Abstract
Activation of thin fibre muscle afferent nerves by metabolic by-products plays a critical role in the initiation and maintenance of the autonomic response to exercise and the metabolic profile of active muscle can influence the response. The purpose of this report was to determine the responsiveness of sensory neurones innervating muscles comprising predominantly glycolytic and oxidative fibres to protons and capsaicin using whole-cell patch clamp methods. Dorsal root ganglion (DRG) neurones from 4- to 6-week-old rats were labelled by injecting the fluorescence tracer DiI into the muscle 3–5 days prior to the recording experiments. The percentage of the DRG neurones innervating glycolytic and oxidative muscle was similar in response to both protons and capsaicin. However, the neurones innervating glycolytic muscle had greater inward current amplitude responses to protons and capsaicin as compared with oxidative muscle. The peak current amplitudes to pH 6.0 were 0.84 ± 0.06 nA (oxidative muscle) versus 1.36 ± 0.07 nA (glycolytic muscle, P < 0.05). The capsaicin-induced current amplitudes were 2.3 ± 0.15 nA (oxidative muscle) versus 3.1 ± 0.21 nA (glycolytic muscle, P < 0.05). Of neurones that responded to pH 6.0 with a sustained current, 88% also responded to capsaicin. Capsaicin exposure enhanced the proton responsiveness in the neurones innervating the muscle; and protons also increased the capsaicin response. These data suggest that (1) receptors mediating protons and capsaicin responses coexist in the DRG neurones innervating muscle; (2) the responsiveness of acidosis and capsaicin can be sensitized by each other; and (3) DRG neurones with nerve endings in a glycolytic muscle developed greater inward current responses to protons and capsaicin than did those with nerve endings in an oxidative muscle.
Metabolite-sensitive receptors on thin-fibre muscle afferent nerves are stimulated when interstitial metabolite concentrations increase with muscle contraction (Kaufman et al. 1984; Kaufman & Forster, 1996). This increases the sympathetic nervous activity and arterial blood pressure via a reflex mechanism (Mark et al. 1985; Victor et al. 1988; Sinoway et al. 1989), and this is accentuated when contraction is performed under ischaemic conditions (Kaufman et al. 1984; Kaufman & Forster, 1996). The augmented response of these ‘metaboreceptors’ with ischaemic contraction is attributed to the generation of a variety of chemicals by local muscle cells (Mitchell et al. 1983). Hydrogen ions are a prominent metabolic by-product in the interstitium of active muscle that induce tissue acidosis with muscle ischaemia and fatigue (Victor et al. 1988; Sinoway et al. 1989). The degree of acid-induced reflex response correlates with the level of tissue pH (Victor et al. 1988; Sinoway et al. 1989).
Acid-sensing ion channels (ASICs) but not ‘capsaicin’ receptors (transient receptor potential vanilloid type 1; TRPV1) on thin-fibre afferent nerves are stimulated by acid ions (Li et al. 2004). However, in rats pretreated with resiniferatoxin (RTX) to destroy muscle afferents containing TRPV1, acid-induced responses are blunted (Li et al. 2004). This suggests that ASICs appears frequently on afferents containing TRPV1. The acid phosphate-evoked response is attenuated to a greater degree with simultaneous TRPV1 and ASIC blocking than with application of the respective blockers (Gao et al. 2006), indicating that ASICs and TRPV1 play an interactive role in mediating the muscle afferent response to acid.
TRPV1 receptors that are found preferentially on chemically sensitive thin-fibre sensory neurones (Ma, 2001) respond to several metabolic products of contraction such as adenosine, free oxygen radicals, and products of lipoxygenases (Szallasi et al. 2007). We examined the interaction between the TRPV1 and ASIC receptors on DRG neurones. Because the chemical milieu surrounding nerve endings residing in glycolytic muscle are probably different from those residing in oxidative muscle we compared the DRG responses from afferent nerves innervating the two respective muscle types. We hypothesized that the DRG neurones innervating muscle would be responsive to both protons and capsaicin, and an interaction between responses to the two chemicals would be seen.
