Abstract
Background
In China, many former plasma donors (FPD) were infected with the human immunodeficiency virus (HIV) in the early-mid 1990s. Highly active antiretroviral therapy (HAART) was provided to FPDs beginning in 2002. The effect of HAART on mortality in this cohort has never been described.
Methods
Retrospective analysis of the national HIV epidemiology and treatment databases, from 1993–2006. All HIV-infected subjects from ten high HIV prevalence counties in six provinces were eligible. Inclusion criteria were: 1) plasma donation history, 2) Western blot positive, 3) clinical diagnosis of AIDS or CD4+ cell count <200/μl at any time, and 4) age ≥18 at AIDS diagnosis.
Results
Of 9059 eligible subjects, 4093 met the inclusion criteria. Mean age was 41 years, 51% were male, 99% were farmers, and 87% were from Henan Province. Overall mortality declined from 27.3/100 person-years in 2001 to 4.6/100 person-years in 2006. Conversely, the proportion of AIDS patient-years on HAART increased from 0% in 2001 to 70.5% in 2006. In a multivariate Cox proportional hazards analysis, the greatest risk factor for mortality was not receiving HAART, with a hazard ratio 2.8, 95% confidence interval 2.4–3.3. Among treated patients, those initiating therapy with lower CD4+ cell counts and higher numbers of opportunistic infections were at greater risk of death.
Conclusions
The national treatment program has significantly reduced the mortality rate among HIV-infected FPDs through the use of generic drugs in a rural treatment setting with limited laboratory monitoring. Treatment success can be improved through increased coverage and earlier initiation of therapy.
Keywords: HIV, China, HAART, mortality, developing country
INTRODUCTION
Although human immunodeficiency virus (HIV) infection was first reported in China in 1985, the magnitude of its spread was not evident until the epidemic among former plasma donors (FPD) across central China was realized. Poor, rural farmers sold plasma to unscrupulous collectors under unsanitary conditions during the early to mid-1990s, causing untold numbers of infection [1]. With the majority of the plasma selling activities, and thus infections, thought to have occurred between 1993–1996 [2–4], large numbers of acquired immunodeficiency syndrome (AIDS) cases developed in this cohort in the early 2000s. In response, the Chinese government initiated the China Comprehensive AIDS Response (China CARES) program to provide free HIV treatment in 127 FPD counties across central China. This program began as a pilot, treating 100 patients in 2002, but was rapidly scaled up to cover over 30,000 patients by the end of 2006 [5–7]. The National Free Antiretroviral Therapy Program (NFATP), built on the foundation laid by China CARES, now stretches across the country, treating over 40,000 patients by the end of 2007.
To date, the effectiveness of highly active antiretroviral therapy (HAART) in China has not been well studied. With few CD4+ T-cell count (CD4 count) and almost no HIV viral load data available in the NFATP, other markers must be used to determine treatment success or failure. Because death records are reliably maintained in China, this study examines the impact of HAART on mortality in several former plasma donating (FPD) regions, regions which have received HAART the longest in China.
METHODS
Patients assessed in this study were identified through two national databases. The first was an epidemiological database of all HIV-infected patients in China reported to the China CDC through the national surveillance system. Included were demographics, route of infection, date of diagnosis, and date of death (if any). The second database was the national treatment database, including all subjects who met the national treatment criteria (WHO stage III or IV disease, CD4 count <200 cells/uL, or total lymphocyte count <1200/mm3) and were provided free treatment [5, 8]. Patients were seen in follow-up every three months and, after each visit, local healthcare providers completed a standardized case report form (CRF) and faxed the form to the China CDC via Datafax. These CRFs were then maintained in an ongoing observational treatment database [5]. For this study, all data obtained from these two databases were validated with the local Center for Disease Control (CDC) in each province.
To evaluate the effect of HAART on mortality, ten counties in six provinces, representing the highest HIV prevalence regions, were selected (Henan: Shangcai County; Anhui: Lixin County; Hubei: Nanzhang County; Shanxi: Jiang, Xinjiang, and Xia Counties; Shandong: Chengwu and Cao Counties, Mudan District; Jilin: Chuanying District). The selected counties were all rural and poor, with the residents being primarily farmers. All known HIV-infected individuals from these ten counties from 1993–2006 were eligible for this study. Inclusion criteria were subjects who: 1) had a plasma (blood) donation history; 2) were confirmed HIV-positive by Western blot; 3) were diagnosed with AIDS either by an AIDS-defining illness or with a CD4 count <200/μl at any time; and 4) were ≥18 years old at the time of AIDS diagnosis.
