Abstract
The renin-angiotensin system in the brain acts to regulate a number of physiological processes. Evidence suggests that angiotensin peptides may act as neurotransmitters, although their biosynthetic pathways are poorly understood. We review evidence for neuronal production of angiotensin peptides, and hypothesize that angiotensin may be synthesized intracellularly in neurons.
Many advances in our understanding of the renin-angiotensin system (RAS) have been made over the last century, and the pace of discovery regarding this hormone system is ever quickening. Within the last two decades, the discovery of a second angiotensin converting enzyme (ACE2), a putative receptor for angiotensin-[1–7], and the (pro)renin receptor have vastly expanded our appreciation for the complex biochemistry and physiological responses elicited by the RAS. These discoveries have also led to many new hypotheses about the function of the RAS and potential novel clinical interventions for the diseases that are mediated through this humoral system.
Most textbooks and historical reviews of the RAS focus on the peripheral system as it exists in the circulation and its role in blood pressure and renal-mediated regulation of plasma osmolarity and volume. Indeed, plasma renin activity (PRA) is the clinical marker for systemic RAS activity. While the systemic functions of the RAS (i.e. stimulating aldosterone synthesis, causing peripheral vasoconstriction, and antinatriuresis) are critical, they fall short of describing and appreciating the paracrine and autocrine functions of the RAS within individual organs. Many organs express components of the RAS, including most notably the heart, kidney, and brain. These organs are extremely important in the regulation of cardiovascular function, and it is not surprising that modulation of RAS activity within these individual organs (even without modulating the circulating, or hormone RAS) can have major consequences on cardiovascular function.
In 1961, Bickerton and Buckley (3) first demonstrated that Ang-II acts directly within the central nervous system to increase blood pressure. It was further demonstrated that central injection of purified Ang-II (specifically around the hypothalamus) resulted in a robust drinking response (7). These reports firmly established a role for central Ang-II in the regulation of blood pressure and hydromineral balance, and implied that Ang-II-sensitive receptors are present within discrete regions of the brain. Other studies documented the presence of renin within the brain, thereby providing evidence for endogenous angiotensin production within the central nervous system (9; 12). Together, these early studies established both the production of, and receptive fields for, Ang-II within the central nervous system.
With these data now in hand, current brain-RAS researchers are left with a discrete set of questions: Since the RAS ultimately functions by stimulating cell surface receptors which induce intracellular signaling mechanisms, where do these signals originate, where do they act, and how are they transduced? In other words, what portions of the brain (both regional and cell specific) synthesize angiotensin peptides, and which portions express receptors for these peptides? What are the functions of the RAS within the brain, in terms of behavioral and physiological regulation? Are angiotensin peptides utilized by neurons as neurotransmitters and/or neuromodulators?
Regulation of peripheral organ system function by the brain is mediated through either direct synaptic contact (such as the innervation of skeletal muscle, cardiac muscle, and various glands), or through neurohormonal signaling (such as vasopressin or ACTH release into the circulation). In both cases, regulation of anatomically and chemically distinct neuronal populations mediates these functions. To understand exactly how the central RAS can modulate physiological functions, then, it should be our first goal to define the molecular and cellular localizations and actions of the RAS within the brain. Determining, for example, whether angiotensin peptides are used as neurotransmitters (versus neuromodulators) is key to our understanding of how the central RAS can control neuronal function.
In their textbook, Cooper, Bloom, and Roth (4) have described a five-point definition for what constitutes a neurotransmitter: 1) the substance must be synthesized in, and released from, the presynaptic neuron, 2) the substance must be released from nerve terminals (into synapses), 3) the substance must cause effects in post-synaptic neurons that reflect the signal carried by the pre-synaptic neuron, 4) the substance’s effects on the post-synaptic neuron must be receptor-mediated and dose-dependent, and 5) an active degradation or reuptake mechanism must exist. Several excellent reviews (most notably (8)) have outlined how Ang-II fulfills many parts of this classic definition, with the notable exception of the first, neuronal synthesis. We will focus on the evidence for neuronal synthesis of Ang-II, the first criteria required to define Ang-II as a neurotransmitter. Further, we examine how the discovery of new components of the RAS, namely an intracellular form of the renin enzyme and the (pro)renin receptor ((P)RR), may help provide mechanisms to achieve neuronal synthesis of Ang-II.
