Abstract
Nitroglycerin (glyceryl trinitrate; GTN) is the most prominent representative of the organic nitrates or nitrovasodilators, a class of compounds that have been used clinically since the late nineteenth century for the treatment of coronary artery disease (angina pectoris), congestive heart failure and myocardial infarction. Medline lists more than 15 000 publications on GTN and other organic nitrates, but the mode of action of these drugs is still largely a mystery. In the first part of this article, we give an overview on the molecular mechanisms of GTN biotransformation resulting in vascular cyclic GMP accumulation and vasodilation with focus on the role of mitochondrial aldehyde dehydrogenase (ALDH2) and the link between the ALDH2 reaction and activation of vascular soluble guanylate cyclase (sGC). In particular, we address the identity of the bioactive species that activates sGC and the potential involvement of nitrite as an intermediate, describe our recent findings suggesting that ALDH2 catalyses direct 3-electron reduction of GTN to NO and discuss possible reaction mechanisms. In the second part, we discuss contingent processes leading to markedly reduced sensitivity of blood vessels to GTN, referred to as vascular nitrate tolerance. Again, we focus on ALDH2 and describe the current controversy on the role of ALDH2 inactivation in tolerance development. Finally, we emphasize some of the most intriguing, in our opinion, unresolved puzzles of GTN pharmacology that urgently need to be addressed in future studies.
Keywords: cyclic GMP, endothelial dysfunction, nitric oxide, organic nitrate, soluble guanylate cyclase, smooth muscle, superoxide, thiol, vascular relaxation
Basic pharmacology of glyceryl trinitrate
The beneficial clinical effect of nitroglycerin (glyceryl trinitrate; GTN) is due to dilation of large coronary arteries, resulting in improved blood supply to the heart and venodilation, resulting in increased venous pooling and consequent reduction of venous return and cardiac preload (Bode-Böger and Kojda, 2005). The combination of increased supply and decreased demand of oxygen provides unique therapeutic benefit in cardiac ischaemia. Small arterial resistance vessels are much less sensitive to organic nitrates, and lowering of arterial blood pressure is relatively minor at therapeutic dosage. However, the antihypertensive effect of nitrovasodilators is significantly potentiated by phosphodiesterase 5 inhibitors, for example, sildenafil, resulting in pronounced, potentially harmful reduction of systemic blood pressure (Speakman and Kloner, 1999; Cheitlin, 2003).
Though nitrovasodilators are considered as being safe and free of serious side effects, their clinical use is limited by reduction of efficacy upon long-term application, resulting in a complete loss of haemodynamic effects after 24–48 h of continuous application. This phenomenon of nitrate tolerance, which caused the so-called Monday disease of workers in dynamite factories in the late nineteenth century (Marsh and Marsh, 2000), is still an unresolved issue in nitrate therapy (Fung, 2004). In stable angina patients, nitrate tolerance is avoided by intermittent application of the drugs, for example, overnight removal of GTN patches (Abrams, 2002), but nitrate tolerance seriously hampers continuous intravenous nitrate therapy of patients with acute heart failure, unstable angina or myocardial infarction (Thadani, 1997; Elkayam et al., 2004).
Early reports on potential pathways of GTN bioactivation
The mechanism by which organic nitrates dilate blood vessels has remained a mystery for almost a century and is still not fully understood. In the mid-1970s Ferid Murad, one of the pioneers of nitric oxide (NO)/cyclic GMP (cGMP) signalling, discovered that NO and a wide variety of compounds releasing NO or related molecules act as activators of soluble guanylate cyclase (sGC) in vascular smooth muscle, resulting in cGMP-mediated vasodilation (Katsuki et al., 1977; Murad, 1999). Today drugs acting through release of NO as active principle, including organic nitrates, sodium nitroprusside and the sydnonimine SIN-1, are grouped under the term nitrovasodilators. In contrast to sodium nitroprusside or SIN-1, GTN and other organic nitrates do not activate sGC in cell-free systems but function as prodrugs that are bioactivated to release NO in vascular smooth muscle. Biotransformation of GTN is not necessarily linked to bioactivation. The pathway of GTN biotransformation yielding NO or a related bioactive species activating sGC is referred to as mechanism-based metabolism or bioactivation, in contrast to clearance-based metabolism unrelated to sGC activation. Cell culture and organ bath studies suggest that GTN bioactivation correlates with formation of 1,2-GDN (glyceryl dinitrate), whereas formation of the 1,3-isomer appears to reflect clearance-based degradation of the nitrate (Fung, 2004; Thatcher et al., 2004).
Non-enzymatic bioactivation
Virtually all reduced thiols react with GTN in a non-enzymatic manner resulting in the formation of roughly stoichiometric amounts of 1,2- and 1,3-GDN together with inorganic nitrite. Although nitrite may exert biological activity and can be reduced or otherwise converted to NO under certain conditions (see below), the reaction of GTN with most thiols is clearance-based and not associated with bioactivation (Chong and Fung, 1991). Notable exceptions are cysteine and certain cysteine derivatives, for example, N-acetylcysteine, which promote NO-mediated activation of purified sGC by GTN in the absence of tissue (Noack and Feelisch, 1991). The mechanism of the non-enzymatic reaction between GTN and cysteine has been thoroughly investigated but is still not fully understood. The challenging proposal according to which the reaction involves formation of the NO donor S-nitrosocysteine as an intermediate (Ignarro and Gruetter, 1980) does not explain why L-cysteine containing peptides, such as glutathione (GSH), which would also form the corresponding S-nitroso derivatives, do not trigger GTN bioactivation. According to an alternative proposal, the reaction involves formation of a trace metal-stabilized cyclic GTN-cysteine adduct as an intermediate (Thatcher and Weldon, 1998; Thatcher et al., 2004). As very high concentrations of L-cysteine (millimolar) and GTN (high micromolar) are required for significant sGC activation, the relevance of this reaction for GTN bioactivation in vivo is questionable. Notwithstanding the specificity of the reaction for non-peptide bound L-cysteine, it is tempting to speculate that direct reduction of GTN to NO by ALDH2, which contains an essential reactive sulphydryl group in the active site (see below), occurs through a similar reaction. In this case, the role of the additional functional group in free L-cysteine (for example, the carboxylate moiety) that is essential for NO formation might be taken over by another amino-acid residue.
The reaction with L-cysteine has remained the sole non-enzymatic pathway of GTN bioactivation for the last three decades. Recently, we have discovered that ascorbate or a reactive intermediate of ascorbate autoxidation reacts with GTN in a non-enzymatic manner to yield NO or a NO-related species that activates purified sGC (Kollau et al., 2007). Although slow reaction kinetics and relatively poor efficiency in terms of cGMP accumulation question the biological relevance of this reaction, there is evidence that ascorbate prevents the development of GTN tolerance in rats and humans, and we found that ascorbate deficiency induces nitrate tolerance in guinea-pigs (see the section on nitrate tolerance below). Thus, at present the contribution of the GTN/ascorbate reaction to GTN bioactivation in blood vessels cannot be definitively excluded.
Enzymatic bioactivation
There has been an extensive search for the enzyme(s) catalysing GTN bioactivation and several candidates have been proposed in the past, including GSH-S-transferase, cytochrome P450, cytochrome P450 reductase and xanthine oxidase (Fung, 2004). These earlier proposals were mainly based on evidence obtained with more or less selective enzyme inhibitors blocking GTN-induced vasodilation, in some cases combined with in vitro experiments showing that the enzymes catalyse conversion of GTN to a species that triggers intracellular cGMP accumulation under certain conditions. However, none of these enzymatic pathways appeared to satisfactorily explain GTN bioactivity (Fung, 2004). In 2002, Stamler and co-workers reported on GTN reductase activity of mitochondrial ALDH (ALDH2) (Chen et al., 2002) and recent in vivo data suggest that ALDH2 is indeed the key enzyme mediating the high-affinity component of GTN action in blood vessels (Chen and Stamler, 2006). Before we discuss this pathway in detail, we would address the puzzling controversy on the identity of the GTN-derived bioactive species that activates sGC in vascular smooth muscle.
Identity of the bioactive species: NO or no NO?
Although NO-mediated activation of sGC is widely considered as the common principle of action of GTN and other nitrovasodilators, several studies reported on a mismatch between GTN bioactivity, that is, vasodilation and/or sGC activation, and the amount of NO released compared with equi-effective concentrations of direct NO donors. These observations led to the proposal that GTN bioactivation results in formation of an as yet unidentified NO-related species which, unlike free NO radical, may activate sGC in a haem-independent manner. In the following, we will discuss this issue with respect to the GTN/cysteine reaction and GTN bioactivation in blood vessels, respectively.
The enigmatic GTN/cysteine reaction
The claimed lack of NO formation in the course of the GTN/cysteine reaction is based on two key observations: (i) lack of correlation between NO release and activation of sGC by varying concentrations of GTN compared with direct NO donors (Artz et al., 2001) and (ii) lack of spectroscopic detection of a nitrosyl–haem complex (indicative for NO binding to the haem) of sGC in the presence of GTN/cysteine (Artz et al., 2002). These negative data led the authors to propose that (i) the GTN/cysteine reaction results in formation of a novel sGC-activating species that is not identical to free NO radical and that (ii) a new pathway of sGC activation exists that is not involving the prosthetic haem group. Obviously, if correct, these conclusions would have revolutionized the NO/cGMP field. However, we found a strict correlation between the amount of NO released from equi-effective concentrations of DEA/NO (2,2-diethyl-1-nitroso-oxyhydrazine) and GTN measured under identical experimental conditions and observed formation of the expected nitrosyl–haem complex of sGC under conditions of sufficiently high GTN turnover, indicating that non-enzymatic NO formation fully accounts for sGC activation by GTN/cysteine (Gorren et al., 2005). In the previous study (Artz et al., 2002), the nitrosyl–haem complex was not observed simply because sGC was present in large, 10- to 100-fold excess over the amount of NO released from GTN. Thus, maximally 1–10% of protein-bound haem could be nitrosylated under these conditions. Such a minor fraction of nitrosylated protein is not detected by the applied spectroscopic techniques. Thus, there is overwhelming evidence suggesting that activation of sGC by GTN/cysteine is mediated by NO binding to the prosthetic haem group of sGC.
Lack of detectable GTN-derived NO in blood vessels
Two laboratories reported on a striking lack of correlation between detectable NO release and GTN bioactivity, questioning the role of free NO radical as a mediator of GTN-induced vasorelaxation. Kleschyov et al. (2003) used an electron paramagnetic resonance spin trapping technique to quantify vascular NO formation in response to GTN and the Ca2+ ionophore A23187. Whereas A23187 caused pronounced vascular NO formation, equi-effective concentrations of GTN (and other organic nitrates) did not increase basal NO production. As NO signals were observed in response to suprapharmacological concentrations of GTN, the authors proposed that NO release may account for the so-called ‘second' GTN pathway, which is activated by high doses of GTN and is apparently resistant to nitrate tolerance, whereas the therapeutically relevant pathway of GTN bioactivation does not involve free NO radical. In line with this study, discrepancies between the effects of GTN and NO donors on mitochondrial O2 consumption, vasoactivity and NO release measured by real-time confocal microscopy have been reported (Núñez et al., 2005). The authors emphasized that their data argue against the idea that NO is a product of mitochondrial GTN bioactivation because it would be difficult to explain how NO could diffuse to sGC without inhibiting cytochrome c oxidase or being detected by the NO-sensitive dye.
