I. Introduction
Although the etiology of multiple sclerosis (MS) remains uncertain, a growing body of research indicates that environmental factors interact with genetic factors to cause disease. Potential environmental risk factors include infectious pathogens and stress (Sospedra and Martin, 2005; Ackerman et al., 2003; Mohr and Pelletier, 2006). Both human and animal research suggests that viral infection is a likely environmental trigger of MS (Challoner et al., 1995; Gilden, 2005; Sospedra and Martin, 2005). Additionally, MS patients frequently report elevated levels of stress prior to initial diagnosis and/or disease exacerbation (Ackerman et al., 2002; Grant et al., 1989; Mohr et al., 2000; Mohr et al., 2004; Warren et al., 1982). Thus, stress may interact with viral infection and genetic vulnerability to determine disease onset and severity.
Theiler's murine encephalomyelitis virus (TMEV) infection is a commonly used animal model of MS (Lipton, 1975; Oleszak et al., 2004). Intracerebral inoculation of TMEV induces a biphasic disease of the central nervous system (CNS). The acute phase of disease is characterized by CNS inflammation as the result of neuronal and glial infection (Njenga et al., 1997), whereas the chronic phase is characterized by immune-mediated demyelination (Lipton, 1975; Oleszak et al., 2004). During the acute phase, mice exhibit mild signs of encephalitis and hind limb impairment similar to polio (Campbell et al., 2001; Johnson et al., 2004; Johnson et al., 2006; Sieve et al., 2004; Sieve et al., 2006). During the chronic phase, susceptible strains of mice progress from minor disruptions in gait, impaired motor coordination, and reduced locomotor activity at the early stages of demyelination to profound dysfunction at the later stages due to axonal loss (McGavern et al., 1999; McGavern et al., 2000).
After infection with TMEV resistant strains of mice mount an effective immune response and clear the virus from the CNS during the acute phase of disease. In contrast, susceptible strains of mice fail to generate an effective immune response during early infection, resulting in viral persistence and subsequent immune-mediated demyelination during the chronic phase (Lipton and Melvold, 1984; Rodriguez et al., 1983; Olezak et al., 2004). Although susceptibility to TMEV-induced demyelination is genetically regulated (Brahic and Bureau, 1998; Brahic et al., 2005), other factors that influence the initial immune response play an important role in determining viral load and the degree of viral persistence, thereby altering disease progression and severity (Aubagnac et al., 1999; Brahic et al., 2005; Rodriguez et al., 1996). For example, depletion of CD8 cells or exposure to chronic social stress prior to infection exacerbates disease (Borrow et al., 1992; Johnson et al., 2004; Johnson et al., 2006).
Our laboratory has previously shown that repeated exposure to social disruption stress (SDR) subsequently exacerbates both the acute and chronic phase of Theiler's murine encephalomyelitis virus infection (Johnson et al., 2004; Johnson et al., 2006). Specifically, we have shown that SDR increases inflammation in the spinal cord and brain of TMEV-infected mice and that this is associated with increased levels of the proinflammatory cytokine interleukin-6 (IL-6) in circulating blood and the development of glucocorticoid resistance (GCR). In association with these increases in inflammation, SDR exacerbates TMEV-induced motor impairment and disrupts viral clearance from the brain. Although the neuroimmune mechanism(s) mediating the adverse effects of SDR on TMEV infection have not been determined, we propose that IL-6 provides a likely candidate because it is increased by both TMEV infection (Chang et al., 2000; Mi et al., 2006; Sato et al., 1997; Theil et al., 2000) and by exposure to SDR (Avitsur et al., 2002; Johnson et al., 2006; Merlot et al., 2003; Merlot et al., 2004a; Stark et al., 2002).
Proinflammatory cytokines play a critical role in orchestrating the immune responses involved in viral clearance during early infection and in demyelination during late disease (Oleszak et al., 2004). During acute TMEV infection, IL-6 and other proinflammatory cytokines are elevated in all strains of mice, but higher levels are observed in susceptible mice compared to resistant mice (Chang et al., 2000; Mi et al., 2006; Sato et al., 1997; Theil et al., 2000). Other research indicates that stressors can increase circulating and central levels of IL-6 and other proinflammatory cytokines (Johnson et al., 2006; Johnson et al., 2005; Merlot et al., 2004a; Merlot et al., 2004 b; Nguyen et al., 1998; Nguyen et al., 2000; O'Connor et al., 2003; Shintani et al., 1995; Shizuya et al., 1998; Stark et al., 2002; Huang et al., 1997). Moreover, prior exposure to a stressor can potentiate or prolong the release of proinflammatory cytokines following immune challenge (Johnson et al., 2002; Johnson et al., 2006; Merlot et al., 2004b; Quan et a., 2001). These findings suggest that exposure to stress can directly alter immune cell function and promote sustained increases in proinflammatory cytokine production. Accordingly, we propose that central increases in IL-6 production may be one mechanism mediating SDR-induced exacerbation of acute and chronic TMEV infection.
The present studies examined the role of IL-6 in the stress-induced exacerbation of acute TMEV infection. To block the effects of stress-induced IL-6 in the brain, we infused a selective neutralizing antibody to IL-6 into the cerebroventricle. This method has been successfully used to neutralize IL-1, IL-6, and TNF-α in brain in both rats and mice (Jean Harry et al., 2003; Nadeau and Rivest, 2000; Nadeau and Rivest, 2003). Mice were pretreated with the neutralizing antibody prior to each of six SDR sessions and IL-6 levels were measured in sera and brain following stressor termination. To determine whether stress-induced increases in IL-6 contribute to the exacerbation of acute TMEV infection, we examined whether infusion of the IL-6 neutralizing antibody during SDR would prevent the subsequent exacerbation of acute TMEV infection. Because other research suggests that SDR-induced increases in inflammation may be attributed to the development of GCR (Avitsur et al., 2001; Stark et al., 2001; Merlot et al., 2004a), we also examined whether the neutralizing antibody altered the development of GCR before and after infection. Finally, to determine whether IL-6 alone was sufficient to exacerbate disease course, we examined whether mice pretreated with intracerebral IL-6 prior to TMEV infection would mimic the adverse effects of SDR. Taken together, these studies tested the hypothesis that activation of central and/or peripheral IL-6 is both necessary and sufficient to exacerbate acute TMEV infection.
2. Materials and Methods
2.1. Animals
Male Balb/cJ mice (23-day-old; Jackson Labs, Bar Harbor, ME) were individually housed. Following recovery from surgery on post-natal day 32, mice were housed three per cage, matching on body weight across cages. Mice were maintained on a 12 h light/dark cycle (lights on at 05:00 h) with ad libitum access to food and water. Intruders were retired male breeders aged 6-8 months. To increase aggressive behavior, intruders were pair-housed with sterilized females. Intruders were screened for aggressive behaviors by placing them in the home cage of another mouse. Intruders who consistently attacked peers within 30 s and adolescent mice within 2 min were used in these experiments. Both BALB c/J and B10 males were used as intruders, with the B10 mice having a slightly faster latency to first attack. No other differences were observed across strains. All animal care protocols were in accordance with the Texas A&M University Laboratory Animal Care and Use Committee (ULACC).
2.2. Experimental designs
2.2.1. Experiment 1: impact of SDR and neutralizing antibody on IL-6 and GCR
A 2 (SDR vs. NON-SDR) × 2 (AbTx vs. vehicle) factorial design was used to determine whether SDR increases central and peripheral levels of IL-6 and whether ICV administration of a neutralizing antibody (AbTx) to IL-6 effectively blocks these effects (N = 24). We also examined whether the neutralizing antibody altered the development of GCR. On pnd 30, either neutralizing antibody or vehicle was infused ICV 4 h prior to each SDR session (17:00-19:00 h). After the final SDR session (pnd 37), all animals were mice were overdosed with ketamine/xylazine, bled from the brachial artery, and perfused with PBS. Spleens were taken for GCR assay, while brain tissue and sera was assessed for IL-6 levels using an ELISA.
2.2.2. Experiment 2: the necessity of IL-6 in mediating the adverse effects of SDR on acute TMEV infection
A 2 (SDR vs. NON-SDR) × 2 (AbTx vs. Vehicle) × 2 (day 7 vs. 21 pi) design was used to determine whether infusion of the neutralizing antibody to IL-6 during the SDR exposure period could subsequently prevent its adverse effects on acute TMEV infection. Starting on pnd 30, neutralizing antibody or vehicle were infused ICV 4 h prior to each SDR session (17:00-19:00 h). After the final SDR session, all mice were infected with TMEV at 21:00 h. Following baseline assessment, TMEV-induced sickness behaviors were assessed in all mice between day 0 and 7 pi, while measures of motor function were only assessed in mice sacrificed at day 21 pi. Mice were sacrificed at day 7 or 21 pi, to assess CNS viral clearance or to histologically analyze CNS inflammation. Those sacrificed on day 7 pi for viral clearance were also tested for GCR.
