Abstract
Background and purpose:
Bridging the gap between preclinical research and clinical trials is vital for drug development. Predicting clinically relevant steady-state drug concentrations (Css) in serum from preclinical animal models may facilitate this transition. Here we used a pharmacokinetic/pharmacodynamic (PK/PD) modelling approach to evaluate the predictive validity of 5-hydroxytryptamine (5-HT; serotonin) transporter (SERT) occupancy and 5-hydroxytryptophan (5-HTP)-potentiated behavioral syndrome induced by 5-HT reuptake inhibitor (SRI) antidepressants in mice.
Experimental approach:
Serum and whole brain drug concentrations, cortical SERT occupancy and 5-HTP-potentiated behavioral syndrome were measured over 6 h after a single subcutaneous injection of escitalopram, paroxetine or sertraline. [3H]2-(2-dimethylaminomethylphenylsulphanyl)-5-methyl-phenylamine ([3H]MADAM) was used to assess SERT occupancy. For PK/PD modelling, an effect-compartment model was applied to collapse the hysteresis and predict the steady-state relationship between drug exposure and PD response.
Key results:
The predicted Css for escitalopram, paroxetine and sertraline at 80% SERT occupancy in mice are 18 ng mL−1, 18 ng mL−1 and 24 ng mL−1, respectively, with corresponding responses in the 5-HTP behavioral model being between 20–40% of the maximum.
Conclusions and implications:
Therapeutically effective SERT occupancy for SRIs in depressed patients is approximately 80%, and the corresponding plasma Css are 6–21 ng mL−1, 21-95 ng mL−1 and 20–48 ng mL−1 for escitalopram, paroxetine and sertraline, respectively. Thus, PK/PD modelling using SERT occupancy and 5-HTP-potentiated behavioral syndrome as response markers in mice may be a useful tool to predict clinically relevant plasma Css values.
Keywords: PK/PD modelling, serotonin reuptake inhibitors, clinical predictions, SERT occupancy, 5-HTP, mice, MADAM
Introduction
The discovery and selection of drug candidates for clinical development rely heavily on the results of preclinical testing. It is essential that the applied preclinical models are predictive not only qualitatively for clinical response within relevant indications but also quantitatively to obtain a reasonable assessment of ‘drugability' for lead candidates. ‘Drugability' is defined as an acceptable level of drug formulation/daily dosage regimen that can maintain relevant drug exposure within the therapeutic window in patients. For prediction of therapeutically relevant drug exposure, a reliable screening model must be established that enables characterization of the relationship between the pharmacokinetics (PK) of the drug and the corresponding pharmacodynamic (PD) response. Ideally, the PD measure should be based on a biomarker that is translatable between the pharmacological response observed in the preclinical model and the therapeutic response in the clinic (Chien et al., 2005).
Major depressive disorder (MDD) is a recurrent psychiatric disease that affects an estimated 121 million people worldwide. It is among the top 10 causes of morbidity and mortality, and is predicted to rival ischaemic heart disease as a primary cause of disability by the year 2020 (Murray and Lopez, 1996; World Health Organization, 1996). The primary symptoms of MDD include daily and recurrent depressed mood, marked loss of interest and pleasure in all or most daily activities, sleep disturbances and difficulty in concentrating (American Psychiatric Association, 2000).
The neurotransmitter 5-hydroxytryptamine (5-HT; serotonin) is a key CNS substrate of MDD. Decreased brain levels of 5-HT are associated with MDD, and increasing brain 5-HT levels have proved to be a highly successful treatment (Coppen et al., 1972; Byerley and Risch, 1985; Jesberger and Richardson, 1985; Asberg et al., 1986; Korpi et al., 1986). The most commonly used antidepressants are selective 5-HT reuptake inhibitors (SRIs) (Wong et al., 1995). SRIs elevate levels of 5-HT in the CNS by blocking 5-HT transporter (SERT)-mediated reuptake of 5-HT. Other pharmacotherapies for treating depression include drugs that increase CNS levels of dopamine and/or noradrenaline, but that also directly block the SERT, such as tricyclic antidepressants, or have secondary effects on 5-HT release through a primary action on noradrenaline and dopamine systems, such as bupropion.
To understand the pharmacological actions of drugs with the potential for treating CNS disorders, including MDD, it is important to characterize how they occupy receptors in the brain. In some cases, a relatively small change in receptor occupancy can lead to significant changes in clinical effects (Farde et al., 1992; Kapur et al., 2003). In the case of SRIs, the site of action is the SERT. Therefore, measuring SERT occupancy by SRIs may be a reliable biomarker for estimating the antidepressant activity of SRIs. The therapeutic effects of several SRIs in patients with MDD are observed at doses that produce approximately 80% SERT occupancy (Meyer et al., 2001, 2004). The in vivo efficacy of SRIs in rodent behavioral tests predictive of antidepressant-like activity has also been associated with high levels of in vivo SERT binding (Larsen et al., 2004; Hirano et al., 2005).
There are several preclinical behavioral tests used to evaluate the antidepressant-like properties of drugs (O'Neil and Moore, 2003). Some of these tests are hypothesized to model symptoms of depression, whereas others are mechanistically specific tests that are useful as complementary or surrogate approaches for assessing activity at a specific target. One test that is not a model of depression-related symptoms, but is useful for indexing the potency of compounds to elevate extracellular levels of 5-HT in the brain, is the 5-hydroxytryptophan (5-HTP)-potentiated behavioral syndrome test.
