Abstract
Introduction:
Subarachnoid hemorrhage (SAH) can trigger immune activation sufficient to induce the systemic inflammatory response syndrome (SIRS). This may promote both extra-cerebral organ dysfunction and delayed cerebral ischemia, contributing to worse outcome. We ascertained the frequency and predictors of SIRS after spontaneous SAH, and determined whether degree of early systemic inflammation predicted the occurrence of vasospasm and clinical outcome.
Methods:
Retrospective analysis of prospectively collected data on 276 consecutive patients admitted to a neurosciences intensive care unit with acute, non-traumatic SAH between 2002 and 2005. A daily SIRS score was derived by summing the number of variables meeting standard criteria (HR >90, RR >20, Temperature >38°C or <36°C, WBC count <4,000 or >12,000). SIRS was considered present if two or more criteria were met, while SIRS burden over the first four days was calculated by averaging daily scores. Regression modeling was used to determine the relationship between SIRS burden (after controlling for confounders including infection, surgery, and corticosteroid use), symptomatic vasospasm, and outcome, determined by hospital disposition.
Results:
SIRS was present in over half the patients on admission and developed in 85% within the first four days. Factors associated with SIRS included poor clinical grade, thick cisternal blood, larger aneurysm size, higher admission blood pressure, and surgery for aneurysm clipping. Higher SIRS burden was independently associated with death or discharge to nursing home (OR 2.20/point, 95% CI 1.27-3.81). All of those developing clinical vasospasm had evidence of SIRS, with greater SIRS burden predicting increased risk for delayed ischemic neurological deficits (OR 1.77/point, 95% CI 1.12-2.80).
Conclusions:
Systemic inflammatory activation is common after SAH even in the absence of infection; it is more frequent in those with more severe hemorrhage and in those who undergo surgical clipping. Higher burden of SIRS in the initial four days independently predicts symptomatic vasospasm and is associated with worse outcome.
Keywords: Subarachnoid hemorrhage, Inflammation, Vasospasm, Sepsis syndrome
INTRODUCTION
Activation of the systemic immune response after subarachnoid hemorrhage (SAH) is frequently manifested by elevated levels of circulating cytokines, the major effectors of systemic inflammation [1]. The clinical manifestations of this process have been termed the Systemic Inflammatory Response Syndrome (SIRS), a constellation of findings originally described in association with sepsis [2]. It is now recognized that SIRS may be seen with a number of non-infectious insults, including trauma and surgery [3, 4]. Its presence is delineated by a combination of abnormal heart rate, respiratory rate, temperature, and leukocyte count [5]. These highly sensitive clinical signs reflect a systemic process associated with endothelial activation and dysfunction [6], which itself alters tissue perfusion, promotes organ failure and worsens outcome. This host response also includes activation of complement and coagulation cascades with potential for thrombosis and impaired microcirculatory flow [7]. High levels of catecholamines are released after SAH and are known to correlate with extra-cerebral organ dysfunction such as myocardial stunning and neurogenic pulmonary edema [8, 9]; they may also play a role in activating the systemic immune response [10].
SIRS is seen in the majority of patients after SAH ,and is associated with extra-cerebral organ dysfunction and worse outcome [11]. Its components, such as fever and leukocytosis have long been associated with adverse events after SAH [12-15]. In addition, an intriguing link between SIRS and cerebral vasospasm has been observed [16], although this study did not control for confounding factors such as surgery, corticosteroid use, and infections. This recent observation complements the growing recognition that inflammation, both local and systemic, plays an important role in the pathogenesis of vasospasm after SAH [17]. Infiltrates of inflammatory cells are seen in the walls of vasospastic arteries [18]. Cytokines and endothelial activation promote changes in smooth muscle cells, while activated leukocytes release potent vasoconstrictors such as endothelin-1 [19]. Agents that block this inflammatory cascade, including corticosteroids and non-steroidal anti-inflammatory agents, have been shown to reduce experimental vasospasm [20]. Thus, a greater degree of early neurogenic inflammatory activation may place patients at higher risk for the subsequent development of disabling cerebral ischemia.
We sought to determine the frequency as well as predictors of SIRS after SAH, and assess whether the early burden of systemic inflammation, after controlling for confounders such as concurrent infection, surgical stress, and the use of corticosteroids, was associated with increased risk of symptomatic vasospasm and poor clinical outcome.
METHODS
Subjects
All patients diagnosed with SAH at our institution are admitted to the Neurology / Neurosurgery Intensive Care Unit (NNICU) for stabilization and management. Data on patients cared for in this unit are prospectively entered into a computerized database (QUiC, Space Labs) by a trained nurse utilizing strict definitions and guidelines, while the NNICU director (MND) performs periodic reviews to ensure reliability. We evaluated all patients with SAH admitted over a three-year period (December 2002 to December 2005) for inclusion. Eligibility criteria consisted of admission to the NNICU within four days of known ictus with head computed tomography (HCT) or lumbar puncture confirming presence of SAH. Subjects were excluded if there was a history of trauma or if a vascular malformation or other non-aneurysmal source of bleeding was discovered. Readmissions to the NNICU were also excluded. The Washington University Human Studies Committee approved the collection of data for this analysis; a waver for individual patient informed consent was obtained.
