Abstract
Pancreatic cancer is the fifth most common cause of cancer death. Identification of defined patient groups based on a prognostic index may improve the prediction of survival and selection of therapy. Many prognostic factors have been identified often based on retrospective, underpowered studies with unclear analyses. Data from 653 patients were analysed. Continuous variables are often simplified assuming a linear relationship with log hazard or introducing a step function (dichotomising). Misspecification may lead to inappropriate conclusions but has not been previously investigated in pancreatic cancer studies. Models based on standard assumptions were compared with a novel approach using nonlinear fractional polynomial (FP) transformations. The model based on FP-transformed covariates was most appropriate and confirmed five previously reported prognostic factors: albumin, CA19-9, alkaline phosphatase, LDH and metastases, and identified three additional factors not previously reported: WBC, AST and BUN. The effects of CA19-9, alkaline phosphatase, AST and BUN may go unrecognised due to simplistic assumptions made in statistical modelling. We advocate a multivariable approach that uses information contained within continuous variables appropriately. The functional form of the relationship between continuous covariates and survival should always be assessed. Our model should aid individual patient risk stratification and the design and analysis of future trials in pancreatic cancer.
Pancreatic ductal adenocarcinoma is a common cause of cancer death and is difficult to treat because clinical presentation is often late, and the disease is resistant to conventional chemotherapy. Long-term survival remains poor with a 5-year survival rate of 0.4–4% (Bramhall et al, 1995; Jemal et al, 2003). Multivariable prognostic models are important for grouping patients into risk sets for predicting survival and treating appropriately. There is currently no prognostic tool in routine use to identify subgroups of pancreatic cancer patients for selection and stratification of treatment and prediction of survival.
Because of its poor prognosis, few prognostic factors may be expected for patients with advanced pancreatic cancer; however, many possible factors have been identified. The majority of prognostic factor studies are questionable in terms of sample size and statistical methods, most based on small retrospective analyses. Literature searches (ISI Web of Science and Ovid Technologies databases) identified 36 prognostic factor studies reporting a total of 34 possible prognostic factors for advanced pancreatic cancer patients (Table 1) grouped as surgical, clinical, laboratory or demographic. Four studies (Johnson et al, 2001; Berlin et al, 2002; Ducreux et al, 2002; Maisey et al, 2002) were randomised controlled trials reporting five prognostic factors from multivariate analyses, namely metastases, tumour site, performance status, alkaline phosphatase and treatment. The remaining 32 studies (Friedman and van den Eeden, 1993; Yasue et al, 1994; Falconer et al, 1995; Lundin et al, 1995; Ishii et al, 1996; Rothenberg et al, 1996; Shibamoto et al, 1996; Cubiella et al, 1999; Storniolo et al, 1999; Halm et al, 2000; Terwee et al, 2000; Trigui et al, 2000; Ueno et al, 2000; Ikeda et al, 2001; Ridwelski et al, 2001; Tas et al, 2001; Tsuruta et al, 2001; Saad et al, 2002; Bachmann et al, 2003; Engelken et al, 2003; Fujino et al, 2003; Karayiannakis et al, 2003; Micke et al, 2003; Ohigashi et al, 2003; Paillaud et al, 2003; Stemmler et al, 2003; Talar-Wojnarowska et al, 2003; Ziske et al, 2003; Gupta et al, 2004; Kuhlmann et al, 2004; Watanabe et al, 2004; Ni et al, 2005) were based on consecutive series of patients, often retrospective, often single-centre, of which 15 studies were based on fewer than 100 patients (Yasue et al, 1994; Ishii et al, 1996; Rothenberg et al, 1996; Halm et al, 2000; Ikeda et al, 2001; Tsuruta et al, 2001; Saad et al, 2002; Karayiannakis et al, 2003; Micke et al, 2003; Ohigashi et al, 2003; Paillaud et al, 2003; Stemmler et al, 2003; Talar-Wojnarowska et al, 2003; Ziske et al, 2003; Gupta et al, 2004). One was based on five observational studies with varied inclusion criteria, inconsistent results and no prospective verification (Terwee et al, 2000). The largest series (2380 patients) identified factors based on univariate analyses and data containing a large proportion (57%) of censored patients (Storniolo et al, 1999).
Table 1. Literature review.
| Type (number) of studies | Number of patients per study | Prognostic factors reported (frequency of reporting) | U or MV analysis | ||
|---|---|---|---|---|---|
| Randomised controlled trial (n=4)3–6 | 207–322 | Surgical | Metastases | (3) | 4 MV |
| Tumour location | (1) | ||||
| Clinical | Performance status | (3) | |||
| Treatment | (1) | ||||
| Laboratory | Alkaline phosphatase | (1) | |||
| Consecutive series >500 patients (n=3)7–9 | 782–2380 | Surgical | Metastases | (1) | 2 MV |
| Stage of disease | (1) | 1 U | |||
| Operation | (1) | ||||
| Clinical | Performance status | (1) | |||
| Diabetes | (1) | ||||
| Pain | (1) | ||||
| Appetite/weight | (1) | ||||
| Jaundice | (1) | ||||
| Treatment | (1) | ||||
| Laboratory | Albumin | (1) | |||
| Demographic | Age | (1) | |||
| Specialist centre | (1) | ||||
| Consecutive series 100–500 patients (n=14)10–23 | 102–450 | Surgical | Metastases | (4) | 13 MV |
| Stage of disease | (2) | 1 U | |||
| Tumour location | (1) | ||||
| Operation | (2) | ||||
| Tumour size | (1) | ||||
| Duodenal invasion | (1) | ||||
| Peridissemination | (1) | ||||
| Ascites | (1) | ||||
| Clinical | Performance status | (2) | |||
| Diabetes | (1) | ||||
| Pain | (1) | ||||
| Appetite/weight | (3) | ||||
| Symptom onset | (1) | ||||
| Treatment | (2) | ||||
| Laboratory | CA242 | (2) | |||
| CA19-9 | (2) | ||||
| Leukocytes | (1) | ||||
| Gamma GT | (1) | ||||
| Albumin | (1) | ||||
| LDH | (1) | ||||
| CRP | (3) | ||||
| Iron | (1) | ||||
| Demographic | Age | (1) | |||
| Consecutive series | 28–95 | Surgical | Metastases | (1) | 8 MV |
| <100 patients (n=15)24–38 | Stage of disease | (1) | 7 U | ||
| Grade of disease | (1) | ||||
| Nodal status | (1) | ||||
| Operation | (1) | ||||
| Tumour size | (2) | ||||
| Fibrosis | (1) | ||||
| Clinical | Performance status Inflammation | (4) | |||
| Appetite/weight | (1) | ||||
| Treatment | (1) | ||||
| Laboratory | CA19-9 | (1) | |||
| VEGF | (7) | ||||
| CEA | (1) | ||||
| Phase angle BIA | (1) | ||||
| SCA | (1) | ||||
| (1) | |||||
MV=multivariate; U=univariate.
An important issue in prognostic factor studies is the nature of the relationship between the factor and survival (functional form). Continuous variables are often simplified at analysis by assuming a linear relationship with log-hazard or by introducing a step function through categorisation (frequently dichotomisation). If the linearity assumption is not correct, the final prognostic model could be misspecified. Misspecification of the functional form may lead to inappropriate conclusions but has not been previously investigated in pancreatic cancer studies. Many researchers avoid this problem by dichotomising, with a consequent loss of power. There is also the risk of important bias when the choice of cutoff is data-driven and the use of different cutoff points across multiple studies hinders direct comparisons.