Notwithstanding the puzzling data obtained by two independent laboratories using essentially different methods for NO detection, there is general agreement that GTN-induced relaxation of blood vessels is sensitive to various agents interfering with NO-mediated sGC activation, including haemoglobin (Martin et al., 1985), superoxide (Cherry et al., 1990) and a haem site inhibitor of sGC (Brunner et al., 1995). Moreover, we found that purified ALDH2 efficiently converts GTN to free NO radical measured with a highly selective electrochemical method (see below). Thus, the active species mediating vascular cGMP accumulation in response to GTN looks like NO, tastes like NO and smells like NO but, for unknown reasons, escapes detection by established analytical methods. The discrepancy could be explained by tight coupling of GTN biotransformation to sGC activation in a locally constrained cellular environment. However, considering the lack of evidence for mitochondrial localization of sGC, this idea is hard to reconcile with the ALDH2 hypothesis of GTN bioactivation. More detailed insights into the molecular mechanisms of vascular GTN bioactivation are required to settle this issue.
Catalytic function of ALDH2
Aldehyde dehydrogenases (EC 1.2.1.3) catalyse the oxidation of a wide spectrum of aliphatic and aromatic aldehyde substrates to the corresponding carboxylic acids with NA(P)D+ serving as electron accepting cofactor (Racker, 1949).The ALDH superfamily consists of 555 genes, including 31 archebacterial, 351 eubacterial and 173 eukaryotic sequences (Hempel et al., 1993; Sophos and Vasiliou, 2003). Analysis of the ALDH gene superfamily in the latest databases revealed that the human genome contains 19 putatively functional genes and three pseudogenes (Vasiliou and Nebert, 2005). The major mammalian isoforms are cytosolic ALDH1 and mitochondrial ALDH2 that are expressed in a variety of tissues, including liver (Nishimura and Naito, 2006; Alnouti and Klaassen, 2008). Expressed as homotetrameric protein with subunits of ∼54 kDa in the mitochondrial matrix, ALDH2 is thought to be essential for the detoxification of acetaldehyde produced during ethanol oxidation. About 40% of the East-Asian population that express a low-activity mutant (Glu487Lys) of ALDH2 (Larson et al., 2007) exhibit significantly lowered alcohol tolerance due to adverse effects of acetaldehyde accumulation (Crabb et al., 1989), and the ALDH inhibitor disulfiram is still approved in many countries as deterrent for the aversive pharmacotherapy of alcohol dependence (Soyka and Roesner, 2006).
Dehydrogenase and esterase activities
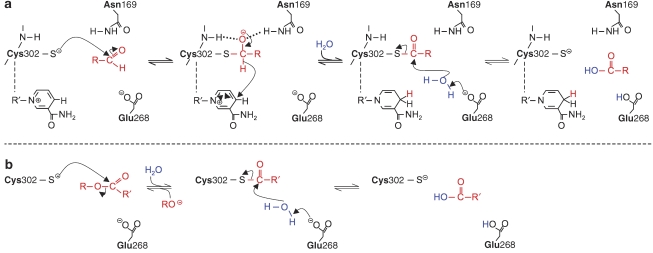
The mechanisms of the classical ALDH2 activities are fairly well understood (Mann and Weiner, 1999; Wymore et al., 2007). As shown in Figure 1a, aldehyde oxidation is thought to involve a nucleophilic reaction of the substrate with a critical cysteine residue in the active site (Cys302 in human ALDH2), resulting in the formation of a thiohemiacetal intermediate (acylation step). The oxyanion of the thiohemiacetal intermediate is stabilized by hydrogen bonds with the side chain of Asn169 and the Cys302 amide group. Subsequently, hydride transfer from NAD+, which is non-covalently bound in close proximity to Cys302, yields a thioester intermediate and NADH, followed by Glu268-aided hydrolysis of the thioester and release of the carboxylic acid product (deacylation step).
Figure 1.
Classical reactions catalysed by aldehyde dehydrogenase (ALDH2). (a) Aldehyde oxidation. (b) Ester hydrolysis.
Besides its classical dehydrogenase activity, ALDH2 catalyses hydrolysis of ester substrates. As shown in Figure 1b, the esterase activity of the enzyme involves the same essential cysteine residue as aldehyde oxidation but is not strictly dependent on the presence of NAD+. The reaction is initiated by nucleophilic attack of the ester substrate at Cys302, resulting in the formation of a thioester and release of the corresponding alcohol. As in the dehydrogenase reaction, hydrolysis of the thioester intermediate occurs through activation of water by Glu268 (Mann and Weiner, 1999).
GTN denitration
In 2002, Chen et al. (2002) screened various cells and tissues for enzymatic denitration of GTN to 1,2-GDN and identified ALDH2 as a major GTN-metabolizing enzyme in cultured RAW 267.4 macrophages. Selective formation of the 1,2-GDN isomer, fairly high GTN affinity (low micromolar) and, most importantly, impaired vasoactivity and cGMP accumulation in isolated blood vessels treated with ALDH inhibitors suggested that ALDH2 may essentially contribute to vascular GTN bioactivation. This was confirmed in subsequent studies showing that cGMP-mediated vasorelaxation to GTN, but not to direct NO donors, was markedly reduced in ALDH2−/− mice (Chen et al., 2005) and in humans expressing the low-activity Glu487Lys mutant of ALDH2 (Mackenzie et al., 2005). These and several other studies (DiFabio et al., 2003; De La Lande et al., 2004; Zhang et al., 2004; Kollau et al., 2005; Huellner et al., 2008) consistently show that vasorelaxation to therapeutically relevant low concentrations of GTN is mediated by ALDH2, whereas a second pathway resistant to nitrate tolerance, ALDH2 inhibition or gene deletion appears to be involved in the low-affinity component of GTN action.
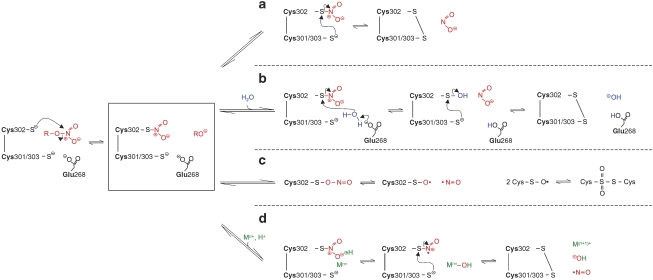
The mechanism of ALDH2-catalysed GTN denitration is not known. According to current view (Chen and Stamler, 2006), the initial step of the reaction resembles that of the classical ALDH2 activities, that is, nucleophilic attack by one of the three nitrogens of GTN (preferentially a terminal nitro group) at Cys302, resulting in the formation of a thionitrate intermediate and release of the corresponding alcohol (preferentially 1,2-GDN). As illustrated in Figure 2, two alternative reactions have been proposed to explain the release of nitrite from the intermediate. Nucleophilic attack of one of the adjacent cysteine residues (Cys301 or Cys303) would result in formation of a disulphide in the active site of the enzyme (pathway a). Alternatively, activation of water by Glu268 would result in hydrolysis of the intermediate yielding nitrite and the sulphenic acid derivative of Cys302, which may undergo S-thiolation (Biswas et al., 2006) to form a cysteinyl disulphide with Cys301/Cys303 (pathway b). Both mechanisms would be compatible with oxidative inactivation of ALDH2 by GTN (see below), but pathway b appears more attractive because it accommodates GTN reduction to the known function of Glu268 in classical ALDH2 catalysis.
Figure 2.
Hypothetical pathways of aldehyde dehydrogenase (ALDH2)-catalysed denitration of glyceryl trinitrate (GTN). (a, b) Possible pathways yielding 1,2-glyceryl dinitrate (GDN) and nitrite (adapted from Chen and Stamler, 2006). (c and d) Possible pathways yielding 1,2-GDN and NO.
GTN-triggered ALDH2 inactivation and protection by reductants
Oxidative inactivation of ALDH2 after one turnover implicates that a reducing cofactor is required for continuous recycling of the active enzyme. As GTN-triggered ALDH2 inactivation is thought to essentially contribute to the development of nitrite tolerance (Münzel et al., 2005), it is of considerable interest to clarify the underlying mechanism and to identify (the) potential endogenous ALDH2 reductant(s). Several early studies showed that ALDHs are inhibited by organic nitrates (Komura, 1974; Towell et al., 1985; Mukerjee and Pietruszko, 1994), but the implications of these observations to the pharmacology of organic nitrates have not been realized before the discovery of ALDH2-catalysed GTN bioactivation by the Stamler laboratory in 2002 (Chen et al., 2002). Mukerjee and Pietruszko (1994) reported that the cytosolic and mitochondrial ALDH isoforms are inactivated by isosorbide dinitrate in a mechanism-based manner; reversibility of enzyme inactivation by 2-mercaptoethanol suggested the involvement of sulphydryl oxidation. Later studies confirmed and extended these observations, showing that the presence of a reductant is essential for sustained ALDH2-catalysed GTN metabolism and bioactivation (Chen et al., 2002; Kollau et al., 2005).
As the dehydrogenase and GTN reductase activities of GTN-inactivated ALDH2 are not restored by GSH, the most abundant low molecular weight thiol in cells, researchers have screened for alternative agents that could function as endogenous reductants. Recently, dihydrolipoic acid, a metabolic reductant present in mitochondria, was shown to partially restore ALDH2 activity in GTN-exposed blood vessels (Wenzel et al., 2007a). However, enzyme reactivation required relatively high concentrations of dihydrolipoic acid, and evidence for a role of endogenous dihydrolipoic acid in GTN bioactivation is still missing. In a recent study (Beretta et al., 2008b), we confirmed the expected inactivation of ALDH2 by GTN and the protective effects of dithiothreitol and, though with lower efficacy, dihydrolipoic acid. However, the activity of the GTN-inactivated enzyme was only partially restored by these reductants, suggesting a significant irreversible component of ALDH2 inactivation. However, our recent findings showing that the enzyme converts GTN to NO in a reaction that is independent of (clearance-based) GTN denitration (see below), indicates that inactivation/reactivation of GTN denitration does not necessarily reflect bioactivation. We are currently investigating this issue in our laboratory.