We defined an opportunistic infection (OI) as only: 1) fever of unknown origin >38°C for >1 month; 2) chronic diarrhea for >1 month; 3) oral candidiasis; or 4) oral hairy leukoplakia. These illnesses were selected because they were easily diagnosed clinically in rural settings. The date of AIDS diagnosis was defined as the earliest date of an AIDS-defining illness or CD4 count <200/μl. The most commonly used regimens were nevirapine with zidovudine or stavudine and didanosine or lamivudine.
The primary endpoint of this study was all-cause mortality. The mortality rate was calculated for the interval 1995–2000 due to the small numbers during these years, then yearly from 2001–2006 by taking the total number of subjects dying within the specified time interval and dividing by the sum of individual person-years for subjects diagnosed with AIDS during the same interval. Those lost-to-follow-up counted as one-half person-year. A similar approach was used to calculate each subject’s person-years on HAART during each time interval. The log-linear model was used to determine whether a decreasing trend for death incidence density existed. Crude AIDS mortality was calculated by demographic characteristics (gender, age at AIDS diagnosis, marriage status, education, occupation, and race) and potential risk factors (number of OIs at HAART initiation and CD4 counts at HAART initiation).
Cox proportional hazard modeling was used to assess the relationship between mortality and potential risk factors. Subjects still alive or lost-to-follow-up in November 2006 were censored. Univariate Cox models were run to assess the unadjusted relationship between mortality and specified covariates of interest for all subjects and stratified by HAART treatment status. Survival time was defined as the time from AIDS diagnosis to either death or censoring. Covariates with a P-value <0.2 from the univariate analysis were entered into a full multivariate Cox model, with stepwise selection used to eliminate non-significant covariates. Because AIDS-related risk factors were only available for subjects on HAART, the analyses for mortality and potential risk factors using Cox proportional hazard models were performed on all subjects, then stratified by treatment status. Data were analyzed using SPSS version 13.0 and SAS version 9.3 (SAS Institute, Cary, NC). All hypothesis testing was based on 2-sided tests with alpha level of 0.05.
RESULTS
Between 1995-November 2006, 9059 HIV-infected individuals were reported from the ten counties, 7576 were Western blot confirmed HIV+ FPDs, and 5314 were diagnosed with AIDS at ≥18 years of age. Of these, 1221 were excluded for logic inconsistencies, leaving 4093 patients in the analysis (Figure 1). The mean age of these subjects was 41 years, 51% were male, 86% were married, and 99% were farmers. Subjects from Henan comprised 87% of the total, consistent with the overall epidemiology of HIV infection among FPDs (Table 1). Of the 4093 total subjects, 2704 (66%) received HAART. The remaining 1389 did not receive treatment largely because treatment was still being scaled up, with patients treated on a first-come-first-served basis. Overall, 716 subjects died during the study period, 15% of those on HAART and 36% of those not (Table 1).
Figure 1.
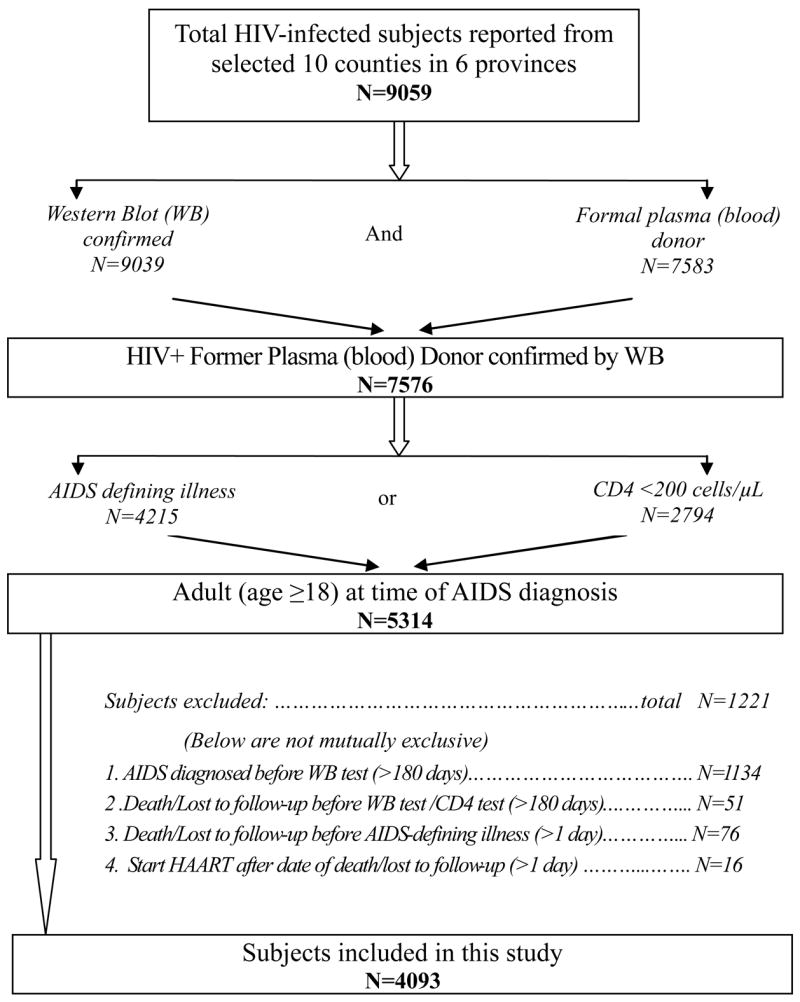
Selection algorithm for the subjects included in the study.
