Abstract
We have previously shown that intranigral transplants of immortalized GABAergic cells decrease the number of kainic acid-induced seizures [5] in an animal model. In the present study, recurrent spontaneous behavioral seizures were established by repeated systemic injections of this excitotoxin into male Sprague Dawley rats. After the seizures had been established, cells were transplanted into the substantia nigra. Animals with transplants of control cells (without hGAD67 expression) or with sham transplants showed a death rate of more than 40% over the 12 weeks of observation, whereas in animals with M213-2O Cl4 transplants, the death rate was reduced to less than 20%. The M213-2O Cl4 transplants significantly reduced the percentage of animals showing behavioral seizures; animals with these transplants also showed a lower occurrence of stage V seizures than animals in the other groups. In vivo and in vitro analyses provided evidence that the GABAergic cells show sustained expression of both GAD67 and hGAD67cDNA, as well as increased GABA levels in the ventral mesencephalon of transplanted animals. Therefore, transplantation of GABA-producing cells can produce long-term alleviation of behavioral seizures in an animal model.
Keywords: Neural transplantation, human glutamate descarboxylase-67 (GAD67), gamma-aminobutyric acid (GABA), temporal lobe epilepsy, animal models, substantia nigra pars reticulata, gabaergic cell line, kainic acid, high performance liquid chromatography, status epilepticus
INTRODUCTION
The earliest studies demonstrating functional effects of fetal tissue transplants employed animal models of Parkinson’s disease [4, 14, 35] and diabetes insipidus [15] and involved transplantation of fetal tissue known to produce neurochemicals that can alleviate these conditions, i.e. dopamine or vasopressin. It has reasonably been presumed that the influence of intracerebral transplants would be spatially limited, and these disorders were targeted because of their known localized pathophysiology. Other disorders, for which the underlying defect is similarly localized, might also be good candidates for neural transplantation-based therapeutic approaches.
Of all disorders that might conceivably be treated by neural transplantation, epilepsy would seem to be ideal: some forms of epilepsy are focal in nature, and in many cases epilepsy is not adequately controlled by drugs. In animal models, generalized or kindled seizures can be suppressed by local injections of GABAergic drugs into the substantia nigra or pyriform cortex [21, 27]. Thus, it seems reasonable that local delivery of an inhibitory neurotransmitter, e.g. GABA, by transplanted cells might be used to reduce excess neuronal excitability in brain regions from which seizures are generated.
The principle of employing localized release of GABA to inhibit seizures was demonstrated in a study by Kokaia and coworkers [28] through the use of implanted GABA-releasing polymers. These polymers produced a localized release of GABA into the brain and suppressed seizures, at least for the few days during which high-level GABA release was sustained. This study suggests that fairly high concentrations of GABA released non-synaptically would be required to obtain functional effects in epilepsy, perhaps in order to obtain high local concentrations comparable to those achieved at synapses [7]. Nevertheless, there has been little progress in developing a transplantation-based therapeutic approach for the treatment of epilepsy, since neural transplantation has not been demonstrated to produce long-term suppression of established seizures in animal models.
Many studies have shown that grafts of fetal noradrenergic, cholinergic, and GABAergic neurons and cells can suppress subsequently induced seizures, in some cases with considerable intervals between the transplantation and seizure induction [1, 3, 12, 13, 29, 30]. Another group of studies of transplantation of fetal brain regions containing GABAergic or noradrenergic neurons indicated only minor effects on established seizures [25, 26, 40, 44], or a short lasting effect [31]. One recent study has shown that intrahippocampal grafts of hippocampal fetal cells treated with neurotrophic factors and a capase inhibitor survive robustly and reduce the frequency of spontaneous motor seizures measured two-months after grafting [37].
Lately, attention has turned to the possibility of employing cell lines engineered to produce high levels of GABA in epilepsy models. For example, Gernert and coworkers [17] found that transplantion of cells modified to express the GABA-synthesizing enzyme GAD65, elevated initial seizure thresholds. Seizure thresholds subsequent to kindling were not significantly elevated, although the transplanted cells continued to express GAD65 at least for the duration of the experiment, i.e. three weeks. Ross and coworkers [38] found that cells engineered to express human GAD67 decreased audiogenic seizures for two weeks. Thompson [42] found that transplantation of cells engineered to express GAD65 into the dentate gyrus increased the threshold and reduced the duration of the after discharge induced by granule cell stimulation and also slowed the appearance of stage V seizures after stimulation of the entorhinal cortex. Using transplants of the same cell line into the substantia nigra, Thompson and Suchomelova [43] were able to produce a short-term (one week) suppression of previously established seizures. Castillo et al. [5] also demonstrated an effect of cells expressing human GAD67 on seizures induced subsequently by kainic acid-induced kindling. None of these studies using engineered cell lines have demonstrated a long-term seizure-suppressant effect.
To develop cells for transplantation in epilepsy, we have taken the approach of first, employing immortalized cells which exhibit some properties of GABAergic cells, similar to the primary cells employed by Löscher and coworkers [31], on the theory that such cells would have the inherent machinery to produce and release GABA [18, 19]. Second, GABA production was enhanced by introducing the GAD67 gene, the presumed non-synaptic GAD isoform, using an episomal Epstein-Barr virus based plasmid. These cells were found to produce relatively high levels of GABA [8] and to suppress audiogenic seizures on a short-term basis [38] and kainic acid-induced seizures eight weeks after transplantation [5]. In this study we sought to determine whether grafts of M213-2O/Cl4 cells could suppress established kainic acid-induced behavioral seizures on a long-term basis.
The kainic acid (KA) animal model of epilepsy produces spontaneous seizures that do not disappear over time. Systemic administration of repeated, low doses of KA, results in a pattern of hippocampal degeneration similar to that observed after a single, high dose of kainate, with improved survival, and increased percentage of animals showing spontaneous seizures [20, 23, 24, 33]. After KA administration, during the acute phase that may last up to 10 hours, the animal presents immobility, facial clonus, sniffing, wet dog shakes, and stage III to V seizures (rated according to Racine’s scale), all leading to status epilepticus (SE). Then, there is an active phase that lasts 10–30 days, during which seizures of brief duration are observed. This phase is followed by a latent phase of 30–90 days, during which no seizures occur. It is during this phase that synaptical reorganization takes place, as evidenced by neurogenesis, synaptogenesis, and up-regulation of transcription factors, among other significant cellular and molecular changes. Finally, there is the chronic phase that lasts from 90 days to several months. Seizures similar to those observed during the acute phase reappear, and they increase in frequency and duration [2, 24, 32].
The aims of the present study were: first, to characterize the effects of the systemic administration of low doses of kainate by evaluating the occurrence of behavioral seizures during the light/dark cycle and observing the duration of the seizures over several months; second, to evaluate, over a three-month period, the effect of intranigral transplants of a GABAergic cell line on already established motor seizures induced by systemic kainate administration; and third, to evaluate the changes in GABA content of cells grown in vitro, and in tissue homogenates obtained from the area of the transplant.
MATERIAL AND METHODS
In vitro experiments
Cell cultures
Briefly, cell lines were cultured in DMEM-F12 (1:1 Gibco, Invitrogen Life Technologies, Carlsbad, CA, U.S.A) with 10% fetal calf serum (FCS, Gibco) and 1% penicillin-streptomycin (Gibco) in 75-cm2 culture flasks at 33°C and 5% CO2. Only M213-2O GAD 67 (M213-2O CL-4) cells were grown in selection medium containing Hygromycin B (200 μg/μl) (Sigma-Aldrich, St. Louis, MO, U.S.A). Some cultures were used to verify the expression of various proteins, glutamate decarboxylase-67 (mouse anti GAD67, Chemicon MAB5401; 1:1000, 1:3000; Chemicon, Temecula, CA, U.S.A.), glial acidic fibrillary protein (rabbit anti GFAP, DAKO Z0334; 1: 1000, 1:3000; DAKO, Hamburg, Germany), doublecortin (guinea pig anti doublecortin Chemicon AB5910; 1: 1000, 1:3000), and β-tubulin (mouse anti beta tubulin E7 Developmental Studies Hybridoma Bank, University of Iowa1; 1:1000, 1:10,000). The secondary antibodies (1:200) were either a Cy™3-conjugated F(ab’)2 fragment of goat anti-mouse IgG (H+L) for GAD67 (Jackson ImmunoResearch, West Grove, PA, U.S.A.); a goat anti-rabbit IgG (H+L) FITC conjugate for GFAP (Zymed, Carlsbad, CA, U.S.A.); a Cy™2-conjugated Affini-Pure goat anti-guinea pig IgG (H+L) for doublecortin (Jackson ImmunoResearch), or a goat anti-mouse IgG FITC conjugate for beta tubulin (Zymed). Briefly, culture medium was aspirated, cells were incubated in 4% paraformaldehyde for 10 min, washed (3×) with phosphate buffered saline (PBS, 0.1 M, pH 7.4), permeabilized with EtOH:acetic acid (95:5 v/v), washed (3x) with PBS, incubated in 10% horse serum (10–40 min), incubated with the first antibody overnight, washed with PBS (3x), and incubated in the secondary antibody for 120 min. The coverslips with the cells attached were carefully retrieved and mounted onto glass slides using a Vectashield/DABCO Mounting medium (Vector Laboratories).