2.2.3. Experiment 3: the sufficiency of IL-6 in exacerbating acute TMEV infection
A 2 (IL-6 vs. Vehicle) × 2 (day 7 vs. 21 pi) design was used to determine whether exposure to IL-6 alone would mimic the negative effects of SDR on acute TMEV infection. IL-6 or vehicle was administered daily beginning at 17:00 h on pnd 30-36. After the final IL-6 treatment, all mice were infected with TMEV at 21:00 h. The same measures of sickness behavior, motor impairment, viral clearance, and CNS inflammation used in Experiment 2 were used in this study.
2.3. Surgery and IL-6 Intracranial Ventricular Treatments
On pnd 26 mice were anesthetized with isoflurane gas (2-5%). Then a 33 gauge guide cannula (Plastics One, Roanoke, VA, C315GS-2/SPC) was stereotaxically implanted into the left lateral ventricle (+1.0 mm lateral to bregma, -0.4 mm rostral to bregma, and 1.75 mm ventral–dorsal from top of the skull) and secured with cyanoacrylic gel according the manufacturer's instructions. During the 6 day recovery period, mice were provided with water treated with acetaminophen (325 mg/ 2000 mL) and food softened with the acetaminophen treated water.
To reverse SDR-induced increases in IL-6 expression, a neutralizing antibody to IL-6 was administered ICV (Experiments 1 and 2). This method has been previously used to neutralize IL-1, IL-6, and TNF-α in brain in both rats and mice (Jean Harry et al., 2003; Nadeau and Rivest, 2000; Nadeau and Rivest, 2003). The neutralizing antibody to IL-6 (polyclonal goat anti-mouse; 10 ng/ 2 µL for 2 min; R&D Systems, Madison, WI; AF-406-NA) or vehicle (goat immunoglobulin, Ig, Santa Cruz Biotechnology, Inc #SC-2342) was infused 1 h before each SDR session using a microinjection pump. To mimic SDR-induced increases in IL-6, exogenous IL-6 was administered ICV (Experiment 3). Mice were treated as described above except that they received mouse IL-6 (200 pg/2 µL for 2 min; R&D Systems; 406-ML-005/CF) or vehicle (saline plus mouse Ig, Santa Cruz Biotechnology, Inc #SC-2025) at 16:00 h.
2.4. Social Disruption Stress (SDR)
Cages of three mice were randomly assigned to either control or SDR groups. Control mice remained undisturbed in their home cages, while SDR mice were exposed to aggressive male intruders. Intruders were introduced into the home cage at the onset of the dark cycle for a period of 2 h for a total of six SDR sessions. SDR occurred for three consecutive sessions, then one night off, followed by three additional consecutive sessions (Stark et al., 2001; Johnson et al., 2004; Johnson et al., 2006). SDR sessions were monitored to ensure that the intruder attacked the residents and that the residents demonstrated submissive behaviors. If intruders did not attack within 10 min of session initiation, they were replaced and the session continued for the remaining 2 h.
2.5. Virus and infection
The BeAn strain of Theiler's virus (obtained from Dr. H.L. Lipton, Dept. of Microbiology-Immunology, Univ. Illinois, Chicago, IL.) was initially propagated in lung tumor (L2) cells (Welsh et al., 1987). Mice were anesthetized with isoflurane (Vedco Inc., St. Joseph, MO) on pnd 37 (day 0 pi, 21:00 h) and inoculated into the right mid-parietal cortex (1.5 mm depth) with 5 × 104 pfu of TMEV in a 20 µl volume (Campbell et al., 2001; McGavern et al., 1999; McGavern et al., 2000; Rose et al., 1998; Theil et al., 2000). Inoculation for all subjects occurred at 21:00 h, following the last SDR session.
2.6. Behavioral measures
Early in TMEV infection a range of sickness behaviors are observed which are associated with CNS inflammation and the secretion of proinflammatory cytokines (Johnson et al., 2004; Johnson et al., 2006; Mi et al., 2006). Similar to cytokine-induced sickness syndrome (Barak et al., 2002a; Barak et al., 2002b; Dantzer and Kelley 2007; Pollak et al., 2000; Watkins et al., 1995), TMEV-induced sickness behaviors include weight loss, decreased motor activity, heightened mechanical hypersensitivity, and anhedonia (i.e., reduced pleasure seeking behavior).
As acute TMEV infection develops in Balb/cJ mice, behavioral signs of motor impairment begin to emerge between day 4 and 21 pi (Johnson et al., 2006; Johnson et al., 2004). Therefore, multiple measures of motor behavior were examined, including hind limb impairment ratings, footprint stride length, locomotor activity in an open field (in the vertical and horizontal planes), and mechanical sensitivity. However, two of these measures also reflect sickness syndrome when assessed immediately following infection, prior to the onset of motor impairment. Specifically, a reduction of open field behavior and heightened mechanical sensitivity on day 1 pi may indicate sickness. Thus, these measures are interpreted as reflecting theoretically distinct constructs at different stages in the disease process.
2.6.1. Sucrose preference
Sucrose preference was used to measure sickness-induced anhedonia (Maier and Watkins, 1998; Pollak et al., 2000). Mice were provided with a 2% sucrose water bottle and tap water bottle beginning at day -11 pi and ending on day 7 pi. The position of the sucrose and water bottles was alternated daily, to prevent any place preference. Beginning on day -7 pi, sucrose preference was calculated by dividing the intake of the sucrose solution by the total fluid intake. Only cages that met the criteria of 60% or more sucrose preference prior to infection on day -1 pi were included in the analysis.
2.6.2. Weight loss
Sickness behavior-related weight loss was assessed from days -1 through 1 pi. Mice were weighed at 9:00 am using a scale sensitive to 0.01 g.
2.6.3. Open field activity
Six optical beam activity monitors (Model RXYZCM-16), equipped with two banks of eight photocells on each wall, were used to measure horizontal and vertical locomotion. These open field boxes are interfaced with a digital-multiplexor and Versamax software (Model DCM-4, Omnitech Electronics, Columbus, OH). Mice were habituated to the chambers for 60 min on pnd 28, prior to baseline data collection on pnd 29. During habituation, activity levels stabilized after the first 30 min to levels comparable to those observed on successive days. Test sessions were conducted in the dark beginning at 15:00 h for 30 min with continual white noise (64 dB) to mask extraneous disturbances. Open field activity was measured to assess both sickness and motor impairment. Using a change from baseline analysis, we expected to observe sickness-induced decreases in open field horizontal activity and center time on day 1 pi, prior to prior to the onset of motor impairment as indicated by other measures. We also expected to observe decreases in both horizontal and vertical activity on days 4, 7, 11, and 18 pi, corresponding to the development of motor impairment on other measures (Johnson et al., 2004; Johnson et al., 2006).
2.6.4. Mechanical sensitivity
Mechanical sensitivity was assessed at baseline and post-infection on days 1, 7, 14, and 20 pi. Mice were placed within one of three translucent circular chambers (750 mL, Rubbermaid) positioned on top of a mesh screen (2 mm gauge). The mesh screen was suspended 6” above the countertop, which allowed administration of mechanical stimuli from below to the hind paws. Mechanical threshold for each hind paw was obtained using the up/down method (Dixon, 1980) with von Frey monofilaments (.0784 - 3.92 mN range; Stoelting, Wooddale, IL). Prior to testing, mice were habituated to the apparatus for 20 min. Starting with the smallest filament, each filament was applied to the left and right hind paws in an ABBA manner in both ascending and descending order. Mice received three trials: ascending, descending, and then ascending again. Sickness-related increases in mechanical sensitivity indicative of allodynia were expected on day 1 pi (Watkins and Maier, 2005). With the development of motor impairment between days 7 and 21 pi, we expected decreases in the hind limb withdrawal response. However, a decrease in mechanical sensitivity may reflect either motor or sensory impairment (Clarke and Harris, 2004).
2.6.5. Hind limb impairment (HLI)
Hind limb impairment was assessed beginning at day -1 pi and continuing through to days 7 or 21 pi. Details of the hind limb impairment scale and protocol have been presented elsewhere (Johnson et al., 2004). A separate numeric score was given for each hind limb, based on the symptoms of impairment the mice display (0=healthy; 1=slight weakness in grip; 2=clear weakness in grip; 3=slight paralysis; 4=moderate paralysis, 5=complete paralysis with muscle tone, 6= complete paralysis with no muscle tone; Johnson et al., 2004). These separate scores were then added together and the combined score was analyzed.
