Abstract
Thiamin pyrophosphate is a required cofactor in all organisms. The biosynthesis of thiamin requires the independently synthesized 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate (HMP-PP) and 5-hydroxyethyl-4-methyl thiazole phosphate (THZ-P) moieties. In bacteria, the pyrimidine moiety is derived from 5-aminoimidazole ribotide (AIR) and ThiC is the only gene product known to be required for this conversion in vivo. We report here the purification and characterization of the ThiC protein from Salmonella enterica. The data showed this protein generated HMP when AIR, S-adenosyl methionine (AdoMet), and an appropriate reducing agent were present. It is further shown that ThiC carries an oxygen labile [Fe-S] cluster essential for this activity.
Thiamin pyrophosphate (TPP) is an essential cofactor formed from 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate (HMP-PP) and thiazole monophosphate (THZ-P) moieties. In bacteria, HMP-PP and THZ-P are independently synthesized and combined by thiamin phosphate (TMP) synthase (ThiE, E.C. 2.5.1.3) yielding TMP prior its conversion to TPP by the TMP kinase (ThiL, E.C. 2.7.4.16) (1) Figure 1A. Synthesis of the THZ moiety has been reconstituted in vitro using components from Gram-positive (B. subtilis) and Gram-negative (E. coli, S. enterica) bacteria (2-7). In contrast, our understanding of the biochemical steps leading to the synthesis of HMP is limited. Genetic and biochemical studies have shown that HMP is derived from 5-aminoimidazole ribotide (AIR) (8-12). In vivo labeling studies showed that all carbon and nitrogen atoms present in HMP are derived from AIR, as illustrated in Figure 1B (8, 9, 13, 14). These labeling data suggested that the conversion from AIR to HMP involves the breakage and reforming of multiple bonds. Despite this complex intramolecular rearrangement, only one gene product (ThiC) has been shown to be essential for this conversion in vivo.
Figure 1.
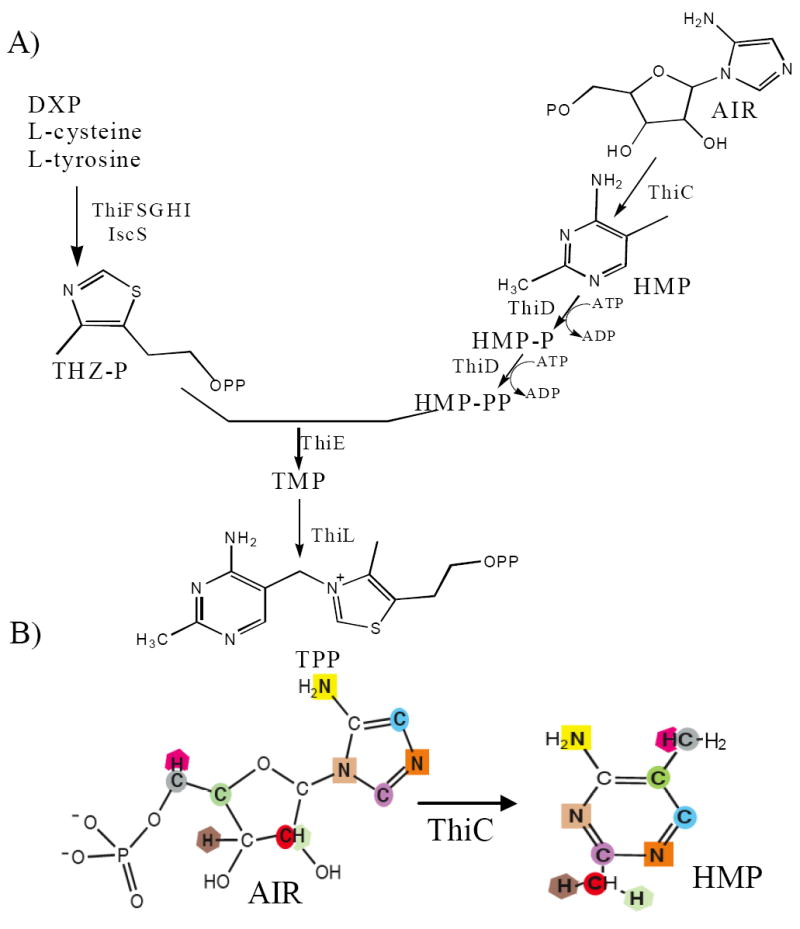
Thiamin biosynthesis in Salmonella enterica. A) Schematic of thiamin synthesis showing the independent formation of THZ-P and HMP-PP prior to thiamin synthase. Gene products involved are indicated by the relevant steps. B) Labeling studies have shown the correlation of atoms present in AIR and HMP as indicated by shape and color (8, 14). Abbreviations: AIR, aminoimidazole ribotide; HMP, 4-amino-5-hydroxymethyl-2-methylpyrimidine; DXP, 1-Deoxy-D-xylulose-5-phosphate; TMP, thiamin phosphate; TPP, thiamin pyrophosphate.
