Abstract
Prodynorphin - derived peptides elicit various pathological effects including neurological dysfunction and cell death. These actions are reduced by N-methyl-D-aspartate receptor (NMDAR) but not opioid receptor antagonists suggesting NMDAR - mediation. Here we show that a conserved epitope (KVNSEEEEEDA) of the NR1 subunit of the NMDAR binds dynorphin peptides (DYNp) non-covalently. Synthetic peptides containing this epitope form stable complexes with DYNp and prevent the potentiation of NMDAR - gated currents produced by DYNp. They attenuate DYNp - evoked cell death in spinal cord and prevent, as well as reverse, DYNp - induced paralysis and allodynia. The data reveal a novel mechanism whereby prodynorphin-derived peptides facilitate NMDAR function and produce neurotoxicity. Furthermore, they suggest that synthetic peptides that bind DYNp, thus preventing their interaction with NMDAR, may be novel therapeutic agents for the treatment of spinal cord injury.
Keywords: NMDA receptor, Dynorphin, neurotoxicity, paralysis, noncovalent interaction
Increased production of the opioid peptide, dynorphin A 1–17 (DYN), following trauma to the spinal cord is implicated in the pathophysiology of spinal cord injury, and in neuropathic pain1–8. Although intrathecal (i.t) infusion of low concentrations of DYN results in opioid-receptor mediated analgesia, higher concentrations produce allodynia (pain behaviour in response to innocuous stimuli), paralysis, and neuronal loss in the spinal cord of experimental animals4–7, 9–10. These actions are not opioid receptor mediated as they are not blocked by opioid receptor antagonists. Moreover, deleterious effects are retained by truncated peptides that lack the N-terminus tyrosine residue that is essential for opioid receptor binding8–10.
DYN and its major biotransformation product, DYN 2–17, bind to the NMDAR in vitro and can potentiate NMDA-evoked currents7,10,11. NMDAR antagonists reduce DYN-induced tactile allodynia, paralysis and cell death suggesting that NMDAR mediates the pronociceptive and neurotoxic actions of DYN. The mechanisms by which DYNp modulate NMDAR function are unknown.
We show that adjacent arginine6,7 (Arg/R) in the DYN molecule enables DYNp to bind noncovalently to a conserved acidic epitope of the NR1 subunit (594–599, EEEEED) via salt bridging12–15. Synthetic peptides (590–600) containing the NR1 epitope form stable complexes with DYN and prevent the potentiation of NMDA-mediated currents elicited by DYN and DYN 2–17 in-vitro. The peptides reduce cell death in spinal cord cultures and their i.t. infusion attenuates the tactile allodynia, flaccid paralysis and loss of spinal cord motoneurons elicited by DYN 2–17. However the really striking effects are: when the peptides are administered after DYN 2–17, paralysis and motor dysfunction are significantly reduced.
Material and Methods
Peptides
The NR1 epitopes KVNSEEEEEDA (590–600) [KVNE5DA], and MLYLLDRFSP FGRFKVNSEE EEEDALTLSS AMWFSWGVLL NSGIGEGAPRS (576–626) were synthesized by the Sequencing and Synthesis Laboratory at the Johns Hopkins School of Medicine. DYN 1–17 (YGGFLRRIRPKLKWNDQ) and 2–17 were purchased from Sigma.
Mass spectrometry
Spectra were acquired on a MALDI DE-Pro,12,14 a TOF_TOF 470015 and a Q-TOF13. The peptides mixtures were made of an equimolar amount of peptides, for a concentration of 1pmole/μl. A saturated solution of 6-Aza-2-Thiothymine in 50% ethanol, was used as matrix. Digestion studies using an equimolar mixture of KVNSE5DA, the 51 mer and DYN were conducted as previously described12.
NMR
NMR experiments were performed on a Bruker Avance 500 MHz spectrometer equipped with a cryoprobe and z-gradient. Peptides were measured alone, to determine their monomeric state. Measured diffusion coefficients were corrected for viscosity due to D2O contents. To examine the interactions between DYN and l-or d-peptide, increasing amounts of KVNSE5DA were added to a 200 μM solution of DYN and the diffusion coefficient of DYN measured. Diffusion data were used as fitting parameters in calculating dissociation constants16.
Electrophysiology
Injection and recording from Xenopus oocytes were conducted as described11,17,18. Oocytes were injected with mRNA encoding NR1 and NR2B or an equal volume of water and perfused with modified Barths medium (pH 7.5).
Spinal cord neuron cultures
Dorsal spinal cord neurons were isolated from embryonic day 14 ICR mouse fetuses and maintained in vitro as previously described8. Dorsal spinal cord neurons in these cultures have been characterized as neurofilament, PGP 9.5, and MAP2 immunoreactive, 19,20 and the vast majority co-express -opioid receptors and NR1 subunits8. Neurons were photographed before and for 72 h following addition of DYN 2–17, KVNSE5DA or their combination. Neuronal losses were characterized by destruction of the cell body as evidenced by nuclear pyknosis, as well as neuritic beading,fragmentation, and subsequent dissolution8.