The 5-HTP-potentiated syndrome is induced by administering drugs that increase extracellular 5-HT levels in combination with the immediate synthetic precursor to 5-HT (5-HTP). Together, these treatments induce a robust behavioral syndrome that has been shown to be specific to drugs that readily occupy the SERT (Larsen et al., 2004). 5-HTP-potentiated behavioral syndrome is quantifiable in rodents by measuring head twitches, hindlimb abduction and body tremor almost immediately after the administration of drug and 5-HTP (Ortmann et al., 1982; Larsen et al., 2004).
Taken together, the preclinical measurement of SERT occupancy and the efficacy in the 5-HTP-potentiated behavioral syndrome may be useful for characterizing the relationship between the SRI primary site of action and SRI in vivo PD effects. These methods used in combination with the measurement of corresponding drug levels in serum would be valuable for establishing a target range of serum levels for early clinical trials.
To further optimize the predictive potential of combining these unique data sets and to correlate the data with the steady state of exposure experienced by chronically treated patients, it is essential to obtain steady-state PK/PD estimates (Kapur et al., 2003). Steady-state conditions can be obtained by long-term drug administration, or they can be simulated from single-dose administration using an appropriate PK/PD model. The latter approach is advantageous because the duration of the experiment is shortened and the PD-effect duration is characterized in relation to the serum elimination half-life of the tested drug, which may aid the selection of an optimal dosage regimen in clinical trials.
The purpose of the present study was to use the SRI antidepressants escitalopram, paroxetine and sertraline to evaluate the clinical predictive validity of using SERT occupancy, 5-HTP-potentiated behavioral syndrome and serum levels of drug in mice using a PK/PD modelling approach. Escitalopram, paroxetine and sertraline were chosen because they are commonly used, structurally diverse and have well-characterized efficacy in humans and animals.
Materials and methods
Animals
Ethical permission for the studies were granted by the animal welfare committee appointed by the Danish Ministry of Justice, and all animal procedures were carried out in compliance with the EC Directive 86/609/EEC and with the Danish law regulating experiments on animals.
Male NMRI/BOM mice (18–25 g; Bomholtgaard, Ry, Denmark) were housed in plastic cages (35 × 30 × 12 cm) in groups of four and were habituated to the animal facilities for at least a week before testing. The temperature (21±2 °C), relative humidity (55±5%) and air exchange (16 times per hour) in the housing room were automatically controlled. The animals had free access to commercial food pellets and tap water and were maintained on a 12 h light/dark cycle (0600/1800 h).
Administration of drugs
Escitalopram and paroxetine were dissolved in saline, whereas sertraline hydrochloride was dissolved in aqueous 20% Solutol HS. Escitalopram, paroxetine and sertraline were injected s.c. using an injection volume of 10 mL kg−1. 5-HTP (Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline and injected i.v. All doses are expressed as mg base per kg body weight (mg kg−1). Serum drug exposure, SERT occupancy and 5-HTP-potentiated behavioral syndrome response were determined 0, 5, 15, 30, 60, 120, 240 and 360 min (including 1440 min for sertraline SERT occupancy assessment) after drug administration.
5-HTP behavioral syndrome
The 5-HTP-potentiated behavioral syndrome test in mice was carried out as described previously (Hyttel et al., 1992). Briefly, at multiple time points (as shown above) after the administration (s.c.) of escitalopram, paroxetine or sertraline, the mice were injected (i.v.) with 5-HTP (100 mg kg−1) and returned immediately to their home cages. The 100 mg kg−1 dose of 5-HTP was chosen because it does not induce 5-HT syndrome by itself. In contrast, a robust, dose-dependent 5-HTP-potentiated behavioral syndrome is observed when 100 mg kg−1 of 5-HTP is injected in combination with SRIs. An experimenter, unaware of the drug treatments, rated stereotypy (lateral head movements), tremor and hindlimb abduction over the 15 min after 5-HTP injection. The behaviours were rated individually, and a score of 0 (not present), 1 (present in a mild-to-moderate degree) or 2 (present in a marked degree) was given to each animal for each behaviour. Thus, the maximum possible score for each mouse was 6. A total of 8–16 mice were used per dose. The data are expressed as the mean±s.e.mean of the 5-HTP syndrome score.
Serotonin transporter occupancy
In vivo binding of [3H]2-(2-dimethylaminomethylphenylsulphanyl)-5-methyl-phenylamine ([3H]MADAM) to the serotonin transporter (SERT) in mouse brain was used to determine SERT occupancy (Larsen et al., 2004). All test compounds were administered s.c. to mice at varying time points prior to treatment with [3H]MADAM. The relevant dose for each drug was chosen based on expected SERT occupancies in the range between 0 and 90% over the time profile. Escitalopram (10 mg kg−1) defined the nonspecific binding and was injected s.c. 30 min before [3H]MADAM in all experiments. Total binding was measured using vehicle-treated mice. Animals received tail-vein injections of 4 μCi [3H]MADAM and were killed by cervical dislocation 15 min later. The cerebral cortex was immediately dissected out, homogenized in ice-cold buffer (50 mM Tris-HCL, 12 mM NaCl, 5 mM KCl, pH 7.5) and filtered through Whatman GF/C filters. The filters were washed with 2 × 5 mL ice-cold buffer. Homogenization and filtration were always completed less than 1 min after cervical dislocation. Samples from homogenates were used for protein determination.