2.6.6. Stride length
Footprint stride length and spread were assessed at day 20 pi using a method similar to McGavern et al. (1999, 2000) and identical to our previous studies (Johnson et al., 2006; Johnson et al., 2004). Footprint has been shown to provide a valid indicator of TMEV-induced inflammation and demyelination within the spinal cord (McGavern et al., 1999; McGavern et al., 2000).
2.7. Tissue preparation
Mice were deeply anesthetized with ketamine (100mg/kg)/ xylazine (5 mg/kg), bled from the brachial artery, and transcardially perfused. For the IL-6 ELISA, viral titer, and glucocorticoid resistance (GCR) assays, mice were perfused with PBS. Brains and spinal cords were weighed, flash frozen in liquid nitrogen, and stored at -80 ºC until ELISA and viral titer assays were performed. In contrast, spleens were used the same day in the GCR assays. For histology, mice were perfused with 10 mL of PBS followed by 10 mL of 10% formalin. Brains and spinal cords were placed in 10% formalin overnight and sectioned as in previous studies (Johnson et al., 2004). To determine whether increases in IL-6 induce splenomegaly and thymic atrophy, spleen and thymus weights were taken in Experiments 2 and 3.
2.8. Immunological and histological measures
2.8.1. IL-6 ELISA assay
IL-6 levels in sera and brain were determined using an ELISA assay (M6000B R&D Systems Madison, WI). Sera and brain tissue were frozen at -80°C between sacrifice and the time of the assay. Brain tissue was homogenized in 1 mL of Dulbecco's Eagle Medium (Sigma) and flash frozen. Homogenized tissue was thawed, centrifuged at 2000 rpm for 5 min, and 50 µL of the supernatant was used to perform the ELISA.
2.8.2. Corticosteroid sensitivity assay
The spleens from mice sacrificed for the viral titer assays were used to determine whether SDR or the IL-6 manipulations altered the sensitivity of splenocytes to GC regulation (as per Stark et al., 2001). Details of the present assay have been published previously (Johnson et al., 2004). Cell survival was assessed following the manufacturer's instructions with the Cell Titer 96 Aqueous non-radioactive proliferation assay kit from Promega (Madison, WI). Color changes were quantified by optical density readings at 490 nm from an EMAX ELISA plate reader (Molecular Devices, Sunnyvale, CA). Mean optical density values for the three replications of each sample were used and the percentage of the corticosteroid-exposed cells versus the lipopolysaccharide (LPS) stimulated cells was determined.
2.8.3. Viral load and clearance
Prior studies have shown that viral titers in CNS are maximal 1-2 weeks pi and cleared to non-detectable levels by 3-4 weeks pi (Welsh et al., 1987; 1989). To evaluate the impact of SDR and the IL-6 manipulations on CNS viral clearance, brains and spinal cords were removed on the day of sacrifice and stored at –80°C. The brains and spinal cords were later homogenized and the viral content of the supernatant was determined with a plaque assay on L2 cells (Welsh et al., 1987). After being incubated at 37°C at 5% CO2 for 72 h, plates were stained with 0.1% crystal violet to visualize the plaques that formed. Plates were scored based on the number of plaques formed on the L2 cells and calculated per gram of tissue (Welsh et al., 1987).
2.8.4. Histological evaluation of CNS inflammation
Acute TMEV infection is characterized by CNS inflammation consisting of meningitis, microgliosis, and perivascular cuffing. To evaluate the impact of SDR and the IL-6 treatments on CNS inflammation, brains and spinal cords were sectioned transversely into 4 and 12 pieces, respectively (Campbell et. al., 2001). Tissues were processed for hematoxylin and eosin (H&E) staining. Two raters, who were blind to experimental condition, scored each section using criteria similar to those used in our previous study (Johnson et al., 2004). Stereo Navigator software was used to quantify the percentage of meningitis, microgliosis, and perivascular cuffing by tracing the affected regions in each section, as well as the total perimeter and total area of each section. Meningitis was calculated as a percentage of total section perimeter, whereas microgliosis and perivascular cuffing (microglia and macrophage infiltrate in the white and/or grey matter) were calculated as a percentage of total section area.
2.9. Statistical analyses
Data are presented as mean ± SEM. Physiological data were analyzed using ANOVA followed by Duncan's New Multiple Range Test post hoc mean comparisons. Theoretically linked sets of behavioral measures (sickness behaviors, motor impairment) were analyzed using MANOVAs and RM-MANOVAs. ANOVAs and post hoc mean comparisons were then conducted to clarify the direction of the effects.
3. Results
3.1.1. Experiment 1: impact of SDR and neutralizing antibody on IL-6 and GCR
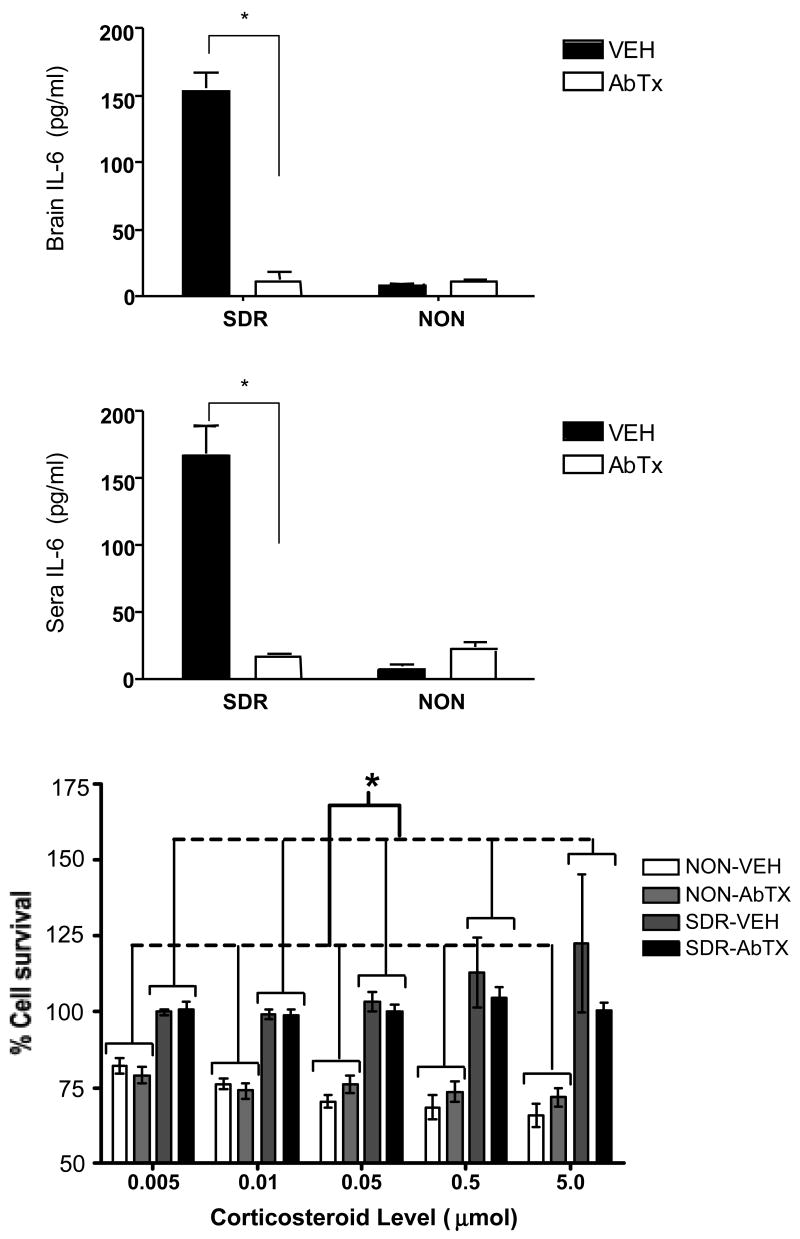
SDR elevated IL-6 in both brain (Fig 1A) and sera (Fig 1B) in the vehicle treated mice, and the neutralizing antibody to IL-6 reversed this effect. An ANOVA confirmed that SDR alone elevated IL-6 in brain, F (1, 26) = 69.248, p ≤ .0001, and in sera, F (1, 26) = 31.331, p ≤ .0001. Moreover, a significant interaction between SDR and the IL-6 neutralizing antibody treatment in brain, IL-6, F (1, 26) = 69.777, p ≤ .0001, and in sera, F (1, 26) = 36.649, p ≤ .0001, indicated that infusion of the neutralizing antibody prevented the SDR-induced increase in free IL-6, ps < .05.
Fig 1.
The impact of SDR and the neutralizing antibody on IL-6 levels in brain (A) and sera (B) in uninfected mice. In addition, the effect of SDR and the IL-6 neutralizing antibody on the development of GCR prior to infection is shown in panel C. The amounts of corticosteroid used to suppress cell survival ranged from .005 µmol to 5 µmol, and the dashed line depicts 80% cell survival. Asterisks (*) indicate significant post hoc differences between groups.