Coupling GTN denitration to sGC activation
The observation that ALDH2-catalysed denitration of GTN yielding inorganic nitrite causes accumulation of cGMP in vascular smooth muscle immediately raised the obvious question for the link between nitrite formation and sGC activation. We confirmed the existence of such a link in a cell-free system consisting of isolated rat liver mitochondria and purified sGC (Kollau et al., 2005, 2008). In the presence but not in the absence of mitochondria, GTN activated sGC and this effect was attenuated by ALDH inhibitors as well as by haemoglobin and superoxide, strongly suggesting that mitochondrial GTN biotransformation results in production of NO that it accessible to sGC outside the mitochondria. The molecular mechanism of NO formation, however, has remained elusive.
Inorganic nitrite as a potential intermediate
As a major metabolite of GTN biotransformation, inorganic nitrite has to be considered as an intermediate of bioactivation. There is experimental evidence indicating that inorganic nitrite serves as a vascular storage pool for NO that contributes to cGMP-dependent hypoxic vasodilation and other NO-mediated biological processes in vivo (Gladwin et al., 2006) but the pathways mediating the NO-like biological activity of inorganic nitrite are not fully understood. There are several pathways leading to conversion of nitrite to NO, which may become biologically relevant in certain conditions. Protonation of nitrite to HNO2 (nitrous acid) results in NO formation through disproportionation of N2O3 according to Equations (1 and 2).
Although homolysis of N2O3 (Equation (2)) is considerably faster than hydrolysis (that is, the back reaction in Equation (1)), the bimolecular conversion of HNO2 to N2O3 is extremely slow at low nitrite concentrations, questioning the biological relevance of the protonation pathway even in most pathologies associated with tissue acidosis (Butler and Ridd, 2004). Alternatively, reduction of nitrite could account for NO formation in mammalian tissues. It is well established that ascorbate reduces nitrite to NO (Mirvish et al., 1972; Archer et al., 1975), but the reaction with thiols is more complex and presumably involves formation of S-nitrosothiols as intermediates (Ignarro and Gruetter, 1980) that release free NO radical through trace metal-catalysed homolytic cleavage or enzymatic reduction (Stamler and Toone, 2002).
Non-enzymatic bioactivation of nitrite becomes significant only at millimolar concentrations of the reactants (Kollau et al., 2007), raising the question for biologically relevant enzymatic nitrite reductase pathways. Xanthine oxidase, which catalyses reduction of nitrite to NO in the presence of NADH or xanthine at anaerobic conditions (Zhang et al., 1998; Godber et al., 2000), was found to reduce organic nitrates to NO in vitro (Millar et al., 1998; O'Byrne et al., 2000; Doel et al., 2001; Li et al., 2005), but there is no evidence that this reaction plays a role in vascular GTN bioactivation. Deoxyhaemoglobin and myoglobin function as allosterically regulated nitrite reductases at low O2 tension (Cosby et al., 2003; Shiva et al., 2007). As deoxyhaemoglobin catalyses reduction of nitrite to NO along the physiological O2 gradient, this reaction could be essentially involved in the regulation of nitrite-dependent hypoxic vasodilation (Gladwin et al., 2006). Though smooth muscle does not contain haemo- or myoglobin, it cannot be excluded that a haem protein with similar function catalyses reduction of GTN-derived nitrite in mitochondria.
Finally, components of the mitochondrial respiratory chain exhibit nitrite reductase activity. On the basis of accumulation of nitroso-haemoglobin in isolated rat liver mitochondria incubated with respiratory substrates in the presence of nitrite, Kozlov et al. (1999) proposed that complex III catalyses nitrite reduction under anaerobic conditions. More recently, it has been suggested that complex IV rather than complex III is responsible for nitrite reduction under hypoxic conditions, because formation of nitrite-derived NO by respiring yeast and rat liver mitochondria was sensitive to cyanide and mimicked by non-physiological reduction of cytochrome c oxidase (Castello et al., 2006). Considering the mitochondrial localization of ALDH2, Chen and Stamler (2002) proposed that GTN-derived nitrite could be reduced by components of the respiratory chain or undergo proton-triggered disproportionation to yield NO and/or nitrosothiols in the mitochondrial intermembrane space. The disproportionation pathway is most likely not efficient enough (Butler and Ridd, 2004), but nitrite reduction coupled to mitochondrial respiration could well be involved. In a study published 20 years ago, nitrite was excluded as a possible intermediate of GTN bioactivation because of a pronounced mismatch between the potencies of GTN and nitrite to cause S-nitrosothiol formation and cGMP accumulation in blood vessel homogenates (Romanin and Kukovetz, 1988). However, this previous study did not consider bioactivation of GTN in the mitochondrial matrix not readily accessible to exogenous nitrite. We tested whether respiratory substrates or mitochondrial poisons affect GTN-induced activation of sGC in the presence of isolated mitochondria but our data did not support the idea that GTN bioactivation is coupled to mitochondrial respiration (Kollau et al., 2005, 2008).
Direct formation of NO by ALDH2
In search for alternative pathways linking the ALDH2 reaction to sGC activation, we speculated that NO might be produced in addition to nitrite during enzymatic GTN reduction. Surprisingly, we found that GTN metabolism by purified ALDH2 did indeed result in formation of NO, measured electrochemically with a Clark-type electrode, that was accompanied by pronounced activation of purified sGC co-incubated with ALDH2 (Beretta et al., 2008a). Formation of NO and sGC activation occurred at virtually identical GTN concentrations (low micromolar) as 1,2-GDN formation and were blocked by substrate-competitive ALDH inhibitors. Like the other activities of ALDH2 (dehydrogenase, esterase and denitration of GTN), formation of NO was abolished by site-directed mutagenesis of Cys302 and was increased in the presence of a reductant (M Beretta and B Mayer, unpublished observations). Taken together, these results suggest that a reaction of GTN with the SH group of Cys302 in the active site of ALDH2 is essential for both 2- and 3-electron reduction of GTN, yielding nitrite and NO radical, respectively. According to preliminary characterization of several ALDH2 mutants, clearance-based GTN denitration (yielding 1,2-GDN and nitrite) and NO formation reflect two independent reactions both taking place in the active site of the enzyme. Thus, the classical GTN reductase activity of ALDH2 studied so far does not necessarily reflect GTN bioactivation. Further studies are needed to clarify whether the two GTN-related activities of the enzyme are differentially regulated in cells.
Other ALDH isoforms, other nitrates
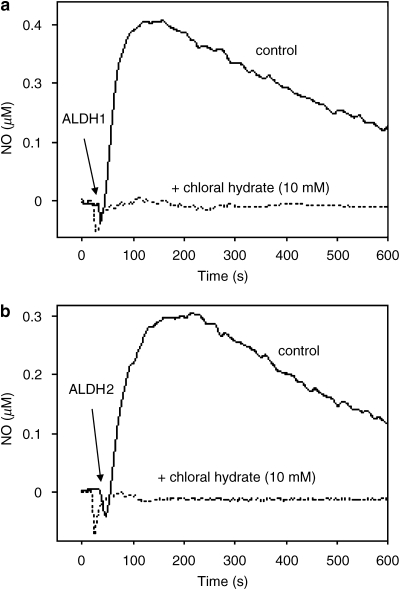
Organic nitrates were shown to inhibit multiple vascular ALDHs (Murphy et al., 2005). The authors found that GTN inhibited both ALDH2 and ALDH3A in mouse aorta, whereas low concentrations (⩽50 μM) of isosorbide mononitrate and isosorbide dinitrate selectively inhibited ALDH3A. Moreover, ALDH2 gene deletion does not affect isosorbide mononitrate and isosorbide dinitrate bioactivation (Chen et al., 2005). Taken together, these observations raise the question whether other ALDH isoforms are capable of catalysing bioactivation of GTN and other nitrovasodilators. We found that purified ALDH1 catalyses GTN denitration and bioactivation (Beretta et al., 2008a). Figure 3 shows formation of NO by purified ALDH1 and ALDH2, measured with a Clark-type electrode, and inhibition by the non-selective ALDH inhibitor chloral hydrate. Even though low GTN affinity questions the role of ALDH1 in GTN bioactivation, the cytosolic or another isoform could be involved in the second, low-Km pathway of GTN vasoactivity and catalyse bioactivation of organic nitrates, such as isosorbide mononitrate and isosorbide dinitrate, which exhibit lower vasodilatory potency than GTN (Daiber et al., 2004).
Figure 3.
Formation of NO from glyceryl trinitrate (GTN) by aldehyde dehydrogenase (ALDH1) (a) and ALDH2 (b). Purified ALDH1 (a) or ALDH2 (b) was incubated with 100 and 10 μM GTN, respectively, in the presence of 2 mM dithiothreitol and 1000 U mL−1 superoxide dismutase (SOD) with and without 10 mM chloral hydrate. Formation of NO was measured with a Clark-type electrode as described in Beretta et al. (2008a).
So far, enzymatic bioactivation of organic nitrates other than GTN has not been studied with purified ALDH2, but reduced potency of pentaerythritol tetranitrate (PETN) to relax blood vessels from ALDH2−/− mice suggests that PETN is also a substrate of the enzyme (Wenzel et al., 2007b). By testing different organic nitrates, the authors found that at least three nitro groups must be present in the molecule to enable bioactivation by ALDH2. Interestingly, PETN was reported to not trigger oxidative stress or ALDH2 inactivation in blood vessels (Daiber et al., 2004; Mollnau et al., 2006), possibly explaining the lack of tolerance development in humans treated with PETN (Jurt et al., 2001). Further studies on bioactivation of PETN by purified ALDH2 should clarify the molecular mechanism underlying the differences between GTN and PETN.
Possible mechanisms of ALDH2-catalysed NO formation
We considered three alternative mechanisms that may explain the observed formation of NO in the course of ALDH2-catalysed GTN biotransformation: (i) conversion of nitrite to NO, (ii) formation of nitroxyl (NO−) as an intermediate and (iii) reaction of GTN with Cys302 similarly to non-enzymatic bioactivation triggered by free cysteine. ALDH2 was found to convert inorganic nitrite to NO in a chloral hydrate-sensitive manner, but millimolar concentrations of nitrite were required for significant sGC activation, most likely excluding conversion of nitrite as relevant mechanism of ALDH2-catalysed GTN bioactivation in vivo (Beretta et al., 2008a). This view is further supported by the apparent independence of the two reactions yielding nitrite and NO, respectively.
It has been shown that vascular GTN biotransformation results in accumulation of hydroxylamine, a stable metabolite formed by reaction of HNO (nitroxyl) with thiols (Booth et al., 2000). The complex chemistry of HNO and its deprotonated form NO− (pKa=11.4), which even occurs in two different spin states, raised considerable confusion about the biological role of this species (Wink et al., 2003; Fukuto et al., 2005). HNO donors such as Angeli's salt or Piloty's acid are potent cGMP-dependent vasodilators (Fukuto et al., 1992) and antiplatelet agents (Zamora et al., 1995; Bermejo et al., 2005). The cGMP-dependent effects of HNO have been attributed to intracellular oxidation of NO− to NO (Zamora et al., 1995; Dierks and Burstyn, 1996), but several recent studies reported that vasodilation to HNO donors was insensitive to NO scavengers, suggesting direct activation of sGC by HNO or NO− (Costa et al., 2001; Wanstall et al., 2001; Irvine et al., 2003; Favaloro and Kemp-Harper, 2007). As it is not known whether the accumulation of hydroxylamine observed in the course of vascular biotransformation of GTN (Booth et al., 2000) was catalysed by ALDH2, it cannot be excluded that the apparent formation of NO we observed was due to oxidation of GTN-derived HNO by superoxide dismutase (SOD) and/or trace metal ions.
