Summary
Peripheral sensory neurons respond to axon injury by activating an importin-dependent retrograde signaling mechanism. How is this mechanism regulated? Here we show that Ran GTPase and its associated effectors RanBP1 and RanGAP regulate the formation of importin signaling complexes in injured axons. A gradient of nuclear RanGTP versus cytoplasmic RanGDP is thought to be fundamental for the organization of eukaryotic cells. Surprisingly, we find RanGTP in sciatic nerve axoplasm, distant from neuronal cell bodies and nuclei, and in association with dynein and importin α. Following injury, localized translation of RanBP1 stimulates RanGTP dissociation from importins and subsequent hydrolysis, thereby allowing binding of newly synthesized importin β to importin α and dynein. Perturbation of RanGTP hydrolysis or RanBP1 blockade at axonal injury sites reduces the neuronal conditioning lesion response. Thus, neurons employ localized mechanisms of Ran regulation to control retrograde injury signaling in peripheral nerve.
Introduction
The cell body of a lesioned neuron must receive accurate and timely information on axonal injury in order to activate repair mechanisms. Early work in Aplysia suggested that retrograde injury signals originating in the axon are transported retrogradely to the cell body in a nuclear localization signal (NLS)-dependent manner (Ambron and Walters, 1996). Nuclear import of proteins is mediated by NLS binding to the importins, soluble transport factors that mediate the translocation of substrates through the nuclear pore complex (Harel and Forbes, 2004; Weis, 2003). We have shown that importins are found in rodent nerve axons and that they enable retrograde transport of injury-signaling proteins. Importin α is found in axons of both control and injured sciatic nerve in constitutive association with dynein. In contrast, importin β protein is not detectable under normal conditions in sciatic nerve axoplasm, although its mRNA is found in intermittent local concentrations throughout the axons (Hanz et al., 2003). Upon lesion, this mRNA is rapidly translated into importin β protein, leading to the formation of importin α/β heterodimers bound to the retrograde motor dynein, thereby creating high affinity NLS binding sites linked to the retrograde transport machinery. Introduction of excess NLS peptides into lesioned DRG axons inhibited conditioning lesion responses in vivo (Hanz et al., 2003). Further work showed that soluble forms of the type III intermediate filament vimentin are also elevated by local translation and then undergo calpain-mediated cleavage in sciatic nerve axoplasm after injury (Perlson et al., 2005; Perlson et al., 2004). Vimentin binds phosphorylated Erk (pErk) in a calcium dependent manner (Perlson et al., 2006), and links the activated MAP kinase to the retrograde transport system via direct binding of vimentin to importin β. Upon arrival in the cell body pErk activates the transcription factor Elk1 (Perlson et al., 2005), thus importins enable coupling of axonal injury to specific transcriptional responses in the cell body. Since activation of axonal importins has far-reaching consequences for the neuron, how might this be regulated?
Classical nuclear transport is tightly regulated by the small GTPase Ran, which cycles between a GTP bound form prevalent in the nucleus, and a GDP bound form in the cytoplasm (Kalab and Heald, 2008; Weis, 2003). This asymmetric distribution regulates cargo interactions with importins, since Ran-GDP does not bind importins, while the GTP form interacts directly with importin β and indirectly via CAS with importin α (Figure S1). The importins are exported from the nucleus in association with Ran-GTP. In the cytosol, competitive binding of RanBP1 releases Ran-GTP from the importins, and rebinding is prevented by RanGAP mediated hydrolysis of Ran to the GDP-bound state (Kalab and Heald, 2008; Poon and Jans, 2005). The fundamental roles of Ran in regulating importin-dependent nuclear import prompted us to ask if it might also be involved in regulating importins in axons. Here we show that the RanGTPase system regulates the formation of retrograde importin signaling complexes in the axons of injured peripheral neurons. Surprisingly, RanGTP is found in axonal cytoplasm in the sciatic nerve, distant from neuronal cell bodies and nuclei, and in association with CAS, importin α, and dynein. Following injury, localized translation of RanBP1 stimulates RanGTP dissociation from importins and subsequent hydrolysis, thereby allowing binding of newly synthesized importin β to importin α and dynein. Thus, localized mechanisms of Ran regulation allow neurons to control importin-cargo interactions at axonal sites distant from the nucleus.
Results
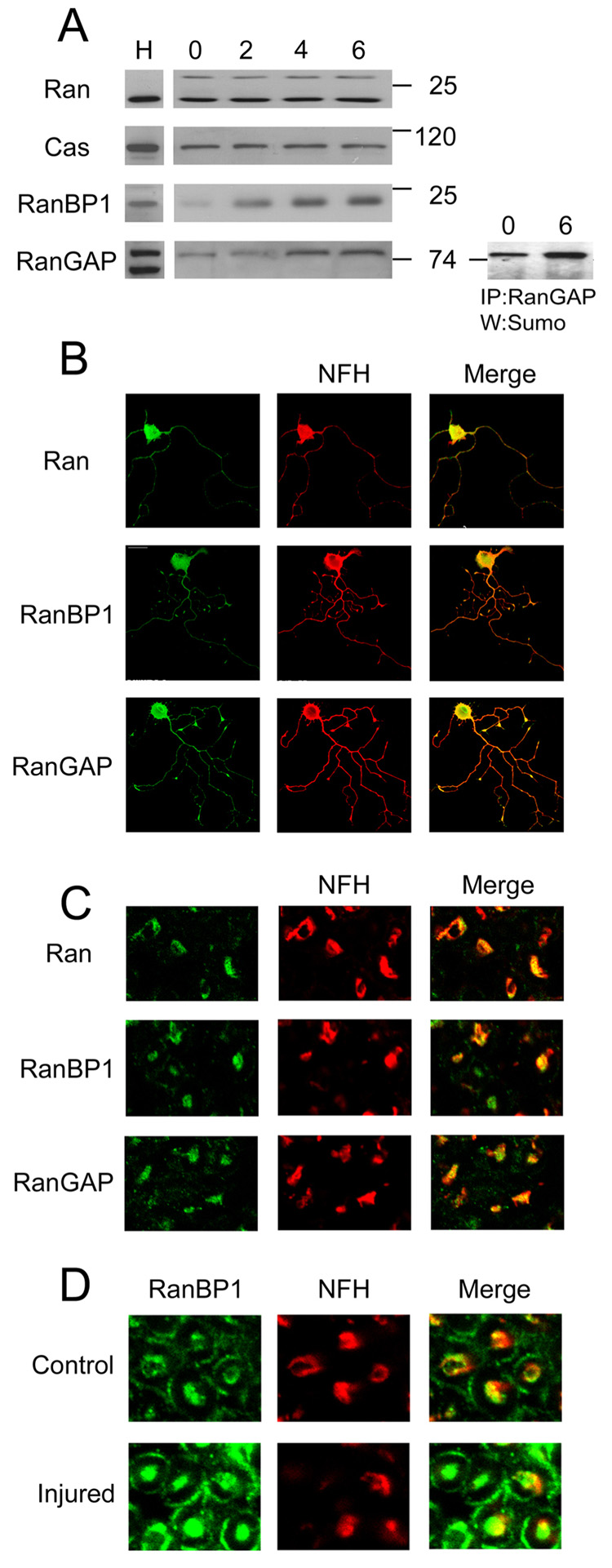
We used Western blots to screen axonal cytoplasm (axoplasm) from adult rat sciatic nerve for the presence of nuclear transport regulating proteins. Axoplasm purity was verified by Western blotting for the RanGEF RCC1, which serves as a nuclear marker, and glial markers such as S100 and GFAP (Hanz et al., 2003; Perlson et al., 2005; Figure S2). Ran and the adaptor protein CAS were both found in sciatic nerve axoplasm, and the levels of these proteins were not markedly affected by injury (Figure 1A). In contrast, RanBP1 levels were very low in uninjured nerve, and significantly up-regulated after lesion. Soluble RanGAP levels in axoplasm also appeared to be regulated by injury, albeit more modestly than RanBP1. A large fraction of the axonal RanGAP migrated as a higher molecular mass band on the blots in both control and injured nerve, and this was found to represent a sumoylated form of the protein (Figure 1A), as previously described in other systems (Pichler and Melchior, 2002). Interestingly, sumoylation was recently shown to regulate retrograde transport of an RNA-binding protein in axons (van Niekerk et al., 2007). In order to verify that the observed proteins are indeed found in axons, we performed immunostaining for Ran, RanBP1 and RanGAP on cross-sections of injured sciatic nerve, and on cultures of regenerating adult sensory neurons from the L4/L5 dorsal root ganglia (DRG), that project axons into the sciatic nerve. As shown in Figures 1B and 1C, all three proteins are found within regenerating neurites in vitro and in injured axons in vivo, whereas RCC1 is restricted to the nuclei of cultured DRG neurons (Figure S2). Higher magnification immunostaining on sciatic nerve cross-sections also supports RanBP1 upregulation within axons after injury (Figure 1D).
Figure 1. Ran and associated proteins are found in neuronal processes in the sciatic nerve.
(A) Western blot analysis of sciatic nerve axoplasm shows similar levels of Ran and CAS before and 2, 4 and 6 hours after injury. RanBP1 is significantly increased and RanGAP is slightly up-regulated over the same time period. Immunoprecipitation of RanGAP and Western with anti-Sumo reveals that a significant proportion of the axonal RanGAP is sumoylated. H denotes Hela cell extract, used as a positive control. 40 µg protein per lane. (B) Immunostaining for Ran, RanBP1, RanGAP and the axonal marker NF-H on adult DRG neurons in culture shows that all three proteins are found in NF-H positive axons. (C) Immunostaining for Ran, RanBP1 and RanGAP in cross-sections of injured sciatic nerve reveals the presence of all three proteins in NF-H positive axons. Magnification 40x. (D) Immunostaining for RanBP1 on cross-sections of control versus injured (6 hr post-lesion) sciatic nerve. Magnification 60x.