Angiotensin Synthesis in the Central Nervous System
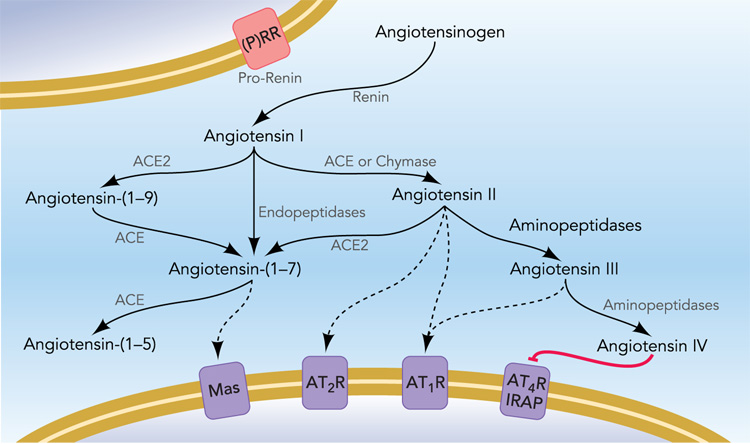
All of the angiotensin peptides are produced by sequential enzymatic cleavage of angiotensinogen, and alternate processing pathways result in the irreversible formation of subsequent peptides (Figure 1). Thus, the minimum requirement to generate all angiotensin peptides is the synthesis of angiotensinogen. The majority of angiotensinogen synthesized within the brain is produced by astrocytes where it is constitutively secreted into the interstitial space and cerebrospinal fluid (5; 51). Glial angiotensinogen has been presumed to be the substrate through which exogenous renin can cause pressor and dipsogenic responses, when the enzyme is administered directly into the brain ventricles (15). Indeed, the importance of glial angiotensinogen is highlighted by the consequences for blood pressure and drinking by the gene’s over-expression or ablation (27; 38). Interestingly, recent studies suggest that glial cells may play more than a simple passive role in the regulation of blood pressure (1), as transduction of glial cells with a constitutively active AT1 receptor in the rostral ventrolateral medulla (RVLM) causes a rise in arterial blood pressure.
Figure 1. The Renin-Angiotensin System.
Recent advances in understanding the complex biochemistry of the RAS have prompted new interest in the significance of alternative peptide products. Alternate processing pathways result in the stimulation of distinct receptor populations on target cells, and thus the presence or absence of individual enzymes in the local environment, and receptor subtypes expressed in target cells, can grossly alter the effects of angiotensin signaling on target cells. Although typically assumed to function entirely in the interstitium, emerging evidence suggests possible mechanisms of intracellular formation of the various peptides. For a more detailed review of the known enzymatic pathways involved, see (47; 48).
Numerous studies have established that neurons are also capable of producing angiotensinogen. Cultured neurons express and secrete angiotensinogen (16; 53), and immunocytochemical localization of angiotensinogen in the brain has demonstrated the presence of angiotensinogen in neurons of the ventrolateral medulla (33), hypoglossal nuclei (52), forebrain, thalamus, hypothalamus, and brain stem (31; 54), magnocellular neurons of the paraventricular nucleus (PVN) (2), and nucleus of the solitary tract (NTS), paraventricular nucleus, RVLM, and subfornical organ (SFO) (56). Moreover, preliminary work by Vinsant, et al. (55) suggests that inhibition of glial angiotensinogen has little effect on neuronal Ang II-staining in central pathways controlling sympathetic activity.
Given the presence of abundant angiotensinogen and assuming there is a source of renin to process angiotensinogen in neurons, is there evidence for neuronal Ang-II staining? Ang-II stained magnocellular and parvocellular neuronal cell bodies are abundant in the PVN and supraoptic nuclei (SON), and Ang-II has also been reported in other hypothalamic nuclei, circumventricular organs, and the NTS (24). Ang-II immunoreactive nerve fibers have been identified in the SFO, median preoptic nucleus (MnPO), SON, PVN, and posterior pituitary (10; 22; 23). Furthermore, Ang-II has been co-localized with γ-aminobutyric acid in nerve terminals near synapses, and some Ang-II is evident in secretory vesicles, which may be synaptic vesicles (35).