According to an attractive alternative explanation, the reaction of GTN with the essential cysteine residue Cys302 in the active site of ALDH2 may proceed similarly to the well-known non-enzymatic reduction of GTN by free cysteine (Gorren et al., 2005). A simple mechanism involving rearrangement of the thionitrate to a sulphenyl nitrite intermediate followed by homolysis yielding NO and sulphenyl radical is shown as pathway c in Figure 2. However, as cysteine containing (poly)peptides such as GSH or serum albumin do not trigger bioactivation, an additional functional group of free cysteine appears to be essential (Thatcher et al., 2004). Conceivably, an adjacent cysteine residue in the active site of ALDH2 could take over this function according to the metal-catalysed pathway d in Figure 2. Whereas pathway c would implicate virtually irreversible inactivation of ALDH2 due to dimerization of sulphenyl radicals, pathway d would yield a disulphide that could be converted back to the active reduced state by low molecular weight thiols or other reductants. At present there is no evidence for the involvement of a redox-active transition metal in the reaction, but this issue certainly deserves further investigation.
Nitrate tolerance
Nitrate tolerance is generally considered as a complex, presumably multifactorial phenomenon leading to reduced responsiveness of blood vessels to GTN and other organic nitrates (Fung, 2004; Münzel et al., 2005). Besides neurohormonal counter-regulation, also classified as pseudotolerance (Bertel, 1988), intrinsic vascular processes appear to be essentially involved in vascular tolerance to organic nitrates.
GTN tolerance and cross-tolerance to NO
For the judgement of the molecular mechanisms that could be involved in the development of vascular nitrate tolerance, it is essential to know whether or not the exposure of blood vessels to organic nitrates leads to cross-tolerance, that is, to desensitization of vasodilator responses to NO donors and/or endothelium-dependent vasodilators. If cross-tolerance does not occur, processes occurring downstream of GTN bioactivation, for example, reduction of NO bioavailability by superoxide or sGC inactivation, are to be excluded because they would affect any type of NO-mediated vasodilation. Unfortunately, there is no consensus in the literature on this issue. In guinea-pigs treated for 3 days with GTN, we observed a pronounced decrease in the vasodilatory response to GTN but no cross-tolerance to the NO donor DEA/NO (Wölkart et al., 2008). Obviously, these findings would be consistent with any mechanism of nitrate tolerance involving impaired GTN bioactivation but not with reduced sGC reactivity as a major cause of tolerance. Numerous previous studies also reported on the absence or only marginal cross-tolerance to GTN in various experimental models, including cultured vascular smooth muscle cells (Mülsch et al., 1989), laboratory animals (Molina et al., 1987; Sakai and Kuromaru, 1987; De Garavilla et al., 1993; Matsumoto et al., 1995; Miller et al., 2000) and isolated human blood vessels (Bohyn et al., 1991; Du et al., 1992). Of note, two studies measuring the vascular (Sage et al., 2000) and antiplatelet (Chirkov et al., 1997) response to GTN in humans in vivo also failed to detect any cross-tolerance to ACh and sodium nitroprusside, respectively. On the other hand, there are reports indicating that endothelium-dependent vasodilation is impaired in blood vessels obtained from GTN-tolerant rodents (Münzel et al., 1995; Laursen et al., 1996a) or from GTN-treated humans undergoing bypass surgery (Schulz et al., 2002). One study provided indirect evidence for GTN-induced endothelial dysfunction by showing abnormal coronary vasoconstriction to ACh measured in vivo in patients exposed to transdermal GTN (Caramori et al., 1998). These intriguingly controversial answers to an apparently simple question may reflect the general confusion in the field of nitrate tolerance.
In the following, we will first describe mechanisms that would be expected to cause cross-tolerance to NO donors and endothelium-dependent vasodilators and then discuss thiol depletion and ALDH2 inactivation as processes that would specifically interfere with GTN vasoactivity. On the basis of identical rates of NO formation detected in control and tolerant blood vessels exposed to GTN, one study concluded that tolerance is not due to impaired GTN bioactivation (Laursen et al., 1996b). However, considering the apparent lack of detectable vascular NO formation in response to GTN (Kleschyov et al., 2003; Núñez et al., 2005), this conclusion may have been premature, and, as described in the following sections, there is a large body of evidence supporting reduced GTN bioactivation as a main cause of vascular nitrate tolerance.
Desensitization of sGC
Exposure of sGC to GTN results in inactivation of the enzyme due to oxidation of the ferrous haem iron that is essential for NO binding (Waldman et al., 1986; Schröder et al., 1988; Gorren et al., 2005). On the basis of reduced sGC activity in GTN-treated cultured vascular smooth muscle cells (Waldman et al., 1986), cultured fibroblasts (Schröder et al., 1988) and isolated coronary arteries (Romanin and Kukovetz, 1989), sGC inactivation was suggested as a possible cause of vascular tolerance to GTN. However, this proposal was questioned by other studies indicating that GTN treatment does not cause cross-tolerance to direct NO donors or endothelium-dependent vasodilators (Kowaluk et al., 1987; Mülsch et al., 1988, 1989). These and several later studies (for review, see Fung, 2004) suggest that desensitization of sGC may become significant only after prolonged exposure of blood vessels to high GTN concentrations exceeding by far the therapeutically relevant submicromolar plasma levels of the drug (Wei and Reid, 1981).
The oxidative stress hypothesis of nitrate tolerance
According to an interesting hypothesis proposed first by Münzel et al. (1995), tolerance to organic nitrates is associated with increased vascular superoxide production, limiting NO bioavailability due to rapid formation of peroxynitrite, a potent cellular oxidant that may further aggravate oxidative stress and endothelial dysfunction (Schulz et al., 2008). Although some studies cast doubt on the oxidative stress hypothesis of nitrate tolerance (Hinz and Schröder, 1998; Csont et al., 2002; Hanspal et al., 2002; Keimer et al., 2003), numerous reports suggest that nitrate tolerance is prevented or at least ameliorated by co-administration of antioxidants. In particular, many studies demonstrated protection against tolerance development by ascorbate (vitamin C) in laboratory animals (Yeates and Schmid, 1992; Bassenge and Fink, 1996; Fink et al., 2000) and humans (Bassenge et al., 1998; Watanabe et al., 1998b, 1998d; McVeigh et al., 2002; Thomas et al., 2007). Determination of vascular superoxide production by electron paramagnetic resonance spin trapping showed that the protective effect of ascorbate correlates with a decrease in GTN-derived superoxide production (Dikalov et al., 1998; Fink et al., 2000). Other antioxidants reported to protect against nitrate tolerance include the β-adrenergic antagonist carvedilol (Watanabe et al., 1998a; Fink et al., 1999; El-Demerdash, 2006), the vasodilator hydralazine (Münzel et al., 1996), statins (Fontaine et al., 2003; Otto et al., 2005), folate (Gori et al., 2001), vitamin E (Watanabe et al., 1998c) and resveratrol (Coskun et al., 2006). Interestingly, virtually all of these antioxidative agents are also known to protect against endothelial dysfunction caused by various oxidative stress-related cardiovascular disorders (Aminbakhsh and Mancini, 1999; Laight et al., 2000; Warnholtz et al., 2001; Schulz et al., 2008). Although this similarity appears to provide strong evidence for superoxide-mediated inactivation of NO as a common mechanism of nitrate tolerance and endothelial dysfunction (Schulz et al., 2008), oxidative inactivation of ALDH2 as major cause of vascular nitrate tolerance offers an attractive alternative explanation for the protective effects of antioxidants (see below).
Potential sources of superoxide in GTN-exposed blood vessels
Besides NADPH oxidase, the key enzyme catalysing vascular superoxide production (Griendling and Ushio-Fukai, 1997), several other pathways may contribute to superoxide generation in GTN-exposed blood vessels (Münzel et al., 2005). An interesting possibility is uncoupling of endothelial NO synthase (eNOS) due to limited availability of the essential cofactor tetrahydrobiopterin (BH4). Under these conditions the enzyme generates superoxide in addition to NO, resulting in peroxynitrite formation (Mayer and Hemmens, 1997; Pfeiffer et al., 1999; Vasquez-Vivar et al., 2003; Li and Shah, 2004). How is GTN thought to affect BH4 levels? It has been proposed (Münzel et al., 2005) that the organic nitrate decreases the rate of endothelial BH4 biosynthesis through downregulation of GTP-cyclohydrolase I, the rate-limiting enzyme of BH4 synthesis, because cDNA microarrays showed that the expression of this gene, among dozens of other genes, was reduced by about 50% after GTN infusion to mice (Wang et al., 2002a). Alternatively, superoxide generated by NADPH oxidase or uncoupled mitochondria (see below) may react with eNOS-derived NO, yielding peroxynitrite, which was shown to oxidize BH4 in vitro (Milstien and Katusic, 1999; Kuzkaya et al., 2003). Thus, a vicious circle is conceivable involving peroxynitrite-triggered BH4 depletion, resulting in eNOS uncoupling, which, in turn, would further increase peroxynitrite formation. Several reports suggest that eNOS-derived superoxide contributes to the development of nitrate tolerance but the conclusion is mainly based on quantification of eNOS-derived superoxide by a questionable chemiluminescence method using lucigenin or lucigenin analogues (Kaesemeyer et al., 2000; Münzel et al., 2000; Gori et al., 2001; Parker et al., 2002). As electron paramagnetic resonance spin trapping is not suitable for superoxide detection under conditions of co-generation of NO due to rapid peroxynitrite formation outcompeting the reaction of superoxide with all traps available today (Pignitter et al., 2006), we used hydroethidine oxidation as a more reliable assay for intracellular superoxide formation but obtained no evidence for significant eNOS-derived superoxide formation in GTN-treated cultured endothelial cells (M Spachinger et al., unpublished observations). There are several additional facts casting doubt on the eNOS uncoupling hypothesis of nitrate tolerance: (i) there is no evidence for BH4 depletion of GTN-tolerant blood vessels in the literature and we found no reduction of BH4 levels in endothelial cells treated with 0.1 mM GTN for 48 h (K Schmidt and B Mayer, unpublished observations), (ii) exposure of endothelial cells with 1 mM of the peroxynitrite donor 3-morpholino-sydnonimine (SIN-1) did not cause a reduction of cellular BH4 levels (K Schmidt and B Mayer, unpublished observations), (iii) though BH4 was shown to improve endothelium-dependent relaxation in GTN tolerant rats, the pterin did not ameliorate impaired vasodilation to GTN (Gruhn et al., 2001) and (iv) eNOS gene deletion did not affect the development of nitrate tolerance in mice (Wang et al., 2002b). Taken together, the evidence against an involvement of eNOS uncoupling in nitrate tolerance appears to prevail over the supportive studies.
