Abstract
The current study investigated how mechanisms of attention that have been well-characterized in the cognitive psychology literature (Lavie, Hirst, De Fockert, & Viding, 2004; Maylor & Lavie, 1998) may be differentially associated with psychopathic traits in non-incarcerated men. Previous research on cognition and psychopathy indicates that primary psychopathic traits are associated with over-focused attention and/or reduced processing of information peripheral to the focus of attention. Conversely, deficits in executive functioning, such as working memory and cognitive control, are implicated in secondary psychopathic traits. Results revealed a significant relationship between traits typically associated with primary psychopathy (e.g., low anxiety, social dominance, fearlessness, callousness) and reduced processing of task-irrelevant distractors, suggesting diminished basic attentional capacity among individuals high on these traits. In contrast, some characteristics linked to secondary psychopathy (e.g., social alienation, cynicism) showed a positive relationship with impaired working memory functioning, indicative of deficits in cognitive control, whereas other traits (i.e., self-centeredness, antagonism) did not. These results suggest that psychopathic traits are differentially related to selective impairments in attentional functioning, which may help explain the observed heterogeneity in psychopathic manifestations.
Keywords: psychopathy, Psychopathic Personality Inventory, selective attention, working memory
Cleckley (1976) conceptualized psychopathy as a constellation of abnormal personality and affective traits (e.g., superficial charm, low neuroticism, shallow affect) that lead to inadequately motivated antisocial behavior. The term primary psychopathy is often used to refer to the dysfunctional personality profile outlined by Cleckley. However, researchers have also identified secondary pathways to a psychopathic lifestyle, such as poor socialization, low intelligence, or an externalizing predisposition (Krueger et al., 2002; Lykken, 1995, Newman & Brinkley, 1997). Despite presumable etiological differences, both subtypes of psychopathy are associated with deficient self-regulation and frequent antisocial behavior. To parse the heterogeneity of psychopathy, researchers have attempted to identify cognitive and affective processes that are differentially associated with primary versus secondary psychopathic offenders. Given growing empirical evidence that psychopathy is a dimensional construct (Edens, Marcus, Lilienfeld, & Poythress, 2006; Murrie et al., 2007), the current study examined associations among psychopathic personality traits and attention in non-incarcerated individuals.
Facets of Psychopathy
The majority of research on psychopathy is based on studies of incarcerated offenders, though increasingly more research is being conducted with non-forensic samples. This trend follows empirical work that suggests psychopathy can be conceptualized as a constellation of extreme manifestations of normal personality traits (Miller, Lynam, Widiger, & Leukefeld, 2001; Widiger & Lynam, 1998) and is dimensional rather than taxonic in nature (Edens et al., 2006; Murrie et al., 2007). Thus, it is important to investigate hypothesized etiological mechanisms at every level of the construct, including in samples with purportedly lower levels of psychopathic traits (e.g. college students, community participants) than are typically found in forensic samples.
Empirical work in both community and forensic samples indicates that psychopathy is multidimensional (Cooke & Michie, 2001; Hicks, Markon, Patrick, Krueger, & Newman, 2004; Newman, MacCoon, Vaughn, & Sadeh, 2005; Poythress & Skeem, 2006). Factor analysis of the widely used Psychopathy Checklist-Revised diagnostic interview (PCL-R; Hare, 2003) suggests psychopathy represents at least two separable facets: an affective-interpersonal factor related to Cleckley's (1976) conceptualization of primary psychopathy and an impulsive-antisocial lifestyle factor associated with secondary psychopathy (Harpur, Hare, & Hakstian, 1989). Similarly, research by Benning, Patrick, Hicks, Blonigen, and Krueger (2003) indicates that the self-report Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996) is also composed of at least two orthogonal dimensions that parallel the affective-interpersonal and social deviance factors of the PCL-R, respectively. However, the PCL-R and the PPI emphasize different aspects of the psychopathic syndrome. The PPI represents a normal personality trait approach to conceptualizing psychopathy, whereas the PCL-R assesses more malicious antisocial traits and behaviors. Nonetheless, research suggests both instruments can be used to reliably assess psychopathic traits in community and incarcerated samples (Lilienfeld & Andrews, 1996; Poythress, Edens, & Lilienfeld, 1998).
Cognitive Deficits in Psychopathy
Laboratory studies have extended work on the assessment of psychopathy by identifying individual facets or subtypes of psychopathy that are differentially associated with affective and cognitive abnormalities. According to proponents of the low-fear model of psychopathy, the syndrome results from deficits in the emotional circuitry of the brain postulated to modulate the experience of fear, including the amygdala, paralimbic system, and orbitofrontal cortex (Birbaumer et al., 2005; Blair, 2005; Kiehl, 2006; Kiehl et al., 2001). Deficiencies in these affective processing areas are thought to underlie the inadequate development of socialization and moral conscience in psychopathy. In support of this contention, empirical work has linked psychopathy with deficits in aversive conditioning (Flor, Birbaumer, Hermann, Ziegler, & Patrick, 2002), anticipation of threat (Hare, 1965), passive avoidance learning (Newman & Kosson, 1986), response reversal (Blair, 2004) and deficient startle reactivity to threatening cues (Levenston, Patrick, Bradley, & Lang, 2000; Patrick, Bradley, & Lang, 1993), outcomes that could result from amygdala dysfunction or impairments in higher order cortical areas innervated by the amygdala (e.g., orbitofrontal cortex).
Despite the prominence of the low-fear hypothesis, other investigations suggest that psychopathic individuals also perform abnormally on tasks that involve the processing of neutral (i.e., non-affective) stimuli (Hiatt, Schmitt, & Newman, 2004; Jutai & Hare, 1983; Newman, Schmitt, & Voss, 1997; Vitale, Brinkley, Hiatt, & Newman, 2007). Thus, some theorists attribute the development and maintenance of psychopathy partly to abnormalities in attentional processing, such as reduced attentional capacity or breadth (Harpur & Hare, 1990; Kosson, 1996), or over-focused attention (Jutai & Hare, 1983; Kosson & Newman, 1986). Seminal research conducted by Jutai and Hare (1983) found that psychopathic offenders showed smaller N100 responses, an event-related potential (ERP) index of attentional processing, to irrelevant tones when they were engaged in an attentionally demanding task (playing a video game) compared to non-psychopathic offenders. This finding is consistent with the hypothesis that psychopathic individuals selectively allocate attentional resources to motivationally salient stimuli, which concomitantly reduces the processing of less relevant stimuli.
Importantly, studies by Newman and colleagues (Hiatt et al., 2004; Newman, 1998) indicate that abnormal attentional processing is specific to a primary psychopathy subtype (i.e., low anxious psychopathic individuals) and not to what they refer to as secondary psychopathy (i.e., high anxious psychopathic individuals). A series of studies (see Newman et al., in press, for a review) indicate that primary or low-anxious psychopathic offenders fail to use peripheral information to regulate their behavior when they are focused on a primary task (e.g., exhibit response perseveration in gambling tasks despite decreased rewards and increased punishment; Newman, Patterson, & Kosson, 1987). According to Gray and McNaughton (2000), the septohippocampal area (i.e., portions of the medial septum, dentate gyrus, hippocampus, subiculum, entorhinal cortex, and posterior cingulate cortex) facilitates the coordination of behavior by detecting and resolving goal conflicts that arise between incoming contextual information (e.g., bottom-up sensory input represented in subcortical areas) that is incompatible with a top-down attentional set. Based on this premise, individuals with damage to the septohippocampal system would repeatedly fail to notice and utilize novel information that emerges outside the focus of attention, even if it has important implications for behavior, such as subtle changes in threat or reward cues that would typically cause individuals to modify their behavior (e.g., inhibit or adapt responding) and reevaluate their goals.
