Abstract
Background and purpose:
To test whether development of enhanced vasoconstriction to 5-hydroxytryptamine (5-HT; serotonin) in SHR was temporally related to hypertension, elevated vascular superoxide (O2−) levels, decreased NO bioavailability, or increased contractile effects of cyclooxygenase or rho-kinase and/or PKC.
Experimental approach:
We examined systolic blood pressure (SBP), vascular O2−, and 5-HT-induced contractile responses of aortic segments from 4- and 8-week-old WKY and SHR.
Key results:
SBP was 35% higher in SHR than WKY at 4 weeks and 60% higher at 8 weeks. Contractile responses to 5-HT were similar in WKY and SHR at 4 weeks, but were markedly augmented in SHR at 8 weeks. The NO synthase inhibitor, L-NAME, enhanced contractile responses to 5-HT markedly in both strains at 4 weeks and in WKY at 8 weeks, but only very modestly in SHR at 8 weeks. These functional differences were associated with higher O2− levels in SHR versus WKY at 8 weeks, but not at 4 weeks. The rho-kinase inhibitor, Y-27632, and the PKC inhibitor, Ro 31-8220, each only modestly attenuated contractions in WKY and SHR in each age group, and their effects in each strain were more pronounced at 8 weeks. The cyclooxygenase inhibitor, indomethacin, had no effect on contractile responses.
Conclusions and implications:
Development of augmented vascular contractile responses to 5-HT in SHR is preceded by hypertension. It is associated with increased vascular O2− levels and reduced modulatory effects of NO, and is unlikely to be due to enhanced activity of rho-kinase, PKC or cyclooxygenase.
Keywords: hypertension, nitric oxide, PKC, reactive oxygen species, rho-kinase
Introduction
Endothelial dysfunction is a common characteristic of advanced cardiovascular diseases associated with reduced bioavailability of the endogenous vasodilator, nitric oxide (NO), such as chronic hypertension. Endothelial NO is a critical modulator of vascular contractility, and its absence—for example, due to physical removal of the endothelium or endothelial NO synthase (eNOS) gene deletion—leads to enhanced vascular contractile responses, particularly to G protein-coupled receptor agonists such as 5-HT (Lamping et al., 1985; Lamping and Faraci, 2003; Budzyn et al., 2004). Increased levels of reactive oxygen species such as superoxide (O2−), which occurs in the vasculature of chronically hypertensive animals (Zalba et al., 2000; Landmesser et al., 2003; Matsuno et al., 2005), can also contribute to compromised NO bioavailability and consequent vascular dysfunctions (Cai and Harrison, 2000).
It is also recognised that ‘Ca2+ sensitisation'—the mechanism of vascular contraction that occurs independently of increasing intracellular Ca2+ levels is another major mechanism that regulates vascular contractility, particularly during hypertension (Soloviev and Bershtein, 1992; Shaw et al., 1997). In particular, the role of rho-kinase—the predominant mediator of Ca2+ sensitisation—in the regulation of vascular tone during hypertension has been studied extensively (Uehata et al., 1997; Chrissobolis and Sobey, 2001; Wehrwein et al., 2004; Jin et al., 2006). Furthermore, although early studies suggested that PKC might also contribute to increased vascular contractility during hypertension (Bruschi et al., 1988; Shibata et al., 1990; Secrest et al., 1991; Soloviev and Bershtein, 1992), very few studies have sought to clarify the relative roles of each kinase in hypertension.
Although many studies have described abnormalities in vascular function in established chronic hypertension (Shaw et al., 1997; Endemann et al., 2002; Jarajapu and Knot, 2005; Northcott et al., 2005), very little is known about the timing of the occurrence of certain abnormalities during its development. Hence, the main aims of this study were to test whether the development of enhanced vasoconstriction to 5-HT, early in genetic hypertension, is temporally related to increased blood pressure (BP), elevated vascular O2− levels, decreased NO bioavailability, or increased contractile effects of rho-kinase and/or PKC.