Another potential site of superoxide production is the mitochondrial respiratory chain. Reversible inhibition of cytochrome c oxidase by NO and inactivation of respiratory complexes by reactive nitrogen species, in particular peroxynitrite and S-nitrosothiols, may enhance mitochondrial superoxide production by uncoupling of the electron transport chain (Brown and Borutaite, 2004, 2007; Erusalimsky and Moncada, 2007). Thus, biotransformation of GTN by ALDH2 may trigger superoxide formation within mitochondria. In support of this idea, long-term infusion of GTN to heterozygous Mn-SOD+/− mice resulted in enhanced tolerance of aortic rings that was associated with increased mitochondrial superoxide production compared with wild type, suggesting that efficient scavenging of mitochondrial superoxide is essential for normal vasodilator response to GTN (Daiber et al., 2005). Another study (Esplugues et al., 2006) showed that mitochondrial O2 consumption by rat and human blood vessels was decreased upon prolonged in vivo or in vitro application of GTN, whereas acute administration of GTN had no effect. As the complex II electron donor succinate normalized decreased O2 consumption rates in GTN-treated cultured endothelial cells, the authors concluded that complex I might be the main target of GTN-derived superoxide and/or peroxynitrite.
Reduced GTN bioactivation due to vascular thiol depletion
The reaction of GTN with thiols was proposed to yield a thionitrate (RSNO2) intermediate, which may react with another RSH to yield the corresponding disulphide (RSSR) and HNO2 (Yeates et al., 1985). This reaction was considered as a key mechanism of GTN bioactivation, possibly through formation of S-nitrosothiols as intermediates (Ignarro and Gruetter, 1980). Accordingly, continuous application of organic nitrates could provoke thiol depletion in vascular smooth muscle cells, resulting in diminished thiol-dependent GTN bioactivation and nitrate tolerance (Needleman and Johnson, 1973). Screening of the available literature shows that this issue is still highly controversial. Despite substantial evidence for an essential role of thiols in GTN bioactivity (Loscalzo, 1985; Stamler et al., 1988; Sellke et al., 1991; Haramaki et al., 2001) and association of nitrate tolerance with reduced cellular thiol levels (Hutter et al., 1988; Boesgaard et al., 1993; Kojda et al., 1993; El-Demerdash, 2006), several studies found no correlation between thiol levels and GTN bioactivity (Gruetter and Lemke, 1985; Romanin and Kukovetz, 1988) or vascular nitrate tolerance (Gruetter and Lemke, 1986; Abdollah et al., 1987; Boesgaard et al., 1994). As described in the next section, the ALDH2 hypothesis of GTN bioactivation adds an interesting novel aspect to this issue.
Role of ALDH2 inactivation in the development of nitrate tolerance
As described above, the reaction of GTN with thiols as proposed by Yeates et al. (1985) was adapted to explain ALDH2-catalysed conversion of GTN to 1,2-GDN and nitrite, with vicinal cysteine residues in the active site of the enzyme serving as sulphydryl reactants that are oxidized to the corresponding disulphide in the course of enzyme turnover. As this mechanism implicates the essential requirement of reductant for sustained catalysis, it is conceivable that prolonged GTN biotransformation causes depletion of this a reductant, resulting in vascular GTN tolerance due to oxidative inactivation of ALDH2. There is general agreement that long-term application of GTN inactivates ALDH2 in rodent (DiFabio et al., 2003; Daiber et al., 2004; Sydow et al., 2004; Esplugues et al., 2006; Chen et al., 2007; Szöcs et al., 2007) and human (Hink et al., 2007) blood vessels. However, the involvement of ALDH2 inactivation in vascular tolerance to GTN is controversial. Bennett and co-workers (DiFabio et al., 2003) concluded that tolerance cannot be due to ALDH2 inactivation because they found that GTN-induced vasodilation was similarly sensitive to ALDH2 inhibitors in non-tolerant and tolerant blood vessels. Although one study confirmed these findings (De La Lande et al., 2004), others reported that ALDH inhibitors attenuated the response to GTN in non-tolerant but not in GTN-tolerant blood vessels (Sydow et al., 2004) and that ALDH2−/− mice do not develop nitrate tolerance (Chen et al., 2005).
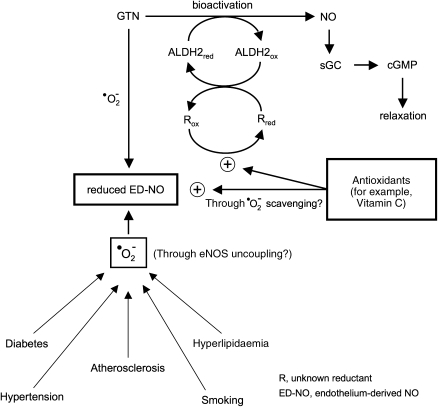
Inactivation of ALDH2 due to depletion of an essential reductant would provide an attractive explanation for the protection against nitrate tolerance by ascorbate and other antioxidants. As illustrated schematically in Figure 4, antioxidants may prevent tolerance development by recycling the ALDH2 reductant, whereas protection against endothelial dysfunction may occur mainly through reduction of oxidative stress by scavenging of superoxide and/or minimizing eNOS uncoupling. This concept of dual antioxidant action would explain prevention of nitrate tolerance by ascorbate in experimental models not associated with cross-tolerance to endothelium-dependent vasodilators. Assuming that a mitochondrial pool of a specific ALDH2 reductant is particularly sensitive to GTN-induced depletion, this concept may also revive a modified version of the thiol depletion hypothesis of nitrate tolerance.
Figure 4.
Nitrate tolerance and endothelial dysfunction: protective effects of antioxidants. Mitochondrial biotransformation of glyceryl trinitrate (GTN) requires recycling of aldehyde dehydrogenase (ALDH2) by an as yet unknown reductant. Depletion of this reductant by prolonged exposure of smooth muscle cells to GTN may lead to ALDH2 inactivation and vascular tolerance to GTN. In some experimental models (see text), nitrate tolerance is accompanied by endothelial dysfunction that may be triggered by GTN-derived superoxide, which limits bioavailability of endothelium-derived NO (ED-NO). Ascorbate and other antioxidants may improve endothelial function in GTN tolerance as well as in cardiovascular pathologies associated with increased vascular superoxide production. In addition, antioxidants may protect against nitrate tolerance through recycling of the reductant essential for GTN bioactivation. The proposed dual mode of action of antioxidants would explain their beneficial effects in experimental models of nitrate tolerance not associated with endothelial dysfunction.
The involvement of ascorbate in GTN vasoactivity is supported by a recent study showing that prolonged ascorbate deprivation of guinea-pigs caused 10- to 100-fold reduction of the vasodilatory potency of GTN without affecting vasodilation to a direct NO donor (Wölkart et al., 2008). As ascorbate-deficient blood vessels remained fully sensitive to ALDH2 inhibitors, and ascorbate deprivation did not reduce the rates of ALDH2-catalysed GTN denitration by liver mitochondria, we concluded that inactivation of ALDH2 does not take place in this model of ascorbate deficiency. However, in continuation of this work we found that GTN biotransformation was significantly attenuated in ascorbate-deficient blood vessels (Wenzl et al., in preparation). Thus, the effect of ascorbate deprivation we observed in guinea-pigs appears to resemble that of prolonged GTN application to rats reported by DiFabio et al. (2003), that is, development of vascular tolerance to GTN associated with reduced ALDH2 activity but unaffected sensitivity of the tolerant blood vessels to ALDH2 inhibitors. This led us to speculate that vascular GTN tolerance might be a consequence of ascorbate depletion. More than 50% reduction of ascorbate levels in plasma of GTN-tolerant guinea-pigs appeared to support this hypothesis (Wölkart et al., 2008), but the levels in arterial and venous blood vessels isolated form GTN-tolerant animals were not significantly different from controls (Wenzl et al., in preparation), suggesting that vascular smooth muscle cells are efficiently protected against ascorbate oxidation by GTN. Further work should clarify the mechanism underlying the puzzling relationship between ascorbate levels and GTN vasoactivity.
Future directions
Though the identification of ALDH2 as a key enzyme of GTN bioactivation certainly represented a significant progress in the field, this discovery may have raised more questions than it has answered. The intriguing failure to detect GTN-derived NO in blood vessels is still unresolved. In our view, direct formation of NO by ALDH2 suggests that the bioactive product of GTN biotransformation is indeed free NO radical. Why does it escape detection by well-established analytical methods? Why does it not affect mitochondrial respiration? Conceivably, GTN-derived NO may be rapidly converted into a more stable, diffusible compound inside mitochondria before being released in the cell cytosol to activate sGC. Though relatively unlikely, we cannot even exclude that conversion of GTN to NO by ALDH represents an in vitro artefact unrelated to GTN bioactivation in vivo. Obviously, proposals on reaction mechanisms remain speculative before unequivocal identification of the relevant bioactive reaction product. Another unresolved issue is the high efficiency of GTN bioactivation in smooth muscle cells compared with other cell types, for example, endothelial cells or fibroblasts, which show only moderate cGMP accumulation in response to high GTN concentrations. In line with this question, we cannot explain why GTN exhibits higher vasodilatory potency in large blood vessels as compared with small resistance arteries. It appears that an unknown mechanism specifically expressed in vascular smooth muscle of large arteries and veins is necessary for efficient coupling of GTN biotransformation to sGC activation. The molecular processes underlying tolerance development are comparably puzzling. Although nitrate tolerance has been known for more than a century (Stewart, 1905), many basic aspects of this phenomenon are still largely unknown or controversial. Does GTN application cause endothelial dysfunction? Does it cause eNOS uncoupling? Does it cause vascular thiol depletion? How do antioxidants exert their protective effects? Why does ascorbate deficiency cause nitrate tolerance? Is tolerance to GTN caused by inactivation of ALDH2? If yes, how is the enzyme recycled back to the active form? If not, what else could be responsible? Future research on these topics may have clinical impact on nitrate therapy and will add significantly to our understanding of cellular NO/cGMP signalling and the molecular physiology of vasodilation.
Acknowledgments
We are supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Austria (P16690, P20669 and W901 DK Molecular Enzymology).
Abbreviations
- ALDH
aldehyde dehydrogenase
- BH4
tetrahydrobiopterin
- cGMP
cyclic GMP
- eNOS
endothelial nitric oxide synthase
- GDN
glyceryl dinitrate
- GTN
glyceryl trinitrate (nitroglycerin)
- sGC
soluble guanylate cyclase
- NO
nitric oxide
- PETN
pentaerythritol tetranitrate
Conflict of interest
The authors state no conflict of interest.