The aim of this study was to evaluate potentially important baseline prognostic factors for survival in advanced pancreatic cancer using prospective data from two randomised controlled trials and a total of 653 patients (Bramhall et al, 2001, 2002). The study investigated clinical, histological, biochemical and demographic variables. A multivariable approach was used accounting for the functional form of the relationship between continuous factors and survival. Models were developed either on the basis of standard assumptions of log linear or step functional relationships with survival, or a novel approach based on nonlinear relationships, using more complex fractional polynomial (FP) transformations: a flexible, parametric method for modelling nonlinear relationships (Royston and Altman, 1994; Altman and Lyman, 1998).
PATIENTS AND METHODS
Data
Two international phase III British Biotech studies (BB128, Bramhall et al, 2001; BB193, Bramhall et al, 2002) randomised 414 and 239 patients with advanced pancreatic cancer, respectively: BB128 randomised patients between marimistat and gemcitabine; BB193 randomised patients between marimistat with gemcitabine and gemcitabine alone. The studies had similar eligibility criteria: histologically or cytologically unresectable pancreatic cancer, within 8 weeks of diagnosis or disease recurrence and Karnofsky performance status of ⩾50% (BB128) or ⩾60% (BB193). Previous therapy for metastatic or locally advanced disease was an exclusion criterion. The primary outcome measure in both studies was survival time calculated from the date of randomisation to the date of death from any cause. Randomisation was stratified by cancer stage (stage I/II, III or IV), Karnofsky performance status (50–70%, 80–100%), sex and study centre. The first stage of data reduction was considering only factors that were clinically relevant and available within an NHS outpatient clinic. Eighteen baseline clinical, histological, biochemical and demographic variables (including trial and randomised treatment group) were considered appropriate for analysis as possible prognostic factors (Table 2).
Table 2. Patient characteristics by trial.
| Variable | BB12839 N=414 (63%) | BB19340 N=239 (37%) | Total N=653 (100%) |
|---|---|---|---|
|
Demographics
| |||
| Age at entry (years) a | |||
| Median | 63 | 62 | 63 |
| Range | 29–89 | 32–85 | 29–89 |
| Ethnic race | |||
| White | 364 (88%) | 226 (95%) | 590 (90%) |
| Black | 27 (6%) | 8 (3%) | 35 (6%) |
| Oriental | 7 (2%) | 0 | 7 (1%) |
| Other | 15 (4%) | 5 (2%) | 20 (3%) |
| Missing | 1 | 0 | 1 |
| Sex | |||
| Male | 228 (55%) | 140 (59%) | 368 (56%) |
| Female | 186 (45%) | 99 (41%) | 285 (44%) |
| Treatment | |||
| Gemcitabine | 103 (25%) | 119 (50%) | 222 (34%) |
| Marimistat | 311 (75%) | 120 (50%) | 431 (66%) |
|
Tumour information
| |||
| Cancer stage | |||
| I | 19 (4%) | 13 (5%) | 32 (5%) |
| II | 45 (11%) | 27 (11%) | 72 (11%) |
| III | 76 (19%) | 28 (12%) | 104 (16%) |
| IV | 268 (66%) | 171 (72%) | 439 (68%) |
| Missing | 6 | 0 | 6 |
| Distant metastases | |||
| M0 | 129 (31%) | 65 (27%) | 194 (30%) |
| M1 | 265 (64%) | 171 (72%) | 436 (67%) |
| Missing | 20 (5%) | 3 (1%) | 23 (3%) |
| Regional lymph nodes | |||
| N0 | 153 (37%) | 90 (38%) | 243 (37%) |
| N1 | 164 (40%) | 87 (36%) | 251 (39%) |
| Missing | 97 (23%) | 62 (26%) | 159 (24%) |
| Primary tumour T stage | |||
| T0 | 5 (1%) | 3 (1.5%) | 8 (1.5%) |
| T1 | 114 (30%) | 44 (20%) | 158 (26%) |
| T2 | 90 (24%) | 54 (25%) | 144 (24%) |
| T3 | 167 (44%) | 113 (53%) | 280 (47%) |
| T4 | 6 (1%) | 1 (0.5%) | 7 (1.5%) |
| Missing | 32 | 24 | 56 |
|
Serum chemistry and haematology
| |||
| Laboratory variables | Median (range), missing | Median (range), missing | Median (range), missing |
| AST (SGOT)a | 24 (6–365), 17 | 26 (9–538), 12 | 25 (6–538), 29 |
| Total bilirubina | 13.7 (3.4–277.0), 16 | 13.7 (3.0–135.1), 8 | 13.7 (3.0–277.0), 24 |
| Alkaline phosphatasea | 136 (36–1660), 16 | 157 (35–2064), 8 | 140 (35–2064), 24 |
| Albumina | 38 (22–47), 17 | 38 (24–47), 8 | 38 (22–47), 25 |
| LDHa | 163 (77–1074), 21 | 169 (29–1495), 11 | 164 (29–1495), 32 |
| BUNa | 9.2 (2.9–34.3), 17 | 9.3 (4.3–27.9), 16 | 9.3 (2.9–34.3), 33 |
| CA19/9a | 686 (5–1 000 000), 17 | 800 (8–1 000 000), 30 | 710 (5–1 000 000), 47 |
| Haemoglobina | 12.5 (5.5–16.1), 28 | 12.4 (8.3–19.1), 13 | 12.4 (5.5–19.1), 41 |
| WBCa | 7.6 (2.3–31.6), 28 | 8.3 (2.4–23.7), 13 | 7.9 (2.3–31.6), 41 |
|
Outcome
| |||
| Event indicator | |||
| Alive | 22 (5%) | 19 (8%) | 41 (6%) |
| Dead | 392 (95%) | 220 (92%) | 612 (94%) |
| Follow-up of alive (months) a | |||
| Median | 20.1 | 19.4 | 20.7 |
| Range | 0.9–24.6 | 1.9–23.3 | 0.9–24.6 |
Continuous measurements.
Statistical analysis
We followed a strategy aimed at maximising model performance and avoiding poorly fitted and overfitted regression models in the development (Harrell et al, 1996) and reporting (McShane et al, 2005) of multivariable prognostic models. Initial analysis was based on standard methodology comparing Kaplan–Meier survival estimates using the log-rank test and estimating univariate hazard ratios for levels of each factor. The hazard of death was assessed in the multivariable setting using Cox proportional hazards regression modelling with variable reduction by backward elimination. The proportional hazards assumption was investigated for each covariate using log cumulative hazard (Collett, 1994) and martingale residual plots, and incorporating a time-dependent covariate (X=factor(LN (survival)−LN (mean survival))) and did not indicate any significant violation.
Ten of the 18 possible prognostic factors were collected as continuous measurements. Continuous data were investigated by assessing three different assumptions of the underlying relationship between survival and predictor, they are: (a) a linear relationship between the predictor and log hazard, (b) a step functional relationship using dichotomised covariates (laboratory measures based on central laboratory reference ranges) and (c) a nonlinear relationships based on either a simple log or more complex nonlinear FP transformation (Royston and Altman, 1994). For the third model, the functional form of each variable was assessed univariately comparing the Akaike's Information Criterion (AIC) (Collett, 1994) of a model based on the simple log transformation with the AIC of a model based on the best fitting FP transformation. First- and second-degree FP transformations (Meier-Hirmer et al, 2003) were considered using a selection level of 0.05 for input of variables based on power values of the polynomial ranging (−2, −1, −0.5, 0 (log), 0.5, 1, 2, 3). The best FP for each predictor was selected if it resulted in a significantly better fit (significantly smaller AIC) than the log transformation. The most appropriate (log or FP) transformation, if any, was applied to each variable and all variables were considered multivariately using Cox proportional hazards regression based on a backward selection method using a nominal significance level of 0.05 for elimination and including trial, sex, cancer stage (stratification factors at randomisation) and randomised treatment in each model.
