in response to P2RX7 agonist treatment was not limited to human monocytes, as treatment of murine RAW 264.7 macrophages with BzATP or high concentrations of ATP induced robust pCREB (Figs. 1C and 2A). A time course of BzATP treatment in RAW 264.7 macrophages was performed to assess the kinetics of CREB phosphorylation. As shown in Figure 1C, BzATP treatment resulted in a more rapid phosphorylation of CREB than that induced by LPS, and this CREB phosphorylation returned to basal levels after 1 h of treatment with either stimulus. The antibody used to detect pCREB also detects nucleotide-induced phosphorylation of ATF-1, a CREB-related transcription factor that is known to heterodimerize with CREB in response to activating stimuli [26, 38]. The nucleotide-induced phosphorylation profile of ATF-1 paralleled that of CREB.
Fig. 1.
Effect of P2RX7 agonists on pCREB in human monocytic cells. (A) Isolated peripheral human blood monocytes were treated with vehicle (2.5 mM HEPES), 100 μM BzATP, or 100 ng/mL LPS for the indicated times. A representative immunoblot displaying pCREB is shown (n=3). The pCREB antibody recognizes pCREB and phosphorylated ATF-1 (pATF1); thus, it is also shown. Immunoblotting was performed with anti-Grb2 antibody as a loading control. (B) THP-1 human monocytes were treated with vehicle (2.5 mM HEPES), 100 μM PMA, 1 mM ATP, or 250 μM BzATP for 15 min. A representative immunoblot displaying pCREB/pATF1 is shown (n=3). Immunoblotting was performed with anti-Grb2 antibody as a loading control. (C) Murine RAW 264.7 macrophages were stimulated with vehicle (2.5 mM HEPES), 250 μM BzATP, or 1 μg/mL LPS for the indicated time-points. Immunoblotting was performed with anti-pCREB/pATF1 and anti-β-tubulin (loading control) antibodies. Results of the three independent experiments were combined and graphed ± sem.
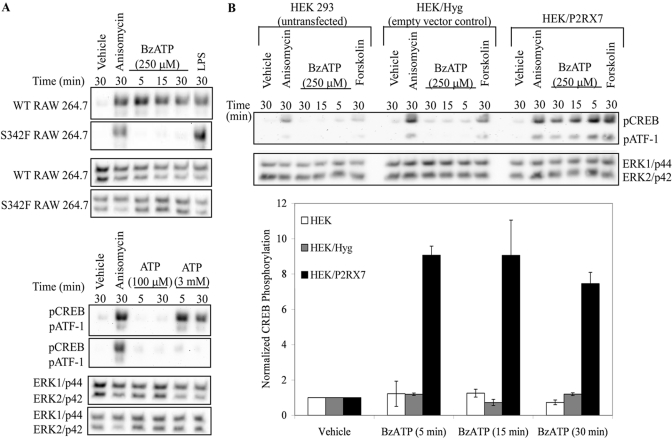
Fig. 2.
Dependence of nucleotide-induced pCREB on P2RX7 function/expression. (A) Wild-type (WT) and serine to phenylalanine mutation (SF) mutant RAW 264.7 macrophages were treated with vehicle (2.5 mM HEPES), 10 μg/mL anisomycin, 1 μg/mL LPS, 250 μM BzATP, or the indicated concentrations of ATP for the time-points shown. A representative immunoblot displaying pCREB/pATF1 is shown (n≥3). Immunoblotting was performed with anti-pan-ERK1/2 antibody as a loading control. (B) Untransfected HEK-293 cells and HEK-293 cells transfected with the pIREShyg (Hyg) or pIRES/hP2RX7 (P2RX7) expression vectors were stimulated with vehicle (2.5 mM HEPES), 10 μg/mL anisomycin, 250 μM BzATP, or 100 μM forskolin for the indicated times and immunoblotted for pCREB/pATF1. Immunoblotting was performed with anti-pan-ERK1/2 antibody as a loading control. Results of the three independent experiments were collated, and BzATP-induced pCREB was graphed ± sem.
CREB phosphorylation is dependent on P2RX7 activation
All members of the P2 receptor family can bind and respond to ATP, albeit with variable affinities. With respect to P2RX7, it can be activated by high (mM) levels of ATP as well as by the pharmacological agonist BzATP. Although BzATP is a potent agonist of P2RX7, BzATP can also activate several other P2 receptors (e.g., P2RX1 and P2RX2) [39, 40]. Therefore, it was critical to evaluate whether the nucleotide-induced CREB phosphorylation events observed in the present studies are the result of P2RX7 activation and not activation of other P2 receptors. To ascertain if nucleotide-induced CREB phosphorylation was mediated via P2RX7-mediated signaling events, a nonfunctional P2RX7 cell line was used. Specifically, RAW 264.7 macrophages possessing a mutant P2RX7 gene containing a serine to phenylalanine mutation in the second transmembrane domain (referred to as S342F or SF) were used as a nonfunctional P2RX7 macrophage model, as this mutation confers attenuated P2RX7 protein expression and function [6, 34, 41]. Following stimulation with P2RX7 agonists (Fig. 2A), CREB was readily phosphorylated in the wild-type macrophages but not in the macrophages containing the SF P2RX7 mutant gene. A higher concentration of ATP was used for the murine macrophage experiments, because of the difference in the EC50 of ATP between human and murine P2RX7 [42]. To further support that P2RX7 activation is responsible for nucleotide-induced CREB phosphorylation, a lower concentration of ATP (100 μM) was also used, as this concentration will not activate P2RX7 but will saturate all other known P2 receptors [15]. CREB phosphorylation was not detectable after stimulation of RAW 264.7 macrophages with 100 μM ATP, which is consistent with this phosphorylation event being dependent on P2RX7-mediated signaling.