Ruptured aneurysms are secured surgically or by endovascular means as early as possible after SAH. Standard NNICU care includes administration of nimodipine to all SAH patients, while corticosteroids are given perioperatively depending on the mode of aneurysm treatment (stopped immediately after coiling, longer duration after complex surgery), and at the discretion of the individual neurosurgeon. A euvolemic state is aggressively maintained in all patients but prophylactic hemodynamic augmentation is not employed. Transcranial doppler (TCD) studies are not used for routine monitoring and cerebral angiograms are only obtained if clinical suspicion dictates. Hemodynamic augmentation for reversal of ischemic neurological deficits consists of raising mean arterial pressure (MAP) by 15-30% after aggressive fluid resuscitation, without intentional hemodilution [21].
Data Collection
The following variables were extracted from the database for this analysis: Demographics including age, gender, past medical history of hypertension, diabetes mellitus, ischemic heart disease, and smoking; Admission status including time from symptom onset to admission, Glasgow Coma Scale (GCS) score on presentation (from which the World Federation of Neurological Surgeons [WFNS] grade was derived [22]); poor grade was considered a WFNS grade of IV or V (equivalent to GCS ≤ 12) on admission. Daily measures of physiological variables included leukocyte count, maximum heart rate and respiratory rate, and highest (or lowest) temperature. The need for intubation and duration of mechanical ventilation were also recorded, as was occurrence of hydrocephalus requiring ventriculostomy. The occurrence and timing of infections including bacteremia, pneumonia, and urinary tract infections (UTI) were also abstracted from the prospectively collected ICU database using pre-specified standard definitions.
All available admission HCTs (obtained within four days of SAH onset and before any aneurysm treatment) were reviewed by a single investigator (RD), who categorized them into grades based on the Fisher classification [23]. The presence of any intraventricular hemorrhage (IVH) was noted and the sum IVH score was calculated on a standard scale from 0 to 12 [24]. Records of all cerebral angiograms were reviewed and the size and location of any ruptured aneurysm was noted. The mode of aneurysm treatment, whether surgical or endovascular, was recorded. Duration of early corticosteroid use was extracted from the central pharmacy database; exposure over the first four days was dichotomized as: 1) Minimal: none or less than one day; 2) Significant: greater than one day. The admission MAP and highest serum glucose in the initial 24 hours was also collected from hospital records.
Outcomes
Vasospasm may be defined a number of ways: it may be based on the demonstration of vessel narrowing on angiography, elevation of TCD velocities, or the emergence of delayed neurological deficits. For this study, we used both clinical and angiographic criteria for vasospasm in our analysis. Symptomatic vasospasm was diagnosed when abnormal neurological status, including altered mentation, diminished level of consciousness, or new focal deficits, developed for which other causes had been excluded. While angiography was not mandatory, in the absence of radiographic support this clinical diagnosis required improvement after hemodynamic augmentation. The degree of angiographic vasospasm was independently extracted from all reports of follow-up cerebral angiograms, and classified as mild, moderate, or severe based on radiologists' interpretation; radiographic vasospasm was considered significant when at least one vessel demonstrated moderate to severe narrowing. The association between our clinical and radiographic definitions of vasospasm was examined to validate the common but imperfect overlap between these two pathophysiologic states.
Clinical outcomes included ICU and hospital mortality, length of stay, and discharge disposition. A good outcome was considered discharge to home or a rehabilitation facility, as opposed to death or discharge to a nursing facility.
Analysis
The daily SIRS score was calculated by summing the number of variables meeting standard criteria for SIRS [5]: 1) Heart rate > 90 beats per minute; 2) Respiratory rate > 20 breaths per minute; 3) Leukocyte count < 4,000 or > 12,000; and 4) Temperature > 38 °C or < 36 °C. SIRS is traditionally diagnosed if two or more criteria are present on a given day [5], although this is a nonspecific and arbitrary delineation. To more accurately estimate the cumulative exposure of the brain to this systemic inflammatory process, a novel variable, the “SIRS burden” was quantified by summing daily scores over the first four days and calculating the mean. This early period was selected to coincide with the time when degree of systemic inflammation may be most relevant to the subsequent development of cerebral vasospasm.
Data was exported from the QUiC database into SPSS for Windows (version 11.5; SPSS, Chicago, IL) for analysis. Initial analysis was performed by dividing all subjects into groups based on presence or absence of SIRS over the first four days, development of clinical and/or angiographic vasospasm, and poor vs. good outcome. For analysis of vasospasm, patients were excluded who died within the first four days of ICU admission. Groups were compared using two-tailed ttests for continuous variables, Mann-Whitney tests for semi-quantitative variables (IVH score, SIRS burden) or chi-squared tests for categorical variables. To determine which variables were independently associated with the selected outcome measures, logistic regression analyses were performed; all relevant variables with p < 0.10 in the univariate analysis for each outcome were entered into a backward stepwise model. For analyses of SIRS and vasospasm, all baseline variables and early interventions (aneurysm treatment, corticosteroid use) were included in these models, while complications of SAH were not. In order to focus on neurogenic SIRS and exclude any infectious or other systemic contribution to the inflammatory burden we specifically controlled for those with early infections (bacteremia, UTI, pneumonia), surgery, and corticosteroid exposure, by forcing these variables into the regression model regardless of their univariate associations. Covariates with p < 0.05 were considered significant and presented in tabular format as odds ratios (OR) with 95% confidence intervals (CI).