The majority of variables had ⩽5% missing values (Table 2). Tumour stage, CA19-9 and WBC had 5–10% missing values, and lymph node status was missing for 24% of patients. Metastases or lymph node status was considered in the analysis as dummy variables using ‘negative’ as a reference level. Primary analysis was based on complete cases and a secondary analysis used multiple imputation to investigate the possible influence of variables with larger amounts of missing data (Rubin, 1987; Schafer, 1997) and provided valid inferential alternative results.
Model fit was assessed comparing AIC statistics, deviance residuals and Kaplan–Meier survival statistics for four predictive groups. The four predictive groups were based on quartiles of linear predictor scores, assessed comparing median survival estimates and hazard ratios. The bootstrap resampling approach described by Harrell et al (1996) was applied to assess the extent of overfitting in the final model, using 200 bootstrap resamples. This approach repeats the model selection methods used in the original model development in a series of bootstrapped resamples, freezing the derived model and applying to the original sample. Model optimism (overfitting) is described by the difference in the rank correlation coefficient relating predicted and observed survival times between the model derived in the bootstrap resample and that from the frozen model applied to the original sample averaged over 200 resamples. This provides an honest estimate of internal validity penalised for overfitting (Harrell et al, 1996).
Analyses were carried out using SAS and R using a two-sided significance level of 0.05 throughout.
RESULTS
Patient characteristics
A total of 653 patients were randomised. The eighteen clinically appropriate factors for analysis are presented in Table 2 and appear balanced across the two studies. On average, patients in the two trials were randomised 20 and 15 days after diagnosis and started treatment the day following randomisation. The average age of patients was 63 years (range 29–89), 368 (56%) were male, 439 (68%) had cancer stage IV disease, 436 (67%) presenting with metastases and 251 (39%) had lymph node involvement.
Survival
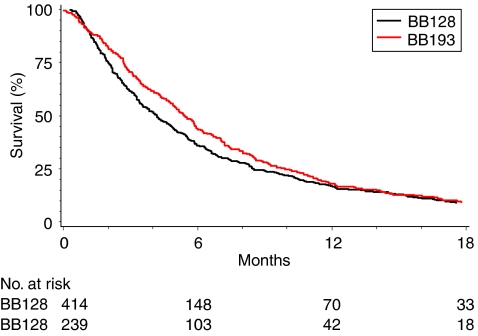
The majority of patients (612, 94%) had died by the time of analysis with a median follow-up time of 21 months for the 41 patients still alive (Table 2, Figure 1). The median survival estimate for the group is 4.7 months (95% CI: 4.2, 5.1) with 12-month survival estimate of 17% (Figure 1). Hazard functions estimated for 1-monthly time intervals to 18 months from trial entry were similar for both trials and reasonably constant over time. No significant survival benefit for marimastat was identified in the BB128 trial (P=0.19) when compared with gemcitabine (Bramhall et al, 2001). Similarly, no significant survival benefit was seen for a combination of gemcitabine and marimistat when compared with gemcitabine alone in the BB193 trial (P=0.95) (Bramhall et al, 2002).
Figure 1.
Survival function by trial.
Univariate analyses
Log-rank analyses (Table 3) indicated that potentially important factors were age (split at median, P=0.036), nodal status (P=0.035), cancer stage (I/II vs III/IV), metastases (both P<0.001) and laboratory measures (split as normal/abnormal according to laboratory reference ranges as per current clinical practise) as AST, alkaline phosphatase, albumin, LDH, WBC (all P<0.001), bilirubin (P=0.002), CA19-9 (P=0.005) and haemoglobin (P=0.009). Trial, treatment (gemcitabine vs marimistat), race (white vs not-white), sex, tumour stage (T0, 1, 2 vs T3, 4) and BUN were not significantly related to survival.
Table 3. Univariate log-rank analyses.
| Patients | Deaths | 12-month survival (%) | Median survival (95% CI) | χ2LR, p (χ2W, p) | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Overall survival | 653 | 612 | 17 | 4.7 (4.2, 5.1) | — | — |
| Trial | ||||||
| BB128 | 414 | 392 | 17 | 4.2 (3.6, 4.8) | 2.28, P=0.13 | 1.0 |
| BB193 | 239 | 220 | 18 | 5.4 (4.8, 6.0) | 0.88 (0.75, 1.04) | |
|
Demographics
| ||||||
| Age group (years) | ||||||
| <=63 | 343 | 320 | 21 | 5.1 (4.3, 5.8) | 4.42, P=0.036 | 1.0 |
| >63 | 310 | 292 | 13 | 4.3 (3.5, 4.9) | (7.55, P=0.006) | 1.18 (1.01, 1.39) |
| Ethnic group | ||||||
| White | 590 | 554 | 18 | 4.6 (4.1, 5.1) | 0.20, P=0.65 | 1.0 |
| Other | 62 | 57 | 12 | 5.2 (3.5, 6.0) | 1.06 (0.80, 1.41) | |
| Sex | ||||||
| Female | 285 | 267 | 18 | 4.9 (4.2, 5.8) | 0.73, P=0.39 | 1.0 |
| Male | 368 | 345 | 17 | 4.5 (3.9, 5.1) | 1.07 (0.91, 1.26) | |
| Treatment | ||||||
| Gemcitabine | 222 | 204 | 18 | 5.5 (4.7, 5.9) | 2.79, P=0.095 | 1.0 |
| Marimistat | 431 | 408 | 17 | 4.2 (3.5, 4.9) | 1.15 (0.98, 1.36) | |
|
Tumour information
| ||||||
| Cancer stage | ||||||
| Early (I/II) | 104 | 92 | 26 | 6.8 (5.7, 8.2) | 14.72, P<0.001 | 1.0 |
| Late (III/IV) | 543 | 514 | 16 | 4.1 (3.5, 4.7) | 1.53 (1.26, 1.86) | |