Newman's model differs from other biopsychological models of psychopathy in that it does not focus exclusively on emotional deficits and dysfunction in affect-related brain regions. However, the septohippocampal system is reciprocally interconnected with several brain regions emphasized in emotion-based models of psychopathy, including the amygdaloid complex, cingulate cortex, and regions of the prefrontal cortex, such as the orbitomedial prefrontal cortex. Newman's theory emphasizes abnormalities in the neural integration of cortical and subcortical areas that have been implicated in psychopathy and aggression by other theorists and emotion researchers (e.g., amygdala, orbitofrontal cortex; Blair, 2005; Davidson, Putnam, & Larson, 2000), although it does not focus on actual dysfunction in these structures. Thus, the current study draws primarily on the model of attentional dysfunction put forth by Newman and colleagues (Newman, 1998; Newman et al., in press). However, the actual neural structures that underlie attentional abnormalities in primary psychopathy are not a focus of the current study.
In contrast to primary psychopathic traits, evidence suggests that secondary psychopathic traits are part of the externalizing spectrum of psychopathology (Krueger et al., 2002) and like other externalizing disorders (e.g., antisocial personality, substance abuse) are associated with deficits in executive functioning and cognitive control. Indeed, deficits in response inhibition and executive functioning have been linked to reactive aggression and the social deviance facet of the PPI (Raine et al., 1998; Sellbom & Verona, 2007). Consistent with this literature, research indicates that deficient functioning of the dorsolateral prefrontal cortex, a region of the brain that mediates working memory functions, such as cognitive control and flexibility, is present in individuals with antisocial personality disorder (Dolan & Park, 2002; Morgan & Lilienfeld, 2000). Similarly, reduced P300 amplitude, an ERP index of working memory functioning that is thought to have neural generators in the lateral prefrontal cortex (Nieuwenhuis, Aston-Jones, & Cohen, 2005) and anterior cingulate cortex (Dien, Spencer, & Donchin, 2003), has been found consistently in externalizing spectrum disorders (e.g., substance abuse, antisocial personality; Iacono, Malone, & McGue, 2003; Patrick et al., 2006). Recent work has demonstrated diminished error-related negativity, an ERP index of conflict monitoring generated by the anterior cingulate (Dehaene, Posner, & Tucker, 1994), among individuals high on externalizing traits (Hall, Bernat, & Patrick, 2007).
Together, the results of these studies suggest that deficits in cognitive control and conflict monitoring are present among individuals with an externalizing vulnerability. The view taken in this paper is that the overlap between secondary psychopathy and other externalizing disorders may explain the deficits in executive functioning and cognitive control that have been observed in some neuropsychological studies on psychopathy (Gorenstein & Newman, 1980; Raine et al., 1998).
Mechanisms of Selective Attention and Cognitive Control
Interestingly, the distinction between selective attention and cognitive control deficits in primary and secondary psychopathy, respectively, parallels basic research conducted in the cognitive psychology literature (Lavie, 1995; Lavie et al., 2004). Lavie and colleagues have identified two mechanisms of attention that are thought to regulate the processing of irrelevant distractors: a perceptual selection mechanism and cognitive control mechanism. According to Lavie et al. (2004), the perceptual selection mechanism is a consequence of processing limitations in the brain and is automatically implemented when perceptual demands exceed sensory processing capabilities. A series of behavioral (Lavie, 1995; Lavie & Tsal, 1994; Maylor & Lavie, 1998) and fMRI (Rees, Frith, & Lavie, 1997; Schwartz et al., 2005) studies revealed that perceptual load (i.e., the number of task-relevant items in a display or the perceptual demands of a task) influences the extent to which irrelevant distractors affect task-related behavior and are perceived at the level of the visual cortex. Specifically, with only few task-relevant stimuli in a display (i.e., low perceptual load), participants demonstrate significant response interference to incompatible target-distractor combinations. In contrast, when a display includes many task-relevant stimuli (i.e., high perceptual load) perceptual capacity is taxed and participants' responses are no longer slowed by incompatible targets and distractors, which is reflected in reduced activation in visual cortex as early as V1 (Schwartz et al., 2005).
Neuroimaging studies indicate that a frontoparietal network, that includes right prefrontal (i.e., frontal eye fields, anterior cingulate gyrus) and parietal cortices, modulates attention under high load and determines when stimuli enter visual awareness (Lavie, 2006; Schwartz et al., 2005). Once this attention network is engaged, the septohippocampal system would be important for monitoring goal conflicts that arise between task-relevant sensory input activated in subcortical areas (e.g., target-distractor response conflict) and goal-directed behavior guided by a top-down attentional set (e.g., rapid and accurate target identification). Thus, Newman's model of septohippocampal dysfunction predicts that primary psychopathic individuals will show less interference from irrelevant distractors, indicated by deficient distractor processing at lower levels of perceptual load, because subcortical representations of response conflict will have less of an impact on attention and ultimately concomitant behavioral responses.
Lavie's cognitive control mechanism presumably operates at a later stage of processing and is involved in suppressing the effects of irrelevant distractors on behavior after they have been perceived and recognized. Unlike the perceptual selection mechanism that is initiated automatically and involves sensory areas, the cognitive control mechanism is actively implemented by executive functions, particularly working memory functioning governed in part by the dorsolateral prefrontal cortex (Lavie et al., 2004). Data indicate that under high working memory demands, individuals exhibit greater response interference from distractors than they do under low working memory demands (Lavie et al., 2004), suggesting that available working memory capacity is critical for suppressing the effects of incompatible distractors in situations of response conflict. Dorsolateral areas of the prefrontal cortex (e.g., inferior frontal gyrus, middle frontal gyrus, precentral gyrus) have been shown to maintain task priorities in situations of response conflict (i.e., when targets and distractors are associated with different behavioral responses) by suppressing behavioral responses to lower-priority, incompatible distractors (De Fockert, Rees, Frith, & Lavie, 2001). Importantly, individuals with secondary psychopathic traits and externalizing disorders show impaired functioning in these same areas of the prefrontal cortex (e.g., Dolan & Park, 2002; Raine et al., 1998).
Present Study
Given the parallels between the mechanisms of attention identified by Lavie and colleagues and the impaired selective attention and cognitive control observed in different variants of psychopathy, the present study examined whether facets of psychopathic personality traits were differentially related to functioning on tasks that assess perceptual selection and cognitive control. To test these associations, we adopted tasks developed by Lavie that provided us with an established theoretical framework for making inferences about the processing stage (e.g., basic perception vs. controlled processes) at which these deficits occur. Based on the extant literature, the current study tested the hypothesis that primary psychopathic traits are associated with deficits in selective attention (Hiatt et al., 2004; Newman, 1998), whereas secondary psychopathic traits are linked to deficits in executive functioning or cognitive control similar to those observed in other externalizing disorders (Morgan & Lilienfeld, 2000; Sellbom & Verona, 2007).
On the Perceptual Load Task, we predicted that individuals scoring high on traits related to primary psychopathy (fearlessness, social dominance, callousness) would exhibit reduced distractor processing at lower levels of perceptual load (e.g., with fewer items in array) than those scoring low on the those traits, given the previous findings by Newman and colleagues (Hiatt et al., 2004; Newman, 1998) of reduced peripheral processing among primary psychopaths. Previous research on the Perceptual Load Task indicates that young adults continue to process peripheral distractors until the most demanding load (i.e., load 6) and that it is almost impossible to ignore distractors at the least demanding load (i.e., load 1; Huang-Pollock et al., 2002; Maylor & Lavie, 1998). Thus, we predicted that primary psychopathic traits would be inversely related to distractor processing at an intermediate level of perceptual load, either load 2 or 4, given our hypothesis that these traits are associated with deficient, but not completely impaired attentional capacity. In contrast, we expected the secondary psychopathic or social deviance traits to be associated with more difficulty rejecting distractors under high working memory demands on the Cognitive Control Task, due to research that suggests externalizing traits are associated with impaired cognitive control.