Methods
All experimental procedures were approved by the Monash University and the University of Melbourne Animal Experimentation Ethics Committees and complied with National Health and Medical Research Council of Australia guidelines. Male Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHR) were studied at 4 weeks (n=47) or 8 weeks (n=48) of age. Rats were housed under a 12-h light/dark cycle with access to food and water ad libitum. Systolic BP (SBP) was measured by tail-cuff plethysmography as described previously (Okuniewski et al., 1998).
In vitro measurement of arterial O2−
Rats were killed by inhalation of 80% CO2:20% O2 and decapitation. Segments of thoracic aorta were excised and cut into segments of equal length (∼5 mm). O2− levels in the presence of NADPH (100 μM) were measured by 5 μM lucigenin-enhanced chemiluminescence as described previously (Paravicini et al., 2004; Miller et al., 2005). Vessels from age-matched WKY and SHR were studied in parallel on the same day. The 4- and 8-week-old rats were studied on separate days. Aortic O2− levels were normalised for dry tissue weight. Some additional experiments were performed using 100 μM L012-enhanced chemiluminescence (Wassmann et al., 2004) to compare basal O2− levels in aortae from 4-week-old WKY and SHR.
In vitro measurement of contractile responses
The thoracic aorta was isolated, cleaned and cut into 3–4 mm wide rings. Aortic segments from 4-week-old rats were mounted at 5 mN passive tension in 5 mL chambers of a small vessel myograph (Model 610 M, Multi Myograph, Denmark) containing Krebs-bicarbonate solution of the following composition (mmol L−1): NaCl 118, KCl 4.5, MgSO4 0.45, KH2PO4 1.03, NaHCO3 25, glucose 11.1 and CaCl2 2.5, bubbled with 5% CO2 in O2 at 37 °C. Tension was continuously recorded on a chart recorder (Model 3721, Yokogawa, Japan). Aortic segments from 8 week-old rats were mounted at 5 mN passive tension in 10 mL organ chambers containing Krebs-bicarbonate solution bubbled with 5% CO2 in O2 at 37 °C. Tension was continuously recorded using a Grass FT03 force transducer and Powerlab Chart computer software (Version 5.2.2).
Following 45 min equilibration, arterial segments were exposed to an isotonic high K+-containing physiological saline solution (KPSS; [K+]KPSS=124 mM). KPSS-induced contraction reached a stable level after 10–20 min. Following washout and return to a stable baseline, segments were precontracted to ∼50% of their KPSS response with phenylephrine (1–3 μM). Relaxation in response to acetylcholine (10 μM) confirmed the presence of functional endothelium. Following washout and return to stable baseline, cumulative concentration–response curves were established to 5-HT (10 nM–0.3 mM).
Effects of NOS, cyclooxygenase, rho-kinase and PKC inhibition on contractile responses
We assessed the effect of 30 min pretreatment with the NO synthase (NOS) inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) on contractile responses to 5-HT. Similarly, the effects of the cyclooxygenase inhibitor, indomethacin (10 μM), or the rho-kinase inhibitor, Y-27632 (1 μM) and/or the PKC inhibitor, Ro 31-8220 (5 μM), on responses to 5-HT were also assessed after 30 min treatment in separate tissues.
Data analysis and statistical procedures
Contractile responses to 5-HT were normalised by expressing them as a percentage of the KPSS response of each arterial segment. Each n represents the number of animals used. All data were normally distributed, and expressed as mean±s.e.mean. Single comparisons were made using Student's paired or unpaired t-test, as appropriate. Multiple comparisons were made using one-way ANOVA, followed by Tukey's post hoc test. GraphPad Prism (version 4) was used to perform all statistical analyses and P<0.05 was considered significant.
Drugs
Y-27632 (R-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide) was obtained from Welfide Corporation (Osaka, Japan). Ro 31-8220 (3-[1-[3-(amidinothio)propyl-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl) maleimide) was obtained from Calbiochem (La Jolla, CA, USA). All other drugs were obtained from Sigma Chemical Co. (St Louis, MO, USA). Ro 31-8220 was dissolved as a stock solution of 1 mM in 100% dimethyl sulphoxide and diluted in de-ionised water. Indomethacin was dissolved in 0.1 M Na2CO3 and diluted in de-ionised water. All other drugs were dissolved and diluted in de-ionised water or saline.