To further support the idea that nucleotide-induced CREB phosphorylation was mediated through P2RX7 activation, signaling downstream of human P2RX7 stably transfected into HEK-293 cells was examined. HEK-293 cells are often used as a model system for studying P2RX7, given that these cells do not endogenously express P2RX receptors [43], although they do express P2RY1 and P2RY2 [44]. When HEK cells expressing P2RX7 were stimulated with BzATP, CREB phosphorylation was detected (Fig. 2B). In contrast, stimulation of untransfected or empty vector, control-transfected HEK cells with BzATP did not induce CREB phosphorylation, which strongly supports the hypothesis that nucleotide-induced CREB phosphorylation is mediated through P2RX7. Anisomycin (activator of p38 MAPK) and forskolin (inducer of cAMP accumulation) were used as positive controls to demonstrate that the cell lines were still able to induce CREB phosphorylation in response to stimuli that promote p38 and cAMP-dependent pathways in the absence of functional P2RX7.
P2RX7-induced CREB phosphorylation is inhibited by MAPK inhibitors
As our data support a model wherein P2RX7 stimulation promotes CREB phosphorylation, we next sought to establish which kinase(s) are upstream of this event. pCREB can be mediated by a variety of kinases. Although protein kinase A (PKA) is the prototypical kinase that induces CREB phosphorylation upon cAMP accumulation in the cell, this kinase pathway is unlikely to be upstream of P2RX7-induced pCREB because of the rapid kinetics of this phosphorylation. For PKA to phosphorylate CREB, the catalytic subunit of PKA must be transported to the nucleus, and in many cell systems, this effect is not maximal until 30 min poststimulation [25, 45]. Furthermore, preliminary evidence from our laboratory suggests that in murine RAW 264.7 macrophages, P2RX7 agonist-induced CREB phosphorylation is not attenuated by the PKA antagonist adenosine 3′,5′-monophosphorothioate isomer of 8-bromo-cAMPS (data not shown).
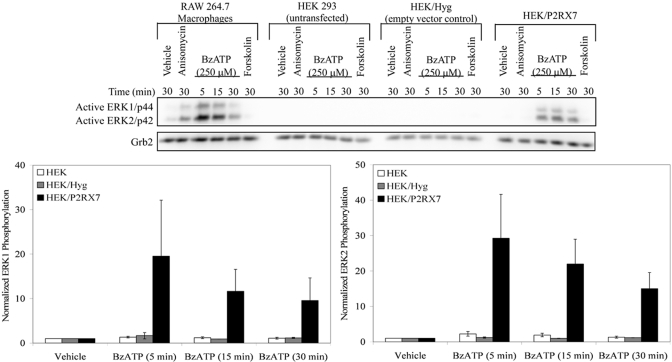
pCREB can be mediated by kinases that are downstream of MAPK-dependent pathways, including ERK1/2 [27, 46]. Studies by us and others [16, 36, 47, 48] provided evidence for the activation of ERK1/2 (p44/42) by endogenous P2RX7s in various cell types. As can be seen in Figure 3, the concept that nucleotides can stimulate ERK1/2 phosphorylation through P2RX7 is supported further by evidence of this phosphorylation event occurring in human P2RX7-transfected HEK-293 cells. Only the cells that express P2RX7 (RAW 264.7 macrophages and HEK/P2RX7) exhibit BzATP-stimulated ERK1 and ERK2 phosphorylation. Therefore, the role of ERK1/2 in the downstream phosphorylation of CREB in response to the P2RX7 agonists was investigated.
Fig. 3.
P2RX7 agonist-induced ERK1/2 phosphorylation. RAW 264.7 macrophages, untransfected HEK-293 cells, and HEK-293 cells that were transfected with the pIREShyg or pIRES/hP2RX7 expression vectors were stimulated with vehicle (2.5 mM HEPES), 10 μg/mL anisomycin, 250 μM BzATP, or 100 μM forskolin for the indicated times and immunoblotted to detect ERK1/2 phosphorylation. Immunoblotting was performed with the anti-Grb2 antibody as a loading control. The results of the three independent experiments were combined, and BzATP-induced ERK1/2 phosphorylation was graphed (mean±sem).
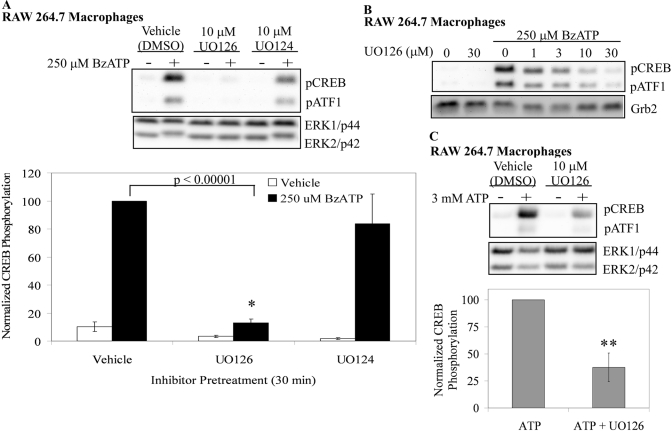
The pharmacological inhibitor (UO126) for the ERK1/2 kinases (MEK1/2) along with the inactive analog for this inhibitor (UO124) were used to inhibit MEK1/2-ERK1/2 signaling in RAW 264.7 macrophages. These inhibitor-pretreated cells were subsequently treated with 250 μM BzATP, and cell lysates were immunoblotted for pCREB. The data in Figure 4A show that UO126 significantly inhibits (P<0.00001) BzATP-induced CREB phosphorylation in RAW 264.7 macrophages, whereas UO124 has no significant effect. The attenuation of P2RX7 agonist-induced CREB phosphorylation by UO126 was seen at all time-points examined (5 and 30 min; data not shown) and was dose-dependent (Fig. 4B), which is consistent with the IC50 of the inhibitor [49, 50]. The dependence of CREB phosphorylation on the P2RX7-induced MEK-ERK cascade was supported further by attenuation of ATP-induced CREB phosphorylation by UO126 (P<0.05) in RAW 264.7 macrophages (Fig. 4C).
Fig. 4.
