Abstract
Background and purpose:
Hypothalamic neuropeptides centrally modulate sexual arousal. However, the role of neuropeptides in peripheral arousal has been ignored. Vascular and non-vascular smooth muscle relaxation in the vagina is important for female sexual arousal. To date, in vitro studies have focused on vaginal strips with no studies on vaginal arteries. The aim of this study was to compare the effects of sexual hypothalamic neuropeptides on rabbit vaginal wall strips and arteries.
Experimental approach:
Tissue bath and wire myography techniques were used to measure isometric tension from strips and arteries, respectively.
Key results:
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) relaxed both preparations, effects that were only antagonized by the VIP/PACAP antagonist VIP6–28 (10 nM) and the PAC1 antagonist PACAP 6–38 (1 μM). The melanocortin agonist α-melanocortin-stimulating hormone (1 μM), but not bremelanotide (1 μM), also relaxed both preparations. Oxytocin and vasopressin contracted vaginal preparations, which could be antagonized by the V1A antagonist SR 49059. Neuropeptide Y (NPY) and the NPY Y1 agonist Leu31, Pro34 NPY only contracted arteries, which was antagonized by the NPY Y1 receptor antagonist BIBP 3226. Melanin-concentrating hormone (MCH; 1 μM) contracted arteries.
Conclusion and implications:
Hypothalamic neuropeptides can exert contractile and relaxant effects on vaginal strips and arteries. NPY Y1, V1A, MCH1 antagonists as well as VIP/PAC1 agonists may have therapeutic potential in both central and peripheral female sexual arousal. Differences in effect of neuropeptides between preparations raise the question of which preparation is important for female sexual arousal.
Keywords: female sexual arousal, vagina, arteries, neuropeptides, contraction, relaxation
Introduction
Extensive preclinical in vivo studies have shown that hypothalamic neuropeptides play an important role in the central control of female sexual behaviour. Neuropeptide Y (NPY; Clark, 1992; Marin-Bivens et al., 1998) and vasopressin (AVP; Pedersen and Boccia, 2006) have all been shown to inhibit female receptive copulatory behaviour, also known as lordosis or receptivity, whereas oxytocin (Arletti and Bertolini, 1985; Caldwell et al., 1986, 1989; Caldwell, 1992; Benelli et al., 1994; Pedersen and Boccia, 2002), pituitary adenylate cyclase-activating polypeptide (PACAP; Apostolakis et al., 2004; Apostolakis et al., 2005), melanin-concentrating hormone (MCH; Gonzalez et al., 1996) and α-melanocortin-stimulating hormone (αMSH; Cragnolini et al., 2000; Gonzalez et al., 1998; Gonzalez et al., 1996; Nocetto et al., 2004; Pfaus et al., 2004) have all been shown to facilitate female sexual receptivity when injected into the paraventricular nucleus, medial preoptic area or ventromedial nucleus.
Surprisingly to date, although most effort has focused on the role of neuropeptides in centrally mediated sexual arousal, there has been little focus on neuropeptides underlying the peripheral control of female sexual arousal. Vascular and non-vascular smooth muscle relaxation play a critical role during sexual arousal and deficits in these mechanisms during sexual arousal may underlie the pathophysiology of female sexual arousal disorder. During sexual arousal, the vaginal blood vessels relax allowing increased blood flow into the vagina, clitoris and external genital organs. The increase in flow leads to increased vaginal lubrication by increased plasma transudation. It is thought that there are increases in genital sensitivity during sexual arousal; however, the underlying mechanisms are poorly understood. The rabbit has generally been used as a preclinical in vivo model to investigate vaginal blood flow as a physiological measure of peripheral sexual arousal (Park et al., 1997, 2001; Min et al., 2001, 2002, 2003; Kim et al., 2002, 2003; Angulo et al., 2004). Most studies have focused on investigating the role of the nitric oxide–cGMP pathway in controlling vaginal smooth muscle tone, demonstrating the ability of phosphodiesterase type-5 inhibitors to relax vaginal and clitoral muscles and potentially facilitate arousal in women (Sandner et al., 2007). To date, only sildenafil has been investigated clinically for effects on female sexual function with disappointing results (Sandner et al., 2007). However, there have been limited studies investigating the effect of neuropeptides on vaginal blood flow with most emphasis on vasoactive intestinal peptide (VIP; Levin, 1991). Likewise, there is a paucity of data using non-vascular vaginal smooth muscle strips to study the functional effects of neuropeptides on sexual arousal. Following histochemical identification of VIP, PACAP, peptide histidine methonine, peptide histidine valine and helospectin in nerves of the vagina (Graf et al., 1995; Hoyle et al., 1996), Ziessen et al. (2002) have demonstrated that each of these neuropeptides can directly relax the rabbit vagina when bath applied to pre-contracted vaginal strips. No in vitro studies, investigating the effect of neuropeptides directly on vaginal arteries contributing to vaginal blood flow, have been performed.