Given the presence of the angiotensin precursor, angiotensinogen, and the effector, Ang-II, in neurons, where is the renin? It is commonly accepted that the first, and rate-limiting step in the production of all subsequent angiotensin peptides is the enzymatic cleavage of angiotensinogen by renin. Therefore, by definition, a local RAS can only exist in the brain if renin is also present or can gain access. Renin-like enzymatic activity was first demonstrated in dog and rat brain homogenates (9; 12). Further studies demonstrated that renin protein and mRNA were present in astrocytes and neurons, in rat, mouse, and human (6; 11; 14; 46). The localization of renin to particular structures and cell types within the brain has proven difficult, though, as its levels within the brain are very low. No doubt this has been a major technical hurdle toward understanding the biosynthesis of Ang-II in the brain, thus leading some to hypothesize that angiotensin peptides may be formed through renin-independent mechanisms. Arguing against a renin-independent mechanism are genetic data using transgenic mouse and rat models which take advantage of the strict species-specificity of the enzymatic reaction between human renin and human angiotensinogen (13; 26). In nearly all of those studies, double transgenic animals exhibit robust cardiovascular and metabolic phenotypes whereas single transgenic animals (over-expressing renin or angiotensinogen but not both) are phenotypically normal. On the other hand, there are no data directly addressing the possibility that the interaction between angiotensinogen and non-renin processing proteases are also species specific. Such a species-specificity between angiotensinogen and non-renin processing enzymes could explain the lack of a phenotype in transgenic animals expressing only human angiotensinogen. Future studies utilizing the renin inhibitor, aliskiren, in animals overexpressing species-matched angiotensinogen could perhaps aid in addressing this question.
Recently, we reported evidence for renin promoter activity in both astrocytes and neurons of mice using transgenic mice expressing a sensitive reporter gene driven by the renin promoter (18). Further, using sensitive reporter transgenes controlled by the renin- and angiotensinogen-promoters, we have demonstrated similar distribution of renin- and angiotensinogen-expressing cells within the SFO, PVN and RVLM (17). Finally, studies utilizing mice overexpressing renin, driven by a highly regulated endogenous promoter encoded in a very large genomic transgene, have provided strong evidence for de novo renin synthesis within the brain (30).
The use of transgenic rats and mice either over-expressing or ablating the synthesis of RAS components in the brain has resulted in a wealth of new detailed information on the role of the RAS in regulating blood pressure and hydromineral balance. Most commonly used rat models include the TGR(mREN27) model expressing the mouse Ren-2 gene, TGR(ASrAogen) expressing an antisense to angiotensinogen under the control of the glial GFAP promoter, and double transgenics expressing both the human renin and angiotensinogen genes. The TGR(ASrAogen) rat perhaps has the greatest potential to address issues of neuronal production of angiotensin peptides. Indeed, Sakima et al (37) suggest that non-glial sources of Ang-II and Ang-(1–7) in the NTS may contribute to arterial pressure responses to anesthesia in these rats, and that in particular, Ang-(1–7) from a non-glial source may have effects on baroreflex sensitivity. In interpreting these findings, one must not only consider that the antisense construct causes a reduction, but not knockout of glial angiotensinogen, but also the potential role of circulating angiotensin in the regulation of the baroreflex within the NTS (34).
Several transgenic models have also been established in mice. In these studies, cell-specific promoters were used to drive expression of human renin, human angiotensinogen, or rat AT1 receptors specifically to either neurons or glial cells (28). Transgenic animals overexpressing the human renin gene alone either centrally or systemically show no phenotypic alterations in Ang-II production or blood pressure, whereas animals harboring both human renin and angiotensinogen exhibit increased brain-specific Ang-II production and potent AT1 receptor-dependent hypertension (27–29). Similarly, neuronal specific overexpression of AT1 receptors causes an increase in arterial pressure in response to exogenous Ang-II (20). The next level of sophistication came from combining the power of cell-specific over-expression (as above) with Cre-recombinase loxP-mediated ablation. This allowed the development of robust genetic platforms to first over-express components of the RAS, and then to ablate them in a tissue-specific, region-specific, or cell-specific manner. We first applied this technique and gathered proof-of-principle for its effectiveness by showing that intravenous delivery of an adenovirus encoding Cre-recombinase (AdCre) could efficiently ablate expression of a “floxed” human angiotensinogen transgene (hAGTloxP) in the liver of transgenic mice (49) and could lower arterial pressure in double transgenic mice carrying human renin and floxed human angiotensinogen (50). This study provided strong evidence that the major source of circulating angiotensinogen is derived from the liver. We also bred the same double transgenic mice with mice expressing Cre-recombinase under a glial-specific promoter and showed that the triple transgenics had lower blood pressure than the hypertensive double transgenic mice, once again illustrating the importance of glial angiotensinogen (40). Davisson then showed that stereotactic delivery of AdCre could effectively cause regional ablation of gene expression in the brain (44; 45). They went on to report that AdCre-mediated SFO-specific ablation of hAGTloxP attenuated the pressor response to ICV injection of human renin (43). Similarly, we studied a double transgenic mouse model which simultaneously expresses hAGTloxP in neurons and glia, and human renin in neurons. These mice exhibit hypertension and an increase in water and salt intake at baseline (36). SFO-specific ablation of human angiotensinogen in this model by AdCre significantly reduced water intake. Although we performed site-specific injection of AdCre into the SFO, we recognize that adenoviruses can be retrogradely transported and thus may result in the ablation of transgene expression in neurons which project to the injection site (44). Consequently, the reduced pressor response to human renin in single transgenic mice and the reduced water intake in the double transgenic mice may be due to loss of human angiotensinogen in the SFO but also in neurons projecting to the SFO. Nevertheless, both studies collectively highlight the importance of local angiotensin production within the brain.
