Abstract
The mechanism of store-operated Ca2+ entry (SOCE) remains one of the intriguing mysteries in the field of Ca2+ signalling. Recent discoveries have resulted in the molecular identification of STIM1 as a Ca2+ sensor in endoplasmic reticulum, Orai1 (CRACM1) as a plasma membrane channel that is activated by the store-operated pathway, and iPLA2β as an essential component of signal transduction from the stores to the plasma membrane channels. Numerous studies have confirmed that molecular knock-down of any one of these three molecules impair SOCE in a wide variety of cell types, but their mutual relations are far from being understood. This report will focus on the functional roles of Orai1, STIM1 and iPLA2β, and will address some specific questions about Orai1 and TRPC1, and their relation to SOC channels in excitable and non-excitable cells. Also, it will analyse the novel role of STIM1 as a trigger for CIF production, and the complex relationship between STIM1 and Orai1 expression, puncta formation and SOCE activation. It will highlight some of the most recent findings that may challenge simple conformational coupling models of SOCE, and will offer some new perspectives on the complex relationships between Orai1, STIM1 and iPLA2β in the SOCE pathway.
Store-operated channels (SOCs) and store-operated Ca2+ entry (SOCE) are activated upon depletion of endoplasmic reticulum (ER) Ca2+ stores (Parekh & Putney, 2005), but the molecular mechanism of SOCE remains one of the intriguing mysteries in the field of Ca2+ signalling. Recent discoveries have resulted in the molecular identification of 1) STIM as a Ca2+ sensor in the endoplasmic reticulum (ER) that is capable of triggering a cascade of reactions leading to SOCE activation, 2) Orai (CRACM) as a plasma membrane (PM) channel that is activated by the store-operated pathway, and 3) iPLA2β as an essential component of signal transduction from the stores to plasma membrane channels. Molecular knock-down of any one of these three molecules (Orai, STIM1 and iPLA2β) was shown to impair SOCE in a wide variety of cell types, but their relationship is far from being understood. Numerous review articles have been published within the last year (Lewis, 2007; Putney, 2007; Hewavitharana et al. 2007; Clapham, 2007) which nicely summarize the new knowledge about STIM1 and Orai1, and discuss currently popular models that suggest their conformational coupling as a mechanism of signal transduction and SOCE activation. This report will highlight some of the most recent findings that may challenge currently popular models of SOCE, and will offer some new perspectives on this complex mechanism.
Orai1 and TRPC1: which gene encodes which channel?
One of the most important recent discoveries was the identification of Orai1 (Feske et al. 2006) or CRACM1 (Vig et al. 2006) as a pore-forming subunit of the CRAC channel (Feske et al. 2006; Vig et al. 2006; Yeromin et al. 2006; Luik et al. 2006; Lorin-Nebel et al. 2007). However, while most of the experts agree on the ability of the Orai1 gene to encode a Ca2+-selective SOC (CRAC) channel, and numerous studies demonstrated that molecular knock-down of Orai1 protein produces dramatic inhibition of ICRAC and SOCE in all non-excitable cells tested so far (Roos et al. 2005; Liou et al. 2005; Zhang et al. 2005; Feske et al. 2006; Spassova et al. 2006; Peinelt et al. 2006; Soboloff et al. 2006a,b; Vig et al. 2006; Peel et al. 2006; Lorin-Nebel et al. 2007; Mignen et al. 2007), the role of Orai1 protein in a variety of excitable cells (in which SOCE is mediated by much less selective cation channels, cat-SOCs) remains an open question. Indeed, most investigators do not believe that the same Orai1 gene could be responsible for the channels with such profoundly different selectivity to Ca2+ as CRAC and cat-SOC. Several recent models proposed cat-SOC to be formed by TRPC1 (Beech, 2005; Ambudkar, 2007), or a complex of Orai1 and TRPC1 subunits (Ambudkar, 2007; Ong et al. 2007; Yuan et al. 2007; Worley et al. 2007), or some other TRPC channels (Liao et al. 2007; Liao et al. 2008). So which gene encodes which channel, and how can the variety of SOC channels with different cation selectivity be created?