Results
Systolic blood pressure and aortic O2− levels
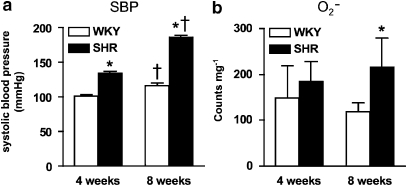
Systolic blood pressure was already higher in SHR than WKY at 4 weeks (P<0.05, unpaired t-test; Figure 1a), and this difference became even greater at 8 weeks (P<0.05, unpaired t-test; Figure 1a). Basal aortic O2− levels did not differ between WKY and SHR at 4 weeks using chemiluminescence assays based on either L-012 (WKY: 1318±196 counts per mg versus SHR: 1094±131 counts per mg; P>0.05, unpaired t-test) or lucigenin (Figure 1b) as a lumiphore. However, aortic O2− levels were significantly augmented at 8 weeks in SHR versus WKY (P<0.05, unpaired t-test; Figure 1b).
Figure 1.
Systolic blood pressure and aortic O2− levels in WKY and SHR. (a) Measurement of systolic blood pressure in WKY and SHR at 4 and 8 weeks. (b) O2− levels in aortic segments from WKY and SHR at 4 and 8 weeks. All values are mean±s.e.mean. n=8 of each strain per group. *P<0.05, unpaired t-test versus WKY, †P<0.05, unpaired t-test versus 4 weeks. SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.
Contractile responses to 5-HT
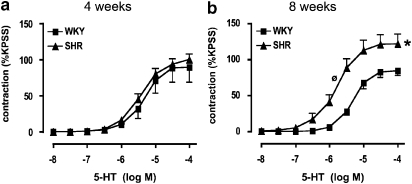
Contractile responses of aortic segments to 5-HT were similar in WKY and SHR at 4 weeks (Figure 2a). However, at 8 weeks, maximum responses to 5-HT were augmented by ∼50% in SHR relative to WKY (P<0.05, unpaired t-test; Figure 2b). There was also increased sensitivity to 5-HT-induced contractile responses in SHR versus WKY at 8 weeks (P<0.05, unpaired t-test; Figure 2b; Table 1). Moreover, absolute contractile responses to KPSS in aorta from 8 week-old rats was virtually identical in WKY (17.5±0.06 mN; n=24) and SHR (16.8±0.07 mN; n=24) (P>0.05, unpaired t-test), confirming that the responses to 5-HT were somewhat selectively augmented at 8 weeks.
Figure 2.
Contractile responses to 5-HT in WKY and SHR. Concentration–response curves to 5-HT in aortic segments from (a) 4-week- and (b) 8-week-old WKY and SHR. All values are mean±s.e.mean. n=15–16 of each strain per group. *P<0.05 versus WKY maximum, ⊘P<0.05 (unpaired t-test) versus WKY log EC50. SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.
Table 1.
Log EC50 values from concentration–response curves to 5-HT in arterial segments from WKY and SHR
| Age | WKY | SHR |
|---|---|---|
| 4 weeks | −5.32±0.07 (15) | −5.32±0.16 (16) |
| 8 weeks | −5.26±0.08 (16) | −5.72±0.10* (16) |
Abbreviations: SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.*P<0.05, unpaired t-test versus WKY.