Novel Players in the RAS: Intracellular Renin
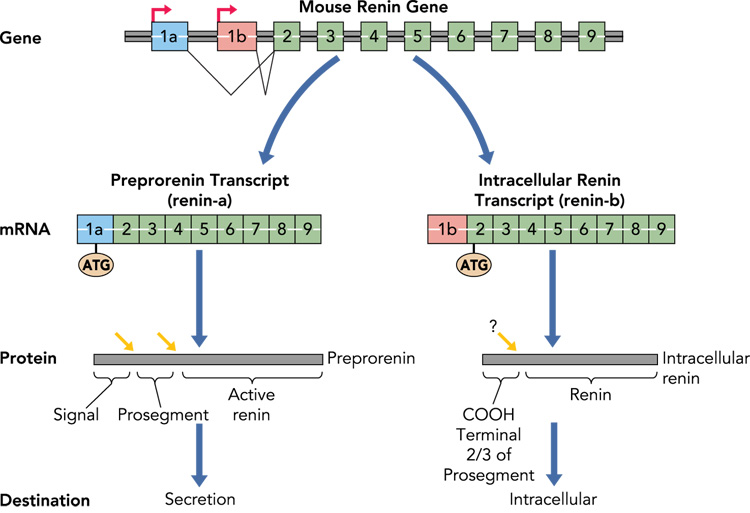
The classical renin mRNA transcript contains nine exons in rodents (ten exons in humans) and has a very short 5’ untranslated region (UTR) (Figure 2). Translation begins at the first ATG codon in exon 1. In juxtaglomerular cells of the kidney, the primary product of renin mRNA translation is preprorenin. The “pre” represents the signal peptide which directs the protein to enter the endoplasmic reticulum with subsequent incorporation of the polypeptide into the secretory pathway. After cleavage of the signal peptide, the remaining protein (prorenin) is sorted into either the constitutive secretory pathway (and is passed directly out of the cell without further processing), or into the regulated pathway where the protein is glycosylated, packaged into secretory granules, and the prosegment removed. The prorenin converting enzyme which processes inactive prorenin to active renin remains unclear. It is also unclear if this pathway is shared in other renin-expressing cell types and tissues including the brain.
Figure 2. Synthesis of Intracellular Renin.
The hypothesized biosynthetic pathway for intracellular renin is shown. Classically, preprorenin is the primary translation product of the renin-A mRNA transcribed from the classical renin promoter and including exon-1a. Preprorenin is processed (red arrows) first by removal of the signal peptide and then by removal of the prosegment. Active renin is subsequently released into the systemic circulation. In the brain, a different renin mRNA termed renin-b is transcribed from an unknown promoter within intron-1 (in mice) or 6.2 kb upstream of the classical renin promoter (in humans) to result in a novel transcript lacking exon-1a, and including exon-1b. There are no ATG sequences in exon-1b in rat, mouse, or human, and thus translation begins at the highly conserved ATG present in exon-2. This product encodes the entire active renin protein and ⅔ of the prosegment. It is unclear, if the prosegment is removed. As the protein lacks a signal peptide, it is unlikely to be secreted. Additional studies are needed to determine if this protein is stable, remains intracellularly, and within which intracellular structures it resides.
We and Lee-Kirsh, et al. reported that the structure of renin mRNA in the brain is different from that transcribed in the kidney (21; 42). Instead of transcription beginning at the classical renin promoter and containing exon-1 (now termed exon-1a), the transcripts in the brain start at a unique transcription start site and contain a new first exon (termed exon-1b, encoding renin-b). Renin-b mRNA initiated at exon-1b splices directly to exon 2 and thus the remainder of the mRNA is identical in brain and kidney Figure 2). Since there is no exon-1a, translation cannot initiate from the “normal” ATG present in exon-1a, and importantly, there are no ATG sequences in exon-1b. Thus, translation must initiate at the next “in frame” ATG which lies in exon-2. Interestingly, this ATG is extremely well conserved throughout evolution, more so than the surrounding sequences in the prosegment. Translation from the exon-2 ATG would result in the production of a protein devoid of the signal peptide and the first ⅓ of the pro-segment. Biochemical studies revealed that renin-b is active, suggesting that the remaining ⅔ of the prosegment is insufficient to inhibit renin activity (21). It is therefore hypothesized that translation of the renin-b transcript produces an intracellular form of active renin. Interestingly, mutations in the renin prosegment caused both an increase in enzymatic activity of prorenin but also decreased secretion in transiently transfected CHO cells (25). This led the authors to conclude that the prosegment was required for efficient expression of renin. Along these lines, it is notable that renin levels in the brain are extremely low. We examined the hypothesis that expression of the intracellular form of renin in the brain would result in increased arterial pressure by comparing two double transgenic mouse models each expressing human angiotensinogen and glial-specific expression of either intracellular renin or secreted renin. Both models exhibited an equivalent increase in arterial pressure that was AT1 receptor-dependent providing evidence for a physiological function of intracellular renin in the brain (19).