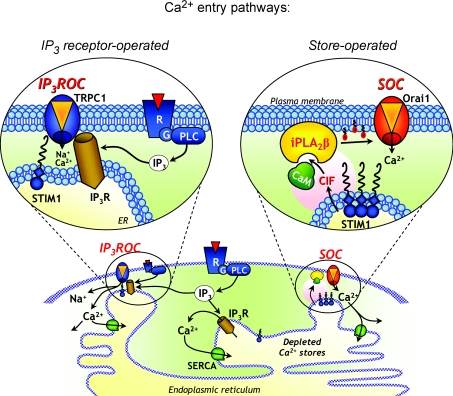
In our recently published article (Zarayskiy et al. 2007) we presented evidence that when Orai1 and TRPC1 proteins are endogenously present in the same cells, they form two totally different channels that can both respond to agonist and IP3-dependent stimulation. Figure 1 summarizes these findings and shows that while Orai1 forms a store-operated channel that is activated exclusively upon depletion of Ca2+ stores (via an iPLA2β-dependent pathway), TRPC1 forms an IP3 receptor-operated channel (IP3ROC) that is activated via its conformational coupling with IP3 receptor. In RBL-2H3 cells endogenously expressing both Orai1 and TRPC1, we unmasked and characterized the whole-cell current through IP3ROC channels, which was hidden behind some familiar fingerprints of ICRAC, a current through the classical Ca2+-selective SOC (CRAC) channels. We discriminated these currents by their molecular identity, selectivity and totally different requirements for store depletion, iPLA2β and conformational coupling to IP3 receptor. Among many differences, we found that only Orai1-encoded CRAC channel, but not TRPC1-encoded IP3ROC channel, requires the presence and functional activity of iPLA2β, and while ICRAC was absent, IIP3ROC could be easily activated in the cells in which iPLA2β was knocked down. In contrast, TRPC1, but not CRAC, channels were fully dependent on activation of IP3R. It is important to mention that despite clear molecular, biophysical and functional differences, both Orai1 and TRPC1 channels can be activated by agonists (and elevation of IP3, but through totally different pathways), and both channels appear to be sensitive to 2-aminoethoxydiphenyl borate (2-APB) (which is widely used as SOC inhibitor). Because of these similarities, Ca2+ entry through TRPC1-dependent IP3ROC can sometimes be confused with Orai1-dependent SOCE. Part of the confusion may arise from the ability of SERCA inhibitors to produce a significant Ca2+ rise in the cytosol, which, in the absence of efficient intracellular Ca2+ buffering, may lead to Ca2+-induced activation of phospholipase C (PLC) and subthreshold IP3 production, which may be enough for secondary activation of TRPC1-encoded IP3ROC. Also, store depletion and iPLA2β-dependent activation of SOCE can trigger activation of some other signalling cascades that can further activate different kinds of ion channels and physiological responses. As one of many examples, Ca2+ entry through CRAC was shown to activate cPLA2 (Chang & Parekh, 2004), which is a major source of arachidonic acid (AA). AA and its products can be further involved in numerous signalling cascades, and can also trigger activation of some other Ca2+-conducting channels, such as ARC channel (Shuttleworth et al. 2004). New knowledge of the specific properties and possible coexistence of Orai1-encoded SOC and TRPC1-encoded IP3ROC should help avoid further confusion about these channels, and open new exciting possibilities for their independent study.
Figure 1. Two Ca2+ entry pathways can be mediated by two distinct channels, Orai1-dependent SOC and TRPC1-dependent IP3ROC.
Both pathways may be activated upon receptor (R) and G-protein (G)-mediated stimulation of phospholipase C (PLC) that leads to IP3 production. The store-operated pathway (on the right) is activated upon depletion of Ca2+ stores, which leads to oligomerization and accumulation of STIM1 in ER membrane in close proximity to plasma membrane. STIM1 triggers production of calcium influx factor (CIF) that displaces inhibitory calmodulin (CaM) from a plasma membrane bound Ca2+-independent phospholipase A2 (iPLA2β), which produces lysophospholipids and activates Orai1-dependent SOC channels that are responsible for the store-operated Ca2+ entry (SOCE). The IP3 receptor-operated pathway (on the left) does not require store depletion, and is activated by IP3-dependent conformational coupling of IP3 receptor (IP3R) and TRPC1-encoded plasma membrane channel (IP3ROC). From Zarayskiy et al. 2007, ©Landes Bioscience.