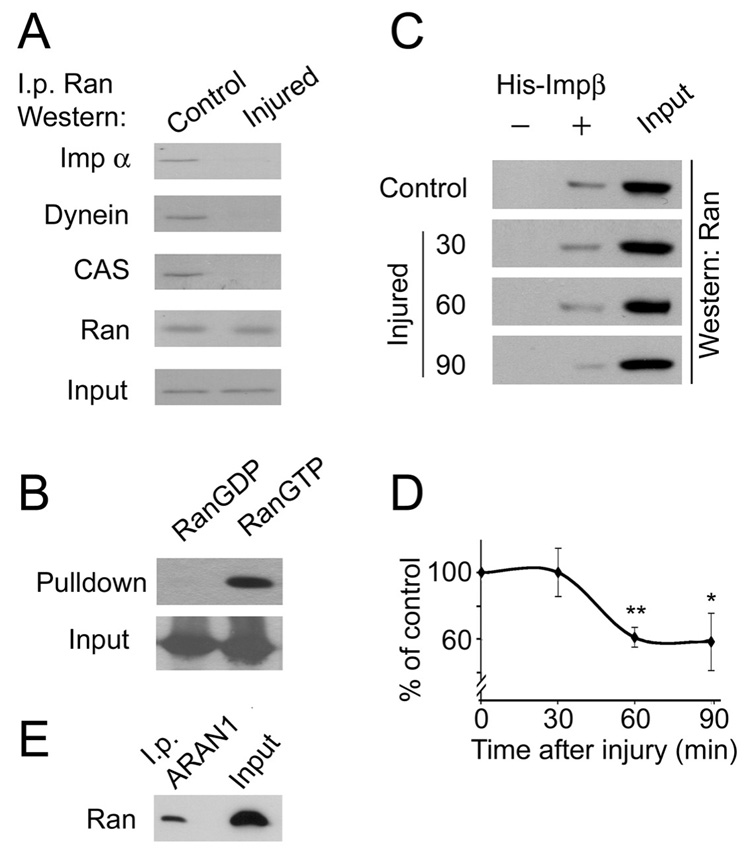
Next we examined the association of Ran with components of the retrograde injury-signaling complex. Throughout these studies we used antibodies against importin α4 and importin β1, referred to hereafter as importin α and importin β for simplicity. Importin α co-precipitates with Ran in control but not in injured nerve (Figure 2A). Importin α was previously shown to be constitutively bound to dynein in uninjured nerve (Hanz et al., 2003), and indeed dynein was also co-precipitated with Ran in control but not in injured nerves (Figure 2A). Ran normally interacts with importin α̣ only via the adaptor CAS, and immunoprecipitation of Ran from sciatic nerve axoplasm shows an association of CAS with Ran in control but not in injured nerves (Figure 2A). Notably, Ran must be in its GTP bound form for interaction with CAS and importin α (Quimby and Dasso, 2003; Weis, 2003). Hence, these data suggest that uninjured sciatic nerve axons contain Ran-GTP in vivo, which is striking given the distance of these processes (~5 cm) from neuronal nuclei. In order to confirm this observation we carried out pull-downs of Ran from axoplasm with exogenously added His-tagged importin β1, after first verifying in vitro that recombinant His-importin β binds specifically to RanGTP, but not RanGDP (Figure 2B). Endogenous axonal Ran-GTP was co-precipitated with His-importin β from control nerve (Figure 2C). The proportion of RanGTP from total Ran was reduced rapidly following lesion, reaching approximately half control levels 60–90 minutes after a crush injury (Figure 2D). We also used the ARAN1 antibody (Hieda et al., 1999) to verify this result. The C-terminal epitope in Ran that is recognized by ARAN1 is exposed only when Ran is bound to importin β. ARAN1 immunoprecipitation of axoplasm charged with importin β confirmed precipitation of Ran-GTP (Figure 2E).
Figure 2. RanGTP in sciatic nerve axoplasm.
(A) Ran is associated with importin α through CAS in control but not in injured nerves. Importin α, dynein intermediate chain and CAS are co-precipitated with Ran in control but not after injury, although equal amounts of Ran were precipitated in all samples. 1 mg protein was used per i.p. Input in this panel is a 10% loading control probed for dynein intermediate chain. (B) Pull-down of Ran by importin β. Purified recombinant importin β and Ran were incubated together after pre-loading of Ran with GDP or with GTP. Importin β was then immunoprecipitated and co-precipitated Ran was monitored by Western blot. Only RanGTP co-precipitated with importin β. Input control in this and all subsequent panels is Ran. (C) Sciatic nerve axoplasm (250 µg) was charged with recombinant importin β, and incubated as described before precipitation of importin β. Ran content in the precipitates was examined by Western blot. (D) Quantification of the experiment described in C shows that levels of RanGTP in axoplasm are significantly reduced following injury (average ± standard deviation, n = 3, * denotes p < 0.05, ** denotes p < 0.01, Student’s T-test). (E) Immunoprecipitation of RanGTP from axoplasm with the ARAN1 antibody. Sciatic nerve axoplasm (200 µg) was precipitated with 10 µg each of His-importin β and ARAN1 antibody. Precipitated RanGTP was visualized on Western blot with rabbit anti-Ran (Abcam).
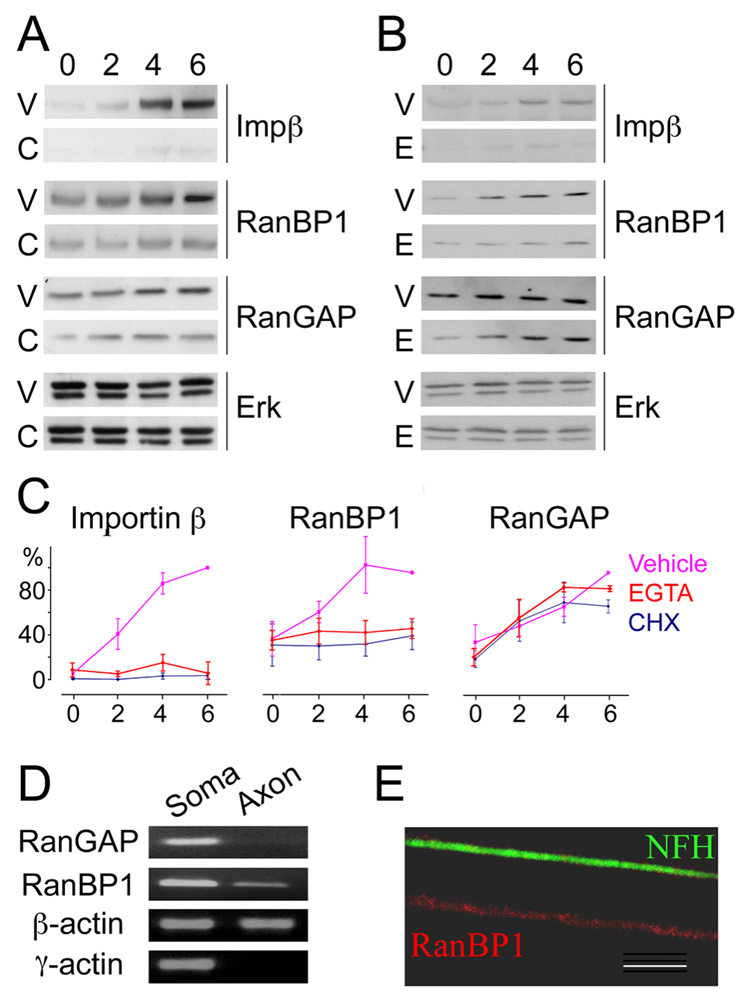
RanBP1 triggers the hydrolysis of Ran-GTP to Ran-GDP by displacing bound importins from Ran-GTP in concert with RanGAP. The low levels of RanBP1 in uninjured axons in vivo should therefore facilitate maintenance of Ran-GTP, and RanBP1 up-regulation upon injury is likely to be important for releasing importins for cargo binding in lesioned axons. We therefore examined the mode of upregulation of RanBP1 protein in axoplasm after injury. Previous studies have shown that a number of different proteins, including importin β1, are synthesized by local translation from axonal mRNA after nerve injury (Wang et al., 2007; Willis et al., 2005; Willis et al., 2007). Injury causes increase in axonal calcium levels (Mandolesi et al., 2004; Petrescu et al., 2007), and calcium was previously shown to regulate binding interactions within the retrograde injury signaling complex (Perlson et al., 2006). As demonstrated in Figure 3, the local synthesis of importin β1 in injured axons can be blocked by the calcium chelator EGTA as well as by the translation inhibitor cycloheximide. Similar findings were observed for the injury-induced elevation in RanBP1 levels, but not for the more modest elevation of soluble RanGAP (Figure 3A–C). Indeed a RanBP1 transcript could be amplified by RT-PCR from isolated DRG axons, while no RT-PCR products were obtained for RanGAP (Figure 3D). Further confirmation for the occurrence of RanBP1 transcripts in axons was also obtained by fluorescent in situ hybridization (FISH) on cultured DRG neurons (Figure 3E and Figure S3).
Figure 3. RanBP1 and importin β are locally synthesized in a calcium-dependent manner after nerve injury.