In addition, GCR developed normally in response to SDR, regardless of neutralizing antibody treatment (Fig 1C). A significant SDR effect, F (1, 24) = 34.508, p < .0001, revealed that the non-stressed mice exhibited a reduction in spleen cell survival compared to the SDR group. This effect was qualified by a marginally significant interaction between SDR and corticosterone concentration, F (4, 96) = 2.208, p = .07, suggesting that the non-stressed mice exhibited a dose-dependent reduction in cell survival, whereas the SDR groups did not. Finally, the neutralizing antibody treatment alone did not alter GCR, F (1, 24) = 0.165, p > .05, nor did it interact with SDR or corticosteroid concentration, all F's ≤ 1.016, p > .05. This suggests that IL-6 is not necessary for the development of GCR following SDR.
3.2. Experiment 2: the necessity of IL-6 in mediating the adverse effects of SDR on acute TMEV infection
3.2.1. Sickness measures
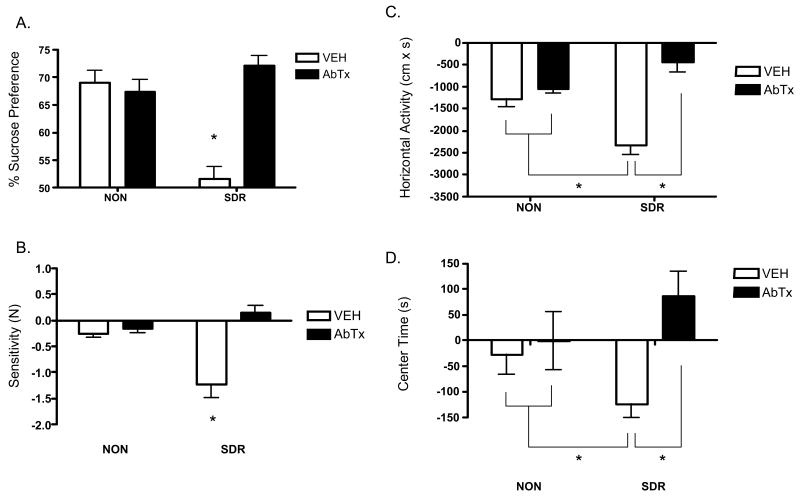
Sickness was assessed within the first 24 h pi by measuring changes in sucrose preference (Fig 2A), mechanical sensitivity (Fig 2B), horizontal (Fig 2C) and center time (Fig 2D) activity in an open field, and body weight (data not shown). A MANOVA revealed significant main effects for both SDR, Wilk's Λ = .263, F (6,35) = 16.388, p < .0001, and neutralizing antibody treatment, Wilk's Λ = .572, F (6,35) = 4.373, p < .0001. However, these effects were qualified by a significant interaction between SDR and neutralizing antibody treatment, Wilk's Λ = .360, F (6,35) = 10.379, p < .0001. Follow up ANOVAs conducted on the sickness data depicted in Figure 2 revealed significant interactions between SDR and neutralizing antibody, all Fs ≥ 4.610, ps ≤ .05. Mean comparisons indicated that SDR increased sickness and that this effect was reversed by the neutralizing antibody to IL-6, all ps < .05. Analyses of body weight revealed an effect of time, F (1, 98) = 214.59, p < .0001, and an interaction between SDR, antibody treatment, and time, F (1, 98) =6.30, p < .01. Mean comparisons indicated that all groups exhibited weight loss post infection (mean loss 0.8-1.0 g; ps < .05), with the exception of the SDR mice treated with the IL-6 antibody.
Fig 2.
The neutralizing antibody to IL-6 prevents the adverse effects of SDR on sickness behaviors during acute TMEV infection (Experiment 2). SDR increased sickness behavior across all measures within 24 h pi, as indicated by a decrease in sucrose preference (A), mechanical thresholds (B), open field horizontal activity (C), and center time (D). Importantly, infusion of the neutralizing antibody to IL-6 during SDR prevented the adverse effects of SDR on sickness behavior during early infection. Asterisks (*) indicate significant post hoc differences between groups.
3.2.2. Motor impairment measures
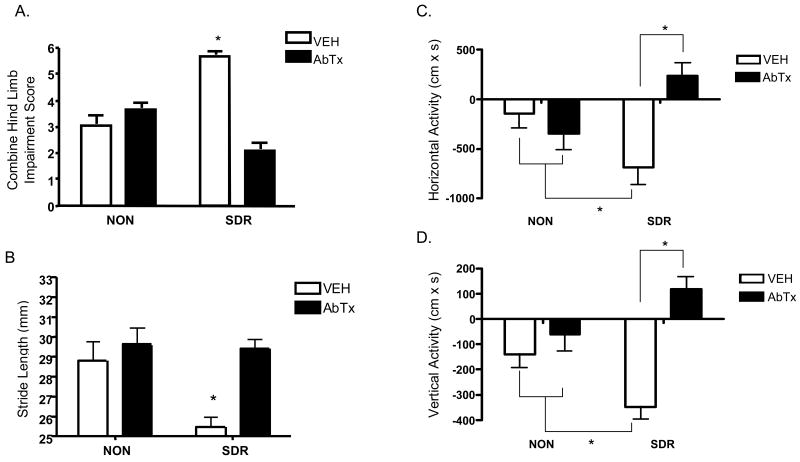
Motor impairment was assessed until day 21 pi by examining hind limb impairment (Fig 3A), stride length (Fig 3B), horizontal (Fig 3C) and vertical (Fig 3D) activity, and mechanical sensitivity. A repeated measures MANOVA revealed significant main effects for both SDR, Wilk's Λ = .256, F (21, 24) = 3.319, p < .01, and neutralizing antibody treatment, Wilk's Λ = .160, F (21, 24) = 5.993, p < .001, as well as a significant interaction between SDR and neutralizing antibody treatment, Wilk's Λ = .161, F (21, 24) = 5.934, p < .0001. Follow up ANOVAs conducted on each measure revealed significant interactions between SDR and neutralizing antibody, all Fs ≥ 5.53, ps ≤.05. Mean comparisons confirmed that SDR exacerbated motoric impairment in the vehicle treated mice, all ps < .05. In contrast, the neutralizing antibody to IL-6 reduced the level of SDR-induced motor impairment to non-stressed levels or better, all ps < .05.
Fig 3.
The neutralizing antibody to IL-6 prevents the adverse effects of SDR on motor impairment during acute TMEV (Experiment 2). SDR exacerbated motor impairment between days 4 and 21 pi, as indicated by increased combined hind limb impairment scores (A), decreased stride length (B), decreased horizontal activity (C), and decreased vertical activity (D) in an open field. These adverse effects were prevented by infusion of the IL-6 neutralizing during SDR. Asterisks (*) indicate significant post hoc differences between groups.
3.2.3. Glucocorticoid resistance
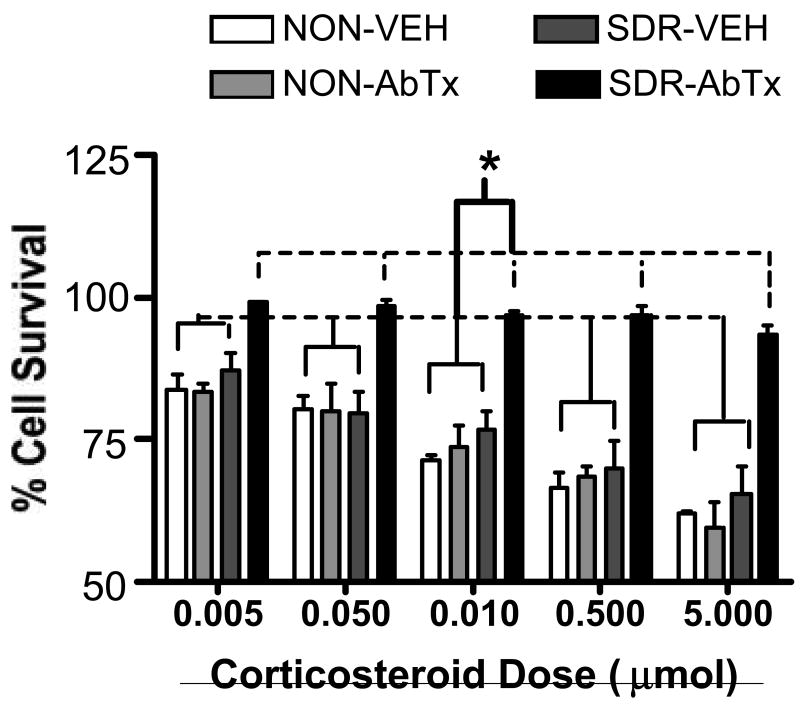
Fig 4 depicts the effects of the IL-6 neutralizing antibody on the development of GCR in mice sacrificed at day 7 pi (Fig 4). An ANOVA revealed significant effects of antibody, SDR, and a significant interaction between antibody and SDR, all F's (1,8) ≥ 19.17, p ≤ .002. The effects of corticosterone concentration and its interaction with antibody and SDR were significant, along with the 3-way interaction between SDR, antibody, and corticosteroid concentration, all Fs (4, 32) ≥ 3.832, p ≤ .01. Post hoc analyses indicated that while the vehicle treated SDR and non-stressed groups showed a significant dose-dependent reduction in cell survival, ps < .05, the antibody treated SDR group did not. The finding that GCR did not develop post-infection in the vehicle treated SDR group is consistent with previous work (Johnson et al., 2004; Merlot et al., 2004b). Interestingly, the persistence of GCR in the antibody treated SDR group suggests that neutralizing IL-6 prior to infection increases vulnerability to GCR following infection, despite less severe behavioral and physiological manifestation of acute infection.