The aims of the study therefore were to (a) investigate the effect of pro-sexual and anti-sexual hypothalamic neuropeptides in the rabbit vagina in vitro, (b) compare potency and efficacy of neuropeptides between vaginal strips and arteries and (c) identify receptor subtypes underlying neuropeptide responses.
Methods
Rabbit vagina preparation
All experiments were carried out in compliance with the UK legislation and subject to local ethical review. Female New Zealand rabbits (∼3 kg) were sacrificed by an overdose of pentobarbitone (Pentoject, Animalcare, York, UK) injected into the marginal ear vein. The vagina was removed and placed into Krebs solution (mM): NaCl 119, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 11, at 37 °C and gassed with 95% O2/5% CO2.
Vaginal strip dissection and mounting
The vagina was dissected into four longitudinal strips from the lower 3 cm of the vagina and then cut in half horizontally to produce four upper and four lower strips. Strips were mounted in 5 mL organ baths containing Krebs solution aerated with 95% O2/5% CO2 and allowed to equilibrate under a resting tension of 19.6 mN for 1 h.
Vaginal artery dissection and mounting
Following removal of the vagina, arteries were isolated and dissected into 2 mm rings. The artery entering the vagina is referred to as the ‘extra-vaginal artery' and the artery within the lower 2 cm of the vagina is referred to as the ‘intra-vaginal artery' (IVA). Using wire myography, isometric recordings were made from vessel rings (i.d. of extra-vaginal artery: 263±15 μM; IVA: 173±3.6 μM) equilibrated for 30 min under a resting tension of 2.94 mN. All experiments were performed in Krebs solution at 37 °C and gassed with 95% CO2/5% O2. Following equilibration, arterial rings were pre-contracted three times using phenylephrine (10 μM) followed by a 30 min washout period before neuropeptide application.
Concentration response curves
To investigate the contractile effect of neuropeptides in vaginal strips and arteries, agonist concentration response curves were obtained by cumulatively applying half-log concentration increments. A washout period for 1 h was followed by either vehicle or antagonist incubation for a further 20 min before repeating a reproducible concentration response curve.
For relaxation experiments, vaginal strips and arteries were pre-contracted with phenylephrine (10–20 μM). Neuropeptide agonist concentration response curves were obtained by cumulative half-log additions. A 1 h washout period was followed by either vehicle or antagonist incubations for 20 min before repeating a reproducible concentration response curve.
Data analysis
Data are expressed as mean±s.e. of the mean (s.e.mean) and n indicates the number of vaginal strips/arterial rings. The EC50, slope and pKB were determined using Labstats (Excel add-in, Pfizer). Experimental data using three antagonist concentrations were analysed to obtain pKB values using the global nonlinear regression method described by Lew and Angus (1995). Advantages offered by this method over the traditional Schild method have been discussed previously (Lew and Angus, 1995), briefly the method does not require the within-tissue control concentration response curves required for standard Schild regression methods. In experiments, where the antagonist was known to be competitive, a single antagonist concentration was used to antagonize the agonist concentration response curve and the Gaddum equation (http://www.pdg.cnb.uam.es/cursos/Barcelona2002/pages/Farmac/Comput_Lab/Guia_Glaxo/chap2d.html#pkb) was used. Statistical significance was determined by Students t-test or ANOVA.
Compounds
Compounds were obtained from the following sources: phenylephrine, AVP, oxytocin, BIBP 3226 and MCH were obtained from Sigma-Aldrich (Gillingham, Dorset, UK). NPY (human, rat), PACAP 6–38, GR231118, NPY (13–36), [cPP1−7, NPY19−23, Ala31, Aib32, Gln34]-h pancreatic polypeptide PYY (3–36), Leu31,Pro34 NPY were supplied by Bachem (St Helens, Merseyside, UK) and VIP, VIP6–28, PACAP 1–27, PACAP 1–38 and αMSH from Tocris (Avonmouth, Bristol, UK). SR 49059, L-368899 and bremelanotide were synthesized as reported in the literature (Serradeil-Le Gal et al., 1993; Thompson et al., 1997; Blood et al., 2001).