Methods
Participants
We recruited 107 males through flyers posted in the community (n = 29) and the Psychology Department Subject Pool (n = 78) to participate in the study.1 These recruitment strategies were used to ensure that individuals who were not college freshmen could also be included in the study. Three participants were removed from analyses, because their error rates on the Perceptual Load Task (n = 2) or the Working Memory Task (n = 1) were more than 4 SDs above the sample mean. We removed four participants from analyses, due to elevated scores on one or more of the PPI validity scales that indicated their responses were either inconsistent or unreliable. To be included in the study, participants needed to obtain an estimated full scale IQ score above 70 on the Shipley Institute of Living Scale, which all participants did.
Table 1 includes demographic information and IQ scores for the final sample, and the community and student subsamples separately. Ninety-five of the final 100 participants were between the ages of 18 to 22 and all were age 30 or younger. The majority of participants identified as Caucasian (69 %), reported an annual household income of $60,001 - $75,000+ (60 %), and were currently enrolled in college (90 %). Of the final 100 participants, five had missing data from either the Working Memory Task (n = 3) or the Perceptual Load Task (n = 2) and thus, were excluded from the analysis of those measures.
Table 1. Demographic Characteristics and Estimated IQ for Final Sample and Statistical Comparisons of Subsamples.
| Total Sample | Community Subsample | Subject Pool Subsample | Subsample Comparisons | |||||
|---|---|---|---|---|---|---|---|---|
| M (SD) | Min/Max | M (SD) | Min/Max | M (SD) | Min/ Max | Test Statistic / P-Value | ||
| Age | 20 (1.9) | 18 / 30 | 19 (1.6) | 18 / 30 | 21 (2.0) | 19 / 27 | t = 4.84 / .00 | |
| Shipley's Estimated IQ | 110 (6.8) | 91 / 123 | 110 (7.2) | 91 / 123 | 110 (5.6) | 99 / 123 | t = .140 / .89 | |
|
|
||||||||
| Frequency (%) | Frequency (%) | Frequency (%) | ||||||
|
|
||||||||
| Ethnicity | ||||||||
| Caucasian | 69 (69.0) | 17 (65.4) | 52 (70.3) | χ2 = 6.02 / .31 | ||||
| African -American | 2 (2.0) | 1 (3.8) | 1 (1.4) | |||||
| Asian | 16 (16.0) | 3 (11.5) | 13 (17.6) | |||||
| Hispanic | 6 (6.0) | 1 (3.8) | 5 (6.8) | |||||
| Other | 7 (7.0) | 5 (15.3) | 3 (4.1) | |||||
| Education | χ2 = 28.1 / .00 | |||||||
| < High School | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| High School Diploma | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Current College | 90 (90.0) | 17 (65.4) | 74 (100.0) | |||||
| Some College | 3 (3.0) | 3 (11.5) | 0 (0.0) | |||||
| Bachelor's Degree | 6 (6.0) | 5 (19.2) | 0 (0.0) | |||||
| Higher Education | 1 (1.0) | 1 (3.8) | 0 (0.0) | |||||
| Household Income | χ2 = 2.64 / .76 | |||||||
| $0 - $30,000 | 18 (18.4) | 5 (19.2) | 13 (18.1) | |||||
| $30,001 - $60,000 | 21 (21.4) | 8 (30.8) | 13 (18.0) | |||||
| $60,001 - $75,000+ | 60 (59.2) | 13 (50.0) | 46 (63.9) | |||||
Note. Total Sample N = 100; Community Sample n = 26; Student Sample n = 74. Statistics in bold indicate significant differences between the community and subject pool subsamples.
Procedure
Prior to beginning the study, we obtained written informed consent from all participants. Completion of the cognitive tasks was counterbalanced across participants so that half of the participants completed the Cognitive Control Task first and half completed the Perceptual Load Task first. All participants completed the PPI after finishing both of the computer tasks. Community members were paid $8 per hour and subject pool participants received course credit.
Psychopathy Facets
The 187-item Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996) was used to measure psychopathic personality traits. It yields eight subscales: Social Potency (i.e., charm, persuasiveness, “Members of the opposite sex find me “sexy” and appealing.”); Fearlessness (i.e., risk taking, “I like my life to be unpredictable, even a little surprising.”); Stress Immunity (i.e., low anxiousness, “I can remain calm in situations that would make many other people panic.”); Machiavellian Egocentricity (i.e., self-centeredness, antagonism, “I become very angry if I do not receive special favors or privileges I feel I deserve.”); Impulsive Nonconformity (i.e., reckless and unconventional behavior, “I would enjoy hitch-hiking my way across the United States with no prearranged plans.”); Carefree Nonplanfulness (i.e., lacking foresight, imprudent, “I often make the same errors in judgment over and over again.”); Blame Externalization (i.e., cynicism, blames others for misfortunes, “A lot of people in my life have tried to stab me in the back.”); and Coldheartedness (i.e., callousness, lacks social closeness, “When someone is hurt by something I say or do, I usually consider that to be their problem.”). Cronbach's αs for the subscales ranged from .78 - .90 in this study.
To ensure that we could derive the same factors as Benning et al. (2003), who recruited male twins from the Minnesota Twin Registry, we conducted a principal axis factor analysis with varimax rotation on the 8 PPI subscales and extracted factor scores via the regression method. Using the scree-plot and eigenvalues greater than 1, we extracted three factors scores that accounted for 52 % of the covariance, replicating the factors identified by Benning et al. (2003), which they referred to as Fearless Dominance (PPI-I), Social Deviance (PPI-II), and Coldheartedness (PPI-Coldheartedness). The first factor we extracted (PPI-II) accounted for 22.4% of the variance (eigenvalue = 2.3) and was characterized by Machiavellian Egocentricity, Blame Externalization, Carefree Nonplanfulness, and Impulsive Nonconformity. The second factor extracted (PPI-Coldheartedness) accounted for 15.9 % of the variance (eigenvalue = 1.7) and was marked by high Coldheartedness. The third factor extracted (PPI-I) accounted for 13.8 % of the variance (eigenvalue = 1.3) and was composed of Social Potency, Fearlessness, and Stress Immunity. PPI-II did not correlate significantly with either the PPI-Coldheartedness factor, r = .00, or PPI-I, r = -.08. Similarly, PPI-I did not correlate significantly with PPI-Coldheartedness, r = -.02, indicating the factor scores were orthogonal. The amount of variance accounted for and the factors extracted are consistent with those derived from Benning et al. (2003), which is not surprising given that both studies recruited community participants.