Pharmacokinetics
At the predetermined time points described above after s.c. drug administration, the mice were killed by cervical dislocation and trunk blood and whole brains were collected. The blood samples were allowed to coagulate for 30 min at room temperature. The brains were homogenized 1:4 (w v−1) in saline (Ultra-turrax T-25; IKA-Labortechnik, Staufen, Germany) and centrifuged at 2200 g for 10 min. Serum and brain supernatants were isolated and frozen at −80 °C until analysis.
Quantitative analysis of drug levels
Serum and brain content of escitalopram, paroxetine and sertraline were determined by liquid chromatography/tandem mass spectrometry (LC-MS/MS). Online sample preparation and LC were performed with turbulent flow chromatography (Cohesive Technologies, Franklin, MA, USA). A dual-column configuration method was used for escitalopram and paroxetine, whereas a monolith column (Chromolith SpeedROD; 50 × 4.6 mm) was used for analytical separation of sertraline, with increased flow rate during the transfer step to 4 mL min−1, reducing total run time to 4 min.
MS/MS detection was done with a Quattro Ultima (Micromass, Manchester, UK) in positive-ion electrospray ionization mode. Escitalopram, paroxetine and sertraline were detected at parent>daughter molecular masses of 324.99>108.95, 305.94>274.71 and 329.93>192.05 Da, using a cone voltage of 50, 25 and 50 V and a collision energy of 25, 13 and 20 eV, respectively. For all samples, sertindole (440.96>112.96 Da, 48 V and 30 eV) was used as internal standard. Nitrogen was used for the auxiliary and nebulizer gases, and argon was used for the collision gas. The peak area correlated linearly with the serum concentration and brain supernatant levels of all drugs (r2 >0.99) in the range of 1.0–500 ng mL−1 (serum) and 0.5–500 ng mL−1 (brain), respectively. The lower limit of quantification (S/N>5) was 2.5 ng mL−1 in serum for all drugs and 0.1, 0.4 and 0.5 ng mL−1 for brain analysis of escitalopram, paroxetine and sertraline, respectively.
PK/PD modelling
PK/PD analyses of the data were performed with PC-compatible software WinNonlin ver. 4.1 (Pharsight Corporation, Mountain View, CA, USA). Choice of models for PK/PD analysis was based on best fit in terms of Akaike criterion and weighted residual sum of squares, using the Gauss–Newton minimization method. A one- or two-compartment model with first-order absorption and elimination was used for PK modelling (Gabrielsson and Weiner, 2001). Derived constants were volume of central compartment/bioavailability (V1/F), absorption rate (k01), elimination rate (k10), distribution rate from central to peripheral compartment (k12) and distribution rate from peripheral back to central compartment (k21). Secondary calculated parameters were serum clearance (CL), maximum serum exposure (Cmax) and time thereto (Tmax) and terminal serum half-life (t1/2β). A sigmoidal Emax model was fitted to the PD data. PK and PD models were linked using an effect-compartment model (Sheiner et al., 1979), where ke0 describes the equilibration rate to the effect compartment. For each drug, the steady-state PD modelling was performed with the pooled collapsed data from all three doses. Total brain exposure of the drugs was determined by non-compartmental analysis, extrapolating terminal half-life to infinity to assess area under the curve (AUC0−inf). Brain/serum ratios were calculated as (AUC0−inf, brain/AUC0−inf, serum).
Materials
Escitalopram oxalate (escitalopram), paroxetine hemioxalate (paroxetine) and sertraline hydrochloride (sertraline) were synthesized in the Department of Medicinal Chemistry, H Lundbeck A/S.
Results
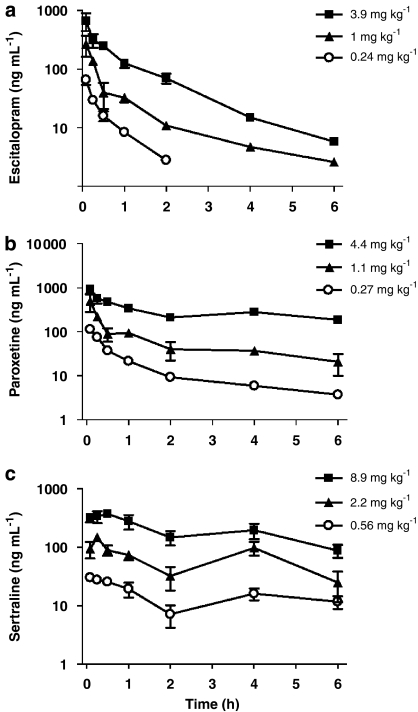
The PK profiles of three doses each of escitalopram, paroxetine and sertraline following a single s.c. administration to mice are presented in Figure 1. The serum concentration–time course for escitalopram and paroxetine was characterized by rapid absorption and a following biexponential decline in serum drug concentrations. Accordingly, best fit to the data was found with a two-compartment PK model with first-order absorption and elimination. The corresponding derived PK parameters are displayed in Table 1. The rapid absorption was reflected in the short Tmax values in the range 0.07–0.09 h for escitalopram and paroxetine and 0.08–0.5 h for sertraline. Terminal serum half-life of escitalopram was consistent across the three doses around 1 h. For paroxetine, a dose-related increase in serum half-life from 1.6 to 6.3 h was observed corresponding to a dose increase from 0.27 to 4.4 mg kg−1.
Figure 1.
Semilogarithmic plot of mean (±s.e.mean) serum concentration–time profiles of (a) escitalopram, (b) paroxetine and (c) sertraline following s.c. administration in mice.
Table 1.