Table 1.
Demographic characteristics by HAART status of 4093 AIDS subjects included in study
| All subjects N (%) | Subjects on HAART (n=2704)
|
Subjects not on HAART (n=1389)
|
|||
|---|---|---|---|---|---|
| Alive N (%) | Died N (%) | Alive N (%) | Died N (%) | ||
| Overall | 4093 | 2352 | 352 | 1025 | 364 |
| Region | |||||
| Henan | 3546 (86.6) | 2019 (85.8) | 312 (88.6) | 952 (92.9) | 263 (72.3) |
| Non-Henan | 547 (13.4) | 333 (14.2) | 40 (11.4) | 73 (7.1) | 101 (27.8) |
| Gender | |||||
| Male | 2080 (50.8) | 1094 (46.5) | 208 (59.1) | 566 (55.2) | 212 (58.2) |
| Female | 2013 (49.2) | 1258 (53.5) | 144 (40.9) | 459 (44.8) | 152 (41.8) |
| Age at Diagnosis (years) | |||||
| Mean +standard deviation | 41.2+8.7 | 41.1+8.3 | 42.8+9.0 | 40.6+8.8 | 42.5+9.9 |
| 18–29 | 299 (7.3) | 164 (7.0) | 20 (5.7) | 88 (8.6) | 27 (7.4) |
| 30–39 | 1784 (43.6) | 1035 (44.0) | 126 (35.8) | 470 (45.9) | 153 (42.0) |
| 40–49 | 1305 (31.9) | 782 (33.3) | 123 (34.9) | 301 (29.4) | 99 (27.2) |
| 50–59 | 611 (14.9) | 332 (14.1) | 74 (21.0) | 138 (13.5) | 67 (18.4) |
| ≥60 | 94 (2.3) | 39 (1.7) | 9 (2.6) | 28 (2.7) | 18 (5.0) |
| Marital Status† | |||||
| Single | 165 (4.0) | 61 (2.6) | 28 (8.0) | 41 (4.0) | 35 (9.7) |
| Married/Live together | 3576 (87.5) | 2058 (87.6) | 310 (88.1) | 887 (86.7) | 321 (88.9) |
| Divorced/Widowed | 344 (8.4) | 230 (9.8) | 14 (4.0) | 95 (9.3) | 5 (1.4) |
| Education‡ | |||||
| No school | 598 (14.9) | 356 (15.5) | 64 (18.3) | 109 (10.9) | 69 (19.2) |
| Primary | 1935 (48.2) | 1095 (47.6) | 175 (50.1) | 473 (47.2) | 192 (53.3) |
| Middle school & above | 1482 (36.9) | 852 (37.0) | 110 (31.5) | 421 (42.0) | 99 (27.5) |
| Occupation | |||||
| Farmer | 4045 (98.8) | 2321 (98.7) | 349 (99.2) | 1019 (99.4) | 356 (97.8) |
| Other | 48 (1.2) | 31 (1.3) | 3 (0.9) | 6 (0.6) | 8 (2.2) |
| Race | |||||
| Han | 4034 (98.6) | 2318 (98.6) | 350 (99.4) | 1006 (98.2) | 360 (98.9) |
| Other | 59 (1.4) | 34 (1.5) | 2 (0.6) | 19 (1.9) | 4 (1.1) |
8 cases missing;
78 cases missing
The all-cause mortality rate and the proportion of AIDS patient-years on HAART over time are shown in Figure 2. Before national treatment was initiated, mortality ranged from 27.3–30.2/100 person-years. Treatment was piloted in 2002, then ramped up in 2003. Compared to the mortality rate of 27.3/100 person-years in 2001, each year from 2003 onwards was significantly lower (P≤0.0004). Conversely, the proportion of patients on HAART increased from 0% of AIDS patient-years in 2001 to 70.5% in November 2006, showing an inverse relationship between mortality and proportion of AIDS patients on HAART.
Figure 2.