In addition, the effect of fibre-type composition on the pressor reflex evoked by static muscle contraction was studied in cats. Static contraction of the soleus muscle (slow twitch oxidative) induced no increase in blood pressure; whereas, contraction of the medial gastrocnemius muscle (mixed fibre type) caused an increase in blood pressure (Petrofsky & Lind, 1980; Petrofsky et al. 1981). However, an increase in blood pressure was observed from red muscles although it was smaller than that from white muscles (Iwamoto & Botterman, 1985). Moreover, static contraction of a predominantly glycolytic muscle evoked a larger pressor response as compared with that elicited by contraction of a primarily oxidative muscle (Wilson et al. 1995). Thus muscle fibre type has an effect on the magnitude of the sensory afferent feedback during exercise. Therefore, we further examined the responsiveness of sensory neurones innervating muscles comprising predominantly glycolytic and oxidative fibres to protons and capsaicin. We hypothesized that the amplitude of inward currents responsive to protons and capsaicin in neurones innervating glycolytic muscle would be greater than that innervating oxidative muscle.
Methods
DiI injection into hindlimb muscle
Male Sprague–Dawley rats (4–6 weeks old) were anaesthetized by inhalation of an isoflurane–oxygen mixture (2–5% isoflurane in oxygen). The skin was incised and pulled away from underlying muscle tissue, and the fluorescent retrograde tracer 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine percholate (DiI; 60 mg ml−1; Molecular Probes, Eugene, OR, USA) was injected into the superficial white and deep red portion of the gastrocnemius muscle. These muscles are composed predominantly of glycolytic and oxidative fibres, respectively (Armstrong & Phelps, 1984). The injection volume was 1 μl, and injection was repeated three times at different locations. The injection needle was placed in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to allow the retrograde tracer to be transported to DRG neurones. All procedures outlined in this study were approved by the Animal Care Committee of this institution.
Isolation of DRG neurones
After anaesthesia, the rats were decapitated. Then DRGs at lumbar 4–6 were removed on the side ipsilateral to muscle injected with DiI, and immediately placed into Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA). The DRGs were then incubated with 1 mg ml−1 collagenase IV (Sigma-Aldrich, St Louis, MO, USA) and 0.5 mg ml−1 trypsin (Sigma-Aldrich) for 30 min at 34°C in a shaking water bath. Then soybean (1.25 mg ml−1; Sigma-Aldrich), a trypsin inhibitor, was added. The cell suspension was centrifuged (150g, 5 min) to remove the supernatant, replenished with DMEM, and plated onto a 35 mm culture dish containing poly l-lysine (50 μg ml−1; Sigma-Aldrich)-precoated coverslips and maintained for at least 60 min before electrophysiological recordings were made within 6 h.
Electrophysiological recordings
Whole-cell recordings were made using fire-polished glass electrodes (2–5 MΩ resistance) pulled from glass capillaries (1.17 mm i.d., 1.5 mm o.d.; Harvard Apparatus) on a model P-97 micropipette puller (Sutter Instruments). The recording chamber was perfused (1–2 ml min−1) with artificial cerebral spinal fluid (aCSF) containing (in mm) 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes and 10 glucose (pH adjusted to 7.4, osmolarity 320 mosmol l−1). Electrodes were filled with a solution containing (in mm) 124 KCl, 13.2 NaCl, 2 MgCl2, 0.3 NaGTP, 1 EGTA, 10 Hepes and 4 Mg-ATP (pH brought to 7.2; osmolarity to 300 mosmol l−1). For pH 4.0 and 6.0, Hepes was replaced by 2-(N-morpholino) ethanesulphonic acid at the same concentration. When the antagonism by capsazepine and amiloride was tested, we removed Ca from aCSF to minimize the desensitization of TRPV1-mediated responses, and increased Mg to 2 mm. All solutions were filtered and made fresh daily. DiI-labelled DRG neurones were visualized using a combination of fluorescence illumination and differential interference contrast (DIC; ×20–40) optics on a Nikon TE2000 inverted microscope. Under DIC, images of cells were displayed on a video monitor. A tight gigaohm seal was subsequently obtained in the selected neuron. The size of cell soma was estimated by calculating the mean of the longest and shortest cross-sectional diameters with the aid of a calibrated eyepiece reticle.