Effect of NOS and cyclooxygenase inhibition on contractile responses to 5-HT
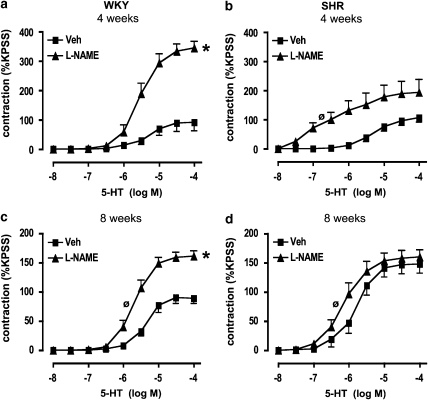
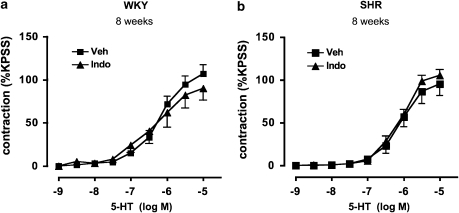
L-NAME markedly increased contractile responses to 5-HT in WKY at both 4 and 8 weeks of age (P<0.05, paired t-test; Figures 3a and c). Furthermore, although L-NAME also augmented the sensitivity to 5-HT-induced contractions in SHR, at both 4 and 8 weeks, this effect was pronounced (∼300-fold) at 4 weeks but minimal (∼2-fold) at 8 weeks (P<0.05, paired t-test; Figures 3b and d; Table 2). In contrast, L-NAME had no overall effect on maximum contractile responses in SHR at either age (Figures 3b and d). Indomethacin had no effect on 5-HT-induced contractile responses in either WKY or SHR at 8 weeks (Figures 4a and b).
Figure 3.
Effect of NOS inhibition on contractile responses to 5-HT. Concentration–response curves to 5-HT in aortic segments from (a and c) WKY and (b and d) SHR at 4 and 8 weeks of age, in the presence of vehicle (Veh; saline) or L-NAME (100 μM). All values are mean±s.e.mean. n=8 per group. *P<0.05, unpaired t-test versus Veh maximum, ⊘P<0.05, unpaired t-test versus Veh log EC50. NOS, nitric oxide synthase; SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.
Table 2.
Log EC50 values from concentration–response curves to 5-HT in arterial segments from WKY and SHR
| Treatment |
4 weeks |
8 weeks |
||
|---|---|---|---|---|
| WKY | SHR | WKY | SHR | |
| Vehicle | −5.36±0.12 (8) | −5.02±0.27 (8) | −5.34±0.07 (8) | −5.79±0.12 (8) |
| L-NAME | −5.52±0.12 (8) | −7.48±0.81* (8) | −5.69±0.08* (8) | −6.12±0.12* (8) |
Abbreviations: SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.*P<0.05, paired t-test versus vehicle.
Figure 4.
Effect of cyclooxygenase inhibition on contractile responses to 5-HT. Concentration–response curves to 5-HT in aortic segments from (a) WKY and (b) SHR at 8 weeks of age, in the presence of vehicle (Veh; 1 mM Na2CO3) or indomethacin (10 μM). All values are mean ±s.e.mean. n=7–8 per group.
Effect of rho-kinase and PKC inhibition on contractile responses to 5-HT
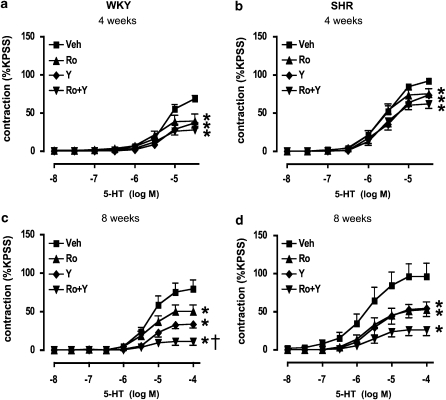
Maximum contractile responses to 5-HT were only modestly reduced by either the rho-kinase inhibitor, Y-27632, or the PKC inhibitor, Ro 31-8220, in both WKY and SHR at 4 weeks of age (P<0.05, one-way ANOVA followed by Tukey's test; Figures 5a and b). Similarly, cotreatment of aortic segments from 4-week-old rats with Y-27632 and Ro 31-8220 also caused only a minimal inhibition of responses (Figures 5a and b). Y-27632 or Ro 31-8220 alone each caused a more pronounced attenuation of contractile responses to 5-HT at 8 weeks of age in both WKY and SHR (Figures 5c and d). Cotreatment with Y-27632 and Ro 31-8220 caused further inhibition of contractile responses than treatment with either inhibitor alone (Figures 5c and d) in WKY (Figure 5c; one-way ANOVA followed by Tukey's test).
Figure 5.