References
- Abdollah A, Moffat JA, Armstrong PW. N-Acetylcysteine does not modify nitroglycerin-induced tolerance in canine vascular rings. J Cardiovasc Pharmacol. 1987;9:445–450. doi: 10.1097/00005344-198704000-00009. [DOI] [PubMed] [Google Scholar]
- Abrams J. How to use nitrates. Cardiovasc Drugs Ther. 2002;16:511–514. doi: 10.1023/a:1022982213484. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (ALDH) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci. 2008;101:51–64. doi: 10.1093/toxsci/kfm280. [DOI] [PubMed] [Google Scholar]
- Aminbakhsh A, Mancini GBJ. Chronic antioxidant use and changes in endothelial dysfunction: a review of clinical investigations. Can J Cardiol. 1999;15:895–903. [PubMed] [Google Scholar]
- Archer MC, Tannenbaum SR, Fan TY, Weisman M. Reaction of nitrite with ascorbate and its relation to nitrosamine formation. J Natl Cancer Inst. 1975;54:1203–1205. doi: 10.1093/jnci/54.5.1203. [DOI] [PubMed] [Google Scholar]
- Artz JD, Schmidt B, McCracken JL, Marletta MA. Effects of nitroglycerin on soluble guanylate cyclase—implications for nitrate tolerance. J Biol Chem. 2002;277:18253–18256. doi: 10.1074/jbc.C200170200. [DOI] [PubMed] [Google Scholar]
- Artz JD, Toader V, Zavorin SI, Bennett BM, Thatcher GRJ. In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics. Biochemistry. 2001;40:9256–9264. doi: 10.1021/bi002885x. [DOI] [PubMed] [Google Scholar]
- Bassenge E, Fink B. Tolerance to nitrates and simultaneous upregulation of platelet activity prevented by enhancing antioxidant state. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:363–367. doi: 10.1007/BF00168641. [DOI] [PubMed] [Google Scholar]
- Bassenge E, Fink N, Skatchkov M, Fink B. Dietary supplement with vitamin C prevents nitrate tolerance. J Clin Invest. 1998;102:67–71. doi: 10.1172/JCI977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta M, Gruber K, Kollau A, Russwurm M, Koesling D, Goessler W, et al. Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases J Biol Chem 2008a. e-pub ahead of print [DOI] [PMC free article] [PubMed]
- Beretta M, Sottler A, Schmidt K, Mayer B, Gorren ACF.Partially irreversible inactivation of mitochondrial aldehyde dehydrogenase by nitroglycerin. Implications for nitrate tolerance 2008b(submitted) [DOI] [PMC free article] [PubMed]
- Bermejo E, Sáenz DA, Alberto F, Rosenstein RE, Bari SE, Lazzari MA. Effect of nitroxyl on human platelets function. Thromb Haemost. 2005;94:578–584. doi: 10.1160/TH05-01-0062. [DOI] [PubMed] [Google Scholar]
- Bertel O. Nitrate tolerance. Schweiz Med Wochenschr. 1988;118:1892–1898. [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Bode-Böger SM, Kojda G. Organic nitrates in cardiovascular disease. Cell Mol Biol. 2005;51:307–320. [PubMed] [Google Scholar]
- Boesgaard S, Aldershvile J, Poulsen HE, Loft S, Anderson ME, Meister A. Nitrate tolerance in vivo is not associated with depletion of arterial or venous thiol levels. Circ Res. 1994;74:115–120. doi: 10.1161/01.res.74.1.115. [DOI] [PubMed] [Google Scholar]
- Boesgaard S, Poulsen HE, Aldershvile J, Loft S, Anderson ME, Meister A. Acute effects of nitroglycerin depend on both plasma and intracellular sulfhydryl compound levels in vivo—effect of agents with different sulfhydryl-modulating properties. Circulation. 1993;87:547–553. doi: 10.1161/01.cir.87.2.547. [DOI] [PubMed] [Google Scholar]
- Bohyn M, Berkenboom G, Fontaine J. Effect of nitrate tolerance and dipyridamole on the response to SIN1 in the human isolated saphenous vein. Cardiovasc Drugs Ther. 1991;5:457–462. doi: 10.1007/BF03029770. [DOI] [PubMed] [Google Scholar]
- Booth BP, Tabrizi-Fard MA, Fung HL. Calcitonin gene-related peptide-dependent vascular relaxation of rat aorta: an additional mechanism for nitroglycerin. Biochem Pharmacol. 2000;59:1603–1609. doi: 10.1016/s0006-2952(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–290. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Brunner F, Stessel H, Kukovetz WR. Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion. FEBS Lett. 1995;376:262–266. doi: 10.1016/0014-5793(95)01297-x. [DOI] [PubMed] [Google Scholar]
- Butler AR, Ridd JH. Formation of nitric oxide from nitrous acid in ischemic tissue and skin. Nitric Oxide Biol Chem. 2004;10:20–24. doi: 10.1016/j.niox.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Caramori PRA, Adelman AG, Azevedo ER, Newton GE, Parker AB, Parker JD. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J Am Coll Cardiol. 1998;32:1969–1974. doi: 10.1016/s0735-1097(98)00456-2. [DOI] [PubMed] [Google Scholar]
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Cheitlin MD. Should the patient with coronary artery disease use sildenafil. Prev Cardiol. 2003;6:161–165. doi: 10.1111/j.1520-037x.2003.01488.x. [DOI] [PubMed] [Google Scholar]
- Chen YR, Nie SD, Shan W, Jiang DJ, Shi RZ, Zhou Z, et al. Decrease in endogenous CGRP release in nitroglycerin tolerance: role of ALDH-2. Eur J Pharmacol. 2007;571:44–50. doi: 10.1016/j.ejphar.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med. 2006;16:259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry PD, Omar HA, Farrell KA, Stuart JS, Wolin MS. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol. 1990;259:H1056–H1062. doi: 10.1152/ajpheart.1990.259.4.H1056. [DOI] [PubMed] [Google Scholar]
- Chirkov YY, Chirkova LP, Horowitz JD. Nitroglycerin tolerance at the platelet level in patients with angina pectoris. Am J Cardiol. 1997;80:128–131. doi: 10.1016/s0002-9149(97)00305-6. [DOI] [PubMed] [Google Scholar]
- Chong S, Fung HL. Biochemical and pharmacological interactions between nitroglycerin and thiols—effects of thiol structure on nitric oxide generation and tolerance reversal. Biochem Pharmacol. 1991;42:1433–1439. doi: 10.1016/0006-2952(91)90456-f. [DOI] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Coskun B, Soylemez S, Parlar AI, Tulga Ulus A, Fehmi Katircioglu S, Akar F. Effect of resveratrol on nitrate tolerance in isolated human internal mammary artery. J Cardiovasc Pharmacol. 2006;47:437–445. doi: 10.1097/01.fjc.0000211798.91023.14. [DOI] [PubMed] [Google Scholar]
- Costa G, Labadía A, Triguero D, Jiménez E, García-Pascual A. Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of NO. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:516–523. doi: 10.1007/s002100100480. [DOI] [PubMed] [Google Scholar]
- Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH22 allele is dominant. J Clin Invest. 1989;83:314–316. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csont T, Csonka C, Ónody A, Görbe A, Dux L, Schulz R, et al. Nitrate tolerance does not increase production of peroxynitrite in the heart. Am J Physiol. 2002;283:H69–H76. doi: 10.1152/ajpheart.00817.2001. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Sulyok S, Coldewey M, Schulz E, Treiber N, et al. Heterozygous deficiency of manganese superoxide dismutase in mice (Mn-SOD+/−): a novel approach to assess the role of oxidative stress for the development of nitrate tolerance. Mol Pharmacol. 2005;68:579–588. doi: 10.1124/mol.105.011585. [DOI] [PubMed] [Google Scholar]
- De Garavilla L, Volberg ML, Pratt PF, Silver PJ, Buchholz RA. Lack of cross-tolerance between nitroglycerin and endothelium-derived relaxing factor-mediated vasoactive agents in spontaneously hypertensive rats. Eur J Pharmacol. 1993;234:77–82. doi: 10.1016/0014-2999(93)90708-p. [DOI] [PubMed] [Google Scholar]
- De La Lande IS, Stepien JM, Philpott AC, Hughes PA, Stafford I, Horowitz JD. Aldehyde dehydrogenase, nitric oxide synthase and superoxide in ex vivo nitrate tolerance in rat aorta. Eur J Pharmacol. 2004;496:141–149. doi: 10.1016/j.ejphar.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Dierks EA, Burstyn JN. Nitric oxide (NO center dot), the only nitrogen monoxide redox form capable of activating soluble guanylyl cyclase. Biochem Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- DiFabio J, Yanbin J, Vasiliou V, Thatcher RJ, Bennett BM. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol Pharmacol. 2003;64:1109–1116. doi: 10.1124/mol.64.5.1109. [DOI] [PubMed] [Google Scholar]
- Dikalov S, Fink B, Skatchkov M, Stalleicken D, Bassenge E. Formation of reactive oxygen species by pentaerithrityltetranitrate and glyceryl trinitrate in vitro and development of nitrate tolerance. J Pharmacol Exp Ther. 1998;286:938–944. [PubMed] [Google Scholar]
- Doel JJ, Godber BLJ, Eisenthal R, Harrison R. Reduction of organic nitrates catalysed by xanthine oxidoreductase under anaerobic conditions. Biochim Biophys Acta. 2001;1527:81–87. doi: 10.1016/s0304-4165(01)00148-9. [DOI] [PubMed] [Google Scholar]
- Du ZY, Buxton BF, Woodman OL. Tolerance to glyceryl trinitrate in isolated human internal mammary arteries. J Thorac Cardiovasc Surg. 1992;104:1280–1284. [PubMed] [Google Scholar]
- El-Demerdash E. Evidences for prevention of nitroglycerin tolerance by carvedilol. Pharmacol Res. 2006;53:380–385. doi: 10.1016/j.phrs.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Elkayam U, Bitar F, Akhter MW, Khan S, Patrus S, Derakhshani M. Intravenous nitroglycerin in the treatment of decompensated heart failure: potential benefits and limitations. J Cardiovasc Pharmacol Ther. 2004;9:227–241. doi: 10.1177/107424840400900403. [DOI] [PubMed] [Google Scholar]
- Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- Esplugues JV, Rocha M, Nuñez C, Bosca I, Ibiza S, Herance JR, et al. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Fink B, Dikalov S, Bassenge E. A new approach for extracellular spin trapping of nitroglycerin-induced superoxide radicals both in vitro and in vivo. Free Rad Biol Med. 2000;28:121–128. doi: 10.1016/s0891-5849(99)00228-2. [DOI] [PubMed] [Google Scholar]
- Fink B, Schwemmer M, Fink N, Bassenge E. Tolerance to nitrates with enhanced radical formation suppressed by carvedilol. J Cardiovasc Pharmacol. 1999;34:800–805. doi: 10.1097/00005344-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fontaine D, Otto A, Fontaine J, Berkenboom G. Prevention of nitrate tolerance by long-term treatment with statins. Cardiovasc Drugs Ther. 2003;17:123–128. doi: 10.1023/a:1025383601304. [DOI] [PubMed] [Google Scholar]
- Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved. Annu Rev Pharmacol Toxicol. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Raat NJH, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, et al. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- Gori T, Burstein JM, Ahmed S, Miner SES, AlHesayen A, Kelly S, et al. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance—a human in vivo study. Circulation. 2001;104:1119–1123. doi: 10.1161/hc3501.095358. [DOI] [PubMed] [Google Scholar]