GABA determination by high performance liquid chromatography of cells grown in vitro. In order to test if cells responded to a depolarizing solution, the M213-2O CL-4 cell line and the control cell line without the hGAD67 transgene, M213-2O (CONTROL) were cultured in 6-well plates until they reached 90% confluence. Media was then aspirated, and cells were washed with sterile saline solution (NaCl 0.9%); 500 μl of a buffer solution (1.0 mM MgCl2; 1.8 mM CaCl2; 3.0 mM NaH2PO4; 140 mM NaCl; 10 mM HEPES: 5 mM glucose, pH 7.4) was added and retrieved after 5 min in order to obtain basal GABA levels; then a similar buffer solution except that it contained 50 mM KCl and 94 mM NaCl was added and retrieved after 15, 30, or 45 minutes. The samples were stored at −70°C until chromatographic analysis. Cells from each well were resuspended in saline, and an aliquot was used to obtain the total cell count and percent viability using the trypan blue dye-exclusion test. The remaining cells were stored at −20°C for total protein determination with the Bradford method.
To determine total GABA content for each cell line, cells were cultured in 25-cm2 flasks. When they reached 90% confluence, 1 ml of 35% sulfosalicilic acid was added. After 20 minutes, cells were separated with a cell sorter, placed into tubes, and stored overnight at 4°C. After centrifuging 5 min at 3,000 rpm (850 g, Sorvall MC-12V, Thermo Scientific, Waltham MA, U.S.A.) the supernatant was obtained and analyzed by HPLC; the pellet was used for protein determination.
GABA was quantified by high performance liquid chromatography (HPLC); briefly, samples were pre-derivatized with O-phthalaldehyde (5 mg OPA in 625 μl methanol, 5.6 ml 0.4 M borate buffer, 25 μl 2-mercaptoethanol, pH 9.5) and separated by means of HPLC using a C18 reverse-phase column (ESA HR 80 column, ESA, Inc., Chelmsford, MA, U.S.A.) with electrochemical detection (Coulochem III, ESA, Inc.). A guard cell (Model 5020, ESA, Inc.) and a coulometric analytical cell (Model 5010, ESA, Inc.) were employed with a 1100 Series chromatograph (Agilent Technlologies Waldbronn, Germany) provided with a 20-μl manual injector (Model 7725i, Rheodyne, Rohnert Park, CA, U.S.A.). The mobile phases were a 50 mM sodium phosphate buffer containing methanol (15 % solution A; 35% solution B; pH 6.7) the elution was performed at room temperature by means of a linear gradient from 0% to 100% solution B (15% to 35% methanol) in 20 min, at a flow rate of 1.4 ml/min. An external standard was used to construct a calibration curve for GABA; the concentration obtained from each sample was expressed in μmol per g of protein.
In vivo experiments
Animals
Male Sprague-Dawley rats from the local animal facility were kept under a normal 12-hour/12-hr light/dark cycle (lights on at 8:00 AM) in individual cages with free access to food and water. Experiments were carried out according to the Norma Oficial Mexicana de la Secretaría de Agricultura (SAGARPA NOM-062-ZOO-1999) which complies with the guidelines in the Institutional Animal Care and Use Committee Guidebook (NIH publication 80-23. Bethesda, MD, USA, 1996) and were approved by the local Committee on Bioethics. Weight of the animals was recorded weekly; animal appearance and sensorimotor function were evaluated at the beginning and at the end of the experiments, as described in Castillo et al. [6]. Briefly the response of the animal to various sensory stimuli, muscle tone, posture, and general appearance were evaluated.
Experiment 1: Effect of KA treatment (see Figure 4 for experimental design)
Figure 4.
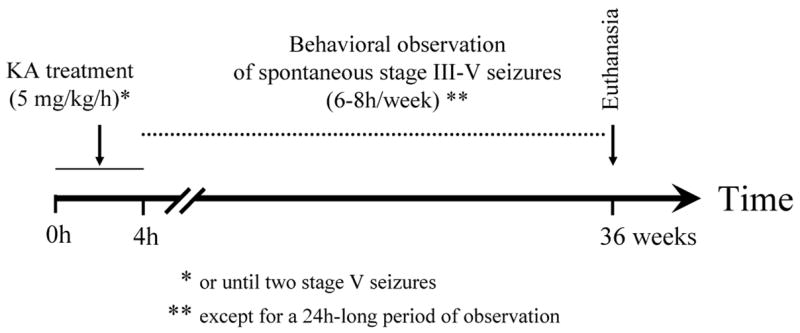
Experiment 1. Effect of KA treatment. Diagram showing the experimental design used to characterize the effects of the systemic administration of low doses of kainate by evaluating the occurrence of behavioral seizures during the light/dark cycle and observing the duration of the seizures over several months.
Kainic Acid Treatment
Rats (n = 51) received ip injections of KA (5 mg/kg/h) dissolved in physiological saline, over the course of 4 h or until two, stage V seizures were presented by the animal (the maximum amount of KA any animal received was 20 mg/kg). If animals showed hyperactivity or if there were more than 10, stage-IV or V seizures, no more KA was injected. Intact animals (n = 15) received vehicle only. This method has been shown to reliably induce spontaneous motor seizures after a latent period [24].
Behavioral rating of seizures
Based on Racine’s observations [36], seizures were defined as follows: stage III when forelimb clonus was observed, stage IV when rearing and forelimb clonus were observed, stage V when animals showed characteristics of the previous stages and also fell over. After the acute phase, behavior was monitored for 1–2 h periods totaling 6–8 h/week, and the incidence of stage III, IV, and V seizures was quantified. In order to determine when during the cycle to carry out observations, a 24-h observation period was scheduled. Based on the results obtained and those of others [23], subsequent observations were done during the light-phase of the cycle.
Experiment 2: Intranigral transplants in KA-treated animals (see Figure 6 for experimental design)
Figure 6.
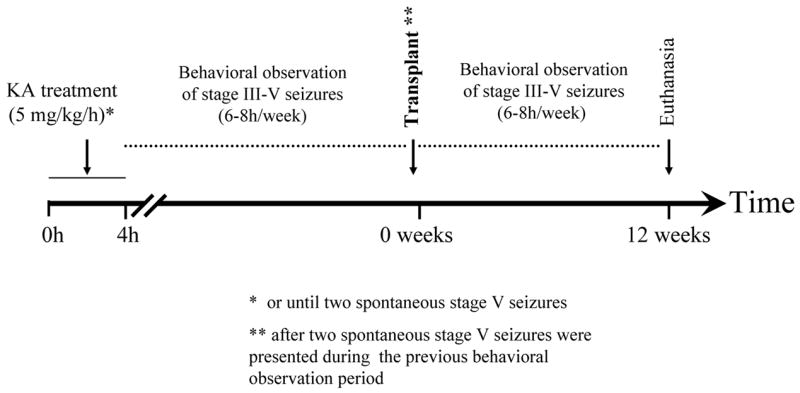
Experiment 2. Intranigral transplants in KA-treated animals. Diagram showing the experimental design used to evaluate, over a three-month period, the effect of intranigral transplants of a GABAergic cell line on already established motor seizures induced by systemic kainate administration. In order to receive a transplant, animals had to show two spontaneous stage V seizures. The latency in weeks to reach this criterion was variable, with a median of 24, and a quartile range of 14. After transplantation, animals were observed for twelve weeks. In addition to rating of seizures, behavioral observations included recording of spontaneous locomotor activity.
Kainic Acid Treatment
Rats (n = 147) received ip injections of KA (5 mg/kg/h) dissolved in physiological saline, over the course of 4 h or until two, stage-V seizures were presented by the animal. Behavioral ratings followed the same criteria as indicated above for experiment 1. Briefly, the incidence of stage III, IV, and V seizures was quantified during the light phase of the daily cycle for 6–8 h/week.
Transplants
Since bisbenzimide (Hoechst 33342) was used as a marker for transplanted cells, it was important to verify that it did not leak and was internalized by the surrounding tissue after transplantation. Therefore, in a preliminary experiment three different concentrations (5, 10, and 15 μM) of the marker (2 μl; 0.5 μl/min) were injected directly into the brain (striatum and substantia nigra), or used to label cells (400, 000 cells/2 μl) that were either intact or lysed before transplantation; at least three animals per condition were used. Seven or fifteen days after transplantation, the animals were sacrificed by decapitation, the brain was sectioned in a cryostat (40 μm), and sections were observed under a fluorescence microscope. At seven days, fluorescent nuclei were evident in the striatum and substantia nigra of animals receiving transplants of intact cells; a halo of fluorescence and some labeled nuclei were observed after direct injection of the marker or after transplantation of lysed cells labeled with the higher concentrations (10 and 15 μM) of the marker. By fifteen days, fluorescence was present only on the sections of animals receiving transplants of intact cells at all concentrations of the marker. Thus, at this time-point, fluorescent nuclei labeled by direct injection of bisbenzimide or by transplantation of lysed cells had disappeared; only the nuclei of intact and labeled cells were visible. This finding indicates that, at the concentration used in the present experiment (5 μM) and at fifteen days after administration, there was no evidence that bisbenzimide had leaked from the transplanted lysed cells and had been internalized by the surrounding host cells, since only nuclei from transplanted intact cells were fluorescent.
In order to receive intranigral transplants, KA-treated animals had to show two spontaneous, stage-V seizures (n = 71). Care was taken to ensure that all animals had an equal chance of being selected for any of the subgroups, so that all subgroups had a similar distribution in terms of latency to reach criterion (two spontaneous, stage-V seizures). Once they reached criterion, animals were assigned to one of these subgroups, (1) SHAM, which received only vehicle; (2) M213-2O CL-4, which received transplants of the GABAergic cell line with the hGAD67 cDNA; (3) CONTROL, which received intranigral transplants of a control cell line, of similar origin but without the hGAD67 cDNA. Care was taken to ensure that all animals had an equal chance of being selected for any of these subgroups, regardless of their latency to reach criterion (two spontaneous, stage-V seizures), so that each subgroup had a similar distribution in terms of latency.