Effect of an ERK1/2 kinase inhibitor on P2RX7-induced CREB phosphorylation in murine macrophages. (A) RAW 264.7 macrophages were pretreated for 15 min with vehicle (DMSO), the MEK1/2 inhibitor UO126 (10 μM), or the MEK1/2 inactive inhibitor analog UO124 (10 μM). The cells were subsequently treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for 15 min. A representative immunoblot displaying pCREB/pATF1 is shown. Immunoblotting was performed with anti-pan-ERK1/2 antibody as a loading control. The results of five independent experiments were collated and are represented as normalized CREB phosphorylation (mean±sem); *, P < 0.00001. (B) RAW 264.7 macrophages were pretreated for 15 min with vehicle (DMSO) or UO126 at concentrations from 1 μM to 30 μM. The cells were subsequently treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for 15 min. A representative immunoblot displaying pCREB/pATF1 is shown (n=3). (C) RAW 264.7 macrophages were pretreated for 15 min with vehicle (DMSO) or 10 μM UO126. The cells were subsequently treated with vehicle (2.5 mM HEPES) or 3 mM ATP for 15 min. The results of three independent experiments were combined and are represented as a percent of ATP-induced CREB phosphorylation (mean±sem); **, P < 0.05.
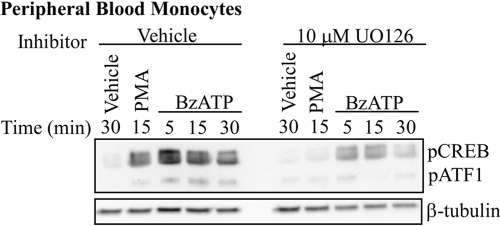
The effect of UO126 on BzATP-induced CREB phosphorylation was also examined in human peripheral blood monocytes (Fig. 5). Consistent with the RAW 264.7 macrophage data, UO126 attenuated BzATP-induced CREB phosphorylation at all time-points examined. In addition, cell viability was not affected by UO126, as assessed by flow cytometry of propidium iodide-stained RAW 264.7 macrophages under all of the treatment conditions (data not shown).
Fig. 5.
Effect of an ERK1/2 kinase inhibitor on P2RX7-induced CREB phosphorylation in primary human monocytes. Isolated human peripheral blood monocytes were pretreated for 15 min with vehicle (DMSO) or 10 μM UO126 and were subsequently stimulated with vehicle (2.5 mM HEPES), 250 μM BzATP, or 10 μM PMA for the indicated time-points. A representative immunoblot displaying pCREB/pATF1 is shown. Immunoblotting was performed with an anti-β-tubulin antibody as a loading control. Analogous results were observed in two separate experiments.
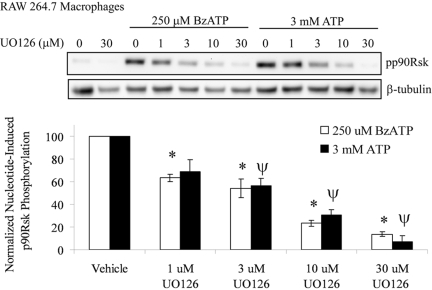
P2RX7 agonist-induced p90Rsk phosphorylation in RAW 264.7 macrophages
The serine/threonine kinase p90Rsk is known to phosphorylate CREB directly [26, 51] and is a downstream substrate for ERK1/2 in many cell systems [52,53,54,55]. Therefore, the phosphorylation status of p90Rsk was measured after ATP or BzATP stimulation of RAW 264.7 macrophages. As shown in Figure 6, treatment of cells with ATP or BzATP leads to a robust phosphorylation of p90Rsk after 15 min of stimulation. Pretreatment of RAW 264.7 cells with increasing doses of the MEK1/2 inhibitor UO126 caused a dose-dependent decrease (P<0.05) in p90Rsk phosphorylation. These data further support a model, where P2RX7 activation induces the MEK/ERK signaling cascade that mediates the phosphorylation of the transcription factor CREB through p90Rsk.
Fig. 6.
P2RX7 agonist-induced p90Rsk phosphorylation in RAW 264.7 macrophages, which were pretreated for 15 min with vehicle (DMSO) or the MEK1/2 inhibitor UO126 at concentrations from 1 μM to 30 μM. The cells were subsequently treated with vehicle (2.5 mM HEPES), 250 μM BzATP, or 3 mM ATP for 15 min. A representative immunoblot displaying p90Rsk phosphorylation (pp90Rsk) is shown. Immunoblotting was performed with anti-β-tubulin antibody as a loading control. The results of three independent experiments were collated and are represented as a percent of nucleotide-induced p90Rsk phosphorylation (mean±sem); *, P < 0.05, as compared with DMSO-pretreated, BzATP-induced p90Rsk phosphorylation; Ψ, P < 0.05, as compared with DMSO-pretreated, ATP-induced p90Rsk phosphorylation.
P2RX7 agonist-induced ERK/CREB activation Is Ca2+-dependent
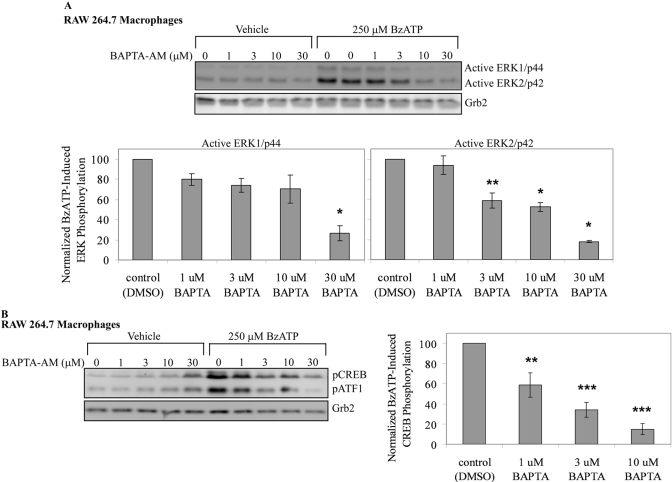
It has been shown by us and others [22, 56,57,58] that P2RX7 stimulation leads to the rapid influx of Ca2+ into cells. Furthermore, other cell models have also revealed that Ca2+ is essential for P2RX7 agonist-mediated MAPK activation. For example, when human 1321N1 cells expressing the recombinant rat P2RX7 are loaded with increasing doses of the intracellular Ca2+ chelator BAPTA-AM, BzATP-induced ERK1/2 phosphorylation is inhibited dose-dependently [36]. In addition to its capacity to lead to MAPK activation, elevations in intracellular-free Ca2+ have been linked to the activation of CREB in many cell systems [26]. Therefore, the role of Ca2+ in P2RX7 agonist-induced ERK1/2 and CREB phosphorylation in RAW 264.7 macrophages was examined.
