Abstract
Background and purpose:
Experiments were designed to determine the modulation by nitric oxide (NO) and endothelium-dependent hyperpolarizations (EDHF-mediated responses) of endothelium-dependent contractions in renal arteries of normotensive and hypertensive rats.
Experimental approach:
Rings, with or without endothelium, of renal arteries of 8-month-old Wistar Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) were suspended in myographs for isometric force recording.
Key results:
ACh evoked relaxations in preparations contracted with phenylephrine. L-NAME (inhibitor of NOS) attenuated (WKY) or abolished (SHR) these relaxations. TRAM-34 plus UCL 1684 (inhibitors of EDHF-mediated responses) did not decrease the relaxation, except in rings of WKY when L-NAME was also present. High concentrations of ACh caused a secondary increase in tension, augmented in rings of WKY by L-NAME or TRAM-34 plus UCL 1684. The increase in tension was prevented by indomethacin. Under baseline tension, ACh induced endothelium-dependent contractions, prevented by indomethacin (COX inhibitor) or terutroban (TP receptor antagonist). The calculated endothelium-dependent contractions were larger in rings of SHR compared with those of WKY. In preparations of SHR, the contractions were augmented by L-NAME in the presence of SC19220 (EP-1 receptor antagonist). In arteries of WKY, the endothelium-dependent contractions were augmented by TRAM-34 plus UCL 1684. The responses were reduced by SC19220.
Conclusions and implications:
In the renal artery of the rat, EDCF-mediated contractions are augmented by hypertension. The endothelium-dependent contractions are facilitated by NOS inhibition (in the presence of an EP-1 receptor antagonist) and by the withdrawal of EDHF-mediated responses.
Keywords: ACh, COX, EP-1 receptors, NOS, TP receptors
Introduction
Endothelial cells release contracting substances (EDCFs) in addition to endothelium-derived relaxing factors (Furchgott and Zawadzki, 1980; Furchgott and Vanhoutte, 1989). Thus, ACh elicits endothelium-dependent contractions in various vascular preparations of different species (Lüscher and Vanhoutte, 1986; Katusic et al., 1988; Tesfamariam et al., 1989; Taddei et al., 1997; Zhou et al., 2001). Metabolites of COX, that activate endoperoxide–thromboxane A2 receptors (TP receptors) on vascular smooth muscle cells, mediate most of those endothelium-dependent contractions (Lüscher and Vanhoutte, 1986; Tesfamariam et al., 1989; Auch-Schwelk et al., 1990; Yang et al., 2004; Tang et al., 2005). The generation of EDCF can be observed under normal conditions (Altiere et al., 1986; Lüscher and Vanhoutte, 1986; Katusic et al., 1988). For example, in isolated canine basilar arteries and in renal arteries of Wistar Kyoto rats (WKY), ACh evokes endothelium-dependent contractions under control conditions, suggesting a physiological role of EDCF in the control of cerebral or renal vascular tone (Katusic et al., 1988; Nishimura et al., 1995; Gao and Lee, 2005). EDCF-mediated contractions become prominent with ageing, hypertension or diabetes (Lüscher and Vanhoutte, 1986; Koga et al., 1988, 1989; Okon et al., 2003; Yang et al., 2004; Shi et al., 2007).
In the isolated aorta of the rat, the inhibition of endothelial NOS optimizes EDCF-mediated responses (Auch-Schwelk et al., 1992; Yang et al., 2004; Tang et al., 2005), indicating a functional antagonism between nitric oxide (NO) and EDCF. In animals and humans, the augmented production of EDCF is associated with endothelial dysfunction and the withdrawal of NO (Auch-Schwelk et al., 1992; Taddei et al., 1997; Yang et al., 2004). Indeed, in the aorta of spontaneously hypertensive rats (SHR), impaired endothelium-dependent relaxations are the consequence of an enhanced production of EDCF (Lüscher and Vanhoutte, 1986), whereas exposure to NO diminishes the release of endothelium-derived contracting prostanoids (Auch-Schwelk et al., 1992; Yang et al., 2004; Tang et al., 2005).
Endothelium-dependent relaxations are not only due to the release of NO, but also due to endothelium-dependent hyperpolarizations (EDHF-mediated responses), which contribute to a varying degree (see Félétou and Vanhoutte, 2006). In rat renal arteries, ACh-induced relaxations are mediated both by NO and by endothelium-dependent hyperpolarizations (Kagota et al., 1999; Jiang et al., 2000; Büssemaker et al., 2003). In the renal artery of the SHR, the EDHF-mediated component of endothelium-dependent relaxations is blunted (Büssemaker et al., 2003). It is unknown whether or not the absence of EDHF-mediated responses facilitates the occurrence of endothelium-dependent contractions.
The present study was designed to compare the EDCF-mediated contractions with ACh, under control conditions, in renal arteries of 8-month-old WKY and SHR. Another purpose of this study was to determine whether or not the occurrence of EDCF-mediated responses is influenced by the absence or presence of NO- or EDHF-mediated responses.