Effects of rho-kinase and PKC inhibition on contractile responses to 5-HT. Concentration–response curves to 5-HT in aortic segments from (a and c) WKY and (b and d) SHR at 4 and 8 weeks of age, in the presence of vehicle (Veh; 0.5% dimethyl sulphoxide), Ro 31-8220 (Ro, 5 μM) or Y-27632 (Y, 1 μM) alone, or in combination. All values are mean±s.e.mean. n=7–8 per group. *P<0.05 versus Veh maximum, †P<0.05 versus Y-27632 maximum; one-way ANOVA followed by Tukey's test. SHR, spontaneously hypertensive rats; WKY, wistar–Kyoto.
Discussion and conclusions
Although many studies have described altered mechanisms of vascular function in SHR once hypertension is established, very few have examined temporal changes in such mechanisms during the developing phases of hypertension. We have investigated the degree to which NO, cyclooxygenase, rho-kinase and PKC influence vascular contractile responses to 5-HT during the developing phase of hypertension in SHR, as compared with normotensive WKY. Although SBP is elevated in SHR at 4 weeks, vascular O2− levels and contractility to 5-HT remain unchanged, relative to age-matched WKY. However, both become markedly enhanced in SHR by 8 weeks of age, in association with further elevation of SBP. In addition, endothelial NO contributes significantly to modulating responses to 5-HT in WKY at both 4 and 8 weeks of age, but only in SHR at 4 weeks, consistent with the possibility that NO is inactivated by O2− by 8 weeks in SHR. Furthermore, rho-kinase and PKC each contribute to vascular contraction in both WKY and SHR, albeit to similar degrees between each strain. Cyclooxygenase activity appears to play little or no role in contractile responses of either WKY or SHR to 5-HT in the first 8 weeks of life.
Blood pressure development in WKY and SHR
The present results indicate that SBP of SHR was already higher than in WKY at 4 weeks of age, consistent with other studies that have also used the tail cuff method of BP measurement (for example, Dickhout and Lee, 1998). This non-invasive method of measuring BP is reported to yield reproducible results similar to those obtained using intra-arterial methods in conscious rats (Widdop and Li, 1997; Ibrahim et al., 2006), and thus is thought to be valid. There is disagreement in the literature as to whether BP of SHR is indeed elevated at 4 weeks of age, or even earlier (Bruno et al., 1979; Cheng et al., 2004; Cruzado et al., 2005). However, several studies have reported that BP of WKY and SHR diverge from between 2 and 6 weeks of age (Morton et al., 1990; Dickhout and Lee, 1998; Christiansen et al., 2002).
Roles of cyclooxygenase or endothelial NO in contractile responses to 5-HT in WKY and SHR
Contractile responses to 5-HT were virtually identical in aortae from 4-week-old WKY and SHR, but became markedly augmented in SHR relative to WKY at 8 weeks. This difference at 8 weeks was apparently not related to augmented cyclooxygenase activity, because indomethacin had no effect on contractions to 5-HT in either WKY or SHR. Furthermore, although the inhibition of NOS by L-NAME caused a significant increase in contractions to 5-HT in all WKY and in 4-week-old SHR (increasing potency by ∼300-fold and approximately doubling the maximum), it increased potency by a modest twofold and clearly had no effect on the magnitude of these responses in 8 week-old SHR. Previous studies have demonstrated that vascular sensitivity to 5-HT is augmented in the absence of endothelial NO, either as a consequence of eNOS gene deletion, physical removal of the endothelium or pharmacological inhibition of eNOS (Lamping et al., 1985; Lamping and Faraci, 2003; Budzyn et al., 2004). The present results are thus consistent with the notion that NO plays a substantial role in modulating contractions of aorta to 5-HT in the normotensive WKY, but not in the genetically hypertensive adult SHR. The lack of modulation of contractile responses by NO is therefore likely to account, at least in part, for the increased reactivity to 5-HT in SHR seen at 8 weeks of age. Furthermore, it is conceivable that this relative lack of functional endothelial NO can be accounted for by increased generation of vascular O2−, and subsequent inactivation of eNOS/NO.