When RAW 264.7 macrophages are pretreated with BAPTA-AM, there is a dose-dependent decrease in ERK1 and ERK2 phosphorylation (e.g., at 30 μM BAPTA-AM, P<0.01), although ERK2 is more sensitive to this inhibition than ERK1 (Fig. 7A). Chelation of Ca2+ also attenuates BzATP-induced CREB phosphorylation in a dose-dependent manner (e.g., at 10 μM BATPA-AM, P<0.003), although higher doses of BAPTA-AM promote increases in basal CREB phosphorylation (Fig. 7B). This increase in basal phosphorylation may be a result of incomplete hydrolysis of BAPTA-AM, which would impede Ca2+ chelation and is known to occur in other systems. This increase in basal CREB phosphorylation at high levels of BAPTA-AM may also be the result of indirect activation of other kinases that can phosphorylate CREB. These data support the concept that P2RX7 agonist-induced MAPK phosphorylation and subsequent CREB phosphorylation are, at least in part, Ca2+-dependent signaling events.
Fig. 7.
Effect of calcium chelator BAPTA on P2RX7 agonist-induced ERK/CREB phosphorylation. (A) RAW 264.7 macrophages were loaded with vehicle (DMSO) or BAPTA-AM at increasing concentrations (1 μM–30 μM) for 30 min at 37°C. Following inhibitor incubation, the cells were washed twice and treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for 5 min and immunoblotted for ERK1/2 phosphorylation. (Note: BzATP-induced ERK1/2 phosphorylation without BAPTA-AM was done in duplicate.) Immunoblotting was performed with anti-Grb2 antibody as a loading control. The results of three independent experiments were combined and are represented as a percent of BzATP-induced ERK1 (left panel) and ERK2 (right panel) phosphorylation (mean±sem). (B) RAW 264.7 macrophages were loaded with vehicle (DMSO) or BAPTA-AM at increasing concentrations (1 μM–30 μM) for 30 min at 37°C. Following inhibitor incubation, the cells were washed twice and treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for 5 min and immunoblotted for pCREB/pATF1. Immunoblotting was performed with anti-Grb2 antibody as a loading control. The results of three independent experiments were collated and are represented as a percent of BzATP-induced CREB phosphorylation (mean±sem); *, P < 0.01; **, P < 0.05; ***, P < 0.003.
P2RX7 agonist-induced CREB/CBP complex formation
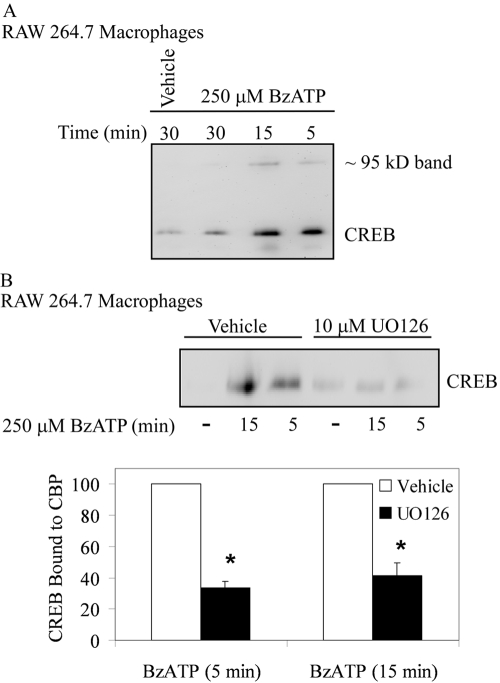
Although phosphorylation of CREB is critical for its transactivation, this event does not always fully correspond to CREB-induced gene expression [59, 60]. For instance, the stimulation of T-cell receptors (TCRs) leads to Ca2+ influx and robust phosphorylation of CREB, yet TCR activation does not promote CREB/CBP complex formation nor target gene induction [59]. The kinase-inducible domain of CREB, once phosphorylated at S133, can associate with the KIX domain of the coactivator CBP to initiate gene transcription [61,62,63,64]. This CREB/CBP interaction has been found in other systems to be sufficient for CREB-induced gene activation [63, 65]. Therefore, we examined the effect of P2RX7 agonists on CREB/CBP formation to test whether P2RX7 agonist-induced CREB phosphorylation leads to the capacity to assemble a functional CREB transcriptional complex.
In these studies, RAW 264.7 macrophages were stimulated with BzATP for 5–30 min, lysed, and incubated with glutathione beads coupled to the CBP-KIX domain. CREB/CBP interactions were then determined by immunoblotting for any CREB that was bound to the beads. As shown in Figure 8A, BzATP treatment promoted a highly inducible interaction between CREB and CBP at 5 min, and this interaction decreased after 30 min. When immunoblotting for CREB, a faint band was seen in the lanes containing the samples obtained from cells treated with BzATP for 5 and 15 min. This band ran at 95–100 kD and may be the result of CREB dimers that were not uncoupled completely in the SDS-PAGE gel. When RAW 264.7 macrophages were pretreated with the MEK1/2 antagonist UO126, BzATP-induced CREB/CBP interactions were inhibited significantly (Fig. 8B; P<0.0005 for 5- and 15-min treatments), supporting a role for the MEK/ERK pathway in CREB activation and CBP complex formation.
Fig. 8.