RESULTS
Of 368 patients with a diagnosis of SAH over the study period, 276 were eligible and included in subsequent analyses. The remainder were excluded based on prolonged or unknown duration from ictus (n=49), or due to the association of bleeding with trauma or a non-aneurysmal source (n=43). Of those included, 90% were admitted within the first 24 hours after symptom onset. SIRS was present on at least one ICU day in 241 (87%) patients, and in 235 (85%) it occurred within the first four days. Criteria for SIRS were present on the day of admission in 145 (53%) of SAH patients. Demographic variables of the entire group and those patients with and without SIRS in the first four days are presented in Table 1.
Table 1.
Factors associated with presence of SIRS in the first four days
| SIRS (n = 235) |
No SIRS (n = 41) |
All (n = 276) |
P value | Multivariate Odds Ratio |
95% CI for OR |
|
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 57.0 ± 13.4 | 49.6 ± 12.3 | 55.9 ± 13.5 | 0.001 | ||
| Sex: Female, n (%) | 151 (64) | 25 (61) | 176 (64) | 0.68 | ||
| Medical history, n (%) | ||||||
| Hypertension | 117 (50) | 14 (34) | 131 (48) | 0.06 | ||
| Diabetes mellitus | 19 (8) | 3 (7) | 22 (8) | 0.99 | ||
| Smoking | 70 (30) | 9 (22) | 79 (29) | 0.30 | ||
| Ischemic heart disease | 17 (7) | 2 (5) | 19 (7) | 0.75 | ||
| WFNS grades IV-V, n (%) | 77 (33) | 3 (7) | 80 (29) | 0.001 | 15.8 | 2.6 – 95.7 |
| Fisher grade, n (%) 1 | 2 (1) | 5 (19) | 7 (3) | .007‡ | 3.1 | 0.95 – 10.0 |
| 2 | 35 (20) | 12 (46) | 47 (23) | |||
| 3 | 98 (55) | 7 (27) | 105 (52) | |||
| 4 | 43 (24) | 2 (8) | 45 (22) | |||
| IVH score (median) | 2 | 0 | 2 | .002 | ||
| IVH present, n (%) | 123 (69) | 10 (37) | 133 (65) | .001 | ||
| Aneurysm location, n (%) | ||||||
| Anterior cerebral artery | 69 (29) | 3 (7) | 72 (26) | < .001‡ | ||
| Internal carotid artery | 65 (28) | 9 (22) | 74 (27) | |||
| Middle cerebral artery | 33 (14) | 1 (2) | 34 (12) | |||
| Posterior circulation | 34 (15) | 8 (20) | 42 (15) | |||
| Angiogram negative | 19 (8) | 17 (42) | 36 (13) | |||
| Aneurysm size, mm (± SD) | 8.9 ± 5.7 | 5.9 ± 3.2 | 8.6 ± 5.6 | 0.02 | 1.2 | 1.04 – 1.40 |
| Aneurysm treatment, n (%)† | ||||||
| None | 7 (3) | 0 | 7 (3) | 0.05 | 6.7 | 1.6 – 27.8 |
| Surgical (clipping) | 121 (60) | 7 (33) | 128 (58) | |||
| Endovascular (coiling) | 73 (37) | 14 (67) | 87 (39) | |||
| Steroid exposure > 1 day | 163 (69) | 19 (46) | 182 (66) | 0.004 | ||
| Bacteremia / Sepsis | 4 (2) | 0 | 4 (1) | NS | ||
| Pneumonia | 6 (3) | 0 | 6 (2) | 0.60 | ||
| Urinary tract infection | 20 (9) | 5 (12) | 25 (9) | 0.39 | ||
| MAP on admission (mm Hg) | 105 ± 20 | 96 ± 20 | 103 ± 20 | 0.01 | 1.04 | 1.01 – 1.07 |
| Peak day #1 glucose (mmol/L) | 195 ± 60 | 170 ± 53 | 191 ± 59 | 0.01 | ||
| SIRS burden (median) | 2.0 | 0.5 | 1.75 | < .001 | ||
| Complications, n (%) | ||||||
| Hydrocephalus | 140 (60) | 13 (32) | 153 (55) | .001 | ||
| Vasospasm: Clinical | 63 (27) | 2 (5) | 65 (24) | .002 | ||
| Vasospasm: Angiographic | 57 (40) | 3 (12) | 60 (36) | 0.02 | ||
| Intubation | 126 (54) | 4 (10) | 130 (47) | < .001 | ||
| MV duration (mean ± SD) | 6.8 ± 8.0 | 4.0 ± 5.3 | 6.7 ± 7.9 | 0.49 | ||
| ICU mortality, n (%) | 38 (16) | 3 (7) | 41 (15) | 0.14 | ||
| Mortality, n (%) | 43 (18) | 4 (10) | 47 (17) | 0.18 | ||
| ICU LOS, d (mean ± SD) | 13.0 ± 10.1 | 5.2 ± 5.2 | 11.8 ± 9.9 | < .001 | ||
| Poor outcome, n (%) | 63 (27) | 5 (12) | 68 (25) | 0.04 | ||
| Discharge home, n (%) | 76 (32) | 33 (81) | 109 (40) | < .001 | ||