Tactile Allodynia
Male Sprague Dawley rats (300–350 g) were anesthetized with Equithesin (3 ml/kg, i.p.) and implanted with an i.t catheter (PE-10 tubing) directed to the lumbar spinal subarachnoid space. Five days later the influence of co-infusion of KVNSE5DA (75 nmol; 10 μl) infusion upon changes in mechanical threshold produced by i.t infusion of DYN 2–17 (0; 5 nmol: 10 μl) was measured with Von Frey filaments. In parallel rats, motor performance on an accelerating rotor rod (Ugo Basile, Comerio, Italy) was tested 30 min and 1–21 days after drug injection21.
Hind-Limb Paralysis
We assessed paralysis induced by DYN 2–17 (75 nmol), KVNSE5DA (250 nmol) or their combination in rats at various times after i.t infusion using a 5 point rating scale (4 normal motor function; 3 paraparesis with ability to support weight and walk with some impairment; 2 paraparesis with ability to make walking movements without supporting weight; 1 severe paraparesis in which animals could make hind limb movements but could not walk; 0 flaccid paralysis with absence of hind limb movement).
Immunocytochemistry
Rats were perfused intracardially with 4 % paraformaldehyde 72 hrs after i.t infusion of vehicle, DYN 2–17 (75 nmol), KVNSE5DA (250 nmol) or their combination. Spinal cord tissue was removed, cryoprotected in 30 % sucrose, and 30 μm serial frozen horizontal sections through the lumbosacral cord immunostained with a rabbit anti- CGRP antiserum (1: 10,000; Bachem AG, Belmont, CA) followed by a Cy-3 conjugated goat anti-rabbit secondary (1:1000; Jackson Labs). After capturing images, sections were counterstained with Cresyl Violet for Nissl staining.
Statistic
Data are expressed as mean ± SE or as a percentage of control values. Electrophysiological data were analyzed using the Student’s t-test and tissue culture data using one– or two-way ANOVA followed by post-hoc comparisons. Tactile allodynia and rotarod performance were analyzed using a two –factor repeated measures ANOVA, and Bonferroni corrections, followed by post-hoc comparisons. Paralysis scores were analyzed using the non-parametric Kruskal Wallis test. The Fisher’s exact test was used to analyze motor neuron loss. For this analysis, spinal cords were scored as + or – for the presence of motor neuron loss. Sections were independently scored by two observers blind to treatment and whose results were completely concordant. P≤ 0.05 was considered significant.
Results
DYNp and NR1 form stable complexes via salt-bridging
Examination of the NMDAR revealed that the NR1 subunit contains five adjacent Glu and an Asp (594–599, EEEEED). This sequence is conserved in all species. Since adjacent guanidinium groups on Arg 6,7 interact with adjacent carboxyl groups of Asp or Glu12–15 resulting in formation of a powerful salt bridge, we hypothesized that the NR1 subunit epitope interacts similarly with DYN. Mass spectrometry confirmed this hypothesis. DYN or DYN 2–17 formed stable complexes with the NR1 epitope (590– 600, KVNSE5DA; Fig 1). Molecular ions [MH+] were seen at amu 1279.3 (KVNE5DA), 1985.3 (DYN 2–17), 2148.5 (DYN), as well as at amu 3263.6 and 3426.8 corresponding to a noncovalent complex of DYN 2–17 or DYN and the NR1 epitope KVNSE5DA.
Fig. 1.
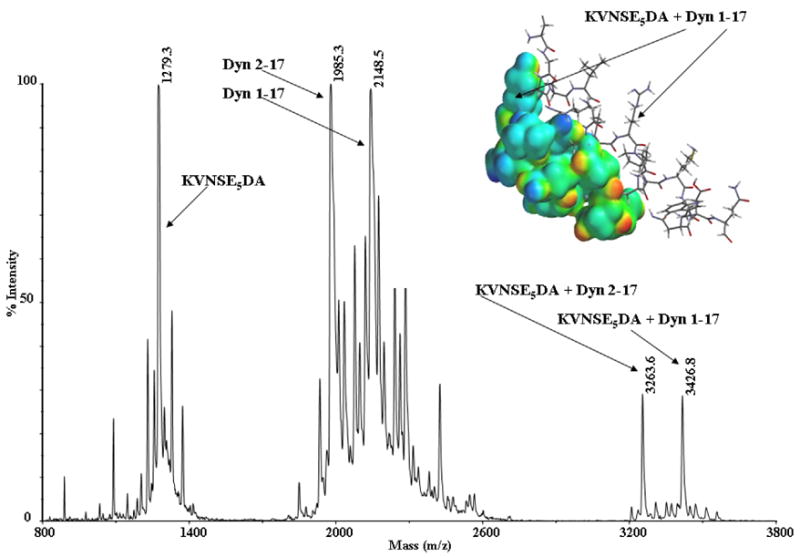
Positive ion mode MALDI Mass spectrum of a solution containing an equimolar mixture of DYN (1pmole), DYN 2–17 (1 pmole), and KVNSE5DA (1 pmole). Complexes of DYN and DYN 2–17 with KVNSE5DA are seen at amu 3426.8 and 3263.6. The insert shows a proposed model of the interaction of DYN and KVNSE5DA.