Solutions were applied locally and rapidly to the neurone of interest using silica 28-gauge syringes of 0.25 mm i.d. (World Precision Instruments) for all chemical tests. The tip of each syringe was placed 100 μm from the cell soma using a manipulator. The gravity-fed solutions were controlled using manual switching of one-way stopcock valves. To investigate the acidosis response, solutions containing protons were buffered to 4.0 and 6.0 with HCl. To investigate the capsaicin response, 1 μm capsaicin was prepared freshly each day from a 1 mm capsaicin stock solution. The effect of capsaicin on DRG neurones was examined in a group of neurones by applying 1 μm capsaicin for 2 s. To determine the effect of proton exposure on capsaicin responsiveness, a group of neurones was treated with a pH 6.0 solution for 10 s, washed for 2 min, and then exposed to 1 μm capsaicin for 2 s. To determine the effect of capsaicin exposure on proton responsiveness, neurones were treated with 1 μm capsaicin for 10 s, washed for 2 min, and then exposed to pH 6.0 for 2 s. In another group of experiments, capsazepine (50 μm) or amiloride (100 μm) was applied 2 min before the application of agonists. Capsazepine and amiloride were used to attenuate capsaicin receptors and ASICs, respectively. One neurone per coverslip was studied and, after each recording, the chamber was washed with ethanol and water to eliminate any residual chemicals. All drugs and chemicals were from Sigma-Aldrich.
Data acquisition and analysis
Signals were recorded with a MultiClamp 700B amplifier (Axon Instruments, Union City, CA, USA), digitized at 10 kHz with a DigiData 1322A, and filtered at 1–2 kHz and saved in a PC-based computer using pClamp 10.1 software (Axon Instruments). The whole-cell configuration was maintained at −60 mV. Seals ranged from 1.5 to 6.0 GΩ. An equilibration period of 5–10 min was allowed after whole-cell access was established and the recording reached a steady state. The recording was then made to measure changes in inward currents evoked by chemical stimuli. Electrical access to the cell was monitored throughout each recording. The recording was abandoned if the monitored input resistance changed > 10%. The magnitude of inward current was determined using Clampfit 10.1 (Axon Instruments). Neurones were considered to be proton or capsaicin sensitive if either chemical elicited an inward current of > 50 pA in peak amplitude.
Experimental data were analysed using one-way repeated measures analysis of variance. As appropriate, Tukey's post hoc tests were used. All values were presented as mean ± s.e.m. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed using SPSS for Windows version 15.0.
Results
A total of 407 neurones were tested in the current study. All DRG neurones used in this report were DiI-labelled. At the end of each experiment, the gastrocnemius muscle was dissected to confirm that DiI was located in the white or red portion of the gastrocnemius muscle.
Acid-induced currents in DRG neurones
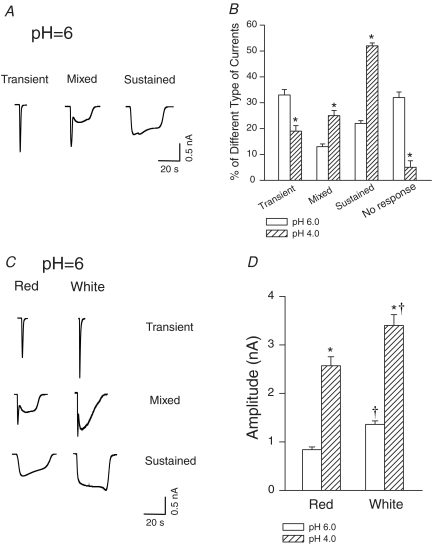
All cells recorded were small and medium sized DRG neurones (diameter < 35 μm). There was no significant difference in cell size responding to pH in DRG neurones innervating red (26.7 ± 1.1 μm) and white muscles (26.8 ± 0.9 μm) of the gastrocnemius. We observed three distinct inward currents evoked by pH 6.0 in DRG neurones tested (Fig. 1A). The first was a transient current that was activated and inactivated rapidly. The second type of response was a mixed current which showed a transient response followed by a sustained response. The third type of response exhibited a sustained inward current that reached a peak slowly and desensitized slowly. Both pH 4.0 and 6.0 induced all three types of responses, but the percentage of the different responses differed at pH 6.0 and 4.0 (Fig. 1B). There was no significant difference in the percentage of DRG neurones innervating red and white muscle in response to proton. However, peak amplitudes of inward currents responsive to acid were greater in the neurones innervating white muscle (3.4 ± 0.22 nA to pH 4.0, n = 37; and 1.36 ± 0.07 nA to pH 6.0, n = 62, P < 0.05 versus red muscle; Fig. 1C and D) than in those innervating red muscle (2.5 ± 0.19 nA to pH 4.0, n = 36; and 0.84 ± 0.06 nA to pH 6.0, n = 33; Fig. 1C and D). Furthermore, pH 4.0 induced greater current amplitude than pH 6.0 in the neurones innervating white and red muscles (Fig. 1D).