Role of vascular O2−
Increased O2− levels have been detected in blood vessels of hypertensive animals (Laursen et al., 1997; Schnackenberg et al., 1998; Kerr et al., 1999; Landmesser et al., 2003), and the generation of O2− is thought to play a major role in the development of hypertension. Interestingly, although SBP was higher in SHR than in WKY at both 4 and 8 weeks of age, higher aortic O2− levels were only detected in SHR at 8 weeks of age. Our data, therefore, suggest that an elevated vascular O2− level (and indeed vascular hypercontractility to 5-HT) does not necessarily precede the development of hypertension in SHR as previously suggested by others (Jameson et al., 1993; Cosentino et al., 1998; Nabha et al., 2005), but may be an effect rather than the cause of elevated BP.
Given that the aorta functions predominantly as a conductance vessel and whose contractility is also known to be greatly influenced by endothelial NO (Lamping et al., 1985; Lamping and Faraci, 2003; Budzyn et al., 2004), a possible extension to the present study could include investigating how contractility and vascular O2− production compare in resistance vessels, where the relative importance of endothelial NO in modulating contractility is diminished (Vanhoutte, 2004). We and others have previously demonstrated that the contractile properties of small resistance arteries such as the mesenteric artery, do indeed differ to those of larger conductance vessels such as the aorta (Asano and Nomura, 2003; Budzyn et al., 2006). Given that such vessels contribute to total peripheral resistance, and therefore BP regulation, it is possible that even more stark functional and biochemical differences could be observed in this vascular region.
Role of rho-kinase and PKC in contractile responses to 5-HT
The contribution of rho-kinase in the regulation of vascular tone is known to become enhanced in several animal models of established chronic hypertension (Uehata et al., 1997; Chrissobolis and Sobey, 2001; Wehrwein et al., 2004; Jin et al., 2006), however, this is the first study to investigate the functional contribution of rho-kinase to contractile responses at such an early stage of its development. The rho-kinase inhibitor, Y-27632, modestly reduced contractile responses to 5-HT in aorta from 4-week-old WKY and SHR, and importantly this occurred to a similar degree in each strain. Similarly modest effects were observed using the PKC inhibitor, Ro 31-8220, in 4-week-old rats. In contrast, at 8 weeks, Y-27632 and Ro 31-8220 each more effectively reduced responses to 5-HT in WKY and SHR, with the effects of each inhibitor being essentially equivalent in each strain. It should be noted that Ro 31-8220 may not be strictly selective for PKC and also may only inhibit certain isoforms of PKC (Davies et al., 2000), and so the data should be interpreted with care. Thus, although the contribution of both rho-kinase and PKC to 5-HT-induced vasoconstriction appears to increase with age, selectively augmented activity of either enzyme is unlikely to account for the selectively enhanced contractility to 5-HT in SHR at 8 weeks, even if this will eventually develop as chronic hypertension progresses (Soloviev and Bershtein, 1992; Satoh et al., 1994).
The possibility that the altered contractility observed in SHR at 8 weeks is also in part due to structural changes within the aorta at this stage of development cannot be ruled out. For instance, it is well documented that contractile properties of the aorta can become altered in parallel with vascular hypertrophy that occurs during the development of hypertension (Sudhir and Angus, 1990; van Gorp et al., 2000). Thus, it would be of interest to investigate whether any such structural changes have occurred in the aorta at the time points studied, and if so, whether these are temporally correlated with the onset of changes in the contractile properties of these vessels.
In summary, the present results suggest that the development of augmented contractile responses to 5-HT in SHR is preceded by hypertension and associated with increased vascular O2− levels, in parallel with reduced modulatory effects of NO, but is probably not due to excessive activity of cyclooxygenase, rho-kinase or PKC—at least during the earlier stages of hypertension.
Acknowledgments
This study was supported by funds from Project grants from the National Health and Medical Research Council of Australia (NHMRC ID 208969, 350477). KB was supported by a Melbourne University Research Scholarship and is currently supported by a CJ Martin Overseas Training Fellowship from the NHMRC. AAM is a Postdoctoral Fellow of the Foundation for High Blood Pressure Research, Australia. CGS is a Senior Research Fellow of the NHMRC.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- O2−
superoxide
- PKC
protein kinase C
- Ro 31-8220
(3-[1-[3-(amidinothio)propyl-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl) maleimide)
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rats
- WKY
Wistar-Kyoto
- Y-27632
(R-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide)
Conflict of interest
The authors state no conflict of interest.