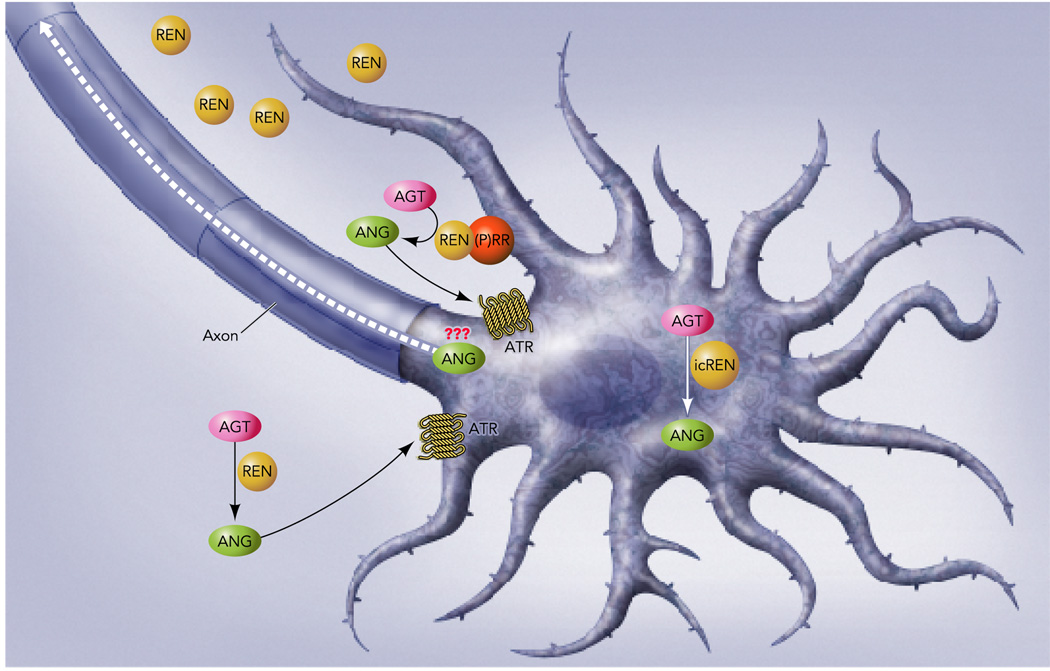
Recent studies in non-neural cell types in culture suggests that intracellular levels of angiotensinogen, renin and Ang-II can be modulated under certain conditions (41). Consequently, this begs the question of whether the identification of this novel brain-specific transcript encoding an intracellular active renin is the missing link in defining Ang-II as a neurotransmitter. Clearly, there are many unresolved questions derived from this observation (Figure 3). Are renin-b mRNA and protein localized in neurons in regions of the brain controlling cardiovascular function? Is renin-b sorted to an intracellular compartment where it could interact with angiotensinogen, ACE, neprilysin, ACE2 or aminopeptidases to form Ang-II, Ang-(1–7), or other smaller angiotensin peptides? Does this intracellular processing of angiotensinogen, if it occurs, result in the production of angiotensin peptides that are sorted to the neurosecretory apparatus?
Figure 3. Neuronal RAS- A Provocative Hypothesis.
The findings reviewed herein suggest the intriguing possibility for the intracellular generation of angiotensin peptides (ANG) derived from the intracellular conversion of angiotensinogen (AGT) by renin-b or intracellular renin (icREN). The fate of intracellular angiotensin peptides synthesized in this way remains undefined. We hypothesize that some mechanism may exist for these peptides to be packaged into neurosecretory bodies and perhaps transported through axons to the synapse. Similarly, we hypothesize that the presence of the prorenin receptor [(P)RR] on the neuronal surface may provide a mechanism for prorenin or renin in the extracellular space to be concentrated in the proximity of angiotensinogen released from either glial cells or neurons. This, in the presence of other angiotensin converting enzymes may lead to the synthesis of angiotensin peptides in the proximity of neuronal angiotensin receptors (ATR).