Previous research with other psychopathy measures, such as the PCL-R, suggests that callousness and unemotionality form a single dimension (i.e., Factor 1 of the PCL-R). Thus, although the Coldheartedness scale fell as a separate factor, we combined it with PPI-I to form a single PPI-Callous-Unemotional (PPI-CU) factor. We created the PPI-CU factor by weighting the two contributing factors (PPI-I and PPI-Coldheartedness) according to the number of subscales that each encompasses [(PPI-I * .75) + (PPI-Coldheartedness *.25)].2
Lavie's Cognitive Tasks
Perceptual Load Task
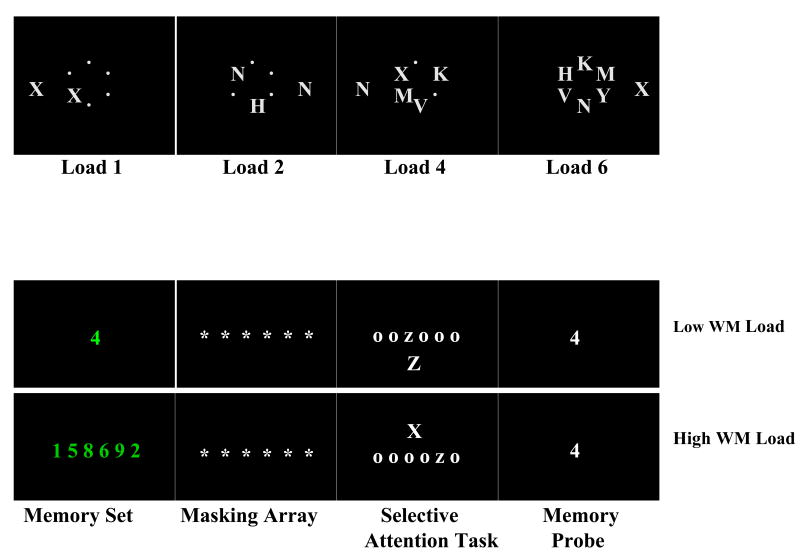
We administered a modified version of the task employed by Maylor and Lavie (1998) using DMDX display software (Forester & Forester, 2003). See Figure 1 (top panel) for a depiction of this task. In each trial, a circle of letters appeared for 200 ms that consisted of one target letter (X or N) and 0, 1, 3 or 5 non-target letters to represent four levels of perceptual load (Loads 1, 2, 4, and 6). Participants were instructed to press “X” (left arrow key) if they saw X in the circle and to press “N” (right arrow key) if they saw N in the circle. Larger distractor letters (X and N) that were either compatible or incompatible with the target also appeared in the periphery to either the right or left of the circle. The distractors letters were irrelevant to completing the task, and participants were instructed to ignore them and focus on the central circle. WRONG appeared in the middle of the screen following incorrect responses. All possible combinations of target, non-target and distractor letters and locations were counterbalanced within each perceptual load, resulting in a total of 96 unique letter displays. Participants completed two blocks of practice trials consisting of 12 trials each, followed by five blocks of 96 randomly presented experimental trials. Previous studies show that young adults are able to ignore incompatible distractors only at Load 6 of this task (Huang-Pollock et al., 2002; Maylor & Lavie, 1998), when perceptual load is fully taxed.
Figure 1.
(Top Panel) Load Examples from the Perceptual Load Task, and (Bottom Panel) Memory Set, Masking Array, Selective Attention Task and Memory Probe Examples from the Cognitive Control Task.
Cognitive Control Task
We administered a modified version of the task employed by Lavie et al. (Experiment 2; 2004) using DMDX display software (Forester & Forester, 2003). As depicted in Figure 1 (bottom panel), each trial consisted of three parts: 1) memory set, 2) selective attention task and 3) memory probe. At the beginning of a trial, a memory set (numbers randomly selected from 1 to 9) appeared centrally and consisted of either 6 digits presented in a horizontal row for 1,500 ms (High Working Memory Load) or 1 digit presented for 750 ms (Low Working Memory Load). Participants were instructed to memorize and mentally rehearse the numbers on the screen. Next, a masking display appeared for 1,250 ms that was followed by a 500 ms fixation cross. Subsequently, a letter display with one lowercase target letter (x or z) and five small circles in a horizontal row appeared for 100 ms. Simultaneously, a larger distractor letter (X or Z), compatible or incompatible, also appeared either above or below the horizontal row (see Figure 1). The distractor letters were irrelevant to completing the task, and participants were instructed to ignore them and press the key (“x” or “z”) that corresponded to the target letter that appeared in the row. After the letter display, the memory probe (i.e., a single digit) appeared centrally on the screen and participants were told to press the “Present” key if the number was one they had memorized at the beginning of the trial or press the “Absent” key if it was not. This memory probe was only used to ensure participants memorized the working memory load digits. WRONG appeared following an incorrect response. All combinations of target and distractor letters and locations were counterbalanced across trials. The memory probe was equally likely to be present or absent, to follow an incompatible or compatible target-distractor combination and to probe each of the six number locations in the high load. Participants completed three blocks of 12 practice trials followed by four blocks of 72 randomly presented experimental trials. Half of the participants started with a low load block and half started with the high load block. Young adults typically show the most difficulty ignoring incompatible distractor letters (and exhibit the longest RTs and most errors in the selective attention portion) under high vs. low working memory load (Lavie et al., 2004).
Dependent Measures
For both tasks, we used mean reaction time (RT) on correct trials and error rate as our dependent variables. Analysis of the RT data from the selective attention portion of the cognitive control task only included trials in which participants responded correctly to both the selective attention task and the memory probe. RTs greater than 3,000 ms and less than 100 ms were excluded from analyses (Maylor & Lavie, 1998).
Data Analysis
First, we analyzed RT and error data from the Perceptual Load Task using a mixed-model repeated measures ANOVA with Load (load 1, load 2, load 4, load 6) and Distractor Compatibility (incompatible, compatible) as the within-subject variables, and the two continuous PPI Factor Scores (PPI-CU, PPI-II) and their interaction as the between-subject variables in the same analysis. Second, we performed another repeated measures ANOVA on RT and error rate from the Cognitive Control Task with Load (low load, high load) and Distractor Compatibility (incompatible, compatible) as the within-subjects variables, and the two continuous PPI Factor Scores and their interaction as the between-subject variables in the same analysis. For purposes of follow-up correlational analyses, RT and error interference scores were used to index the amount of response interference generated by distractors. We calculated interference scores by subtracting the dependent variable (RT or error rate) on compatible trials from the analogous dependent variable on incompatible trials. In addition to p-values, we also report an effect size estimate using partial eta squared (i.e., equivalent to ΔR2 from multiple regression models). We used Greenhouse-Geisser corrections for violations of the sphericity assumption.
Results
Descriptive Statistics for the PPI
Table 2 reports the means, standard deviations, and range of scores for the two PPI factors (PPI-CU and PPI-II), total, and subscale scores. The descriptive statistics for the PPI total score in the present study are not markedly different from those reported in an undergraduate sample (M = 368, SD = 43.5, range = 261 - 471; Sellbom & Verona, 2007) and Benning et al.'s (2003) community sample (M = 348, SD = 30.6, range = 266 – 441; as reported in Blonigen, Carlson, Krueger, & Patrick, 2003), though the mean for the current sample is slightly higher. All subscale means for the current sample were higher than or equal to those reported for the community sample used in Benning et al. (2003).
Table 2. Descriptive Statistics for the Psychopathic Personality Inventory and Statistical Comparisons of Subsamples.
| Total Sample | Community Subsample | Student Subsample | Subsample Comparisons | ||||
|---|---|---|---|---|---|---|---|
| M (SD) | Min / Max | M (SD) | Min / Max | M (SD) | Min / Max | Test Statistic / P-Value | |
| PPI-CU | 0.0 (0.7) | -2.0 / 1.8 | 0.0 (0.8) | -2.0 / 1.4 | 0.0 (0.7) | -1.5 / 1.8 | t = -0.4 / .68 |
| PPI-I | 0.0 (0.9) | -2.1 / 2.5 | -0.1 (1.0) | -1.7 / 1.8 | 0.0 (0.9) | -2.1/ 2.5 | t = -0.7 / .48 |
| PPI-Coldheartedness | 0.0 (1.0) | -2.7 / 2.6 | 0.1 (1.2) | -2.7 / 2.6 | 0.0 (0.9) | -2.0 / 2.4 | t = 0.8 / .44 |
| PPI-II | 0.0 (0.9) | -2.3 / 3.1 | 0.1 (0.9) | -0.1 / 3.1 | 0.0 (0.9) | -2.3 / 1.6 | t = 0.7 / .46 |
|
| |||||||
| PPI-Total Score | 380 (37.2) | 267 / 477 | 380 (36.5) | 329 / 470 | 380 (37.8) | 267 / 477 | t = -1.0 / .33 |
| Machiavellian Egocentricity | 70 (14.0) | 35 / 101 | 69 (10) | 56 / 98 | 70 (15.0) | 35 / 101 | t = -0.5 / .60 |
| Blame Externalization | 35 (8.5) | 19 / 62 | 33 (8.0) | 19 / 52 | 36 (8.6) | 19 / 62 | t = -1.6 / .11 |
| Carefree Nonplanfulness | 39 (8.2) | 22 / 65 | 40 (7.6) | 30 / 65 | 39 (8.4) | 22 / 61 | t = 0.7 / .52 |
| Impulsive Nonconformity | 39 (7.7) | 24 / 60 | 42 (7.5) | 28 / 58 | 38 (7.5) | 24 / 60 | t = 2.3 / .02 |
| Fearlessness | 51 (9.4) | 28 / 73 | 52 (8.8) | 34 / 66 | 51 (9.7) | 28 / 73 | t = 0.6 / .55 |
| Social Potency | 64 (12.0) | 37 / 90 | 63 (13) | 38 / 90 | 65 (12.0) | 37 / 86 | t = -0.8 / .44 |
| Stress Immunity | 32 (5.5) | 17 / 43 | 31 (6.6) | 17 / 42 | 32 (5.0) | 21 / 43 | t = -1.0 / .32 |
| Coldheartedness | 45 (8.4) | 24 / 67 | 46 (10) | 24 / 67 | 45 (7.8) | 37 / 65 | t = 0.8 / .44 |
Note. Total Sample N = 100; Community Sample n = 26; Student Sample n = 74. Statistics in bold indicate significant differences between the community and subject pool subsamples.