Fig 4.
Impact of SDR and neutralizing antibody treatment on GCR in TMEV infected mice at day 7 pi (Experiment 2). Although SDR resulted in GCR prior to infection and the neutralizing antibody to IL-6 did not alter this effect (Fig 1C), only the neutralizing antibody treated SDR mice exhibited GCR at day 7 pi. The corticosteroid levels that were used to suppress survival ranged from .005 µmol to 5 µmol. Asterisks (*) indicate significant post hoc differences compared to control (LPS-stimulated only) cells.
3.2.4. Splenomegaly and thymic atrophy
Consistent with prior research (Avitsur et al., 2001), analyses of spleen weights confirmed a main effect for SDR, F (1,32) = 39.325, p < .0001, indicating that SDR increased spleen weights (data not shown). Analyses of thymus weights revealed a significant interaction between SDR and neutralizing antibody at day 7 pi, F (1,32) = 5.777, p < .05. Post hoc comparisons indicated that SDR significantly reduced thymus weights and that this effect was reversed by neutralizing antibody, ps < .05 (data not shown).
3.2.5. Viral clearance
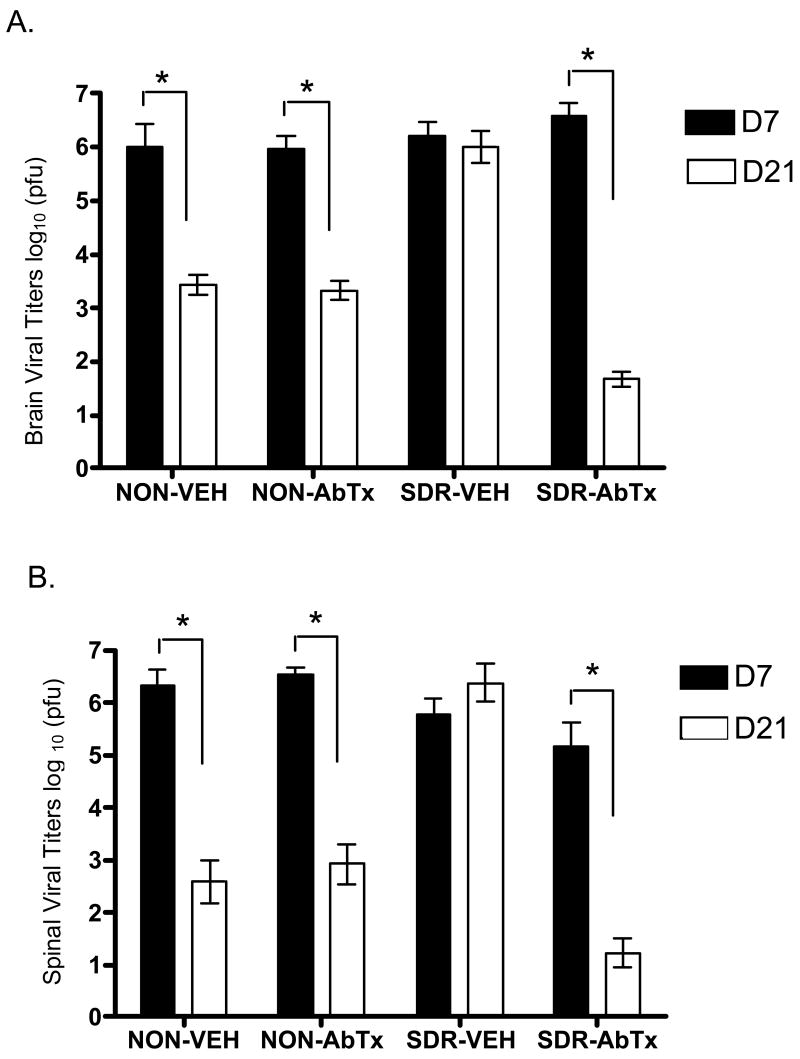
To assess the process of viral clearance in spinal cord and brain, viral titers were measured in two subsets of mice sacrificed at day 7 and 21 pi (Fig 5). ANOVAs revealed significant effects of day pi and antibody in both spinal cord and brain, all F's (1,40) ≥ 28.41, p ≤ .0001, and an effect of SDR in brain, F (1,40) = 5.47, p < .02. The critical 3-way interaction between SDR, antibody, and day pi was significant in both spinal cord and brain, both F's (1, 40) ≥ 22.706, p ≤ .0001. The remaining interaction terms were significant, all Fs (1,40) ≥ 17.08, p ≤ .0002, with the exception of the interaction between SDR and day pi in brain. Post hoc comparisons indicated a significant reduction in viral titers occurred between day 7 to day 21 pi in the non-stressed groups and in the antibody treated SDR group, all ps < .05, while titers in the vehicle treated SDR group did not change over time. This suggests that SDR disrupted viral clearance in spinal cord and brain and this effect was prevented by infusion of the neutralizing antibody to IL-6.
Fig 5.
Viral titers were measured in spinal cord (A) and brain (B) at days 7 and 21 pi. Viral titers are expressed as log10 plaque forming units (pfu) per gram of tissue. A reduction of viral load over time indicates viral clearance. SDR disrupted viral clearance in spinal cord and brain in the vehicle treated mice, but this effect was prevented by the Il-6 neutralizing antibody treatment. Significant post hoc differences between groups are indicated with asterisks (*).
3.2.6. Inflammation
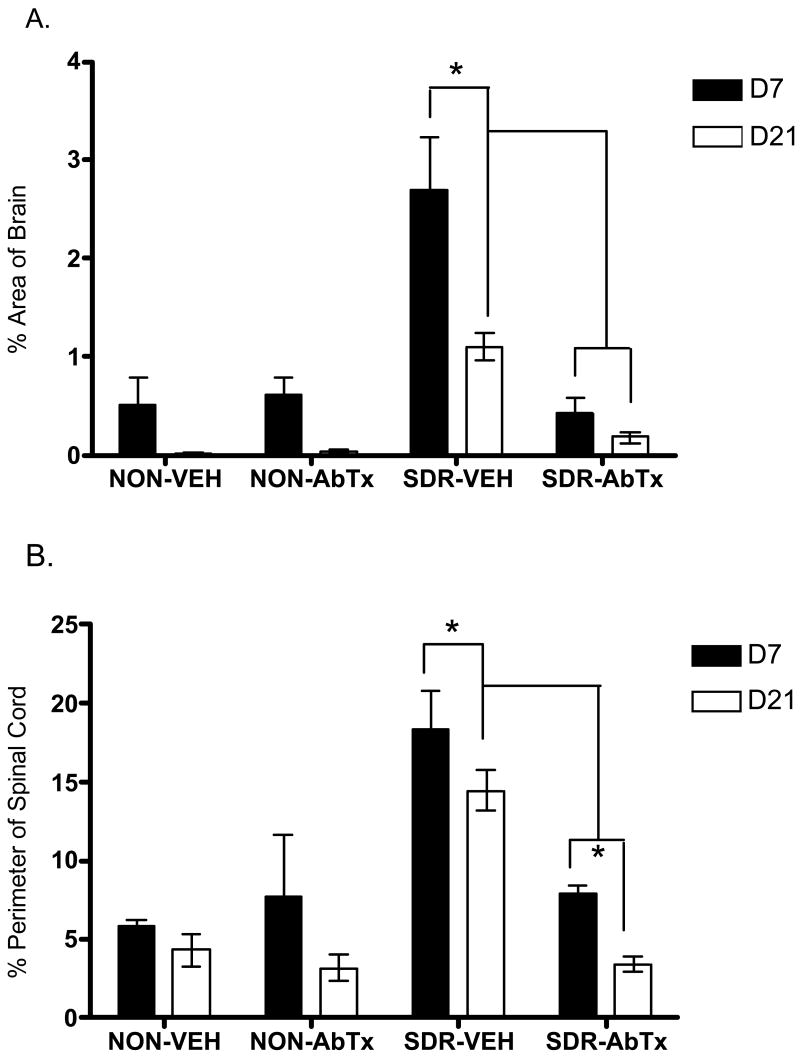
Consistent with previous findings (Johnson et al., 2004), SDR caused greater inflammation in both spinal cord and brain (Fig 6). ANOVAs confirmed significant effects of SDR, antibody, and day pi, all Fs (1, 40) ≥ 8.03, p ≤ .01, as well as significant interactions between SDR and antibody in both spinal cord (meningitis) and brain (microgliosis and perivascular cuffing), both F's (1, 40) ≥ 19.091, p < .0001. There was also a significant 3-way interaction between SDR, antibody, and day pi in brain, F (1, 40) = 4.41, p < .04. Post hoc comparisons indicated that SDR alone significantly elevated infection-related inflammation in spinal cord and brain on both days 7 and 21 compared to the other three groups. In addition, the vehicle treated SDR group showed greater inflammation compared to non-stressed groups, at day 7 pi, but not at day 21 pi. Moreover, this SDR-induced increase in CNS inflammation was prevented by IL-6 neutralizing antibody treatment during stress exposure, all ps < .05.