P2RX7 agonist-induced CREB/CBP complex formation. (A) RAW 264.7 macrophages were treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for the indicated time-points. Cells were lysed with 3× GST lysis buffer, and equal amounts of protein were incubated with GST-KIX beads for 18 h. The proteins that were pulled down with the GST-KIX beads were immunoblotted for CREB. A representative blot from three independent experiments is shown. (B) RAW 264.7 macrophages were pretreated for 15 min with vehicle (DMSO) or 10 μM UO126 and then were treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for the indicated time-points at 37°C. Cells were lysed with 3× GST lysis buffer, and equal amounts of protein were incubated with GST-KIX beads for 18 h. Pulled-down proteins were immunoblotted for CREB. The results of independent experiments (n=3- to 5-min treatment; n=4 for 15-min treatment) were collated and are represented as a percent of BzATP-induced CREB phosphorylation (mean±sem); *, P < 0.0005.
Attenuation of CREB activation leads to down-regulation of P2X7 agonist-induced c-Fos protein expression
Previous studies have shown that CREB becomes phosphorylated at Ser133, when cells are stimulated by a wide range of extracellular stimuli, including many growth factors, in a cAMP-independent manner [26]. In the case of growth factors, CREB plays a critical role in mediating nerve growth factor (NGF) induction of c-fos gene transcription in a Ser133-dependent manner [66]. Furthermore, NGF-induced CREB phosphorylation was found to be dependent on ERK activation [51]. Given that our data support that P2RX7 agonist-induced CREB phosphorylation is also dependent on ERK1/2 activation, it was hypothesized that P2RX7 activation leads to the generation of c-Fos protein.
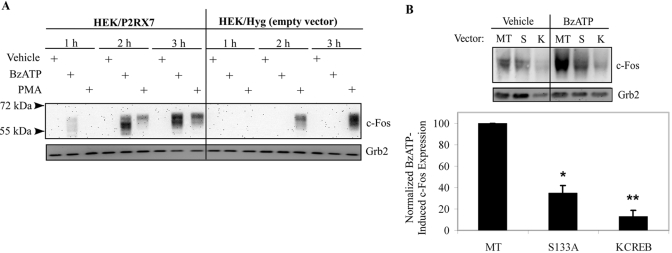
To test this hypothesis, HEK-293 cells stably expressing pIREShyg or pIRES/hP2RX7 expression vectors were stimulated with vehicle (2.5 mM HEPES), 250 μM BzATP, or 1 μg/mL PMA (a well-known inducer of c-Fos protein expression) for 1–3 h and immunoblotted for total c-Fos protein. When HEK cells expressing P2RX7 were stimulated with BzATP, we observed that c-Fos protein expression was induced robustly after 2 h of stimulation (Fig. 9A). In contrast, stimulation of empty vector control-transfected HEK cells with BzATP did not induce detectable c-Fos protein, which supports the idea that BzATP-mediated c-Fos expression is mediated through P2RX7. When blotting for c-Fos, multiple bands were observed, and it has been suggested that the higher bands represent post-transcriptional modifications to c-Fos, such as phosphorylation or sumolyation [67, 68].
Fig. 9.
Attenuation of CREB activation leads to down-regulation of P2RX7 agonist-induced c-Fos protein expression. (A) HEK-293 cells transfected with the pIREShyg or pIRES/hP2RX7 expression vectors were stimulated with vehicle (2.5 mM HEPES), 250 μM BzATP, or 1 μg/mL PMA for the indicated times and immunoblotted for total c-Fos protein. Immunoblotting was performed with an anti-Grb2 antibody as a loading control. Analogous results were observed in three separate experiments. (B) HEK-293 cells stably transfected with the pIRES/hP2RX7 expression vector were transiently transfected with MT, S133A (S), or KCREB (K) CREB dn vectors. Twenty-four hours post-transfection, cells were treated with vehicle (2.5 mM HEPES) or 250 μM BzATP for 2 h and immunoblotted for total c-Fos protein expression. Immunoblotting was performed with an anti-Grb2 antibody as a loading control. The results of four independent experiments were collated and are represented as a percent of BzATP-induced c-Fos expression over basal c-Fos expression (mean±sem); *, P < 0.05; **, P < 0.005, as compared with MT-transfected, BzATP-treated c-Fos expression.
To test the importance of P2RX7 agonist-induced CREB activation on the induction of the transcriptional regulator c-Fos, we used two separate dnCREB plasmids. HEK-293 cells stably expressing P2RX7 were transiently transfected with MT, S133A (mutation at S133 of CREB that blocks phosphorylation, thus preventing transcription), or KCREB (mutation in the DNA-binding domain of CREB that forms an inactive dimer with endogenous CREB) vectors. Twenty-four hours post-transfection, cells were treated with vehicle or 250 μM BzATP for 2 h and immunoblotted for total c-Fos protein expression. As shown in Figure 9B, expression of CREB dominant-interfering mutants significantly attenuate BzATP-induced c-Fos protein expression (P<0.05 for S133A; P<0.005 for KCREB). Together, the data strongly supports an important role for P2RX7 activation in CREB-induced protein expression.
DISCUSSION
In the present report, we provide multiple lines of evidence that extracellular nucleotides induce rapid CREB activation through the nucleotide receptor P2RX7 in monocytic cells. These observations are noteworthy, given that the promoter region of many P2RX7-modulated proinflammatory genes, such as IL-1β, TNF-α, iNOS, IL-6, and COX-2 contain consensus sites for CREB binding. Other transcription factors, such as other CREB family members and AP-1, also have CREB consensus-binding sites; thus, P2RX7-induced CREB activation could lead to the induction of many additional proteins that are regulated by these factors. In support of this notion, our data reveal that stimulation of P2RX7 leads to the expression of c-Fos protein, which itself is a transcription factor of the AP-1 family that can bind to 12-O-tetradecanoylphorbol-13-acetate (or PMA) response elements (5′-TGAG/CTCA-3′) present in the promoter regions of numerous proinflammatory mediators, including TNF-α, IL-1, IL-6, IL-8, and MCP-1 [69,70,71].
In addition, other studies support a role for CREB activation in inflammatory mediator release by monocytic cells in response to other stimuli, such as bacterial LPS and bovine type I collagen [28,29,30]. Interestingly, it has also been reported that in RAW 264.7 macrophages, LPS-induced COX-2 expression and IL-1β release were regulated by the same MAPK cascades as LPS-induced pCREB [27].