On the day of transplantation, cells were harvested with trypsin-EDTA (Gibco), pelleted, and incubated in 5 μM bisbenzimide for 30 min, washed three times, pelleted, and resuspended in DMEM-F12 without serum. The cell suspension was maintained on ice until transplantation, and an aliquot of it was used to determine cell viability (95–98%) with the trypan blue dye-exclusion essay. Previous studies [5] have determined that at this concentration bisbenzimide has no effect on cell viability measured 30 min or 4 weeks after incubation. Before surgery rats were anesthetized with ketamine (70 mg/kg) and xylazine (6 mg/kg) and mounted on a stereotaxic frame. Two trephine holes were made with a dental burr in the skull at these coordinates: AP −5.8, ML ±2.0 for Bregma and DV −7.2 for the dura [34]. Bilateral transplantation of 40, 000 bisbenzimide-labeled cells/3 μl into the substantia nigra, pars reticulata was made using a Hamilton syringe (10 μl). Cells are slowly delivered using a microinjection unit (David Kopf Instruments, Tujunga, CA, U.S.A.); the syringe was kept in place for 2 additional minutes and then slowly retracted.
Behavioral rating of seizures after transplantation
Behavioral ratings followed the same criteria as indicated above for experiment 1. The incidence of stage III, IV, and V seizures, defined according to Racine’s observations [38], was quantified during the light phase of the daily cycle for 6–8 h/week, totaling 24–32 h/month. Between transplantation and euthanasia, each animal was observed 72–96 h.
Spontaneous locomotor activity
To evaluate the general condition of the animals, nocturnal activity (12 h) was monitored using an automated system (Digiscan Animal Activity Monitors, Accuscan Inc., Columbus OH, U.S.A.). Animals were placed in an acrylic box (40 × 40 × 30 cm) with access to food and water for 12 hours during the dark phase of the light/dark cycle. The locomotor variables analyzed were total distance traveled and number of vertical movements per hour (rearing).
Histology
At the end of the experiments animals were anesthetized with an overdose of sodium pentobarbital (≈50 mg/kg) and intracardially perfused with physiological saline followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). The brain was removed and kept in paraformaldehyde for 24 h, and then transferred to a 35% sucrose solution at 4°C until sectioning. Forty-μm coronal sections were obtained using a freezing microtome, and they were observed under a fluorescence microscope (UV fluorescence excitation/emission 350/461 nm). The same sections were then stained with cresyl violet. Serial sections were used for immunocytochemistry or immunofluorescence with anti-GAD67, anti-GFAP, anti-doublecortin, and anti-beta tubulin. Antibodies used were the same as those described for the in vitro experiments. Briefly, free-floating slices were incubated overnight at 4°C with the primary antibodies diluted in PBS with 2% non-specific serum. The sections were rinsed four times in PBS for a total time of 60 min, then incubated for 120 min with the secondary antibody in PBS, and mounted onto gelatinized glass slides using glycerol as mounting medium.
In addition to histological analysis, the mesencephalic tissue of some animals was processed to detect the expression of hGAD67 cDNA by RT-PCR. Thus, the presence of the transplant was verified either by histology, or by RT-PCR. Briefly, animals were decapitated, the ventral mesencephalon was dissected, and the tissue was immediately frozen in liquid nitrogen and kept at −70°C until processing. Total RNA extraction with Trizol (Invitrogen) was done according to manufacturer’s specifications. Reverse transcription was done using 10 μg of the total RNA obtained (Superscript, Invitrogen), and 2 μl of the resulting reaction was used for subsequent polymerase chain reactions (PCRs). PCRs were performed using a sense oligonucleotide corresponding to a fragment of the pREP10 vector (5′-TATCATGTCTGGATCGGTAC-3′) and an antisense oligonucleotide corresponding to part of the hGAD67 gene (5′-CACAAAGCCTCAGGGGTGTG-3′). Conditions used for the PCRs were: initial denaturation for 2 min at 94°C, followed by 25 cycles of denaturation for 30 s at 95°C, annealing for 20 s at 62°C, extension for 30s at 72°C, and a final extension step for 5 min at 72°C. Amplified DNA fragments were fractionated on 2% agarose gels.
Experiment 3: GABA content in tissue homogenates
A separate group of animals (n = 25) was transplanted in order to measure GABA content in tissue homogenates. Animals received intranigral transplants (40,000 or 400,000 cells/2 μl) at the aforementioned coordinates. Animals were further assigned to one of three groups (1) INTACT; (2) CONTROL, which received grafts of a control cell line, of similar origin but without the hGAD67 cDNA; (3) M213-2O CL-4, which received grafts of the GABAergic cell line with the hGAD67 cDNA. At 12 weeks after transplant, animals received an ip injection of the GAD inhibitor 3-mercaptopropionic acid (1.2 mmol/kg), and were quickly decapitated. The brain was rapidly removed, and the ventral mesencephalon was obtained; tissue was homogenized with 85% methanol, and after centrifugation, the supernatant was analyzed for GABA by HPLC as described above, and the pellet was used for protein determination. Samples were analyzed by HPLC as described above.
Statistical analyses
For locomotor activity data, and GABA content, single factor or factorial analysis of variance was used, followed by post-hoc Fisher’s LSD tests, with the Bonferroni correction. If the data did not meet specifications required for parametric analysis, non-parametric analysis of variance was used (Kruskal-Wallis) followed by comparisons between groups (Mann-Whitney U). The unit of analysis for behavioral motor seizures was the group and not the individual, therefore data are expressed as percent of animals showing behavioral seizures within each group. Percentage data was analyzed using the chi-square test, and for survival analysis, the Gehan’s Wilcoxon test was employed. A significance level of <0.05 was chosen, and STATISTICA (StatSoft, Tulsa OK, U.S.A.) software was utilized for all statistical tests.
RESULTS
In vitro experiments
In vitro immunofluorescence staining showed that CONTROL and M213-2O CL-4 cells expressed β-tubulin, and were negative for GFAP and doublecortin. GAD67 immunofluorescence was evident only in M213-2O CL-4 cells (Figures 1–2).
Figure 1.
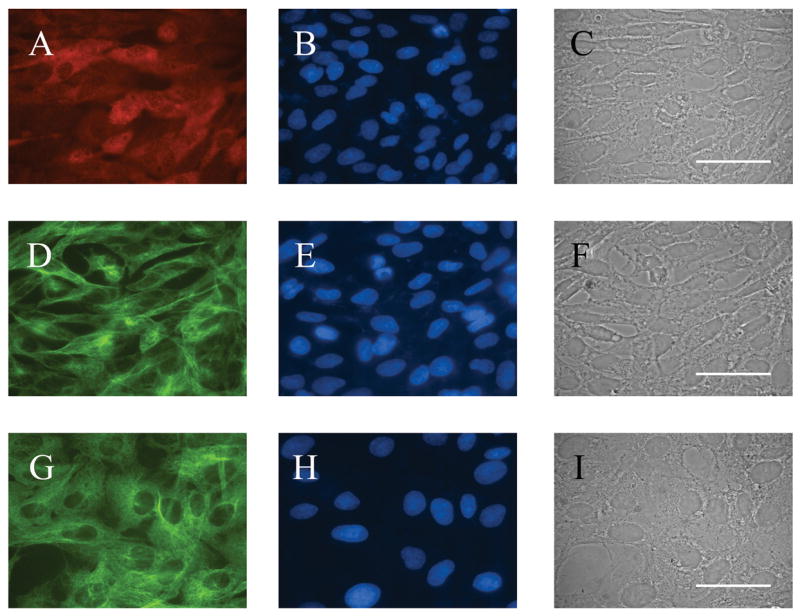
Morphological appearance and protein expression in the cell line transfected with hGAD67 cDNA (clone M213-2O CL-4) (A–F) and in CONTROL cell line (G–I) A. GAD-67 (monoclonal antibody) indirect immunofluorescence. B, E and H. bisbenzimide fluorescence of cells shown in A, D and G. C,F and I. Phase contrast photomicrograph of cells shown in A, D and G. D and G. Beta-tubulin expression. Clone M213-20 CL-4 was grown in selection medium (Hygromycin B, 200 μg/μl) with 10% FCS at 33°C and 5% CO2. CONTROL cells were grown in the same conditions without Hygromycin. Bar = 50 μm.
Figure 2.
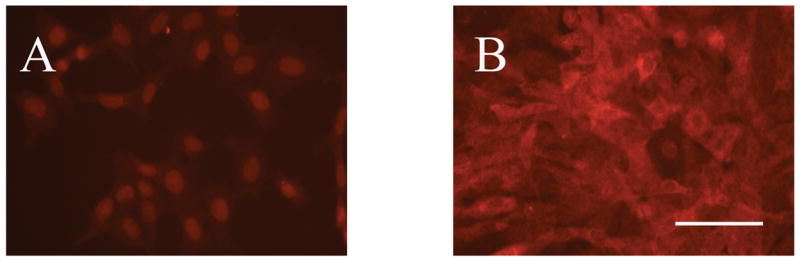
Differential GAD67 indirect immunofluorescence in clone M213-2O CL-4 and CONTROL cells using a Cy™3-conjugated secondary antibody. A. CONTROL cell line. B. clone M213-2O CL-4. Notice the differential level of expression of the protein in the two cell lines. Bar = 100 μm.