| Metastases | ||||||
| M0 | 194 | 176 | 30 | 6.8 (5.9, 8.4) | 35.47, P<0.001 | 1.0 |
| M1 | 436 | 414 | 12 | 3.5 (3.2, 4.0) | 1.69 (1.43, 1.99) | |
| Missing | 23 | 22 | 17 | 5.5 (4.9, 7.5) | 1.35 (0.88, 2.09) | |
| Lymph nodes | ||||||
| N0 | 243 | 226 | 20 | 5.5 (4.8, 6.0) | 6.73, P=0.035 | 1.0 |
| N1 | 251 | 240 | 18 | 4.5 (3.5, 5.4) | 1.19 (1.00, 1.43) | |
| Missing | 159 | 146 | 11 | 3.8 (3.2, 4.9) | 1.29 (1.04, 1.59) | |
| Tumour stage | ||||||
| Early (0/1/2) | 310 | 291 | 16 | 4.3 (3.7, 4.9) | 1.63, P=0.44 | 1.0 |
| Late (3/4) | 287 | 268 | 18 | 4.9 (4.2, 5.8) | 0.91 (0.77, 1.08) | |
| Missing | 56 | 53 | 18 | 5.8 (3.5, 7.9) | 0.87 (0.66, 1.16) | |
|
Serum chemistry and haematology
| ||||||
| AST (SGOT) | ||||||
| Normal | 538 | 499 | 19 | 5.1 (4.6, 5.7) | 14.17, P<0.001 | 1.0 |
| Abnormal | 86 | 84 | 12 | 2.8 (2.2, 3.9) | (5.99, P=0.014) | 1.55 (1.18, 2.04) |
| Total bilirubin | ||||||
| Normal | 464 | 429 | 20 | 5.1 (4.7, 5.7) | 9.32, P=0.002 | 1.0 |
| Abnormal | 165 | 159 | 11 | 3.8 (3.3, 4.4) | (6.27, P=0.012) | 1.32 (1.09, 1.61) |
| Alkaline phosphatase | ||||||
| Normal | 442 | 411 | 20 | 5.5 (5.0, 6.1) | 20.20, P<0.001 | 1.0 |
| Abnormal | 187 | 177 | 13 | 3.1 (2.6, 3.5) | (56.05, P<0.001) | 1.49 (1.23, 1.81) |
| Albumin | ||||||
| Normal | 583 | 544 | 19 | 5.1 (4.6, 5.6) | 31.37, P<0.001 | 1.0 |
| Abnormal | 45 | 43 | 7 | 1.5 (1.0, 2.7) | (74.34, P<0.001) | 2.36 (1.49, 3.72) |
| LDH | ||||||
| Normal | 543 | 505 | 20 | 5.2 (4.8, 5.8) | 37.05, P<0.001 | 1.0 |
| Abnormal | 78 | 75 | 5 | 2.1 (1.5, 2.8) | (36.16, P<0.001) | 2.08 (1.50, 2.88) |
| BUN | ||||||
| Normal | 407 | 382 | 20 | 5.1 (4.3, 5.7) | 3.43, P=0.064 | 1.0 |
| Abnormal | 213 | 199 | 13 | 4.4 (3.5, 5.1) | (5.28, P=0.022) | 1.17 (0.98, 1.40) |
| CA19/9 | ||||||
| Normal | 98 | 86 | 28 | 6.3 (4.8, 8.0) | 7.74, P=0.005 | 1.0 |
| Abnormal | 508 | 481 | 16 | 4.6 (4.0, 5.1) | (4.84, P=0.028) | 1.38 (1.12, 1.70) |
| Haemoglobin | ||||||
| Normal | 79 | 77 | 8 | 3.7 (3.3, 5.1) | 6.88, P=0.009 | 1.0 |
| Abnormal | 533 | 495 | 20 | 4.9 (4.4, 5.6) | (10.64, P=0.001) | 0.73 (0.55, 0.95) |
| WBC | ||||||
| Normal | 483 | 446 | 21 | 5.5 (4.9, 5.9) | 34.36, P<0.001 | 1.0 |
| Abnormal | 129 | 126 | 8 | 2.9 (2.4, 4.0) | (46.52, P<0.001) | 1.78 (1.40, 2.26) |
HR=hazard ratio; LR=log-rank statistic; W=Wald χ2 statistic.
Multivariate analyses
Three Cox proportional hazards regression models were developed (Table 4) using 556 patients (520 deaths) with complete data (excluding patients with missing data) based on the assumption of (a) a linear relationship between continuous covariates and log hazard, (b) a step functional dichotomisation of continuous covariates and (c) a nonlinear transformation of continuous covariates. All three models included trial, sex, cancer stage (stratification factors at randomisation) and randomised treatment group.
Table 4. Cox proportional hazards regression models, n=556 patients, 520 deaths.
| Variable | χ 2 | P-value | HR (95% CI) | |
|---|---|---|---|---|
| (a) Model 1 – ‘Linear’ covariates | ||||
|
Full model
| ||||
| AIC=5558.4 | ||||
| Stratification factors | Trial | 7.3 | 0.007 | 0.76 (0.63, 0.93) |
| Cancer stagea | 0.0001 | 0.99 | 1.00 (0.69, 1.44) | |
| Sex | 3.8 | 0.050 | 0.82 (0.68, 1.00) | |
| TRT | 2.6 | 0.11 | 1.17 (0.97, 1.42) | |
| Independent factors | Age | 3.0 | 0.081 | 1.01 (1.00, 1.02) |
| Albumin | 22.9 | <0.001 | 0.94 (0.92, 0.96) | |
| Alkaline phosphatase | 24.3 | <0.001 | 1.00 (1.00, 1.00) | |
| AST | 5.3 | 0.022 | 1.00 (0.99, 1.00) | |
| Bilirubin | 0.7 | 0.40 | 1.00 (1.00, 1.01) | |
| BUN | 2.3 | 0.13 | 1.02 (0.99, 1.05) | |
| CA19/9 | 0.9 | 0.34 | 1.00 (1.00, 1.00) | |
| Ethnic | 0.0009 | 0.98 | 1.01 (0.74, 1.36) | |
| Haemoglobin | 0.01 | 0.91 | 1.00 (0.94, 1.08) | |
| LDH | 19.3 | <0.001 | 1.00 (1.00, 1.00) | |
| METSb | 8.5 | 0.004 | 1.50 (1.14, 1.96) | |
| Nodesb | 0.4 | 0.51 | 1.08 (0.86, 1.37) | |
| Tumour stageb | 0.03 | 0.86 | 1.02 (0.84, 1.23) | |
| WBC | 10.2 | 0.001 | 1.04 (1.02, 1.07) | |
|
Final model
| ||||
| AIC=5557.1 | ||||
| Stratification factors | Trial | 8.1 | 0.005 | 0.76 (0.63, 0.92) |
| Cancer stagea | 0.008 | 0.93 | 1.01 (0.74, 1.38) | |
| Sex | 3.8 | 0.051 | 0.84 (0.70, 1.00) | |
| TRT | 3.2 | 0.073 | 1.19 (0.98, 1.44) | |
| Independent factors | Albumin | 41.0 | <0.001 | 0.94 (0.92, 0.96) |
| LDH | 13.3 | <0.001 | 1.00 (1.00, 1.00) | |
| METSb | 10.5 | 0.001 | 1.50 (1.17, 1.92) | |
| WBC | 11.7 | <0.001 | 1.04 (1.02, 1.07) | |
| Alkaline phosphatase | 21.1 | <0.001 | 1.00 (1.00, 1.00) | |
| (b) Model 2 – ‘Categorical’ covariates | ||||
|
Full model
| ||||
| AIC=5582.3 | ||||
| Stratification factors | Trial | 5.8 | 0.016 | 0.79 (0.65, 0.96) |
| Cancer stagea | 0.0001 | 0.99 | 1.00 (0.70, 1.44) | |
| Sex | 2.1 | 0.14 | 0.87 (0.72, 1.05) | |
| TRT | 3.3 | 0.07 | 1.19 (0.99, 1.44) | |
| Independent factors | Age | 2.2 | 0.13 | 1.15 (0.96, 1.39) |
| Albumin | 17.6 | <0.001 | 2.08 (1.48, 2.93) | |