Formation of HMP was reported in cell-free extracts of an E. coli strain that overproduced ThiC (14). In the cited study, the ThiC-dependent conversion of AIR to the pyrimidine was measured by in situ conversion of the product to TMP, which was quantified by a thiochrome assay (5). The ThiC-dependent activity detected in the cell-free extract was low, and increased by the addition of dialyzed cell-free extract of a thiC mutant strain. These studies indicated that AIR, AdoMet, one of the following: NAD+, NADH, or NADPH, and an additional cellular component were required for optimal ThiC activity. Purification of ThiC resulted in loss of all activity, and no evidence for an [Fe-S] cluster was found (14). Recently the UV-visible spectrum of a plant-encoded ThiC was used to suggest the presence of an [Fe-S] cluster associated with the protein (15).
In this report we describe the purification and functional assay of the Salmonella enterica ThiC protein. We show that, when purified anoxically, ThiC contained an oxygen labile [Fe-S] cluster that was essential for ThiC to convert AIR to HMP.
Strain DM7474 (E.coli; SG-13009 pREP4 pMD34) was used to produce ThiC-His6. When purified under anoxic conditions 12 mg of ThiC protein sample was obtained from 30g of cells. The ThiC protein sample (6 mg/ml) had a brown color with a UV-visible spectrum characteristic of Fe-S cluster proteins, including a shoulder at 410 nm (Figure 2A green). The signal at 410 nm decreased when the sample was exposed to oxygen (Fig 2A red), or when dithionite was added to an anoxic sample (data not shown). The protein contained 3.8±0.6 and 3.0±0.8 mol of iron and sulfide respectively, per mol of protein. The theoretical A400/A280 ratio of [4Fe-4S] cluster/subunit proteins is f=(0.16-0.19). The A400/A280 ratio of four independently reconstituted ThiC preparations was f=0.17±0.3, consistent with the presence of a [4Fe-4S] cluster/subunit ThiC (ε280=75.33 mM-1cm-1). A typical ThiC preparation had > 90% purity as judged by visualization on an SDS-PAGE gel (supplementary material).
Figure 2.