Results
Neuropeptide-evoked contractions in rabbit vascular and non-vascular smooth muscle
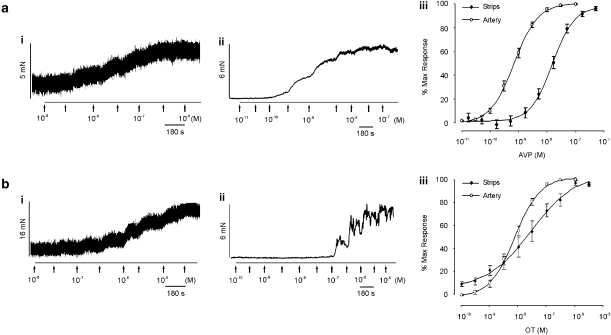
To investigate the effect of neuropeptides in the rabbit vagina, neuropeptides were added to the organ bath. AVP (0.01–100 nM) and oxytocin (0.1 nM–3 μM) elicited concentration-dependent contractions in upper vaginal strips with a maximum contraction of 5.7±0.7 mN (n=28) and 12.4±2.2 mN (n=13), respectively (Figures 1a and b). Neither peptide contracted the lower 1.5 cm vaginal strips. The AVP-induced contraction was 20-fold more potent than oxytocin as summarized in Table 1. As shown in Figure 1, AVP (0.01 nM-100 nM) and oxytocin (0.1 nM–10 μM) also contracted all regions of the rabbit vaginal artery with a maximum contractile response of 6.3±0.5 mN (n=53) and 5.7±0.5 mN (n=49), respectively. As in the vaginal strips, AVP was more potent than oxytocin (see Table 2). Both neuropeptides were more potent in the vaginal arteries compared to strips of vaginal smooth muscle, with AVP being 15-fold and oxytocin 3-fold more potent (Figure 1 aiii and biii, Table 2).
Figure 1.
Effect of oxytocin (OT) and vasopressin (AVP) on rabbit vaginal strips and arteries in vitro. Panel (a) shows an experimental trace of increasing concentrations of AVP on (i) vaginal strips and (ii) vaginal arteries. Panel (a) iii is the average concentration response curve to AVP in both tissues. Panel (b) is the same as (a), but in the presence of oxytocin.
Table 1.
Neuropeptide-induced contraction in rabbit vaginal strips
| Peptide | EC50 (nM) | Slope | Emax (mN) | n |
|---|---|---|---|---|
| AVP | 9.5±1.8 | 1.5±0.2 | 5.7±0.7 | 28 |
| Oxytocin | 206±83.2 | 0.8±0.1 | 12.4±2.2 | 13 |
| NPY | NE | NE | NE | 6 |
| MCH | NE | NE | NE | 8 |
Abbreviations: AVP, vasopressin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NE, no effect.
Table 2.
Neuropeptide-induced contraction in rabbit vaginal artery
| Peptide | EC50 (nM) | Slope | Emax (mN) | n |
|---|---|---|---|---|
| AVP | 0.6±0.1 | 1.1±0.1 | 6.3±0.5 | 53 |
| Oxytocin | 76.9±6.4 | 1.4±0.1 | 5.7±0.5 | 49 |
| NPY | 26.9±4 | 0.9±0.1 | 4.3±0.7 | 25 |
| MCH | >1000 | — | 3.7±0.6 | 13 |
Abbreviations: AVP, vasopressin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y.
In contrast to AVP and oxytocin, NPY failed to elicit a concentration-dependent contraction in vaginal strips (n=6; P>0.05). However, NPY potently elicited a large concentration-dependent contraction in vaginal arteries (Table 2). The NPY-induced contractions were greater in the IVA (4.3 ± 0.7 mN, n=25) compared to the extra-vaginal artery (0.7±0.2 mN, n=9; P <0.05).
Similar to NPY, the endogenous MCH receptor agonist MCH had no effect on vaginal strips (n=8) but elicited a large contraction in arterial rings at high concentrations (1 μM, Emax=3.7±0.6 mN, n=13).
Neuropeptide-evoked relaxations in rabbit vascular and non-vascular smooth muscle
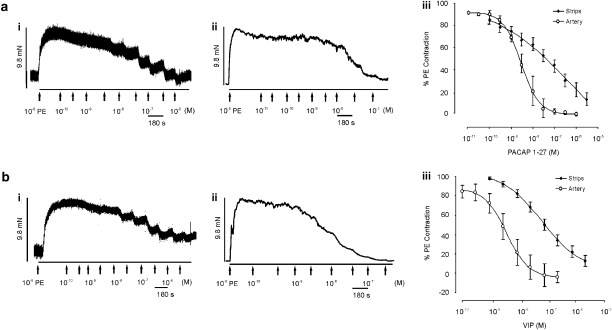
As previously shown by Ziessen et al. (2002), VIP (0.1 nM–3 μM) and PACAP 1–27 (0.1 nM–3 μM) relaxed pre-contracted vaginal strips (Figure 2). VIP and PACAP 1–27 elicited a maximum relaxation of 87±5.3% (n=18) and 95.9±10.5% (n=12), respectively. Both concentration response curves were shallow (Table 3). VIP (n=36) and PACAP 1–27 (n=11) also fully relaxed pre-contracted vaginal arteries (Table 4; Figure 2). Both neuropeptides response had a slope close to unity and were >20-fold more potent than relaxations observed in non-vascular tissue strips. PACAP 1–38 failed to relax either non-vascular or vascular smooth muscle (n=7; P>0.05).
Figure 2.