| Alkaline phosphatase | 0.3 | 0.57 | 1.07 (0.85, 1.34) | |
| AST | 1.5 | 0.22 | 1.20 (0.90, 1.59) | |
| Bilirubin | 5.0 | 0.025 | 1.28 (1.03, 1.58) | |
| BUN | 1.9 | 0.17 | 1.14 (0.94, 1.39) | |
| CA19/9 | 7.8 | 0.005 | 1.43 (1.11, 1.85) | |
| Ethnic | 0.5 | 0.48 | 0.89 (0.66, 1.22) | |
| Haemoglobin | 2.2 | 0.14 | 0.82 (0.62, 1.07) | |
| LDH | 22.8 | <0.001 | 2.07 (1.54, 2.79) | |
| METSb | 13.3 | <0.001 | 1.64 (1.26, 2.14) | |
| Nodesb | 2.3 | 0.13 | 1.20 (0.95, 1.51) | |
| T stageb | 0.06 | 0.80 | 0.98 (0.81, 1.18) | |
| WBC | 9.2 | 0.002 | 1.42 (1.13, 1.78) | |
|
Final model
| ||||
| AIC=5583.2 | ||||
| Stratification factors | Trial | 7.0 | 0.008 | 0.77 (0.64, 0.94) |
| Cancer stagea | 0.9 | 0.36 | 1.16 (0.85, 1.58) | |
| Sex | 1.8 | 0.18 | 0.89 (0.74, 1.06) | |
| TRT | 3.4 | 0.065 | 1.19 (0.99, 1.44) | |
| Independent factors | Albumin | 24.2 | <0.001 | 2.30 (1.65, 3.21) |
| LDH | 25.1 | <0.001 | 2.05 (1.55, 2.72) | |
| METSb | 11.9 | <0.001 | 1.54 (1.21, 1.97) | |
| WBC | 10.3 | 0.001 | 1.44 (1.15, 1.79) | |
| Bilirubin | 8 .4 | 0 .004 | 1.34 (1.10, 1.64) | |
| CA199 | 9 .4 | 0 .002 | 1.48 (1.15, 1.89) | |
| (c) Model 3–‘Transformed’ covariates | ||||
|
Full model
| ||||
| AIC=5509.6 | ||||
| Stratification factors | Trial | 12.5 | <0.001 | 0.70 (0.57, 0.85) |
| Cancer stagea | 0.2 | 0.68 | 0.93 (0.64, 1.33) | |
| Sex | 4.3 | 0.038 | 0.82 (0.67, 0.99) | |
| TRT | 3.0 | 0.082 | 1.19 (0.98, 1.44) | |
| Independent factors | ||||
| Linear | Age | 2.3 | 0.13 | 1.01 (1.00, 1.02) |
| Linear | Albumin | 20.8 | <0.001 | 0.94 (0.92, 0.97) |
| Linear | Alkaline phosphatase | 11.3 | <0.001 | 1.00 (1.00, 1.00) |
| Linear | AST | 7.5 | 0.0062 | 1.00 (0.99, 1.00) |
| 1st degree FP | Log (bilirubin) | 3.0 | 0.083 | 1.16 (0.98, 1.37) |
| Linear | BUN | 3.2 | 0.075 | 1.02 (1.00, 1.05) |
| 2nd degree FP | CA1990.5 | 36.3 | <0.001 | 1.03 (1.02, 1.03) |
| 2nd degree FP | CA1990.5 × log(CA199) | 30.9 | <0.001 | 1.00 (1.00, 1.00) |
| Categorical | Ethnic | 0.1 | 0.74 | 0.95 (0.70, 1.29) |
| Linear | Haemoglobin | 0.3 | 0.61 | 0.98 (0.92, 1.05) |
| 1st degree FP | Log (LDH) | 12.3 | <0.001 | 1.72 (1.27, 2.32) |
| Categorical | METSb | 6.1 | 0.014 | 1.41 (1.07, 1.85) |
| Categorical | Nodesb | 1.4 | 0.24 | 1.15 (0.91, 1.46) |
| Categorical | Tumour stageb | 0.3 | 0.60 | 1.05 (0.87, 1.28) |
| Linear | WBC | 10.5 | 0.0012 | 1.05 (1.02, 1.07) |
|
Final model
| ||||
| AIC=5510.2 | ||||
| Stratification factors | Trial | 15.1 | <0.001 | 0.68 (0.56, 0.83) |
| Cancer stagea | 0.001 | 0.97 | 1.0 (0.73, 1.36) | |
| Sex | 3.5 | 0.061 | 0.84 (0.70, 1.01) | |
| TRT | 3.16 | 0.075 | 1.19 (0.98, 1.44) | |
| Independent factors | ||||
| Linear | Albumin | 41.4 | <0.001 | 0.94 (0.92, 0.95) |
| 1st degree FP | Log (LDH) | 12.8 | <0.001 | 1.70 (1.27, 2.27) |
| Categorical | METSb | 4.0 | 0.047 | 1.29 (1.00, 1.66) |
| Linear | WBC | 10.0 | 0.002 | 1.04 (1.02, 1.07) |
| Linear | Alkaline phosphatase | 14 .6 | <0 .001 | 1.00 (1.00, 1.00) |
| Linear | AST | 5 .2 | 0 .023 | 1.00 (0.99, 1.00) |
| Linear | BUN | 5 .0 | 0 .026 | 1.03 (1.00, 1.06) |
| 2nd degree FP | CA199 0.5 | 33 .3 | <0 .001 | 1.02 (1.02, 1.03) |
| 2nd degree FP | CA1990.5 × log(CA199) | 28.4 | <0.001 | 1.00 (1.00, 1.00) |
FP=fractional polynomial; HR=hazard ratio.
Stage (I/II vs III/IV).
Metastases (negative vs positive), Nodes (negative vs positive), Tumour stage (I/II vs III/IV): missing data included in analysis as a separate ‘dummy’ variable using lower level as the reference level.
The ‘linear’ model (Table 4, Model 1) identified five highly significant prognostic factors, namely albumin, alkaline phosphatase, LDH, WBC and metastases. The ‘categorical’ model (Table 4, Model 2) identified six highly significant prognostic factors, namely LDH, albumin, metastases, WBC, CA19-9 and bilirubin. Univariate analysis of the 10 continuous variables identified that nonlinear transformations were appropriate for 3 variables in their relationship with survival: bilirubin and LDH both as log transformations and CA19-9 as a second-degree FP transformation (CA19-90.5+(CA19-90.5 × log(CA19-9))). The seven remaining continuous covariates were analysed assuming a linear relationship with log hazard, as in Model 1. The ‘transformed’ model (Table 4, Model 3) identified eight prognostic factors. Five factors were highly significant with P<0.01, namely albumin, CA19-9, LDH, alkaline phosphatase and WBC with AST, BUN and metastases being more borderline in the model (P=0.023, 0.026 and 0.047, respectively).
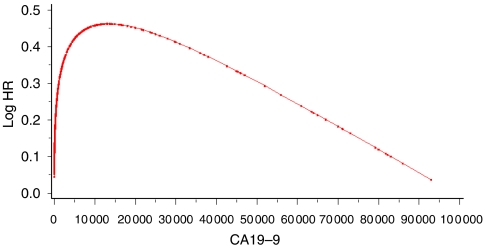
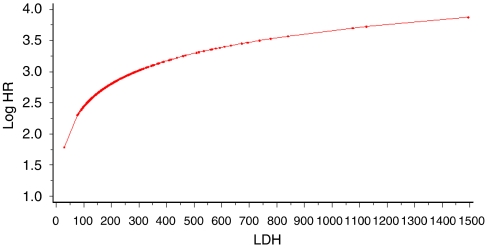
Nonlinear transformations were appropriate for two variables, LDH and CA19-9, and the estimated log hazard ratio functions are shown graphically in Figures 2 and 3. The second-degree FP function for CA19-9 estimates increasing risk up to an approximate CA19-9 value of 14 000 and then decreases with increasing CA19-9. The log function for LDH estimates increasing risk for increasing values of LDH.