Perceptual Load Task
Table 3 contains means and standard deviations for RT and error rate in the perceptual load task. Analyses revealed that participants responded more slowly and inaccurately as the number of items in the display increased, RT, F(1.4, 93) = 550.2, p = .000, ηp2 = .86, and error rate, F(1.9, 93) = 274.1, p = .000, ηp2 = .75. Participants also responded more slowly and inaccurately to incompatible relative to compatible distractors, RT, F(1, 93) = 19.6, p = .000, ηp2 = .17, and error rate, F(1, 93) = 16.0, p = .000, ηp2 = .15. In support of Lavie's (1995) perceptual load theory, the analyses revealed a Perceptual Load × Distractor Compatibility interaction for RT, F(2.7, 93) = 8.05, p = .000, ηp2 = .08, but not error rate ( p > .10). Follow-up analyses revealed significant RT interference effects at perceptual loads 1, 2, and 4, Fs(1, 93) > 7.19, ps < .009, but not perceptual load 6 (p > .19).
Table 3. Mean Reaction Time and Total Error Rate by Load and Distractor Compatibility for the Perceptual Load Task and Cognitive Control Task.
| Perceptual Load | Distractor Compatibility | Reaction Time (ms) | Error Rate |
|---|---|---|---|
| 1 | Incompatible | 627 (84.1) | .059 (.047) |
| Compatible | 606 (83.8) | .038 (.032) | |
| 2 | Incompatible | 685 (90.5) | .058 (.049) |
| Compatible | 674 (90.7) | .047 (.038) | |
| 4 | Incompatible | 834 (134) | .092 (.060) |
| Compatible | 822 (130) | .086 (.056) | |
| 6 | Incompatible | 916 (158) | .187 (.083) |
| Compatible | 924 (159) | .176 (.080) | |
|
| |||
| Working Memory Load | Distractor Compatibility | Reaction Time (ms) | Error Rate |
|
|
|
|
|
| Low Load | Incompatible | 848 (202) | .069 (.074) |
| Compatible | 807 (196) | .056 (.059) | |
| High Load | Incompatible | 859 (183) | .071 (.084) |
| Compatible | 808 (179) | .057 (.066) | |
Note. Standard deviations are presented in parentheses. N = 98 for the Perceptual Load Task. N = 97 for the Cognitive Control Task.
In addition to replicating the findings of Lavie and colleagues, the analysis yielded the predicted PPI-CU × Perceptual Load × Distractor Compatibility three-way interaction for RT, F(2.7, 93) = 2.96, p = .037, ηp2 = .03, but not for error rate (p > .74). Follow-up analyses revealed a significant PPI-CU × Distractor Compatibility interaction for load 4, F(1, 93) = 5.63, p = .02, ηp2 = .06, but not for loads 1, 2 and 6 (ps > .49). This effect is mirrored in the correlational analyses presented in Table 2. There was a significant inverse relationship between PPI-CU and the amount of interference generated from the distractors at perceptual load 4, suggesting that PPI-CU is associated with diminished distractor processing at a lower level of perceptual load than would be expected based on previous research (Huang-Pollock et al., 2002; Maylor & Lavie, 1998). Given a significant interaction emerged for RT, but not error rate, there was no evidence that the findings can be attributed to a speed-accuracy tradeoff.
The Perceptual Load × Distractor Compatibility interactions with PPI-II (ps > .20) did not reach statistical significance for RT or error rate. Correlations between PPI-II and RT interference scores are also provided in Table 4. To confirm that PPI-CU and PPI-II exhibited differential relationships with RT interference at perceptual load 4, we tested the difference in magnitude for the correlations between each PPI factor and RT interference at load 4. Results of a Fisher's Z test indicated that the correlation between PPI-CU and RT interference was significantly stronger than that between PPI-II and RT interference at load 4 (see Table 4), z = -2.38, p = .017. This suggests that the reduced RT interference effect is specific to the PPI-CU factor that is composed of the callous, social dominance and unemotional traits of the psychopathic syndrome.
Table 4. Correlations between Reaction Time Interference and PPI factors by Load Condition for Perceptual Load Task and Cognitive Control Task.
| Reaction Time Interference
Incompatible – Compatible |
||||
|---|---|---|---|---|
| PPI Factors/ Subscales | Perceptual Load 1 | Perceptual Load 2 | Perceptual Load 4 | Perceptual Load 6 |
| PPI-CU | -.06 | .07 | -.25*a | .06 |
| Social Potency | .12 | .00 | -.13 | .06 |
| Fearlessness | -.09 | .08 | -.01 | -.06 |
| Stress Immunity | -.08 | .03 | -.26** | .09 |
| Coldheartedness | .00 | .08 | -.08 | -.17 |
| PPI-II | .10 | .11 | .09 a | -.02 |
| Machiavellian Egocentricity | .06 | .17 | -.01 | -.07 |
| Blame Externalization | .14 | .16 | .10 | -.07 |
| Carefree Nonplanfulness | .09 | .07 | .00 | .07 |
| Impulsive Nonconformity | .02 | -.03 | .04 | .10 |
|
|
|
|||
| PPI Factors/ Subscales | Low Working Memory Load | High Working Memory Load | ||
|
|
|
|
||
| PPI-CU | -.10 | -.09 | ||
| Social Potency | -.06 | -.02 | ||
| Fearlessness | -.12 | -.23* | ||
| Stress Immunity | -.02 | -.05 | ||
| Coldheartedness | -.07 | -.05 | ||
| PPI-II | -.20 | .02 | ||
| Machiavellian Egocentricity | -.02 | .02 | ||
| Blame Externalization | -.04 | .25* | ||
| Carefree Nonplanfulness | -.31** | -.00 | ||
| Impulsive Nonconformity | -.21* | -.09 | ||
Note. n = 98 for all perceptual load correlations. n = 97 for all working memory load correlations. PPI = Psychopathic Personality Inventory.
indicates p < .01.
indicates p < .05. The letter a indicates the correlations between the factor scores (PPI-CU and PPI-II) and RT are significantly different, p = .017
Cognitive Control Task
Load manipulation check
Working Memory Load (low, high) × Probe Type (present, absent) repeated measures analysis conducted on the memory probe portion of the task suggested that responses were slower, F(1, 96) = 255.5, p = .000, ηp2 = .72, and more inaccurate, F(1, 96) = 28.4, p = .000, ηp2 = .23, under high load (RT: M = 1079, SD = 23.4; error rate: M = .088, SD = .007) than low load (RT: M = 814.6, SD = 18.2; error rate: M = .054, SD = .005). Thus, the memory load manipulation was successful at taxing working memory capacity. Probe type also had a significant effect on RT, F(1, 96) = 83.35, p = .000, ηp2 = .45, with participants responding slower to absent (M = 989, SD = 21.7) than present probes (M = 905, SD = 17.7).