References
- Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. Hypertens Res. 2003;26:97–106. doi: 10.1291/hypres.26.97. [DOI] [PubMed] [Google Scholar]
- Bruno L, Agar S, Weller D. Absence of a prehypertensive stage in post-natal Kyoto hypertensive rats. Jpn Heart J. 1979;20 Suppl 1:90–92. [Google Scholar]
- Bruschi G, Bruschi ME, Capelli P, Regolisti G, Borghetti A. Increased sensitivity to protein kinase C activation in aortas of spontaneously hypertensive rats. J Hypertens Suppl. 1988;6:S248–S251. doi: 10.1097/00004872-198812040-00075. [DOI] [PubMed] [Google Scholar]
- Budzyn K, Marley PD, Sobey CG. Chronic mevastatin modulates receptor-dependent vascular contraction in eNOS-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R342–R348. doi: 10.1152/ajpregu.00156.2004. [DOI] [PubMed] [Google Scholar]
- Budzyn K, Paull M, Marley PD, Sobey CG. Segmental differences in the roles of rho-kinase and protein kinase C in mediating vasoconstriction. J Pharmacol Exp Ther. 2006;317:791–796. doi: 10.1124/jpet.105.100040. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Chen JJ, Yen MH. The expression of heme oxygenase-1 and inducible nitric oxide synthase in aorta during the development of hypertension in spontaneously hypertensive rats. Am J Hypertens. 2004;17:1127–1134. doi: 10.1016/j.amjhyper.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ Res. 2001;88:774–779. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- Christiansen RE, Roald AB, Tenstad O, Iversen BM. Renal hemodynamics during development of hypertension in young spontaneously hypertensive rats. Kidney Blood Press Res. 2002;25:322–328. doi: 10.1159/000066792. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, et al. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: Effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens. 2005;18:81–87. doi: 10.1016/j.amjhyper.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhout JG, Lee RM. Blood pressure and heart rate development in young spontaneously hypertensive rats. Am J Physiol. 1998;274:H794–H800. doi: 10.1152/ajpheart.1998.274.3.H794. [DOI] [PubMed] [Google Scholar]
- Endemann D, Touyz RM, Yao G, Schiffrin EL. Tyrosine kinase inhibition attenuates vasopressin-induced contraction of mesenteric resistance arteries: alterations in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;40:123–132. doi: 10.1097/00005344-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Ibrahim J, Berk BC, Hughes AD. Comparison of simultaneous measurements of blood pressure by tail-cuff and carotid arterial methods in conscious spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Hypertens. 2006;28:57–72. doi: 10.1080/10641960500386817. [DOI] [PubMed] [Google Scholar]
- Jameson M, Dai FX, Luscher T, Skopec J, Diederich A, Diederich D. Endothelium-derived contracting factors in resistance arteries of young spontaneously hypertensive rats before development of overt hypertension. Hypertension. 1993;21:280–288. doi: 10.1161/01.hyp.21.3.280. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H1917–H1922. doi: 10.1152/ajpheart.01012.2004. [DOI] [PubMed] [Google Scholar]
- Jin L, Ying Z, Hilgers RH, Yin J, Zhao X, Imig JD, et al. Increased RhoA/Rho-kinase signaling mediates spontaneous tone in aorta from angiotensin II-induced hypertensive rats. J Pharmacol Exp Ther. 2006;318:288–295. doi: 10.1124/jpet.105.100735. [DOI] [PubMed] [Google Scholar]
- Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Faraci F. Enhanced vasoconstrictor responses in eNOS deficient mice. Nitric Oxide. 2003;8:207–213. doi: 10.1016/s1089-8603(03)00028-4. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Marcus ML, Dole WP. Removal of the endothelium potentiates canine large coronary artery constrictor responses to 5-hydroxytryptamine in vivo. Circ Res. 1985;57:46–54. doi: 10.1161/01.res.57.1.46. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- Morton JJ, Beattie EC, Griffin SA, MacPherson F, Lyall F, Russo D. Vascular hypertrophy, renin and blood pressure in the young spontaneously hypertensive rat. Clin Sci (Lond) 1990;79:523–530. doi: 10.1042/cs0790523. [DOI] [PubMed] [Google Scholar]