Western blot analyses of axoplasm (40 µg protein per lane for importin β or RanGAP, 100 µg for RanBP1) shows that the upregulation of importin β and RanBP1 is blocked by (A) injection of the translation inhibitor cycloheximide (CHX) or (B) the calcium chelator EGTA to the sciatic nerve concomitantly with nerve crush. RanGAP upregulation is not influenced by either reagent. Erk is used as a loading control. (C) Densitometric quantification of the experiments shown in panels A and B, Vehicle in pink, EGTA in red, and CHX in black; all normalized as % of the highest value observed in each experiment (n = 3). (D) RT-PCR on isolated DRG axons amplifies RanBP1 transcript from axons, but not RanGAP mRNA. RT-PCR for β-actin (a known axonal transcript) and γ-actin (a soma-restricted transcript) provide positive and negative controls, respectively. (E) Fluorescent in situ hybridization (FISH) on DRG cultures reveals the presence of RanBP1 transcript (red) in an axon identified by immunostaining for neurofilament heavy chain (NFH, green). The panel shows high magnification of a single axon segment (scale bar 5 µm), note the granular signal for RanBP1 mRNA. For additional FISH images see Supplementary Figure 2.
Transport of specific transcripts into axons is usually controlled by untranslated sequences (UTR) that interact with RNA transport proteins (Bassell and Kelic, 2004). We therefore carried out 3’RACE RT-PCR on RNA extracted from DRG’s and from axoplasm to identify and clone RanBP1 3’UTR sequences. Two main variants of RanBP1 3’UTR were identified, a short form of 94 bases and a longer 302 bases sequence (Figure 4A and Figure S4). These UTR sequences did not contain any known localization motifs, hence we tested their capacity to induce axonal localization of a reporter gene. DRG neurons were transfected with constructs containing RanBP1 UTR sequences fused to a GFP-encoding open reading frame, and FISH was carried out to localize the GFP transcripts. Axonal localization of transcript was observed in approximately 55% of neurons transfected with constructs containing the long UTR, while transcripts containing the short UTR were mainly restricted to the cell body (Figure 4B).
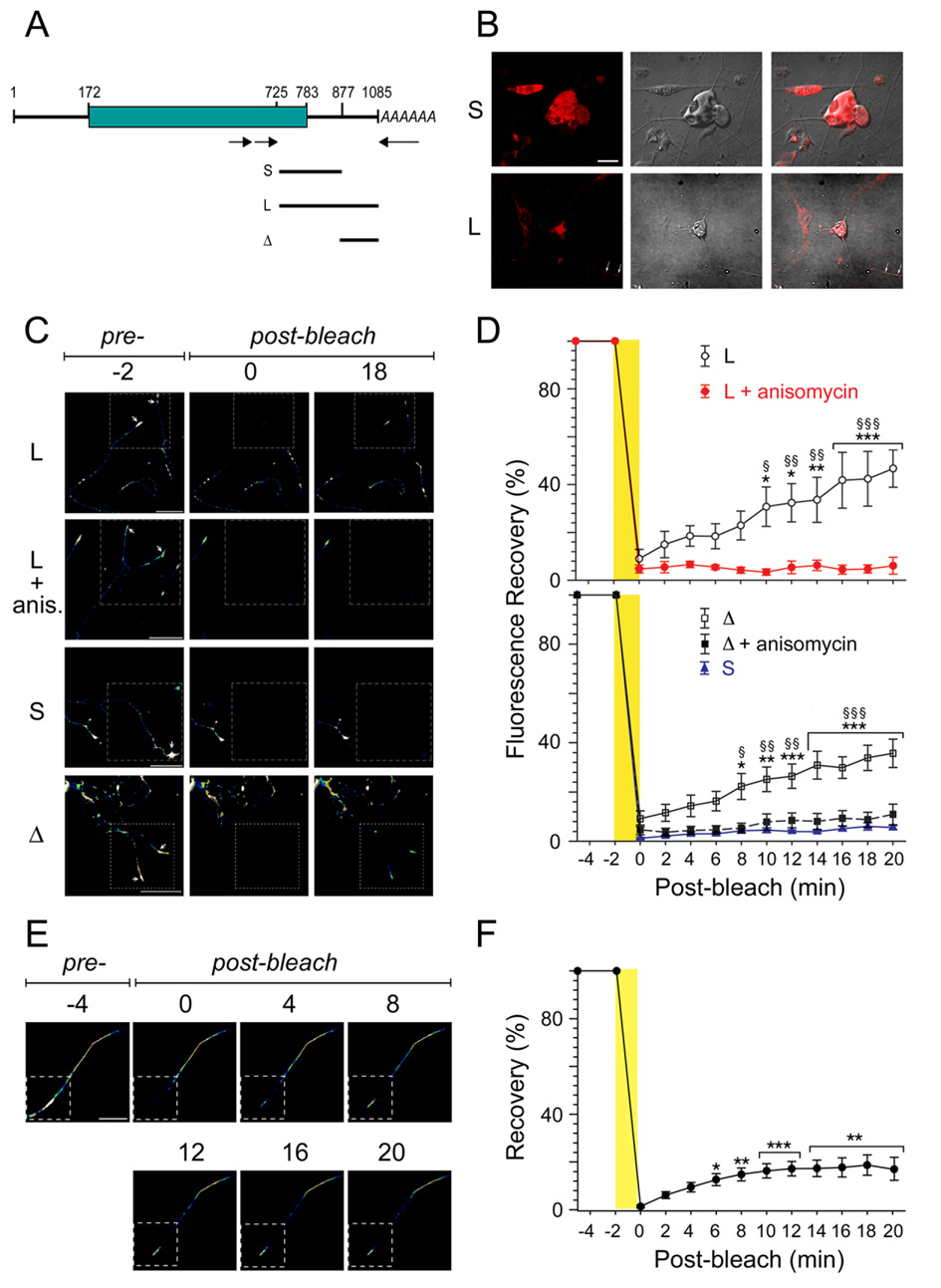
Figure 4. A long variant 3’UTR targets RanBP1 to the axon.
(A) Schematic of RanBP1 transcripts, with open reading frame denoted by the green box. The lines under the schematic delineate regions subcloned for constructs containing long (L) or short (S) 3’UTR variants, or the differential segment (Δ) found in the long but not in the short UTR. (B) Representative confocal images of in situ hybridization on adult DRG neurons with a GFP riboprobe following Amaxa nucleofection with the indicated constructs. White arrows indicate signal in the neurites. Scale bar is 20 µm. (C) Representative images from time-lapse sequences of photobleach experiments before (−2 min) and after photobleaching (0 and 18 min) of adult DRG neurons transfected with the indicated constructs. The boxed regions represent the area subjected to photobleaching with recovery monitored over 18 min. (More detailed image series are shown in Figure S5 and in the supplemental movies). Arrows indicate growth cones. Anis. indicates transfected neurons that were treated with 1 µM anisomycin immediately prior to pre-bleach imaging (see also Figure S5). (D) Fluorescence intensity over multiple time-lapse sequences. Average recoveries (% of pre-bleach levels) ± SEM are shown (n = 4–6). Significant recovery after photobleaching assessed by two-way ANOVA at each time point vs. 0 min post-bleach, * denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001. Time points of significant differences of axonal fluorescence compared with cultures treated with anisomycin and those transfected with RanBP short 3’UTR are indicated by §§ for p<0.01 or §§§ for p<0.001. (E) FRAP Analyses in Cut Axons. Representative images from a cut axon FRAP sequence from DRG neurons transfected with myr-dzGFP fused to the long RanBP1 3’UTR. The GFP fluorescence recovers in the distal bleached portion of the axon (arrows) without any transition of GFP signals from the proximal unbleached regions. Scale bar is 25 µm. (F) Quantification of fluorescence after photobleaching in cut axons (n=10). Significant recovery after photobleaching assessed by one-way ANOVA at each time point vs. 0 min post-bleach, ** denotes p<0.01, *** denotes p<0.001.
In order to further verify axon localization with RanBP1 UTR sequences, both UTR variants were fused to a previously described destabilized and myristoylated GFP reporter (Aakalu et al., 2001; Smith et al., 2005). Importantly, fusion of the myristoylation sequence to GFP limits diffusion of newly synthesized protein, allowing its use as a reliable reporter of localized protein synthesis after photobleaching (Aakalu et al., 2001; Willis et al., 2007). An additional construct comprised the differential segment between the long and short UTR forms (schematic in Figure 4A). All three constructs were transfected into cultured DRG neurons for FRAP (fluorescence recovery after photobleaching) analyses. In these experiments a portion of the axon expressing the fluorescent GFP reporter is bleached, and recovery of fluorescence is monitored over a period of 20 minutes. This time scale allows observation of recovery due to localized synthesis, but is not long enough for transport or diffusion of myristoylated reporter protein from the soma to the bleached region (Aakalu et al., 2001 and data not shown). Fluorescence recovery was observed for constructs containing the long form of RanBP1 3’UTR, but not for the short UTR variant (Figure 4C, D, Figure S5, and movie MS1 and movie MS3). The average pre-bleach fluorescent signal in distal axons was approximately two-fold higher for the long form of RanBP1 3’UTR as compared with the short UTR variant (36.2 ± 2.5 vs. 17.4 ± 0.5 pixels/µm; P = 0.001). The recovery observed for the long 3’UTR construct was blocked upon incubation with the translation inhibitor anisomycin, consistent with the new fluorescence signal arising from local translation in the axon (Figure 4C, D, Figure S5, movie MS2). Moreover, the calculated time for complete recovery to pre-bleach fluorescence intensities for dzGFP fused to the full length RanBP1 UTR is 49 min (± 2.9 min) based on linear regression (correlation coefficient = 0.98), versus 478 ± 62.9 min (correlation coefficient = 0.88) for the identical dzGFP reporter fused to the short RanBP1 3’UTR. Since the only difference between these two constructs is the axon-localizing segment in the long UTR, the rapid recovery must arise from axonally-localized transcript. Similar results were obtained with the differential segment construct (Figure 4C, D, Figure S5, movie MS4 and movie MS5), confirming that axon targeting motifs are localized within the distal segment of the longer form of RanBP1 3’UTR.