Main analyses
The repeated measures ANOVA that included working memory load, distractor compatibility (within subjects) and both of the PPI factors (between subjects) as independent variables yielded significant main effects of distractor compatibility on RT, F(1, 93) = 94.5, p = .000, ηp2 = .50, and error rate, F(1, 93) = 9.12, p = .003, ηp2 = .09, reflecting slower and more inaccurate responses to incompatible than compatible distractors (see Table 3 for Ms and SDs for RT and error rate). Consistent with the findings of Lavie et al. (2004), participants displayed significantly greater RT interference from the distractors under high load (M = 50.5, SD = 53.3) than low load (M = 40.5, SD = 52.2), Working Memory Load × Distractor Compatibility interaction, F(1, 93) = 3.95, p = .05, ηp2 = .04. No significant correlations emerged between mean RT and total errors for either working memory load (ps >. 43), indicating there was no speed-accuracy tradeoff.
In addition to replicating the findings of Lavie et al. (2004), we also found the expected PPI-II × Working Memory Load × Distractor Compatibility interaction for RT, F(1, 93) = 4.54, p = .036, ηp2 = .05, but not error rate (p > .20). Follow-up analyses revealed a significant PPI-II × Distractor Compatibility interaction for the low load, F(1, 93) = 4.67, p = .033, ηp2 = .05, but not high load condition (p > .99). That is, PPI-II was negatively correlated with RT in low load and uncorrelated with RT in high load (see Table 4). However, an exploratory investigation of the correlations between RT interference and each of the subscales that contribute to PPI-II for low and high working memory load suggests a more complicated picture. The correlations displayed in Table 4 indicate that PPI-Blame Externalization (alienation, cynicism, hostility) shows the expected relationship with RT distractor processing under high working memory load (i.e., a positive association with RT interference from distractors under high load, but not low load), suggesting that those high on this trait show difficulties in performance under conditions requiring higher cognitive control. On the other hand, PPI-Carefree Nonplanfulness and PPI-Impulsive Nonconformity demonstrated an inverse relationship with distractor processing under low working memory load, indicating that these traits are associated with rapid responding under less complex situations. Thus, post-hoc analyses suggest that the relationship of PPI-II with RT interference on the cognitive control task is driven by the differential associations of its subscales with distractor processing under varying loads.
A comparison of the magnitude of the difference between the RT interference correlations for PPI-CU and PPI-II using a Fisher's Z test indicated that the correlations were not significantly different for either the high or low working memory load condition, which suggests the two main facets were not differentially associated with performance on this task. However, particular subscales that composed PPI-II were uniquely associated with distractor rejection under high working memory. That is, Blame Externalization showed a significantly stronger correlation with RT interference under high working memory load than the PPI-CU factor, z = 2.37, p = .017, suggesting that individuals high on Blame Externalization in particular displayed greater difficulty with cognitive control than individuals scoring high on the affective-interpersonal traits of psychopathy. None of the other correlations for the individual PPI-II subscales showed a significant difference in magnitude under high or low working memory load compared to PPI-CU. PPI-CU did not interact significantly with working memory load or distractor compatibility (ps > .27) for RT or error rate (see Table 2 for RT correlations).
Discussion
The results of the current study were generally consistent with our hypotheses in that psychopathic personality traits linked to primary and secondary psychopathy were differentially related to abnormal perceptual selection and cognitive control, respectively. In particular, traits typically associated with primary psychopathy (i.e., PPI-CU) were associated with reduced distractor processing at a lower level of perceptual load than low levels of these traits. This finding suggests that individuals with primary psychopathic traits may have diminished early perceptual processing capabilities, which may explain their tendency to screen out environmental information that is irrelevant to goal attainment, such as distress cues that engender empathy or threatening consequences that deter antisocial behavior. Conversely, high levels of secondary psychopathic traits (i.e., PPI-II) were related to both less response interference from distractors under low working memory load and greater response interference from distractors under high working memory load. Individuals with these traits may respond impulsively and have trouble maintaining cognitive control in complex situations, which could explain their risk-taking tendencies and problems with frustration and anger regulation.
On the Perceptual Load Task, high scores on PPI-CU were related to diminished distractor processing at a less perceptually demanding load (i.e., perceptual load 4) than participants as a whole who continued to show interference from the distractors until the most perceptually demanding condition (i.e., perceptual load 6) (cf. Huang-Pollock et al., 2002; Maylor & Lavie, 1998). Interestingly, this is the first study to identify individual differences in distractor processing under high levels of perceptual load, which challenges research that suggests individual differences may only be important for determining distractibility at low levels of perceptual load (Forster & Lavie, 2007). Although the performance by the high PPI-CU scorers may seem adaptive and not a “deficit”, cognitive researchers have observed similar findings among young children and older adults and have attributed their reduced processing of distractors at low levels of perceptual load to underdeveloped attentional capacity for perceptual selection and the deleterious effects of aging on processing capacity, respectively (Huang-Pollock et al., 2002; Maylor & Lavie, 1998). Individuals with primary psychopathic personality traits seem to exhibit a similar decrease in perceptual processing capacity.
This finding may be a result of the affective poverty (e.g., low neuroticism) that is central to the disorder. From a developmental perspective, individuals with low levels of anxiety may allocate relatively fewer attentional resources to monitoring their environment for threatening stimuli, which in turn may influence their capacity to perceive peripheral information overtime. Thus, a long-term consequence of psychopathic individual's tendency to devote less attentional resources to processing contextual information may be overall reduced perceptual capacity relative to individuals who are motivated by anxiety or fear to regularly monitor peripheral stimuli for threatening cues. In support of this contention, deficient contingency monitoring and peripheral information processing has been repeatedly observed in incarcerated individuals with primary psychopathic traits (Jutai & Hare, 1983; Newman, Patterson, & Kosson, 1987).
Although the neuroanatomical basis for this deficit is currently unknown, the brain mechanisms implicated in both the emotion- and attention-based models of psychopathy are plausible contributors to the reduced perceptual capacity observed in this study. More specifically, the capacity of the frontoparietal attention network (i.e., right prefrontal cortex, frontal eye fields, anterior cingulate, parietal cortex) engaged by the perceptual load task may be reduced in individuals high on primary psychopathic traits as a consequence of chronic hypoactivation in the amygdala and thus reduced reciprocal connections with cortical regions that modulate attention, such as the prefrontal cortex. Given this theoretical model, it is interesting to note that we observed differences in attentional functioning on a task that did not involve affective stimuli, suggesting that if amygdala deficits/ fearlessness can account for performance on this task (as some emotion-based models would assume), it may be due to the long-term outcome of these deficits on attentional processing. Alternatively, the attentional abnormality observed in this study may reflect deficits in septohippocampal system functioning or the combined effect of amygdala and septohippocampal system dysfunction, given their reciprocal interconnections. Based on Newman's (1998) theory, the reduced peripheral processing observed among those high on primary psychopathic traits could be interpreted as a consequence of poor integration of bottom-up inputs (i.e., task-irrelevant distractors) coming from visual areas into an established top-down attentional set (i.e., focusing only on targets). Since this model is not based primarily on an emotion deficit, it provides an explanation for the abnormal selective attention observed in a task with relatively neutral stimuli (perceptual load task) that does not rely on the long-term of effects of limbic system dysfunction. However, it does not preclude the possibility of a more complex interaction between deficits in the amygdala and septohippocampal system. More research is needed to clarify the neural substrates that govern the development of attenuated attentional capacity in primary psychopathy, including work that uses psychophysiological and neural imaging techniques to determine whether the abnormalities in perceptual processing observed in this study via behavioral indices can be detected at earlier stages of processing, such as sensory brain potentials.