Shorter neurotoxic fragments8 of DYN (containing Arg 6,7) also formed salt bridges with the epitope. Fragments that lack Arg 6,7, and are not neurotoxic did not. A larger epitope of NR1 (570–621, MH+ 5742.5) containing KVNSE5DA also formed noncovalent complexes with DYN 2–17 and DYN at amu 7726.8 and 7890, again confirming complex formation. Complexes were observed after chymotryptic digestion of a solution containing an equimolar mixture of the 51 mer peptide (aa residues 570–621) of the NR1 epitope and DYN, indicating that once formed, complexes are stable and resistant to enzymatic attack (Table 1). The fragments forming complexes show that the DYN and NMDA residues likely to be involved in the interaction are RRIRPK and NSEEEEED respectively. In addition, collision induced dissociation by both MALDI and ESI15 of the noncovalent complex of KVNSE5DA and DYN did not result in fragmentation of the complex. Instead high fluency for MALDI and high collision voltage for ESI were needed to partially dissociate the complex.
Table 1.
Chymotryptic fragments generated from the digest of the complex formed by the 51 mer epitope of the NMDA NR1 and DYN
| Dyn aa residues + NR1 51mer aa residues | MW of Noncovalent Complex fragments | MH+ |
|---|---|---|
| 5-16 + 15-25 | 1594.9 + 1278.3 | 2874.2 |
| 6-17 + 15-25 | 1609.9 + 1278.3 | 2889.2 |
| 3-17 + 17-25 | 1927.3 + 1050.9 | 2978.2 |
| 5-17 + 15-25 | 1723.1 + 1278.3 | 3002.4 |
| 2-17 + 17-25 | 1984.3 + 1050.9 | 3036.2 |
| 2-17 + 16-24 | 1984.3 + 1079.0 | 3064.3 |
| 1-17 + 18-25 | 2147.5 + 936.8 | 3085.3 |
| 1-16 + 16-25 | 2019.4 + 1150.1 | 3170.5 |
| 1-17 + 17-25 | 2147.5 + 1079.0 | 3199.4 |
| 1-16 + 15-24 | 2019.4 + 1207.2 | 3227.6 |
| 1-17 + 15-24 | 2147.5 + 1207.2 | 3355.7 |
| 1-17 + 15-25 | 2147.5 + 1278.3 | 3426.8 |
| 5-17 + 16-29 | 1723.1 + 1564.6 | 3288.7 |
| 2-11 + 14-32 | 1199.5 + 2129.3 | 3329.8 |
| 5-17 + 8-21 | 1723.1 + 1672.8 | 3396.9 |
| 5-17 + 16-31 | 1723.1 + 1722.7 | 3446.8 |
| 5-17 + 12-26 | 1723.1 + 1751.8 | 3475.9 |
| 1-17 + 12-26 | 2147.5 + 1751.8 | 3900.3 |
| 1-17 + 14-38 | 2147.5 + 2892.2 | 5040.7 |
| 1-17 + 1-25 | 2147.5 + 3022.3 | 5170.8 |
To calculate the dissociation constant of the KVNSE5DA-DYN complex we performed NMR diffusion measurements. Diffusion coefficients measured at 100 μM and 200 μM concentrations were 2.14 ± 0.01 × 10−10 m2/s for DYN and 2.62 ± 0.02 × 10−10 m2/s for l-and d-KVNSE5DA. The values are in agreement with expected values for randomly coiled monomeric peptides of the same molecular weight16. Thus, at low concentrations the three peptides are found in aqueous solutions as randomly coiled monomers. NMR spectroscopy revealed a Kd of 0.25 ± 0.05 mM for DYN–l-KVNSE5DA and 2.4 ± 0.2 mM for DYN–d-KVNSE5DA indicating slow dissociation of the complexes. The diffusion coefficient for complexes of DYN and either l-or d-KVNSE5DA was 1.46 ± 0.03 10−10 m2/s.