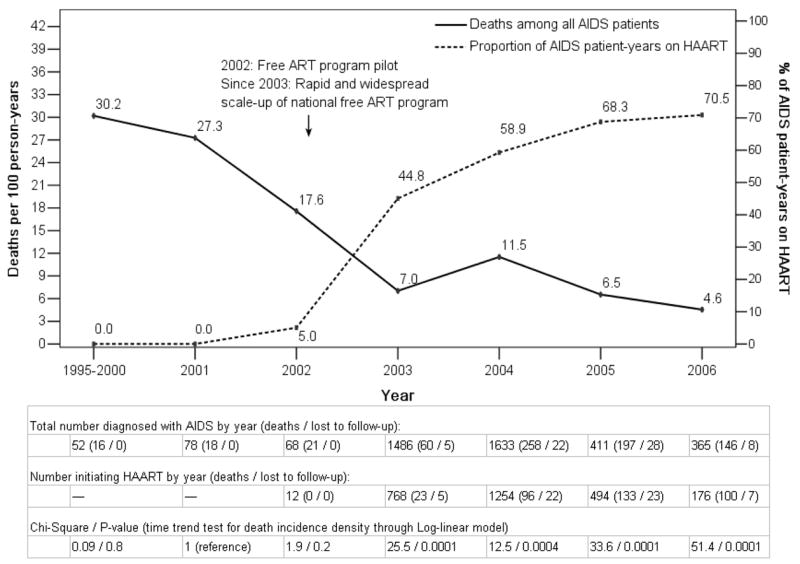
Mortality rate and proportion of AIDS patient-years on HAART over time for subjects included in the study.
In univariate Cox proportional hazard models of risk factors associated with mortality, the greatest risk factor was lack of HAART, with a 2.9 fold increased risk of death compared to those on HAART (Table 2). Other factors significantly associated with increased mortality included being single (hazard ratio [HR]=2.7, compared to married), age ≥50 (HR=1.6), male (HR=1.4), and less education (HR=1.4). Being divorced/widowed was protective against mortality (HR=0.27) compared with being married. Each of these factors remained significant in the overall multivariate model with similar HRs (Table 3). When the overall multivariate model was stratified by treatment status, however, marital status and education became insignificant (P>0.05) in the treatment arm but remained significant in the untreated arm. In the multivariate model of mortality among treated patients, male gender (HR=1.9) and age ≥50 (HR=1.7) remained significant. Additional significant risk factors included ≥2 OIs (HR=2.3), compared to no OIs at treatment initiation, and CD4 count <50/μl (HR=6.9) or 50–99/μl (HR=3.5) at treatment initiation, compared to ≥200/μl (Table 3). Thus, the patients on HAART who survive the best are women less than 50 years old who are treated before any OIs develop and have CD4 counts ≥100/μl when starting treatment.
Table 2.
Unadjusted association of mortality and risk factors by HAART status
| All Subjects (deaths=716)
|
Subjects on HAART (deaths=352)
|
Subjects not on HAART (deaths=364)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths (%) | HR (95% CI) | P value | Deaths (%) | HR (95% CI) | P value | Deaths (%) | HR (95% CI) | P value | |
| Gender | |||||||||
| Male | 420 (20.2) | 1.4 (1.2–1.7) | <.0001 | 208 (16.0) | 1.6 (1.3–2.0) | <.0001 | 212 (27.3) | 1.1 (0.9–1.4) | 0.2 |
| Female | 296 (14.7) | 1.0 | 144 (10.3) | 1.0 | 152 (24.9) | 1.0 | |||
| Age at AIDS Diagnosis (years) | |||||||||
| <50 | 548 (16.2) | 1.0 | 269 (12.0) | 1.0 | 279 (24.5) | 1.0 | |||
| ≥50 | 168 (23.8) | 1.6 (1.3– 1.9) | <.0001 | 83 (18.3) | 1.6 (1.3–2.1) | <.0001 | 85 (33.9) | 1.5 (1.2–2.0) | 0.001 |
| Marriage | |||||||||
| Single | 63 (38.2) | 2.7 (2.1–3.5) | <.0001 | 28 (31.5) | 3.1 (2.1–4.6) | <.0001 | 35 (46.1) | 1.9 (1.3–2.7) | <.0001 |
| Divorced/Widowed | 19 (5.5) | 0.3 (0.2–0.5) | <.0001 | 14 (5.7) | 0.4 (0.2–0.7) | 0.001 | 5 (5.0) | 0.2 (0.1–0.4) | <.0001 |
| Married/Live together | 631 (17.7) | 1.0 | 310 (13.1) | 1.0 | 321 (26.6) | 1.0 | |||
| Education | |||||||||
| Primary school & less | 500 (19.2) | 1.4 (1.2–1.7) | <.0001 | 239 (14.1) | 1.2 (1.0–1.5) | 0.08 | 261 (31.0) | 1.7 (1.4–2.2) | <.0001 |
| Middle school & above | 209 (14.1) | 1.0 | 110 (11.4) | 1.0 | 99 (19.0) | 1.0 | |||