As noted above, 100% of dzGFP from the long RanBP1 3’UTR construct is locally synthesized in a time period that allows for less than 10% recovery from other sources. Nonetheless, to establish this point beyond doubt, the question whether cell body synthesis could play a role was addressed by FRAP experiments on cut axons physically disconnected from cell bodies (Figure 4E, F). Lesioned DRG axons retain protein synthetic capacity for several hours in vitro after severing their connection to the cell body (Willis et al., 2005; Zheng et al., 2001). After cutting the axon with a glass microelectrode the proximal and distal cut ends retract slightly over 5–10 minutes (Zheng et al., 2001), such that the distal cut axon is clearly separated from the proximal segment by a few microns. Recovery of myr-dzGFP-3’UTR-RanBP1 in the cut axons occurs with similar initial kinetics as observed for whole axons (Figure 4E, F). The reduced recovery rate observed at later time points is most likely due to the fact that neuronal processes rapidly degenerate after separation from the cell body by this cutting approach (Aakalu et al., 2001). Even so, the calculated time to full recovery based on the initial kinetics is 122 min (± 22 min), well within the range for physiological significance (see discussion).
To further address the possibility of anterograde transport of dzGFP from proximal regions, we transfected DRG neurons with myr-dzGFP-3’UTR-RanBP1 and Kif5C560-dTomato, a kinesin mutant that selectively accumulates in axonal growth cones through anterograde transport (Jacobson et al., 2006). Photobleached growth cones from these neurons showed statistically significant recovery of myr-GFP but not of Kif5C560-Tomato over 20 minutes after the bleach (Figure S6A). Extrapolation from these data indicate that the earliest time point one could start seeing new Kif5c560-dTomato arriving in the bleached region is 64 minutes – at which time one would already have observed complete recovery of dzGFP-long-RanBP1-UTR from local translation. Finally, we devised a 'double-bleach' FRAP sequence to directly address the possibility of lateral movement of the myristoylated reporter protein within the axoplasmic membrane as well as other modes of transport of myr-dzGFP. In this experiment, terminal segments of the distal axons in intact neurons were photobleached as before and then the proximal half of the photobleached region was continuously bleached while monitoring potential recovery in the distal portion. We carried out such double-bleach experiments on neurons co-transfected with both dzGFP-long-RanBP1-UTR and the Kif5c560-dTomato reporter, and observed clear recovery of the locally translated dzGFP (Figure S6B, C, movie MS7) compared to no recovery for the Kif motor reporter (Figure S6C). The complete recovery time for dzGFP extracted from these data is 148 ± 11.6 min (correlation coefficient = 0.96). Since any GFP molecules moving down the axon would have been fully photobleached prior to reaching this region, we conclude that the distal recovery must be due to localized translation of myr-dzGFP localized via the 3’-RanBP1 UTR sequence. Taken together, these experiments show conclusively that the complete FRAP recovery of the construct fused to the long form of RanBP1 3’UTR is due to local synthesis.
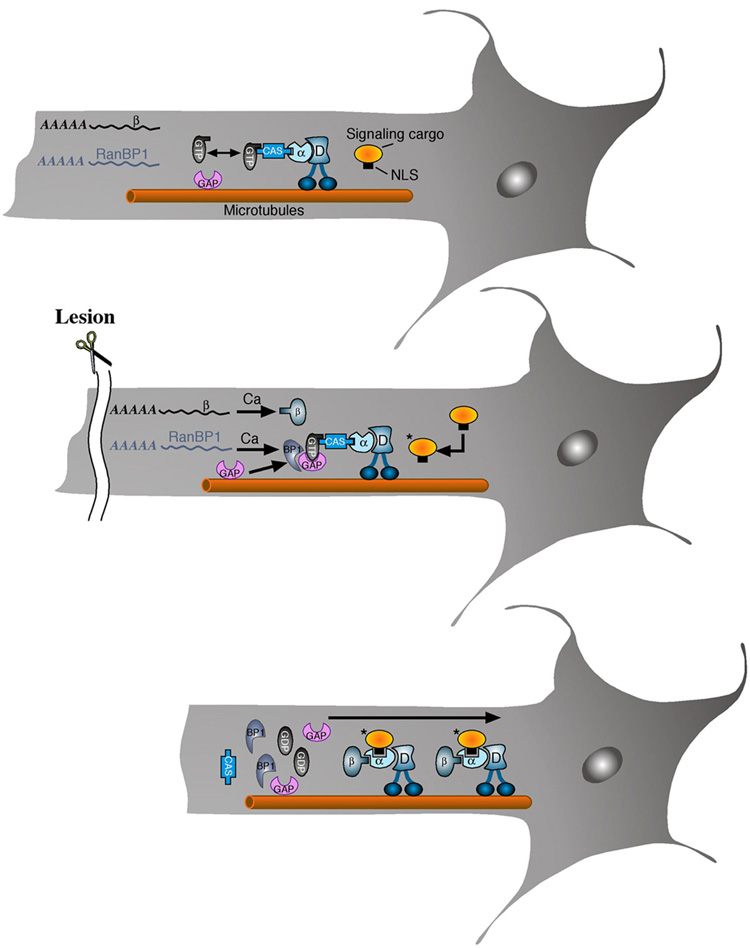
The results described above suggest that retrograde injury signaling may be regulated by the Ran system in peripheral nerve. Under normal conditions, Ran-GTP bound to axonal CAS and importins will prevent importin α and β interaction and binding of cargo proteins (Herold et al., 1998). Importin β1 and RanBP1 are found in the axon as mRNA’s, and axonal RanBP1 mRNA is targeted to processes by a specific 3’UTR sequence. Following lesion, localized translation of these mRNAs leads to upregulation of the corresponding proteins. The parallel and more modest upregulation of soluble RanGAP in axoplasm may be due to changed sequestration with membrane or cytoskeleton (Pay et al., 2002; Seewald et al., 2003), although this remains to be determined. The newly synthesized RanBP1 stimulates disassociation of RanGTP and RanGAP synergized hydrolysis, thus allowing formation of a cargo-binding complex of importin α with de novo synthesized importin β (Figure 5).
Figure 5. Schematic model of Ran regulation of axonal retrograde signaling after nerve lesion.
Under normal conditions (upper panel), Ran-GTP bound to axonal CAS and importins will prevent importin α and β interaction and binding of cargo proteins. Importin β1 and RanBP1 are found in the axon as mRNAs. Following lesion (middle panel), localized translation of these mRNAs leads to upregulation of the corresponding proteins. The newly synthesized RanBP1 stimulates disassociation of RanGTP and RanGAP synergized hydrolysis, thus allowing formation of a cargo-binding complex of importin α with de novo synthesized importin β (lower panel).
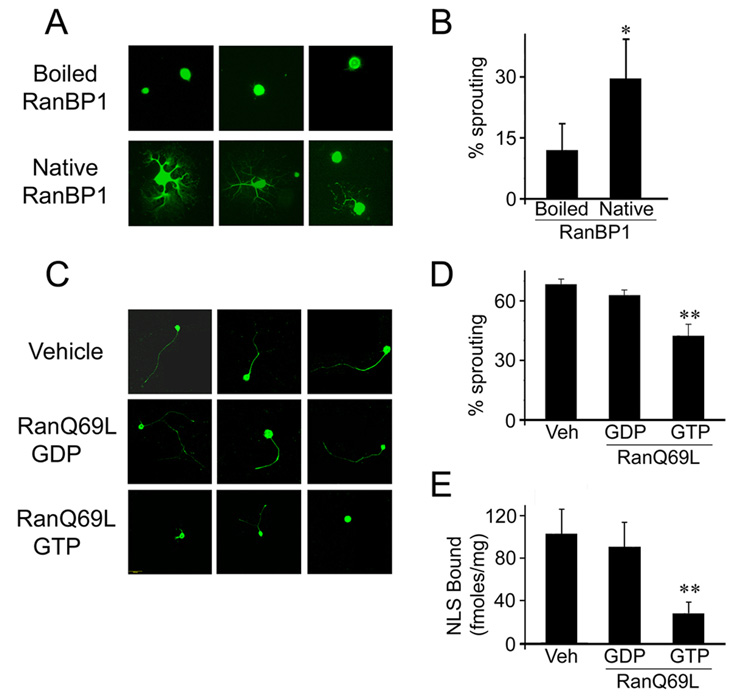
In order to test the model outlined in Figure 5, we searched for ways to specifically perturb Ran dynamics and functions in axons, without interfering with critical roles of the Ran system in nuclear import. An initial gain of function experiment was conducted by trituration of RanBP1 protein into DRG neurons during preparation for culture. This method was previously shown to induce protein uptake into the lesioned axonal stump, thus allowing rapid assessment of effects on process outgrowth (Hanz et al., 2003; Perlson et al., 2005). Indeed trituration of native RanBP1 by this method induced robust neurite extension in comparison to controls within the first 24 hours of culture (Figure 6A, B). Control neurons, or neurons triturated with heat-inactivated RanBP1, extended few or no neurites early in the culture, but at later time points of 48 hr or more exhibited outgrowth similar to that found in RanBP1-triturated cells (data not shown). Thus, increasing the levels of RanBP1 at the site of injury causes a transient acceleration of the injury response, as predicted by the model.
Figure 6. Modulating the Ran system affects neuronal injury responses.