Figure 1. Whole-cell patch clamp methods show that acid induced currents in dorsal root ganglion (DRG) neurones innervating the white portion (predominantly glycolytic) and the red portion (predominantly oxidative) of the gastrocnemius muscle.
A and B, original traces of three distinct inward currents evoked by pH 6.0 in DRG neurones, and the different percentages of the three types of currents responsive to pH 6.0 and 4.0 in DRG neurones. C and D, original traces and average data show that a greater peak amplitude of inward currents responsive to low pH was seen in the neurones innervating white muscle than in those innervating red muscle. pH 4.0 induced greater amplitude of inward currents in the neurones innervating white and red muscle as compared with pH 6.0. *P < 0.05, versus pH 6.0. †P < 0.05, versus red muscle.
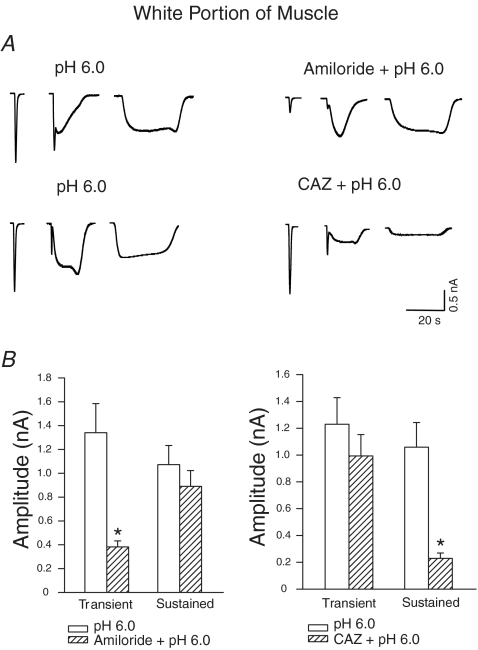
We examined the sensitivity of proton-evoked currents to amiloride and capsazepine. The transient proton currents and the transient component of the mixed proton currents were attenuated by amiloride, a blocker of ASICs. When amiloride (100 μm) was applied 2 min before the application of pH 6.0, the amplitude of the transient current was reduced by 71% (n = 16) in the DRG neurones innervating the red portion of the muscle (data not shown); and by 72% (n = 16) in the DRG neurones innervating the white portion of the gastrocnemius (Fig. 2A and B).
Figure 2. Effects of prior blocking of acid-sensing ion channels (ASICs) and TRPV1 capsaicin receptors with amiloride and capsazepine on acid-evoked currents in DRG neurones innervating the white portion of the gastrocnemius muscle.
A, original traces. B, amiloride attenuated the transient proton currents and the transient component of the mixed proton currents; capsazepine (CAZ) attenuated sustained proton-induced currents and the sustained part of the mixed currents. *P < 0.05, versus pH 6.0 applied alone.
The transient currents were not significantly affected by 50 μm capsazepine, a TRPV1 receptor inhibitor (CAZ; Fig. 2A and B). In contrast, sustained proton-induced currents and the sustained part of the mixed currents were attenuated by capsazepine. When capsazepine (50 μm) was applied 2 min before the application of acidic solution (pH 6.0), sustained proton-activated currents were reduced by 78% (n = 13) in the DRG neurones innervating the red portion (data not shown); and by 77% (n = 13) in the DRG neurones innervating the white portion of the gastrocnemius, but were not considerably affected by 100 μm amiloride (Fig. 2A and B).