Materials and methods
Tissue preparation and isometric force measurement
All animal procedures were approved by the Committee on the Use of Live Animals for Teaching and Research (CULATR) of the University of Hong Kong. The study was conducted in 8- to 9-month-old male SHR and aged-matched normotensive WFY (400–450 g). On the day of the experiments, the animals were anaesthetized with an i.p. injection of pentobarbitone sodium (50 mg kg−1). The carotid artery was cannulated (0.6 mm ID) for blood pressure measurement. The cannula was connected to a pressure transducer (Gould P10EZ, Spectramed, Oxnard, CA, USA) and the signal displayed on a chart recorder (Gould, Recording System Division, Cleveland, OH, USA). The mean arteries blood pressure of SHR and WKY was 183±19 and 106±5 mm Hg (n=5, P<0.05), respectively. The kidneys were excised and placed in a cold modified Krebs–Ringer bicarbonate solution of the following composition (mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3 and 11.1 glucose (control solution). The main branches of the renal arteries, with an outer diameter of 450–500 μm, were isolated. Rings (2 mm in length) were cleaned of fat and connective tissue and suspended in a Halpern-Mulvany myograph (model 610M, Danish Myo Technology A/S, Arhus, Denmark). In some preparations, the endothelium was removed by the perfusion of the artery, prior to preparing the rings, with 1 mL of a saponin solution (500 μg mL−1) for 20 s (Shi et al., 2006). The rings were suspended between two stainless steel wires with a diameter of 40 μm in an organ chamber filled with control solution kept at 37 °C and aerated with 95% O2 and 5% CO2. One of the wires was connected to a movable holder supporting a tension transducer (FT03, Grass Instrument Co., Quincy, MA, USA) so that the isometric force measurements could be collected by a data acquisition system (PowerLab 4SP, ADInstruments, Colorado Springs, CO, USA). An optimal load of 5 mN (determined in preliminary experiments (data not shown)) was applied to the rings, which were allowed to equilibrate for 1 h.
Experimental protocols
After stabilization, the segments were exposed twice to KCl (60 mM). This concentration produced a maximal response to potassium and served as reference contractions.
Preparations without endothelium of WKY and SHR were exposed to increasing concentrations of U46619 (TP receptor agonist, 1 nM–1 μM; Tang et al., 2005), in the absence or presence of SC19220 (EP-1 receptor antagonist, 0.1 mM; Michel et al., 2007) or phenylephrine (α1-adrenergic receptor agonist, 1–0.1 nM; Shi et al., 2006) in half-log increments.
In arteries of WKY or SHR, the response induced by ACh (0.1 nM–0.1 mM (in a half-log increment)) was assessed during phenylephrine (1 μM)-induced contractions in the absence or presence of indomethacin (non-selective COX inhibitor, 10 μM; Shi et al., 2006). The response to ACh during phenylephrine-induced contraction was also examined in rings incubated 30 min before administration of ACh with L-NAME (Nω-nitro-L-arginine methyl ester, NOS inhibitor, 10 μM; Shi et al., 2006) or with TRAM-34 plus UCL 1684 (intermediate- and small-conductance Ca2+-activated K+ channel blocker, respectively, 10 μM; Gluais et al., 2005) in the absence or presence of indomethacin. Some rings of WKY were incubated with L-NAME and with TRAM-34 plus UCL 1684 and SC19220.
Quiescent preparations with or without endothelium of WKY and SHR under baseline tension were exposed to increasing concentrations of ACh (10 nM–0.1 mM) in half-log increments in the absence or presence of indomethacin or terutroban (selective TP receptor antagonist, 0.1 μM; Simonet et al., 1997). Some rings, with or without endothelium, were incubated 30 min before administration of ACh, with SC19220 or with L-NAME plus SC19220 in the absence or presence of indomethacin or terutroban. In rings with or without endothelium, ACh-induced contractions were also assessed in the presence of TRAM-34 plus UCL 1684 in the absence or presence of SC19220.
Data analysis
The results are given as means±s.e.mean; n is the number of animals from which arteries were taken. Changes in isometric tension under baseline tension were expressed as absolute values in mN. The response caused by ACh during contractions to phenylephrine (relaxations and increases in tension) was expressed as a percentage of the phenylephrine-induced increases in tension. A nonlinear sigmoid regression analysis for each concentration–response curve was traced on GraphPad PRISM (GraphPad Software Inc., San Diego, CA, USA), allowing calculation of Emax (maximal amplitude) and pD2 values (negative logarithm of the concentration of agonist which evoked 50% of the maximal response). The area under the curve of sigmoid regression of changes in tension in quiescent preparations was calculated in GraphPad PRISM. The calculated EDCF-mediated response is defined as the area under the curve from rings with endothelium minus the one from the rings without endothelium from the same arteries. Statistical analysis was performed by a two-way ANOVA for multiple comparisons followed by a Bonferroni test in the case of significant ANOVA and Student's t-test for unpaired observations. P<0.05 was considered to indicate statistically significant differences.