WFNS, World Federation of Neurological Surgeons; LOS, length of stay; MV, mechanical ventilation
Comparisons made between Fisher grade 3 and other grades, Angiogram negative vs. aneurysm found
Of those with aneurysms found on angiography
A SIRS burden of <1 was present in 37 patients (13%), 1-2 in 109 (40%), 2-3 in 92 (33%), and 3-4 in 38 (14%). The median daily SIRS burden over the first four days was 1.75. It was significantly higher in those presenting with WFNS grade IV or V (2.4 vs. 1.7 for grades I to III), those with hydrocephalus (2.0 vs. 1.7), and in those requiring intubation (2.3 vs. 1.5). It was lower in the group who were subsequently discharged home compared to those who died or were discharged elsewhere (1.5 vs. 2.3). SIRS was infrequent in cases of angiogram negative SAH, whose SIRS burden was also significantly lower than in those with aneurysmal bleeding (1.0 vs. 2.0). All these differences between groups were significant at p ≤ .001.
Few patients suffered infections in the first four days of ICU admission, and there was no statistically significant association between having bacteremia, pneumonia, or UTI and presence of SIRS early after SAH (Table 1). Even after excluding those with any early infection (n = 35), the incidence of SIRS remained at 85% and the median SIRS burden remained 1.75. There was no association between any of these infections and the occurrence of vasospasm or clinical outcome. Corticosteroids were given to 211 (76%) with a median duration of 4 days (mean 4.8 ± 5.8d). Steroid use was more common in those developing SIRS over the first four days, as was mean duration of exposure (5.2 vs. 2.9 days, p = 0.02). However, in multivariate analysis, only surgical treatment of the aneurysm, along with clinical grade, Fisher grade, admission MAP, and aneurysm size were independently associated with presence of SIRS. As would be expected if surgery induced a systemic inflammatory response, SIRS was no more common on admission in those later undergoing aneurysmal clipping, but was a significant predictor of SIRS when examined over the entire four day period perioperatively.
Vasospasm
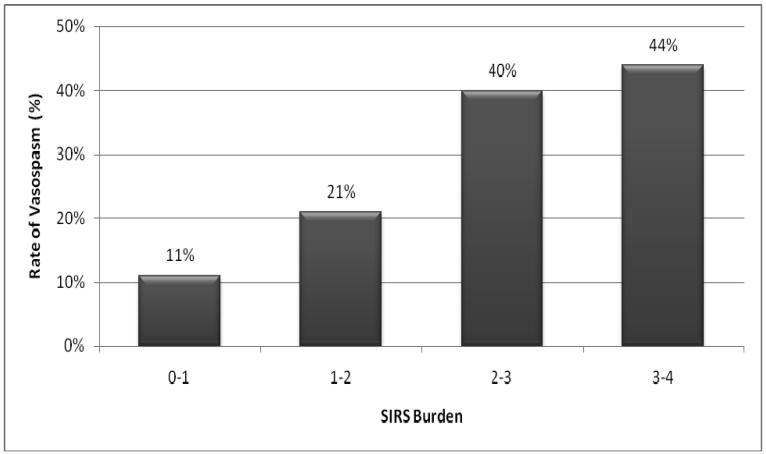
Symptomatic vasospasm developed in 64 (26%) of the 245 patients surviving beyond the first four ICU days. 100% of patients with vasospasm showed evidence of SIRS, all but two in the critical first four days; median SIRS burden was also significantly greater in this group (Table 2). Those with SIRS burden of 2 or greater had a 38% incidence of vasospasm, compared to 16% in those with a smaller burden (OR 3.1, 95% CI 1.7-5.6). This dose-dependence of SIRS burden on risk of vasospasm is shown in Figure 1. Multivariate analysis found that SIRS burden was a strong independent predictor of vasospasm, along with thick cisternal blood on HCT (Fisher grade 3) and undergoing surgery. Age, gender, and past medical history did not differ between those developing and not developing vasospasm. There was a close association between delayed ischemic deficits and significant angiographic narrowing (χ-squared, p < .001). Of those with symptoms, 54 of 64 had significant radiologic narrowing while only 5 further patients had angiographic vasospasm without clinical correlation. Multivariate analysis found that Fisher grade and surgical clipping were independent predictors of angiographic vasospasm, but unlike the association seen with symptomatic vasospasm, SIRS burden was not independently associated with angiographic changes.