Fig 6.
Inflammation in brain (A) and in spinal cord (B) was elevated by SDR (SDR-Vehicle) across days 7 and 21 pi, but this SDR-induced increase in inflammation was prevented by IL-6 neutralizing antibody treatment (SDR-AbTx). Inflammation in brain is expressed as the percent of area with microgliosis and perivascular cuffing, whereas inflammation in spinal cord is expressed as the percent of section perimeter with meningitis. Significant post hoc differences are indicated by asterisks (*) for comparisons across groups and by “†” for comparisons of the neutralizing antibody condition collapsing across day pi.
3.3. Experiment 3: the sufficiency of IL-6 in exacerbating acute TMEV infection
MANOVAs revealed that the IL-6 treatment did not significantly alter sickness or motor impairment, all ps > .05. However, ANOVA found significant main effects of IL-6 on mechanical sensitivity, F (1, 22) = 8.277, p < .01, and on viral titers in spinal cord, F (1, 20) = 17.350, p < .001, indicating that IL-6 increased allodynia and reduced viral load in spinal cord, ps < .05. In contrast, the IL-6 treatment did not alter viral titers in brain, nor did it alter inflammation in brain and spinal cord, all Fs (1, 20) ≤ 1.467, p >.05. As expected, there was a significant effect for day pi on viral titers and inflammation in both brain and spinal cord, all Fs (1,20) >561.49, p < .001. However, the IL-6 treatment did not interact with day pi, all Fs (1,20) < .53 p > .05. This indicates that while viral titers and inflammation decreased from day 7 to day 21 pi, the IL-6 treatment did not alter the magnitude of either effect. All other differences were non-significant, all Fs (1,20) ≤ 1.47, p >.05. Because these findings were largely negative, these data are not shown.
4. Discussion
We have previously shown that repeated exposure to SDR exacerbates both the acute and chronic phase of TMEV infection (Johnson et al., 2004; Johnson et al., 2006). The present findings suggest that IL-6 plays an important role in mediating the adverse effects of SDR on disease course. In Experiment 1, the proinflammatory effects of SDR were confirmed by increases in both central and peripheral levels of IL-6 and by the induction of GCR in uninfected mice. This study also established that pretreatment with a selective neutralizing antibody to IL-6 effectively blocked SDR-induced elevations of central and circulating IL-6, without affecting the development of GCR. Experiment 2 demonstrated that intracranial infusion of the IL-6 neutralizing antibody during SDR attenuated the exacerbation of TMEV-induced sickness behaviors, motor impairment, CNS viral titers, and CNS inflammation. This suggests that stress-induced elevations of IL-6 mediate the adverse effects of SDR on acute TMEV infection. Intracerebral infusion of exogenous IL-6 the week prior to TMEV infection did not, however, mimic the adverse effects of SDR (Experiment 3). Collectively, these findings suggest that IL-6 is necessary but not sufficient to exacerbate acute TMEV infection.
4.1. Repeated SDR elevates central IL-6 in the absence of immune challenge
Several laboratories have reported stress-induced increases in CNS proinflammatory cytokine responses (Deak et al., 2003, Deak et al., 2005; Johnson et al., 2002; Johnson et al., 2004; Merlot et al., 2003; Merlot et al., 2004a; Minami et al, 1991; Nguyen et al., 1998; Nguyen et al., 2000; O'Connor et al., 2003; Quan et al., 2001; Shintani et al., 1995; Shizuya et al., 1998). For instance, exposure to prolonged restraint stress has been shown to increase expression of IL-6 in hypothalamus (Shizuya et al., 1997; Shizuya et al., 1998). Likewise, exposure to six sessions of SDR increases expression of IL-1β and TNF-α in brain following LPS challenge (Quan et al., 2001). Although prior studies reported SDR-induced increases of IL-6 in plasma and in LPS stimulated splenocytes (Avitsur et al., 2001; Merlot et al., 2004a; Stark et al., 2001; Stark et al., 2002), to our knowledge the present study provides the first demonstration that SDR increases IL-6 levels in brain.
4.2. SDR-induced IL-6 subsequently exacerbates TMEV-induced sickness behaviors
We have previously shown that prior exposure to SDR exacerbates behavioral signs of TMEV-induced motor impairment (Johnson et al., 2004; Johnson et al., 2006). The present study extended our behavioral analysis to include a range of sickness behaviors assessed during the first 24 hours post-infection, prior to the onset of motor impairment. Exposure to SDR decreased sucrose preference, suggesting that the stressor reduced pleasure seeking behavior (i.e., anhedonia). In addition, sensitivity to mechanical stimulation was increased, suggesting that the stressor heightened TMEV-induced allodynia. SDR also exacerbated the infection-related reduction in exploratory behavior. These findings extend prior research by showing that TMEV-induced neuroinflammation induces a sickness behavior syndrome (Barak et al., 2002a; Dantzer and Kelley, 2007; Maier & Watkins et al., 1998; Pollak et al., 2000). Consistent with our previous work, SDR increased hind limb impairment, decreased stride length, and decreased locomotor activity as TMEV-induced motor impairment intensified between days 4 and 21 post-infection. Importantly, these stress-induced exacerbations of sickness behavior and motor impairment were either attenuated or blocked by pretreatment with the IL-6 neutralizing antibody, suggesting that IL-6 partially mediates the adverse behavioral effects of SDR. These findings demonstrate the critical role of stress–induced IL-6 in the exacerbation of TMEV-associated behavioral syndrome.
4.3. Glucocorticoid resistance is not mediated by SDR-induced increases in IL-6
Previous research suggests that SDR-induced increases in inflammation may be attributed to the development of GCR (Avitsur et al., 2001; Stark et al., 2001; Merlot et al., 2004a). Therefore, we examined whether the IL-6 neutralizing antibody altered the development of GCR before and after infection. As expected, SDR reduced the sensitivity of spleen cells to the anti-inflammatory effects of corticosterone in uninfected mice (GCR), but the neutralizing antibody did not alter the development of GCR. This finding is consistent with prior research showing that GCR developed normally in IL-6 knock out mice exposed to SDR (Stark et al., 2002). Thus, despite the co-occurrence of increased IL-6 and GCR following SDR, IL-6 is not necessary for the induction of GCR.
Following infection, GCR did not persist in the vehicle treated SDR mice, which is consistent with previous studies (Johnson et al., 2004; Merlot et al., 2004b). Merlot has suggested that infection may reduce the sensitivity of spleen cells to stress, thereby preventing the development of GCR (Merlot et al., 2004b). However, GCR was observed in the TMEV infected SDR mice treated with the IL-6 neutralizing antibody. Despite the presence of GCR, these mice showed less severe signs of inflammation compared to the SDR mice treated with vehicle. This suggests that the presence of GCR post-infection does not provide an accurate indicator of inflammation during TMEV infection. Finally, while SDR mice treated with IL-6 neutralizing antibody exhibited GCR prior to infection, they subsequently exhibited reduced disease severity. This indicates that the induction of GCR prior to infection is not sufficient to exacerbate acute TMEV. Taken together, these findings suggest that SDR-induced increases in inflammation during TMEV infection are not attributable to the development of GCR prior to or during infection.