Chemicals
ACh chloride, indomethacin, Nω-nitro-L-arginine methyl ester, phenylephrine hydrochloride, SC19220, TRAM-34 and UCL 1684 were purchased from Sigma-Aldrich (St Louis, MO, USA). 9,11-Dideoxy-9α,11α-epoximethano prostaglandin F2α (U46619) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Terutroban (S18886, Triptiol) was a kind gift from the Institut de Recherches Servier (Suresnes, France). All drugs were dissolved in deionized water except indomethacin (sodium bicarbonate 5 mM), SC19220 (100% DMSO (dimethyl sulphoxide)), TRAM-34 (100% DMSO), U46619 (100% ethanol) and UCL 1684 (100% DMSO). Further dilutions were performed in deionized water. Concentrations are expressed as final molar concentrations in the bath solution.
Results
Control vascular responses
Potassium
The reference contractions to KCl (60 mM) averaged 10.5±1 and 9.5±1 mN in arteries of WKY with and without endothelium, respectively. In preparations of SHR, this potassium stimulus evoked contractions that were not significantly different compared with those in WKY arteries (10.5±0.5 and 9.5±0.5 mN in rings with and without endothelium, respectively).
Phenylephrine
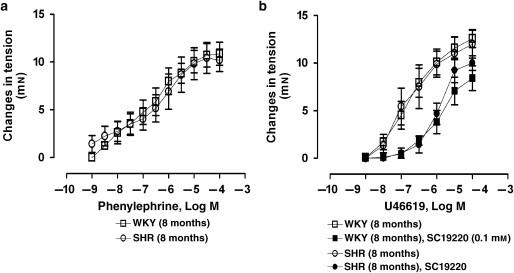
The α1-adrenergic agonist, from 1 nM to 0.1 mM, caused concentration-dependent contractions that were comparable in rings without endothelium of WKY and SHR (Figure 1a). At 1 μM, it caused comparable increases in tension in rings with endothelium of WKY and SHR (Table 1).
Figure 1.
Concentration-dependent contractions to (a) phenylephrine (1 nM–0.1 mM) or (b) U46619 (1 nM–1 μM) in quiescent renal arteries without endothelium of WKY and SHR in the absence or presence of SC19220 (0.1 mM).
Table 1.
Maximal (Emax) relaxations and secondary increases in tension caused by ACh and negative logarithm (pD2) of the concentration of ACh that evoked 50% of either the maximal relaxations or secondary increases in tension in renal arteries of WKY and SHR during contractions to phenylephrine (PE; 1 μM)
| PE 1 μM (mN) |
Relaxations to Ach |
Secondary increases in tension to ACh |
|||
|---|---|---|---|---|---|
| Emax (% of PE) | pD2 (−log M) | Emax (% of PE) | pD2 (−log M) | ||
| WKY | |||||
| Control solution | 10.7±0.7 | 80±2 | 7.5±0.2 | 27±10 | 4.8±0.2 |
| +Indomethacin | 8.0±2.4 | 89±4 | 7.7±0.1 | No increase | — |
| L-NAME | 11.5±1.1 | 56±1* | 8.0±0.1 | −30±9* | 5.4±0.2* |
| +Indomethacin | 7.5±1.0# | 72±9# | 7.4±0.3 | No increase | — |
| TRAM-34+UCL 1684 | 6.0±1.0* | 75±6 | 7.5±0.1 | −43±29* | 5.6±0.2* |
| + L-NAME+SC19220 | 9.3±2.0 | No relaxation | — | −118±22* | 5.5±0.4 |
| +Indomethacin | 6.0±1.7 | 92±5# | 7.7±0.1 | No increase | — |
| SHR | |||||
| Control solution | 12.7±0.8 | 66±9 | 7.1±0.2 | −27±6* | 5.2±0.1 |
| +indomethacin | 5.1±0.7# | 91±4# | 7.7±0.1† | No increase | — |
| L-NAME | 10.7±1.6 | No relaxation | — | −64±23 | 4.8±0.3 |
| +Indomethacin | 9.1±0.6 | 35±6† | 6.9±0.3 | −2±7#,† | 4.6±0.3 |
| TRAM-34+UCL 1684 | 5.5±0.9† | 78±5 | 7.7±0.4 | −84±27 | 5.4±0.3 |
| +Indomethacin | 5.0±1.3 | 94±3# | 7.3±0.1 | No increase | — |
Abbreviations: L-NAME, Nω-nitro-L-arginine methyl ester; PE, phenylephrine; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Rings were incubated in control solution, in a solution containing L-NAME or in solutions containing TRAM-34 plus UCL 1684 in the absence or presence of indomethacin. Values are means±s.e.mean; n=5–7 per group. The negative signs indicate secondary increases in tension above the initial level of PE-induced contraction.
*P<0.05 compared with rings of WKY incubated in the control solution; †P<0.05 compared with rings of SHR incubated in the control solution; #P<0.05 compared with rings of the same group in the absence of indomethacin (all with Student's t-test for unpaired observations).
U46619
The TP receptor agonist (1 nM–1 μM) elicited similar concentration-dependent contractions in rings without endothelium of WKY and SHR (Figure 1b).