Capsaicin-induced currents in DRG neurones
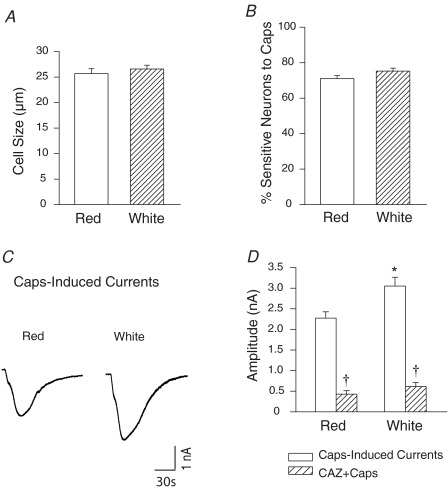
The size of the capsaicin-sensitive DRG neurones innervating the red (25.7 ± 0.9 μm, n = 68) and white muscles (26.6 ± 0.7 μm, n = 59) of the gastrocnemius was similar (Fig. 3A). In addition, the percentage of the DRG neurones innervating red and white muscles responding to capsaicin was similar (71% versus 75%; Fig. 3B). Importantly, the magnitude of the peak inward currents was greater in the neurones innervating white muscle than in those innervating red muscle (Fig. 3C). Figure 3D also shows the average amplitude of capsaicin-induced currents innervating red (2.3 ± 0.15 nA, n = 48); and white muscle (3.1 ± 0.21 nA, n = 44, P < 0.05). We further examined the effect of capsazepine on evoked currents in this group of experiments (Fig. 3D). With prior exposure of 50 μm of capsazepine, capsaicin-induced currents in DRG neurones were blocked by 81% (n = 14) in the red muscle group; and by 80% (n = 19) in white muscle group.
Figure 3. Capsaicin-induced currents in DRG neurones innervating the white and red portion of the gastrocnemius muscle.
A, there is no difference in the size of the DRG neurones innervating red and white muscles in response to capsaicin (Caps). B, percentage of the DRG neurones innervating the red and white muscles responsive to capsaicin was similar. C and D, original traces and average data show that a peak amplitude of inward currents was greater in the neurones innervating the white muscle than that in those innervating the red muscle. With prior exposure to 50 μm capsazepine (CAZ), capsaicin-induced currents in DRG neurones were attenuated in red and white muscle groups. *P < 0.05 compared to red. †P < 0.05, Caps versus Caps with prior CAZ.
Overlapping response to protons and capsaicin in DRG neurones
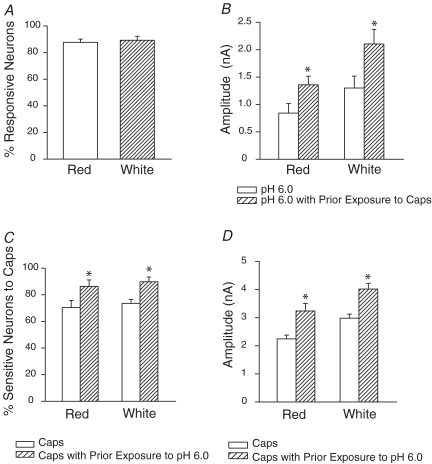
We further examined whether the same neurone responding to protons also responded to capsaicin. Thus we applied pH 6.0 for 10 s, waited for 2 min, and then applied 1 μm capsaicin. Responsiveness to protons and capsaicin in the DRG neurones innervating either red or white muscle overlapped. Among DRG neurones innervating white muscle that responded to pH 6.0 with sustained inward currents, 89% responded to capsaicin 2 min later (Fig. 4A). The overlap was similar for DRG neurones innervating red muscle for which 88% of the neurones that responded to pH 6.0 with a sustained inward current also responded to capsaicin. A few of DRG neurones that exhibited no response to protons responded to capsaicin.
Figure 4. Overlapping response to low pH and capsaicin in DRG neurones.