4.4. Exposure to repeated SDR subsequently exacerbates acute TMEV infection
Stressor exposure produces an exaggerated proinflammatory cytokine response to subsequent immune challenge (Johnson et al., 2002; Johnson et al., 2006; Merlot et al., 2004b; Quan et al., 2001), and this response depends on elevations of central proinflammatory cytokine expression during stressor exposure (Johnson et al., 2004). In light of these data, it is tempting to speculate that SDR exacerbates TMEV infection through cross-sensitization or priming of virus-induced cytokine expression during acute infection. In particular, SDR-induced increases in central IL-6 during the time of stressor exposure period may sensitize virus-induced pro-inflammatory cytokine expression, thereby exacerbating TMEV-associated behavioral syndrome, dysregulation of antiviral responses, and CNS inflammation. Consistent with this hypothesis, blocking IL-6 activity with the neutralizing antibody during the time of stressor exposure prevented these adverse effects. Additional support is provided Quan et al. (2001) who found that SDR increased proinflammatory cytokine expression and inflammatory histopathology in brain and other organs when mice were later challenged with a septic dose of LPS.
The present findings suggest that SDR-induced increases in IL-6 mediate subsequent increases in TMEV-induced inflammatory histopathology in CNS. It remains unclear, however, whether this is due to the cross-sensitization of virus-induced pro-inflammatory cytokine expression. While SDR-induced IL-6 may play a critical role in sensitizing virus-induced cytokine expression, it is also possible that repeated exposure to SDR may down regulate IL-6 and other proinflammatory cytokines. Supporting this view, others have shown that exposure to chronic social stress down regulates IL-1β mRNA levels in hippocampus (Bartolomucci et al., 2003) and that repeated restraint stress down regulates IL-6 mRNA expression in hypothalamus and midbrain (Miyahara et al., 2000). In addition, circulating levels of IL-6 have been shown to decline after repeated exposure to SDR, even though they remain elevated, relative to control mice, after the final SDR session (Merlot et al., 2004a). If central pro-inflammatory responses habituate to repeated SDR exposure, it is possible that virus-induced cytokine responses may be blunted during immune challenge. To resolve this issue, future studies will need to determine whether SDR up regulates or down regulates TMEV-induced cytokine expression.
4.5. The role of IL-6 in TMEV infection
Understanding the role of IL-6 in TMEV infection is complex, probably due to its pleiomorphic effects. An additional complexity is that TMEV investigators use different strains of virus and different strains of mice. However, in general IL-6 and other proinflammatory cytokines are induced both in vitro (Olson et al., 2001; Palma et al., 2003;) and in vivo (Begolka et al., 1998; Chang et al., 2000; Mi et al., 2006; Sato et al., 1997) following infection with TMEV. Both astrocytes (Palma et al., 2003) and microglia (Olson et al., 2001) have been shown to secrete IL-6 after in vitro TMEV infection. Virus-infected microglia have also been shown to be able to present both endogenous and viral antigen to CD4+ T cells (Mack et al., 2003; Olson et al., 2001), thus stress-induced alterations in IL-6 secretion by microglia cells may influence antigen presentation. Other evidence suggests that IL-6 plays an important role in the development of immune responses by enhancing antibody production, T cell activation, growth and differentiation, and cytotoxic T cell differentiation and expression (Kishimoto, 2003).
In vivo studies have shown that IL-6 plays a protective role in preventing neurodegeneration and demyelination. Recombinant human IL-6 administered during early infection with Theiler's virus protected against the later development of TVID (Rodriguez at al., 1994). IL-6 appeared to mediate protection through enhanced antibody production and increased viral clearance from the CNS. In a recent study, IL-6 was shown to prevent anterior horn cell damage in IL-6 -/- mice generated on a TVID-susceptible background – H-2q (Pavelko et al., 2003). The mechanism of action was hypothesized to be through the neuroprotective effects of IL-6 rather than its immunomodulatory functions. Interestingly, this effect was not observed in IL-6-/- mice generated on a TVID-resistant background – H-2b (Pavelko et al., 2003). These mice had similar viral clearance profiles and did not exhibit persistent infection and demyelination compared to IL-6 +/+ mice.
Although IL-6 may be protective during TMEV infection, the present findings suggest that SDR-induced increases in central IL-6 during the time of stressor exposure have a negative impact on disease course by exacerbating inflammatory histopathology and disrupting viral clearance. The proinflammatory influence of SDR-induced IL-6 may alter early immunological events in viral infection and have cascading effects on later infection. The initial response to the virus influences the innate immune response, which in turn influences the development of the specific immune response (Biron 1998, 1999). Thus, SDR-induced increases in IL-6 may have cascading effects that dysregulate central inflammatory and antiviral responses during early infection, which in turn can shape T and B cell responses to the virus and myelin during the chronic phase of disease.
4.6. Conclusion
A growing body of evidence suggests that stress and neuroimmune activation contribute to the pathogenesis of neurodegenerative diseases (Ackerman et al., 2003; Biondi & Zannino, 1997; Buscioglio et al., 1998; Rosch, 1999; McGeer & McGeer, 2004; Mei-Tal et al., 1970; Mohr et al., 2004; Mohr & Pellitier, 2006; Morale et al., 2001; Perry et al., 2003; Stein-Behrens et al., 1994). For example, stressful life events have been found to precede and predict the development of new brain lesions in MS patients (Mohr et al., 2000). Importantly, chronic social stressors at home and at work were implicated, as well as disruptions in daily routine. There are also reports that proinflammatory cytokines may play an important role in mediating MS disease severity (e.g., Al-Omaishi et al., 1999; Begolka et al., 1998). Although stress and inflammatory processes are implicated in the pathogenesis of diseases such as MS (Ackerman et al., 1998; Ackerman et al., 2002; Mohr et al., 2000, 2006; Nguyen et al., 2002), the mechanisms underlying disease exacerbation remain unknown. The present study provides the first demonstration that social stress-induced increases in IL-6 may mediate the deleterious effects of stress in an animal model of MS.
Acknowledgments
This research was supported by NRSA 5F31NS50476-2 to RRJ, and NMSS RG3128 and NINDS RO1 NS39569 to CJRW and MWM. We thank Lou Tassinary and Jim Grau for serving on Robin Johnson's dissertation committee. We also thank Colin Young, Andrew Steelman, Jennifer Bizon, and Laura Koehly for their technical assistance. Krishna Patel, Sarah Young, and Josh McKay also served supporting roles in the collection of this data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med. 1998;60:484–491. doi: 10.1097/00006842-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, Baum A. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64:916–920. doi: 10.1097/01.psy.0000038941.33335.40. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Stover A, Heyman R, Anderson BP, Houck PR, Frank E, Rabin BS. Relationship of cardiovascular reactivity, stressful life events, and multiple sclerosis disease activity. Brain Behav Immun. 2003;17:141–151. doi: 10.1016/s0889-1591(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Al-Omaishi J, Bashir R, Gendelman HE. The cellular immunology of multiple sclerosis. J Leukocyte Biology. 1999;65:444–452. doi: 10.1002/jlb.65.4.444. [DOI] [PubMed] [Google Scholar]
- Aubagnac S, Brahic M, Bureau JF. Viral load and a locus on chromosome 11 affect the late clinical disease caused by Theiler's virus. J Virol. 1999;73:7965–7971. doi: 10.1128/jvi.73.10.7965-7971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Barak O, Goshen I, Ben-Hur T, Weidenfeld J, Taylor AN, Yirmiya R. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein120. Brain Res. 2002a;933:98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- Barak O, Weidenfeld J, Goshen I, Ben-Hur T, Taylor AN, Yirmiya R. Intracerebral HIV-1 glycoprotein 120 produces sickness behavior and pituitary-adrenal activation in rats: role of prostaglandins. Brain Behav Immun. 2002b;166:720–735. doi: 10.1016/s0889-1591(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull. 2003;62:173–178. doi: 10.1016/j.brainresbull.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immun. 1998;161:4437–4446. [PubMed] [Google Scholar]
- Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol. 1990;30:201–212. doi: 10.1016/0165-5728(90)90104-u. [DOI] [PubMed] [Google Scholar]
- Biondi M, Zannino LG. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychother Psychosom. 1997;66:3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- Biron CA. Role of early cytokines, including alpha and beta interferons (IFN a/h), in innate and adaptive immune responses to viral infections. Immunology. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Biron CA. Initial and innate responses to viral infections—a pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJ, Nash AA. The role of CD8+T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73(Pt 7):1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Borrow P, Welsh CJ, Nash AA. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler's murine encephalomyelitis. Immunol. 1993;80:502–506. [PMC free article] [PubMed] [Google Scholar]