Table 2.
Factors associated with symptomatic vasospasm and poor outcome
| Vasospasm (n = 64) |
No Vasospasm (n = 181) |
P value |
Odds Ratio (95% CI) |
Poor Outcome (n = 68) |
Good Outcome (n = 208) |
P value |
Odds Ratio (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 53.3 ± 10.9 | 55.7 ± 13.6 | 0.16 | 62.1 ± 15.4 | 53.9 ± 12.1 | < .001 | 1.04 (1.01-1.07) | |
| Sex : female, n (%) | 43 (67) | 111 (61) | 0.40 | 50 (74) | 126 (61) | 0.05 | ||
| Hypertension, n (%) | 29 (45) | 83 (46) | 0.94 | 40 (59) | 91 (44) | 0.03 | ||
| WFNS grades IV-V, n (%) | 21 (33) | 35 (20) | 0.03 | 42 (62) | 38 (18) | < .001 | ||
| Fisher grade 3, n (%) | 45 (80) | 48 (38) | < .001 | 6.2 (2.8-14.0) | 32 (58) | 73 (49) | 0.01 | |
| IVH score (median) | 2.0 | 1.0 | 0.01 | 4.0 | 1.0 | < .001 | ||
| IVH present, n (%) | 43 (77) | 72 (56) | 0.01 | 49 (88) | 84 (56) | < .001 | ||
| Angiogram negative, n (%) | 2 (3) | 33 (19) | 0.003 | 2 (4) | 34 (16) | 0.02 | ||
| Aneurysm size, mm (± SD) | 9.0 ± 5.8 | 8.2 ± 5.5 | 0.34 | 10.1 ± 6.7 | 8.2 ± 5.1 | 0.03 | ||
| Aneurysm treatment, n (%)† | ||||||||
| Surgical | 44 (72) | 80 (56) | 0.03 | 3.6 (1.7-7.7) | 24 (57) | 104 (60) | 0.73 | |
| Endovascular | 17 (28) | 65 (44) | 18 (43) | 69 (40) | ||||
| MAP on admission | 107 ± 22 | 102 ± 17 | 0.16 | 109 ± 23 | 103± 18 | 0.13 | ||
| Peak day #1 glucose | 191 ± 45 | 184 ± 57 | 0.38 | 218 ± 66 | 182 ± 54 | < .001 | ||
| Steroid exposure > 1 day | 49 (77) | 124 (69) | 0.22 | 37 (54) | 145 (70) | 0.02 | ||
| SIRS on arrival, n (%) | 40 (63) | 83 (46) | 0.02 | 51 (75) | 94 (45) | < .001 | ||
| SIRS in first 4 days, n (%) | 62 (97) | 145 (80) | .001 | 63 (93) | 172 (83) | 0.04 | ||
| SIRS burden (median) | 2.375 | 1.67 | < .001 | 1.77¶ (1.12-2.80) | 2.375 | 1.75 | < .001 | 2.20¶ (1.27-3.81) |
| Hydrocephalus, n (%) | 53 (83) | 73 (40) | < .001 | 54 (79) | 99 (48) | < .001 | ||
| Vasospasm: angiographic‡ | 54 (84) | 5 (5) | < .001 | 15 (40) | 44 (21) | 0.016 | ||
| Vasospasm: clinical‡ | 16 (42) | 48 (23) | 0.015 | |||||
| Intubation, n (%) | 40 (63) | 62 (34) | < .001 | 61 (90) | 69 (33) | < .001 | 8.64 (2.77-26.9) | |
| MV duration (mean ± SD)‡ | 8.1 ± 7.8 | 7.9 ± 9.2 | 0.92 | 8.9 ± 5.5 | 7.6 ± 9.8 | 0.47 | ||
| ICU mortality, n (%) | 6 (10) | 7 (4) | 0.10 | |||||
| Mortality, n (%) | 8 (13) | 9 (5) | 0.08 | |||||
| ICU LOS, d (mean ± SD)‡ | 19.8 ± 7.5 | 10.4 ± 9.6 | < .001 | 16.7 ± 8.6 | 12.2 ± 10.1 | 0.01 | ||
| Poor outcome, n (%) | 16 (25) | 22 (12) | 0.02 | |||||
| Discharge home, n (%) | 9 (14) | 100 (55) | < .001 | |||||
WFNS, World Federation of Neurological Surgeons; LOS, length of stay; MV, mechanical ventilation
Of those who underwent treatment for a ruptured aneurysm
Per point increase in mean SIRS burden
Excluding those dying early before period of vasospasm risk
Figure 1.
Rate of symptomatic vasospasm associated with increasing SIRS burden
Outcomes
Poor outcome was seen in 68 patients (25%), and was associated with expected prognostic variables such as age, poor neurologic grade, and thick cisternal blood on HCT (Table 2). However, even after controlling for these factors, SIRS burden was the strongest independent predictor of poor outcome, along with respiratory failure requiring intubation. Significantly longer lengths of stay were seen in those with SIRS (Table 1), while SIRS on admission was strongly associated with overall mortality (24% if SIRS present vs. 9% if absent, p = .001).