Analysis of the Cognitive Control Task revealed a selective impairment under the high working memory load for individuals elevated on certain PPI-II subscales, particularly Blame Externalization. These findings suggest that taxing working memory capacity differentially affects the ability of individuals with high levels of alienation and hostility to control the effects of incompatible distractors on their behavioral responses. Diminished cognitive control may contribute to the chronic irresponsibility, substance use, and emotional dysregulation observed in individuals with high levels of these traits. It should be noted that the Blame Externalization subscale of the PPI is conceptually distinct from what is assessed in the PCL-R item “lack of remorse”. The PCL-R assesses the psychopath's inability to take responsibility for criminal actions, whereas PPI Blame Externalization assesses feelings of distrust and resentment and correlates with negative affect traits such as alienation (r = .70) and aggression (r = .34) (Benning et al., 2003). Research has found associations between low agreeableness (i.e., antagonism) similar to Blame Externalization and deficient prefrontal activation using hemodynamic measures (Haas, Omura, Constable, & Canli, 2007).
A subset of PPI-II traits, especially those measuring impulsivity and sensation-seeking (Impulsive Nonconformity and Carefree Nonplanfulness), were differentially related to faster responding (but not error rate) under low working memory load. This finding suggests that impulsivity may be advantageous in fairly simple tasks, with no gross cognitive abnormalities observed among persons with impulsive and nonplanful traits. However, most real-life endeavors involve substantial complexity and dual-tasking, which may explain why these traits were not related to rapid responding under high working memory loads (i.e., individuals high on these traits needed to slow their responding under high cognitive load in order to respond correctly). Given the differential relationships of the social deviance factor subscales of the PPI with performance on the cognitive control task, it may be important in future research on cognitive functioning in psychopathy to examine these personality traits both in isolation and as an aggregate factor. Indeed, our findings for PPI-II are consistent with work in the personality psychology literature, which suggests that narrowly construed facets show more predictive validity than broader dimensions (Paunonen, Haddock, Forsterling, & Keinonen, 2003). It is important to note, however, that the follow-up analyses with the individual PPI-II subscales on the Cognitive Control Task were exploratory in nature and conducted in an attempt to understand the unexpected association between PPI-II with RT interference under low working memory load. Thus, the findings with the individual subscales should be interpreted with caution until further research is conducted.
Strengths, Weaknesses, and Future Directions
This study has several strengths. One contribution is its ability to demonstrate hypothesized relationships between a widely-used self-report measure of psychopathy and mechanisms of attention, research that is currently lacking. Secondly, we attempted to advance current cognitive models of psychopathy by adopting an up-to-date theory on mechanisms of selective attention developed in the cognitive psychology literature. Importantly, this study is one of the few to examine individual difference moderators of the perceptual load theory advanced by Lavie and colleagues (1995). Additionally, we used a well-validated assessment instrument to examine the multidimensional nature of psychopathic traits, which allowed us to investigate the combined cognitive effects that contribute to the manifestation and development of the disorder.
In addition to these strengths, the current study also has limitations. One potential concern is how closely the Perceptual Load task employed resembles cognitive demands that arise in real world situations. In support of the ecological validity of this task, Forster and Lavie (2007) have demonstrated an association between distractibility on a variant of the perceptual load task in a laboratory setting and distractability in everyday life. Higher interference in response to distractors has been linked to attentional deficits that occur in the natural environment, which suggests that the decreased interference from distractors under high perceptual load observed in high PPI-CU scorers is also representative of how these individuals process information in everyday life. Psychopathic individuals' tendency to myopically focus on obtaining a goal or reward regardless of contradicting peripheral information (e.g., high probability of arrest, distress cues that engender empathy) is an example of how this type of abnormal selective attention may relate to psychopathic behavior as it occurs in the real world.
Secondly, although we recruited from both the general community and the college campus, participants were mostly college students who likely had relatively intact cognitive functions. Despite the potentially restricted range of our sample, we still detected differences on the tasks that were consistent with research in incarcerated individuals (Hiatt et al., 2004; Vitale et al., 2007). Additional replication in incarcerated or forensic samples is still needed to ascertain how these attentional mechanisms operate in more severe manifestations of the psychopathic syndrome. Furthermore, the magnitude of the relationships we found between the psychopathy factors and the cognitive tasks were relatively small (rs ∼ .25), which limits the implications of these findings. However, the effect sizes obtained are comparable to those reported in other investigations of affective or cognitive abnormalities in forensic samples (e.g., Benning, Patrick, & Iacono, 2005; Bernat, Hall, Steffen, & Patrick, 2007). We also did not include female participants, which further limits the generalizability of our findings to non-incarcerated men. Prior studies on the generalizability of laboratory findings to psychopathic females has been equivocal, with some cognitive abnormalities observed with males replicating among females (e.g., abnormal selective attention; Vitale et al., 2007) while others do not (response perseveration; Vitale & Newman, 2001). Importantly, abnormal selective attention has been demonstrated among both male and female psychopathic offenders (Hiatt et al., 2004 and Vitale et al., 2007, respectively), which increases the likelihood that our findings generalize to females.
Given that the community and student samples were compensated differently (money versus course credit, respectively) it should be considered that the form of compensation each received may have influenced task performance (e.g., increased or decreased motivation), though the compensation was non-contingent on performance. Furthermore, the stimuli in this study were limited to the visual modality, leaving open the possibility that our findings are modality specific. In relation to psychopathy, there is no theoretical reason to expect the results would change if the stimuli were presented aurally, and Jutai & Hare (1983) found a similar effect (reduced processing of peripheral stimuli) when the distractor was an auditory stimulus. Despite some weaknesses, the current results provide insight into how selective attention and cognitive control may contribute to the development and maintenance of distinct psychopathic traits.
Acknowledgments
This research was supported by an NIMH National Research Service Award MH19554 to the University of Illinois and was conducted while Naomi Sadeh was a predoctoral trainee in the Cognitive Psychophysiology Training Program of the Department of Psychology, University of Illinois at Urbana-Champaign. Thanks are extended to Howard Berenbaum, John Curtin, Shabnam Javdani, and Keith Bredemeier for comments on earlier versions of this manuscript.
Footnotes
Comparison of the subsamples on demographic variables (age, ethnicity, household income, education level), estimated WAIS Full Scale IQ, PPI subscales, and PPI factor scores revealed significant differences between the two groups only on age, F(1, 98) = 23.5, p = .000, education level, χ2(3, 100) = 28.1, p = .000, and PPI Impulsive Nonconformity, F(1, 98) = 5.27, p = .024. Community participants were on average older, slightly more educated, and reported higher levels of PPI Impulsive Nonconformity. Age and education did not correlate with interference on either task. However Impulsive Nonconformity correlated negatively with RT interference under low load on the Cognitive Control Task, r = -.21, p = .049. Thus, we examined whether the main findings replicated within each subsample and found the pattern and direction of results were similar across the two subsamples [Perceptual Load Task: PPI-CU and RT interference load 4, rs = -.31 and -.23; Cognitive Control Task: PPI-II and RT interference low load, rs = -.36 and -.11; for the community and subject pool samples, respectively]. Given this, we combined the subsamples in all other analyses.