- Brahic M, Bureau JF. Genetics of susceptibility to Theiler's virus infection. BioEssays. 1998;20:627–633. doi: 10.1002/(SICI)1521-1878(199808)20:8<627::AID-BIES5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Ann Rev Microbiol. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Andersen JK, Schipper HM, Gilad GM, McCarty R, Marzatico F, Toussaint O. Stress, aging, and neurodegenerative disorders. Molecular mechanisms. Ann NY Acad Sci. 1998;851:429–443. doi: 10.1111/j.1749-6632.1998.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJ. The effects of restraint stress on the neuropathogenesis of Theiler's virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JR, Zaczynska E, Katsetos CD, Platsoucas CD, Oleszak EL. Differential expression of TGF-beta, IL-2, and other cytokines in the CNS of Theiler's murine encephalomyelitis virus-infected susceptible and resistant strains of mice. Virology. 2000;278:346–360. doi: 10.1006/viro.2000.0646. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Harris J. The organization of motor responses to noxious stimuli. Brain Res Rev. 2004;46:163–72. doi: 10.1016/j.brainresrev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immunol. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D'Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Brown GW, Harris T, McDonald WI, Patterson T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. J Alzheimers Dis. 2002;4:179–189. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- Huang QH, Takaki A, Arimura A. Central noradrenergic system modulates plasma interleukin-6 production by peripheral interleukin-1. Am J Physiol. 1997;273:R731–738. doi: 10.1152/ajpregu.1997.273.2.R731. [DOI] [PubMed] [Google Scholar]
- Jean Harry G, Bruccoleri A, Lefebvre d'Hellencourt C. Differential modulation of hippocampal chemical-induced injury response by ebselen, pentoxifylline, and TNFalpha-, IL-1alpha-, and IL-6-neutralizing antibodies. J Neurosci Res. 2003;73:526–536. doi: 10.1002/jnr.10653. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002;27:353–365. doi: 10.1016/s0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Prentice TW, Bridegam P, Young CR, Steelman AJ, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity and onset of the chronic phase of Theiler's virus infection. J Neuroimmunol. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Storts R, Welsh TH, Jr, Welsh CJ, Meagher MW. Social stress alters the severity of acute Theiler's virus infection. J Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6. In: Thomson Angus W, Lotze Michael T., editors. The Cytokine Handbook. 4th. Elsevier Science; London: 2003. [Google Scholar]
- Lipton HL. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infectious Immunity. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Zoecklein L, Sathornsumetee S, Rodriguez M. Assessment of hindlimb gait as a powerful indicator of axonal loss in a murine model of progressive CNS demyelination. Brain Res. 2000;877:396–400. doi: 10.1016/s0006-8993(00)02710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann NY Acad Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Mei-Tal V, Meyerowitz S, Engel GL. The role of psychological process in a somatic disorder: multiple sclerosis. 1 The emotional setting of illness onset and exacerbation. Psychosom Med. 1970;32:67–86. doi: 10.1097/00006842-197001000-00006. [DOI] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Importance of fighting in the immune effects of social defeat. Physiol Behav. 2003;80:351–357. doi: 10.1016/j.physbeh.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice: activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004a;7:55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Immune alterations induced by social defeat do not alter the course of an on-going BCG infection in mice. Neuroimmunomodulation. 2004b;11:414–418. doi: 10.1159/000080152. [DOI] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJR. Restraint stress decreases virus-induced pro-inflammatory cytokine expression during acute Theiler's virus infection. J Neuroimmunol. 2006;178:59–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Young CR, Storts R, Steelman A, Meagher MW, Welsh CJR. Stress alters pathogenecity and facilitates systemic dissemination of Theiler's virus. Microbial Pathogenesis. 2006;41:149–156. doi: 10.1016/j.micpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Satoh M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;123:254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- Miyahara S, Komori T, Fujiwara R, Shizuya K, Yamamoto M, Ohmori M, Okazaki Y. Effects of repeated stress on expression of interleukin-6 (IL-6) and IL-6 receptor mRNAs in rat hypothalamus and midbrain. Life Sci. 2000;66:PL93–98. doi: 10.1016/s0024-3205(99)00626-8. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Bacchetti P, Boudewyn AC, Huang L, Marrietta P, Cheuk W, Dee B. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurol. 2000;55:55–61. doi: 10.1212/wnl.55.1.55. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. Brit Med J. 2004;328:731–735. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Pelletier D. A temporal framework for understanding the effects of stressful life events on inflammation in patients with multiple sclerosis. Brain Behav Immun. 2006;20:27–36. doi: 10.1016/j.bbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Morale C, Brouwer J, Testa N, Tirolo C, Barden N, Dijkstra CD, Amor S, Marchetti B. Stress, glucocorticoids and the susceptibility to develop autoimmune disorders of the central nervous system. Neurol Sci. 2001;22:159–162. doi: 10.1007/s100720170016. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga MK, Asakura K, Hunter SF, Wettstein P, Pease LR, Rodriguez M. The immune system preferentially clears Theiler's virus from the gray matter of the central nervous system. J Virol. 1997;71:8592–8601. doi: 10.1128/jvi.71.11.8592-8601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Njenga MK, Asakura K, Hunter SF, Wettstein P, Pease LR, Rodriguez M. The immune system preferentially clears Theiler's virus from the gray matter of the central nervous system. J Virol. 1997;71:8592–8601. doi: 10.1128/jvi.71.11.8592-8601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler's virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kwon D, Clipstone NA, Kim BS. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J Virol. 2003;77:6322–6331. doi: 10.1128/JVI.77.11.6322-6331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelko KD, Howe CL, Drescher KM, Gamez JD, Johnson AJ, Wei T, Ransohoff RM, Rodriguez M. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J Neurosci. 2003;23:481–92. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Y, Ovadia H, Goshen I, Gurevich R, Monsa K, Avitsur R, Yirmiya R. Behavioral aspects of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;104:31–36. doi: 10.1016/s0165-5728(99)00257-x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, McKinney CW, Leibowitz JL. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J Immunol. 1994;153:3811–3821. [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, Njenga MK, Logan WC, Wettstein PJ. The balance between persistent virus infection and immune cells determines demyelination. J Immunol. 1996;157:5699–5709. [PubMed] [Google Scholar]
- Rosch PJ. Stress and Graves' disease. Lancet. 1992;339:428. [PubMed] [Google Scholar]
- Rose JW, Hill KE, Wada Y, Kurtz CI, Tsunoda I, Fujinami RS, Cross AH. Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler's virus infection. J Neuroimmunol. 1998;81:82–89. doi: 10.1016/s0165-5728(97)00162-8. [DOI] [PubMed] [Google Scholar]
- Sato S, Reiner SL, Jensen MA, Roos RP. Central nervous system cytokine mRNA expression following Theiler's murine encephalomyelitis virus infection. J Neuroimmunol. 1997;76:213–223. doi: 10.1016/s0165-5728(97)00059-3. [DOI] [PubMed] [Google Scholar]
- Rubio N, De Felipe C, Torres C. Theiler's murine encephalomyelitis virus-binding activity on neural and non-neural cell lines and tissues. J Gen Virol. 1990;71:2867–2872. doi: 10.1099/0022-1317-71-12-2867. [DOI] [PubMed] [Google Scholar]
- Rubio N, Sierra A. Interleukin-6 production by brain tissue and cultured astrocytes infected with Theiler's murine encephalomyelitis virus. Glia. 1993;9:41–47. doi: 10.1002/glia.440090106. [DOI] [PubMed] [Google Scholar]
- Sato S, Reiner SL, Jensen MA, Roos RP. Central nervous system cytokine mRNA expression following Theiler's murine encephalomyelitis virus infection. J Neuroimmunol. 1997;76:213–223. doi: 10.1016/s0165-5728(97)00059-3. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997;61:135–140. doi: 10.1016/s0024-3205(97)00608-5. [DOI] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The expressions of mRNAs for interleukin-6 (IL-6) and the IL-6 receptor (IL-6R) in the rat hypothalamus and midbrain during restraint stress. Life Sci. 1998;62:2315–2320. doi: 10.1016/s0024-3205(98)00212-4. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJ, Meagher MW. Chronic restraint stress during early Theiler's virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJ, Meagher MW. Sex-dependent effects of chronic restraint stress during early Theiler's virus infection on the subsequent demyelinating disease in CBA mice. J Neuroimmunol. 2006;177:46–62. doi: 10.1016/j.jneuroim.2006.04.020. [DOI] [PubMed] [Google Scholar]
- So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki A, Huang QH, Somogyvari-Vigh A, Arimura A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomod. 1994;1:335–342. doi: 10.1159/000097185. [DOI] [PubMed] [Google Scholar]
- Theil DJ, Tsunoda I, Libbey JE, Derfuss TJ, Fujinami RS. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler's virus infections. J Neuroimmunol. 2000;104:22–30. doi: 10.1016/s0165-5728(99)00251-9. [DOI] [PubMed] [Google Scholar]
- Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case-control evidence of a relationship. J Chronic Dis. 1982;35:821–831. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Welsh CJ, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68(Pt 6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Blakemore WF, Tonks P, Borrow P, Nash AA. Theiler's murine encephalomyelitis virus infection in mice: a persistent viral infection of the central nervous system which induces demyelination. In: Dimmock N, editor. Immune responses, Virus Infection and Disease. Oxford University Press; 1989. pp. 125–147. [Google Scholar]