DISCUSSION
Sudden arterial hemorrhage within the subarachnoid space precipitates acute brain injury, initiating a complex cascade of both cerebral and systemic events [25]. Not only does a local inflammatory response propagate from blood breakdown, but a systemic state of inflammation is triggered. This may result either directly from the action of liberated cytokines, or indirectly through release of high levels of circulating catecholamines which promote immune activation [26]. The acutely injured brain releases significant quantities of interleukin-6 (IL-6), as evidenced by the much higher levels measured in the jugular vein compared to a peripheral artery after SAH [27]. The extent of this cerebral cytokine release correlates with severity of brain injury and prognosis [28]. Furthermore, IL-6 is a major stimulus for hepatic production of acute-phase proteins [29]. This acute-phase response is reflected in the elevated levels of C-reactive protein (CRP) found in the systemic circulation early after SAH, with higher peaks in those developing delayed ischemic deficits [30].
SIRS is a clinically-defined syndrome that serves as a simple and highly sensitive surrogate for identifying this systemic inflammatory activation [5]. It has been shown to occur frequently following SAH [11, 16], and may itself reflect a process that contributes to harmful sequelae such as vasospasm and extra-cerebral organ dysfunction, promoting poor outcome. In this study we confirmed that SIRS is present in most people after SAH, even in the absence of infection, and frequently occurs in the critical first four days prior to the development of delayed cerebral ischemia. Its frequency parallels the severity of the cerebral insult, being more common and of greater degree in higher grade radiographic and clinical SAH. The surge in ICP and sympathetic nervous system activation may both contribute to this strong relationship between severity of SAH and degree of SIRS.
SIRS was more frequent in those undergoing aneurysm surgery. The relationship between surgical stress and inflammatory response is well known [4], but has not been well described in the neurosurgical population. That surgery promotes inflammation is even more notable in that we found a relationship between those undergoing surgical clipping and increased risk of symptomatic cerebral ischemia. There is controversy in the literature over any differential risk of vasospasm between modes of aneurysm treatment, although a recent meta-analyses of six studies found a similar trend towards higher rates of symptomatic vasospasm in those undergoing surgery [31]. Our findings support an inflammatory mechanism by which surgery might contribute to adverse outcomes including vasospasm, and deserves further study.
We did not simply define SIRS as present or absent, which does not capture the continuous spectrum of systemic inflammation after SAH. Instead we estimated SIRS burden as a novel clinical measure of the intensity of this systemic response, as quantified by the mean number of SIRS criteria being met over the first four days. We found that this semi-quantitative measure strongly predicted hospital disposition after SAH, with patients having a higher burden being very unlikely to be discharged home and more likely to suffer a poor outcome. Moreover, the presence of SIRS early after SAH, especially with a higher burden, was associated with symptomatic vasospasm independent of traditional risk factors and such confounders as surgery and corticosteroid exposure; vasospasm was extremely unlikely in those not exhibiting signs of early systemic activation. This lends further credence to the hypothesis that an activated inflammatory process not only reflects severe SAH but also may be detrimental to vascular function and cerebral perfusion, thereby contributing to the development of symptomatic cerebral ischemia [17].
Levels of inflammatory cytokines are known to rise in parallel to the increase in blood flow velocities in basal cerebral vessels indicative of emerging vasospasm [32]. Complement activation associated with systemic inflammation accelerates red blood cell lysis and can liberate more spasmogenic proteins into the CSF space [33], while activation of the coagulation system can promote thrombosis and impair microcirculatory flow [7]. Endothelial dysfunction may further impair regulation of cerebral blood flow in this vulnerable state [34]. Loss of cerebral autoregulation and microcirculatory dysfunction may be more strongly related to systemic inflammatory activation, as opposed to local CSF inflammation; this may explain why SIRS burden was a powerful predictor of symptomatic cerebral ischemia while not being independently associated with large vessel angiographic narrowing. Clinical vasospasm is a complex multifactorial disorder involving changes at multiple levels of the cerebral vasculature, and correlates imperfectly with angiographic changes alone [35].
There are a number of limitations to a study of this kind. Although data was collected prospectively into an ICU database, retrospective review and analysis for this study depended entirely on the range and quality of available data. The SIRS criteria are extremely sensitive for this systemic response to a variety of insults, but lack specificity to any one cause. However, our research suggests that SIRS most frequently stems from the primary nervous system insult rather than reflecting early infectious triggers. Further, the high frequency of SIRS in this population means that the absence of SIRS is a much better predictor of a low risk group, with almost uniformly good outcome and minimal risk of vasospasm, than the presence of SIRS absolutely predicting a poor outcome. A higher SIRS burden may provide better positive predictive value in this regard (Figure 1), but the use of this novel score requires further validation in both sepsis and SAH populations.
Further research is needed to test and refine the hypothesis generated here in a more rigorous prospective manner. We did not measure serum markers of inflammation, such as CRP or procalcitonin [36], or look for microalbuminuria, a marker of endothelial dysfunction [37]. Such testing may allow a more precise estimation of this potentially detrimental process.