Although CREB regulation is well recognized to play a role in macrophage function, little is known concerning the effect of extracellular nucleotides on the activation of CREB. In this regard, Feng et al. [72] examined the effect of a few nucleotides on pCREB and observed that stimulation of mast cells with BzATP induced this phosphorylation event 30 min poststimulation, although the data were not shown. In addition, Potecuk et al. [73] proposed that BzATP-induced CREB phosphorylation may be ERK1/2-dependent in microglial cells, but this study only examined BzATP-induced pCREB using 10 μM UO126 at one unspecified time-point. Considering that BzATP, although a potent agonist of P2RX7, can also activate other P2RX receptors [39, 40], it was critical to rigorously test that these nucleotide-driven events are mediated through P2RX7-dependent signaling events. The present study strongly supports a role for the P2RX7 in the phosphorylation of CREB and the CREB-related transcription factor ATF-1 in addition to CREB/CBP complex formation, which together, are sufficient for CREB-dependent gene expression. This report is the first study to show that P2RX7 activation is responsible for nucleotide-induced CREB phosphorylation in multiple monocytic cell systems. The involvement of P2RX7 over other P2R receptors that are coexpressed in monocytic cells was strongly supported by the use of the P2RX7-defective macrophage mutants as well as heterologous expression of human P2RX7 in HEK-293 cells. Our data also support a role for ERK1/2 in P2RX7 agonist-induced CREB phosphorylation in RAW 264.7 macrophages as well as primary human-derived blood monocytes. It is noteworthy that this is the first study that has shown that the activation of ERK1/2 was necessary for P2RX7 agonist-induced CREB/CBP complex formation, which further supports the role of this MAPK in P2RX7 agonist-induced CREB activation.
The present study also supports a role for Ca2+ in P2RX7 agonist-induced ERK/CREB phosphorylation in monocytic cells. It has been established in other systems that Ca2+-induced CREB activation can result from Ca2+-stimulated activation of protein tyrosine kinase 2 (Pyk2) or Ras protein-specific guanine nucleotide-releasing factor (Ras-GRF) [26]. The tyrosine kinase Pyk2 has been shown to be phosphorylated in response to BzATP in astrocytoma cells overexpressing rat P2RX7 [36], which supports a role for this kinase in P2RX7 signaling. Additionally, our laboratory has previously shown that P2RX7 activation leads to Ras activation in macrophages [16], and Ras-GRF is directly upstream of Ras activation [26]. Therefore, calcium-dependent activation of Pyk2 or Ras may be involved in P2RX7 agonist-induced CREB phosphorylation, but further studies are needed.
Although CBP is a CREB-binding protein, it also serves as a coactivator for numerous other transcription factors, such as STATs, AP-1, and NF-κB [62, 74]. Numerous studies have found that CBP interaction with one transcription factor results in a functional antagonism of other transcription factors as a result of direct competition for CBP binding [75, 77], suggesting that CBP is a limiting factor in the cell. Numerous transcription factors use CBP as a coactivator; thus, it is a critical nuclear factor in gene transcription [62, 74, 78]. Therefore, the BzATP-induced CREB/CBP interaction supports that CREB is using this coactivator for gene expression.
Our laboratory and others have reported that stimulation of cells with P2RX7 agonists promotes the degradation of IκBα and subsequent nuclear translocation of NF-κB at 1 h, but these data do not provide direct evidence of transcriptional activation. Parry and Mackman [79] reported that activation of CREB competes for limiting amounts of CBP in monocytic cells, inhibiting NF-κB-mediated transcription without preventing nuclear translocation. One of the first genes that is activated upon NF-κB nuclear translocation is IκBα. Interestingly, our lab reported previously that P2RX7 agonists delay the reappearance of IκBα in LPS-treated RAW 264.7 macrophages [16], and this delay may be explained by CREB/CBP interactions that sequester the CBP that NF-κB needs to initiate gene transcription.
Furthermore, c-Jun, another member of the AP-1 family of transcription factors, is activated in murine macrophages in response to P2RX7 agonists [80], but at least 50 min are required for this event to occur. The rapid and transient nature of CREB activation by P2RX7 agonists may account for this delay in c-Jun activation, and CBP is bound to CREB only for a short time (5–30 min). This is important for short- and long-term gene transcription, as it has been observed recently that short-term stimulation of P2RX7s does not lead to apoptosis [81,82] but actually induces cell proliferation [82]. Because of the presence of ectoATPases on the surface of cells, this short-term stimulation is physiologically possible. A role for ERK/CREB activation has been implicated in proliferation induced by oxidative stress [83, 84], which is another inflammatory mediator; thus, it is possible that the observed increase in cell survival after a short exposure to P2RX7 agonists is through the ERK/CREB signaling cascade. Thus, P2RX7 agonist-induced CREB activation may be important in the role of P2RX7 in MGC formation, as the cells within granulomas can proliferate [85], and increased MGC formation has been linked to increased P2RX7 expression [10, 13].
Lastly, the induction of c-Fos protein expression after P2RX7 activation supports the idea that P2RX7 stimulation alone can lead to the induction of protein expression. Our report and the findings that P2RX7 regulates the expression of iNOS and early growth response factor-1 support the idea that P2RX7 is a gene transcription mediator [85, 86]. Recent mRNA microarray analysis comparing P2RX7 agonist-treated PBMC from tuberculosis patients with P2RX7 agonist-treated cells from control patients revealed differential gene expression, suggesting a potentially complex role of P2RX7 in gene regulation [88].
In summary, these data support an important role for P2RX7 activation in CREB-induced protein expression and are one of the first reports to describe the activation of protein expression after P2RX7 activation without the addition of another proinflammatory stimulus. In light of recent evidence that P2RX7 stimulation does not necessarily lead to cell death, it appears that further investigation into other genes that are up-regulated in response to P2RX7 agonists is warranted.