Results from the new transgenic mice which lack either the Orai1 (Vig et al. 2008) or the TRPC1 (Dietrich et al. 2007b; Liu et al. 2007) gene brought new insights into the physiological roles of Orai1 and TRPC1-encoded channels. Orai1−/− mice appeared to have severe abnormalities, with a dominant phenotype of impaired mast cell function, and strong potential for malfunctioning of other cells and organs that may be affected by Orai1 protein deficiency. In contrast, TRPC1−/− mice appeared to be rather healthy. The only major defect found so far was in its salivary gland function (Liu et al. 2007). Agonist induced activation of Ca2+ entry and poorly selective cation currents (which are thought to be mediated by cat-SOC in these cells) were found to be significantly compromised in TRPC1−/− animals. However, cat-SOC and SOCE appeared to be fully normal in vascular SMC, and TRPC1−/− mice did not show any noticeable flaws in their vascular function (Dietrich et al. 2007a). Thus, TRPC1 did not appear to be important for SOCE in vascular SMC, in which the role of cat-SOC and SOCE is well established by the work done in many different laboratories. Following all these studies, we were not surprise to find that the molecular knock-down of TRPC1 did not affect SOCE in both non-excitable RBL cells (Zarayskiy et al. 2007) and excitable vascular SMC (Yang & Bolotina, unpublished observations). However, a significant line of evidence suggests that the mechanism of SOCE activation can be identical in excitable and non-excitable cells types, which brings us back to the question on the molecular identity of the cat-SOC channels.
The majority of the experts rule out the possibility that Orai1 by itself may encode the channels with such profound differences in selectivity as Ca2+-selective CRAC and poorly cation selective cat-SOC channels. However, our most recent studies of SMC (as a model for cat-SOC) and RBL cells (as a model for CRAC) brought us to a rather unexpected discovery, which may help resolve the molecular nature of cat-SOC channels. We obtained new evidence that the Orai1 gene may in fact encode both CRAC and cat-SOC channels and molecular knock-down of Orai1 protein equally impairs ICRAC in RBL and IcatSOC in vascular SMC. Most importantly, we discovered that RNA editing may be a molecular mechanism that can produce a post-transcriptional modification of Orai1 selectivity, and adjust it to the specific needs of excitable and non-excitable cells (Zarayskiy et al., 2008). Thus, mounting evidence make us believe that Orai1 may encode not only Ca2+-selective CRAC in non-excitable cells, but also cat-SOC channels in a variety of excitable cells. At the same time, TRPC1 can encode an important, but totally different, agonist-activated channel that is regulated via conformational coupling with the IP3 receptor.
STIM1 and its new role as a trigger for CIF production
STIM1 is a protein predominantly located in the ER membrane; it has one transmembrane domain and an EF hand motif in its N terminus that may allow STIM1 to bind Ca2+ in the ER lumen and to function as a low affinity Ca2+ sensor in the stores (for a most recent review see Lewis, 2007). Upon Ca2+ depletion, STIM1 has been shown to lose Ca2+ from its EF hand, oligomerize and accumulate into punctate structures in the ER membrane located in close proximity (10–25 nm) to the plasma membrane, followed by SOCE activation (Liou et al. 2005; Mercer et al. 2006; Wu et al. 2006; Baba et al. 2006; Luik et al. 2006; Stathopulos et al. 2006; Liou et al. 2007; Ross et al. 2007; Li et al. 2007; Muik et al. 2008). The role of STIM1 in SOCE was originally discovered in Drosophila S2 (Roos et al. 2005) and in HeLa cells (Liou et al. 2005), and was confirmed by numerous other studies which showed that the molecular knock-down of STIM1 results in the disappearance of SOCE in virtually all cell types tested so far. Although it is now clear that STIM1 is an essential component of the SOCE pathway, the exact mechanism of its involvement in SOCE is far from being understood.