Serum pharmacokinetic constants and parameter values (coefficient of variation for estimate) for escitalopram and paroxetine from compartmental modelling of single-dose serum concentration–time data following s.c. administration to mice and corresponding derived secondary PK parameters. PK parameters for sertraline were calculated by non-compartmental modelling (except where noteda)
| Drug | Dose (mg kg−1) | V1/F (l kg−1) | k01 (h−1) | k10 (h−1) | k12 (h−1) | k21 (h−1) | CL/F (l h−1 kg−1) | Tmax (h) | Cmax (ng mL−1) | t1/2β (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Escitalopram | 0.24 | 2.4 (94) | 30 (295) | 3.1 (118) | 3.2 (209) | 2437 (94) | 7.6 (34) | 0.07 (133) | 65 (22) | 0.9 (123) |
| 1.0 | 1.5 (4202) | 12 (4215) | 5.2 (4207) | 5.8 (4748) | 1466 (4202) | 7.6 (16) | 0.09 (10) | 264 (2) | 1.2 (54) | |
| 3.9 | 2.9 (3312) | 30 (7439) | 3 (3420) | 9.9 (5426) | 2918 (3312) | 8.8 (108) | 0.05 (2641) | 726 (929) | 0.8 (32) | |
| Paroxetine | 0.27 | 1.7 (11) | 25 (29) | 1.7 (17) | 2.3 (28) | 1.2 (33) | 2.9 (11) | 0.09 (12) | 114 (2) | 1.6 (30) |
| 1.1 | 0.9 (1439) | 14 (1488) | 2.9 (1445) | 9.5 (1625) | 1.4 (44) | 2.6 (22) | 0.08 (34) | 494 (4) | 2.3 (43) | |
| 4.4 | 3.5 (63) | 30 (207) | 0.4 (97) | 3.7 (139) | 1.7 (79) | 1.3 (55) | 0.08 (101) | 931 (9) | 6.3 (77) | |
| Sertraline | 0.56 | NA | NA | NA | NA | NA | 3.5 | 0.08 | 30.0 | NA |
| 2.2 | NA | NA | NA | NA | NA | 5.0 | 0.3 | 148 | NA | |
| 2.2a | 12 (22) | 3.6 (23) | 0.7 (26) | 0.9 (51) | 0.7 (32) | 8.9 (5) | 0.4 (2) | 98 (0.7) | 2.9 (20) | |
| 8.9 | NA | NA | NA | NA | NA | 6.5 | 0.5 | 373 | NA |
Abbreviations: NA, not applicable; PK, pharmacokinetic.
Additional (compartmentally modelled) PK profile for modelling of SERT occupancy data.
A secondary peak was found in the PK profiles of sertraline around 4 h. This precluded the fit of a simple compartmental model to the data, and due to the limited data set, more advanced modelling, such as enterohepatic recirculation models, was not considered scientifically justified. Thus, the basic PK properties of sertraline were described by non-compartmental modelling, with the exception of a PK profile made for modelling sertraline SERT occupancy. An excellent fit was found for a two-compartment model with first-order absorption and elimination (Table 1).
Brain/serum distribution of all the three drugs was suggested to be independent of the studied dose ranges, and mean ratio was 3.6, 1.1 and 7.4 for escitalopram, paroxetine and sertraline, respectively (Table 2).
Table 2.
Brain pharmacokinetic parameter values for escitalopram, paroxetine and sertraline from non-compartmental modelling of single-dose brain concentration–time data following s.c. administration to mice and corresponding brain/serum ratios based on AUC0−inf
| Drug | Dose (mg kg−1) | AUC0−inf (ng h g−1) | Tmax (h) | Cmax (ng mL−1) | t1/2β (h) | Brain/serum ratio |
|---|---|---|---|---|---|---|
| Escitalopram | 0.24 | 103 | 1.0 | 59 | 0.6 | 3.3 |
| 1.0 | 380 | 0.5 | 237 | 0.8 | 2.9 | |
| 3.9 | 2075 | 0.5 | 2007 | 0.9 | 4.6 | |
| Paroxetine | 0.27 | 97 | 1.0 | 30 | 1.3 | 0.9 |
| 1.1 | 642 | 1.0 | 158 | 1.8 | 1.3 | |
| 4.4 | 4045 | 0.5 | 1104 | 2.0 | 1.0 | |
| Sertraline | 0.56 | 1188 | 1.0 | 356 | 2.2 | 7.6 |
| 2.2 | 2896 | 2.0 | 828 | 1.5 | 6.6 | |
| 8.9 | 10782 | 0.5 | 2954 | 1.6 | 8.0 |
Abbreviation: AUC, area under the curve.