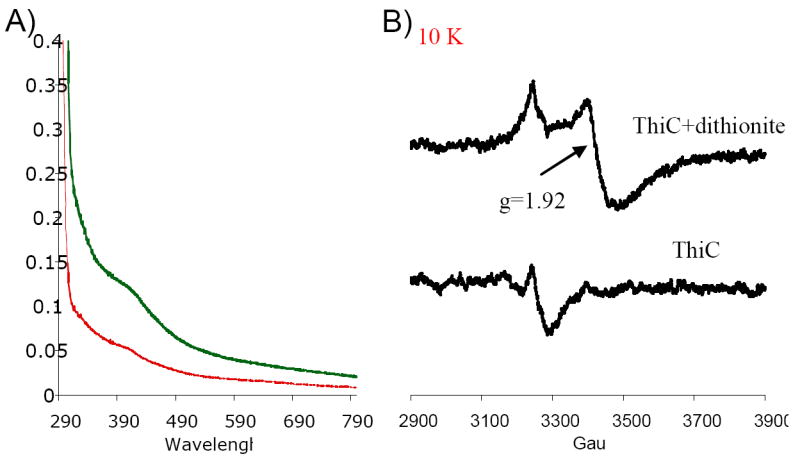
Spectroscopic analysis detects an Fe-S cluster in ThiC. A) UV-Visible spectra. The UV-Visible spectra of ThiC (10 μM) as purified (green line), and after 5 hours exposure to oxygen (red line) are shown. B) EPR spectra. ThiC (100 μM) was incubated with 2.7 mM dithionite (10 min) and the EPR spectra were recorded at 10 K. A signal at g=1.92 is only present upon reduction with dithionite. Field center 3300 Gauss; scan width 400 Gauss; microwave frequency 9.26 GHz; microwave power 1mW; modulation frequency 100kHz, modulation amplitude 16 Gauss; time constant 0.3s; scan time 240s; and gain 8000.
When purified under oxic conditions, the ThiC protein sample (4 mg/mL) was colorless and had no spectroscopic feature that would indicate the presence of an Fe-S cluster (data not shown). Together these results suggested that ThiC contained an oxygen labile Fe-S cluster. All subsequent manipulations of the protein were performed under anoxic conditions (<2 ppm O2) unless otherwise stated.
The ThiC protein was reduced with 2.7 mM dithionite, transferred to an EPR tube and frozen in liquid nitrogen. The spectra recorded at 10 K had a signal at g=1.92, characteristic of [4Fe-4S] cluster proteins reduced to the (+1) state (Figure 2B) (16).
Purified ThiC was assayed in vitro for activity. ThiC (11 nmol) was reduced with dithionite (2.7 mM) for 30 minutes, and substrates were added in the following order: AdoMet (1.2 mM), AIR (2 mM), MgCl2 (2.6 mM), and ATP (1.6 mM) to a final volume of 100 μL. The reaction was incubated at 37 °C for 8 hours, boiled for 2 minutes, and cleared by centrifugation (16,000 × g, 2 min at 4 °C). The supernatant was removed from the anaerobic chamber and tested for its ability to support the growth of a strain lacking ThiC (DM7185) (17). Figure 3A shows results from a representative bioassay, with authentic HMP as a control.
Figure 3.
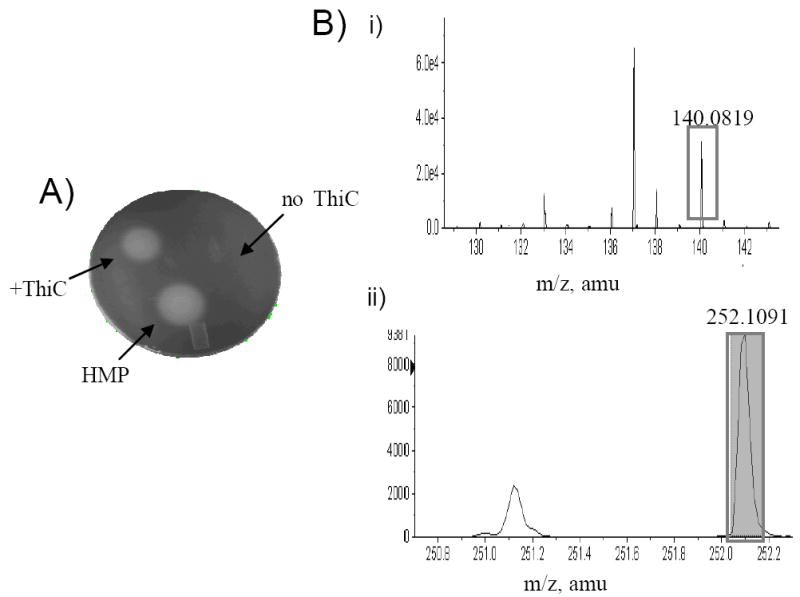
The ThiC reaction generates HMP. A) Bioassay of reaction products. A layer of soft agar seeded with a culture of strain DM7185 (thiC) was spread on minimal medium. Reaction mixtures from an assay with or without ThiC protein as indicated, were spotted on the agar and incubated for 18 hr. at 37 °C. HMP was spotted as a control. Turbid zones indicated growth. B) HPLC-MS analysis of reaction products. Units on the y axis correspond to intensity counts. i) In the HPLC elution profile, at 6.49 minutes there was a peak with the accurate mass of HMP (m/z=140.0819). ii) Likewise at 2.79 minutes a peak with accurate mass of 5’-deoxyadenosine (m/z=252.10) was detected. Both signals were compared to authentic standards. In addition to ThiC, the mixture reaction contained: 0.8 mM NADPH, Fpr (12 mM), FldA (10 mM), 2 mM AIR, 1.2 mM AdoMet, 1.6 mM ATP, 2.6 mM MgCl2