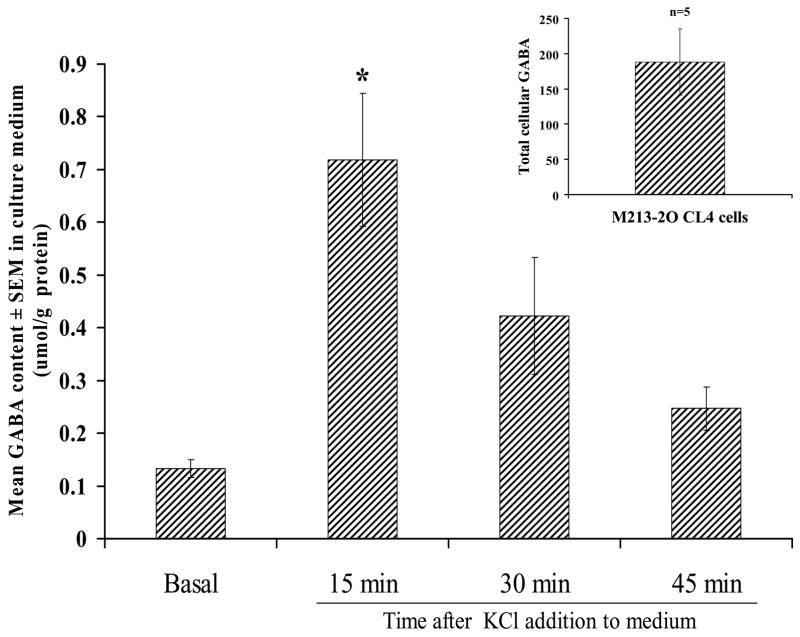
GABA was detectable only in M213-2O CL-4 cells, and their average total GABA content was 188 ± 46.80 μmol/g of protein (n = 4). In response to a depolarizing stimulus, GABA levels in the medium were significantly higher after a 15-min exposure to 50 mM KCl, whereas after a 30- or 45-min exposure, GABA levels were no different from basal levels (Figure 3) [Time effect, F(3, 26) = 7.28, p < 0.01; followed by Fisher’s LSD comparisons, p < 0.01].
Figure 3.
In vitro GABA levels in the culture medium before and 15, 30, and 45 minutes after exposure to 50 mM KCl in clone M213-2O CL-4. Each bar represents mean ± SEM of seven experiments. Insert indicates total cellular content of GABA in this cell line. Data are expressed in μmol/g protein. In the CONTROL cell line GABA was not detectable.
* p < 0.01 different from basal, and 45-min levels according to Fisher’s LSD tests.
In vivo experiments
Experiment 1: Effect of KA treatment
Fifty-seven percent of Sprague-Dawley rats treated with KA (n = 29) survived the first month after treatment, when they no longer lost weight or showed any other overt symptoms (Figure 5B). The 24-h observation period of stage III, IV, and V seizures indicated more animals showed seizures during the light phase (Figure 5A), with an even distribution throughout the daylight hours. After KA administration there was a latent period of several weeks before spontaneous behavioral seizures occurred; once the first seizure took place, their incidence increased. The insert in Figure 5A shows the percent of animals presenting stage V seizures during a 9-month period, and how this percent increased over time until it leveled off at 50–60% from the sixth month after KA administration. From all KA-treated animals, 8% were not found to have seizures, while none of the INTACT (vehicle-treated) animals were found to show spontaneous behavioral seizures at any time. It is important to note that during the 9-month observation period, KA-treated animals continued to die; death was not preceded by any apparent pre-mortem state such as sudden weight loss or incapacitation. Survival analysis indicated a significant difference in survival compared to untreated animals (Gehan’s Wilcoxon test statistic = 4.92, p < 0.01) (Figure 5B). After an initial period of weight loss following KA treatment, all animals recovered and show similar rates of weight gain (data not shown).
Figure 5.
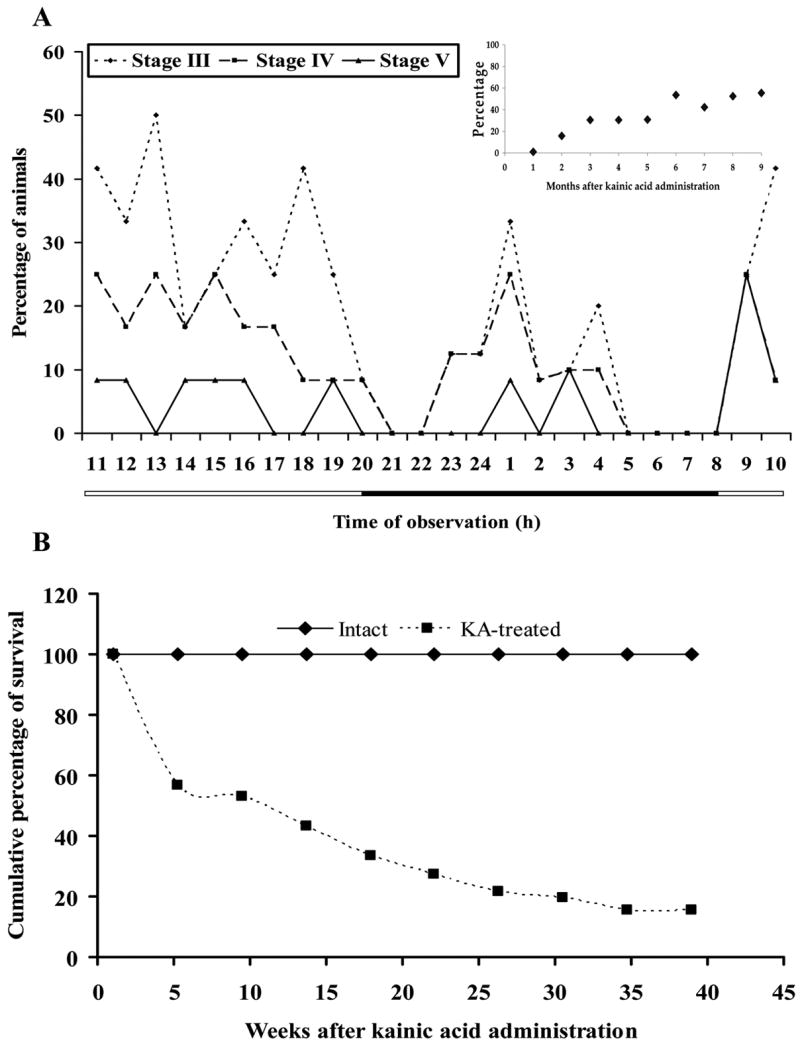
Experiment 1. Effect of KA treatment. Graphs show the effects of kainic acid (KA) treatment on behavior and survival. A. Percentage of animals treated with kainic acid (5mg/kg/hr) showing stages III, IV, and V spontaneous seizures during the light-dark cycle, indicated by the horizontal bar. This representative figure was obtained from observations made nine weeks after treatment, when approximately 30% of the animals already showed spontaneous stage V seizures (see insert). The insert indicates the percentage of animals presenting spontaneous stage V seizures during a 9-month period of observation; which leveled off at about 6 months after KA administration, and no decrease was observed afterwards. B. Severity of the effects of KA treatment as evidenced by cumulative survival curves for KA-treated and INTACT animals for the duration of the experiment. Significant differences were found between groups according to Gehan′s Wilcoxon test (p < 0.05).
Experiment 2: Intranigral transplants in KA-treated animals with spontaneous seizures
After KA-treatment, seventy-one animals survived and reached criterion, i.e. two stage V seizures, and were transplanted. Latency to criterion in weeks showed a mode of 16, a median of 24, and a quartile range of 14.
Histology
Figures 7 and 8 show the location of transplants of M213-2O CL-4 and CONTROL cell lines labeled with bisbenzimide, and located within the SNpr. Transplants were found in the anterior portion of the SNpr and reached the limit of the posterior SNpr (coordinates from Bregma −4.8 to −5.8 mm; [41]; no tumors or host tissue necrosis was observed. Transplanted cells were smaller that host cells, they were usually found in clusters, and in some sections cells were apparent along the cannula tract. GAD67 immunoreactivity was present in some labeled cells (Figure 7F), while macrophages were occasionally observed around the grafts. Transplants were usually well integrated into the host tissue, with very little GFAP immunoreactivity surrounding them. The transplants were negative for this marker (data not shown).
Figure 7.
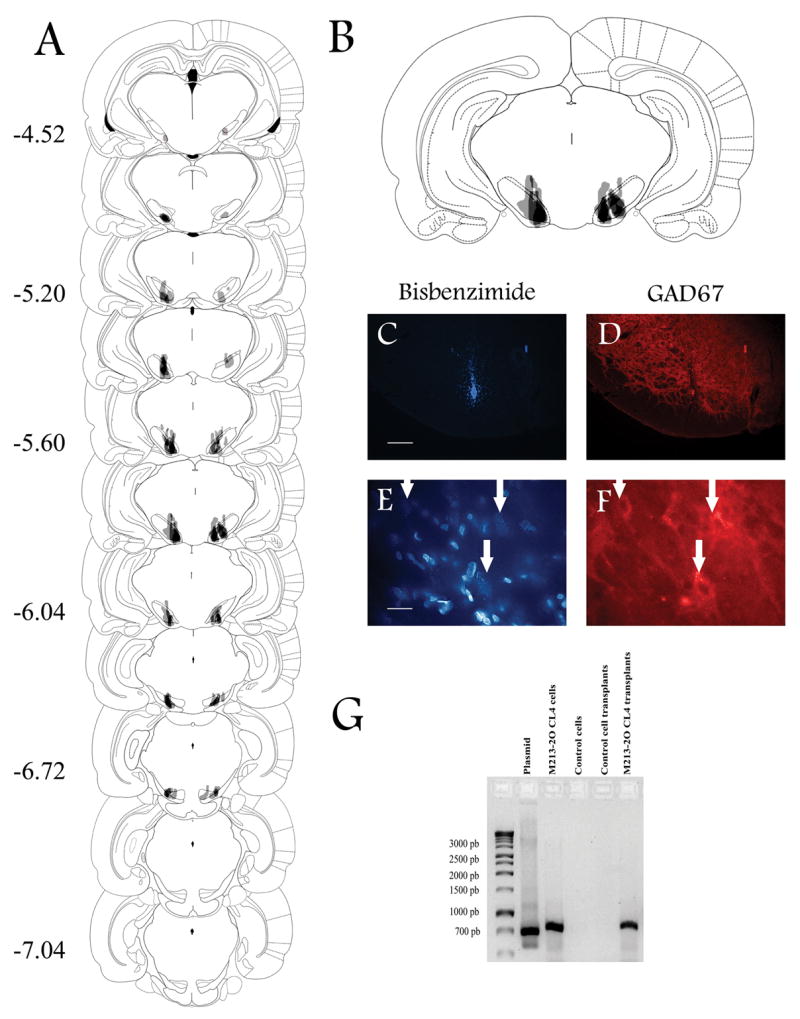
Experiment 2. Intranigral transplants in KA-treated animals. Representative coronal brain drawings and photomicrographs showing the location of intranigral transplants of the M213-2O CL-4 cell line in KA-treated animals. A. The majority of transplants were located between −5.20 and −6.04 mm from bregma as indicated by numbers on the left according to Paxinos and Watson (1986). Darker shading indicates areas of overlap among animals in each group. B. Amplification of the coronal section showing the greatest overlap among animals. Only animals with evidence of bilateral transplants within the substantia nigra pars reticulata (SNpr) were included in this figure, and in the behavioral analysis. C and E. Coronal brain sections at the level of the SNpr from M213-2O CL-4 transplanted cells labeled with bisbenzimide. D and F. Indirect immunofluorescence for GAD67 in the same sections as in C and E, using a Cy™3-conjugated secondary antibody. GAD67 immunoreactivity was evident in the SNpr (D), and it was present in some transplanted cells, especially in transplants of this cell line (F). Arrows indicate the same location within the transplant site. Bar = 200 μm (C and D), and 20 μm (E and F). G. Expression of hGAD67 cDNA amplified by RT-PCR in cultures of M213-2O CL-4, and mesencephalic tissue of M213-2O CL-4 transplanted animals at 12 weeks post-transplant. No expression was found in cultures of the CONTROL cell line, or in the tissue of animals transplanted with this cell line.