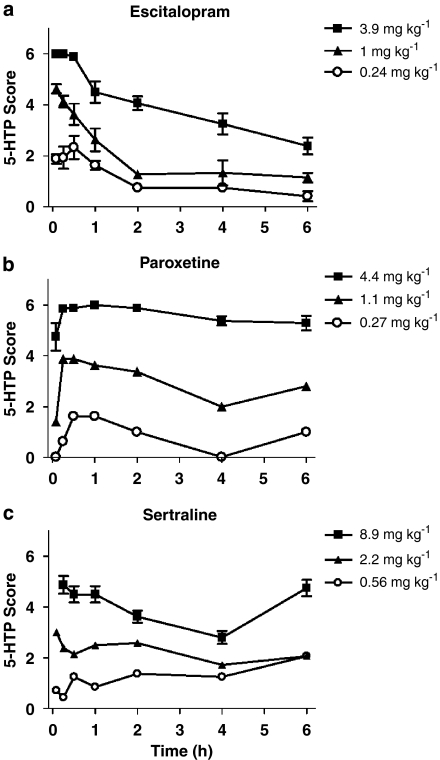
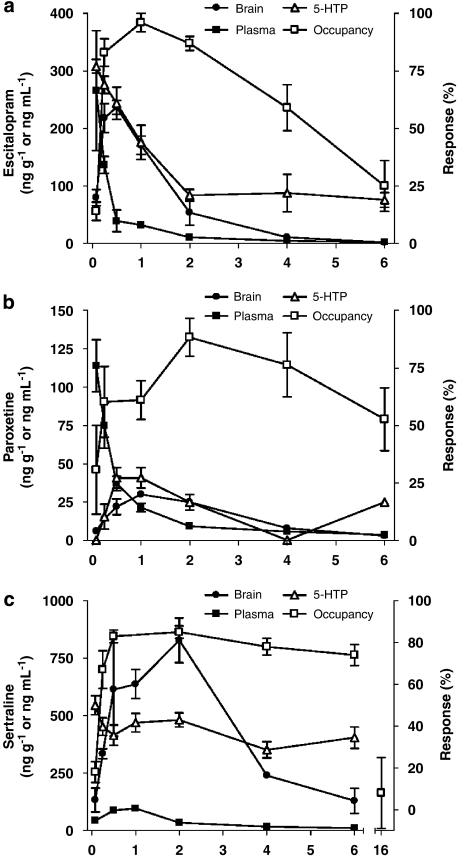
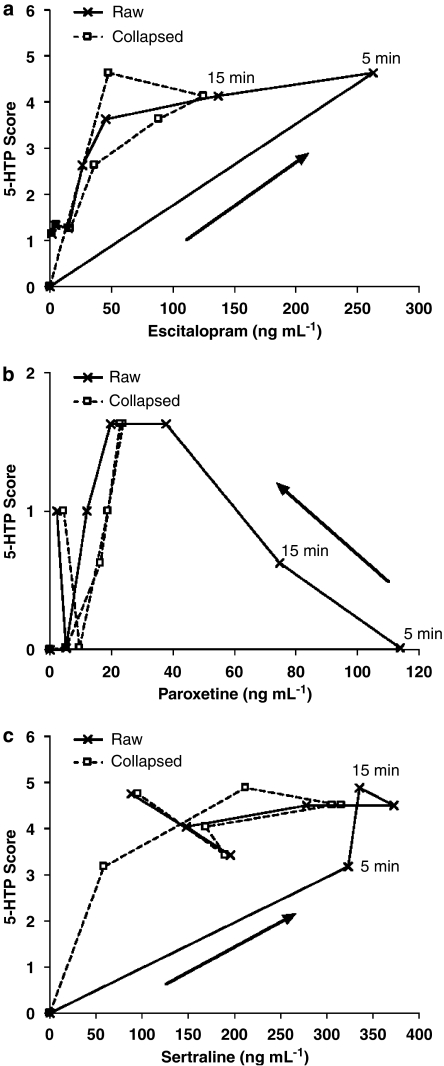
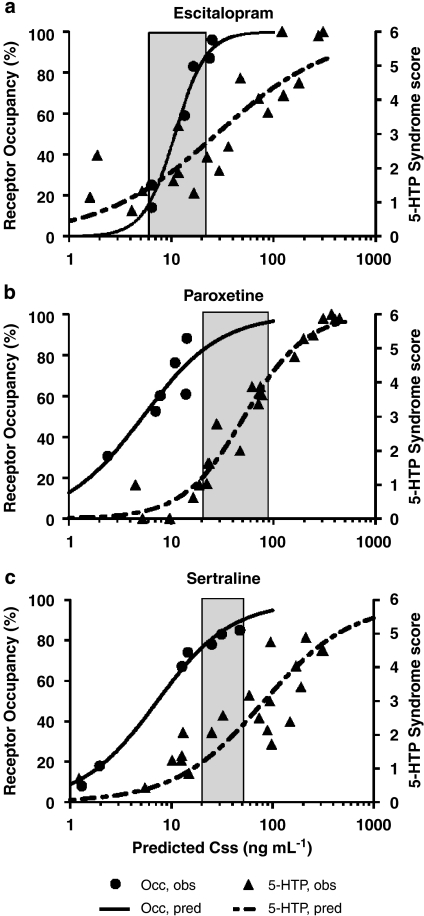
The corresponding pharmacological responses to each drug treatment in terms of 5-HTP-potentiated behavioral syndrome score are presented in Figure 2. Hysteresis was observed between changes in drug serum levels and corresponding SERT occupancy and pharmacological response at non-steady-state conditions for all three SRIs (Figure 3). Thus, an effect-compartment model (Sheiner et al., 1979) was applied to collapse the hysteresis and predict the steady-state relationship between drug exposure and corresponding PD response. For the 5-HTP-potentiated syndrome score after treatment with escitalopram and sertraline, a semicompartmental model was used providing initial assessment of ke0 for best collapse of the hysteresis curve at individual doses (Figure 4). A common compartmental PK/PD-link model did not provide robust assessment of ke0. For paroxetine, the 5-HTP-potentiated syndrome score and all PK/PD assessments using SERT occupancy as a PD marker were analysed by a compartmental PK/PD-link model to collapse the acute hysteresis data into predicted steady-state assessments. The predicted steady-state data from each dose were pooled to establish the PD relationship. Best fit to all predicted steady-state data sets was found with a sigmoidal Emax model, locking maximum response at 100% for SERT occupancy and 6 for 5-HTP-potentiated syndrome score (Figure 5). The corresponding parameters from the modelling are collected in Table 3. Steady-state EC80 for SERT occupancy was estimated to 18, 18 and 24 ng mL−1 for escitalopram, paroxetine and sertraline, respectively. Corresponding 5-HTP behavioral score at 80% SERT occupancy was 42, 17 and 23%, respectively. High SERT occupancy (93–94%) was needed to obtain 50% behavioral response in the 5-HTP model for all the three drugs.