ACh
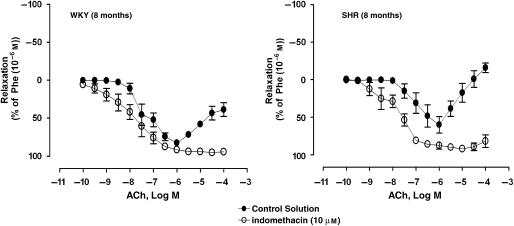
In arteries of WKY or SHR, ACh (0.1 mM–1 μM) evoked concentration-dependent relaxations during contractions evoked by 1 μM phenylephrine (Figure 2). The Emax of ACh-induced relaxations and the pD2 values were similar in rings of both experimental groups (Table 1). Higher concentrations of ACh (3 μM–0.1 mM) evoked secondary concentration-dependent increases in tension (Figure 2). The amplitude of the increases in tension was significantly greater in preparations of SHR compared with those of WKY (Table 1).
Figure 2.
Concentration-dependent relaxations to ACh (0.1 nM–0.1 mM) in arteries contracted with phenylephrine (Phe; 1 μM) of WKY (8 months) (left panel) and SHR (8 months) (right panel) incubated in control solution, in the absence or presence of indomethacin (10 μM). Values are mean±s.e.mean, n=5–7 per group.
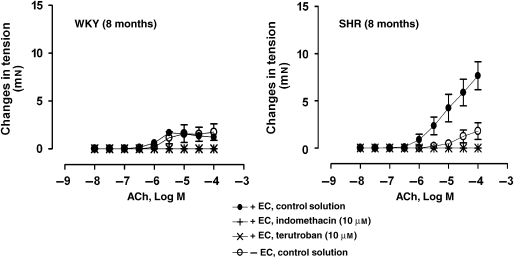
In quiescent preparations, with and without endothelium, of WKY and SHR, ACh (10 nM–0.1 mM) evoked concentration-dependent contractions (Figure 3). In arteries of SHR, the contractions caused by ACh were absent in rings without endothelium (Figure 3).
Figure 3.
Concentration-dependent contractions to ACh (10 nM–0.1 mM) in quiescent arteries, with or without endothelium, of WKY (8 months) (left panel) and SHR (8 months) (right panel), incubated in control solution or in the presence of indomethacin (10 μM) or terutroban (10 μM). Values are mean±s.e.mean, n=5 per group.
Inhibition of COX and antagonism of prostanoid receptors
The inhibitor of COX, indomethacin (10 μM), significantly decreased the increases in tension to 1 μM phenylephrine in rings of SHR (Table 1). In rings of SHR, the concentration–relaxation curve to ACh, administered during phenylephrine-induced contractions, was shifted significantly to the left in the presence of indomethacin (Figure 2 and Table 1). Indomethacin inhibited the secondary increases in tension caused by higher concentrations of ACh in arteries of WKY and SHR (Figure 2 and Table 1). Indomethacin abolished the contractions caused by ACh in quiescent rings of WKY and SHR (Figure 3).
The TP receptor antagonist terutroban (0.1 μM) abolished the contractions evoked by U46619 in rings without endothelium of WKY and SHR (data not shown). It abolished the contractions to ACh in quiescent preparations of WKY and SHR (Figure 3).
The EP-1 receptor blocker, SC19220 (0.1 mM), caused a significant shift to the right of the concentration–response curve to U46619 in rings without endothelium of WKY and SHR (Figure 1).
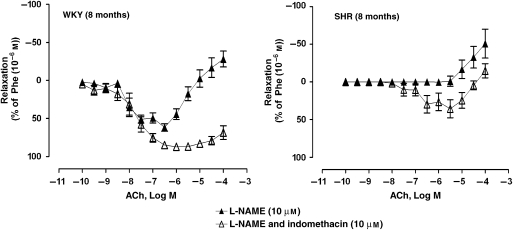
Inhibition of NOS
L-NAME (10 μM) significantly inhibited the amplitude of ACh-evoked relaxations (during contractions to phenylephrine) in arteries of WKY (Figure 4 and Table 1). In rings of SHR, no relaxations were obtained with the muscarinic agonist in the presence of L-NAME. In preparations of WKY, the Emax of the secondary increases in tension and the pD2 values were significantly higher in the presence of L-NAME (Table 1). In arteries of WKY, the addition of indomethacin restored the relaxations to basal conditions and significantly inhibited the secondary increases in tension (Figure 4 and Table 1). In rings of SHR, indomethacin attenuated the impaired ACh-induced relaxations and the augmented secondary increases in tension due to L-NAME (Figure 4 and Table 1).
Figure 4.
Concentration-dependent relaxations to ACh (0.1 nM–0.1 mM) in arteries contracted with phenylephrine (1 μM) of WKY (8 months) (left panel) and SHR (8 months) (right panel) incubated with L-NAME (10 μM) in the absence or presence of indomethacin (10 μM). Values are mean±s.e.mean, n=5 per group.