Effect of pituitary adenylate cyclase-activating polypeptide (PACAP 1–27) and vasoactive intestinal peptide (VIP) on rabbit vaginal strips and arteries in vitro. Panel (a) shows an experimental trace of increasing concentrations of PACAP 1–27 on (i) vaginal strips and (ii) vaginal arteries, pre-contracted with phenylephrine (PE). Panel (a) iii shows the average concentration response curve to PACAP 1–27 in both tissues. Panel (b) is the same as (a), but in the presence of VIP.
Table 3.
Neuropeptide-induced relaxation in rabbit vaginal strips
| Peptide | EC50 (nM) | Slope | Emax (mN) | n |
|---|---|---|---|---|
| VIP | 80.9±5.4 | 0.60±0.3 | 87.0±5.3% | 18 |
| PACAP 1–27 | 116±82.3 | 0.4±0.2 | 95.9±10.5% | 12 |
| αMSH | >1000 | — | 36±6.7% | 12 |
Abbreviations: αMSH, α-melanocortin-stimulating hormone; PACAP, pituitary adenylate cyclase-activating polypeptide; VIP, vasoactive intestinal peptide.
Table 4.
Neuropeptide-induced relaxation in rabbit vaginal arteries
| Peptide | EC50 (nM) | Slope | Emax (mN) | n |
|---|---|---|---|---|
| VIP | 4.8±0.2 | 1.1±0.1 | 97.9±5.5% | 36 |
| PACAP 1-27 | 3.7±0.3 | 1±0.1 | 100.4±0.7% | 11 |
| αMSH | >1000 | — | 43.6±10.2% | 12 |
Abbreviations: αMSH, α-melanocortin-stimulating hormone; PACAP, pituitary adenylate cyclase-activating polypeptide; VIP, vasoactive intestinal peptide.
The non-selective melanocortin agonist, αMSH, relaxed both vaginal strips and arteries; an effect that was not concentration-dependent and was only elicited at 1 μM (36±6.7% (n=12) in vaginal strips and 43.6±10.2% (n=12) in arteries). In contrast, no effect was observed with another non-selective melanocortin agonist, bremelanotide (1 μM, n=4–5; P>0.05).
Characterization of receptor subtype(s) underlying AVP and oxytocin-induced contractions in non-vascular and vascular smooth muscle
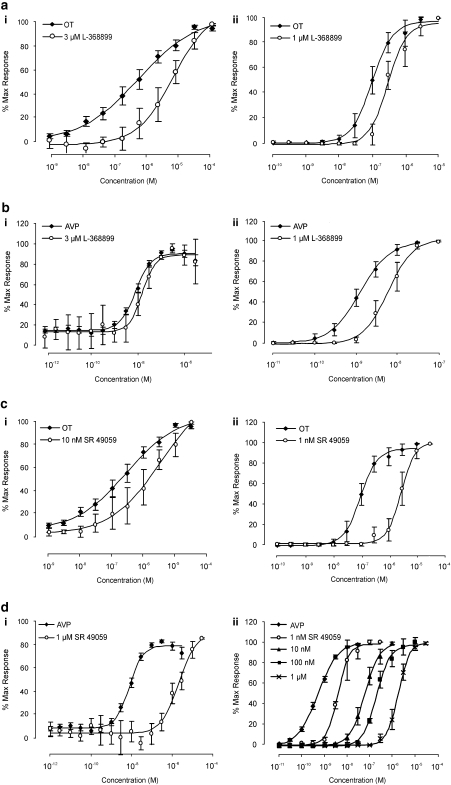
To determine whether oxytocin was acting through oxytocin receptors on vascular and non-vascular smooth muscle, the potent oxytocin antagonist L-368899 was used (Ki=3.6 nM; Thompson et al., 1997). L-368899 (10–100 nM) failed to shift the oxytocin concentration response curve in low concentrations. Only at high, non-selective, micromolar concentrations were there effects in vaginal arteries (oxytocin, EC50 75±3.9 nM; oxytocin and 1 μM L-368899 EC50 300±43 nM, n=10; P<0.01; Figure 3a) and in strips (oxytocin EC50 206.4±83.2 nM; oxytocin and 3 μM L-368899 EC50 1.8±0.8 μM, n=10; P<0.05; Figure 3a). As observed with oxytocin, only 1 μM L-368899 antagonized the AVP concentration response curves in vaginal arteries (AVP EC50 1.2±0.07 nM; AVP and 1 μM L-368899 EC50 6.2±0.5 nM, n=4, P<0.05), but failed to affect AVP at concentrations up to 3 μM in vaginal strips (n=4; Figure 3b).
Figure 3.
Effect of OT and AVP in the presence of L-368899 and SR 49059 in vagina strips and arteries. The left hand column shows the effect of both OT and AVP in the presence of either the OT antagonist L-368899 (a and b) or the V1A antagonist SR 49059 (c and d) on vagina strips. Graphs in the right hand column are the same as the left hand column but on vagina arteries.