Figure 2.
Time course of the effects of (a) escitalopram, (b) paroxetine and (c) sertraline administered s.c. on 5-HTP-potentiated behaviours in mice. Data represent the mean (±s.e.mean) for 8–16 mice per group. 5-HTP, 5-hydroxytryptophan.
Figure 3.
Time profile of mean (±s.e.mean) brain and serum concentrations, SERT occupancy and 5-HTP-potentiated syndrome score following (a) escitalopram (1.0 mg kg−1, s.c.), (b) paroxetine (0.27 mg kg−1, s.c.) or (c) sertraline (2.2 mg kg−1, s.c.) administration in mice. Data are drug concentration in ng g−1 (brain) or ng mL−1 (serum), or percent response (5-HTP, occupancy). 5-HTP, 5-hydroxytryptophan; SERT, serotonin transporter.
Figure 4.
Plot of the relationship between serum concentration and 5-HTP-potentiated syndrome score at individual time points in mice following the s.c. administration of (a) escitalopram (1.0 mg kg−1, s.c.), (b) paroxetine (0.27 mg kg−1, s.c.) or (c) sertraline (2.2 mg kg−1, s.c.). The hysteresis of non-steady-state raw data (Raw) is shown, as well as the results representing predicted steady-state relationship following collapse (Collapsed) with an effect-compartment model (escitalopram and paroxetine: ke0=5 h−1; sertraline: ke0=0.8 h−1). 5-HTP, 5-hydroxytryptophan.
Figure 5.
Predicted (pred) steady-state pharmacodynamic relationship between cortical SERT occupancy (Occ) and 5-HTP-potentiated syndrome score (5-HTP) in the mouse for (a) escitalopram, (b) paroxetine and (c) sertraline. Symbols are observed (obs) response values at predicted steady-state serum concentrations (Css), and the lines are modelled curves to the data using a sigmoidal Emax model. The shaded area represents the range of clinically observed serum concentrations. 5-HTP, 5-hydroxytryptophan; SERT, serotonin transporter.
Table 3.
Predicted pharmacodynamic parameters (with coefficient of variation for) for escitalopram, paroxetine and sertraline at steady-state conditions for SERT occupancy and 5-HTP-potentiated behavioral syndrome score
| Drug | Response | EC50 (ng mL−1) | EC80 (ng mL−1) | Emax | γ | ke0 (h−1) | SERT occupancy at 5-HTP EC50 (%) | 5-HTP score at 80% SERT occupancy (%) |
|---|---|---|---|---|---|---|---|---|
| Escitalopram | SERT occ | 11 (13) | 18 | 100 | 2.8 (23) | 0.4 | 94 | |
| 5-HTP score | 28 (81) | — | 100 | 0.8 (37) | 5.0 | 42 | ||
| Paroxetine | SERT occ | 5.3 (139) | 18 | 100 | 1.1 (95) | 0.4 | 93 | |
| 5-HTP score | 52 (13) | — | 100 | 1.4 (15) | 0.8/0.8/3.0a | 17 | ||
| Sertraline | SERT occ | 7.1 (20) | 24 | 100 | 1.2 (14) | 1.0 | 94 | |
| 5-HTP score | 80 (40) | — | 100 | NA | 1.0/0.0/5.0a | 23 |
Abbreviations: 5-HTP, 5-hydroxytryptophan; NA, not applicable; SERT, serotonin transporter.
Acute non-steady-steady data were collapsed with an effect-compartment model. A sigmoidal Emax model was fitted to the pooled data from the three doses. Emax was locked at 100% for all fits.
Low/medium/high dose, respectively.
Discussion
We demonstrate the use of a PK/PD model linking drug exposure, a translational biomarker (SERT occupancy) and pharmacological response (5-HTP-potentiated syndrome) in mice for the prediction of serum steady-state concentrations (Css) of SRI antidepressant drugs in humans. A key finding of the present study is that the predicted Css for each SRI tested is within the range of known Css achieved in humans who are given therapeutic doses.
In the present study, the PK profile of escitalopram is suggested to be dose-independent across the dose range of 0.24–3.9 mg kg−1 (s.c.), and a simple two-compartment model was fitted simultaneously to all three tested doses (0.24, 1.0 and 3.9 mg kg−1). Therefore, derived constants may be useful for simulation of PK profiles following alternative dosage regimens within the tested range.
Clearance and bioavailability of paroxetine decreases, and corresponding half-life increases with increasing doses, suggesting a saturation of hepatic elimination particularly at the highest dose tested (4.4 mg kg−1). This may be related to the potent inhibition of CYP2D6, which is involved in the elimination of paroxetine, and leads to saturable clearance at therapeutically relevant doses in humans (Harvey and Preskorn, 1996). To establish a model that adequately describes the PK profile of paroxetine independently of dose, a Michaelis–Menten type elimination model may be relevant. However, for the purposes of the present investigation, an adequate description of the PK/PD properties of paroxetine was found using a simplified two-compartment model with first-order elimination, fitted individually to each dose.