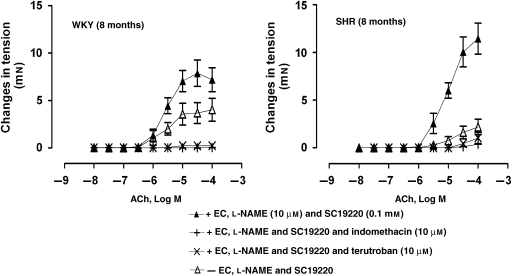
Experiments in quiescent rings were performed in the simultaneous presence of L-NAME and SC19220 to prevent the increase in tension above the initial level induced by the NOS inhibitor (Michel et al., 2007). In quiescent rings, with and without endothelium, of WKY or SHR, ACh evoked contractions in the presence of L-NAME plus SC19220 (Figure 5). In arteries of SHR, the contractions caused by ACh were absent in rings without endothelium (Figure 5).
Figure 5.
Concentration-dependent contractions to ACh (10 nM–0.1 mM) in quiescent arteries, with or without endothelium of WKY (8 months) (left panel) and SHR (8 months) (right panel), incubated with L-NAME (10 μM) and SC19220 (0.1 mM) in the absence or presence of indomethacin (10 μM) or terutroban (10 μM). Values are mean±s.e.mean, n=5–6 per group.
Inhibition of EDHF-mediated responses
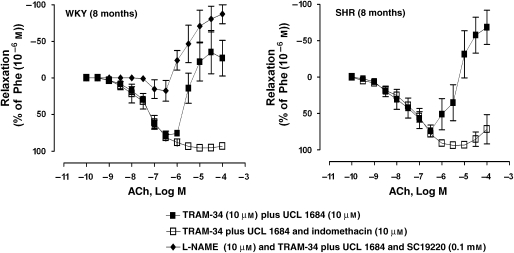
The inhibitors of EDHF-mediated responses, TRAM-34 plus UCL 1684 (10 μM), significantly decreased the increases in tension to 1 μM phenylephrine in rings of WKY and SHR (Table 1). The relaxations induced by ACh during contractions to phenylephrine were absent in WKY arteries with endothelium, incubated with L-NAME, TRAM-34 plus UCL 1684 and SC19220 (Figure 6 and Table 1). The secondary ACh-induced increases in tension were significantly augmented in the presence of TRAM-34 plus UCL 1684 (Figure 6 and Table 1). Indomethacin inhibited the increases in tension in rings of WKY and SHR incubated with TRAM-34 plus UCL 1684 (Figure 6 and Table 1).
Figure 6.
Concentration-dependent relaxations to ACh (0.1 nM–0.1 mM) in renal arteries contracted with phenylephrine (1 μM) of WKY (8 months) (left panel) and SHR (8 months) (right panel), incubated with TRAM-34 (10 μM) plus UCL 1684 (10 μM), in the absence or presence of indomethacin (10 μM). Rings of WKY were also incubated with L-NAME, TRAM-34 plus UCL 1684 and SC19220 (0.1 mM). Values are mean±s.e.mean, n=5 per group.
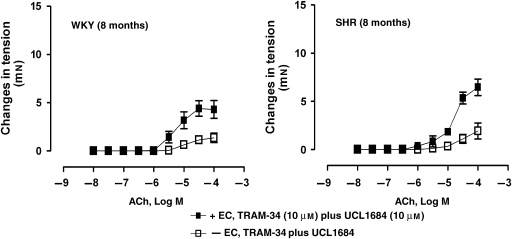
In quiescent preparations of WKY and SHR rings, incubated with TRAM-34 plus UCL 1684, ACh evoked concentration-dependent contractions that were prevented in rings without endothelium (Figure 7).
Figure 7.
Concentration-dependent contractions to ACh (10 nM–0.1 mM) in quiescent rings, with or without endothelium, of WKY (8 months) (left panel) and SHR (8 months) (right panel), incubated with TRAM-34 plus (10 μM) plus UCL 1684 (10 μM). Values are mean±s.e.mean, n=5 per group.
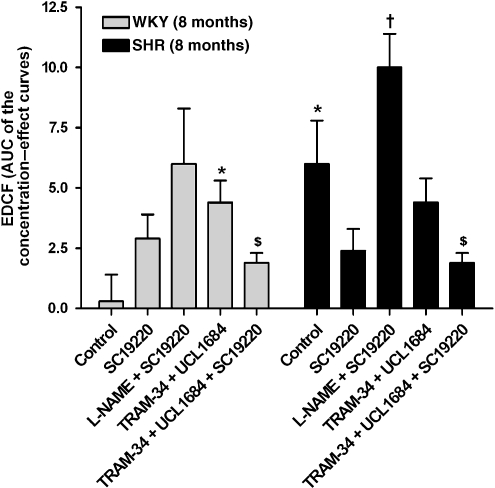
Calculated EDCF-mediated responses (subtracted area under the curve values)
Under control conditions, the calculated EDCF-mediated contractions were significantly larger in preparations of SHR compared with WKY and were not significantly different in the absence and presence of SC19220 (Figure 8). In arteries of SHR, the calculated EDCF-mediated responses were significantly higher in the presence of L-NAME plus SC19220 compared with SC19220 alone. In arteries of WKY, but not in those of SHR, the calculated EDCF-mediated response in the presence of TRAM-34 plus UCL 1684 was significantly higher than that of control rings. In both groups, these contractions were inhibited by SC19220.