| Occupation | |||||||||
| Farmer | 705 (17.4) | 1.0 | 349 (13.1) | 1.0 | 356 (25.9) | 1.0 | |||
| Other | 11 (22.9) | 1.3 (0.7–2.4) | 0.3 | 3 (8.8) | 0.6 (0.2–2.0) | 0.4 | 8 (57.1) | 3.1 (1.5–6.3) | 0.02 |
| Race | |||||||||
| Han | 710 (17.6) | 1.0 | 350 (13.1) | 1.0 | 360 (26.4) | 1.0 | |||
| Other | 6 (10.2) | 1.1 (0.5–2.4) | 0.9 | 2 (5.6) | 1.1 (0.3–4.3) | 0.9 | 4 (17.4) | 1.0 (0.4–2.7) | 1.0 |
| On HAART | |||||||||
| Yes | 292 (12.4) | 1.0 | NA | NA | |||||
| No | 292 (25.8) | 2.9 (2.5– 3.4) | <.0001 | ||||||
| # OIs at HAART initiation † | |||||||||
| None | NA | 28 (5.7) | 1.0 | ||||||
| 1 | 116 (10.5) | 1.7 (1.1– 2.5) | 0.01 | NA | |||||
| ≥2 | 208 (18.9) | 3.1 (2.1– 4.7) | <.0001 | ||||||
| CD4 Counts at HAART initiation (cells/μL) | |||||||||
| 0–49 | NA | 38 (19.7) | 7.4 (4.2–13.1) | <.0001 | NA | ||||
| 50–99 | 16 (9.0) | 3.2 (1.6–6.4) | 0.001 | ||||||
| 100–199 | 19 (3.9) | 1.3 (0.7–2.5) | 0.4 | ||||||
| ≥200 | 17 (3.7) | 1.0 | |||||||
Opportunistic Infections (OI) include fever, diarrhea, thrush, and oral hairy leukoplakia.
NA: the covariate was not considered for proportional hazard models.
Table 3.
Adjusted association of mortality and risk factors by HAART status
| All Subjects1 |
Subjects on HAART2 |
Subjects not on HAART3 |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | NS | |||||
| Male | 1.3 (1.1–1.6) | <.0001 | 1.9 (1.2–3.0) | 0.004 | ||
| Female | 1.0 | 1.0 | ||||
| Age at AIDS Diagnosis (years) | ||||||
| <50 | 1.0 | 1.0 | 1.0 | |||
| ≥50 | 1.6 (1.3–1.9) | <.0001 | 1.7 (1.1–2.9) | 0.03 | 1.4 (1.1–1.8) | 0.01 |
| Marriage | NS | |||||
| Single | 2.3 (1.8–3.0) | <.0001 | 2.1 (1.5–3.0) | <.0001 | ||
| Divorced/Widowed | 0.3 (0.2–0.4) | <.0001 | 0.2 (0.1–0.4) | <.0001 | ||
| Married/Live together | 1.0 | 1.0 | ||||
| Education | NS | |||||
| Primary school & less | 1.5 (1.2–1.7) | <.0001 | 1.7 (1.3–2.2) | <.0001 | ||
| Middle school & above | 1.0 | 1.0 | ||||
| On HAART | NS | NA | ||||
| Yes | 1.0 | |||||
| No | 2.8 (2.4–3.3) | <.0001 | ||||
| # OI symptoms at HAART initiation | NA | NA | ||||
| None | 1.0 | |||||
| 1 | 1.5 (0.7–3.3) | 0.3 | ||||
| ≥2 | 2.3 (1.1–4.8) | 0.03 | ||||
| CD4 Counts at HAART initiation | NA | NA | ||||
| 0–49 | 6.9 (3.9–12.2) | <.0001 | ||||
| 50–99 | 3.5 (1.8–6.9) | <.0001 | ||||
| 100–199 | 1.3 (0.7–2.5) | 0.4 | ||||
| ≥200 | 1.0 | |||||
The initial full model included gender, age at AIDS diagnosis, marriage, education, and on HAART;
The initial full model included gender, age at AIDS diagnosis, marriage, education, number of OIs at HAART initiation, and CD4 counts at HAART initiation.
The initial full model included gender, age at AIDS diagnosis, marriage, education, and occupation.
NA: the covariate was not considered for proportional hazard models.
NS: the covariate was either insignificant, not entered into initial full model, or deleted from the model selection procedure.