While most of research has focused on STIM1 accumulation in puncta and its interactions with Orai1 (which will be discussed below), we decided to look at the early ER-delimited events and examine the role of STIM1 from the perspective of a diffusible messenger model. Calcium influx factor (CIF) is known to be produced in the cells upon depletion of Ca2+ stores, and its production in the ER stores upon the drop of intraluminal Ca2+ concentration was further confirmed in our recent studies (Csutora et al. 2006, 2008). Although the molecular identity of CIF is still unknown, its presence and biological activity were detected by numerous groups in a wide variety of cell types ranging from yeast to human (for review see Bolotina & Csutora, 2005). The physiological target of CIF (iPLA2β, which will be described below), and the mechanism of CIF-induced activation of SOCE (illustrated in Fig. 1) were identified (Smani et al. 2004), but very little is known about the molecular mechanism of CIF production in the stores.
Looking at the early events in ER that follow store depletion and precede puncta formation, we discovered (Csutora et al. 2008) that CIF production is tightly coupled with STIM1 expression and requires functional integrity of glycosylation sites in its intraluminal SAM domain. Molecular knock-down or overexpression of STIM1 resulted in a corresponding impairment or amplification of CIF production. We demonstrated that CIF production is one of the earliest STIM1-dependent events in the ER lumen, which precedes STIM1 accumulation in puncta and activation of SOCE. We also found that the inherent deficiency in SOCE in the NG115 cell line can be the result of deficiency in STIM1 protein and CIF production. Expression of wild-type STIM1 in these cells was sufficient to fully rescue their ability to produce CIF and SOCE. We have established NG115 cells as a very useful model for studying the behaviour of exogenously expressed STIM1 (and its mutants) without involvement of endogenous STIM1 background. Using his model we discovered that STIM1 with mutated glycosylation sites in its SAM domain does not trigger CIF production, and fails to activate SOCE (Csutora et al. 2008), but remains fully capable of sensing store depletion and accumulating in puncta (Gwozdz et al. unpublished observations).
Taken together, these data demonstrate a novel role for STIM1 as a trigger for CIF production in the ER, which seems to be essential for SOCE activation. When the free Ca2+ concentration in the ER lumen drops and Ca2+ is lost from its EF hand, the change in STIM1 conformation may allow glycosylation sites in its SAM domain to interact with (and trigger activation of) CIF-generating machinery in ER. Subsequent accumulation of STIM1 into puncta in close proximity to the plasma membrane may allow effective and fast delivery of CIF to its plasma membrane target, iPLA2β. CIF-induced activation of iPLA2β can further transduce the signal to Orai1, leading to Ca2+ entry. Importantly, without CIF production mere translocation of STIM1 to the vicinity of plasma membrane may not be sufficient for SOCE activation. Thus, STIM1-dependent activation of CIF production may go hand-in-hand with STIM1's ability to sense intraluminal Ca2+ and to accumulate in the vicinity of the plasma membrane, providing important molecular, functional and structural prerequisites for SOCE activation.
STIM1 and Orai1: what is their relationship, and what does it mean for SOCE activation?
The ability of STIM1 and Orai1 to accumulate and colocalize in punctate structures along the plasma membrane (for recent review see Lewis, 2007), and the reported coimmunoprecipitation of overexpressed STIM1 and Orai1, fuelled the return of the direct conformational coupling models of SOCE. Originally, conformational coupling models suggested a direct coupling of SOC channel with the IP3 receptor (IP3R) (Irvine, 1990; Petersen & Berridge, 1996). This idea got strong support from the reported coimmunoprecipitation and functional coupling of some TRPC channels with IP3R (Kiselyov et al. 1998, 1999; Boulay et al. 1999; Rosado & Sage, 2000, 2001), but these models were abandoned after demonstration that triple IP3R knockout DT40 chicken B-cells (in which all three known types of IP3R were deleted) have a totally normal SOCE (Prakriya & Lewis, 2001; Bakowski et al. 2001; Ma et al. 2001). Recently, the conformational coupling idea returned, but instead of the IP3R and TRPC, new models propose direct conformational coupling and signal transduction from STIM1 to Orai1 to be a mechanism for SOCE activation (Hisatsune & Mikoshiba, 2005; Huang et al. 2006; Lopez et al. 2006; Soboloff et al. 2006b; Spassova et al. 2006; Yeromin et al. 2006). This idea was strongly supported by several important findings. First, coimmunoprecipitation of overexpressed STIM1 and Orai1 was shown in S2 cells (Yeromin et al. 2006) and HEK293 cells (Vig et al. 2006). Second, overexpression of both proteins resulted in remarkable amplification (50–100 fold) of ICRAC (so-called monster CRAC) (Peinelt et al. 2006; Soboloff et al. 2006b). Third, Förster resonance energy transfer (FRET) between STIM1 and Orai1 labelled with fluorescent proteins was reported (Muik et al. 2008), indicating their very close spatial proximity. Fourth, overexpression of the C-terminus of STIM1 was reported to be sufficient for SOCE activation (Huang et al. 2006). All these data strongly supported the idea of direct conformational coupling of STIM1 to Orai1 as a rather straightforward mechanism of signal transduction and Orai1 activation. However these studies neither ruled out nor considered the possibility that intermediate steps and molecular components may be required for their co-localization, mutual interaction and signal transduction from ER to PM.