We have begun to take steps toward answering these questions. For example, we have recently determined that Neuro-2a neuroblastoma cells express renin-b mRNA and we are screening other neuronal cell types for expression of this renin isoform. We have also embarked on an ambitious project using gene targeting in C57BL/6 embryonic stem cells to take advantage of the structural differences in the gene encoding the renin-a and renin-b mRNAs to generate separate “null” and “flox” alleles of the renin-a and renin-b mRNAs derived from the mouse Ren-1c gene. Some of these mice have already been obtained and their analysis should provide novel tools and insights that will allow us to dissect the relative importance of these two renin isoforms in the brain.
Novel Players in the RAS: The (Pro)Renin Receptor
In addition to the direct synthesis of intracellular active renin, the discovery of a receptor for (pro)renin provides a possible mechanism by which neurons could either concentrate or potentially sequester extra-neuronal sources of renin. The (pro)renin receptor, first identified by Nguyen, et al. (32), binds both renin and prorenin, non-enzymatically activates prorenin to increase its angiotensinogen-cleaving catalytic efficiency, and activates intracellular signaling pathways. Of particular interest, is the observation that this receptor is expressed at high levels within the brain. Is it possible that through binding to neuronal (pro)renin receptors, local renin levels may be substantially increased near (or potentially within) neurons, independent of the original source of the prorenin or renin (Figure 3)? Perhaps, this could provide a mechanism to concentrate renin directly in the proximity of neuronal AT1 receptors and provide an immediate highly localized source of Ang-II derived from angiotensinogen released from either neurons or glial cells. As of now, there are few data implicating a role for this receptor in the brain. As above, many questions would need resolution. Are (pro)renin receptors localized on neurons in close proximity to AT1 receptors? Are they necessary for the production of Ang-II or other angiotensin peptides in the brain? Do they increase the catalytic activity of prorenin, or transduce a signal within neurons in response to the binding of renin as a ligand? Do they concentrate renin and provide a mechanism for uptake of the enzyme into neurons? Recent data from Shan, et al. (39) provide evidence for the presence of (pro)renin receptors in neurons from neonatal rats. They further report that the addition of renin, in the presence of losartan, to cultured neurons resulted in a potent decrease in action potential frequency that was that was attributed to the (pro)renin receptor.
Summary and Conclusions
We have reviewed evidence for the neuronal production of the precursor and primary enzyme responsible for angiotensin production which collectively support the hypothesis that angiotensins may act as neurotransmitters. Further, we hypothesize that newly recognized components of the RAS, namely renin-b and the (pro)renin receptor, may perhaps be the missing links which are required for the local or intracellular synthesis of neuronal Ang-II. Together, these hypotheses provide a novel theoretical framework for experiments into the neurotransmitter and neuromodulatory roles of angiotensins within the brain.
References
- 1.Allen AM, Dosanjh JK, Erac M, Dassanayake S, Hannan RD, Thomas WG. Expression of Constitutively Active Angiotensin Receptors in the Rostral Ventrolateral Medulla Increases Blood Pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. [DOI] [PubMed] [Google Scholar]
- 2.Aronsson M, Almasan K, Fuxe K, Cintra A, Harfstrand A, Gustafsson JA, Ganten D. Evidence for the existence of angiotensinogen mRNA in magnocellular paraventricular hypothalamic neurons. Acta Physiol Scand. 1988;132:585–586. doi: 10.1111/j.1748-1716.1988.tb08370.x. [DOI] [PubMed] [Google Scholar]
- 3.Bickerton RK, Buckley JP. Evidence for a central mechanism in angiotensin-induced hypertension. Proc Soc Exp Biol Med. 1961;106:834–837. [Google Scholar]
- 4.Cooper JR, Bloom FE, Roth RH. Biochemical basis of neuropharmacology. Oxford: University Press; 1996. [Google Scholar]
- 5.Deschepper CF, Bouhnik J, Ganong WF. Colocalization of angiotensinogen and glial fibrillary acidic protein in astrocytes in rat brain. Brain Res. 1986;374:195–198. doi: 10.1016/0006-8993(86)90411-7. [DOI] [PubMed] [Google Scholar]