We would like to thank one of the anonymous reviewers who suggested we combine the PPI-Coldheartedness and the PPI-I fearless-dominance factors. When analysis of the cognitive tasks was conducted using the three factor solution instead (PPI-I, PPI-II, PPI-Coldheartedness), the results for PPI-I did not differ substantially from the results for the composite PPI-CU factor (i.e., the three-way interaction on the Perceptual Load Task was significant). In contrast, no significant results emerged for the PPI-Coldheartedness factor on either of the tasks. We presented the findings for the two-factor (PPI-CU, PPI-II) rather than the three-factor (PPI-I, PPI-II, PPI-Coldheartedness) model to increase the theoretical validity and parsimony of our analyses.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/neu/
References
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the Psychopathic Personality Inventory: Validity and implications for clinical assessment. Psychological Assessment. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Hall JR, Steffen BV, Patrick CJ. Violent offending predicts P300 amplitude: Controlling for IQ, age, and task performance. International Journal of Psychophysiology. 2007;66:161–167. doi: 10.1016/j.ijpsycho.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: A functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35:1–12. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity. 5th. St. Louis, MO: Mosby; 1976. [Google Scholar]
- Cooke D, Michie C. Refining the construct of psychopathy: Towards a hierarchical model. Psychological Assessment. 2001;13:171–188. [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Cognitive Brain Research. 2003;17(3):637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dolan M, Park I. The neuropsychology of antisocial personality disorder. Psychological Medicine. 2002;32:417–427. doi: 10.1017/s0033291702005378. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG. Psychopathic, not Psychopath: taxometric evidence for the dimensional structure of psychopathy. Journal of Abnormal Psychology. 2006;115:131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Forester KI, Forester JC. DMDX: A windows display program with millisecond accuracy. Behavior Research Methods, Instruments, and Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Forster S, Lavie N. High perceptual load makes everybody equal: eliminating individual differences in distractibility with load. Psychological Science. 2007;18:377–381. doi: 10.1111/j.1467-9280.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87:301–315. [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Function of the Septo-Hippocampal System. Oxford University Press; 2000. [Google Scholar]
- Hare RD. Temporal gradient of fear arousal in psychopaths. Journal of Abnormal Psychology. 1965;70:442–445. doi: 10.1037/h0022775. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Psychopathy Checklist-Revised. 2nd. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Harpur T, Hare RD. Psychopathy and attention. In: Enns J, editor. The development of attention: Research and theory. Amsterdam: North Holland; 1990. pp. 429–444. [Google Scholar]
- Harpur T, Hare RD, Hakstian A. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment. 1989;1:6–17. [Google Scholar]
- Haas B, Omura K, Constable R, Canli T. Is automatic emotion regulation associated with agreeableness? Psychological Science. 2007;18:130–132. doi: 10.1111/j.1467-9280.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt KD, Schmitt WA, Newman JP. Stroop tasks reveal abnormal selective attention in psychopathic offenders. Neuropsychology. 2004;18:50–59. doi: 10.1037/0894-4105.18.1.50. [DOI] [PubMed] [Google Scholar]
- Hicks B, Markon K, Patrick C, Krueger R, Newman JP. Identifying psychopathy subtypes based on personality structure. Psychological Assessment. 2004;16:276–288. doi: 10.1037/1040-3590.16.3.276. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C, Carr T, Nigg J. Development of selective attention: Perceptual load influences early versus late attentional selection in children and adults. Developmental Psychology. 2002;38:363–375. [PubMed] [Google Scholar]
- Iacono W, Malone S, McGue M. Substance use disorders, externalizing psychopathology and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jutai JW, Hare D. Psychopathy and selective attention during performance of a complex perceptual-motor task. Psychophysiology. 1983;20:146–151. doi: 10.1111/j.1469-8986.1983.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kosson DS. Psychopathy and dual-task performance under focusing conditions. Journal of Abnormal Psychology. 1996;105:391–400. doi: 10.1037//0021-843x.105.3.391. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Newman JP. Psychopathy and the allocation of attentional capacity in a divided-attention situation. Journal of Abnormal Psychology. 1986;95:257–263. [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, De Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: Emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109:373–385. [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lykken D. The antisocial personalities. Hillsdale, NJ: Lawrence Earlbaum Associates; 1995. [Google Scholar]
- Maylor E, Lavie N. The influence of perceptual load on age differences in selective attention. Psychology of Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- Miller JD, Lynam DR, Widiger TA, Leukefeld C. Personality disorders as extreme variants of common personality dimensions: Can the Five-Factor Model adequately represent psychopathy? Journal of Personality. 2001;69:253–276. doi: 10.1111/1467-6494.00144. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld O. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–156. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Murrie DC, Marcus DK, Douglas KS, Lee Z, Salekin RT, Vincent G. Youth with psychopathy features are not a discrete class: a taxometric analysis. Journal of Child Psychology and Psychiatry. 2007;48:714–723. doi: 10.1111/j.1469-7610.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- Newman JP. Psychopathic behavior: An information processing perspective. In: Cooke DJ, Hare RD, Forth A, editors. Psychopathy: Theory, Research and Implications for Society. The Netherlands: Kluwer Academic Publishers; 1998. pp. 81–104. [Google Scholar]
- Newman JP, Brinkley CA. Reconsidering the low-fear explanation for primary psychopathy. Psychological Inquiry. 1997;8:236–245. [Google Scholar]
- Newman JP, MacCoon DG, Buckholtz J, Bertsch J, Hiatt KD, Vaughn LJ. Deficient integration of top-down and bottom-up influences on attention in psychopaths: Potential contribution of the septal-hippocampal system. In: Barch D, editor. Cognitive and Affective Neuroscience of Psychopathology. Oxford University Press; in press. [Google Scholar]
- Newman JP, MacCoon DG, Vaughn LJ, Sadeh N. Validating a distinction between primary and secondary psychopathy with measures of Gray's BIS and BAS Constructs. Journal of Abnormal Psychology. 2005;114:319–323. doi: 10.1037/0021-843X.114.2.319. [DOI] [PubMed] [Google Scholar]
- Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. Journal of Abnormal Psychology. 1987;96:145–8. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- Newman JP, Schmitt WA, Voss W. The impact of motivationally neutral cues on psychopathic individuals: Assessing the generality of the response modulation hypothesis. Journal of Abnormal Psychology. 1997;106:563–575. doi: 10.1037//0021-843x.106.4.563. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision Making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone S, Iacono W, Krueger RF, McGue M. P300 amplitude as indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Paunonen SV, Haddock G, Forsterling F, Keinonen M. Broad versus narrow personality measures and the prediction of behavior across cultures. European Journal of Personality. 2003;17:413–433. [Google Scholar]
- Poythress N, Edens J, Lilienfeld S. Criterion-related validity of the Psychopathic Personality Inventory in a prison sample. Psychological Assessment. 1998;10:426–430. [Google Scholar]
- Poythress N, Skeem J. Disaggregating psychopathy: Where and how to look for subtypes. In: Patrick, editor. Handbook of psychopathy. New York: Guilford; 2006. pp. 172–192. [Google Scholar]
- Raine A, Meloy R, Bihrle S, Stoddard J, LaCasse L, Buchsbaum M. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murders. Behavioral Sciences and Law. 1998;16:319–332. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith C, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278(5343):1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan R, Driver J. Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Sellbom M, Verona E. Neuropsychological correlations of psychopathic traits in a non-incarcerated sample. Journal of Research in Personality. 2007;41:276–294. [Google Scholar]
- Vitale JE, Brinkley CA, Hiatt KD, Newman JP. Abnormal selective attention in psychopathic female offenders. Neuropsychology. 2007;21:301–312. doi: 10.1037/0894-4105.21.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale JE, Newman JP. Response perseveration in psychopathic females. Journal of Abnormal Psychology. 2001;110:644–647. doi: 10.1037//0021-843x.110.4.644. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Lynam DR. Psychopathy and the Five-Factor Model of Personality. In: Millon T, et al., editors. Psychopathy: Antisocial, criminal, and violent behavior. New York: Guilford; 1998. pp. 171–187. [Google Scholar]
- Zachary RA. Shipley Institute of living scale, revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]