Modulation of the inflammatory response is a target of much research in sepsis-associated SIRS. In accordance with our findings, drugs that modify systemic immune activation may also have potential to ameliorate the effects of delayed cerebral ischemia after SAH. Therapies aimed at systemic complement depletion or blocking adhesion molecules and leukocyte migration have been promising in animal studies of vasospasm [38-40]. In this observational study, corticosteroids were not associated with a reduced risk of vasospasm. In fact, those treated with steroids had a higher incidence of SIRS, largely driven by an association of both with surgical interventions. Adrenergic-blocking agents, which temper the intense sympathetic activation after SAH, and statins, which stabilize endothelial function and reduce CRP, have been shown in small human studies to significantly reduce vasospasm and improve outcome after SAH [41-43]. In fact, statins upregulate nitric oxide synthesis, reduce adhesion molecule expression and inhibit leukocyte migration in experimental SAH [44]; their promising acute effects may largely be mediated through their modulation of the inflammatory cascade [45]. A recent randomized clinical trial confirmed that statins reduced inflammatory cytokines and markers of neutrophil activation after coronary bypass surgery, another non-infectious trigger for SIRS [46]. Evaluating their impact more precisely on the post-hemorrhagic inflammatory response studied here may help us better understand their role in the prevention of cerebral vasospasm.
CONCLUSIONS
Systemic inflammatory activation assessed by SIRS criteria is common after SAH, with a greater burden in those with more severe brain injury. The early burden of systemic inflammation was not only independently associated with poor outcome, but predicted subsequent development of symptomatic vasospasm. This corroborates experimental evidence that systemic inflammation may be pivotal in the pathogenesis of vasospasm, and provides a simple clinical marker of this complex process for further investigation.
Acknowledgments
Financial support: Supported by NIH-N535906 (MND)
References
- 1.Gruber A, Rossler K, Graninger W, Donner A, Illievich U, Czech T. Ventricular cerebrospinal fluid and serum concentrations of sTNFR-I, IL-1ra, and IL-6 after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2000;12:297–306. doi: 10.1097/00008506-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC, Balk RA, Cerra FB, Fein AM, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 3.Napolitano LM, Ferrer T, McCarter RJ, Jr., Scalea TM. Systemic inflammatory response syndrome score at admission independently predicts mortality and length of stay in trauma patients. J Trauma. 2000;49:647–652. doi: 10.1097/00005373-200010000-00011. discussion 652-643. [DOI] [PubMed] [Google Scholar]
- 4.Ni Choileain N, Redmond HP. Cell response to surgery. Arch Surg. 2006;141:1132–1140. doi: 10.1001/archsurg.141.11.1132. [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Fink MP, Marshall JC, Abraham E, et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. 2001. [DOI] [PubMed] [Google Scholar]
- 6.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 7.Malham GM, Souter MJ. Systemic inflammatory response syndrome and acute neurological disease. Br J Neurosurg. 2001;15:381–387. doi: 10.1080/02688690120082378. [DOI] [PubMed] [Google Scholar]
- 8.Dilraj A, Botha JH, Rambiritch V, Miller R, van Dellen JR. Levels of catecholamine in plasma and cerebrospinal fluid in aneurysmal subarachoid hemorrhage. Neurosurgery. 1992;31:42–51. doi: 10.1227/00006123-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Naredi S, Lambert G, Eden E, et al. Increased sympathetic nervous system activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31 doi: 10.1161/01.str.31.4.901. [DOI] [PubMed] [Google Scholar]
- 10.Moynihan J, Kruszewska B, Madden K, Callahan T. Sympathetic nervous system regulation of immunity. J Neuroimmunol. 2004;147:87–90. doi: 10.1016/j.jneuroim.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Gruber A, Reinprecht A, Illievich U, et al. Extracerebral organ dyfsunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit Care Med. 1999;27:505–514. doi: 10.1097/00003246-199903000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira-Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001;56:1299–1304. doi: 10.1212/wnl.56.10.1299. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson D, Stephenson S. Leukocytosis and subarachnoid hemorrhage. Surg Neurol. 1984;21:132–134. doi: 10.1016/0090-3019(84)90330-6. [DOI] [PubMed] [Google Scholar]
- 14.Weir B, Disney L, Grace M, Roberts P. Daily trends in white blood cell count and temperature after subarachnoid hemorrhage from aneurysm. Neurosurgery. 1989;25:161–165. doi: 10.1097/00006123-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 15.McGirt MJ, Mavropoulos JC, McGirt LY, et al. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98:1222–1226. doi: 10.3171/jns.2003.98.6.1222. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32:1989–1993. doi: 10.1161/hs0901.095646. [DOI] [PubMed] [Google Scholar]