Acknowledgments
This work was supported by National Institutes of Health Grants HL56396 and AI50500. M. L. G. was supported by the Hematology Training 524 Program NIH 5 T32 HL07899-09, University of Wisconsin. We thank Dr. Jennifer Nyborg (Colorado State University) for permitting our use of the GST-KIX construct, the laboratory of Dr. Randal Tibbetts (University of Wisconsin) for providing purified GST-KIX, and members of the Bertics laboratory for critical review of the manuscript.
References
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signal. 2005;1:205–209. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B S, North R A. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Proctor R A, Denlinger L C, Leventhal P S, Daugherty S K, van de Loo J W, Tanke T, Firestein G S, Bertics P J. Protection of mice from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci USA. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux D G, Stam E, Petrushova N, Koller B H, Griffiths R J, Gabel C A. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Guerra A N, Fisette P L, Pfeiffer Z A, Quinchia-Rios B H, Prabhu U, Aga M, Denlinger L C, Guadarrama A G, Abozeid S, Sommer J A, Proctor R A, Bertics P J. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- Labasi J M, Petrushova N, Donovan C, McCurdy S, Lira P, Payette M M, Brissette W, Wicks J R, Audoly L, Gabel C A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Fernando S L, Saunders B M, Sluyter R, Skarratt K K, Goldberg H, Marks G B, Wiley J S, Britton W J. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2006;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiozzi P, Sanz J M, Ferrari D, Falzoni S, Aleotti A, Buell G N, Collo G, Di Virgilio F. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Okamoto H, Horio T. Heightened ability of monocytes from sarcoidosis patients to form multi-nucleated giant cells in vitro by supernatants of concanavalin A-stimulated mononuclear cells. Clin Exp Immunol. 2001;126:151–156. doi: 10.1046/j.1365-2249.2001.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, Chiozzi P, Di Virgilio F. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177:7257–7265. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Chiozzi P, Ferrari D, Buell G, Di Virgilio F. P2X(7) receptor and polykarion formation. Mol Biol Cell. 2000;11:3169–3176. doi: 10.1091/mbc.11.9.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North R A, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- North R A, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Aga M, Johnson C J, Hart A P, Guadarrama A G, Suresh M, Svaren J, Bertics P J, Darien B J. Modulation of monocytes signaling and pore formation in response to agonists of the nucleotide receptor P2X7. J Leukoc Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- Aga M, Watters J J, Pfeiffer Z A, Weipz G J, Sommer J A, Bertics P J. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kB signaling pathways in murine 264.7 macrophages. Am J Physiol Cell Physiol. 2004;286:C923–C930. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi O R, Di Virgilio F. Extracellular ATP triggers IL-1 β release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Grahames C B, Michel A D, Chessell I P, Humphrey P P. Pharmacological characterization of ATP- and LPS-induced IL-1β release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R J, Stam E J, Downs J T, Otterness I G. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;154:2821–2828. [PubMed] [Google Scholar]
- Guerra A N, Gavala M L, Chung H S, Bertics P J. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti M, Sturla L, Giovine M, Benatti U, De Flora A. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-α in lipopolysaccharide-treated RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, Adam D, Bulfone-Paus S. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 β in RAW264 macrophages. J Immunol. 2000;164:3018–3025. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- Wadleigh D J, Reddy S T, Kopp E, Ghosh S, Herschman H R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- Cho M K, Cho Y H, Lee G H, Kim S G. Induction of cyclooxygenase-2 by bovine type I collagen in macrophages via C/EBP and CREB activation by multiple signaling pathways. Biochem Pharmacol. 2004;67:2239–2250. doi: 10.1016/j.bcp.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Eliopoulos A G, Dumitru C D, Wang C C, Cho J, Tsichlis P N. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis A E, Wang Z Q, Cecchini M G, Hofstetter W, Felix R, Fleisch H A, Wagner E F. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Roy S, Charboneau R, Cain K, DeTurris S, Melnyk D, Barke R A. Deficiency of the transcription factor c-fos increases lipopolysaccharide-induced macrophage interleukin 12 production. Surgery. 1999;126:239–247. [PubMed] [Google Scholar]
- Van Orden K, Giebler H A, Lemasson I, Gonzales M, Nyborg J K. Binding of p53 to the KIX domain of CREB binding protein. A potential link to human T-cell leukemia virus, type I-associated leukemogenesis. J Biol Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- Denlinger L C, Sommer J A, Parker K, Gudipaty L, Fisette P L, Watters J W, Proctor R A, Dubyak G R, Bertics P J. Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function. J Immunol. 2003;171:1304–1311. doi: 10.4049/jimmunol.171.3.1304. [DOI] [PubMed] [Google Scholar]
- Korpi-Steiner N L, Bates M E, Lee W M, Hall D J, Bertics P J. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- Gendron F P, Neary J T, Theiss P M, Sun G Y, Gonzalez F A, Weisman G A. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol. 2003;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- Lo C J. MAPK regulation of prostaglandin E2 production by lipopolysaccharide-stimulated macrophages is not dependent on nuclear factor κB. J Surg Res. 2003;113:189–194. doi: 10.1016/s0022-4804(03)00186-0. [DOI] [PubMed] [Google Scholar]
- Liu F, Thompson M A, Wagner S, Greenberg M E, Green M R. Activating transcription factor-1 can mediate Ca(2+)- and cAMP-inducible transcriptional activation. J Biol Chem. 1993;268:6714–6720. [PubMed] [Google Scholar]