As L-368899 did not potently antagonize the oxytocin-induced contractions in vaginal tissues, it was possible that oxytocin was acting through the vasopressin 1A (V1A) receptor. The selective non-peptide V1A antagonist SR 49059 (10 nM; Ki=1.6; Serradeil-Le Gal et al., 1993) caused a fivefold shift of the oxytocin concentration response curve in vaginal strips (pKB=8.7, n=5) with an apparent pKB=10.2 (n=6) in vaginal arteries as shown in Figure 3c. Similarly, AVP-induced contractions were potently antagonized by SR 49059 in vaginal strips (apparent pKB=8.15, n=5; Figure 3d) and vaginal arteries (pKB=9.63, n=8; Figure 3d).
Identification of receptor subtype underlying NPY-induced contraction in the rabbit vaginal artery
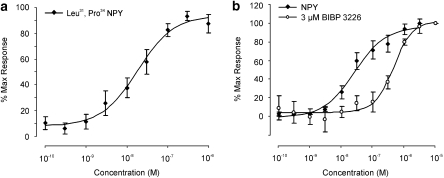
A range of NPY selective agonists were tested on the rabbit vaginal artery including NPY (13–36) (Y2 agonist), GR231118 (Y4 agonist), [cPP1−7, NPY19−23, Ala31, Aib32, Gln34]-h pancreatic polypeptide (Y5 agonist) and the non-selective agonist PYY (3–36). All failed to elicit a contraction (1 μM; n=3–4; P>0.05). However, the Y1 agonist, Leu31, Pro34 NPY induced a concentration-dependent contraction that was of similar amplitude to that of NPY (EC50 38.9±14.1 nM, slope 1.1±0.2, Emax 4.5±1.6 mN, n=11; Figure 4a). To fully investigate the presence of NPY Y1 receptors, the selective peptide NPY Y1 antagonist BIBP 3226 was tested against NPY in the IVA only (rabbit pKB=6.98; Doods et al., 1995). BIBP 3226 competitively inhibited the NPY contractile response (apparent pKB=7, slope 0.7, n=3–9; Figure 4b).
Figure 4.
Characterization of neuropeptide Y (NPY) Y1 receptors in vaginal arteries. Panel (a) is the concentration response curve to the NPY Y1 agonist Leu31, Pro34 NPY, in vaginal arteries. Panel (b) shows the effect of the peptide NPY Y1 antagonist BIBP 3226 (3 μM) on the concentration response curve to NPY.
Characterization of receptor subtype(s) underlying VIP and PACAP-induced relaxations in non-vascular and vascular smooth muscle
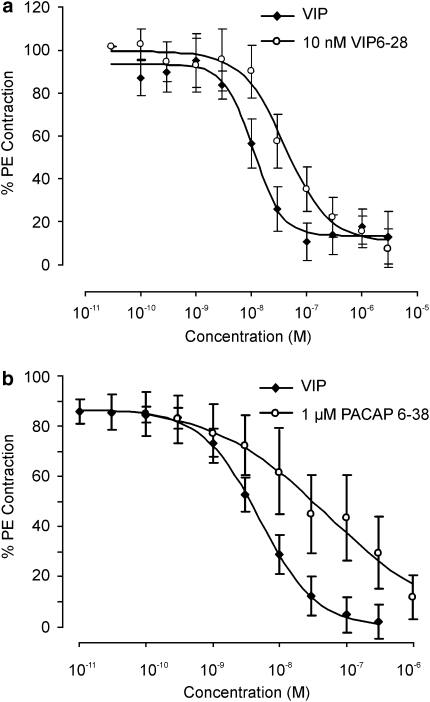
The selective peptide PAC1 receptor antagonist PACAP 6–38 (1 μM) and VIP/PACAP antagonist VIP6–28 (10 nM) failed to block either PACAP 1–27 or VIP-induced relaxations in rabbit vaginal strips (n=6; P>0.05) and extra-vaginal artery (n=8; P>0.05). In contrast, in the IVA both VIP6–28 (10 nM) and PACAP 6–38 (1 μM) caused about a four-fold rightward shift in the VIP EC50 (n=5–8; Figure 5; P>0.05).
Figure 5.
Inhibition of vasoactive intestinal peptide (VIP)-induced relaxations with VIP6–28 and pituitary adenylate cyclase-activating polypeptide (PACAP 6–38) in vaginal arteries. Panel (a) shows the effect of the VIP/PACAP antagonist VIP6–28 (10 nM) on the concentration response curve to VIP in vaginal arteries pre-contracted with phenylephrine (PE). Panel (b) is the same as (a), but in the presence of the PAC1 antagonist PACAP 6–38 (1 μM).