The qualitative results from the bioassay were extended by HPLC/MS (MALDI-TOF) analysis of the reaction product(s). An ion signal with accurate mass m/z=140.0819 was detected in the reaction mixture containing ThiC (Figure 3B). This ion was identical to a control standard of HMP both in mass and retention time and was dependent on ThiC. The amount of HMP formed was low compared to the amount of substrate provided. Consistently, spin quantitation analysis showed a 1:100 correspondence between the amount of [4Fe-4S]+1 cluster observed and the protein present suggesting that only 1% of the protein had a reduced cluster. This result could indicate that the majority of the protein population is not loaded with a [4Fe-4S] cluster, and/or the clusters are not being efficiently reduced
In this study we demonstrated that ThiC was able to convert AIR to HMP, and required AdoMet to do so. The data indicated the ThiC protein contained an oxygen labile [Fe-S] cluster that was essential for activity and explained the need for strictly anoxic conditions in all manipulations. Under standard conditions (10 K), and in the presence of dithionite, the cluster in ThiC had a signal typical of a [4Fe-4S]+1 cluster.
In chemically defined system ThiC required AIR and AdoMet for product formation. In addition, a suitable reducing system such as Fpr/FldA/NADPH, or dithionite was necessary to generate active ThiC. HMP was detected as the final product of the ThiC reaction. The formation of HMP-P could not be eliminated since the phosphate group may be unstable in the acidic conditions used for purification. Previous studies are most consistent with HMP-P being the true product of the ThiC reaction (18, 19) and future studies are needed to clarify this.
The requirement of AdoMet for ThiC activity narrows the possible mechanisms involved in the reaction. ThiC does not contain the characteristic CXXXCXXC motif that defines members of the radical AdoMet superfamily of proteins. However, it contains a C(S/T)MCXXXXC motif near the C-terminus of the protein that is conserved in every ThiC homolog representing over 100 diverse organisms (17). A single additional cysteine is similarly conserved. Further, the primary sequence of ThiC has a conserved motif rich in glycine residues GIVSRGGS. Glycine-rich sequences have been suggested to encode the amino acids necessary to bind AdoMet in members of the AdoMet superfamily of proteins (20). AdoMet is not acting as a methylating agent in thiamin biosynthesis (14), and may be either a substrate or cofactor needed to generate the 5’-deoxyadenosyl radical. Analysis of the reaction mixture detected the ThiC-dependent formation of 5’deoxyadenosine (by mass spectroscopy, Figure 3B) and methionine (by bioassay), suggesting AdoMet is being cleaved in the reaction. Further studies aimed at the quantification of both products of this cleavage will help in defining the specific role of AdoMet in the ThiC catalyzed reaction.
SUPPORTING INFORMATION AVAILABLE
Detailed materials and methods are provided. This material is available free of charge via internet at http://pubs.acs.org.