Secondary peaks in serum concentrations were observed for all three sertraline PK profiles generated in parallel with the behavioral data. Enterohepatic recirculation or precipitation of drug after s.c. administration may account for the secondary peaks. Under these conditions, it would be ideal to define the PK/PD relationship of sertraline in the same animal. Thus, an additional PK profile was generated using blood samples and SERT occupancy assessments in the same mouse.
A clear hysteresis was observed between changes in drug exposure and corresponding response to escitalopram and paroxetine, and partly to sertraline. A similar hysteresis has been reported for escitalopram in rats using extracellular serotonin levels within the hippocampus as a response marker (Bundgaard et al., 2007). This phenomenon is typically associated with relatively slow permeation over the blood–brain barrier or low receptor binding ‘on/off' rates (Yassen et al., 2005).
In the present investigation, the brain PK profile tended to overlap with the response to all three tested SRIs, suggesting that the distribution rate between serum and brain is the rate-limiting step that determines the degree of hysteresis. These findings underline the importance of assessing the PD properties of drugs in steady-state conditions, rather than measuring a plasma concentration–response relationship at a single time point in non-steady-state conditions. A similar lag between decline in escitalopram serum concentration and SERT occupancy has been reported for therapeutically relevant doses of escitalopram in humans (Klein et al., 2007), consistent with the present PK/PD findings in mice.
In preclinical animal models predictive of antidepressant and anxiolytic activity, there is a strong association between serum/plasma concentration of SRIs and pharmacological response (Larsen et al., 2004; Hirano et al., 2005). In depressed patients, this association is less clear (Baumann, 1996; Lundmark et al., 2000). A major reason underlying this discrepancy may be the large placebo response and narrow window of the common scoring systems (for example, Montgomery–Asberg Depression Rating Scale and Hamilton Rating Scale for Depression) used for clinical antidepressant response evaluation. Consequently, relationships between graduated drug exposure and response have been difficult to define in the clinic. However, a relationship between dose and PD response for the SRIs citalopram and escitalopram in depressed patients has been reported (Bech et al., 2002; Bech et al., 2004).
In contrast to the unclear correlation between drug exposure and clinical depression scores, 76–85% SERT occupancy has been consistently associated with therapeutic doses of SRIs in patients with MDD (Meyer et al., 2001, 2004). The steady-state trough serum concentrations related with therapeutically effective doses and SERT occupancies in patients with MDD range from 6 to 21 ng mL−1, 21 to 95 ng mL−1 and 20 to 48 ng mL−1 for escitalopram, paroxetine and sertraline, respectively (Sogaard et al., 2005; Klein et al., 2007). Using the PK/PD modelling approach described in the present study, the predicted Css of escitalopram, paroxetine and sertraline at 80% SERT occupancy in mouse brain cortex was 18 ng mL−1, 18 ng mL−1 and 24 ng mL−1, respectively.
Interspecies differences in major active metabolites and serum protein binding may hamper accuracy of human predictions from preclinical data, if these are not accounted for in the PK/PD modelling. To our knowledge, no significant interspecies differences have been published for these three model drugs in terms of serum protein binding or major pharmacologically active metabolites, and have thus not been accounted for in the present predictions. However, the successful clinical predictions based on the mouse SERT PK/PD model suggest that this is not a major issue for the three model drugs.
For paroxetine and sertraline, the Hill slope (γ) of the fitted sigmoidal curves approached unity, corresponding to a single binding site on the SERT. A steeper Hill slope (2.8) was found for the fitted curve to escitalopram SERT occupancy. Besides binding to the high-affinity site of SERT, escitalopram has been suggested to modulate the allosteric binding site of SERT (Chen et al., 2005). This could have contributed to the apparently higher Hill slope for escitalopram, but needs further investigation to verify.
In the 5-HTP potentiation test in mice, the relationship between SERT occupancy and efficacy is somewhat different. The behavioral response at 80% SERT occupancy is 20–40% of the maximum possible response. Therefore, relatively high SERT occupancy levels (>80%) are needed to obtain a robust response in the 5-HTP-potentiated syndrome test, and clinically relevant serum exposure of SRIs can be identified within the lower range of activity.
The 5-HTP potentiation test is not a model of MDD or symptoms related to MDD. However, it is a useful gauge of the potential antidepressant activity of compounds, based on the well-known association between brain 5-HT levels and MDD. One drawback of the test is that supratherapeutic levels of brain 5-HT may be necessary for the manifestation of the quantifiable behavioral syndrome in mice. This requirement may contribute to the low behavioral scores at clinically relevant (that is, approximately 80%) SERT occupancy levels.
The present study has evaluated the feasibility of using a simple single-dose PK/PD model in mice to predict therapeutically relevant steady-state plasma levels of SRIs in patients suffering from MDD. On the basis of evaluation of three widely prescribed antidepressants, escitalopram, paroxetine and sertraline, the findings suggest that estimates of clinically relevant plasma exposure and corresponding SERT occupancy can be made from single-dose PK/PD modelling in mice. Receptor occupancy is, for many targets, an acknowledged translatable biomarker between animals and humans (Kapur et al., 2003; Olsen et al., 2008), and our results indicate that it may be a valuable drug discovery tool for use in PK/PD modelling for selection of future drug candidates.
Acknowledgments
We wish to thank Anne Grumstrup Sørensen, Lisbet Petri, Nina Guldhammer and Christian Spang Pedersen for skilful technical assistance.
Abbreviations
- 5-HTP
5-hydroxytryptophan
- Css
concentration at steady state
- MADAM
2-(2-dimethylaminomethylphenylsulphanyl)-5-methyl-phenylamine
- MDD
major depressive disorder
- PD
pharmacodynamic
- PK
pharmacokinetic
- SERT
serotonin transporter
- SRI
serotonin reuptake inhibitor
Conflict of interest
The authors state no conflict of interest.