- Nabha L, Garbern JC, Buller CL, Charpie JR. Vascular oxidative stress precedes high blood pressure in spontaneously hypertensive rats. Clin Exp Hypertens. 2005;27:71–82. doi: 10.1081/ceh-200044267. [DOI] [PubMed] [Google Scholar]
- Northcott CA, Hayflick J, Watts SW. Upregulated function of phosphatidylinositol-3-kinase in genetically hypertensive rats: a moderator of arterial hypercontractility. Clin Exp Pharmacol Physiol. 2005;32:851–858. doi: 10.1111/j.1440-1681.2010.04276.x. [DOI] [PubMed] [Google Scholar]
- Okuniewski R, Davis EA, Jarrott B, Widdop RE. A comparison of the development of renal hypertension in male and female rats. Clin Sci (Lond) 1998;95:445–451. [PubMed] [Google Scholar]
- Paravicini TM, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke. 2004;35:584–589. doi: 10.1161/01.STR.0000112974.37028.58. [DOI] [PubMed] [Google Scholar]
- Satoh S, Kreutz R, Wilm C, Ganten D, Pfitzer G. Augmented agonist-induced Ca2+ sensitisation of coronary artery contraction in genetically hypertensive rats. Evidence for altered signal transduction in the coronary smooth muscle cells. J Clin Invest. 1994;94:1397–1403. doi: 10.1172/JCI117475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- Secrest RJ, Williams P, Bonjouklian R, Modlin D, Firman K, Turk J, et al. Hypotensive properties of the protein kinase inhibitor, staurosporine, in normotensive and spontaneously hypertensive rats. Clin Exp Hypertens A. 1991;13:219–234. doi: 10.3109/10641969109042060. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Ohanian J, Heagerty AM. Calcium sensitivity and agonist-induced calcium sensitization in small arteries of young and adult spontaneously hypertensive rats. Hypertension. 1997;30:442–448. doi: 10.1161/01.hyp.30.3.442. [DOI] [PubMed] [Google Scholar]
- Shibata R, Morita S, Nagai K, Miyata S, Iwasaki T. Effects of H-7 (protein kinase inhibitor) and phorbol ester on aortic strips from spontaneously hypertensive rats. Eur J Pharmacol. 1990;175:261–271. doi: 10.1016/0014-2999(90)90563-l. [DOI] [PubMed] [Google Scholar]
- Soloviev AI, Bershtein SA. The contractile apparatus in vascular smooth muscle cells of spontaneously hypertensive rats possess increased calcium sensitivity: the possible role of protein kinase C. J Hypertens. 1992;10:131–136. doi: 10.1097/00004872-199202000-00004. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Angus JA. Contractile responses to alpha 1-adrenoceptor stimulation during maturation in the aorta of the normotensive and spontaneously hypertensive rat: relation to structure. Clin Exp Pharmacol Physiol. 1990;17:69–82. doi: 10.1111/j.1440-1681.1990.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- van Gorp AW, Schenau DS, Hoeks AP, Boudier HA, de Mey JG, Reneman RS. In spontaneously hypertensive rats alterations in aortic wall properties precede development of hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H1241–H1247. doi: 10.1152/ajpheart.2000.278.4.H1241. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium-dependent hyperpolarizations: the history. Pharmacol Res. 2004;49:503–508. doi: 10.1016/j.phrs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Northcott CA, Loberg RD, Watts SW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther. 2004;309:1011–1019. doi: 10.1124/jpet.103.062265. [DOI] [PubMed] [Google Scholar]
- Widdop RE, Li XC. A simple versatile method for measuring tail cuff systolic blood pressure in conscious rats. Clin Sci (Lond) 1997;93:191–194. doi: 10.1042/cs0930191. [DOI] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]