A, the neurones that responded to pH 6.0 with a sustained inward current also responded to capsaicin. The overlap was similar for DRG neurones innervating the red and white muscle. B, prior exposure to capsaicin increased the amplitude of the proton-evoked currents in DRG neurones innervating the red and white muscles. *P < 0.05 compared with pH 6.0 without exposure to capsaicin. C and D show that prior exposure to protons increased the proportion of DRG neurones responding to capsaicin, and increased the amplitude of the capsaicin-induced currents in the neurones. *P < 0.05 compared with capsaicin without exposure to pH 6.0.
Next we examined whether proton responsiveness was affected by capsaicin exposure. We compared the proton responsiveness of DRG neurones previously exposed to capsaicin to that of neurones exposed to protons only. Prior treatment with capsaicin altered the responsiveness of 72% of the DRG neurones innervating red muscle, and 73% of those innervating white muscle, to pH 6.0. Exposure to capsaicin increased the proportion of sustained currents of DRG neurones innervating white muscle that responded to pH 6.0 from 22% to 46%, and from 21% to 45% for neurones innervating red muscle; exposure to capsaicin increased the amplitude of the proton-evoked currents by ∼61% (n = 17) in the neurones innervating red muscles and by ∼62% (n = 19) in the neurones innervating white muscles (Fig. 4B). These data indicate that the functional ion channels that protons and capsaicin activate to evoke a sustained inward current are not completely overlapping. However, capsaicin can increase the proton responsiveness of DRG neurones.
We also examined whether capsaicin responsiveness was affected by proton exposure. Prior treatment with pH 6.0 altered the responsiveness to capsaicin of 65% of DRG neurones innervating red muscle and 66% of those innervating white muscle. Exposure to protons increased the proportion of DRG neurones innervating red muscle that responded to capsaicin from 70% to 86%, and from 74% to 90% for neurones innervating white muscle (Fig. 4C), and increased the magnitude of the capsaicin-induced currents by ∼44% (n = 15) in the neurones innervating red muscles and by ∼35% (n = 16) in the neurones innervating white muscles (Fig. 4D).
Discussion
Consistent with the previous findings (Li et al. 2004; Gao et al. 2006), the present study shows that receptors mediating proton and capsaicin responses coexist in the DRG neurones innervating muscle. The responsiveness to acidosis and capsaicin was sensitized by each other, and the amplitudes of inward currents responsive to protons and capsaicin were greater in the neurones innervating muscle comprising predominantly glycolytic fibres than in those innervating muscle comprising predominantly oxidative fibres. This study provides evidence at a cellular level that sensory neurones with nerve endings in different fibre types respond differently to a given level of metabolic stimulation.
The muscle pressor reflex is an important contribution to the cardiovascular and sympathetic nervous responses seen during exercise (Coote et al. 1971; McCloskey & Mitchell, 1972; Mitchell et al. 1977). The afferent arm of this reflex is composed of thinly myelinated and unmyelinated group III and IV muscle afferents (Kaufman & Forster, 1996), which respond to mechanical deformation of the muscle afferent receptive fields and to metabolic stimulation (Kaufman et al. 1983, 1984). Group III afferents are more mechanically sensitive than group IV afferents (Kaufman & Forster, 1996), but both are stimulated or sensitized by muscle metabolic by-products such as lactate acid, prostaglandins and ATP produced in contracting skeletal muscle (Rotto et al. 1990; Sinoway et al. 1993; Li & Sinoway, 2002; Hanna & Kaufman, 2004). With increasing muscle work, ischaemic muscle enhances production of metabolites and the reflex autonomic responses are augmented.