Discussion and conclusions
Novel findings in this study are (a) AVP and oxytocin contract both rabbit vaginal strips and arteries through V1A receptors, (b) AVP and oxytocin have a greater affinity for receptors present in vaginal artery than non-vascular smooth muscle, (c) V1A antagonists are equipotent in vascular and non-vascular smooth muscle, (d) NPY does not contract vaginal strips but potently contracts vaginal arteries through NPY Y1 receptors, (e) MCH contracts vaginal arteries and not non-vascular vaginal wall, (f) PACAP 1–27 and VIP relax pre-contracted vaginal strips and arteries through different receptor subtypes, (g) PACAP and VIP have a greater affinity for arteries than vaginal strips and (h) αMSH, but not bremelanotide, relaxes both vascular and non-vascular vaginal smooth muscle.
There is much published work showing that oxytocin through the oxytocin receptor has the ability to increase female receptivity (Witt and Insel (1991); Caldwell et al., 1994; Pedersen and Boccia, 2002). In addition, it has been reported that plasma oxytocin levels fluctuate throughout the menstrual cycle in women and significantly relates to genital lubrication; implying its role in peripheral activation of sexual function (Salonia et al., 2005). However, in contrast, in this study, oxytocin contracted both vaginal strips and arteries implying oxytocin peripherally would dampen blood flow. The lack of potency of oxytocin and failure of a selective oxytocin antagonist to antagonize oxytocin implies oxytocin-induced contractions are not through the oxytocin receptor. These findings are supported by Maggi et al. (1988), who by using oestrogenized rabbit vaginal strips showed that oxytocin only contracted the tissue at micromolar concentrations. In addition, binding experiments revealed oxytocin receptors are not present in the rabbit vagina. In this study, the potent contractile effect of AVP and potent antagonism of both oxytocin and AVP by SR 49059 implies the presence of a V1A receptor, which is anti-arousal. Recently, it has been shown that V1A antagonism in the hypothalamus is pro-sexual (Pedersen and Boccia, 2006). Together, these data support the hypothesis that V1A antagonists have the potential to enhance sexual arousal both centrally and peripherally.
It is also well known that NPY released from the forebrain into the hypothalamus is anti-sexual. However, the receptor subtype underlying the central actions of NPY remains to be elucidated. Peripherally, NPY has been shown to modulate uterine blood flow (Tenmoku et al., 1988) and immunohistochemical studies have also shown that NPY is found in abundance in the human vagina (Jorgensen et al., 1989; Hoyle et al., 1996). However, in this study, NPY failed to contract vaginal strips. Interestingly, NPY did contract vaginal arteries through the NPY Y1 receptor. NPY-induced contractions were greater in the IVA, which may be due to an increase in the density of NPY Y1 receptors with decreasing size of vessel. These data suggest NPY Y1 antagonists would have the ability to enhance vaginal blood flow.
Further distinction between vascular and non-vascular smooth muscle pharmacology was observed with MCH, which only contracted vaginal arteries. The receptor subtype underlying the response to MCH is presumably the MCH1 receptor, as rabbit and other non-primates do not express functional MCH2 receptors (Tan et al., 2002). Further evaluation awaits the availability of a commercial selective MCH1 antagonist. The present data contrast with the role of central MCH, which is believed to enhance female sexual behaviour (Gonzalez et al., 1996, 1998).
As previously reported application of exogenous VIP and PACAP 1–27 relaxed the vaginal wall (Ziessen et al., 2002). In contrast, PACAP 1–38 failed to have an effect. VIP and PACAP 1–27 were also significantly more potent in the IVA compared to the vaginal wall, which may be attributed to increased receptor density, different coupling efficiency or a difference in receptor subtypes. The blood vessel data support a clinical case study showing that VIP increases vaginal blood flow and lubrication in healthy women (Levin, 1991). Lack of blockade of both agonists with classical peptide PAC1 and VIP/PACAP antagonists within the vaginal strips, although these antagonists were efficacious in the IVA, may imply different receptor subtypes. Emerging research in this novel class B receptor family have shown that multiple splice variants exist, which also have differential signal-transduction pathways (Spengler et al., 1993; Dickson et al., 2006). In addition to PAC1/VIP receptor agonism enhancing peripheral sexual arousal, it has recently been shown that PACAP acting through PAC1 receptors within the hypothalamus can increase sexual arousal (Apostolakis et al., 2005).
Activation of central melanocortin receptors has been proposed to restore female sexual arousal, both preclinically in rats and also in ongoing clinical trials in women with female sexual arousal disorder. The non-selective peptide melanocortin agonist bremelanotide increased solicitative proceptive copulatory behaviours (hops and darts) in rats and increased desire, arousal and satisfaction in proof-of –concept studies (http://www.palatin.com/pdfs/bremelanotide.pdf; Pfaus et al., 2004). This study is the first report of the ability of αMSH to directly relax vascular and non-vascular smooth muscle; however, this relaxation effect was not reproduced by bremelanotide. This conflicting data may be due to species differences in potency and efficacy of melanocortin agents. Alternatively, a non-selective novel mechanism of action for αMSH may have been revealed, which warrants further investigation.