- 6.Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension. 1986;8:544–548. doi: 10.1161/01.hyp.8.6.544. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970;210:457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- 9.Fischer-Ferraro C, Nahmod VE, Goldstein DJ, Finkielman S. Angiotensin and renin in rat and dog brain. J Exp Med. 1971;133:353–361. doi: 10.1084/jem.133.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuxe K, Ganten D, Hoekfelt T, Bolme P. Immunohistochemical evidence for the existence of angiotensin II-containing nerve terminal in the brain and spinal cord in the rat. Neurosci Lett. 1980;2:229–239. doi: 10.1016/0304-3940(76)90020-3. [DOI] [PubMed] [Google Scholar]
- 11.Ganten D, Fuxe K, Phillips MI, Mann JFE, Ganten U. The Brain Isorenin-Angiotensin System: Biochemistry, Localization, and Possible Role in Drinking and Blood Pressure Regulation. In: Ganong WF, Martini L, editors. Frontiers in Neuroendocrinology. New York: Raven Press; 1978. pp. 61–99. [Google Scholar]
- 12.Ganten D, Minnich JL, Granger P, Hayduk K, Brecht HM, Barbeau A, Boucher R, Genest J. Angiotensin-forming enzyme in brain tissue. Science. 1971;173:64–65. doi: 10.1126/science.173.3991.64. [DOI] [PubMed] [Google Scholar]
- 13.Hatae T, Takimoto E, Murakami K, Fukamizu A. Comparative studies on species-specific reactivity between renin and angiotensinogen. Mol Cell Biochem. 1994;131:43–47. doi: 10.1007/BF01075723. [DOI] [PubMed] [Google Scholar]
- 14.Hermann K, Raizada MK, Sumners C, Phillips MI. Presence of renin in primary neuronal and glial cells from rat brain. Brain Res. 1987;437:205–213. doi: 10.1016/0006-8993(87)91637-4. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki H, Takasaki K, Furukawa T. Exaggerated pressor response to centrally administered renin in freely moving, spontaneously hypertensive rats. Eur J Pharmacol. 1987;138:351–357. doi: 10.1016/0014-2999(87)90473-0. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Rassoli A, Raizada MK. Angiotensinogen gene expression in neuronal and glial cells in primary cultures of rat brain. J Neurosci Res. 1988;19:287–290. doi: 10.1002/jnr.490190302. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of rennin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension. 2004;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of Renin Expressing Cells in the Brain Using a REN-eGFP Transgenic Model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 20.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- 21.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 22.Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactive pathways in the central nervous system of the rat: evidence for a projection from the subfornical organ to the paraventricular nucleus of the hypothalamus. Clin Exp Hypertens A. 1984;6:1915–1920. doi: 10.3109/10641968409046101. [DOI] [PubMed] [Google Scholar]
- 23.Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactivity in the neural afferents and efferents of the subfornical organ of the rat. Brain Res. 1984;321:209–215. doi: 10.1016/0006-8993(84)90174-4. [DOI] [PubMed] [Google Scholar]
- 24.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- 25.Mercure C, Thibault G, Lussier-Cacan S, Davignon J, Schiffrin EL, Reudelhuber TL. Molecular analysis of human prorenin prosegment variants in vitro and in vivo. J Biol Chem. 1995;270:16355–16359. doi: 10.1074/jbc.270.27.16355. [DOI] [PubMed] [Google Scholar]
- 26.Merrill DC, Thompson MW, Carney C, Schlager G, Robillard JE, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated Blood Pressure in Transgenic Mice with Brain-Specific Expression of Human Angiotensinogen Driven by the Glial Fibrillary Acidic Protein Promoter. Circ Res. 2001;89:365–372. doi: 10.1161/hh1601.094988. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto S, Cassell MD, Sigmund CD. Glial- and neuronal-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem. 2002;277:33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto S, Cassell MD, Sigmund CD. Neuron-Specific Expression of Human Angiotensinogen in Brain Causes Increased Salt Appetite. Physiol Genomics. 2002;9:113–120. doi: 10.1152/physiolgenomics.00007.2002. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto S, Cassell MD, Sigmund CD. The Brain Renin-Angiotensin System in Transgenic Mice Carrying a Highly Regulated Human Renin Transgene. Circ Res. 2002;90:80–86. doi: 10.1161/hh0102.102272. [DOI] [PubMed] [Google Scholar]
- 31.Mungall BA, Shinkel TA, Sernia C. Immunocytochemical localization of angiotensinogen in the fetal and neonatal rat brain. Neuroscience. 1995;67:505–524. doi: 10.1016/0306-4522(95)00044-j. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. Journal of Clinical Investigation. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palkovits M, Mezey E, Fodor M, Ganten D, Bahner U, Geiger H, Heidland A. Neurotransmitters and neuropeptides in the baroreceptor reflex arc: connections between the nucleus of the solitary tract and the ventrolateral medulla oblongata in the rat. Clin Exp Hypertens. 1995;17:101–113. doi: 10.3109/10641969509087058. [DOI] [PubMed] [Google Scholar]
