Abstract
Previous studies have suggested that agonists may increase functionally perfused capillary volume by modulation of blood-excluding glycocalyx volume, but direct evidence for this association is lacking at the moment. Using intravital microscopic visualization of mouse cremaster muscle, we determined the effects of bradykinin (10−5 m) and sodium nitroprusside (10−6 m) on capillary tube haematocrit and glycocalyx barrier properties. In control C57Bl/6 mice (n = 10), tube haematocrit in capillaries (n = 71) increased (P < 0.05) from 8.7 ± 0.3% during baseline to 21.2 ± 1.2 and 22.2 ± 0.9% during superfusion with bradykinin and nitroprusside, respectively. In parallel, the exclusion zone of FITC-labelled 70 kDa dextrans decreased (P < 0.05) from 0.37 ± 0.01 μm during baseline to 0.17 ± 0.01 μm with bradykinin and 0.15 ± 0.01 μm with nitroprusside. Bradykinin and nitroprusside had no effect on dextran exclusion and tube haematocrit in capillaries (n = 55) of hyperlipidemic ApoE3-Leiden mice, which showed impaired exclusion of 70 kDa dextrans (0.05 ± 0.02 μm; P < 0.05 versus C57Bl/6) and increased capillary tube haematocrit (23 ± 0.8%; P < 0.05 versus C57Bl/6) under baseline conditions, indicating glycocalyx degradation. Our data show that vasodilator substances increase functionally perfused capillary volume and that this effect is associated with a reduction in glycocalyx exclusion of 70 kDa dextrans. Modulation of glycocalyx volume might represent a novel mechanism of perfusion control at the capillary level.
The endothelial glycocalyx forms a highly hydrated mesh of polysaccharide structures and adsorbed plasma proteins on the luminal side of the vessel wall (Pries et al. 2000; Tarbell & Pahakis, 2006). Recent estimations suggest that the glycocalyx may occupy ∼1.5 l of the systemic vasculature in healthy subjects (Nieuwdorp et al. 2006). These estimations are based on the exclusion of circulating blood by the glycocalyx domain, as originally observed in intravital microscopy studies of muscle microcirculation in rodents (Vink & Duling, 1996). Thus, under resting conditions the glycocalyx has been demonstrated to significantly exclude red blood cells (RBCs) and labelled macromolecules and to greatly retard flowing plasma (Vink & Duling, 1996; Pries et al. 2000; Smith et al. 2003; Weinbaum et al. 2007). These exclusion properties allow the glycocalyx to reduce functionally perfused capillary volume (Vink & Duling, 1996). As a result, the glycocalyx has been demonstrated to contribute to the low tube haematocrit (i.e. 20–50% of systemic values) that is found in capillaries (Klitzman & Duling, 1979; Sarelius & Duling, 1982; Desjardins & Duling, 1987, 1990) and to influence microvascular resistance (Pries et al. 1997; VanTeeffelen et al. 2005b).