DISCUSSION
The effect of the China NFATP on mortality of AIDS patients has not previously been reported. The FPD cohort was selected for this analysis because HAART in China was begun in this population and thus they have the longest duration of follow-up, to date. Ten of the highest HIV prevalence counties from six provinces were selected, including Shangcai County in Henan Province where the plasma donating activities in the mid-1990s were concentrated, and all known adult AIDS patients were included. The results of this study demonstrate a five-fold decrease in mortality from before 2002 (27–30/100 person-years) to 2005–2006 (5–6/100 person-years). Of note, this reduction in mortality was achieved primarily through the use of Chinese-produced generic antiretroviral drugs. In a multivariate Cox proportional-hazard regression, the most significant factor associated with mortality was no HAART, with a 2.8 fold increased risk of death. For patients not on HAART, additional risk factors for death were age ≥50, being single, and having a primary school education or less. For those on HAART, additional mortality risk factors were increased numbers of OIs and lower CD4 counts at treatment initiation.
All-cause mortality was used instead of AIDS-related mortality because only a few deaths were confirmed as non-AIDS related. Of the 716 deaths during the study, 609 (85.1%) were due to AIDS-related causes, 97 (13.5%) to missing/unknown causes, and 10 (1.4%) to non-AIDS causes, such as accidents or suicides. Non-AIDS deaths were included because 10 deaths would not have a significant effect on the mortality rate and, because stigma against HIV was prevalent in FPD regions [9–11], it is difficult to exclude AIDS as a factor in suicides. For missing/unknown deaths, the vast majority were felt to be related to AIDS because this was an otherwise young and healthy population. However, if these deaths were all considered non-AIDS instead, the calculated mortality rate in 2005–2006 (all missing/unknown deaths were from these two years) would be even lower, further emphasizing the effect of HAART.
The reduction in mortality achieved by the NFATP is consistent with reductions seen in other countries [12–20]. This reduction of HIV-associated mortality in China is remarkable for several reasons. First, the vast majority of the antiretroviral drugs used in China are Chinese-produced generic drugs, including zidovudine, stavudine, didanosine, nevirapine, and a limited amount of indinavir. First-line antiretroviral regimens were composed of these initial four drugs until 2005, when branded lamivudine became available. A limited amount of branded efavirenz subsequently also became available for second line regimens. Thus, with initial HAART regimens and two of three drugs in subsequent regimens composed of Chinese generics, the reduced mortality among treated patients demonstrates the benefits of these drugs, although we do not have comparative data against other branded or generic drugs.
A second reason why the mortality reduction in China is remarkable is the structure of the NFATP, which is administered through a low-technology, community-based treatment model using rural healthcare workers [5]. Because China’s HIV epidemic is predominantly based in rural areas, treatment was scaled up using the already existing rural clinics. A national physician training program was established in 2003 to train physicians from provincial to county levels, with the curriculum content geared to the level of physician [6]. Although difficulties were encountered during the initial scale-up in 2003 and 2004 with insufficient education and experience of the clinicians, lack of counseling of the patients, poor adherence, and drug side effects, the overall success of this community-based treatment model can now be appreciated. Under appropriate circumstances, as also demonstrated in other countries, rural healthcare workers can be trained to administer HAART successfully [19, 21].
Finally, a third aspect of China’s NFATP is the general lack of laboratory monitoring of treated patients, particularly in years 2003–2005, because CD4 and viral load testing were not provided through the NFATP and patients generally could not afford them. Some provinces began providing free CD4 testing in 2006 and this continues to be scaled up. Viral load testing, however, is still rarely done. Despite this lack of consistent laboratory monitoring, clear reductions in mortality are seen. Whether or not routine CD4 and viral load testing would have improved the mortality rates even more is not clear. The NFATP does recognize the importance of such monitoring, however, and is working to scale up testing.
In the multivariate Cox proportional hazards regression analysis of risk factors associated with death, not being on HAART was the single greatest risk factor with a HR=2.8, as well as the only readily modifiable factor. The other risk factors significant in the overall model included gender, age, marital status, and educational level. When stratified by treatment status, those not on HAART had the same significant demographic risk factors except for gender, showing that the untreated patients primarily drive the demographic risk factors in the overall model. Increased age and decreased educational level have been previously associated with increased mortality [22–24]. Reports of gender differences in HIV mortality have been mixed, with studies reporting higher male mortality [23], female mortality [25], or no difference [22, 26, 27]. Why single people are at higher risk and divorced or widowed people at lower risk of mortality compared to married people or people living together is not clear and may be related to the small numbers in each category. In the stratified analysis of those on HAART, increased numbers of OIs and decreased CD4 counts at HAART initiation, as well as decreased duration of treatment, were all significantly associated with mortality. Our finding of worse outcomes when HAART is initiated later is consistent with other studies [18, 19, 28].