Previous studies have investigated the influence of fibre type on the cardiovascular responses to static contraction (Petrofsky & Lind, 1980; Petrofsky et al. 1981; Frisk-Holmberg et al. 1983; Fallentin et al. 1985; Iwamoto & Botterman, 1985; Wilson et al. 1995). Static contraction of the predominantly fast-twitch muscle triceps brachii increased arterial blood pressure, whereas contraction of the primarily slow-twitch muscle biceps brachii failed to evoke a pressor response (Fallentin et al. 1985). In a similar fashion, contraction of the slow-twitch oxidative soleus did not evoke a pressor reflex in anaesthetized cats, whereas contraction of the medial gastrocnemius (mixed fibre type) increased blood pressure (Petrofsky & Lind, 1980; Petrofsky et al. 1981). Thus static contraction of highly oxidative muscle fails to alter blood pressure. Contrary to these findings, an increase in blood pressure was observed from red muscle in cats although it was smaller than that from white muscles (Iwamoto & Botterman, 1985). A prior investigation used chronic low-frequency electrical stimulation of the tibial nerve in the rabbit to convert the predominant muscle types (Wilson et al. 1995). The reflex pressor responses evoked by static contraction of oxidative muscle are less, compared with the changes elicited by contraction of glycolytic muscle. The data suggest that muscle fibre type has a profound effect on the magnitude of the sensory afferent feedback during exercise.
The goal of this report was to use cellular electrophysiology to examine the responsiveness of sensory neurones innervating different muscle fibre types as the metabolic milieu of the afferent was changed. The fluorescence tracer DiI was injected into two distinct types of muscle to label DRG neurones: the superficial white portion of the gastrocnemius muscle comprising predominantly glycolytic fibres and the deep red portion of the gastrocnemius comprising predominantly oxidative fibres. We then employed whole-cell patch clamp methods to examine the induced inward current activities of sensory neurones innervating glycolytic and oxidative muscle. Protons and capsaicin were given in concentrations based on data obtained from previous studies. Ischaemic muscle contraction increases interstitial hydrogen ion production, and accumulation of hydrogen ions during ischaemia reduces interstitial pH (Victor et al. 1988; Sinoway et al. 1989; MacLean et al. 2000). Furthermore, numerous by-products in active muscle can activate TRPV1 receptors (Szallasi et al. 2007). The receptors that mediate protons and capsaicin responses appear at the peripheral terminals and cell body of the sensory afferent neurones (Szallasi et al. 2007). The receptor activity in the DRG cell body has generally been used to reflect its effect in the nerve endings (Tsuzuki et al. 2003; Puntambekar et al. 2004). The data show that the amplitudes of inward currents responsive to protons and capsaicin were greater in the neurones innervating glycolytic muscle than in neurones subserving a more oxidative region of the gastrocnemius. This provides cellular evidence suggesting that sensory neurones innervating muscle with different oxidative capacities adapt to the metabolic milieu of the muscle region that they subserve.
We observed that DRG neurones had an overlapping response to protons and capsaicin. The overlap was similar in the DRG neurones innervating glycolytic and oxidative muscle. This supports the hypothesis that receptors mediating protons and capsaicin responses coexist in the DRG neurones, and this coexistence is not modified by the muscle type that sensory nerve subserves.
Amiloride attenuated the transient proton currents and the transient component of the mixed proton currents, whereas capsazepine attenuated sustained proton-induced currents and the sustained part of the mixed currents. In addition, pH 6.0 induced a greater percentage of transient proton currents than pH 4.0. Thus the level of pH is probably essential in determining whether hydrogen ions stimulate ASICs or TRPV1 on thin-fibre afferent nerves when the reflex responses are evoked in in vivo experiments (Li et al. 2004). Nevertheless, ASICs are likely to appear frequently on afferents containing TRPV1 because hydrogen ions stimulate ASICs but acid-induced responses are blunted after muscle afferents containing TRPV1 receptors are destroyed (Li et al. 2004).
TRPV1 receptors that capsaicin activates are present preferentially on chemically sensitive thin-fibre sensory neurones which respond to a number of metabolic stimuli such as adenosine, free oxygen radicals, and products of lipoxygenases (Ma, 2001; Szallasi et al. 2007). Roughly 9 out of 10 DRG neurones responsive to lower pH with a sustained current also responded to exogenous capsaicin. The proton responsiveness was enhanced with prior exposure to capsaicin, and the capsaicin-induced response was increased with prior exposure to pH 6.0. This suggests that the responsiveness of acidosis and capsaicin can be augmented by each other. Our data support the suggestion that ASICs and TRPV1 are likely to play an interactive role in processing the muscle afferent response to acid ions (Gao et al. 2006). In this study (Gao et al. 2006), acid phosphate-evoked responses were attenuated to a greater degree when simultaneous TRPV1 and ASIC blockade was performed than when TRPV1 or ASIC blockers were given alone.