KVNSE5DA prevents DYNp facilitation of NMDAR function
Voltage clamp recording methods were used to measure glutamate-evoked currents in Xenopus oocytes expressing NR1/NR2B receptors. In the presence of glycine, application of glutamate (50 μM) rapidly activated inward currents and this was prevented by the NMDAR channel blocker MK-801. As reported17, glutamate (50 μM) elicited little current in the absence of added glycine. However, addition of either 10 μM DYN (Fig 2A), DYN or DYN 2–17 (Fig 2B) to the bathing solution significantly increased the amplitude of glutamate-evoked currents. Co-application of increasing concentrations of the synthetic peptides, d- or l- KVNSE5DA, reduced the potentiation of glutamate currents evoked by DYNp in a concentration dependent manner (Fig 2C) whereas co-application of a scrambled sequence of KVNSE5DA, (EKEVENESEAD) did not. KVNSE5DA did not modify [3H] TCP binding to the NMDAR in cortical membranes indicating that KVNSE5DA does alter channel number or affinity.
Fig. 2.
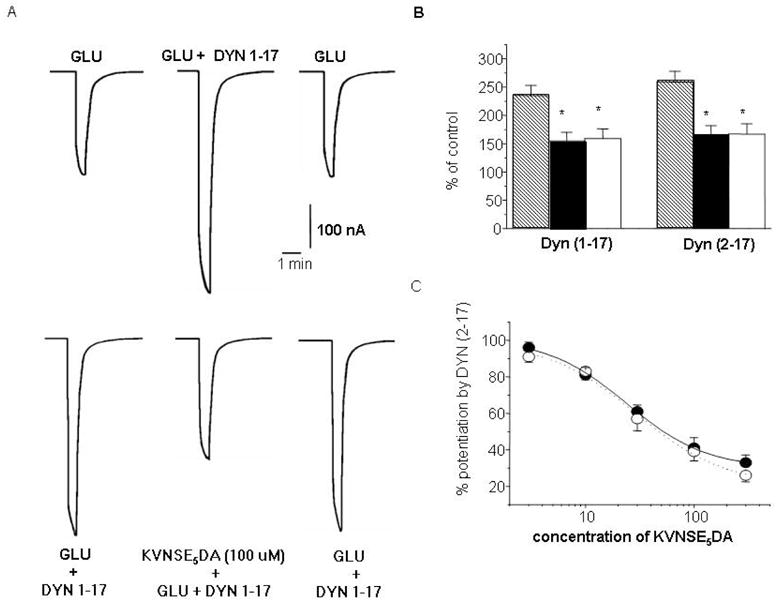
KVNSE5DA prevents the potentiation of glutamate activated currents produced by DYN and DYN 2–17 in Xenopus oocytes expressing NR1 and NR2B subunits. Potentiation of glutamate induced currents by DYN is reversibly reduced by co-application of d-KVNSE5DA (100μM) (A). Co-application of either d- (black symbols) or l-KVNSE5DA (white symbols) significantly inhibits the potentiation of glutamate activated currents induced by DYN or DYN 2-17. [* p≤ 0.05, n = 7–11; Student’s t-test] (B). The inhibitory effect of l- and d-KVNSE5DA upon DYN 2–17 is concentration-dependent (C).
KVNSE5DA exerts neuroprotective effects in-vitro and in-vivo
Increased DYN following spinal cord trauma is thought to contribute to neuronal death associated with secondary injury 18–20, 22–25. To determine whether KVNSE5DA prevents the cytotoxic actions of DYN 2–17, we isolated neurons from spinal cords of embryonic mice and used time-lapse photography to examine neuronal viability following exposure to DYN 2–17 alone and in combination with KVNSE5DA8,25. In these and subsequent experiments, we used DYN 2–17 rather than DYN to avoid confounds resulting from concurrent activation of spinal cord opioid receptors. Continuous exposure of cultures to DYN 2–17 (1–100 μM) induces profound neuronal loss, which is significantly attenuated by NMDAR antagonists but not opioid receptor antagonists8. KVNSE5DA (100 uM) prevented cell loss induced by 10 μM DYN 2–17 (p<0.05) and significantly reduced cell loss produced by 100 μM DYN 2–17 (p<0.05, Fig. 3A).
Fig. 3.