Figure 8.
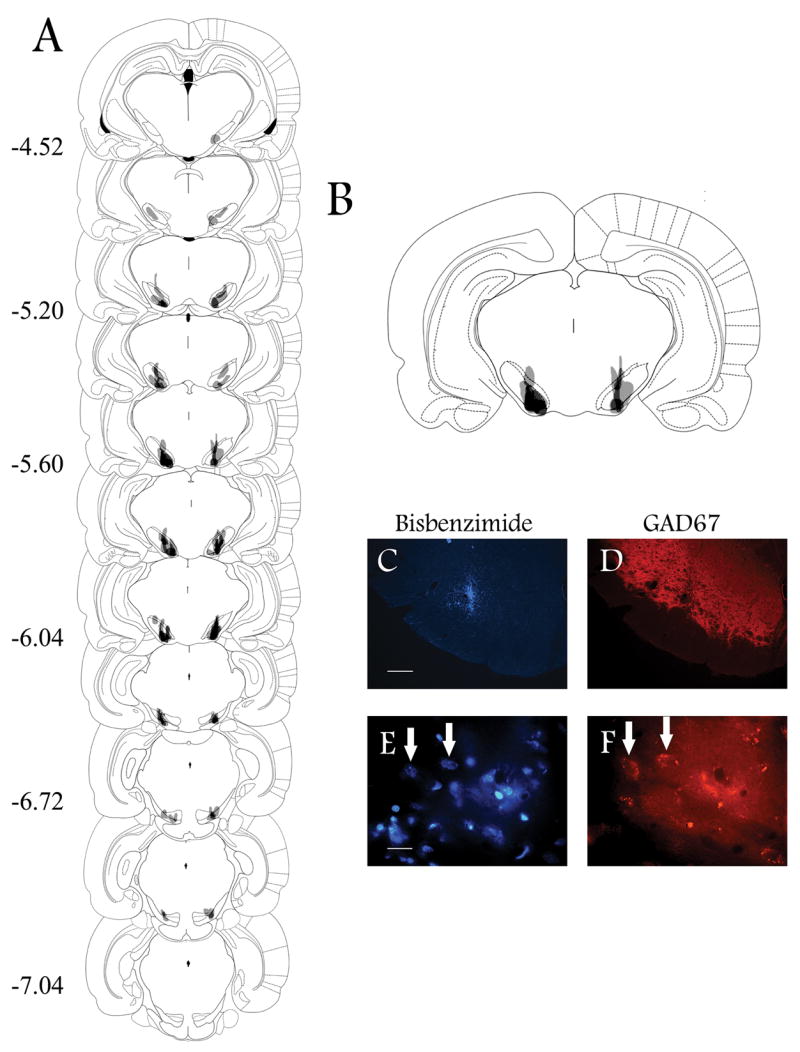
Experiment 2. Intranigral transplants in KA-treated animals. Representative coronal brain drawings and photomicrographs showing the location of intranigral transplants of the CONTROL (213-2O) cell line in KA-treated animals. A. The majority of transplants were located between −5.20 and −6.04 mm from bregma as indicated by numbers on the left according to Paxinos and Watson (1986). Darker shading indicates areas of overlap among animals in each group. B. Amplification of the coronal section showing the greatest overlap among animals. Only animals with evidence of bilateral transplants within the Snpr were included in this figure, and in the behavioral analysis. C and E. Coronal brain sections at the level of the SNpr from CONTROL cells labeled with bisbenzimide, and GAD67 immunoreactivity (D, F) in the same section, using a Cy™3-conjugated secondary antibody. GAD67 immunoreactivity was evident in the SNpr (D) and it was present in some transplanted cells (F). Arrows indicate same location within the transplant site. Bar = 200 μm (C and D), and 20 μm (E and F).
The presence of the transplant was also verified by hGAD67 cDNA expression using RT-PCR in M213-2O CL-4 transplanted animals. Figure 7G shows bands of about 700 bp in the ventral mesencephalon from M213-2O CL-4 transplanted animals 12 weeks after transplantation. The plasmid, as well as RNA extracted from cell cultures of M213-2O CL-4 cells, served as positive controls. No signal was detected in the CONTROL cells or in tissue obtained from animals transplanted with this cell line.
Behavioral observations
The most evident observation made was that more animals with M213-2O CL-4 transplants survived for longer periods (Figure 9). Survival analyses indicated a significant difference in the cumulative percentage of survival between M213-2O CL-4 and CONTROL group (Gehan’s Wilcoxon test statistic = 1,86, p < 0.05) and between M213-2O CL-4 and SHAM groups (Gehan’s Wilcoxon test statistic = 2.085, p < 0.05).
Figure 9.
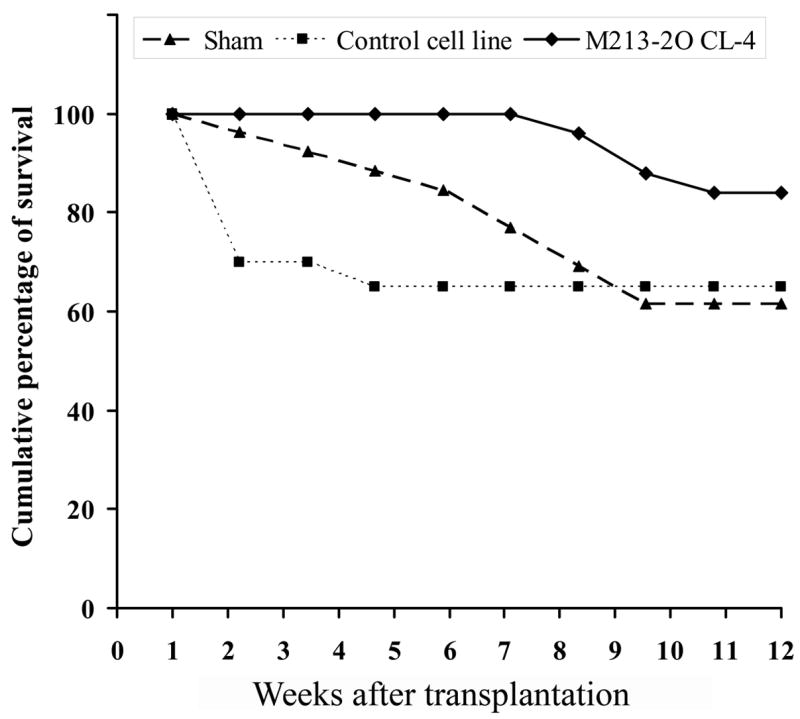
Experiment 2. Intranigral transplants in KA-treated animals. Cumulative survival curves for Sham group, CONTROL cell line transplanted, and M213-2O CL-4 transplanted groups, showing the effect of intranigral transplants on survival of KA-treated animals presenting spontaneous seizures. These data represent survival at ten time-points after transplantation over a period of 12 weeks. A significant difference was observed between M213-2O CL-4 and CONTROL groups, and between M213-2O CL-4 and SHAM groups according to Gehan′s Wilcoxon tests (p < 0.05).
In terms of spontaneous locomotor activity, the treatment with KA significantly increased total distance traveled and rearing [F(3,53) = 5.81; p < 0.05; F(3,53) = 4.18; p < 0.05, respectively; data not shown]; the INTACT group, which received vehicle only, was significantly different from all other groups for total distance traveled, and from the CONTROL group for rearing (Fisher’s LSD tests, p≤0.003). Animals were evaluated again at four and twelve weeks after transplantation; locomotor behavior was similar at these two time-points; therefore, only the 12-week data are presented. There was no group effect for total distance traveled, and no difference with respect to the pre-transplant measure. For rearing, there was a significant group effect [F(3,40) = 3.75; p < 0.05]; the M213-2O CL-4 group showed less rearing activity at twelve weeks after transplant compared to the pre-transplant measure [F(1,40) = 3.54; p < 0.05].