Figure 2.
Estimated functional form for CA19-9. Dots indicate actual data values.
Figure 3.
Estimated functional form for LDH. Dots indicate actual data values.
Model comparison
In all three models, albumin, LDH and WBC were highly statistically significant and influential prognostic factors. Metastases were also an important variable but its parameter estimate and overall significance were reduced in the ‘transformed’ model when continuous covariates were included in a more appropriate format. In both the ‘linear’ and ‘transformed’ models, alkaline phosphatase was also a highly significant and influential prognostic factor. CA19-9 was also a highly significant and influential prognostic factor in both the ‘categorical’ and ‘transformed’ models. This variation is largely explained by the nonlinear relation of CA19-9 to survival (Figure 2), which could explain why it was considered important when dichotomised but not when included as linear. When considered as a transformed second-degree FP, its significance was much greater. Bilirubin was selected as a highly significant factor in the ‘categorical’ model but was not included in either the ‘linear’ or ‘transformed’ models. AST and BUN were only selected as prognostic in the ‘transformed’ model.
Model performance
The AIC statistic was smallest for the ‘transformed’ model (Table 4, Model 3), indicating a better fit to the data. Deviance residuals for this model were plotted against the linear predictor and were randomly scattered centred around a residual value of zero ranging between −3.86 and 3.33, which suggests the data have not been mis-modelled.
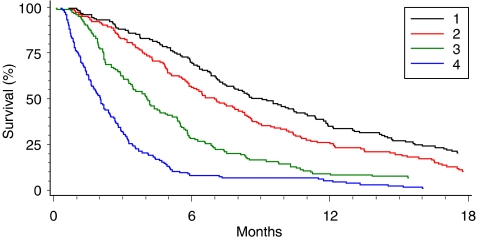
Patients were split into four groups based on quartiles of the distribution of linear predictor scores from the ‘transformed’ model. Kaplan–Meier survival estimates (Figure 4) show four distinct predictive groups with descending median survival estimates of 9.1 (95% CI: 7.4, 10.9), 7.0 (95% CI: 5.9, 8.3), 4.0 (95% CI: 3.4, 4.9) and 2.0 (95% CI: 1.6, 2.4) months. The hazard ratios for groups 2, 3 and 4 using predictive group 1 as the baseline were 1.35 (95% CI: 1.09, 1.66), 2.08 (95% CI: 1.64, 2.64) and 4.21 (95% CI: 3.11, 5.68), respectively.
Figure 4.
Survival by predictive group.
When assessing model validity, the R2 measure of model fit was estimated as 0.30. The bootstrap resampled estimate of R2 of 0.26 described model optimism (overfitting) under 5% and gives an improved estimate of model accuracy.
Multiple imputation allowed all 653 patients to be included in the modelling process and confirmed all the variables included in the ‘transformed’ model with increased significance for metastases (P=0.001), and the model also included nodal status (P=0.016) that had been excluded from all models prior to imputation, suggesting a strong link with other variables already in the model.
DISCUSSION
Large, prospective, phase III randomised controlled trials aim to provide robust statistical evidence for new treatment combinations. Stratification is important to control for known important variability in the data. Generally, patients with pancreatic cancer are not clinically separated into prognostic groups, with the exception of surgical status, before consideration for treatment. This study investigated potentially important baseline prognostic factors for survival as possible stratification variables for randomisation and analysis. Data from 653 patients included in two international randomised controlled trials in advanced pancreatic cancer (Bramhall et al, 2001, 2002) were analysed investigating multiple clinical, histological, biochemical and demographic variables in the form of both binary and continuous measurements. Valid statistical analyses are necessary to make best use of the data and optimise clinical results. As such, a multivariable approach was used to account for the functional form of the relationship between continuous prognostic variable factors and survival. Misspecification of functional form may lead to inappropriate conclusions but has not been previously investigated in pancreatic cancer studies. Continuous variables are often simplified by assuming a linear relationship between predictor and log hazard, that is the log risk increases or decreases linearly as the value of the factor increases, which may not be appropriate. Dichotomisation of continuous data is common but is problematic and unnecessary. As the variability in outcome within groups is ignored by categorisation, the variability between groups may be significantly underestimated as patients close to the cut point are analysed as being very different rather than being very similar, resulting in a serious reduction of statistical power to detect relationships between predictors and outcome, residual confounding and serious bias (Altman and Royston, 2006; Royston et al, 2006a). Regression using FPs of continuous covariates has been used in data from breast cancer (Sauerbrei et al, 1999) and metastatic renal carcinoma (Royston et al, 2006b) trials. Our study supported these in showing that this approach allowed important additional prognostic information to be extracted with less sophisticated approaches missed. FPs provide a flexible, parametric approach for modelling nonlinear relationships, making full use of the information available within each variable and as such can provide a clearer insight into the nature of the underlying relationship (Royston and Altman, 1994; Altman and Lyman, 1998).
Pancreatic ductal adenocarcinoma is the fifth most common cause of death from cancer in the Western world (Bramhall et al, 1995; Parkin et al, 2001; Jemal et al, 2003). It is particularly difficult to treat because of its remote location, late presentation and resistance to conventional chemotherapy. Long-term survival remains poor with a 5-year survival rate between 0.4 and 4% (Bramhall et al, 1995; Jemal et al, 2003). Resection is associated with improved survival but this is only possible in approximately 10% of patients (Sener et al, 1999; Alexakis et al, 2004). Although significant improvements in surgical outcome have been obtained with increasing specialisation (Neoptolemos et al, 1997; Birkmeyer et al, 1999), further benefits are anticipated by identifying high-risk groups. A validated prognostic index would identify subgroups of patients for specific treatments and predict survival, but there is no tool in routine use. Also, many possible prognostic factors have been identified in advanced pancreatic cancer (Table 1), most derived from retrospective studies based on small numbers of patients resulting in analyses that may be underpowered. A total of 34 possible factors were identified from 36 studies (4 randomised controlled trials; Johnson et al, 2001; Berlin et al, 2002; Ducreux et al, 2002; Maisey et al, 2002) and 32 consecutive series (Friedman and van den Eeden, 1993; Yasue et al, 1994; Falconer et al, 1995; Lundin et al, 1995; Ishii et al, 1996; Rothenberg et al, 1996; Shibamoto et al, 1996; Cubiella et al, 1999; Storniolo et al, 1999; Halm et al, 2000; Terwee et al, 2000; Trigui et al, 2000; Ueno et al, 2000; Ikeda et al, 2001; Ridwelski et al, 2001; Tas et al, 2001; Tsuruta et al, 2001; Saad et al, 2002; Bachmann et al, 2003; Engelken et al, 2003; Fujino et al, 2003; Karayiannakis et al, 2003; Micke et al, 2003; Ohigashi et al, 2003; Paillaud et al, 2003; Stemmler et al, 2003; Talar-Wojnarowska et al, 2003; Ziske et al, 2003; Gupta et al, 2004; Kuhlmann et al, 2004; Watanabe et al, 2004; Ni et al, 2005) of which 15 had <100 patients (Yasue et al, 1994; Ishii et al, 1996; Rothenberg et al, 1996; Halm et al, 2000; Ikeda et al, 2001; Tsuruta et al, 2001; Saad et al, 2002; Karayiannakis et al, 2003; Micke et al, 2003; Ohigashi et al, 2003; Paillaud et al, 2003; Stemmler et al, 2003; Talar-Wojnarowska et al, 2003; Ziske et al, 2003; Gupta et al, 2004), including demographic, clinical (including, performance status, weight loss and treatment), surgical (including, palliative procedures, site and stage of disease) and laboratory (including, CA19-9, LDH, alkaline phosphatase and albumin). Further concerns include the inadequate use of statistical methods, model comparison when different factors are being investigated and the differing format of factors across studies.