Intravital microscopy studies from Duling and co-workers in cremaster muscle originally showed that capillary tube haematocrit may increase during agonist and metabolic stimulation and they suggested the possibility that a reduction in glycocalyx exclusion was involved. Thus, tube haematocrit was found to be about 4-fold higher during muscle activity and adenosine superfusion compared to the resting condition (Klitzman & Duling, 1979), while after treatment of the glycocalyx with heparinase, adenosine did not provoke a significant change in the already elevated capillary tube haematocrit anymore (Desjardins & Duling, 1990). Using a simple two-compartment model with RBCs and plasma flowing in the central core of the capillary and a relatively slow moving plasma layer near the vessel wall representing the glycocalyx, the authors calculated that the increase in capillary tube haematocrit could be explained by a reduction in glycocalyx thickness of 1.2 μm during contractions and adenosine (Klitzman & Duling, 1979; Desjardins & Duling, 1990). Direct visualization of exclusion zones for fluorescently labelled large molecular weight dextrans and red blood cells in cremaster capillaries by the Duling laboratory and our own studies indicated glycocalyx dimensions that were smaller than 1.2 μm, i.e. in the range of about 0.2–0.7 μm (Vink & Duling, 1996; Henry & Duling, 1999, 2000; Vink et al. 2000; Vink & Duling, 2000; Platts et al. 2003; Platts & Duling, 2004; Rubio-Gayosso et al. 2006). Nevertheless, Platts & Duling (2004) demonstrated that during superfusion of the muscle with adenosine, exclusion of FITC-labelled 70 kDa dextrans by the glycocalyx was rapidly and profoundly reduced (Platts & Duling, 2004). This observation has prompted the hypothesis that adenosine can ‘recruit’ capillary volume for perfusion by modulating the accessibility of the glycocalyx for flowing blood (Desjardins & Duling, 1987; Van Teeffelen et al. 2007). Unfortunately, concomitant measures of capillary haemodynamics were not made in the study of Platts & Duling (Platts & Duling, 2004), and it is uncertain therefore how the increase in glycocalyx accessibility for 70 kDa dextrans would influence capillary perfusion. In this respect it is worth mentioning that, in contrast to the profound change in dextran exclusion, RBC exclusion appeared modestly impaired at pharmacological concentrations of adenosine only (Platts & Duling, 2004).
In the present study we therefore aimed at determining the potential of vasodilator-induced modulation of glycocalyx accessibility to increase capillary perfusion. We evaluated the effects of an endothelium-dependent dilator, bradykinin, and an endothelium-independent dilator, sodium nitroprusside, on both capillary tube haematocrit and exclusion properties of the glycocalyx. In mouse cremaster muscle, exclusion of RBCs and fluorescently labelled 70 kDa dextrans was determined together with measurements of RBC flux and velocity, and capillary anatomical diameter, under baseline conditions and in the presence of dilator in the superfusate. Since it has been proposed that the glycocalyx layer is affected by RBC velocity with a decrease in its thickness resulting from increases in velocity or shear rate (Klitzman & Duling, 1979; Sarelius & Duling, 1982; Pries et al. 1997), we put particular focus on the relation between capillary tube haematocrit/glycocalyx exclusion and RBC velocity during baseline and vasodilator administration. Measurements were done in healthy C57Bl/6 mice and in hyperlipidemic ApoE3-Leiden mice, used as an experimental model of atherogenic degradation of the glycocalyx. Atherogenic degradation of the glycocalyx in cremaster capillaries was recently shown after intravenous bolus administration of oxidized lipoproteins (Constantinescu et al. 2001).
Methods
General surgery and anaesthesia
All procedures and protocols were approved by, and carried out according to, the guidelines of the Animal Care and Use committee of the Academic Medical Center. Experiments were performed on male mice (25–30 g body wt). C57Bl/6 mice (n = 10) were obtained from Charles River Europe. ApoE3-Leiden mice (n = 10), transgenic strain 2, were obtained from the Gaubius Laboratorium (TNO-PG Leiden, the Netherlands) and were cross-bred with C57Bl/6 mice (van Vlijmen et al. 1994); transgenic animals of the F10–F11 generation, identified by PCR analysis of genomic DNA from the ear pavilion, were used for the current experiments (VanTeeffelen et al. 2005a). C57Bl/6 mice received standard rat/mouse chow (AM-2, Hope Farms; Woerden, the Netherlands), and ApoE3-Leiden mice were placed at the age of 8 week on a cholesterol-enriched high-fat diet (0.5% cholate, 15% cocoa butter, 1% cholesterol, 40.5% sucrose, 10% corn starch, 1% corn oil and 4.7% cellulose; HFC 0.5% diet, Hope Farms) for 3 months. This diet was shown to result in an increase in plasma cholesterol and triglyceride levels in this period of time (VanTeeffelen et al. 2005a).