(A) Representative pictures of adult DRG neurons 24 hr in culture after trituration with 250 µg of recombinant RanBP1 or of heat-inactivated Ran-BP1 as control. (B) Quantification of the fraction of neurite-bearing cells (average ± standard deviation, n = 4), * denotes p < 0.05 (One-tailed T-test). (C) The GTP-loaded form of the non-hydrolyzable Ran mutant RanQ69L inhibits the conditioning lesion response in sensory DRG neurons. 32µg of RanQ69L-GTP or RanQ69L-GDP or vehicle control were injected to the sciatic nerve concomitantly with a conditioning lesion. Five days later L4–L5 DRG neurons were placed in culture, and neuronal outgrowth was examined after 18 hr in vitro. (D) Quantification of the experiments shown in C. The percent of outgrowth of DRG neurons from animals treated with RanQ69L-GTP was significantly lower than neurons from animals treated with either RanQ69L-GDP or vehicle (average ± standard deviation, n = 6). ** denotes p < 0.01 (Student’s T-test). (E) RanQ69L reduces formation of the retrograde importins complex in sciatic nerve. The experiment was carried out as detailed above, with the addition of 30µg of biotinylated NLS peptide in the injection. Axoplasm was extracted six hours later, subjected to dynein immunoprecipitation followed by quantification of bound NLS biotin by a streptavidin-HRP ELISA (Student’s T-test, p < 0.01, n = 3).
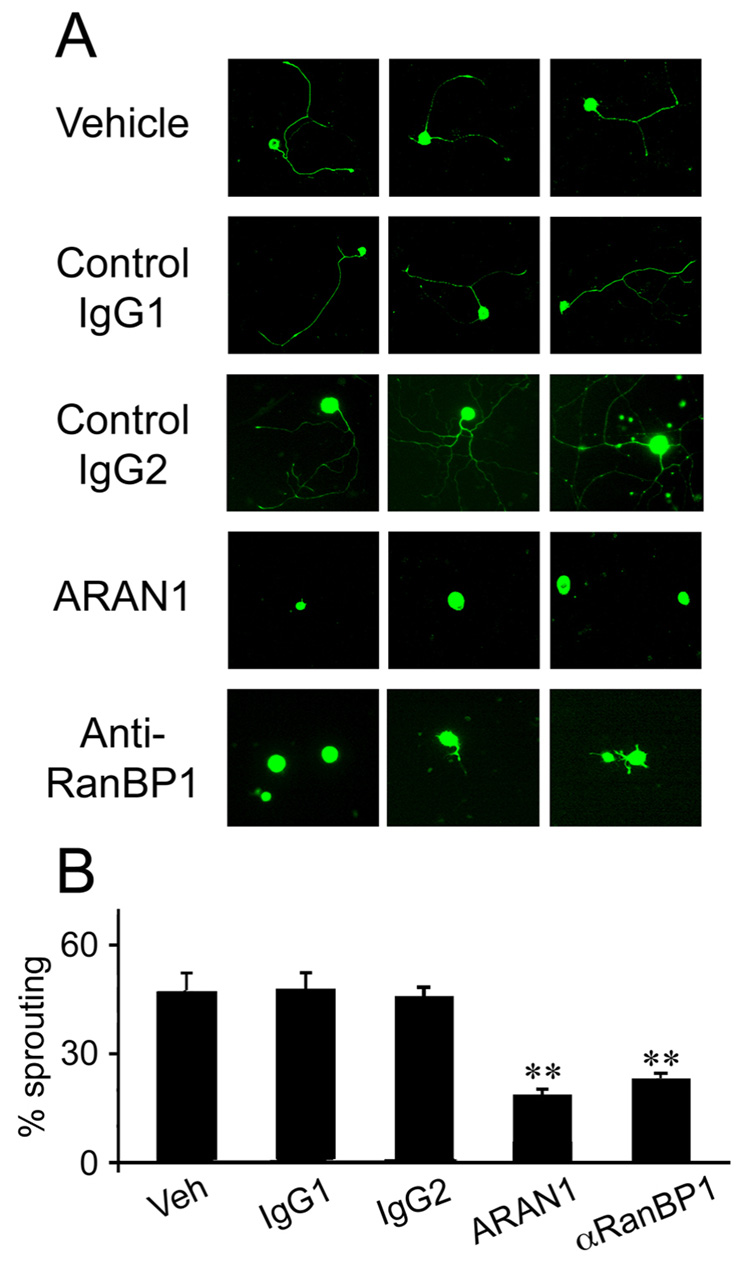
Injection of proteins into sciatic nerve axons concomitantly with injury should enable perturbation of the system in a spatially and temporally restricted manner, as shown by injection of fluorescent reporter proteins or the RanQ69L mutant (Figure S7). We observed uptake of fluorescent streptavidin (Figure S7) or IgG (data not shown) into approximately 50% of the axons near the lesion site in crushed nerve. Levels of the introduced proteins decreased significantly within a few hours, as shown by immunostaining on cross-sections of the nerve (Figure S7A, B) and by Western blots on axoplasm (Figure S7C, D); indicating that this approach allows transient and local interference with the system. We therefore tested the effects of introducing the well-characterized RanQ69L mutant, which binds GTP but cannot hydrolyze it to GDP (Stewart et al., 1998; Weis et al., 1996). Introduction of excess RanQ69L-GTP to the axon should therefore reduce interaction of importins α and β, while RanQ69L-GDP should not interfere with formation of the importins complex. RanQ69L preloaded with either GTP or GDP was injected to the sciatic nerve concomitantly with a conditioning lesion (Smith and Skene, 1997). Five days later L4/L5 DRG neurons from the treated animals were placed in culture, and axonal outgrowth was assessed after 18 hours in vitro. The degree of outgrowth of DRG neurons from animals treated with RanQ69L-GTP was significantly lower than neurons from animals treated with either RanQ69L-GDP or vehicle (Figure 6C, D). ELISA quantification of the NLS-binding capacity associated with axoplasm dynein under these different treatments confirmed that application of RanQ69L-GTP markedly reduced the cargo binding capacity of the retrograde complex in lesioned sciatic nerve axons (Figure 6E). Finally, we examined the involvement of endogenous Ran in regulation of retrograde injury signaling by testing the effects of blocking antibody injections in the conditioning lesion paradigm in sciatic nerve. Two antibodies were tested, an anti-RanBP1 antibody previously shown to interfere with RanBP1 function (Guarguaglini et al., 2000), and the anti-Ran antibody ARAN1 that competes with the binding of RanBP1 to Ran-GTP, and should therefore prevent the disassembly of the RanGTP/CAS/importin α complex. Injection of the anti-RanBP1 antibody or ARAN1 to sciatic nerve concomitantly with a crush injury significantly reduced the level of conditionally lesioned neuronal outgrowth in comparison to vehicle or irrelevant antibody controls (Figure 7A, B). It is noteworthy that all the in vivo function-perturbing experiments carried out with RanQ69L or with antibodies are done by introducing the reagent to axons in vivo a number of days before readout of the eventual effect on neurite-extension characteristics of the cell body. Since the half-life of such reagents is measured in hours in vivo (Figure S7), it follows that a perturbation at the initial stages of formation of the complex is sufficient to affect retrograde injury signaling.
Figure 7. Regulation of retrograde injury signaling by the Ran system.
Anti-RanBP1 or anti-Ran (ARAN1) antibodies inhibit the conditioning lesion response in sensory DRG neurons. Rat sciatic nerves were injected with vehicle control or 10–20 µg of antibody, concomitantly with a crush lesion. IgG1 and IgG2 are two unrelated control antibodies (anti-biotin and anti-GFP, respectively). Five days later L4–L5 DRG neurons were placed in culture. (A) Representative images of neurons after 18 hr in culture. (B) Quantification of the fraction of neurite-extending cells (n = 3). ** denotes significant difference from controls at p < 0.01 (Student’s T-test).
Discussion
We have shown that Ran is found in both GTP and GDP bound forms in sciatic nerve axons, and that RanGTP is in association with CAS, importin α and dynein in non-injured neurons. Upon axonal injury, local translation of RanBP1 together with recruitment of axonal RanGAP facilitates dissociation of Ran from the importin α - dynein complex, and hydrolysis of RanGTP to GDP. This allows binding of newly translated importin β to importin α on dynein, thus creating a retrograde injury-signaling complex ready to bind cargo. In vivo perturbation of this mechanism by introducing a GTP-loaded non-hydrolysable Ran mutant or blocking antibodies to Ran or RanBP1 to sciatic nerve concomitantly with injury inhibits the conditioning lesion response of DRG sensory neurons. These findings reveal a mechanism that regulates initiation of retrograde injury signaling in peripheral sensory neurons, and unveil a new function for Ran in regulating transport in the cytoplasm.
Ran Discriminates Different Modes of Retrograde Signaling
A number of candidate retrograde injury signals have been suggested in the literature, including Erk1/2, p38 MAPK, jun kinase (Jnk), protein kinase A, protein kinase G, and the transcription factors STAT3 and ATF2/3 (Hanz and Fainzilber, 2006 and references cited therein). How does this diversity of cargos link to the retrograde transport machinery? Apart from classical NLS-targeted cargos that should bind the importin α/β complex on dynein, other cargo proteins can bind at distinct sites. For example, calpain cleavage fragments of the intermediate filament vimentin bind directly to importin β and in parallel to phosphorylated Erks, thus linking the latter to importin-mediated retrograde transport (Perlson et al., 2005). Other candidate injury signals may link to dynein by importin-independent mechanisms. Cavalli et al. (2005) have proposed the protein Sunday driver (Syd) as a linker of activated Jnk3 to injury signaling, due to an apparently enhanced interaction between Syd and dynactin after injury. Dynactin is also important for transport of growth factor signaling endosomes on dynein, which is critical for neuronal survival and maintenance (Bronfman et al., 2007; Ibanez, 2007). Thus, different non-exclusive modes have been described for dynein to interact with different cargos. Variability in subunit composition within the dynein complex might also allow for differential cargo-binding (Pfister et al., 2006), leading to different combinations of signals being transported in different cell types. Clearly regulation of cargo binding to the dynein motor is critical for neuronal function, and RanGTP binding to importin α and CAS on dynein provides the system with a ‘safety catch’ that prevents formation of importin complexes, hence preventing inappropriate retrograde transport of importin-dependent cargos. The localized upregulation of Ran hydrolyzing machinery provides tight spatiotemporal control of system activation by releasing the Ran ‘safety catch’. Furthermore, Ran regulation of retrograde signaling allows discrimination between importin-dependent and importin-independent cargos, thus providing an additional layer of flexibility to dynein-dependent retrograde signaling.