Figure 8.
Calculated endothelium-derived contracting factor (EDCF)-mediated response to ACh (10 nM–0.1 mM) in quiescent rings of WKY (8 months) and SHR (8 months). The calculated EDCF-mediated response is defined as the area under the sigmoid regression curves of changes in tension of rings with endothelium minus that of rings without endothelium. Rings were incubated in control solution, in solution containing L-NAME (10 μM) or TRAM-34 (10 μM) plus UCL 1684 (10 μM) in the absence or presence of SC19220 (0.1 mM). Values are mean±s.e.mean; n=5–6 per group. *P<0.05 compared with rings of WKY incubated in the control solution; †P<0.05 compared with rings of SHR in the presence of SC19220; $P<0.05 compared with rings in the presence of TRAM-34 plus UCL 1684 (all with Student's t-test for unpaired observations).
Discussion
The present study demonstrates that in rat renal arteries, enhanced EDCF-mediated contractions are associated with hypertension. These arteries exhibit EDCF-mediated contractions with two components. One is exclusively TP receptor-dependent and can be optimized in arteries of hypertensive rats by the withdrawal of NO. The other may involve concomitant the activation of EP-1 and TP receptors and may be due to the withdrawal of EDHF resulting from hypertension.
ACh induces the release of endothelium-derived relaxing factors that evoke the relaxation of the underlying vascular smooth muscles (Furchgott and Zawadzki, 1980; Förstermann and Neufang, 1984; Furchgott and Vanhoutte, 1989). Both EDHF and NO contribute to the vasodilatation elicited by ACh in intermediate-sized rat renal arteries (Kagota et al., 1999; Jiang et al., 2000; Büssemaker et al., 2003). In the present experiments, the effect of acute incubation with L-NAME, a NOS inhibitor, shows that NO participates in ACh-induced relaxations in renal arteries of WKY. EDHF induces hyperpolarization in the smooth muscle cells of this preparation (Büssemaker et al., 2003). TRAM-34 plus UCL 1684 (intermediate- and small-conductance Ca2+-activated K+ channel blockers, respectively) block the EDHF component (Andersson et al., 2000; Eichler et al., 2003; Gluais et al., 2005). In arteries of WKY and SHR, TRAM-34 plus UCL 1684 had no effect on relaxations to ACh. In preparations of WKY, the contribution of EDHF to endothelium-dependent relaxation was detected only when NOS was inhibited with L-NAME (and in the presence of SC19220). Despite the absence of measurement of the membrane potential, these observations confirm that EDHF-induced responses are curtailed by the ACh-stimulated released of NO (Olmos et al., 1995; Bauersachs et al., 1996; McCulloch et al., 1997). In arteries from SHR, the inhibitor of NOSs abolished the response, indicating that the relaxations are fully NO-dependent. This is in line with a blunted EDHF-mediated response reported in renal arteries of aged SHR (Büssemaker et al., 2003).
With high concentrations of ACh, in preparations previously contracted with phenylephrine, secondary increases in tension occur in the aorta and renal arteries of hypertensive rats and these are prevented by the COX inhibitor, indomethacin, the TP receptor antagonist, terutroban, or the removal of endothelium, suggesting that they are due to the release of EDCF (Lüscher and Vanhoutte, 1986; Koga et al., 1988; Lüscher et al., 1988; Fu-Xiang et al., 1992; Kagota et al., 1999; Zhou et al., 2001; Yang et al., 2004; Tang et al., 2005). In the present study, relaxations of the rat renal artery in response to ACh were also followed by secondary increases in tension at higher concentrations of the muscarinic agonist. In preparations of hypertensive rats, these secondary increases in tension were augmented. Although the contractions caused by phenylephrine were smaller in the presence of indomethacin, the effect of this inhibitor on the increases in tension suggests the involvement of COX products in the secondary increases in tension in response to ACh. Judging from these results, the ACh-induced release of EDCF can be held responsible for the secondary increases in tension caused by the muscarinic agonist in the rat renal artery.
In canine cerebral arteries or rat renal arteries, ACh induces, in the absence of NOS inhibition, EDCF-mediated contractions that require the release of COX metabolites and activation of TP receptors (Katusic et al., 1988; Shirahase et al., 1988; Nishimura et al., 1995; Gao and Lee, 2005). Similarly, in the present quiescent preparations of renal arteries of WKY and SHR, ACh evoked contractions in the absence of an inhibitor of NOS. The contractions were prevented by the removal of endothelium in arteries of the SHR, demonstrating their endothelium-dependent nature. They were abolished in both groups by the COX inhibitor indomethacin or the TP receptor antagonist terutroban, confirming earlier observations in the aorta of the same species (Lüscher and Vanhoutte, 1986; Yang et al., 2004; Tang et al., 2005). These observations confirm the release of EDCF. The comparison of the calculated endothelium-dependent contractions demonstrates that EDCF-mediated contractions are increased with hypertension.