For three months following transplantation Stage III, IV, and V behavioral seizures were monitored daily. The data presented include those animals in which the transplants were located within the SNpr (all but two animals in each group). Twelve weeks after transplantation the percentage of animals in group M213-2O CL-4 showing Stage III seizures was significantly smaller than that in the SHAM group (67% vs. 94%, respectively, χ2 = 6.94, p < 0.05) (Figure 10B). This significant difference was also observed for Stage IV seizures, at four and twelve weeks post transplantation (60% vs. 92%; 60% vs. 94%; χ2 = 6.94; χ2 = 5.02; p < 0.05 respectively; Figures 10A, B). Similar effects were observed for Stage V seizures at four and twelve weeks post transplant (55% vs. 96%; 38% vs. 75%; χ2 = 10.04; χ2 = 4.48; p < 0.05 respectively; Figures 10A, B). The CONTROL group, which received a cell line without the hGAD67 cDNA, showed a significant difference only in the percentage of animals presenting Stage V seizures at four-weeks post transplant (65% vs. 96%; χ2 = 7.46; p < 0.05; Figure 10A).
Figure 10.
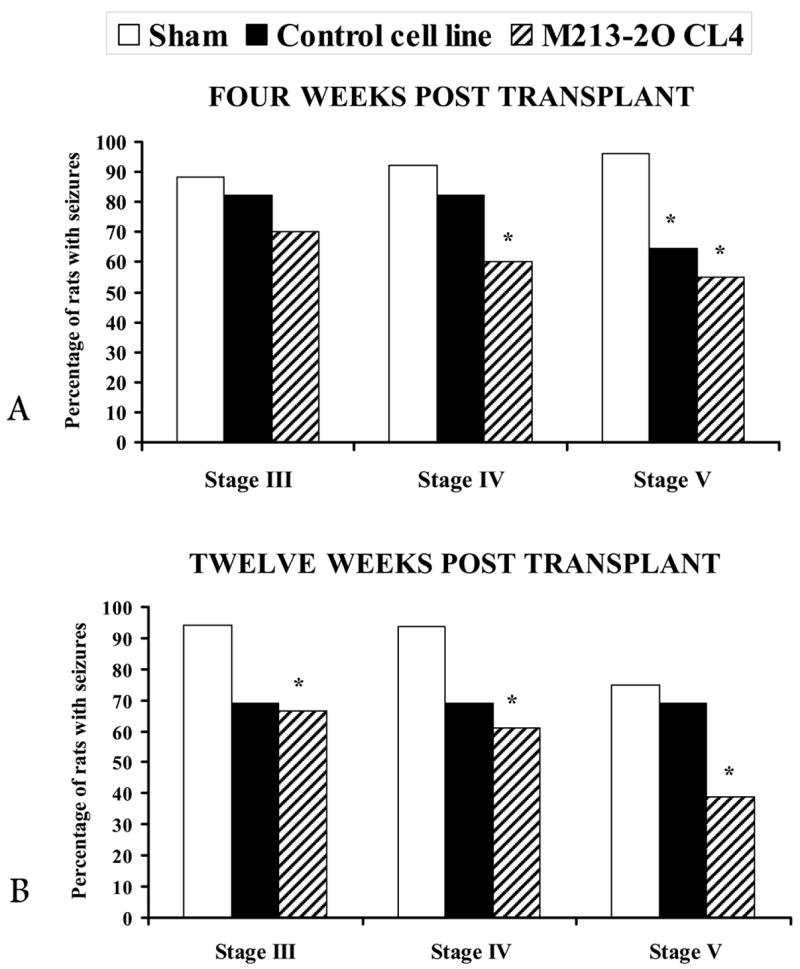
Experiment 2. Intranigral transplants in KA-treated animals. Percentage of animals showing stage III, IV, and V seizures (see text for details) at 4 and 12 weeks after SHAM, CONTROL, and M213-2O CL-4 transplants. Note that four weeks (A) and twelve weeks (B) after the M213-2O CL-4 transplant, there was a significant reduction in the percentage of animals showing stage V seizures. In contrast, in animals with CONTROL cell line transplants, the effect was of shorter duration.
* p < 0.05, compared to SHAM, χ2 tests.
Experiment 3: GABA content in tissue homogenates
The GABA content in tissue homogenates from ventral mesencephalon was increased only in those animals with M213-2O CL-4 transplants (40 and 400 × 103 cells) at 12 weeks after transplantation compared to the corresponding CONTROL; the number of transplanted cells had no effect on GABA tissue levels, which were similar after intranigral transplants of 40,000 or 400,000 cells (Figure 11, inset) [Group effect, F (1, 16) = 54.70, p < 0.01; Number effect, F (1, 16) = 0.53, p > 0.01; Group × Number effect, F (1, 16) = 0.041, p>0.01; followed by Fisher’s LSD comparisons, p < 0.01]. Since there was no effect of the number of transplanted cells, data from all transplanted animals in each transplant group were combined, and a one-way ANOVA was calculated including the INTACT group. There was a significant effect of group [Group effect, F (2, 22) = 33.47, p < 0.01], and significant differences between group M213-20 CL4 and the INTACT and CONTROL groups were found (Fisher’s LSD comparisons, p < 0.01) (Figure 11).
Figure 11.
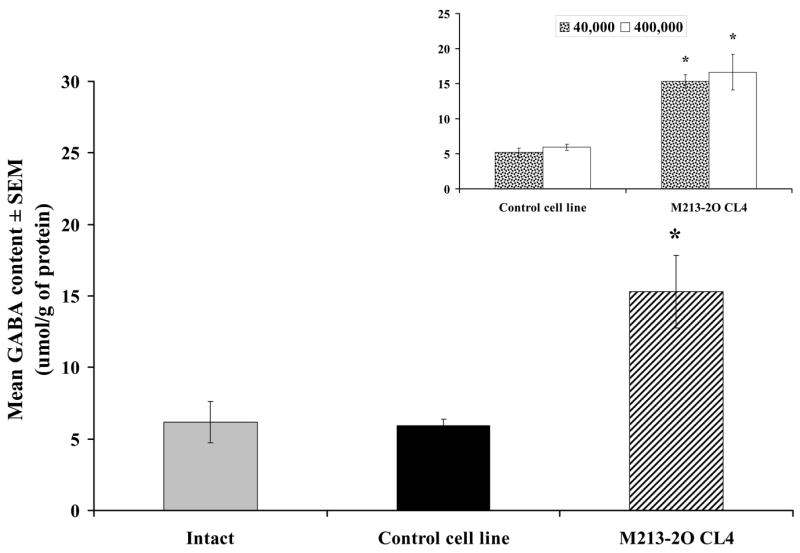
Experiment 3. GABA content in tissue homogenates. GABA content (μmol/g of protein) in ventral mesencephalon twelve weeks after CONTROL and M213-2O CL-4 transplants. The GABA content was significantly higher in animals with M213-2O CL-4 transplants (n = 10) than in INTACT (n = 5) and CONTROL groups (n = 10). In contrast, the number of transplanted cells did not have any effect on GABA content. The insert shows the GABA content (μmol/g of protein) in animals transplanted with 40,000 cells and 400,000 cells. Asterisks indicate differences with the corresponding CONTROL group.
* p<0.01 different from INTACT and CONTROL groups according to Fisher’s LSD tests.
DISCUSSION
This study evaluated the ability of intranigral transplants of GABAergic cells to reduce spontaneous behavioral seizures induced by previous systemic administration of KA, for a period up to three months after transplantation. The results show that intranigral transplants of the hGAD67 cDNA containing cell line (M213-2O CL-4) resulted in a lasting reduction in the percentage of animals with stage V seizures, four and twelve weeks after transplantation, compared to SHAM animals. These transplants also reduced to zero the number of animals with an index of occurrence for stage V seizures greater than 20, increased the survival rate throughout the three-month long evaluation, and led to higher GABA levels in the ventral mesencephalon. This is the first study to show that intranigral transplants of a GABAergic cell line can reduce spontaneous motor seizures on a long-term basis, and also improve survival of the transplanted animals.
The results also show that the CONTROL cell line had a short-term effect at the fourth week after transplantation on stage V seizures induced by KA treatment, reducing the number of animals exhibiting generalized motor seizures. This finding seems to indicate that the mere presence of transplanted cells within the substantia nigra could modify nigral function, such that the frequency of seizures is reduced [13]. Alternatively, the reduction in the percentage of animals showing seizures in the CONTROL group may be due to the loss of those animals with more seizures, and not to a beneficial effect of the transplant per se. Indeed, when looking into the behavior of those animals in the CONTROL group that died before the twelfth week after transplantation, it was found that they showed a higher percentage of stage V seizures, than those in group M213-20 CL-4.
It must be noted that in experiment one it was shown that after KA administration, and as others have also found [2, 24, 32, 37] there was a latent period of several weeks before spontaneous seizures occurred, and once the first seizure took place, their incidence increased. Therefore, the decrease in the percentage of transplanted animals showing severe tonic-clonic seizures cannot be attributed to spontaneous remission.
KA treatment increased locomotor activity during the dark phase of the daily cycle in all treated groups when compared to an intact group. After transplantation, activity in terms of total distance traveled remained elevated in all groups. Group M213-2O CL-4 showed a significant decrease of rearing behavior at twelve weeks after transplantation, which may very well be a consequence of excessive nigral inhibition by the GABAergic transplant. However, we cannot conclude that the effect was due to the nigral transplant without first replicating this finding, because the reduction was not observed in a different measure of exploratory activity. This behavioral evaluation also indicated that animals did not show other behavioral abnormalities in addition to the spontaneous motor seizures, but instead showed normal levels of activity in comparison to an intact, non KA-treated group.