Localized Translation of RanBP1 is Key for Activation of Retrograde Injury Signaling
The ‘safety catch’ mechanism for axonal Ran is based on low hydrolysis of importin-bound RanGTP under normal conditions, when axonal RanBP1 is low. Following injury, localized upregulation of RanBP1 and RanGAP can ‘release the catch’ by catalyzing displacement and hydrolysis of Ran. RanBP1 influences Ran-RanGAP interactions by order of magnitude increases of the association and subsequent hydrolysis rates (Seewald et al., 2003). Strict control of axonal RanBP1 levels by targeted mRNA transport and localized translation upon need should therefore be ideal for localized and dramatic changes in Ran-GTP hydrolysis at a defined site in the axon. The calcium dependence of this process likely enables its activation by calcium influx resulting from nerve injury, and potentially also from other stimuli.
The relative contributions of local axonal translation versus transport of cell body synthesis products to the axonal protein ensemble has been the topic of much debate (Wang et al., 2007 and references cited therein). Much evidence has accumulated in recent years in support of both mechanisms, and indeed it has become clear that they are complementary and that the degree of importance of one versus the other will differ between different proteins and different physiological states of the neuron. The principle utility of local translation to the retrograde injury-signaling mechanism seems to be in allowing the cell to maintain a signaling mechanism in a dormant state until needed, as well as enabling rapid activation of the mechanism. This is exemplified strikingly in the FRAP data of Figures 4 and Supplementary Figure 6, with full local translation reporter fluorescence recovery over ~50 minutes, compared to essentially no recovery of an anterograde transport reporter within this time frame. RanBP1-induced hydrolysis of RanGTP to RanGDP occurs within 60 minutes of injury in vivo (Figure 2), thus the FRAP results show that localized translation provides a sufficiently rapid mode of RanBP1 up-regulation in this case. Moreover the functional perturbations by injecting antibodies or dominant negative Ran (RanQ69L) at the lesion site shown in Figure 6 and Figure 7 are dependent on reagents with a half-life of less than 2 hours in the injured axons, as shown in Supplementary Figure S7. The injury site in our experiments in rat sciatic nerve is approximately 5 centimeters distant from the corresponding neuronal cell bodies. The fastest anterograde transport velocities reported in the literature are ~ 4.5 µm/sec (Kaether et al., 2000). This velocity is beyond that achievable by a single motor, and seems to arise from multiple motors coordinating transport of an individual cargo (Kural et al., 2005). Even if such uniquely rapid transport mechanisms are activated after nerve injury, the maximal distance covered at these velocities will be approximately ~1 cm/hr, under an optimistic 60%/40% estimate of the go/stop ratio. These calculations rule out the possibility that diffusion or motor-driven transport of RanBP1 from the cell body can account for events initiated within minutes after injury and essentially completed within two hours at this 5 cm distant lesion site. Finally, although local axonal translation capacity is thought to be tenfold less per volume unit than that of the cell body (Lee and Hollenbeck, 2003), the data of Hanz et al. (2003) and of Figure 6E suggest that the concentration of functional importin complexes in axoplasm reaches ~0.2 pM, which is three to six orders of magnitude less than concentrations of importin β in cell body cytoplasm of non-neuronal cells (Yang and Musser, 2006). Given stochiometric equivalency of RanBP1 and importins, this suggests that local translation capacity in peripheral axons is more than sufficient to account for physiologically relevant levels of local up-regulation of RanBP1 upon injury.
RanGTP in Axons: Implications and Speculations
One of the most striking aspects of the above data is that Ran-GTP is found in axonal cytoplasm in the sciatic nerve. The strict nuclear localization of RCC1, the only known RanGEF, coupled with RanBP1 and RanGAP in the perinuclear region of the cytoplasm, creates a nuclear RanGTP versus cytoplasmic RanGDP gradient in cells, the steepness of which is determined by cytoplasmic RanBP1 and RanGAP levels (Gorlich et al., 2003). The occurrence of RanGTP in axons might be explained by two mechanisms, both entirely speculative at this time. Facilitated transport of RanGTP from the nucleus in parallel with its protection from hydrolysis might be one such mechanism. Alternatively, a hitherto unrecognized RanGEF might regenerate RanGTP in the axon. In this context it is interesting to note that RCC1 in plants has so far not been identified by sequence homology (Meier, 2007), so the existence of very divergent RanGEF’s is not inconceivable. Notably, splice variants of RCC1 might be found at low levels in the cytoplasm under certain conditions (Hood and Clarke, 2007). A number of cytoplasmic proteins containing RCC1-like domains have been described, although RanGEF activity has not been established for any of them. The multidomain GEF protein alsin/ALS2 is of particular interest due to its linkage to motor neuron disease (Hadano et al., 2007). A recent study identified a homozygous missense mutation in the RCC1 domain of alsin in a patient suffering from juvenile primary lateral sclerosis, and showed mislocalization and cytotoxicity of the mutant protein in neurons (Panzeri et al., 2006). However, we were not able to demonstrate in vitro RanGEF activity of recombinant alsin (data not shown), and the provocative hypothesis of an axonal RanGEF requires further study.
General Roles for Importins and Ran in Neurons
While we have established a role for importins in the transport of injury signals from axonal lesion sites to the cell body in injured peripheral sensory neurons (Hanz et al., 2003; Perlson et al., 2005), others have demonstrated roles for importins in cytoplasmic transport of synaptic signals in central neurons (Thompson et al., 2004), and in tiling of photoreceptor axons in development of the Drosophila visual system (Ting et al., 2007). In addition, a switch in the subtypes of importin α expressed in embryonic stem cells may be critical for neuronal differentiation (Yasuhara et al., 2006), since the importin α switch may dictate changes in the spectrum of cargo transcription factors imported into the nucleus (Shmidt et al., 2007; Yasuhara et al., 2006; Yasuhara and Yoneda, 2007). The Ran interactor RanBPM also influences neuronal differentiation in conjunction with TAF4 in embryonic cortical neural stem cells (Brunkhorst et al., 2005). Most recently, RanBPM was implicated in axon guidance as a modulator of semaphorin 3A signaling via an interaction with Plexin-A receptors (Togashi et al., 2006). Intriguingly, Ran is known to regulate microtubule dynamics and organization during spindle formation in mitosis (Ciciarello et al., 2007; Kalab et al., 2002; Pay et al., 2002), thus RanBPM and Ran might link axon guidance receptors to microtubule functions in growth cones and in axons. Clearly, Ran and its associated effectors and interactors are involved in neuronal physiology from the tip of the growth cone to the nuclear center of the cell body. We suspect that axonal regulation of the GTP-bound state of Ran will prove to be critical for the development and maintenance of neuronal projections under normal conditions as well as in response to injury.
Experimental Procedures
Nerve injury and axoplasm preparation
Adult male Wistar rats were anesthetized and nerve crush and axoplasm preparations in nuclear transport buffer (NTB, 20mM Hepes/KOH pH=7.3, 110mM Kac, 5mM MgAc, 0.5mM EGTA) were as previously described (Hanz et al., 2003; Perlson et al., 2005).
Antibodies and immunofluorescence
The hSRPγ antibody against importin-α4 was a kind gift from Dr. Karsten Weis (UC Berkeley). Goat anti-RanGAP was a kind gift from Dr. Frauke Melchior (University of Göttingen). Rabbit anti-Ran was from Abcam (Cat. #31118); mouse anti-importin-β1 clone 3E9 was from Affinity Bioreagents; mouse anti-dynein intermediate chain clone 74.1 was from Chemicon (MAB1618), rabbit anti-NF-H was from Chemicon (AB1989); mouse anti-NF-H clone N52 was from Sigma; goat anti-RCC1 (SC-1162), Goat anti RanBP1 (SC-1159) and goat anti CAS (SC-1708) were from Santa Cruz; Mouse Ran clone 20 and Mouse CAS clone 24 were obtained from Transduction Labs. Anti-Sumo-1 monoclonal 21C7 was purchased from Zymed. The ARAN1 antibody was prepared as previously described (Hieda et al., 1999). Cultured DRG neurons were fixed with 3% paraformaldehyde for immunostaining. Control and injured sciatic nerve segments were fixed in 4% paraformaldehyde, frozen and sectioned at 15µm thickness.
Immunoprecipitation
For immunoprecipitations, axoplasm from control or lesioned nerve was precleared for 1hr with Protein G Sepharose. Following overnight incubation with primary antibody (quantity as per manufacturer’s specifications), complexes were incubated with protein G Sepharose beads for 2hr at 4°C and then precipitated and washed with NTB. Proteins were eluted by boiling and subjected to Western blot. When cross-linked antibody was used, samples were eluted with stripping buffer (0.1M NaCl, 0.15M glycine, pH 3). Precipitation with ARAN1 antibody was carried out using 10 µg of ARAN1 for 200 µg axoplasm. For immunoprecipitation of RanGTP from axoplasm, 200 µg axoplasm were mixed with 10 µg his-importin β and 10 µg ARAN1 and incubated at 4°C for 2 hours, followed by precipitation of the complex with 20 µg Protein G Sepharose.