In SHR aorta, NO inhibits the EDCF-mediated response (Auch-Schwelk et al., 1992; Yang et al., 2004; Tang et al., 2005). In the renal artery, L-NAME induces EP-1 and TP receptors-dependent increases above the initial tension (Michel et al., 2007). In the present study, the tension of WKY and SHR rings was stable in the presence of L-NAME, when SC19220, a presumed selective EP-1 receptor antagonist, was added to prevent these increases. In the rat renal artery, ACh elicited contractions in the presence of L-NAME and SC19220. In rings of SHR, this response was also prevented by the removal of endothelium, inhibited by indomethacin or blocked by terutroban, demonstrating the involvement of TP receptors, confirming earlier conclusions (Auch-Schwelk et al., 1990; Yang et al., 2004; Tang et al., 2005). The comparison of the calculated endothelium-dependent contractions in the presence of SC19220 showed that an NOS inhibitor augments a TP receptor-dependent EDCF-mediated contraction in arterial preparations of hypertensive rats.
The withdrawal of NO enhances the action of EDCF (Auch-Schwelk et al., 1992; Yang et al., 2004; Tang et al., 2005), but it is unknown whether or not the absence of EDHF-mediated responses also favours the occurrence of endothelium-dependent contractions. TRAM-34 plus UCL 1684 augmented COX-dependent secondary increases in tension in arteries of WKY. In quiescent preparations of WKY, TRAM-34 and UCL 1684 enhanced EDCF-mediated responses. This response was EP-1 receptor-dependent. In the femoral arteries of diabetic rats, the activation of both TP and EP-1 receptors is involved in the EDCF-mediated contractions (Shi et al., 2007). In the present study, SC19220 attenuated the response to U46619, a supposed selective TP receptor agonist, whereas terutroban blocked it in renal arteries without endothelium of WKY and SHR. As far as SC19220 can be regarded as a selective EP-1 receptor blocker (Tang et al., 2008), these observations suggest that, in this artery, U46619 leads to the activation of both TP and EP-1 receptors. Alternatively, activation of TP receptors may induce the formation of prostaglandin E2 in vascular smooth muscles (Bolla et al., 2004) or, as with most of the G-protein-coupled receptors, a part of the pool of the TP receptors in rat renal arteries may be dimerized with EP-1 receptors (Wilson et al., 2004; Laroche et al., 2005; Milligan, 2007). The present observations, in the renal artery of normotensive rats, suggest that the pharmacological blockade of EDHF-mediated responses increased the release of EDCF, inducing the activation of EP-1 receptors. By contrast, in renal arteries of SHR, TRAM-34 and UCL 1684 did not modify the increases in tension. Moreover, in quiescent arteries of mature SHR, TRAM-34 and UCL 1684 did not enhance the EDCF-mediated response, suggesting that, under basal conditions, the increased EDCF-mediated contractions, observed in arteries of the hypertensive strain, are caused by a deficiency in EDHF.
In conclusion, ACh-induced endothelium-dependent contractions are triggered by two pathways in the rat renal artery. The withdrawal of NO augments a response, exclusively TP receptor-dependent in arteries of hypertensive rats. Hypertension favours the occurrence of EDCF-mediated contractions due to the combined activation of EP-1 and TP receptors. This facilitated occurrence may be due to the deficiency in EDHF-mediated response caused by the spontaneous hypertensive process.
Potential implications and limitations
Both EDHF and NO, released by the endothelium, participate in the relaxations of the surrounding smooth muscle of the main branch of the renal artery (Büssemaker et al., 2003). In vitro studies demonstrated that EDCF is also released by the endothelium under normal conditions in this artery (Nishimura et al., 1995; Gao and Lee, 2005). Despite the absence of confirmation in vivo, the present observations suggest that the balance between these factors may modulate renal vascular tone. In patients with essential hypertension, renal blood flow is increased (Hollenberg, 1984). EDCF participates in the moment-to-moment changes of the forearm blood flow of patients with essential hypertension (Taddei et al., 1998). In addition to major factors associated with essential hypertension, such as increased release of the adrenergic neurotransmitter or of angiotensin II (Hollenberg, 1984; Grisk and Rettig, 2004), EDCF may play a role in the local dysregulation of renal arterial tone in hypertension.
Acknowledgments
This work was supported, in part, by the FRM (Fondation pour la Recherche Médicale, France) (Grant SPE20050303429 to FSM) and the Research Centre of Heart, Brain, Hormone and Healthy Aging of the University of Hong Kong and the Research Grant council of Hong Kong (PMV).
Abbreviations
- EDCF
endothelium-derived contracting factor
- EDHF
endothelium-derived hyperpolarizing factor
- EP
prostaglandin E2/prostanoid
- L-NAME
Nω-nitro-L-arginine methyl ester
- SHR
spontaneously hypertensive rat
- TP
thromboxane A2/prostanoid
- WKY
Wistar Kyoto rat
Conflict of Interest
The authors state no conflict of interest.