In our most recent (unpublished) work we looked at the relationship between STIM1 and Orai1 from this new perspective, and tested several important predictions of the conformation coupling models. In contrast to what would be expected, if direct interaction of STIM1 with Orai1 underlies puncta formation and ICRAC activation, we found that: (1) STIM1 can form puncta independently of Orai1 expression and there is no strict correlation between STIM1 and Orai1 accumulation in puncta, (2) STIM1 expression may be neither required nor sufficient for ICRAC and SOCE activation, and (3) without CIF production mere translocation of STIM1 to the vicinity of plasma membrane is not sufficient for SOCE activation (Csutora et al. 2008). Strikingly, we found that normal ICRAC can be activated even in the cells in which STIM1 was knocked down. In this case, cell deficiency in STIM1 could be easily compensated by a direct activation of iPLA2β by cell dialysis with CIF (which is a simple short-cut in the SOCE pathway, which we described in our earlier publications (Smani et al. 2004; Csutora et al. 2006, 2008). These data extend our findings on the role of STIM1 as a trigger for CIF production (Csutora et al. 2008), and strongly suggest that STIM1 may be upstream, and Orai1 downstream, from CIF and iPLA2β, which may be required as functional transducers of the signal from STIM1 in the ER to Orai1 in the plasma membrane (Fig. 1). The need for additional intermediate(s) between STIM1 and Orai1 was strongly supported by recent findings from Dr Balla's group (Varnai et al. 2007), which demonstrated that STIM1 and Orai1 colocalization does not occur in narrow (4–6 nm) junctions, but happens only in the areas where a larger 12–14 nm gap exists between the ER and the plasma membrane. To explain these observations, the need for an additional linker between STIM1 and Orai1 was postulated by the authors.
Thus, although direct coupling of ER-resident STIM1 to PM-resident Orai1 may be rightfully considered as the most straightforward mechanism for signal transduction, there is a growing body of evidence for the presence of additional structural and/or functional linker(s) between STIM1 and Orai1. It is important to emphasize that the apparent requirement for molecular intermediate(s) between STIM1 and Orai1 does not contradict any of the studies that demonstrated their close spatial and functional proximity, including reported FRET between STIM1 and Orai1, as colocalization of fluorescently tagged proteins within ∼10 nm space between ER membrane and plasma membrane may allow energy transfer (Liou et al. 2007; Muik et al. 2008) even without their direct physical interaction.
The idea that additional molecules and functional steps may be involved in signal transduction from STIM1 in the ER to Orai1 in the PM, and new experimental evidence (Csutora et al. 2008; Gwozdz et al. unpublished observations) which places STIM1 upstream and Orai1 downstream from CIF and iPLA2β (Fig. 1) further highlight the important role of iPLA2β in the SOCE mechanism. So, what is so special about this element of the SOCE pathway?
Ca2+-independent phospholipase A2β (iPLA2β): what makes it fit into SOCE pathway?