Local protein synthesis in axoplasm
Sciatic nerves were injected with 15µl of 10µg/ml of cycloheximide or 100 mM EGTA. Axoplasm was resolved on SDS-PAGE gel and blotted for RanBP1, RanGAP and importin β.
Pure axonal preps and RT-PCRs
Isolation of DRG axons and cell bodies was carried out as previously described (Zheng et al., 2001). 200ng of RNA from cell body and axons was used as template for Reverse transcription and PCR. RanBP1 primers were: GCCGCCAAGAGGACAGTC and CATGAGAAGGCGGATGGT; for RanGAP GGGCAAGGGTCTCAAACT and AGCCTCGCACTCCATCAG. β-actin and γ-actin served as positive and negative controls, as previously described (Zheng et al., 2001).
Fluorescent In Situ Hybridization
FISH for endogenous RanBP1 was performed according to the methods of Bassell and colleagues (Bassell et al., 1998) with minor modifications. Two oligonucleotide probes complementary to RanBP1 (at positions 299–345 and 457–506) were designed using Oligo 6 analysis software and checked for homology to other mRNAs by BLAST. Probes were synthesized with amino group modifications at four positions each, and labeled with digoxigenin (DIG) succinamide ester per manufacturer’s instructions (Roche Applied Science). 18 h cultures of 7 d injury-conditioned DRGs were fixed in buffered 4% paraformaldehyde, equilibrated in 1x SSC with 40% formamide, and incubated at 37°C for 12 h in hybridization buffer [40% formamide, 0.4% BSA, 20 mM ribonucleotide vanadyl complex, salmon testes DNA (10 mg/ml), E. coli tRNA (10 mg/ml), and 10 mM sodium phosphate in 1X SSC] containing 20 ng probe. Hybridization was detected by immunofluorescence using Cy3-conjugated mouse anti-digoxigenin (1:1000; Jackson ImmunoResearch); neurofilament protein was detected by co-labeling with chicken anti-NFH (1:1000; Chemicon) followed by FITC conjugated anti-chicken antibody (1:500; Jackson ImmunoRes). Oligonucleotide probes complementary to β-actin mRNA (at positions 3187–3138 and 3446–3495) were used as a positive control and scrambled probes were used to control for non-specific binding. FISH/IF signals were analyzed on an inverted Leica TCS/SP2 confocal microscope.
FISH for GFP reporter constructs was carried out with a GFP riboprobe, prepared by PCR using the following primers: forward 5’-AGTGCTTCAGCCGCTACCC-3’, reverse 5’-CGGTCACGAACTCCAGCA-3’. The resulting 415 bp product was subcloned into a pGEM vector, linearized with BamHI, and the DIG-tagged RNA probe was transcribed in vitro using T7 polymerase. The probe was used for hybridization at 50 µg per slide. Hybridization was conducted for 5 hr at 55°C in wet chambers, followed by overnight incubation with an anti-DIG monoclonal antibody (1:500, Enzo, Roche). Signal was detected by 45 min incubation with an anti-mouse Cy5 labeled secondary antibody (1:1000, Jackson), with imaging by confocal microscopy as described above.
Fluorescence recovery after photobleaching (FRAP)
Dissociated DRG cultures from adult Sprague Dawley rats were transfected with myr-dzGFP-RanBP1 or Kif5C560-Tomato plasmids prior to plating using an Amaxa Nucleofection system. FRAP was performed using a Leica TCS-SP2 confocal microscope fitted with an enviromental chamber to cells at 37°C, 5% CO2. 48–72 h after transfection, GFP-expressing neurons were chosen for FRAP analyses. 40x oil immersion objective (numerical aperture = 0.7) was used for imaging with the pinhole of the confocal set to 4 airy units to ensure that the entire thickness of axons (2–4 µm diameter) was exposed to laser emission. All experiments employed the 488 nm laser line for GFP excitation and photobleaching with energies as indicated below. Excitation and photobleaching for Tomato was performed with 543 nm laser line set to 100% and 50% energy, respectively. GFP emission was collected with a band filter set to 498–530 nm with PMT energy, offset, and gain matched for all collection sets; collection of tomato signals was with band filter set to 595–640 nm. Prior to photobleaching, neurons were imaged every 30 sec for 15 min with 15% laser power. A region of interest (ROI) of the terminal axon was then exposed to 75% laser power for 40 frames at 3.2 sec intervals. Recovery of GFP emission was then monitored every 30 sec over 30 min using 15% laser power. The raw data from multiple time-lapse experiments was used to calculate from matched images for the bleached ROI. In some experiments, cultures were pre-treated with 1 µM anisomycin immediately before imaging (i.e., at 'pre-bleach') as indicated.
A double bleach FRAP sequence was used to fully remove any GFP derived from proximal sources over the course of recovery. The advanced time-lapse module of the LSC was customized for this FRAP sequence. For this, an ROI that included the growth cone and more proximal axon shaft was imaged every 30 sec for 5.5 min at 7% laser power (Lapse 1). This ROI was roughly twice the size of the typical FRAP experiments outlined above. The entire ROI was then photobleached by 40 successive scans (typical duration was 58–60 sec) at maximum speed with 100% laser power (Lapse 2). Photobleaching was confirmed by rapidly acquiring a single image at 7% laser power (Lapse 3). Photobleaching was then maintained in the proximal ½ of the ROI (i.e., axonal shaft excluding terminal axon) for 15 successive scans at 2 sec intervals with 40–70% power (Lapse 4). The remainder of the ROI from Lapse 1 (i.e., terminal axon) was not subjected to bleaching during this Lapse 4. Upon completion of Lapse 4, recovery of fluorescence was monitored by imaging the entire ROI at 7% laser power (Lapse 5). Lapses 4 and 5 were then repeatedly cycled for 100 times, to give a 30 min duration for post-bleaching.
FRAP Analyses of severed axons
To eliminate the neuronal cell body's contribution to fluorescence recovery after photobleaching, axons were disconnected from the cell body by mechanical transection. Briefly, a micropipette was drawn from a glass capillary using a Sutter Instruments P87 pipette puller (South San Francisco, CA) and mounted on a motorized micromanipulator attached to the stage of an inverted microscope. The tip of the mounted micropipette was placed in contact with the bottom of the cover slip and quickly moved across the axon shaft (left to right). Complete transaction was verified both by DIC and compression of axonal GFP signals upon retraction of the cut ends. These pre- and post-transection DIC and fluorescent images were taken with 15% power on 488 nm laser. After verifying complete transection, the field of view was zoomed 3–4 times for FRAP sequences. Pre-bleach images of the distal axon were then taken every 30 sec for 5 min at 15% laser power. The distal segment of the cut axon was then photobleached at 100% laser power at maximum scanning speed for 40 successive scans. Fluorescence recovery was monitored at 30 sec intervals with 15 % laser power for 30 min. Only the transected axons that showed no obvious disintegration (varicosity or blebbing) or detachment from the bottom of the culture vessel were utilized for computing the ROI mean value and pooled for analysis.
Quantification of FRAP and statistical analysis
Image processing and analysis was performed using ImageJ software (NIH, Bethesda, MD). To calculate the mean fluorescence intensity within an ROI that encompassed the terminal axon with growth cone, total fluorescence intensity of the ROI for each time was divided by overall area of the ROI. The percentage of fluorescence recovery at each time point after photobleaching was then normalized to the baseline of the mean fluorescence intensity that had been measured within the ROI of the very first image after photobleaching (0 min) and averaged for all FRAP analyses in a transfection or a treatment. For each construct tested, FRAP was analyzed on at least 3 neurons per well and repeated over two transfection runs. Data was analyzed using GraphPad Prism 4 software package (San Diego, CA). 2-way ANOVA was used to compare the time for the recovery between transfections and between treatments followed by Bonferroni post-hoc multiple comparisons. All values were expressed as mean ± SEM and significance was set at p < 0.05.
Conditioning lesion
Sciatic nerve conditioning lesion was performed as previously (Hanz et al., 2003), with concomitant injection of 2.2µg of ARAN1, irrelevant (anti-biotin) antibody or vehicle per nerve. In another set of experiments 32µg of RanQ69L-GTP or RanQ69L-GDP or vehicle were introduced per nerve. Five days after injury, dissociated cultures were prepared from the L4–5 DRG's as described (Hanz et al., 2003). After 18hr cultures were fixed in 3% paraformaldehyde, stained with NF-H and length of the longest axon per neuron was measured. Between 50–250 cells were measured for each experimental repeat.
Trituration of DRG neurons
DRG neurons were triturated in 0.5 ml HBSS containing 250 µg of recombinant RanBP1 (Cytoskeleton RN07). The same amount of RanBP1 was boiled 10 minutes and used as a negative control. Neurite outgrowth was scored after 24 and 48 hours in culture. Between 50–100 cells were measured for each experimental repeat.
Quantification of NLS binding of dynein bound complexes
Rat sciatic nerves were lesioned by crush and injected with 30µg biotinylated NLS (biotin-CTPPKKKRKV) together with 32µg of RanQ69L-GTP or RanQ69L-GDP or vehicle. After 6hr the rats were sacrificed and sciatic nerve axoplasm was precipitated on anti-dynein, followed by incubation with 100ng of streptavidin-HRP. Beads were washed and resuspended in NTB buffer and HRP activity was assayed as described (Hanz et al., 2003).