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 2000. 4th edn, American Psychiatric Press, Inc: Washington
- Asberg M, Eriksson B, Martensson B, Traskman-Bendz L, Wagner A. Therapeutic effects of serotonin uptake inhibitors in depression. J Clin Psychiatry. 1986;47:23–35. [PubMed] [Google Scholar]
- Baumann P. Pharmacokinetic–pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1996;31:444–469. doi: 10.2165/00003088-199631060-00004. [DOI] [PubMed] [Google Scholar]
- Bech P, Tanghoj P, Andersen HF, Overo K. Citalopram dose–response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology (Berl) 2002;163:20–25. doi: 10.1007/s00213-002-1147-6. [DOI] [PubMed] [Google Scholar]
- Bech P, Tanghoj P, Cialdella P, Andersen HF, Pedersen AG. Escitalopram dose–response revisited: an alternative psychometric approach to evaluate clinical effects of escitalopram compared to citalopram and placebo in patients with major depression. Int J Neuropsychopharmacol. 2004;7:283–290. doi: 10.1017/S1461145704004365. [DOI] [PubMed] [Google Scholar]
- Bundgaard C, Jorgensen M, Mork A. An integrated microdialysis rat model for multiple pharmacokinetic/pharmacodynamic investigations of serotonergic agents. J Pharmacol Toxicol Methods. 2007;55:214–223. doi: 10.1016/j.vascn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Byerley WF, Risch SC. Depression and serotonin metabolism: rationale for neurotransmitter precursor treatment. J Clin Psychopharmacol. 1985;5:191–206. [PubMed] [Google Scholar]
- Chen F, Larsen M, Sβnchez C, Wiborg O. The S-enantiomer of R, S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors. Eur Neuropsychopharmacol. 2005;15:193–198. doi: 10.1016/j.euroneuro.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Chien JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V. Pharmacokinetics/pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J. 2005;7:E544–E559. doi: 10.1208/aapsj070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen A, Prange AJ, Jr, Whybrow PC, Noguera R. Abnormalities of indoleamines in affective disorders. Arch Gen Psychiatry. 1972;26:474–478. doi: 10.1001/archpsyc.1972.01750230084016. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Gabrielsson J, Weiner D. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts & Applications 2001Swedish Pharmaceutical Press: Stockholm; 3 edn [Google Scholar]
- Harvey AT, Preskorn SH. Cytochrome P450 enzymes: interpretation of their interactions with selective serotonin reuptake inhibitors. Part II. J Clin Psychopharmacol. 1996;16:345–355. doi: 10.1097/00004714-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, et al. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel J, Bogeso KP, Perregaard J, Sanchez C. The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J Neural Transm Gen Sect. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- Jesberger JA, Richardson JS. Neurochemical aspects of depression: the past and the future. Int J Neurosci. 1985;27:19–47. doi: 10.3109/00207458509149132. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Klein N, Sacher J, Geiss-Granadia T, Mossaheb N, Attarbaschi T, Lanzenberger R, et al. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT study. Psychopharmacology. 2007;191:333–339. doi: 10.1007/s00213-006-0666-y. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kleinman JE, Goodman SI, Phillips I, DeLisi LE, Linnoila M, et al. Serotonin and 5-hydroxyindoleacetic acid in brains of suicide victims. Comparison in chronic schizophrenic patients with suicide as cause of death. Arch Gen Psychiatry. 1986;43:594–600. doi: 10.1001/archpsyc.1986.01800060088011. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Brennum LT, Egebjerg J, Sanchez C, Halldin C, Andersen PH. Selectivity of (3)H-MADAM binding to 5-hydroxytryptamine transporters in vitro and in vivo in mice; correlation with behavioural effects. Br J Pharmacol. 2004;141:1015–1023. doi: 10.1038/sj.bjp.0705693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark J, Bengtsson F, Nordin C, Reis M, Walinder J. Therapeutic drug monitoring of selective serotonin reuptake inhibitors influences clinical dosing strategies and reduces drug costs in depressed elderly patients. Acta Psychiatr Scand. 2000;101:354–359. doi: 10.1034/j.1600-0447.2000.101005354.x. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy–lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- O'Neil MF, Moore NA. Animal models of depression: are there any. Hum Psychopharmacol. 2003;18:239–254. doi: 10.1002/hup.496. [DOI] [PubMed] [Google Scholar]
- Olsen C, Brennum L, Kreilgaard M. Using pharmacokinetic–pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur J Pharmacol. 2008;584:318–327. doi: 10.1016/j.ejphar.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Ortmann R, Bischoff S, Radeke E, Buech O, ini-Stula A. Correlations between different measures of antiserotonin activity of drugs. Study with neuroleptics and serotonin receptor blockers. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:265–270. doi: 10.1007/BF00498511. [DOI] [PubMed] [Google Scholar]
- Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- Sogaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–1406. doi: 10.1177/0091270005280860. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected. Harvard School of Public Health: Cambridge; 1996. [Google Scholar]
- Yassen A, Olofsen E, Dahan A, Danhof M. Pharmacokinetic–pharmacodynamic modeling of the antinociceptive effect of buprenorphine and fentanyl in rats: role of receptor equilibration kinetics. J Pharmacol Exp Ther. 2005;313:1136–1149. doi: 10.1124/jpet.104.082560. [DOI] [PubMed] [Google Scholar]