iPLA2 (or PLA2 group VI) (Balsinde & Dennis, 1997; Winstead et al. 2000) is a family of intracellular phospholipases that do not require Ca2+ for their activation and function. At least five isoforms of iPLA2 have already been identified (Balsinde & Balboa, 2005), which utilize catalytic serine to perform the hydrolysis of the middle (sn-2) ester bond of substrate phospholipids, producing free fatty acids and lysophospholipids. One particular isoform, iPLA2β (group VIA), demonstrates the most complex structure and function. It has multiple splice variants (Turk & Ramanadham, 2004), can undergo caspase cleavage and post-translational modifications (Song et al. 2004), and has several unique features which puzzled researchers for many years. One of them is its Ca2+-independence (the ability to function in the presence of strong Ca2+ chelators), and at the same time its ability to bind the Ca2+–CaM complex. The signature feature of iPLA2β is that it contains a CaM-binding domain in the C-terminus, which, along with the IQ motif, forms a pocket, enabling CaM to bind and inhibit iPLA2β activity (Wolf & Gross, 1996b; Jenkins et al. 2001). Removal of CaM from iPLA2β results in its activation. An analysis of the known iPLA2β sequences (human, rat, mouse, hamster and Drosophila) revealed that CaM binding domains represent one of the most highly conserved regions in the entire iPLA2β protein. Based on the molecular and biochemical studies (Wolf & Gross, 1996a; Mancuso et al. 2000; Jenkins et al. 2001) it is believed that in the absence of CaM the active site of iPLA2β interacts with the CaM-binding domain leading to a catalytically competent enzyme. Binding of CaM disrupts this interaction, resulting in the loss of iPLA2β activity.
A few years ago, we discovered that iPLA2β is an important molecular determinant of SOCE (Smani et al. 2003, 2004), and can be a physiological target for CIF, which we found to displace inhibitory CaM from iPLA2β. This results in activation of iPLA2β, which triggers a PM-delimited cascade of reactions that lead to activation of SOC and Ca2+ entry (Fig. 1). For all the details of this pathway we will refer the readers to our earlier review (Bolotina & Csutora, 2005), and the papers that described the role of iPLA2β in SMC, platelets, Jurkat T lymphocytes, RBL-2H3, neuroblastoma/glioma, and some other cell types (Smani et al. 2003, 2004; Csutora et al. 2006; Zarayskiy et al. 2007). The role of iPLA2β in SOCE was further confirmed by many other investigators in a growing number of cell types, including astrocytes (Singaravelu et al. 2006), keratinocytes (Ross et al. 2007), skeletal muscle (Boittin et al. 2006), fibroblasts (Martinez & Moreno, 2005), prostate cancer cells (Vanden Abeele et al. 2004), and others. In all these studies molecular knock-down and/or functional inhibition of iPLA2β caused full impairment of SOCE. It is important to emphasize that genetic screening of Drosophila melanogaster performed and published by Vig et al. (2006) picked up not only STIM1 and Orai1, but also an orthologue of iPLA2β encoded by the CG6718 gene, which has a very high level of homology (up to 85% in main structural domains) to the human iPLA2β. Along with STIM1 and Orai1, knock-down of iPLA2β (CG6718) showed a dramatic impact on SOCE activation that was remarkably identical to that of STIM1 (see supplemental data in Vig et al. 2006).
Conclusion
Recent developments in the SOCE field have resulted in major breakthrough discoveries that have opened new exciting areas for future study. Orai1, STIM1 and iPLA2β proved to be the essential components of the SOCE mechanism, and further studies are required to elucidate how these three molecules can work together to create fast, effective and specific transduction of the signal from the depleted stores to the plasma membrane channels. There is no surprise that the SOCE mechanism emerges as a rather complex phenomenon that may require concerted action and close interaction of all currently known and still to be discovered molecules. It may involve components of different models that historically have been thought to be mutually exclusive, but in the end may come together in one multidimensional SOCE mechanism.