We developed three prognostic models, two based on standard assumptions of log linear or step functional relationships with survival and a novel approach based on nonlinear relationships using more complex FP transformations. The model based on transformed covariates (Table 4, Model 3) was the best-fitting model, better utilising the information within nonlinear covariates. This model confirmed five previously reported prognostic factors, namely albumin, CA19-9, alkaline phosphatase, LDH and metastases; and also identified three additional possible prognostic factors not previously reported: WBC, AST and BUN. Nonlinear transformations were appropriate for two variables indicating strong nonlinear effects on survival: CA19-9 as a second-degree FP and LDH under a log transformation. Importantly, the effect of CA19-9 was not apparent in the ‘linear’ model, the effect of alkaline phosphatase was not apparent in the ‘categorical’ model and the effects of AST and BUN were not apparent in either the ‘linear’ or ‘categorical’ models, indicating how the significant effect of these variables may go unrecognised due to simplistic assumptions made in statistical modelling.
Shrinkage represents the degree to which a plot of predicted and observed values is flattened from the 45° line attributable to overfitting. Overfitting leads to inflated estimates of model fit and is a potentially important source of bias in prognostic models. Overfitting may be minimised through sensible model selection, which for survival models implies avoiding attempting to fit models with more than 1 candidate variable (degree of freedom) for each 10 events of interest (e.g., death) included in the analysis. The degree to which overfitting is present in the fitted model may be estimated either directly through validation in an external data set or through a bootstrap process. In practise, it is rare for an external data set to be available, and if data are scarce, it becomes attractive to use all available data to derive the prognostic model. Thus, bootstrap resampling approaches may become the model validation methods of choice. In our model, bootstrap resampling methods (Harrell et al, 1996) suggested minimal optimism. As shrinking estimators will not increase the real discrimination of the model, and the degree of overfitting estimated for the model is minimal, rescaling the model estimates appears neither helpful nor necessary.
A model based on multiple imputation methods (Rubin, 1987; Schafer, 1997) to control for missing covariate data selected an additional variable nodal status as prognostic (P=0.016), which had been excluded from all models prior to imputation. The true importance of this variable requires further investigation, suggesting a strong link with other variables already in the model. All prognostic models ideally require external validation to determine the generality across different data sets, and our results may be seen as provisional until replicated on independent data. Performance status and tumour size at randomisation are well-documented factors (Table 1) but unfortunately were not available in this data set and should be included in any external validation.
This research was based on data from two large, phase III randomised controlled trials representative of patients with advanced pancreatic cancer with a high event rate, long follow-up and an overall 1 year survival rate of 17% (Bramhall et al, 2001, 2002). Analyses were based on a multivariable approach and utilised the information contained within continuous variables appropriately. The functional form of the relationship between continuous covariates and survival should always be assessed when investigating potential prognostic value. Models were based on information readily available in clinic and once validated should have the ability to aid decision-making by identifying patients with borderline disease for surgery and patients for inclusion into clinical trials or off-study treatment, especially since a greater number of palliative and more toxic treatments are becoming available and being trialed in this disease.
Acknowledgments
We are grateful to British Biotech for providing the individual patient data and to all patients who took part in these trials. We are grateful to Cancer Research UK who supported this research under a Population and Behavioural Sciences project grant (reference: C19491/A6150).
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP (2004) Current standards of surgery for pancreatic cancer. Brit J Surg 91: 1410–1427 [DOI] [PubMed] [Google Scholar]
- Altman DG, Lyman GH (1998) Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat 52: 289–303 [DOI] [PubMed] [Google Scholar]
- Altman DG, Royston P (2006) The cost of dichotomising continuous variables. BMJ 332: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MO, Alderson D, Peters TJ, Bedford C, Edwards D, Wotton S, Harvey IM (2003) Influence of specialization on the management and outcome of patients with pancreatic cancer. Br J Surg 90(2): 171–177 [DOI] [PubMed] [Google Scholar]
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Bowen Benson A (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trials E2297. J Clin Oncol 20(15): 3270–3275 [DOI] [PubMed] [Google Scholar]
- Birkmeyer JD, Warshaw AL, Finlayson SRG, Grove MR, Tosteson ANA (1999) Relationship between hospital volume and late survival after pancreatoduodenectomy. Surgery 126: 178–183 [PubMed] [Google Scholar]
- Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP (1995) Treatment and survival in 13 560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 82(1): 111–115 [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JAC (2001) Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomised trial. J Clin Oncol 19: 3447–3455 [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JAC (2002) A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 87: 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D (1994) Modelling Survival Data in Medical Research. London: Chapman and Hall [Google Scholar]
- Cubiella J, Castells A, Fondevila C, Sans M, Sabater L, Navarro S, Fernandez-Cruz L (1999) Prognostic factors in nonresectable pancreatic adenocarcinoma: a rationale to design therapeutic trials. Am J Gastroenterol 94(5): 1271–1278 [DOI] [PubMed] [Google Scholar]
- Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R, Bossett JF, Merouche Y, Raoul JL, Ychou M, Adenis A, Berthault-Cvitkovic F, Luboinski M (2002) A randomised trial comparing 5-FU with 5-FU plus cisplatin in advanced pancreatic carcinoma. Ann Oncol 13: 1185–1191 [DOI] [PubMed] [Google Scholar]
- Engelken FJF, Bettschart V, Rahman MQ, Parks RW, Garden OJ (2003) Prognostic factors in the palliation of pancreatic cancer. Eur J Surg Oncol 29(4): 368–373 [DOI] [PubMed] [Google Scholar]
- Falconer JS, Fearon KCH, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC (1995) Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer 75(8): 2077–2082 [DOI] [PubMed] [Google Scholar]
- Friedman GD, van den Eeden SK (1993) Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol 22(1): 30–37 [DOI] [PubMed] [Google Scholar]
- Fujino Y, Suzuki Y, Ajiki T, Tanioka Y, Ku YS, Kuroda Y (2003) Predicting factors for survival of patients with unresectable pancreatic cancer: a management guideline. Hepatogastroenterol 50(49): 250–253 [PubMed] [Google Scholar]
- Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA (2004) Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr 92(6): 957–962 [DOI] [PubMed] [Google Scholar]
- Halm U, Schumann T, Schiefke I, Witzigmann H, Mossner J, Keim V (2000) Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 82(5): 1013–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387 [DOI] [PubMed] [Google Scholar]