The primary limitation of this study is that it is based on observational data and thus may be subject to inherent biases. Without a non-treatment control group as comparison, how the baseline mortality rate would have changed without HAART is not known. Because the vast majority of infections occurred from 1993–1995, the decline in mortality beginning in 2002 may simply reflect the natural history of HIV infection in this cohort with the majority of those infected already having died. However, a decline in deaths due to natural history would be a decline in the absolute numbers of deaths, not a decline in the mortality rate. A decline in mortality rate can only be achieved through altering the actual disease process and the only large-scale intervention that altered the disease process at that time was HAART. Finally, our post-treatment mortality rate is consistent with mortality rates among treated AIDS patients reported world-wide and has been statistically stable since 2003. Thus HAART is the only reasonable explanation for the decline in mortality rate seen.
Another limitation is that before 2003, the numbers of reported cases of HIV were relatively small and thus may not be representative of overall mortality trends in these 10 counties. In 2003 and 2004, mass HIV screenings among the FPD cohort were done in several provinces, identifying an estimated >90% of the infected FPD in those areas. Data after 2003 are therefore much more stable and representative. Even though the mortality rate of AIDS patients before 2003 may not be precise, the overall mortality trend from the pre-HAART to the post-HAART years will not change significantly. Furthermore, because most deaths among AIDS patients before 2003 were not identified as such, the actual mortality rate may be even higher than what we report here, emphasizing the beneficial effect of HAART.
One final limitation is that some patients may be misclassified as AIDS patients when, in fact, they have not yet reached that threshold. This group would primarily include those who did not have pre-treatment CD4 counts and thus met treatment criteria through clinical diagnosis of an AIDS-defining OI. In 2003 and 2004, treatment was just being scaled up with all HIV patients clamoring for treatment whether or not they met treatment criteria. Due to pressure from patients, some were prescribed HAART before treatment criteria were met. The effect of this, however, would be to minimize the impact of HAART on mortality. Thus the significant difference in mortality between pre- and post-HAART eras despite this bias further demonstrates the robustness of our results. Another possible misclassification could occur if HAART-treated patients were less sick than untreated patients, creating a selection bias that could explain the difference in HR between the two groups. In fact, the treated group was sicker than the untreated group in median CD4 count (treated: 163/ml at treatment vs. untreated: 206/ml most recent, P<0.0001) and in the proportion with any OI at diagnosis (treated: 69% vs. untreated: 31%, P<0.0001). Because treated patients were sicker than untreated patients, the true impact of HAART was actually larger than our HR estimate.
The decline in mortality among FPD AIDS patients in China reported in this analysis validates the beneficial impact of the NFATP, a program built on Chinese generic drugs and community-based treatment by rural healthcare providers. Although this success is noteworthy, much work remains to be done to build upon this initial success. Treatment must continue to be scaled up to cover everyone in need. Patients eligible for treatment must be identified and treated before they develop OIs or have CD4 counts that are too low. The availability of laboratory monitoring measures such as CD4 and viral load testing must also continue to be scaled up. Additional second-line drugs are critically important as patients gradually fail their initial regimens. Some preliminary national HIV resistance surveillance has been done and these results need to be correlated with treatment records to inform national policy on the availability of second-line drugs. As patients live longer due to HAART, other co-morbidities will become factors. Studies have shown extremely high rates of hepatitis C virus co-infection among FPDs [29–31]. How to follow and treat these patients before they progress to end-stage liver disease in rural China is a significant challenge. Finally, research on patients currently being treated must continue to improve treatment options and opportunities. The Chinese government has invested a significant amount of money on providing national free HIV treatment over the last several years and this investment is now demonstrating tangible returns.
Acknowledgments
We acknowledge the work of the staff of the local county CDCs, who spent many hours working with us in obtaining, verifying, and cleaning the data used in this study. This study was supported by the applied research program on AIDS prevention and treatment of the China Ministry of Health (WA-2003-03 and WA-2006-03), the China National Basic Research Program (973 program, 2006CB504201), and the U.S. National Institutes of Health, ICOHRTA grant (U2R TW006918).
Footnotes
Conflict of Interest Statement
All authors declare no conflicts of interest.
References
- 1.He N, Detels R. The HIV epidemic in China: history, response, and challenge. Cell Res. 2005 Nov–Dec;15(11–12):825–32. doi: 10.1038/sj.cr.7290354. [DOI] [PubMed] [Google Scholar]
- 2.Zhang K. The natural history of HIV infection among paid blood donors in Henan Province. Chin J AIDS STD. 2006;12(4):291–3. [Google Scholar]
- 3.Liu Q. The current status, special characteristics, social impact, and effect of policies of the AIDS epidemic in Henan Province. J US-China Public Admin. 2007;4(2):28–34. [Google Scholar]
- 4.Blood products regulation act. Central People’s Government of the People’s Republic of China, December 30, 1996.