References
- Altiere RJ, Kiritsy-Roy JA, Catravas JD. Acetylcholine-induced contractions in isolated rabbit pulmonary arteries: role of thromboxane A2. J Pharmacol Exp Ther. 1986;236:535–541. [PubMed] [Google Scholar]
- Andersson DA, Zygmunt PM, Movahed P, Andersson TL, Högestätt ED. Effects of inhibitors of small- and intermediate-conductance calcium-activated potassium channels, inwardly-rectifying potassium channels and Na(+)/K(+) ATPase on EDHF relaxations in the rat hepatic artery. Br J Pharmacol. 2000;129:1490–1496. doi: 10.1038/sj.bjp.0703226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15 6 Part 2:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusiæ ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- Bolla M, You D, Loufrani L, Levy BI, Levy-Toledano S, Habib A, et al. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–1269. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büssemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984;103:65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Fu-Xiang D, Jameson M, Skopec J, Diederich A, Diederich D. Endothelial dysfunction of resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1992;20 Suppl 12:S190–S192. doi: 10.1097/00005344-199204002-00053. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lee RM. Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br J Pharmacol. 2005;146:1061–1068. doi: 10.1038/sj.bjp.0706423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Félétou M. Role of SK(Ca) and IK(Ca) in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisk O, Rettig R. Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc Res. 2004;61:238–246. doi: 10.1016/j.cardiores.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK. Renal hemodynamics in essential and renovascular hypertension. Influence of captopril. Am J Med. 1984;76:22–28. doi: 10.1016/0002-9343(84)90879-9. [DOI] [PubMed] [Google Scholar]
- Jiang F, Li CG, Rand MJ. Mechanisms of nitric oxide-independent relaxations induced by carbachol and acetylcholine in rat isolated renal arteries. Br J Pharmacol. 2000;130:1191–1200. doi: 10.1038/sj.bjp.0703408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagota S, Tamashiro A, Yamaguchi Y, Nakamura K, Kunitomo M. Excessive salt or cholesterol intake alters the balance among endothelium-derived factors released from renal arteries in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1999;34:533–539. doi: 10.1097/00005344-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid, and acetylcholine in canine basilar arteries. Stroke. 1988;19:476–479. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Ageing suppresses endothelium-dependent relaxation and generates contraction mediated by the muscarinic receptors in vascular smooth muscle of normotensive Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens Suppl. 1988;6:S243–S245. doi: 10.1097/00004872-198812040-00073. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Laroche G, Lépine MC, Thériault C, Giguère P, Giguère V, Gallant MA, et al. Oligomerization of the alpha and beta isoforms of the thromboxane A2 receptor: relevance to receptor signaling and endocytosis. Cell Signal. 2005;17:1373–1383. doi: 10.1016/j.cellsig.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Diederich D, Weber E, Vanhoutte PM, Bühler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension. 1988;11 6 Part 2:573–578. doi: 10.1161/01.hyp.11.6.573. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Bottrill FE, Randall MD, Hiley CR. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br J Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel FS, Man RY, Vanhoutte PM. Increased spontaneous tone in renal arteries of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H1673–H1681. doi: 10.1152/ajpheart.00289.2007. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Usui H, Kurahashi K, Suzuki A. Endothelium-dependent contraction induced by acetylcholine in isolated rat renal arteries. Eur J Pharmacol. 1995;275:217–221. doi: 10.1016/0014-2999(95)00023-e. [DOI] [PubMed] [Google Scholar]
- Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40:520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- Olmos L, Mombouli JV, Illiano S, Vanhoutte PM. cGMP mediates the desensitization to bradykinin in isolated canine coronary arteries. Am J Physiol. 1995;268 2 Part 2:H865–H870. doi: 10.1152/ajpheart.1995.268.2.H865. [DOI] [PubMed] [Google Scholar]
- Shi Y, Feletou M, Ku DD, Man RY, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318:276–281. doi: 10.1124/jpet.105.099739. [DOI] [PubMed] [Google Scholar]
- Shirahase H, Usui H, Kurahashi K, Fujiwara M, Fukui K. Endothelium-dependent contraction induced by nicotine in isolated canine basilar artery—possible involvement of a thromboxane A2 (TXA2) like substance. Life Sci. 1988;42:437–445. doi: 10.1016/0024-3205(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Simonet S, Descombes JJ, Vallez MO, Dubuffet T, Lavielle G, Verbeuren TJ. S 18886, a new thromboxane (TP)-receptor antagonist is the active isomer of S 18204 in all species, except in the guinea-pig. Adv Exp Med Biol. 1997;433:173–176. doi: 10.1007/978-1-4899-1810-9_35. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, et al. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EH, Jensen BL, Skott O, Leung GP, Feletou M, Man RY, et al. The role of prostaglandin E and thromboxane–prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc Res. 2008;78:130–138. doi: 10.1093/cvr/cvm112. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257 5 Part 2:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J Biol Chem. 2004;279:53036–53047. doi: 10.1074/jbc.M405002200. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Félétou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Kosaka H, Tian RX, Abe Y, Chen QH, Yoneyama H, et al. L-Arginine improves endothelial function in renal artery of hypertensive Dahl rats. J Hypertens. 2001;19:421–429. doi: 10.1097/00004872-200103000-00010. [DOI] [PubMed] [Google Scholar]