References
- Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans. 2007;35:96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current ICRAC in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Dennis EA. Function and inhibition of intracellular calcium-independent phospholipase A2. J Biol Chem. 1997;272:16069–16072. doi: 10.1074/jbc.272.26.16069. [DOI] [PubMed] [Google Scholar]
- Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg UT. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119:3733–3742. doi: 10.1242/jcs.03184. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Csutora P. CIF and other mysteries of the store-operated Ca2+-entry pathway. Trends Biochem Sci. 2005;30:378–387. doi: 10.1016/j.tibs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): Evidence for roles of TRP and IP3R in store-depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. J Biol Chem. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Csutora P, Peter K, Zarayskiy V, Kilic H, Park KM, Bolotina VM. Novel role of STIM1 as a trigger for CIF production. J Biol Chem. 2008;283:14524–14531. doi: 10.1074/jbc.M709575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csutora P, Zarayskiy V, Peter K, Monje F, Smani T, Zakharov S, Litvinov D, Bolotina VM. Activation mechanism for CRAC current and store-operated Ca2+ entry: calcium influx factor and Ca2+-independent phospholipase A2β-mediated pathway. J Biol Chem. 2006;281:34926–34935. doi: 10.1074/jbc.M606504200. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos YS, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007b;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos YS, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007a;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Mikoshiba K. Novel compartment implicated in calcium signaling – is it an ‘induced coupling domain’? Sci STKE. 2005;2005:pe53. doi: 10.1126/stke.3132005pe53. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Irvine RF. Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates – a possible mechanism. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2β. Implications for structure and function. J Biol Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva GN, Kuo T, Pessah I, Mignery GA, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed hTRPC1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Lorin-Nebel C, Xing J, Yan X, Strange K. CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologues and is essential for ovulation and fertility. J Physiol. 2007;580:67–85. doi: 10.1113/jphysiol.2006.124883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- Martinez J, Moreno JJ. Role of Ca2+-independent phospholipase A2 and cytochrome P-450 in store-operated calcium entry in 3T6 fibroblasts. Biochem Pharmacol. 2005;70:733–739. doi: 10.1016/j.bcp.2005.04.045. [DOI] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium-selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2007;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CCH, Berridge MJ. Capacitative calcium entry is colocalised with calcium release in Xenopus oocytes: evidence against a highly diffusable calcium influx factor. Pflugers Arch. 1996;432:286–292. doi: 10.1007/s004240050135. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr New molecular players in capacitative Ca2+ entry. J Cell Sci. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Coupling between inositol 1,4,5-triphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000;350:631–635. [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-triphosphate receptor type II and human transient receptor potential (TRP1) channels in human platelets. Biochem J. 2001;356:191–198. doi: 10.1042/0264-6021:3560191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Whitaker M, Reynolds NJ. Agonist-induced calcium entry correlates with STIM1 translocation. J Cell Physiol. 2007;211:569–576. doi: 10.1002/jcp.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Lohr C, Deitmer JW. Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes. J Neurosci. 2006;26:9579–9592. doi: 10.1523/JNEUROSCI.2604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani T, Zakharov S, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Leno E, Csutora P, Trepakova ES, Bolotina VM. Ca2+-independent phospholipase A2 is a novel determinant of store-operated Ca2+ entry. J Biol Chem. 2003;278:11909–11915. doi: 10.1074/jbc.M210878200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006a;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006b;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Song H, Hecimovic S, Goate A, Hsu FF, Bao S, Vidavsky I, Ramanadham S, Turk J. Characterization of N-terminal processing of group VIA phospholipase A2 and of potential cleavage sites of amyloid precursor protein constructs by automated identification of signature peptides in LC/MS/MS analyses of proteolytic digests. J Am Soc Mass Spectrom. 2004;15:1780–1793. doi: 10.1016/j.jasms.2004.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Turk J, Ramanadham S. The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2β) in beat cells. Can J Physiol Pharmacol. 2004;82:824–832. doi: 10.1139/y04-064. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F, Lemonnier L, Thebault S, Lepage G, Parys J, Shuba Y, Skryma R, Prevarskaya N. Two types of store-operated Ca2+ channels with different activation modes and molecular origin in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2004;279:30326–30337. doi: 10.1074/jbc.M400106200. [DOI] [PubMed] [Google Scholar]
- Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- Vig M, Dehaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A2: structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Wolf MJ, Gross RW. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J Biol Chem. 1996a;271:30879–30885. doi: 10.1074/jbc.271.48.30879. [DOI] [PubMed] [Google Scholar]
- Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. J Biol Chem. 1996b;271:20989–20992. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarayskiy V, Monje F, Peter K, Csutora P, Khodorov BI, Bolotina VM. Store-operated Orai1 and IP3R-operated TRPC1 channel: Separation of the Siamese twins. Channels. 2007;1:246–252. doi: 10.4161/chan.4835. [DOI] [PubMed] [Google Scholar]
- Zarayskiy VV, Yang B, Peter K, Herbert AG, Bolotina VM. ADAR-mediated RNA editing of Orai1 is a key to different selectivity of store-operated channels. Biophys J, Suppl. 2008;94a Abstract, 2938-Pos. 2008 Biophysical Society Meeting Abstracts. [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]