In this study, we verified the expression of GAD67 in the transplanted cells, both in vitro and in vivo, through indirect immunofluorescence and by RT-PCR twelve weeks after transplantation. Immunofluorescence using a monoclonal antibody (Chemicon MAB5401, 1:1000) was observed consistently in tissue culture of M213-2O Cl-4 cells and in brain sections but not in CONTROL cells. From all the sections analyzed, it was clear that not all transplanted cells, which were detected by means of the nuclear marker bisbenzimide, expressed GAD67 in vivo. This observation does not seem to be the result of an overall down-regulation of the protein, since GABA levels were significantly increased in the ventral mesencephalon of animals transplanted with the GABAergic cell line. Instead it indicates that in vivo, where the cells are no longer under the control of a selection medium, the expression of the enzyme is more heterogeneous than in vitro. Alternatively, the fluorescent marker used to track the transplanted cells (Hoechst 33342) may be labeling cells other than those transplanted. This is unlikely since preliminary experiments showed that either direct administration of the fluorescent marker, or transplantation of lysed cells resulted in only short-term fluorescence (7 days), indicating that the fluorescent label that could have been taken up by surrounding host cells is not enough to account for the fluorescence observed three months after transplantation.
The GABA content in homogenates of ventral mesencephalon increased at twelve weeks after transplantation of only the GABAergic cell line (M213-2O CL-4) suggesting that the behavioral effect observed in this group at twelve weeks is related to this increase in GABA. The increase in GABA content was independent of the number of cells transplanted. This finding may indicate that regardless of the number of cells initially transplanted, the host brain may have the ability to support only the survival of a certain maximum number of cells that continue to synthesize GABA, resulting in comparable GABA levels. Alternatively, the increase in GABA content could be explained as a result of changes in the host tissue brought about only by the GABAergic cell line. This seems improbable since the GABAergic cell line was derived from the control cell line, and their difference lies in the presence of the plasmid containing the hGAD67 cDNA. Only by using microdialysis to measure GABA release in vivo at the transplant site and correlating the levels of GABA with the number of transplanted cells will this issue be resolved.
The results of other studies also suggest that transplant size is not related to functional effects. Loscher et al., [31] used grafts of fetal striatal GABAergic neurons into the substantia nigra in kindling epilepsy, and found that the number of viable transplanted cells decreased over time, but that there was no significant correlation between the decrease in seizures and transplant size or number of cells, which expressed a protein found in striatal cells and were transplanted into the substantia nigra. Zaman and Shetty [45] quantified absolute graft cell survival of fetal cells in CA3 using bromodeoxyuridine immunostaining at 1 year post transplantation. They found that 36% of the cells survived for one year, and that this result paralleled the degree of cell survival observed at 1 month (i.e., 31%) in an earlier study [46]. Apparently then, only a maximum number of cells can survive in the host brain regardless of how many are transplanted, and if they survive initially, they can do so for extended periods. This maximum number may be regulated by the conditions of the host brain and other factors that may influence graft survival and its functional effects. Among those factors that improve graft survival are fibroblast growth factor-2 (FGF-2) and brain-derived neurotrophic factor (BDNF) as described in a recent study by Rao et al., [37]. These authors also found that combining these growth factors with a caspase inhibitor, significantly improved graft survival and behavioral effects.
The significant reduction in number of animals presenting stage 5 seizures, twelve weeks after transplantation of the GABAergic cell line, appears to be related to the increase in GABA levels in homogenates of ventral mesencephalon at the same time, as discussed above. In the entorhinal cortex-kindling model, Thompson [42] observed increased GABA levels in the hippocampus three and ten days after transplantation of a cell line that produces GABA under the control of doxycycline. They found that behavioral seizures and afterdischarges were reduced only in the animals with transplants of the GABA cells. GABA levels in vivo and in vitro decreased when the cells were treated with doxycycline, and so did the behavioral effects. In an earlier study using a model of spontaneous seizures (lithium pilocarpine model) the authors observed similar effects with the same cell line [43]. Kokaia et al. [28] observed a reduction in generalized seizures in rats previously kindled in the amygdala when GABA-releasing polymer matrices were implanted bilaterally, dorsal to the substantia nigra. The effect lasted until GABA release from the polymer matrices was reduced. These results indicate that increased GABA levels in the area of the transplant may underlie the behavioral effects observed in various animal models of generalized seizures. However, these studies do not indicate whether synaptic integration with the host, and regulated GABA release are needed in order to obtain sustained suppression of seizures.
As discussed above, positive results in terms of reduction of electrophysiological and behavioral parameters have been observed after transplantation of GABA cells into various brain regions. We selected the substantia nigra as a target for transplantation, given the positive results previously observed in the GAERS model, and in models of motor seizures after intranigral GABA agonist administration [10, 11, 27], and after GABAergic transplants [13, 28, 31, 41, 43]. It has been proposed that the substantia nigra exerts a seizure-gating function, but the exact mechanisms underlying these effects are not well understood [16]. By inhibiting endogenous nigral cells, intranigral transplants of GABAergic cells could attenuate the inhibitory effect of the SNpr on anticonvulsant areas of the brain and could thus raise the seizure threshold, or block convulsive seizures [9, 16]. These transplants would not be expected to restore the normal hippocampal circuitry as intrahippocampal CA3 fetal grafts appear to do [39], but to act more as a remote control system of epileptic seizures [9]. Some studies using intrahippocampal transplants of GABAergic cell lines have found positive effects in a model of kindling [42], but others have found no evidence for cessation of seizures or improvement in behavior using the KA model [22] RR352987891ES[37] have recently reported amelioration with fetal hippocampal grafts in the KA model. A conclusion as to which is the best target for transplantation in epilepsy models can only be reached with more studies, and by directly addressing this question.
In conclusion, this study, the first to evaluate the effects of intranigral GABAergic transplants in the KA model of spontaneous seizures for up to three months, showed that the transplants reduced the number of behavioral motor seizures, and that the effects observed at three months correlate with an increase in GABA levels observed in the mesencephalon of animals with the GABAergic transplants, indicating that intranigral GABAergic transplants may exert their positive effects by increasing local GABA levels in the substantia nigra. In vivo and in vitro analyses provide evidence that the GABAergic cells showed sustained expression of GAD67 and hGAD67 cDNA. These results indicate that seizures can be controlled by localized delivery of an inhibitory neurotransmitter using modified cells as vehicle, and they bring forward the development of a therapeutic alternative for curing intractable forms of epilepsy.
Acknowledgments
The authors acknowledge the technical assistance of J. Mejía, A. Márquez, A. Falcón, L. González, L. Lara, and E. N. Hernández; Leonor Casanova executive coordinator of graduate studies; M. García head of the Animal Care Facility; Dr. Michael C. Jeziorski, Dr. Aurea Orozco, and M.C. Patricia Villalobos for advice on detection of the transgene; and Dr. D. Pless for editing of the manuscript. This work was supported by grants IN225305 from PAPIIT-DGAPA, 46161-M and 41477-Q from CONACyT, and in part by the NIDA Intramural program, NIH, DHHS. C.G. Castillo was supported by a fellowship from CONACyT (153810) and DGEP-UNAM.
Footnotes
Claudia Castillo Martín del Campo
Centro de Biología Molecular Severo Ochoa, CSIC-UAM
Cantoblanco, 28049 Madrid, España.
Tel +34-911-964-650; fax +34-911-964-420 Email: cgcastillo@cbm.uam.es
María Soledad Mendoza-Trejo
Departmento de Neurobiología Conductualy Cognitiva
Instituto de Neurobiología, Universidad Nacional Autónoma de México
Blvd. Jurquilla 3001, Querétaro, Qro., 76230, México
Tel +52-442-238-1061; fax +52-442-238-1046.
Email: solecito75@yahoo.com
Manuel B. Aguilar Ramirez
Departamento de Neurobiología
Celulary Molecular,
Instituto de Neurobiología Instituto de Neurobiología, Universidad Nacional Autónoma de México
Blvd. Jurquilla 3001, Querétaro, Qro., 76230, México
Tel +52-442-238-1043; fax +52-442-238-1046. Email: maguilar@servidor.unam.mx
William J. Freed
National Institute On Drug Abuse, NIH, DHHS
Nathan Shock Drive, Baltimore MD 21224, USA
Tel +410-550-1405
Email: WFREED@intra.nida.nih.gov
Magda Giordano Noyola
Departmento de Neurobiología Conductualy Cognitiva
Instituto de Neurobiología
Universidad Nacional Autónoma de México Boulevard Juriquilla #3001
Querétaro, Qro., 76230.
Tel +52-442-238-1061; fax +52-442-238-1046.
Email: giordano@servidor.unam.mx
Developed by Klymkowsky, M.; Developmental Studies Hybridoma Bank under the auspices of NICHD and University of Iowa, Departement of Biological Sciences, Iowa City IA 52242).