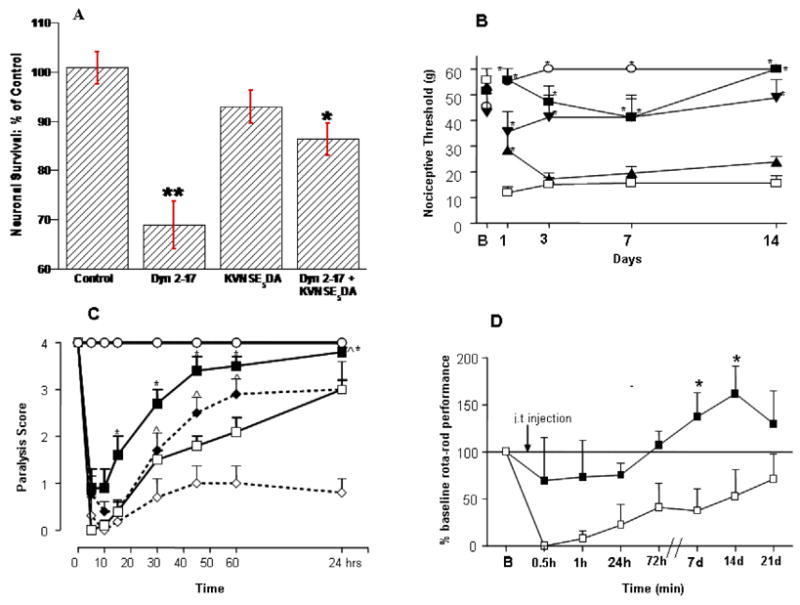
KVNSE5DA attenuates the neurotoxic actions of DYN 2–17-in the spinal cord (A). DYN 2–17 (100 μM) induced significant cell death in cultured spinal cord neurons at 72 h (p< 0.01, F (1,8)= 6.9, ANOVA; **p < 0.01 vs controls; Duncan test), which was significantly attenuated by co-administering an equimolar concentration of KVNSE5DA(*p < 0.01 vs. DYN 2–17; Duncan test).; KVNSE 5DA alone had no effect. i.t. infusion of DYN 2–17 (5 nmol) produces long-lasting tactile allodynia which is prevented by co- infusion of l-KVNSE5DA (p <0.01, F(4,135) = 15.8, ANOVA; * p < 0.05 relative to Veh+DYN 2–17, Student-Newman-Keuls test: SNK). ○ Veh (n=7), □ Veh + DYN 2-17 (n=6), ▲ l-KVNSE5DA 30 nmol + DYN 2–17 (n=5), ▼ l- KVNSE5DA 100 nmol + DYN 2–17, ■ l- KVNSE5DA 250 nmol + DYN 2–17 (n=8). (C) DYN 2–17 induced motor paralysis is reduced by co- or post-infusion of l- KVNSE5DA (250 nmol). l-KVNSE5DA (250 nmol) was infused with DYN 2–17 (75 nmol) or 5 min after DYN 2–17 (100 nmol) infusion (p≤ 0.01, *^ p< 0.05 relative to Veh + DYN 2–17; Kruskal-Wallis test). ○ Control (n=9), □ Veh + 75 nmol DYN 2–17 (n=21), ■ l- KVNSE5DA 250 nmol + 75 nmol DYN 2–17 (n=20). ⋄ DYN 100 nmol, ◆ l- KVNSE5DA 250 nmol + 100 nmol DYN (D) l-KVNSE5DA (250 nmol) also prevents the impairment of motor function (rotor-rod performance) that is apparent 21 days after DYN 2–17 (75 nmol) infusion (p< 0.01, F(1,89 ) = 9.7 , ANOVA; * p< 0.05, SNK), □ Vehicle + DYN 2–17 (n=5), ■ l-KVNSE5DA 250 nmol + l-KVNSE5DA (n=5).
The i.t infusion of DYN or DYN 2–17 induces long-lasting allodynia9,26,27. To determine whether KVNSE5DA protects spinal cord neurons in vivo, we examined whether its co- infusion prevents tactile allodynia elicited by 5 nmol DYN 2–17. We observed a significant and long lasting decrease in nociceptive withdrawal threshold in all rats that received DYN 2–17. However, when DYN 2–17 was co-administered with KVNSE5DA (250 nmol), nociceptive thresholds did not differ from controls (Fig. 3B).
Because higher doses of DYN or DYN 2–17 induce hind limb paralysis and loss of neuronal cell bodies in the lumbosacral spinal cord, we evaluated whether KVNSE5DA could prevent motor dysfunction produced by DYN 2–17. Rats received an i.t. injection of either DYN 2–17 (75 nmol), KVNSE5DA (250 nmol), or both. We assessed the presence or absence of hind-limb paralysis over 24 h using a 5-point scale27. All DYN 2–17 treated rats exhibited hind limb paralysis within 5 min of infusion. However when KVNSE5DA was administered with DYN 2–17 or 5 min after DYN 2–17, the magnitude and duration of paralysis was significantly reduced (Fig 3C; p<0.01). By contrast, co-administration of a scrambled sequence of KVNSE5DA did not affect paralysis produced by DYN 2–17. Similarly, when the Glu and Asp residues of KVNSE5DA were replaced by Ala, no alteration in DYN 2–17 induced paralysis was seen. In other rats we infused DYN 2–17 alone or with l-KVNSE5DA and assessed motor performance for 21 days using the rotating rod test21. Performance was significantly impaired in rats that had received DYN 2–17. However, no significant deficit occurred in animals that had received DYN 2–17 with KVNSE5DA (Fig 3D; p<0.05).