In summary, muscle sensory neurones are responsive to capsaicin and hydrogen ions. The responses are seen in DRG neurones innervating oxidative and glycolytic muscle. However, the magnitudes of the inward current from neurones innervating oxidative muscle are less, as compared with glycolytic muscle. This suggests that the magnitude of the DRG neurones' responses to activation of proton and capsaicin receptors is different in afferents subserving different fibre types. We speculate that this will lead to different reflex responses when the afferents serving these two types of muscle are engaged. Muscle fibre type is likely to have a profound effect on the magnitude of the sensory afferent engagement during exercise. Prior exposure to pH 6.0 sensitized the responsiveness to capsaicin, and the proton responsiveness was enhanced by prior exposure to capsaicin. This supports the suggestion that ASICs and TRPV1 play an interactive role in the processing of the muscle afferent response to by-products in active muscle.
Acknowledgments
The authors express gratitude to Dr Victor Ruiz-Velasco in the Department of Anaesthesia for providing excellent technical assistance, and Jennie Stoner for outstanding secretarial skills. This study was supported by NIH R01 HL075533 (J. Li), R01 HL078866 (J. Li), AHA Established Investigator Award 0840130 N (J. Li), and R01 HL060800 (L. Sinoway).
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;229:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallentin N, Sidenius B, Jorgensen K. Blood pressure, heart rate, and EMG in low level static contractions. Acta Physiol Scand. 1985;125:265–275. doi: 10.1111/j.1748-1716.1985.tb07715.x. [DOI] [PubMed] [Google Scholar]
- Frisk-Holmberg M, Essen B, Fredrikson M, Strom G, Wibell L. Muscle fibre composition in relation to blood pressure response to isometric exercise in normotensive and hypertensive subjects. Acta Med Scand. 1983;213:21–26. doi: 10.1111/j.0954-6820.1983.tb03683.x. [DOI] [PubMed] [Google Scholar]
- Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and acid sensing ion channel mediates acid phosphate activation of muscle afferent nerves. J Appl Physiol. 2006;100:421–426. doi: 10.1152/japplphysiol.00659.2005. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Botterman BR. Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol. 1985;58:1676–1682. doi: 10.1152/jappl.1985.58.5.1676. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. Reflexes controlling circulatory, ventilatory and airway responses to exercise; pp. 381–447. [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. The muscle pressor reflex: The potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- Ma QP. Vanilloid receptor homologue, VR1, is expressed by both A- and C-fiber sensory neurons. Neuroreport. 2001;12:3693–3695. doi: 10.1097/00001756-200112040-00018. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;278:R563–R571. doi: 10.1152/ajpregu.2000.278.3.R563. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanism, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol Heart Circ Physiol. 1977;233:H374–H378. doi: 10.1152/ajpheart.1977.233.3.H374. [DOI] [PubMed] [Google Scholar]
- Petrofsky JS, Lind AR. The blood pressure response during isometric exercise in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol Occup Physiol. 1980;44:223–230. doi: 10.1007/BF00421621. [DOI] [PubMed] [Google Scholar]
- Petrofsky JS, Phillips CA, Sawka MN, Hanpeter D, Lind AR, Stafford D. Muscle fiber recruitment and blood pressure response to isometric exercise. J Appl Physiol. 1981;50:32–37. doi: 10.1152/jappl.1981.50.1.32. [DOI] [PubMed] [Google Scholar]
- Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci. 2004;24:3663–3671. doi: 10.1523/JNEUROSCI.4773-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Ase A, Seguela P, Nakatsuka T, Wang CY, She JX, Gu JG. TNP-ATP-resistant P2X ionic current on the central terminals and somata of rat primary sensory neurons. J Neurophysiol. 2003;89:3235–3242. doi: 10.1152/jn.01171.2002. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. erratum appears in J Clin Invest (1988) 82, following 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, Mitchell JH. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbit. J Appl Physiol. 1995;79:1744–1752. doi: 10.1152/jappl.1995.79.5.1744. [DOI] [PubMed] [Google Scholar]