- Ikeda W, Okada S, Tokuuye K, Ueno H, Okusaka T (2001) Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer 91(3): 490–495 [DOI] [PubMed] [Google Scholar]
- Ishii H, Okada S, Nose H, Yoshimori M, Aoki K, Okusaka T (1996) Prognostic factors in patients with advanced pancreatic cancer treated with systemic chemotherapy. Pancreas 12(3): 267–271 [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ (2003) Cancer statistics, 2003. CA Cancer J Clin 53: 5–26 [DOI] [PubMed] [Google Scholar]
- Johnson CD, Puntis M, Davidson N, Todd S, Bryce R (2001) Randomised, dose-finding phase III study of lithium gamolenate in patients with advanced pancreatic adenocarcinoma. Br J Surg 88: 662–668 [DOI] [PubMed] [Google Scholar]
- Karayiannakis AJ, Bolanaki H, Syrigos KN, Asimakopoulos B, Polychronidis A, Anagnostoulis S, Simopoulos C (2003) Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett 194(1): 119–124 [DOI] [PubMed] [Google Scholar]
- Kuhlmann KFD, de Castro SMM, Wesseling JG, ten Kate FJW, Offerhaus GJA, Busch ORC, van Gulik TM, Obertop H, Gouma DJ (2004) Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer 40: 549–558 [DOI] [PubMed] [Google Scholar]
- Lundin J, Roberts PJ, Kuusela P, Haglund C (1995) Prognostic significance of serum CA 242 in pancreatic cancer. A comparison with CA 19-9. Anticancer Res 15(5B): 2181–2186 [PubMed] [Google Scholar]
- Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, Iveson T, O'Brien M, Tebbutt N, Harrington A, Hill M (2002) Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 20(14): 3130–3136 [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumour marker prognostic studies (REMARK). Br J Cancer 93: 387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Hirmer C, Ortseifen C, Sauerbrei W (2003) Multivariable Fractional Polynomials in SAS. SAS Institute, SAS Circle: Cary. NC, USA [Google Scholar]
- Micke O, Bruns F, Kurowski R, Horst E, deVries AF, Hausler JW, Willich N, Schafer U (2003) Predictive value of carbohydrate antigen 19-9 in pancreatic cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys 57(1): 90–97 [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Russell RCG, Bramhall S, Theis B (1997) Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. Br J Surg 84: 1370–1376 [PubMed] [Google Scholar]
- Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, Xu DK, Zhao P (2005) The clinical value of serum CEA, CA19-9 and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol 31: 164–169 [DOI] [PubMed] [Google Scholar]
- Ohigashi H, Ishikawa O, Yokayama S, Sasaki Y, Yamada T, Imaoka S, Nakaizumi A, Uehara H (2003) Intra-arterial infusion chemotherapy with angiotensin-II for locally advanced and nonresectable pancreatic adenocarcinoma: further evaluation and prognostic implications. Ann Surg Oncol 10(8): 927–934 [DOI] [PubMed] [Google Scholar]
- Paillaud E, Bories PN, Aita SL, Scherman E, Jeanfaivre V, Lejonc JL, Campillo B (2003) Prognostic value of dietary intake and inflammation on survival in patients with advanced cancer: relationship with performance status, pain, and digestive disorders. Nutr Cancer 45(1): 30–35 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37(Suppl 8): S4–S66 [DOI] [PubMed] [Google Scholar]
- Ridwelski K, Meyer F, Ebert M, Malfertheiner P, Lippert H (2001) Prognostic parameters determining survival in pancreatic carcinoma and, in particular, after palliative treatment. Digest Dis 19(1): 85–92 [DOI] [PubMed] [Google Scholar]
- Rothenberg ML, Moore ML, Cripps MC, Anderson JS, Portenoy RK, Burris HA, Green MR, Tarassoff PG, Brown TD, Casper ES, Storniolo AM, Von Hoff DD (1996) A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol 7: 347–353 [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG (1994) Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Stat 43(3): 429–467 [Google Scholar]
- Royston P, Altman DG, Sauerbrei W (2006a) Dichotomising continuous predictors in multiple regression: a bad idea. Stat Med 25: 127–141 [DOI] [PubMed] [Google Scholar]
- Royston P, Reitz M, Atzpodien J (2006b) An approach to estimating prognosis using fractional polynomials in metastatic renal carcinoma. Br J Cancer 94: 1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (1987) Multiple Imputation for Nonresponse in Surveys. Wiley: New York [Google Scholar]
- Saad ED, Machado MC, Wajsbrot D, Abramoff R, Hoff PM, Tabacof J, Katz A, Simon SD, Gansl RC (2002) Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer 32(1): 35–41 [DOI] [PubMed] [Google Scholar]
- Sauerbrei W, Royston P, Bojar H, Schmoor C, Schumacher M (1999) Modelling the effects of standard prognostic factors in node-positive breast cancer. Br J Cancer 79: 1752–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL (1997) Analysis of Incomplete Multivaraite Data. Chapman and Hall: London [Google Scholar]
- Sener SF, Fremgen A, Menck HR, Winchester DP (1999) Pancreatic cancer: a report of treatment and survival trends for 100 313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 189: 1–7 [DOI] [PubMed] [Google Scholar]
- Shibamoto Y, Manabe T, Ohshio G, Sasai K, Nishimura Y, Imamura M, Takahashi M, Abe M (1996) High-dose intraoperative radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 34(1): 57–63 [DOI] [PubMed] [Google Scholar]
- Stemmler J, Stieber P, Szymala AM, Schalhorn A, Schermuly MM, Wilkowski R, Helmberger T, Lamerz R, Stoffregen C, Niebler K, Garbrecht M, Heinemann V (2003) Are serial CA 19-9 kinetics helpful in predicting survival in patients with advanced or metastatic pancreatic cancer treated with gemcitabine and cisplatin? Onkologie 26(5): 462–467 [DOI] [PubMed] [Google Scholar]
- Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R (1999) An investigational new drug treatment program for patients with gemcitabine. Cancer 85(6): 1261–1268 [PubMed] [Google Scholar]
- Talar-Wojnarowska R, Gasiorowska A, Strzelczyk J, Janiak A, Malecka-Panas E (2003) Prognostic factors in the operative and palliative treatment of pancreatic cancer. Neoplasma 50(5): 383–387 [PubMed] [Google Scholar]
- Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E (2001) Prognostic factors in pancreatic carcinoma – serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 24(6): 547–550 [DOI] [PubMed] [Google Scholar]
- Terwee CB, Van Dijkum EJMN, Gouma DJ, Bakkevold KE, Klinkenbijl JHG, Wade TP, van Wagensveld BA, Wong A, van der Meulen JHP (2000) Pooling of prognostic studies in cancer of the pancreatic head and periampullary region: the triple-P study. Eur J Surg 166(9): 706–712 [DOI] [PubMed] [Google Scholar]
- Trigui B, Barrier A, Flahault A, Huguier M (2000) Prognostic factors in advanced pancreatic carcinoma. Multivariate analysis and predictive score of survival. Ann Chir 125(7): 625–630 [DOI] [PubMed] [Google Scholar]
- Tsuruta K, Okamoto A, Egawa N, Kamisawa T, Karasawa K, Takahashi T (2001) Survival benefits of adjuvant chemotherapy with oral doxifluridine (5-DFUR) following radiotherapy in patients with unresectable pancreatic cancer. J Surg Oncol 78: 202–207 [DOI] [PubMed] [Google Scholar]
- Ueno H, Okada S, Okusaka T, Ikeda M (2000) Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Onkologie 59(4): 296–301 [DOI] [PubMed] [Google Scholar]
- Watanabe I, Sasaki S, Konishi M, Nakagohri T, Inoue K, Oda T, Kinoshita T (2004) Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas 28(2): 160–165 [DOI] [PubMed] [Google Scholar]
- Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, Kurimoto K, Ohashi Y (1994) Prognostic values of preoperative and postoperative CEA and CA19-9 levels in pancreatic cancer. Pancreas 9(6): 735–740 [DOI] [PubMed] [Google Scholar]
- Ziske C, Schlie C, Gorschluter M, Glasmacher A, Mey U, Strehl J, Sauerbruch T, Schmidt-Wolf IGH (2003) Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer 89(8): 1413–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]