Motor dysfunction produced by DYN and DYN 2–17 is associated with a loss of motoneurons in the spinal cord18. To determine whether KVNSE5DA antagonizes this effect, we examined serial horizontal sections of the lumbar spinal cords of rats using CGRP immunohistochemistry as a selective marker of motoneurons28 and Nissl staining to monitor microglial infiltration. Fig. 4A shows that significant loss of motoneurons occurred in 9 of 12 rats, 72 h after DYN 2–17 infusion. Although i.t administered drugs are expected to be uniformly distributed, motoneuron loss was, in fact, patchy (Fig. 4A-C) precluding comparison of the magnitude of cell loss across animals. However, the protective effect of co-administered KVNSE5DA was readily apparent. Only 2 of 13 animals showed motoneuron loss (Fig. 4D). Furthermore, the reduction of motoneuron loss exactly paralleled attenuation of paralysis (Fig. 4D).
Fig. 4.
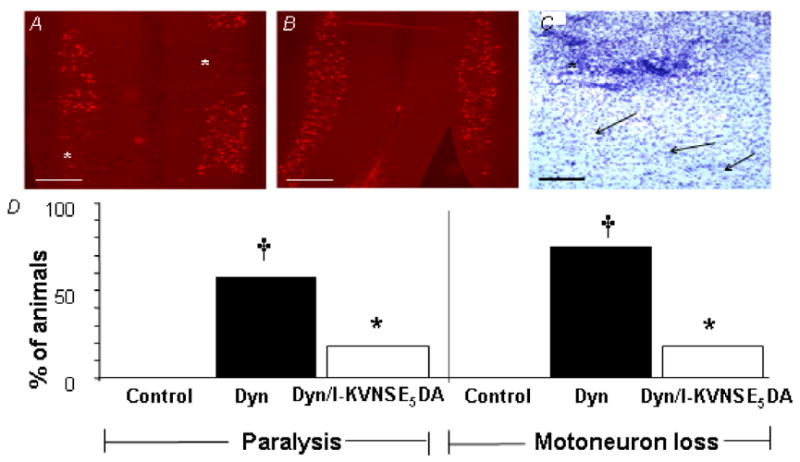
KVNSE5DA reduces DYN 2–17-induced paralysis and motor neuron loss. (A) CGRP immunostaining of motor neurons in horizontal section of DYN 2–17-treated rat illustrates patchy loss of motor neurons (*). (B) Co-administration of l-KVNSE5DA (250 nmol) significantly protected against motor neuron loss. (C) Cresyl Violet Nissl stain illustrates shows microglial invasion (*) into an area of DYN 2–17-treated tissue where there is profound motor neuron loss. This area is adjacent to intact ventral horn tissue, with normal complement of motor neurons (arrows). Scale bars a,b: 500μm; c:100μm. (D) Percentage of animals showing paralysis (score <3) or motor neuron loss, 72 h after infusion of vehicle(control; n=13), DYN 2–17 (DYN; n=12) or DYN + l-KVNSE5DA (DYN/ l-KVNSE5DAt; n=11) (†).
Discussion
DYN and other opioid peptides are potent analgesics by virtue of their N-terminal Tyr. High concentrations of DYN or N-truncated cleavage products of DYN, which lack the opioid address sequence are excitotoxic producing neurological dysfunction and neuronal loss1–9. Although increased DYN expression and a resulting enhancement of excitatory amino acid transmission have been implicated in the pathophysiology of spinal cord injury and nerve injury-induced neuropathic pain2–9, the mechanisms underlying the neurotoxic effects of DYN have not been delineated.
Our studies demonstrate that DYN and DYN 2–17 bind noncovalently to a linear conserved acidic region of the NR1 subunit via salt bridging [a Coulombic interaction] 12–15, 29,30. They further show that the Coulombic interaction of DYN with a short linear epitope (5 Glu and 1 Asp residues) of the NR1 that is contained within the much larger NMDAR complex is sufficient to facilitate NMDAR function and neurotoxicity. Although the critical role of such a short epitope in modulating protein function is at first glance surprising, recent studies have shown that short stretches of oppositely charged residues lead to receptor heteromerization15,29,30.
Previous work has shown that DYN and its major biotransformation product DYN 2–17 potentiate NMDA-mediated cationic currents11. Our in-vitro studies demonstrate that co-application of KVNSE5DA with either of these prodynorphin derived peptide prevents this potentiation. KVNSE5DA was also effective in reducing DYN 2–17 evoked cell death in spinal cord cultures. Importantly, the protective effects of this decoy peptide were also observed in-vivo. The i.t. infusion of KVNSE5DA prevented the tactile allodynia and motor impairment produced by i.t. infusion of DYN 2–17. It also reduced the hindlimb paralysis and motoneuron loss in the spinal cord produced by infusion of a higher concentration of DYN 2–17, and reduced motoneuron loss in the spinal cord. Only peptides that contained the sequence of cognate amino acids corresponding to the NR1 epitope bound DYN and DYN 2–17 and prevented the pathological actions of DYN 2–17. Scrambled peptides or peptides where the Glu and Asp residues were replaced with Ala did not. We hypothesize that salt bridge formation12–15,29,30 between adjacent Arg 6–7 in DYN and adjacent Glu of the NR1 epitope results in sequestration of toxic C-terminal peptides derived from exogenously or endogenously released DYN and that this mechanism underlies the neuroprotective effects of KVNSE5DA.