In conclusion, increased understanding of the physiology and pharmacology of female sexual arousal is key to helping us understand the underlying pathophysiology of female sexual dysfunction. At present, female sexual arousal is subdivided into subjective (mental) and physical (genital) arousal. Currently, the pathophysiology of female sexual dysfunction is unclear and may be a result of a genital deficit, as in male erectile dysfunction, or a psychogenic/centrally mediated deficit such as anxiety and depression.
Therapeutic options would be to (a) increase genital arousal that is, increased genital blood flow/lubrication; (b) increase central subjective arousal that is, subjective arousal, increased desire and improved satisfaction or (c) increase both genital and central subjective arousal. When one combines the data from this study with behavioural data, it is possible to suggest that NPY Y1 and MCH1 antagonists may be helpful for restoring genital arousal. V1A antagonists as well as MCH and oxytocin agonists may be helpful in restoring subjective arousal, whereas melanocortin, VIP/PACAP agonists, V1A and NPY1 antagonists may be useful for the restoration of both genital and subjective arousal. Owing to our poor understanding of female sexual dysfunction, it maybe necessary to test a number of these hypotheses in women to find an effective therapy for the treatment of female sexual dysfunction and restoration of normal sexual function.
Abbreviations
- αMSH
α-melanocortin-stimulating hormone
- AVP
vasopressin
- IVA
intra-vaginal artery
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
- PACAP
pituitary adenylate cyclase-activating polypeptide
- V1A
vasopressin 1A
- VIP
vasoactive intestinal peptide
Conflict of interest
The authors state no conflict of interest.
References
- Angulo J, Cuevas P, Cuevas B, Gupta S, Saenz De Tejada I. Antidepressant-induced inhibition of genital vascular responses is reversed by vardenafil in female rabbits. J Pharmacol Exp Ther. 2004;310:141–149. doi: 10.1124/jpet.103.063362. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Lanz R, O'Malley BW. Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol. 2004;18:173–183. doi: 10.1210/me.2002-0386. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Riherd DN, O'Malley BW. PAC1 receptors mediate pituitary adenylate cyclase-activating polypeptide- and progesterone-facilitated receptivity in female rats. Mol Endocrinol. 2005;19:2798–2811. doi: 10.1210/me.2004-0387. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6:247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, Arletti R. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27:245–250. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Blood CH, Shadiack AM, Bernstein JK, Herbert GW. Compositions and methods for treatment of sexual dysfunction WO 2001/000224. 2001.
- Caldwell JD. Central oxytocin and female sexual behavior. Ann N Y Acad Sci. 1992;652:166–179. doi: 10.1111/j.1749-6632.1992.tb34353.x. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA. Medial preoptic area oxytocin and female sexual receptivity. Behav Neurosci. 1989;103:655–662. doi: 10.1037//0735-7044.103.3.655. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm Behav. 1994;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Prange AJ, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Clark JT. Benextramine, a putative neuropeptide Y receptor antagonist, attenuates the termination of receptivity. Physiol Behav. 1992;52:965–969. doi: 10.1016/0031-9384(92)90378-f. [DOI] [PubMed] [Google Scholar]
- Cragnolini A, Scimonelli T, Celis ME, Schioth HB. The role of melanocortin receptors in sexual behavior in female rats. Neuropeptides. 2000;34:211–215. doi: 10.1054/npep.2000.0815. [DOI] [PubMed] [Google Scholar]
- Dickson L, Sharkey J, McCulloch J, Finlayson K. Maxadilan may discriminate between PAC1 receptor splice variants. Neurosci Abstract. 2006;726.21 [Google Scholar]
- Doods HN, Weinen W, Eentzeroth M, Rudolf K, Eberlein W, Engel W, et al. Pharmacological characterization of the selective nonpeptide neuropeptide Y1 receptor antagonist BIBP 3226. JPET. 1995;275:136–142. [PubMed] [Google Scholar]
- Gonzalez MI, Baker BI, Hole DR, Wilson CA. Behavioral effects of neuropeptide E-I (NEI) in the female rat: interactions with alpha-MSH, MCH and dopamine. Peptides. 1998;19:1007–1016. doi: 10.1016/s0196-9781(98)00045-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Graf AH, Schiechl A, Hacker GW, Hauser-Kronberger C, Steiner H, Arimura A, et al. Helospectin and pituitary adenylate cyclase-activating polypeptide in the human vagina. Regul Pept. 1995;55:277–286. doi: 10.1016/0167-0115(94)00116-f. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996;188:633–644. [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JC, Sheikh SP, Forman A, Norgard M, Schwartz TW, Ottsen B. Neuropeptide Y in the human female genital tract: localization and biological action. Am J Physiol. 1989;257:E220–E227. doi: 10.1152/ajpendo.1989.257.2.E220. [DOI] [PubMed] [Google Scholar]