- 34.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008 doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 35.Pickel VM, Chan J. Co-localization of angiotensin II and gamma-aminobutyric acid in axon terminals in the rat subfornical organ. Neurosci Lett. 1995;193:89–92. doi: 10.1016/0304-3940(95)11673-k. [DOI] [PubMed] [Google Scholar]
- 36.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakima A, Averill DB, Kasper SO, Jackson LM, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor Reflex Regulation in Anesthetized Transgenic Rats with Low Glial-Derived Angiotensinogen. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00984.2006. in press, published online. [DOI] [PubMed] [Google Scholar]
- 38.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci U S A. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol. 2008;93:701–708. doi: 10.1113/expphysiol.2008.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherrod M, Davis DR, Zhou X, Cassell MD, Sigmund CD. Glial-specific ablation of angiotensinogen lowers arterial pressure in renin and angiotensinogen transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1763–R1769. doi: 10.1152/ajpregu.00435.2005. [DOI] [PubMed] [Google Scholar]
- 41.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 42.Sinn PL, Sigmund CD. Identification of Three Human Renin mRNA Isoforms Resulting from Alternative Tissue-Specific Transcriptional Initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- 43.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic Ablation of Angiotensinogen in the Subfornical Organ of the Brain Prevents the Central Angiotensinergic Pressor Response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- 44.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: comparison of two viral vector systems. Hypertension. 2002;39:603–608. doi: 10.1161/hy0202.103295. [DOI] [PubMed] [Google Scholar]
- 45.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of Cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25–32. doi: 10.1152/physiolgenomics.00048.2004. [DOI] [PubMed] [Google Scholar]
- 46.Speck G, Poulsen K, Unger T, Rettig R, Bayer C, Scholkens B, Ganten D. In vivo activity of purified mouse brain renin. Brain Res. 1981;219:371–384. doi: 10.1016/0006-8993(81)90300-0. [DOI] [PubMed] [Google Scholar]
- 47.Speth RC, Karamyan VT. Brain angiotensin receptors and binding proteins. Naunyn Schmiedebergs Arch Pharmacol. 2008 doi: 10.1007/s00210-007-0238-7. [DOI] [PubMed] [Google Scholar]
- 48.Speth RC, Karamyan VT. The significance of brain aminopeptidases in the regulation of the actions of angiotensin peptides in the brain. Heart Fail Rev. 2008 doi: 10.1007/s10741-007-9078-2. [DOI] [PubMed] [Google Scholar]
- 49.Stec DE, Davisson RL, Haskell RE, Davidson BL, Sigmund CD. Efficient liver-specific deletion of a floxed human angiotensinogen transgene by adenoviral delivery of cre-recombinase in vivo. J Biol Chem. 1999;274:21285–21290. doi: 10.1074/jbc.274.30.21285. [DOI] [PubMed] [Google Scholar]
- 50.Stec DE, Keen HL, Sigmund CD. Lower blood pressure in floxed angiotensinogen mice after adenoviral delivery of cre-recombinase. Hypertension. 2002;39:629–633. doi: 10.1161/hy0202.103418. [DOI] [PubMed] [Google Scholar]
- 51.Stornetta RL, Hawelu Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- 52.Tham M, Sim MK, Tang FR. Location of renin-angiotensin system components in the hypoglossal nucleus of the rat. Regul Pept. 2001;101:51–57. doi: 10.1016/s0167-0115(01)00260-9. [DOI] [PubMed] [Google Scholar]
- 53.Thomas WG, Greenland KJ, Shinkel TA, Sernia C. Angiotensinogen is secreted by pure rat neuronal cell cultures. Brain Res. 1992;588:191–200. doi: 10.1016/0006-8993(92)91575-y. [DOI] [PubMed] [Google Scholar]
- 54.Thomas WG, Sernia C. Immunocytochemical localization of angiotensinogen in the rat brain. Neuroscience. 1988;25:319–341. doi: 10.1016/0306-4522(88)90029-2. [DOI] [PubMed] [Google Scholar]
- 55.Vinsant S, Chappel MC, Ferrario CM, Ganten D, Diz DI. Low Glial Angiotensinogen is not Associated with Deficits in Angiotensin Peptides in Neuronal Pathways in Transgenic ASrAogen Rats. FASEB Journal. 2005;19:A1188. Abstract. [Google Scholar]
- 56.Yang G, Gray TS, Sigmund CD, Cassell MD. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res. 1999;817:123–131. doi: 10.1016/s0006-8993(98)01236-0. [DOI] [PubMed] [Google Scholar]