- 5.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005 Nov–Dec;15(11–12):877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Hsu M, Yu L, Wen Y, Pan J. Initiation of the National Free Antiretroviral Therapy Program in Rural China. In: Kaufman J, Kleinman A, Saich T, editors. AIDS and Social Policy in China. Cambridge, MA: Harvard University Asia Center; 2006. [Google Scholar]
- 7.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. Aids. 2007 Dec;21(Suppl 8):S143–8. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FJ, editor. China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; Jan, 2005. [Google Scholar]
- 9.Liu H, Hu Z, Li X, Stanton B, Naar-King S, Yang H. Understanding interrelationships among HIV-related stigma, concern about HIV infection, and intent to disclose HIV serostatus: a pretest-posttest study in a rural area of eastern China. AIDS Patient Care STDS. 2006 Feb;20(2):133–42. doi: 10.1089/apc.2006.20.133. [DOI] [PubMed] [Google Scholar]
- 10.Cao X, Sullivan SG, Xu J, Wu Z. Understanding HIV-related stigma and discrimination in a “blameless” population. AIDS Educ Prev. 2006 Dec;18(6):518–28. doi: 10.1521/aeap.2006.18.6.518. [DOI] [PubMed] [Google Scholar]
- 11.Qian HZ, Wang N, Dong S, et al. Association of misconceptions about HIV transmission and discriminatory attitudes in rural China. AIDS Care. 2007 Nov;19(10):1283–7. doi: 10.1080/09540120701402814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 14.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001 Jul 3;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Chan KC, Wong KH, Lee SS. Universal decline in mortality in patients with advanced HIV-1 disease in various demographic subpopulations after the introduction of HAART in Hong Kong, from 1993 to 2002. HIV Med. 2006 Apr;7(3):186–92. doi: 10.1111/j.1468-1293.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 16.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 17.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998 Nov 28;352(9142):1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006 Sep 15;43(6):770–6. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 19.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006 Aug 16;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004 Apr 9;18(6):887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zaba B, Marston M, Crampin AC, et al. Age-specific mortality patterns in HIV-infected individuals: a comparative analysis of African community study data. AIDS (London, England) 2007 Nov;21(Suppl 6):S87–96. doi: 10.1097/01.aids.0000299415.67646.26. [DOI] [PubMed] [Google Scholar]
- 23.Nyirenda M, Hosegood V, Barnighausen T, Newell ML. Mortality levels and trends by HIV serostatus in rural South Africa. AIDS (London, England) 2007 Nov;21(Suppl 6):S73–9. doi: 10.1097/01.aids.0000299413.82893.2b. [DOI] [PubMed] [Google Scholar]
- 24.Joy R, Druyts EF, Brandson EK, et al. Impact of Neighborhood-Level Socioeconomic Status on HIV Disease Progression in a Universal Health Care Setting. J Acquir Immune Defic Syndr. 2008 Jan 11; doi: 10.1097/QAI.0b013e3181648dfd. [DOI] [PubMed] [Google Scholar]
- 25.Braga P, Cardoso MR, Segurado AC. Gender differences in survival in an HIV/AIDS cohort from Sao Paulo, Brazil. AIDS patient care and STDs. 2007 May;21(5):321–8. doi: 10.1089/apc.2006.0111. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Hoyos S, Rodriguez-Arenas MA, Garcia de la Hera M, et al. Progression to AIDS and death and response to HAART in men and women from a multicenter hospital-based cohort. Journal of women’s health (2002) 2007 Sep;16(7):1052–61. doi: 10.1089/jwh.2007.0437. [DOI] [PubMed] [Google Scholar]
- 27.Lutalo T, Gray RH, Wawer M, et al. Survival of HIV-infected treatment-naive individuals with documented dates of seroconversion in Rakai, Uganda. AIDS (London, England) 2007 Nov;21(Suppl 6):S15–9. doi: 10.1097/01.aids.0000299406.44775.de. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS (London, England) 2005 Dec 2;19(18):2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Xiang K, Tang H, et al. Molecular epidemiology of human immunodeficiency virus type 1 and hepatitis C virus in former blood donors in central China. AIDS Res Hum Retroviruses. 2008 Jan;24(1):1–6. doi: 10.1089/aid.2007.0144. [DOI] [PubMed] [Google Scholar]
- 30.Xu JQ, Wang JJ, Han LF, et al. Epidemiology, clinical and laboratory characteristics of currently alive HIV-1 infected former blood donors naive to antiretroviral therapy in Anhui Province, China. Chin Med J (Engl) 2006 Dec 5;119(23):1941–8. [PubMed] [Google Scholar]
- 31.Qian HZ, Vermund SH, Kaslow RA, et al. Co-infection with HIV and hepatitis C virus in former plasma/blood donors: challenge for patient care in rural China. Aids. 2006 Jun 26;20(10):1429–35. doi: 10.1097/01.aids.0000233577.33973.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]