Although our data are consistent with the hypothesis that the pathological actions of PDYNp result, in part, from their ability to bind to the NR1 subunit non-covalently, other targets must also be considered. DYNp stimulate glutamate release in the spinal cord and this action may contribute to their neurotoxicity. Furthermore, the NR1 subunit is widely expressed in spinal cord, yet neuronal loss induced by the i.t. infusion of DYN 2–17 is most pronounced in motoneurons and exhibits a patchy distribution. This pattern of loss cannot be attributed to inadequate tissue penetration by DYN because neurons in spinal cord segments rostral and caudal to the catheter tip were affected, while adjacent neurons appeared intact. Regional differences in the coupling of NR1 to the NR2A-D or NR3 subunits may confer enhanced susceptibility to the neurotoxic actions of DYN and DYN 2–176,8,10. Since, other membrane proteins possess stretches of adjacent acidic amino acids similar to the NR1 subunit, DYN sequestration by KVNSE5DA may also prevent targeting of DYN to these proteins.
In summary, synthetic peptides that bind DYN and DYN 2–17 noncovalently, and prevent their interaction with the NR1 subunit, are neuroprotective. These peptides attenuate the neurotoxic effects of DYN 2–17 in the spinal cord and improve motor function even when administered after DYN 2–17. In view of the documented involvement of DYN systems in brain and spinal cord injury as well as in persistent pain, the present findings suggest that pharmaceuticals based on the structure of these peptides may be protective in various neuropathological states. Clearly, additional studies are needed to define the structure-activity-relationships of such “decoy” peptides, dosing regimens that produce maximal protection when administered post-insult and their potency following systemic (e.g. intranasal application). Their ability to reverse paralysis produced by exogenous DYN 2–17 and to improve motor function indicates that such studies are warranted. By reducing the deleterious consequences resulting from injury-induced increases in prodynorphin derived peptides31, KVNSE5DA may afford neuroprotection and pain relief without the spectrum of side effects common to NMDAR antagonists.
Acknowledgments
We gratefully acknowledge Jody Franklin (Johns Hopkins School of Medicine) for peptide synthesis, Dr. I. Singh, and S. Chen for technical assistance, Dr. Y. Haysahi (MIT) for providing the NR1 and NR2B plasmids, and ONDCP for instrument grants. This research was funded by the NIH/NIDA IRP.
Footnotes
Animal Welfare: All protocols were approved by the NIH/NIDA Animal Care and Use Committee.
References
- 1.Caudle RM, Manes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 2.Hauser KF, Aldrich JV, Anderson K, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of Dynorphin in Trauma and Disease. Front Biosci. 2005;10:216–35. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- 4.Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985;232:27–32. [PubMed] [Google Scholar]
- 5.Caudle RM, Isaaac L. Intrathecal dynorphin 1–13 results in an irreversible loss of the tail flick reflex in rats. Brain Res. 1987;435:1–6. doi: 10.1016/0006-8993(87)91579-4. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi R, Faden AI. Competitive and non-competitive, NMDA antagonists limit dynorphin A induced rat hindlimb paralysis. Brain Res. 1990;507:1–5. doi: 10.1016/0006-8993(90)90512-a. [DOI] [PubMed] [Google Scholar]
- 7.Caudle RM, Isaac L. A novel interaction between dynorphin 1–13 and an N-methyl-D-aspartate site. Brain Res. 1988;443:329–332. doi: 10.1016/0006-8993(88)91628-9. [DOI] [PubMed] [Google Scholar]
- 8.Hauser KF, Knapp PE, Turbek CS. Structure-activity analysis of dynorphin A toxicity in spinal cord neurons: intrinsic neurotoxicity of dynorphin A and its carboxyl-terminal, nonopioid metabolites. Exp Neurol. 2001;168:78–87. doi: 10.1006/exnr.2000.7580. [DOI] [PubMed] [Google Scholar]
- 9.Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP, Jr, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- 10.Shukla VK, Prasad JA, Lemaire S. Nonopioid motor effects of dynorphin A and related peptides: structure dependence and role of the N-methyl-D- aspartate receptor. J Pharmacol Exp Ther. 1997;283:604–610. [PubMed] [Google Scholar]