- Kim NN, Min K, Huang YH, Goldstein I, Traish AM. Biochemical and functional characterization of alpha-adrenergic receptors in the rabbit vagina. Life Sci. 2002;71:2909–2920. doi: 10.1016/s0024-3205(02)02162-8. [DOI] [PubMed] [Google Scholar]
- Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. Role of the nitric oxide-cyclic GMP pathway in regulation of vaginal blood flow. Int J Impot Res. 2003;15:355–361. doi: 10.1038/sj.ijir.3901038. [DOI] [PubMed] [Google Scholar]
- Levin R. VIP, vagina, clitoral and periurethral glans–an update on human female genital arousal. Exp Clin Endocrinol. 1991;98:61–69. doi: 10.1055/s-0029-1211102. [DOI] [PubMed] [Google Scholar]
- Lew MJ, Angus JA. Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol Sci. 1995;16:328–337. doi: 10.1016/s0165-6147(00)89066-5. [DOI] [PubMed] [Google Scholar]
- Maggi M, Genazzani AD, Giannini S, Torrisi C, Baldi E, Di Tomaso M, et al. Vasopressin and oxytocin receptors in vagina, myometrium, and oviduct of rabbits. Endocrinol. 1988;122:2970–2980. doi: 10.1210/endo-122-6-2970. [DOI] [PubMed] [Google Scholar]
- Marin-Bivens CL, Kalra SP, Olster DH. Intraventricular injection of neuropeptide Y antisera curbs weight gain and feeding, and increases the display of sexual behaviors in obese Zucker female rats. Regul Pept. 1998;75–76:327–334. doi: 10.1016/s0167-0115(98)00085-8. [DOI] [PubMed] [Google Scholar]
- Min K, Munarriz R, Berman J, Kim NN, Goldstein I, Traish AM, et al. Hemodynamic evaluation of the female sexual arousal response in an animal model. J Sex Marital Ther. 2001;27:557–565. doi: 10.1080/713846801. [DOI] [PubMed] [Google Scholar]
- Min K, Munarriz R, Kim NN, Goldstein I, Traish A. Effects of ovariectomy and estrogen and androgen treatment on sildenafil-mediated changes in female genital blood flow and vaginal lubrication in the animal model. Am J Obstet Gynecol. 2002;187:1370–1376. doi: 10.1067/mob.2002.126641. [DOI] [PubMed] [Google Scholar]
- Min K, Munarriz R, Yerxa BR, Goldstein I, Shaver SR, Cowlen MS, et al. Selective P2Y2 receptor agonists stimulate vaginal moisture in ovariectomized rabbits. Fertil Steril. 2003;79:393–398. doi: 10.1016/s0015-0282(02)04677-0. [DOI] [PubMed] [Google Scholar]
- Nocetto C, Cragnolini AB, Schioth HB, Scimonelli TN. Evidence that the effect of melanocortins on female sexual behavior in preoptic area is mediated by the MC3 receptor; Participation of nitric oxide. Behav Brain Res. 2004;153:537–541. doi: 10.1016/j.bbr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Park K, Ahn K, Lee S, Ryu S, Park Y, Azadzoi KM. Decreased circulating levels of estrogen alter vaginal and clitoral blood flow and structure in the rabbit. Int J Impot Res. 2001;13:116–124. doi: 10.1038/sj.ijir.3900655. [DOI] [PubMed] [Google Scholar]
- Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, Azadzoi KM. Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int J Impot Res. 1997;9:27–37. doi: 10.1038/sj.ijir.3900258. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin maintains as well as initiates female sexual behavior: effects of a highly selective oxytocin antagonist. Horm Behav. 2002;41:170–177. doi: 10.1006/hbeh.2001.1736. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience. 2006;139:843–851. doi: 10.1016/j.neuroscience.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc Natl Acad Sci USA. 2004;101:10201–10204. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sandner P, Hütter J, Tinel H, Ziegelbauer K, Bischoff E. PDE5 inhibitors beyond erectile dysfunction. Int J Impot Res. 2007;19:533–543. doi: 10.1038/sj.ijir.3901577. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, Christophe B, et al. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holshoer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, et al. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Tenmoku S, Otteson B, O'Hare MM, Shiekh S, Bardrum B, Hansen B, et al. Interaction of NPY and VIP in regulation of myometrial blood flow and mechanical activity. Peptides. 1988;9:269–275. doi: 10.1016/0196-9781(88)90259-8. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, et al. Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab Dispos. 1997;25:1113–1118. [PubMed] [Google Scholar]
- Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinol. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- Ziessen T, Moncada S, Cellek S. Characterisation of the non-nitregic NANC relaxation responses in the rabbit vaginal wall. Br J Pharmacol. 2002;135:546–554. doi: 10.1038/sj.bjp.0704481. [DOI] [PMC free article] [PubMed] [Google Scholar]