Production of recombinant proteins
A construct for bacterial overexpression of His-tagged RanQ69L was kindly provided by Dr. Ziv Reich (Weizmann Institute). Induction was done with 4mM IPTG for 3hr at 30°C. Proteins were purified over a Nickel-NTA Agarose column. The recombinant proteins were equilibrated in loading buffer (200mM PIPES pH=6.6,10mM EDTA, 10% glycerol, 1mM DTT). The RanQ69L proteins were loaded with either GTP or GDP. 1mg of protein was incubated for 30min at 4°C with loading buffer containing 2.5mM GTP or GDP, followed by 15min at room temperature. Then MgCl2 was added to final concentration of 25mM.
RanGTP pull down from axoplasm
300 µg axoplasm (3–4 µg/µl) were mixed with 10 µg of recombinant His tagged importin-β and incubated for 1 hour in 4°C. Following the incubation, 10 µl of NiNTA agarose beads (Qiagen) were added to the axoplasm-importin mixture and incubated with rotation for 1.5 hour in 4°C. Beads were washed and the bound fraction was eluted with 300 mM imidazol. The eluate was then separated on 10% SDS PAGE and immunodetected with Ran antibody (BD Transduction Labs monoclonal mouse antibody cat #610341, 50 ng/ml).
Recombinant RanGTP-importin β binding assay
4 µg of importin-β and 1 µg of Ran, pre-charged with GTP or GDP, were incubated an 4°C for 1 hour in final volume of 100 µl. After the incubation, 1 µg of importin-β antibody (Sigma clone 31h4 cat#I2534) was added for another 1.5 hours. Then the antibody was precipitated by 10 µl protein G sepharose (Amersham) with rotation of 1 hour at 4°C. Beads were washed, the bound fraction was eluted by boiling with SDS sample buffer and separated on 10% SDS PAGE with subsequent immunodetection with anti-Ran (BD Transduction Labs monoclonal mouse antibody cat #610341, 50 ng/ml).
Supplementary Material
Acknowledgements
Supported by funding from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Minerva Foundation, the European Union FP6 NEST Axon Support project, the USA-Israel Binational Science Foundation (2003208), and NIH/NINDS (R01-NS041596, R01-NS049041, and F32-NS048721). M.F. is the incumbent of the Chaya Professorial Chair in Molecular Neuroscience. We gratefully thank Gary Banker for the Kif5c plasmid and helpful discussions, Erin Schuman for the dzGFPmyr plasmid, Karsten Weis and Frauke Melchior for antibodies, and Geoff Daniels (Leica Microsystems) for assistance in devising the double-bleach FRAP protocols. We appreciate very much the expert assistance of Zehava Levy, Rotem Ben-Tov Perry and Vladimir Kiss; and valuable comments and advice from Ziv Reich, Michael Elbaum, Eran Perlson and Jennifer Coleman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Ambron RT, Walters ET. Priming events and retrograde injury signals - a new perspective on the cellular and molecular biology of nerve regeneration. Molecular Neurobiology. 1996;13:61–79. doi: 10.1007/BF02740752. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Kelic S. Binding proteins for mRNA localization and local translation, and their dysfunction in genetic neurological disease. Curr Opin Neurobiol. 2004;14:574–581. doi: 10.1016/j.conb.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman FC, Escudero CA, Weis J, Kruttgen A. Endosomal transport of neurotrophins: Roles in signaling and neurodegenerative diseases. Dev Neurobiol. 2007;67:1183–1203. doi: 10.1002/dneu.20513. [DOI] [PubMed] [Google Scholar]
- Brunkhorst A, Karlen M, Shi J, Mikolajczyk M, Nelson MA, Metsis M, Hermanson O. A specific role for the TFIID subunit TAF4 and RanBPM in neural progenitor differentiation. Mol Cell Neurosci. 2005;29:250–258. doi: 10.1016/j.mcn.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciarello M, Mangiacasale R, Lavia P. Spatial control of mitosis by the GTPase Ran. Cell Mol Life Sci. 2007;64:1891–1914. doi: 10.1007/s00018-007-6568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich D, Seewald MJ, Ribbeck K. Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. Embo J. 2003;22:1088–1100. doi: 10.1093/emboj/cdg113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarguaglini G, Renzi L, D'Ottavio F, Di Fiore B, Casenghi M, Cundari E, Lavia P. Regulated Ran-binding protein 1 activity is required for organization and function of the mitotic spindle in mammalian cells in vivo. Cell Growth Differ. 2000;11:455–465. [PubMed] [Google Scholar]
- Hadano S, Kunita R, Otomo A, Suzuki-Utsunomiya K, Ikeda JE. Molecular and cellular function of ALS2/alsin: implication of membrane dynamics in neuronal development and degeneration. Neurochem Int. 2007;51:74–84. doi: 10.1016/j.neuint.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Hanz S, Fainzilber M. Retrograde signaling in injured nerve--the axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Herold A, Truant R, Wiegand H, Cullen BR. Determination of the functional domain organization of the importin alpha nuclear import factor. J Cell Biol. 1998;143:309–318. doi: 10.1083/jcb.143.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda M, Tachibana T, Yokoya F, Kose S, Imamoto N, Yoneda Y. A monoclonal antibody to the COOH-terminal acidic portion of Ran inhibits both the recycling of Ran and nuclear protein import in living cells. J Cell Biol. 1999;144:645–655. doi: 10.1083/jcb.144.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood FE, Clarke PR. RCC1 isoforms differ in their affinity for chromatin, molecular interactions and regulation by phosphorylation. J Cell Sci. 2007;120:3436–3445. doi: 10.1242/jcs.009092. [DOI] [PubMed] [Google Scholar]
- Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Heald R. The RanGTP gradient - a GPS for the mitotic spindle. J Cell Sci. 2008;121:1577–1586. doi: 10.1242/jcs.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- Lee SK, Hollenbeck PJ. Organization and translation of mRNA in sympathetic axons. J Cell Sci. 2003;116:4467–4478. doi: 10.1242/jcs.00745. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. Faseb J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- Meier I. Composition of the plant nuclear envelope: theme and variations. J Exp Bot. 2007;58:27–34. doi: 10.1093/jxb/erl009. [DOI] [PubMed] [Google Scholar]
- Panzeri C, De Palma C, Martinuzzi A, Daga A, De Polo G, Bresolin N, Miller CC, Tudor EL, Clementi E, Bassi MT. The first ALS2 missense mutation associated with JPLS reveals new aspects of alsin biological function. Brain. 2006;129:1710–1719. doi: 10.1093/brain/awl104. [DOI] [PubMed] [Google Scholar]
- Pay A, Resch K, Frohnmeyer H, Fejes E, Nagy F, Nick P. Plant RanGAPs are localized at the nuclear envelope in interphase and associated with microtubules in mitotic cells. Plant J. 2002;30:699–709. doi: 10.1046/j.1365-313x.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perlson E, Medzihradszky KF, Darula Z, Munno DW, Syed NI, Burlingame AL, Fainzilber M. Differential Proteomics Reveals Multiple Components in Retrogradely Transported Axoplasm After Nerve Injury. Mol Cell Proteomics. 2004;3:510–520. doi: 10.1074/mcp.M400004-MCP200. [DOI] [PubMed] [Google Scholar]
- Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, Fainzilber M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006;364:938–944. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Petrescu N, Micu I, Malek S, Ouardouz M, Stys PK. Sources of axonal calcium loading during in vitro ischemia of rat dorsal roots. Muscle Nerve. 2007;35:451–457. doi: 10.1002/mus.20731. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM. Genetic Analysis of the Cytoplasmic Dynein Subunit Families. PLoS Genet. 2006;2:e1. doi: 10.1371/journal.pgen.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3:381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Quimby BB, Dasso M. The small GTPase Ran: interpreting the signs. Curr Opin Cell Biol. 2003;15:338–344. doi: 10.1016/s0955-0674(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Seewald MJ, Kraemer A, Farkasovsky M, Korner C, Wittinghofer A, Vetter IR. Biochemical characterization of the Ran-RanBP1-RanGAP system: are RanBP proteins and the acidic tail of RanGAP required for the Ran-RanGAP GTPase reaction? Mol Cell Biol. 2003;23:8124–8136. doi: 10.1128/MCB.23.22.8124-8136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmidt T, Hampich F, Ridders M, Schultrich S, Hans VH, Tenner K, Vilianovich L, Qadri F, Alenina N, Hartmann E, et al. Normal brain development in importin-alpha5 deficient-mice. Nat Cell Biol. 2007;9:1337–1338. doi: 10.1038/ncb1207-1337. author reply 1339. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kent HM, McCoy AJ. The structure of the Q69L mutant of GDP-Ran shows a major conformational change in the switch II loop that accounts for its failure to bind nuclear transport factor 2 (NTF2) J Mol Biol. 1998;284:1517–1527. doi: 10.1006/jmbi.1998.2204. [DOI] [PubMed] [Google Scholar]
- Thompson KR, Otis KO, Chen DY, Zhao Y, O'Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O'Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Schmidt EF, Strittmatter SM. RanBPM contributes to Semaphorin3A signaling through plexin-A receptors. J Neurosci. 2006;26:4961–4969. doi: 10.1523/JNEUROSCI.0704-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci U S A. 2007;104:12913–12918. doi: 10.1073/pnas.0611562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev Neurobiol. 2007;67:1166–1182. doi: 10.1002/dneu.20511. [DOI] [PubMed] [Google Scholar]
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Weis K, Dingwall C, Lamond AI. Characterization of the nuclear protein import mechanism using Ran mutants with altered nucleotide binding specificities. Embo J. 1996;15:7120–7128. [PMC free article] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Musser SM. Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol. 2006;174:951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2006;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- Yasuhara N, Yoneda Y. Normal brain development in importin-alpha5 deficient-mice. Nat Cell Biol. 2007;9:1339. doi: 10.1038/ncb1207-1337. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.