- Gorren ACF, Russwurm M, Kollau A, Koesling D, Schmidt K, Mayer B. Effects of nitroglycerin/L-cysteine on soluble guanylate cyclase: evidence for an activation/inactivation equilibrium controlled by nitric oxide binding and haem oxidation. Biochem J. 2005;390:625–631. doi: 10.1042/BJ20050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Ushio-Fukai M. NADH/NADPH oxidase and vascular function. Trends Cardiovasc Med. 1997;7:301–307. doi: 10.1016/S1050-1738(97)00088-1. [DOI] [PubMed] [Google Scholar]
- Gruetter CA, Lemke SM. Dissociation of cysteine and glutathione levels from nitroglycerin-induced relaxation. Eur J Pharmacol. 1985;111:85–95. doi: 10.1016/0014-2999(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Gruetter CA, Lemke SM. Effects of sulfhydryl reagents on nitroglycerin-induced relaxation of bovine coronary artery. Can J Physiol Pharmacol. 1986;64:1395–1401. doi: 10.1139/y86-236. [DOI] [PubMed] [Google Scholar]
- Gruhn N, Aldershvile J, Boesgaard S. Tetrahydrobiopterin improves endothelium-dependent vasodilation in nitroglycerin-tolerant rats. Eur J Pharmacol. 2001;416:245–249. doi: 10.1016/s0014-2999(01)00879-2. [DOI] [PubMed] [Google Scholar]
- Hanspal IS, Magid KS, Webb DJ, Megson IL. The effect of oxidative stress on endothelium-dependent and nitric oxide donor-induced relaxation: implications for nitrate tolerance. Nitric Oxide Biol Chem. 2002;6:263–270. doi: 10.1006/niox.2001.0412. [DOI] [PubMed] [Google Scholar]
- Haramaki N, Ikeda H, Takajo Y, Katoh A, Kanaya S, Shintani S, et al. Long-term smoking causes nitroglycerin resistance in platelets by depletion of intraplatelet glutathione. Arterioscler Thromb Vasc Biol. 2001;21:1852–1856. doi: 10.1161/hq1001.097021. [DOI] [PubMed] [Google Scholar]
- Hempel J, Nicholas H, Lindahl R. Aldehyde dehydrogenases: widespread structural and functional diversity within a shared framework. Prot Sci. 1993;2:1890–1900. doi: 10.1002/pro.5560021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink U, Daiber A, Kayhan N, Trischler J, Kraatz C, Oelze M, et al. Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human blood vessels. J Am Coll Cardiol. 2007;50:2226–2232. doi: 10.1016/j.jacc.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Hinz B, Schröder H. Vitamin C attenuates nitrate tolerance independently of its antioxidant effect. FEBS Lett. 1998;428:97–99. doi: 10.1016/s0014-5793(98)00506-7. [DOI] [PubMed] [Google Scholar]
- Huellner MW, Schrepfer S, Weyand M, Weiner H, Wimplinger I, Eschenhagen T, et al. Inhibition of aldehyde dehydrogenase type 2 attenuates vasodilatory action of nitroglycerin in human veins FASEB J 2008. e-pub ahead of print 13 February 2008 [DOI] [PubMed]
- Hutter J, Schmidt M, Rittler J. Effects of sulfhydryl-containing compounds on nitroglycerin-induced coronary dilatation in isolated working rat hearts. Eur J Pharmacol. 1988;156:215–222. doi: 10.1016/0014-2999(88)90324-x. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Gruetter CA. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Kemp-Harper BK. NO activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- Jurt U, Gori T, Ravandi A, Babaei S, Zeman P, Parker JD. Differential effects of pentaerythritol tetranitrate and nitroglycerin on the development of tolerance and evidence of lipid peroxidation: a human in vivo study. J Am Coll Cardiol. 2001;38:854–859. doi: 10.1016/s0735-1097(01)01414-0. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Ogonowski AA, Jin LM, Caldwell RB, Caldwell RW. Endothelial nitric oxide synthase is a site of superoxide synthesis in endothelial cells treated with glyceryl trinitrate. Br J Pharmacol. 2000;131:1019–1023. doi: 10.1038/sj.bjp.0703665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S, Arnold WP, Mittal CK, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cycl Nucl Res. 1977;3:23–35. [PubMed] [Google Scholar]
- Keimer R, Stutzer FK, Tsikas D, Troost R, Gutzki FM, Frölich JC. Lack of oxidative stress during sustained therapy with isosorbide dinitrate and pentaerythrityl tetranitrate in healthy humans: a randomized, double-blind crossover study. J Cardiovasc Pharmacol. 2003;41:284–292. doi: 10.1097/00005344-200302000-00018. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin. Circulation. 2003;93:104–112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- Kojda G, Meyer W, Noack E. Influence of endothelium and nitrovasodilators on free thiols and disulfides in porcine coronary smooth muscle. Eur J Pharmacol. 1993;250:385–394. doi: 10.1016/0014-2999(93)90025-d. [DOI] [PubMed] [Google Scholar]
- Kollau A, Beretta M, Gorren ACF, Russwurm R, Koesling D, Schmidt K, et al. Bioactivation of nitroglycerin by ascorbate. Mol Pharmacol. 2007;72:191–196. doi: 10.1124/mol.107.035642. [DOI] [PubMed] [Google Scholar]
- Kollau A, Beretta M, Russwurm M, Koesling D, Keung WM, Schmidt K, et al. Mitochondrial nitrite reduction coupled to soluble guanylate cyclase activation 2008(submitted) [DOI] [PubMed]
- Kollau A, Hofer A, Russwurm M, Koesling D, Keung WM, Schmidt K, et al. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura S. Effects of ethyleneglycol dinitrate and related compounds on ethanol preference and ethanol metabolism. Acta Pharmacol Toxicol. 1974;35:145–154. doi: 10.1111/j.1600-0773.1974.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Kowaluk EA, Poliszczuk R, Fung HL. Tolerance to relaxation in rat aorta: comparison of an S-nitrosothiol with nitroglycerin. Eur J Pharmacol. 1987;144:379–383. doi: 10.1016/0014-2999(87)90392-x. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols—implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- Laight DW, Carrier MJ, Änggard EE. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc Res. 2000;47:457–464. doi: 10.1016/s0008-6363(00)00054-7. [DOI] [PubMed] [Google Scholar]
- Larson HN, Zhou J, Chen Z, Stamler JS, Weiner H, Hurley TD. Structural and functional consequences of coenzyme binding to the inactive Asian variant of mitochondrial aldehyde dehydrogenase: roles of residues 475 and 487. J Biol Chem. 2007;282:12940–12950. doi: 10.1074/jbc.M607959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen JB, Boesgaard S, Poulsen HE, Aldershvile J. Nitrate tolerance impairs nitric oxide-mediated vasodilation in vivo. Cardiovasc Res. 1996a;31:814–819. doi: 10.1016/0008-6363(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Mülsch A, Boesgaard S, Mordvintcev P, Trautner S, Gruhn N, et al. In vivo nitrate tolerance is not associated with reduced bioconversion of nitroglycerin to nitric oxide. Circulation. 1996b;94:2241–2247. doi: 10.1161/01.cir.94.9.2241. [DOI] [PubMed] [Google Scholar]
- Li H, Cui H, Liu X, Zweier JL. Xanthine oxidase catalyzes anaerobic transformation of organic nitrates to nitric oxide and nitrosothiols: characterization of this mechanism and the link between organic nitrate and guanylyl cyclase activation. J Biol Chem. 2005;280:16594–16600. doi: 10.1074/jbc.M411905200. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985;76:703–708. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, et al. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol. 2005;25:1891–1895. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Weiner H. Differences in the roles of conserved glutamic acid residues in the active site of human class 3 and class 2 aldehyde dehydrogenases. Prot Sci. 1999;8:1922–1929. doi: 10.1110/ps.8.10.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N, Marsh A. A short history of nitroglycerin and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- Matsumoto T, Takahashi M, Nakae I, Kinoshita M. Vasorelaxing effect of S-nitrosocaptopril on dog coronary arteries: no cross-tolerance with nitroglycerin. J Pharmacol Exp Ther. 1995;275:1247–1253. [PubMed] [Google Scholar]
- Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Hamilton P, Wilson M, Hanratty CG, Leahey WJ, Devine AB, et al. Platelet nitric oxide and superoxide release during the development of nitrate tolerance—effect of supplemental ascorbate. Circulation. 2002;106:208–213. doi: 10.1161/01.cir.0000021600.84149.78. [DOI] [PubMed] [Google Scholar]
- Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- Miller MR, Megson IL, Roseberry MJ, Mazzei FA, Butler AR, Webb DJ. Novel S-nitrosothiols do not engender vascular tolerance and remain effective in glyceryl trinitrate-tolerant rat femoral arteries. Eur J Pharmacol. 2000;403:111–119. doi: 10.1016/s0014-2999(00)00572-0. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Mirvish SS, Shubik P, Wallcave L, Eagen M. Ascorbate-nitrite reaction—possible means of blocking formation of carcinogenic N-nitroso compounds. Science. 1972;177:65. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- Molina CR, Andresen JW, Rapoport RM, Waldman S, Murad F. Effect of in vivo nitroglycerine therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987;10:371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wenzel P, Oelze M, Treiber N, Pautz A, Schulz E, et al. Mitochondrial oxidative stress and nitrate tolerance—comparison of nitroglycerin and pentaerithrityl tetranitrate in Mn-SOD+/− mice. BMC Cardiovasc Disord. 2006;6 doi: 10.1186/1471-2261-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee N, Pietruszko R. Inactivation of human aldehyde dehydrogenase by iosorbide dinitrate. J Biol Chem. 1994;269:21664–21669. [PubMed] [Google Scholar]
- Mülsch A, Busse R, Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol. 1988;158:191–198. doi: 10.1016/0014-2999(88)90066-0. [DOI] [PubMed] [Google Scholar]
- Mülsch A, Busse R, Winter I, Bassenge E. Endothelium- and sydnonimine-induced responses of native and cultured aortic smooth muscle cells are not impaired by nitroglycerin tolerance. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:568–574. doi: 10.1007/BF00167263. [DOI] [PubMed] [Google Scholar]
- Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- Münzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, et al. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase—a new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel T, Li H, Mollnau H, Hink U, Matheis E, Hartmann M, et al. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- Münzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance—a novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling (Nobel lecture) Angew Chem Int Ed Engl. 1999;38:1857–1868. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1856::AID-ANIE1856>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Murphy TC, Arntzen R, Picklo MJ., Sr Nitrate-based vasodilators inhibit multiple vascular aldehyde dehydrogenases. Cardiovasc Toxicol. 2005;5:321–332. doi: 10.1385/ct:5:3:321. [DOI] [PubMed] [Google Scholar]