- Evans R J, Lewis C, Buell G, Valera S, North R A, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- Bianchi B R, Lynch K J, Touma E, Niforatos W, Burgard E C, Alexander K M, Park H S, Yu H, Metzger R, Kowaluk E, Jarvis M F, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer Z A, Guerra A N, Hill L M, Gavala M L, Prabhu U, Aga M, Hall D J, Bertics P J. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell I P, Simon J, Hibell A D, Michel A D, Barnard E A, Humphrey P P. Cloning and functional characterization of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- Humphreys B D, Dubyak G R. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- Schachter J B, Sromek S M, Nicholas R A, Harden T K. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Deak M, Clifton A D, Lucocq L M, Alessi D R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx L M, Armstrong J N, Feighan D, Wei T, Yong V W, Ransohoff R M, MacVicar B A. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters J J, Sommer J A, Pfeiffer Z A, Prabhu U, Guerra A N, Bertics P J. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1β production. J Biol Chem. 2002;277:9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Goueli S A, Hsiao K, Lu T, Simpson D. UO126: a novel, selective and potent inhibitor of MAP kinase kinase (MEK) Promega Notes. 1998;69:6–10. [Google Scholar]
- Xing J, Kornhauser J M, Xia Z, Thiele E A, Greenberg M E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt R R, Ferrell J E., Jr The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhai Q, Luo Y, Dorf M E. RANTES-mediated chemokine transcription in astrocytes involves activation and translocation of p90 ribosomal S6 protein kinase (RSK) J Biol Chem. 2002;277:19042–19048. doi: 10.1074/jbc.M112442200. [DOI] [PubMed] [Google Scholar]
- Roux P P, Richards S A, Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol. 2003;23:4796–4804. doi: 10.1128/MCB.23.14.4796-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S H, Lin L B, Hung A C, Kuo J S. ATP-stimulated Ca2+ influx and phospholipase D activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J Neurochem. 1999;73:334–343. doi: 10.1046/j.1471-4159.1999.0730334.x. [DOI] [PubMed] [Google Scholar]
- Gudipaty L, Humphreys B D, Buell G, Dubyak G R. Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol. 2001;280:C943–C953. doi: 10.1152/ajpcell.2001.280.4.C943. [DOI] [PubMed] [Google Scholar]
- North R A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Brindle P, Nakajima T, Montminy M. Multiple protein kinase A-regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci USA. 1995;92:10521–10525. doi: 10.1073/pnas.92.23.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Odom D T, Koo S H, Conkright M D, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker J R, Emerson B, Hogenesch J B, Unterman T, Young R A, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- Kwok R P, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Wagner B L, Bauer A, Schutz G, Montminy M. Stimulus-specific interaction between activator-coactivator cognates revealed with a novel complex-specific antiserum. J Biol Chem. 2000;275:8263–8266. doi: 10.1074/jbc.275.12.8263. [DOI] [PubMed] [Google Scholar]
- Cardinaux J R, Notis J C, Zhang Q, Vo N, Craig J C, Fass D M, Brennan R G, Goodman R H. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol Cell Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty D D, Bonni A, Greenberg M E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Gu D, Beltran W A, Li Z, Acland G M, Aguirre G D. Clinical light exposure, photoreceptor degeneration, and AP-1 activation: a cell death or cell survival signal in the rhodopsin mutant retina? Invest Ophthalmol Vis Sci. 2007;48:4907–4918. doi: 10.1167/iovs.07-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossis G, Malnou C E, Farras R, Andermarcher E, Hipskind R, Rodriguez M, Schmidt D, Muller S, Jariel-Encontre I, Piechaczyk M. Down-regulation of c-Fos/c-Jun AP-1 dimer activity by sumoylation. Mol Cell Biol. 2005;25:6964–6979. doi: 10.1128/MCB.25.16.6964-6979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- Roebuck K A, Carpenter L R, Lakshminarayanan V, Page S M, Moy J N, Thomas L L. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- Guo R F, Lentsch A B, Sarma J V, Sun L, Riedemann N C, McClintock S D, McGuire S R, Van Rooijen N, Ward P A. Activator protein-1 activation in acute lung injury. Am J Pathol. 2002;161:275–282. doi: 10.1016/S0002-9440(10)64179-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Mery A G, Beller E M, Favot C, Boyce J A. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- Potucek T D, Crain J M, Watters J J. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Goodman R H, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Fronsdal K, Engedal N, Slagsvold T, Saatcioglu F. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem. 1998;273:31853–31859. doi: 10.1074/jbc.273.48.31853. [DOI] [PubMed] [Google Scholar]
- Sheppard K A, Phelps K M, Williams A J, Thanos D, Glass C K, Rosenfeld M G, Gerritsen M E, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-κB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- Janknecht R, Hunter T. Versatile molecular glue. Transcriptional control. Curr Biol. 1996;6:951–954. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- Parry G C, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- Humphreys B D, Rice J, Kertesy S B, Dubyak G R. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem. 2000;275:26792–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Falzoni S, Chiozzi P, Sanz J M, Ferrari D, Buell G N. ATP receptors and giant cell formation. J Leukoc Biol. 1999;66:723–726. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- Mackenzie A B, Young M T, Adinolfi E, Surprenant A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem. 2005;280:33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- Arany I, Megyesi J K, Reusch J E B, Safirstein R L. CREB mediates ERK-induced survival of mouse renal tubular cells after oxidant stress. Kidney Int. 2005;68:1573–1582. doi: 10.1111/j.1523-1755.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- Barlow C A, Shukla A, Mossman B T, Lounsbury K M. Oxidant-mediated cAMP response element binding protein activation: calcium regulation and role in apoptosis of lung epithelial cells. Am J Respir Cell Mol Biol. 2006;34:7–14. doi: 10.1165/rcmb.2005-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H Y, Nikolic-Paterson D J, Mu W, Atkins R C. Local macrophage proliferation in multinucleated giant cell and granuloma formation in experimental Goodpasture’s syndrome. Am J Pathol. 1995;147:1214–1220. [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Fisette P L, Denlinger L C, Guadarrama A G, Sommer J A, Proctor R A, Bertics P J. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- Stefano L, Rossler O G, Griesemer D, Hoth M, Thiel G. P2X(7) receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca(2+) rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213:36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- Franco-Martinez S, Nino-Moreno P, Bernal-Silva S, Baranda L, Rocha-Meza M, Portales-Cervantes L, Layseca-Espinosa E, Gonzalez-Amaro R, Portales-Perez D. Expression and function of the purinergic receptor P2X7 in patients with pulmonary tuberculosis. Clin Exp Immunol. 2006;146:253–261. doi: 10.1111/j.1365-2249.2006.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]