Acknowledgments
We thank George Reed for generously making his EPR equipment available to us and for valuable insights; Perry Frey for his valuable advice; Susan Wang and Russell Poyner for advice on use of the EPR spectrometer, and Frank Ruzicka for suggestions in analyzing the EPR data. We thank Paola Mera and Jorge Escalante-Semerena for purified Fpr and the plasmid used to over-express FldA. Plasmid pMD34 was constructed by Michael Dougherty. This work was supported by competitive grant GM47296 from the NIH and funds from a 21st Century Scientists Scholars Award from the J.M. McDonnell fund.
References
- 1.Settembre E, Begley TP, Ealick SE. Curr Opin Struct Biol. 2003;13:739–47. doi: 10.1016/j.sbi.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Kriek M, Martins F, Leonardi R, Fairhurst SA, Lowe DJ, Roach PL. J Biol Chem. 2007;282:17413–23. doi: 10.1074/jbc.M700782200. [DOI] [PubMed] [Google Scholar]
- 3.Dorrestein PC, Huili Zhai H, Taylor SV, McLafferty FW, Begley TP. J Am Chem Soc. 2004;126:3091–6. doi: 10.1021/ja039616p. [DOI] [PubMed] [Google Scholar]
- 4.Kambampati R, Lauhon CT. J Biol Chem. 2000;275 doi: 10.1074/jbc.M002680200. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Dorrestein PC, Zhai H, Kinsland C, McLafferty FW, Begley TP. Biochemistry. 2003;42:12430–8. doi: 10.1021/bi034902z. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SV, Kelleher NL, Kinsland C, Chiu HJ, Costello CA, Backstrom AD, McLafferty FW, Begley TP. J Biol Chem. 1998;273:16555–60. doi: 10.1074/jbc.273.26.16555. [DOI] [PubMed] [Google Scholar]
- 7.Xi J, Ge Y, Kinsland C, McLafferty FW, Begley TP. Proc Natl Acad Sci USA. 2001;98:8513–8. doi: 10.1073/pnas.141226698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estramareix B, David S. Biochim Biophys Acta. 1990;1035:154–160. doi: 10.1016/0304-4165(90)90110-i. [DOI] [PubMed] [Google Scholar]
- 9.Estramareix B, Therisod M. J Am Chem Soc. 1984;106:3857–3860. [Google Scholar]
- 10.Newell PC, Tucker RG. Biochemistry Journal. 1968;106:271–277. doi: 10.1042/bj1060271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell PC, Tucker RG. Nature. 1967;215:1384–1385. doi: 10.1038/2151384a0. [DOI] [PubMed] [Google Scholar]
- 12.Newell PC, Tucker RG. Biochemistry Journal. 1968;106:279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estramareix B, Lesieur M. Biochim Biophys Acta. 1969;192:375–7. [PubMed] [Google Scholar]
- 14.Lawhorn BG, Mehl RA, Begley TP. Organ Biomol Chem. 2004;2:2538–46. doi: 10.1039/B405429F. [DOI] [PubMed] [Google Scholar]
- 15.Raschke M, Burkle L, Muller N, Nunes-Nesi A, Fernie AR, Arigoni D, Amrhein N, Fitzpatrick TB. Proc Natl Acad Sci U S A. 2007;104:19637–42. doi: 10.1073/pnas.0709597104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condon C, Cammack R, Patil DS, Owen P. J Biol Chem. 1985;260:9427–34. [PubMed] [Google Scholar]
- 17.Dougherty MJ, Downs DM. Microbiology. 2006;152:2345–53. doi: 10.1099/mic.0.28926-0. [DOI] [PubMed] [Google Scholar]
- 18.Zilles JL, Croal LR, Downs DM. J Bact. 2000;182:5606–5510. doi: 10.1128/jb.182.19.5606-5610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddick JJ, Kinslan C, Nicewonger R, Christian T, Downs DM, Winkler ME, Begley TP. Tetrahedron. 1998;54:15983–15991. [Google Scholar]
- 20.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Nucleic Acids Res. 2001;29:1097–106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon AP, Taylor S, Campobasso N, Chiu HJ, Kinsland C, Reddick JJ, Xi J. Archives of Microbiology. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed materials and methods are provided. This material is available free of charge via internet at http://pubs.acs.org.