- 17.Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–135. doi: 10.1227/01.neu.0000068863.37133.9e. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JT, Schianchi PM. Cerebral artery spasm. A histological study at necropsy of the blood vessels in cases of subarachnoid hemorrhage. J Neurosurg. 1978;48:515–525. doi: 10.3171/jns.1978.48.4.0515. [DOI] [PubMed] [Google Scholar]
- 19.Fassbender K, Hodapp B, Rossol S, Bertsch T. Endothelin-1 in subarachnoid hemorrhage: an acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000;31:2971–2975. doi: 10.1161/01.str.31.12.2971. al. e. [DOI] [PubMed] [Google Scholar]
- 20.Chyatte D. Prevention of chronic cerebral vasospasm in dogs with ibuprofen and high-dose methylprednisolone. Stroke. 1989;20:1021–1026. doi: 10.1161/01.str.20.8.1021. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Dacey RG, Jr, Diringer MN. Safety of hypertensive hypervolemic therapy with phenylephrine in the treatment of delayed ischemic deficits after subarachnoid hemorrhage. Stroke. 1995;26:2260–2266. doi: 10.1161/01.str.26.12.2260. [DOI] [PubMed] [Google Scholar]
- 22.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457. doi: 10.1136/jnnp.51.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21:1156–1161. doi: 10.1161/01.str.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 25.Sebha FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- 26.Naredi S, Lambert G, Friberg P, et al. Sympathetic activation and inflammatory response in patients with subarachnoid haemorrhage. Intensive Care Med. 2006;32:1955–1961. doi: 10.1007/s00134-006-0408-y. [DOI] [PubMed] [Google Scholar]
- 27.McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997;78:520–523. doi: 10.1093/bja/78.5.520. [DOI] [PubMed] [Google Scholar]
- 28.Minambres E, Cemborain A, Sanchez-Velasco P, et al. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit Care Med. 2003;31:933–938. doi: 10.1097/01.CCM.0000055370.66389.59. [DOI] [PubMed] [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 30.Rothoerl RD, Axmann C, Pina A-L, Woertgen C, Brawanksi A. Possible role of C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006;18:68–72. doi: 10.1097/01.ana.0000181693.30750.af. [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira JG, Beck J, Ulrich C, Rathert J, Raabe A, Seifert V. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurosurg Rev. 2007;30:22–30. doi: 10.1007/s10143-006-0045-5. discussion 30-21. [DOI] [PubMed] [Google Scholar]
- 32.Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70:534–537. doi: 10.1136/jnnp.70.4.534. al. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson JW, Kwun BD, Teramura A, et al. Immunological reaction against the aging human subarachnoid erythrocyte. A model for the onset of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1989;71:718–726. doi: 10.3171/jns.1989.71.5.0718. [DOI] [PubMed] [Google Scholar]
- 34.Bowton DL, Bertels NH, Prough DS, Stump DA. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit Care Med. 1989;17:399–403. doi: 10.1097/00003246-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Suarez JI, Qureshi AI, Yahia AB, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–1355. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor E, Venkatesh B, Mashongonyika C, Lipman J, Hall J, Thomas P. Serum procalcitonin and c-reactive protein as markers of sepsis and outcome in patients with neurotrauma and subarachnoid haemorrhage. Anaesth Intensive Care. 2004;32:465–470. doi: 10.1177/0310057X0403200402. [DOI] [PubMed] [Google Scholar]
- 37.Gosling P, Czyz J, Nightingale P, Manji M. Microalbuminuria in the intensive care unit: Clinical correlates and association with outcomes in 431 patients. Crit Care Med. 2006;34:2158–2166. doi: 10.1097/01.CCM.0000228914.73550.BD. [DOI] [PubMed] [Google Scholar]
- 38.Bavbek M, Polin R, Kwan AL, Arthur AS, Kassell NF, Lee KS. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke. 1998;29:1930–1935. doi: 10.1161/01.str.29.9.1930. discussion 1935-1936. [DOI] [PubMed] [Google Scholar]
- 39.Clatterbuck RE, Gailloud P, Ogata L, et al. Prevention of cerebral vasospasm by a humanized anti-CD11/CD18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J Neurosurg. 2003;99:376–382. doi: 10.3171/jns.2003.99.2.0376. [DOI] [PubMed] [Google Scholar]
- 40.German JW, Gross CE, Giclas P, Watral W, Bednar MM. Systemic complement depletion inhibits experimental cerebral vasospasm. Neurosurgery. 1996;39:141–145. doi: 10.1097/00006123-199607000-00028. discussion 145-146. [DOI] [PubMed] [Google Scholar]
- 41.Lynch JR, Wang H, McGirt MJ, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 42.Neil-Dwyer G, Walter P, Cruickshank JM. Beta-blockade benefits patients following a subarachnoid hemorrhage. Eur J Clin Pharmacol. 1985;28(Suppl):25–29. doi: 10.1007/BF00543706. [DOI] [PubMed] [Google Scholar]
- 43.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 44.McGirt MJ, Lynch JR, Parra A, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 45.McGirt MJ, Pradilla G, Legnani FG, et al. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. 2006;58:945–951. doi: 10.1227/01.NEU.0000210262.67628.7E. discussion 945-951. [DOI] [PubMed] [Google Scholar]
- 46.Chello M, Patti G, Candura D, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–667. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]