- Needleman P, Johnson EM. Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709–715. [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet. 2006;21:357–374. doi: 10.2133/dmpk.21.357. [DOI] [PubMed] [Google Scholar]
- Noack E, Feelisch M. Molecular mechanisms of nitrovasodilator bioactivation. Basic Res Cardiol. 1991;86 Suppl 2:37–50. doi: 10.1007/978-3-642-72461-9_5. [DOI] [PubMed] [Google Scholar]
- Núñez C, Víctor VM, Tur R, Alvarez-Barrientos A, Moncada S, Esplugues JV, et al. Discrepancies between nitroglycerin and NO-releasing drugs on mitochondrial oxygen consumption, vasoactivity, and the release of NO. Circ Res. 2005;97:1063–1069. doi: 10.1161/01.RES.0000190588.84680.34. [DOI] [PubMed] [Google Scholar]
- O'Byrne S, Shirodaria C, Millar T, Stevens C, Blake D, Benjamin N. Inhibition of platelet aggregation with glyceryl trinitrate and xanthine oxidoreductase. J Pharmacol Exp Ther. 2000;292:326–330. [PubMed] [Google Scholar]
- Otto A, Fontaine D, Fontaine J, Berkenboom G. Rosuvastatin treatment protects against nitrate-induced oxidative stress. J Cardiovasc Pharmacol. 2005;46:177–184. doi: 10.1097/01.fjc.0000167010.98177.78. [DOI] [PubMed] [Google Scholar]
- Parker JO, Parker JD, Caldwell RW, Farrell B, Kaesemeyer WH. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39:1199–1203. doi: 10.1016/s0735-1097(02)01729-1. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Mayer B, Hemmens B. Nitric oxide: chemical puzzles posed by a biological messenger. Angew Chem Int Ed Engl. 1999;38:1715–1731. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1714::AID-ANIE1714>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Pignitter M, Gorren ACF, Nedeianu S, Schmidt K, Mayer B. Inefficient spin trapping of superoxide in the presence of nitric-oxide: implications for studies on nitric-oxide synthase uncoupling. Free Rad Biol Med. 2006;41:455–463. doi: 10.1016/j.freeradbiomed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Racker E. Aldehyde dehydrogenase, a diphosphopyridine nucleotide-linked enzyme. J Biol Chem. 1949;177:883–892. [PubMed] [Google Scholar]
- Romanin C, Kukovetz WR. Guanylate cyclase activation by organic nitrates is not mediated via nitrite. J Mol Cell Cardiol. 1988;20:389–396. doi: 10.1016/s0022-2828(88)80130-5. [DOI] [PubMed] [Google Scholar]
- Romanin C, Kukovetz WR. Tolerance to nitroglycerin is caused by reduced guanylate cyclase activation. J Mol Cell Cardiol. 1989;21:41–48. doi: 10.1016/0022-2828(89)91491-0. [DOI] [PubMed] [Google Scholar]
- Sage PR, De la Lande IS, Stafford I, Bennett CL, Phillipov G, Stubberfield J, et al. Nitroglycerin tolerance in human vessels: evidence for impaired nitroglycerin bioconversion. Circulation. 2000;102:2810–2815. doi: 10.1161/01.cir.102.23.2810. [DOI] [PubMed] [Google Scholar]
- Sakai K, Kuromaru O. Nitrate tolerance: comparison of nicorandil, isosorbide dinitrate, and nitroglycerin in anesthetized dogs. J Cardiovasc Pharmacol. 1987;10:S17–S24. [PubMed] [Google Scholar]
- Schröder H, Leitman DC, Bennett BM, Waldman SA, Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther. 1988;245:413–418. [PubMed] [Google Scholar]
- Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antiox Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tsilimingas N, Rinze R, Reiter B, Wendt M, Oelze M, et al. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- Sellke FW, Tomanek RJ, Harrison DG. -Cysteine selectively potentiates nitroglycerin-induced dilation of small coronary microvessels. J Pharmacol Exp Ther. 1991;258:365–369. [PubMed] [Google Scholar]
- Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact. 2003;143–144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Soyka M, Roesner S. New pharmacological approaches for the treatment of alcoholism. Expert Opin Pharmacother. 2006;7:2341–2353. doi: 10.1517/14656566.7.17.2341. [DOI] [PubMed] [Google Scholar]
- Speakman MT, Kloner RA. Viagra and cardiovascular disease. J Cardiovasc Pharmacol Ther. 1999;4:259–267. doi: 10.1177/107424849900400407. [DOI] [PubMed] [Google Scholar]
- Stamler J, Cunningham M, Loscalzo J. Reduced thiols and the effect of intravenous nitroglycerin on platelet aggregation. Am J Cardiol. 1988;62:377–380. doi: 10.1016/0002-9149(88)90962-9. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Toone EJ. The decomposition of thionitrites. Curr Opin Chem Biol. 2002;6:779–785. doi: 10.1016/s1367-5931(02)00383-6. [DOI] [PubMed] [Google Scholar]
- Stewart DD. Tolerance to nitroglycerin. JAMA. 1905;44:1678–1679. [Google Scholar]
- Sydow K, Daiber A, Oelze M, Chen ZP, August M, Wendt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöcs K, Lassègue B, Wenzel P, Wendt M, Daiber A, Oelze M, et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol. 2007;42:1111–1118. doi: 10.1016/j.yjmcc.2007.03.904. [DOI] [PubMed] [Google Scholar]
- Thadani U. Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure. Cardiovasc Drugs Ther. 1997;10:735–742. doi: 10.1007/BF00053031. [DOI] [PubMed] [Google Scholar]
- Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO release: contemporary aspects in biological and medicinal chemistry. Free Rad Biol Med. 2004;37:1122–1143. doi: 10.1016/j.freeradbiomed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Thatcher GR, Weldon H. NO problem for nitroglycerin: organic nitrate chemistry and therapy. Chem Soc Rev. 1998;27:331–337. [Google Scholar]
- Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans. Evidence of a free radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- Towell J, Garthwaite T, Wang R. Erythrocyte aldehyde dehydrogenase and disulfiram-like side effects of hypoglycemics and antianginals. Alcohol Clin Exp Res. 1985;9:438–442. doi: 10.1111/j.1530-0277.1985.tb05579.x. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Rad Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- Waldman SA, Rapoport RM, Ginsburg R, Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol. 1986;35:3525–3531. doi: 10.1016/0006-2952(86)90622-2. [DOI] [PubMed] [Google Scholar]
- Wang EQ, Lee WI, Brazeau D, Fung HL. cDNA microarray analysis of vascular gene expression after nitric oxide donor infusions in rats: implications for nitrate tolerance mechanisms. AAPS PharmSci. 2002a;4:E10. doi: 10.1208/ps040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EQ, Lee WI, Fung HL. Lack of critical involvement of endothelial nitric oxide synthase in vascular nitrate tolerance in mice. Br J Pharmacol. 2002b;135:299–302. doi: 10.1038/sj.bjp.0704532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnholtz A, Mollnau H, Oelze M, Wendt M, Münzel T. Antioxidants and endothelial dysfunction in hyperlipidemia. Curr Hyperten Rep. 2001;3:53–60. doi: 10.1007/s11906-001-0081-z. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of carvedilol on the prevention of nitrate tolerance in patients with chronic heart failure. J Am Coll Cardiol. 1998a;32:1194–1200. doi: 10.1016/s0735-1097(98)00392-1. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of ascorbate on the preventive effect of nitrate tolerance in patients with congestive heart failure. Circulation. 1998b;97:886–891. doi: 10.1161/01.cir.97.9.886. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of supplemental vitamin E on attenuation of the development of nitrate tolerance. J Cardiol. 1998c;31:173–181. [PubMed] [Google Scholar]
- Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of the preventive effect of supplemental oral vitamin C on attenuation of development of nitrate tolerance. J Am Coll Cardiol. 1998d;31:1323–1329. doi: 10.1016/s0735-1097(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Wei JY, Reid PR. Relation of time course of plasma nitroglyerin levels to echocardiographic arterial pressure and heart rate changes after sublingual administration of nitroglycerin. Am J Cardiol. 1981;48:778–782. doi: 10.1016/0002-9149(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Hink U, Oelze M, Schuppan S, Schaeuble K, Schildknecht S, et al. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity: implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007a;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Hink U, Oelze M, Seeling A, Isse T, Bruns K, et al. Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2−/− mice. Br J Pharmacol. 2007b;150:526–533. doi: 10.1038/sj.bjp.0707116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Katori T, Mancardi D, Thomas DD, Ridnour L, et al. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: a novel redox paradigm. Am J Physiol. 2003;285:H2264–H2276. doi: 10.1152/ajpheart.00531.2003. [DOI] [PubMed] [Google Scholar]
- Wölkart G, Wenzl MV, Beretta M, Stessel H, Schmidt K, Mayer B.Vascular tolerance to nitroglycerin in ascorbate deficiency Cardiovasc Res 2008. e-pub ahead of print 21 May 2008 [DOI] [PubMed]
- Wymore T, Deerfield DW, II, Hempel J. Mechanistic implications of the cysteine-nicotinamide adduct in aldehyde dehydrogenase based on quantum mechanical/molecular mechanical simulations. Biochemistry. 2007;46:9495–9506. doi: 10.1021/bi700555g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates RA, Laufen H, Leitold M. The reaction between organic nitrates and sulfhydryl compounds. A possible model system for the activation of organic nitrates. Mol Pharmacol. 1985;28:555–559. [PubMed] [Google Scholar]
- Yeates RA, Schmid M. Total prevention of the development of in vitro tolerance to organic nitrates. Experiments with antioxidants. Arzneimittelforschung. 1992;42:297–302. [PubMed] [Google Scholar]
- Zamora R, Grzesiok A, Weber H, Feelisch M. Oxidative release of nitric oxide accounts for guanylyl cyclase stimulating, vasodilator and anti-platelet activity of Piloty's acid: a comparison with Angeli's salt. Biochem J. 1995;312:333–339. doi: 10.1042/bj3120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen ZP, Cobb FR, Stamler JS. Role of mitochondrial aldehyde dehydrogenase in nitroglycerin-induced vasodilation. Circulation. 2004;110:750–755. doi: 10.1161/01.CIR.0000138105.17864.6B. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MCR. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]