- 11.Zhang L, Peoples RW, Oz M, Harvey-White J, Weight FF, Brauneis U. Potentiation of NMDA mediated responses by dynorphin at low extracellular glycine concentrations. J Neurophysiol. 1997;78:582–90. doi: 10.1152/jn.1997.78.2.582. [DOI] [PubMed] [Google Scholar]
- 12.Woods AS, Huestis MA. A study of peptide-peptide interaction by MALDI. JASMS. 2001;12:88–96. doi: 10.1016/S1044-0305(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 13.Woods AS, Koomen J, Ruotolo B, Gillig KJ, Russell DH, Fuhrer K, Gonin M, Egan T, Schultz JA. A study of peptide-peptide interactions using MALDI ion-mobility o-TOF and ESI-TOF mass spectrometry. JASMS. 2002;13:166–169. doi: 10.1016/S1044-0305(01)00348-8. [DOI] [PubMed] [Google Scholar]
- 14.Woods AS. The mighty arginine, the stable quaternary amines, the powerful aromatics and the aggressive phosphate: their role in the noncovalent minuet. J of Proteome Research. 2004;3:478–484. doi: 10.1021/pr034091l. [DOI] [PubMed] [Google Scholar]
- 15.Woods AS, Ferre S. The amazing stability of the arginine-phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielsson J, Jarvet J, Damberg P, Gräslund A. The Alzheimer beta-peptide shows temperature-dependent transitions between left-handed 3-helix, beta-strand and random coil secondary structures. Biochemistry. 2004;43:6261. doi: 10.1111/j.1742-4658.2005.04812.x. [DOI] [PubMed] [Google Scholar]
- 17.Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 4580;17:90. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long JB, Petras JM, Mobley WC, Holaday JW. Neurological dysfunction after intrathecal injection of dynorphin A (1–13) in the rat. II Nonopioid mechanisms mediate loss of motor, sensory and autonomic function. J Pharmacol Exp Ther. 1988;246:1167–74. [PubMed] [Google Scholar]
- 19.Faden AI, Molineaux CJ, Rosenberger JG, Jacobs TP, Cox BM. Endogenous opioid immunoreactivity in rat spinal cord following traumatic injury. Ann Neurol. 1985;17:386–390. doi: 10.1002/ana.410170414. [DOI] [PubMed] [Google Scholar]
- 20.Hauser KF, Foldes JK, Turbek CS. Dynorphin A (1–13) neurotoxicity in vitro: Opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp Neurol. 1999;160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 22.Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh TK, Head VA, Faden AI. Alterations in regional concentrations of endogenous opioids following traumatic brain injury in the cat. Brain Res. 1987;425:225–233. doi: 10.1016/0006-8993(87)90505-1. [DOI] [PubMed] [Google Scholar]
- 24.Tachibana T, Miki K, Fukuoka T, Arakawa A, Taniguchi M, Maruo S, Noguchi K. Dynorphin mRNA expression in dorsal horn neurons after traumatic spinal cord injury: temporal and spatial analysis using in situ hybridization. J Neurotrauma. 1998;15:485–494. doi: 10.1089/neu.1998.15.485. [DOI] [PubMed] [Google Scholar]
- 25.Hauser KF, Foldes JK, Turbek CS. Dynorphin A (1–13) neurotoxicity in vitro: Opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp Neurol. 1999;160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin. J Pharmacol Exp Ther. 2001;299:6–11. [PubMed] [Google Scholar]
- 27.Long JB, Rigamonti DD, deCosta B, Rice KC, Martinez-Arizala A. Dynorphin A-induced rat hindlimb paralysis and spinal cord injury is not altered by the k opioid antagonist nor-binaltorphimine. Brain Res. 1989;497:155–162. doi: 10.1016/0006-8993(89)90982-7. [DOI] [PubMed] [Google Scholar]
- 28.Piehl F, Arvidsson U, Hokfelt T, Cullheim S. Calcitonin gene-related peptide-like immunoreactivity in motoneuron pools innervating different hind limb muscles in the rat. Exp Brain Res. 1993;96:291–303. doi: 10.1007/BF00227109. [DOI] [PubMed] [Google Scholar]
- 29.Woods AS, Ciruela F, Fuxe K, Agnati LF, Lluis C, Franco R, Ferré S. The role of electrostatic interaction in receptor-receptor heteromerization. J of Mol Neurosci. 2005;26:125–132. doi: 10.1385/JMN:26:2-3:125. [DOI] [PubMed] [Google Scholar]
- 30.Jackson SN, Wang HY, Yergey A, Woods AS. Phosphate stabilization of intermolecular interactions. J Proteome Res. 2006;5:122–126. doi: 10.1021/pr0503578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tachibana T, Miki K, Fukuoka T, Arakawa A, Taniguchi M, Maruo S, Noguchi K. Dynorphin mRNA expression in dorsal horn neurons after traumatic spinal cord injury: temporal and spatial analysis using in situ hybridization. J Neurotrauma. 1998;15:485–49. doi: 10.1089/neu.1998.15.485. [DOI] [PubMed] [Google Scholar]