References
- 1.Barry DI, Wanscher B, Kragh J, Bolwig TG, Kokaia M, Brundin P, et al. Grafts of fetal locus coeruleus neurons in rat amygdala-piriform cortex suppress seizure development in hippocampal kindling. Exp Neurol. 1989;106:125–32. doi: 10.1016/0014-4886(89)90085-x. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42(Suppl 3):5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- 3.Bengzon J, Brundin P, Kalen P, Kokaia M, Lindvall O. Host regulation of noradrenaline release from grafts of seizure-suppressant locus coeruleus neurons. Exp Neurol. 1991;111:49–54. doi: 10.1016/0014-4886(91)90049-i. [DOI] [PubMed] [Google Scholar]
- 4.Björklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–60. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 5.Castillo CG, Mendoza S, Freed WJ, Giordano M. Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid-induced seizures in the rat. Behav Brain Res. 2006;171:109–15. doi: 10.1016/j.bbr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Castillo CG, Montante M, Dufour L, Martinez ML, Jimenez-Capdeville ME. Behavioral effects of exposure to endosulfan and methyl parathion in adult rats. Neurotoxicol Teratol. 2002;24:797–804. doi: 10.1016/s0892-0362(02)00268-4. [DOI] [PubMed] [Google Scholar]
- 7.Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends in neurosciences. 1996;19:163–71. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- 8.Conejero-Goldberg C, Tornatore C, Abi-Saab W, Monaco MC, Dillon-Carter O, Vawter M, et al. Transduction of human GAD67 cDNA into immortalized striatal cell lines using an Epstein-Barr virus-based plasmid vector increases GABA content. Exp Neurol. 2000;161:453–61. doi: 10.1006/exnr.1999.7258. [DOI] [PubMed] [Google Scholar]
- 9.Depaulis A, Moshe SL. The basal ganglia and the epilepsies: translating experimental concepts to new therapies. Epileptic Disord. 2002;4(Suppl 3):S7–8. [PubMed] [Google Scholar]
- 10.Depaulis A, Snead OC, 3rd, Marescaux C, Vergnes M. Suppressive effects of intranigral injection of muscimol in three models of generalized non-convulsive epilepsy induced by chemical agents. Brain Res. 1989;498:64–72. doi: 10.1016/0006-8993(89)90399-5. [DOI] [PubMed] [Google Scholar]
- 11.Depaulis A, Vergnes M, Marescaux C, Lannes B, Warter JM. Evidence that activation of GABA receptors in the substantia nigra suppresses spontaneous spike-and-wave discharges in the rat. Brain Res. 1988;448:20–9. doi: 10.1016/0006-8993(88)91097-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferencz I, Kokaia M, Elmer E, Keep M, Kokaia Z, Lindvall O. Suppression of kindling epileptogenesis in rats by intrahippocampal cholinergic grafts. Eur J Neurosci. 1998;10:213–20. doi: 10.1046/j.1460-9568.1998.00033.x. [DOI] [PubMed] [Google Scholar]
- 13.Fine A, Meldrum BS, Patel S. Modulation of experimentally induced epilepsy by intracerebral grafts of fetal GABAergic neurons. Neuropsychologia. 1990;28:627–34. doi: 10.1016/0028-3932(90)90038-p. [DOI] [PubMed] [Google Scholar]
- 14.Freed WJ, Perlow MJ, Karoum F, Seiger A, Olson L, Hoffer BJ, et al. Restoration of dopaminergic function by grafting of fetal rat substantia nigra to the caudate nucleus: long-term behavioral, biochemical, and histochemical studies. Ann Neurol. 1980;8:510–9. doi: 10.1002/ana.410080508. [DOI] [PubMed] [Google Scholar]
- 15.Gash D, Sladek JR, Jr, Sladek CD. Functional development of grafted vasopressin neurons. Science. 1980;210:1367–9. doi: 10.1126/science.7434031. [DOI] [PubMed] [Google Scholar]
- 16.Gernert M, Fedrowitz M, Wlaz P, Loscher W. Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy. Eur J Neurosci. 2004;20:2377–86. doi: 10.1111/j.1460-9568.2004.03699.x. [DOI] [PubMed] [Google Scholar]
- 17.Gernert M, Thompson KW, Loscher W, Tobin AJ. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol. 2002;176:183–92. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- 18.Giordano M, Takashima H, Herranz A, Poltorak M, Geller HM, Marone M, et al. Immortalized GABAergic cell lines derived from rat striatum using a temperature-sensitive allele of the SV40 large T antigen. Exp Neurol. 1993;124:395–400. doi: 10.1006/exnr.1993.1213. [DOI] [PubMed] [Google Scholar]
- 19.Giordano M, Takashima H, Poltorak M, Geller HM, Freed WJ. Constitutive expression of glutamic acid decarboxylase (GAD) by striatal cell lines immortalized using the tsA58 allele of the SV40 large T antigen. Cell Transplant. 1996;5:563–75. doi: 10.1177/096368979600500506. [DOI] [PubMed] [Google Scholar]
- 20.Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- 21.Halonen T, Tortorella A, Zrebeet H, Gale K. Posterior piriform and perirhinal cortex relay seizures evoked from the area tempestas: role of excitatory and inhibitory amino acid receptors. Brain Res. 1994;652:145–8. doi: 10.1016/0006-8993(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa T, Kondziolka D, Choi SJ, Balzer J, Dixon EC, Fellows-Mayle W, et al. Hippocampal neurotransplantation evaluated in the rat kainic acid epilepsy model. Neurosurgery. 2004;55:191–8. 98–200. doi: 10.1227/01.neu.0000126881.40748.93. [DOI] [PubMed] [Google Scholar]
- 23.Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- 24.Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 25.Holmes GL, Thompson JL, Huh K, Holmes C, Carl GF. Effect of neural transplants on seizure frequency and kindling in immature rats following kainic acid. Brain Res Dev Brain Res. 1991;64:47–56. doi: 10.1016/0165-3806(91)90208-z. [DOI] [PubMed] [Google Scholar]
- 26.Holmes GL, Thompson JL, Huh K, Stuart JD, Carl GF. Effects of neural transplantation on seizures in the immature genetically epilepsy-prone rat. Exp Neurol. 1992;116:52–63. doi: 10.1016/0014-4886(92)90175-p. [DOI] [PubMed] [Google Scholar]
- 27.Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–40. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- 28.Kokaia M, Aebischer P, Elmer E, Bengzon J, Kalen P, Kokaia Z, et al. Seizure suppression in kindling epilepsy by intracerebral implants of GABA- but not by noradrenaline-releasing polymer matrices. Exp Brain Res. 1994;100:385–94. doi: 10.1007/BF02738399. [DOI] [PubMed] [Google Scholar]
- 29.Kokaia M, Cenci MA, Elmer E, Nilsson OG, Kokaia Z, Bengzon J, et al. Seizure development and noradrenaline release in kindling epilepsy after noradrenergic reinnervation of the subcortically deafferented hippocampus by superior cervical ganglion or fetal locus coeruleus grafts. Exp Neurol. 1994;130:351–61. doi: 10.1006/exnr.1994.1214. [DOI] [PubMed] [Google Scholar]
- 30.Lindvall O, Bengzon J, Brundin P, Kalen P, Kokaia M. Locus coeruleus grafts in hippocampal kindling epilepsy: noradrenaline release, receptor specificity and influence on seizure development. Prog Brain Res. 1990;82:339–46. doi: 10.1016/s0079-6123(08)62621-7. [DOI] [PubMed] [Google Scholar]
- 31.Loscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Mathern GW, Cifuentes F, Leite JP, Pretorius JK, Babb TL. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993;87:326–39. doi: 10.1016/0013-4694(93)90186-y. [DOI] [PubMed] [Google Scholar]
- 33.Meier CL, Dudek FE. Spontaneous and stimulation-induced synchronized burst afterdischarges in the isolated CA1 of kainate-treated rats. J Neurophysiol. 1996;76:2231–9. doi: 10.1152/jn.1996.76.4.2231. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in stereotaxic coordenates. 2. Academic Press; 1986. [Google Scholar]
- 35.Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–7. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 36.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure Electroencephalogr. Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 37.Rao MS, Hattiangady B, Rai KS, Shetty AK. Strategies for promoting anti-seizure effects of hippocampal fetal cells grafted into the hippocampus of rats exhibiting chronic temporal lobe epilepsy. Neurobiology of disease. 2007;27:117–32. doi: 10.1016/j.nbd.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross KC, Waldman BC, Conejero-Goldberg C, Freed W, Coleman JR. Transplantation of M213-2O cells with enhanced GAD67 expression into the inferior colliculus alters audiogenic seizures. Exp Neurol. 2002;177:338–40. doi: 10.1006/exnr.2002.7987. [DOI] [PubMed] [Google Scholar]
- 39.Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens JR, Phillips I, Freed WJ, Poltorak M. Cerebral transplants for seizures: preliminary results. Epilepsia. 1988;29:731–7. doi: 10.1111/j.1528-1157.1988.tb04227.x. [DOI] [PubMed] [Google Scholar]
- 41.Thompson K, Anantharam V, Behrstock S, Bongarzone E, Campagnoni A, Tobin AJ. Conditionally immortalized cell lines, engineered to produce and release GABA, modulate the development of behavioral seizures. Exp Neurol. 2000;161:481–9. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- 42.Thompson KW. Genetically engineered cells with regulatable GABA production can affect afterdischarges and behavioral seizures after transplantation into the dentate gyrus. Neuroscience. 2005;133:1029–37. doi: 10.1016/j.neuroscience.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Thompson KW, Suchomelova LM. Transplants of cells engineered to produce GABA suppress spontaneous seizures. Epilepsia. 2004;45:4–12. doi: 10.1111/j.0013-9580.2004.29503.x. [DOI] [PubMed] [Google Scholar]
- 44.Willing AE, Saporta S, Lixian J, Milliken M, Poulos S, Bowersox SS, et al. Preliminary study of the behavioral effects of LBS-neuron implantation on seizure susceptibility following middle cerebral artery occlusion in the rats. Neurotox Res. 2002;4:111–8. doi: 10.1080/10298420290015908. [DOI] [PubMed] [Google Scholar]
- 45.Zaman V, Shetty AK. Fetal hippocampal CA3 cell grafts transplanted to lesioned CA3 region of the adult hippocampus exhibit long-term survival in a rat model of temporal lobe epilepsy. Neurobiology of disease. 2001;8:942–52. doi: 10.1006/nbdi.2001.0440. [DOI] [PubMed] [Google Scholar]
- 46.Zaman V, Turner DA, Shetty AK. Prolonged postlesion transplantation delay adversely influences survival of both homotopic and heterotopic fetal hippocampal cell grafts in Kainate-lesioned CA3 region of adult hippocampus. Cell Transplant. 2001;10:41–52. [PubMed] [Google Scholar]