At the beginning of an experiment, mice were anaesthetized with an i.p. injection of ketamine hydrochloride (125 mg kg−1) and medetomidine (0.2 mg kg−1), and tracheotomised to ensure airway patency (VanTeeffelen et al. 2005a). Depth of anaesthesia was maintained according to stability of blood pressure, respiration rate, and lack of toe pinch reflex by supplemental administration (about every hour) of anaesthetic (ketamine: 15 mg kg−1, i.p.; medetomidine: 35 μg kg−1, i.p.). To counter effects of decreases in blood pressure and heart rate induced by medetomidine, atropine was administered at 1 h intervals during the experiment (initial dose: 0.5 mg kg−1, s.c.; maintenance: 0.125 mg kg−1, i.p.). The right carotid artery and jugular vein were canulated for monitoring systemic blood pressure and administration of fluorescent dextrans, respectively. Oesophageal temperature was maintained at ∼37°C by radiant heat. No fluid was supplemented during the experiment except for the anaesthesia and dextrans. At the end of the experimental procedures, a blood sample was collected in a heparinized capillary (50 μl) using tail bleeding for determination of systemic haematocrit, followed by an overdose of ketamine.
Mouse cremaster preparation and video microscopy
The mouse was placed in a supine position on a custom-built animal platform and the right cremaster muscle was prepared (Constantinescu et al. 2001; VanTeeffelen et al. 2005a). Briefly, an incision was made through the skin and the muscle dissected from the surrounding connective tissue. The exposed muscle was positioned on a clear silicon pedestal and longitudinally incised from the apex to the inguinal canal with minimal disruption of the vascular supply. After severing the deferential artery and vein, the testis and epididymis were dissected away and repositioned in the abdominal cavity. The cremaster muscle was spread radially on the pedestal and pinned at the edges. The muscle was continuously (∼5 ml min−1) superfused at 34°C with a bicarbonate-buffered physiological salt solution (PSS) of the following composition (mm): 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 20 NaHCO3 and equilibrated with 5% CO2–95% N2 to obtain a pH of ∼7.4.
Following surgery, the completed preparation was transferred to the stage of an intravital microscope (Olympus BHM), coupled to a cooled intensified CCD video camera (GenIV ICCD, Princeton Instruments). Microvessels were observed using bright-field (100 W Hg lamp) microscopy (condensor: Olympus MA20, numerical aperture (NA): 0.4) or epi-illuminated (100 W Hg lamp) for the examination of fluorescent dextrans. Bright-field images were made with a 435 nm band-pass interference filter (blue light) in the light path; fluorescent tracers were visualized using appropriate filters for FITC and Texas Red. Cremaster muscle capillaries were examined with a ×60 water immersion objective lens (Olympus; LUMPlanFL, NA 0.9), and arterioles were examined with a ×20 objective lens (Olympus; MSPlan 20, NA 0.4). Images were displayed on a Philips CM 8833-II video monitor and recorded using a SVHS video tape recorder (JVC BR-S611E) and a time coding interface unit (JVC SA-F911E) for post hoc data analysis.
Experimental protocol
The preparation was equilibrated for 30 min, during which time the arteriolar network was scanned for the presence of vasomotor tone. This evaluation of tone was based on the diameter range for these vessels established in a previous study (VanTeeffelen et al. 2005a), while in case of doubt the presence of tone was checked for by a few drops of sodium nitroprusside (SNP; 10−5m; Sigma) on the preparation. During the equilibration period, a schematic diagram was drawn to identify sites for data collection. FITC-labelled 70 kDa dextrans (FITC-dextran 70; Sigma) and Texas Red-labelled 40 kDa dextrans (TR-dextran 40; Invitrogen) were intravenously injected as a bolus of 0.1 ml at a concentration of 10 mg ml−1 in saline. Previous intravital microscopic studies have shown that under control conditions FITC-dextran 70 is significantly excluded from the endothelial surface in capillaries by the glycocalyx, whereas TR-dextran 40 appears to have unimpaired access to the entire glycocalyx domain (Vink & Duling, 2000; Platts & Duling, 2004). Cremaster capillaries from different microscopic fields were randomly chosen for examination and recorded on videotape using trans- and epi-illumination. Trans-illumination was used to determine endothelial surface position and velocity, flux and width of RBCs. Epi-illumination was used to determine the distribution of FITC-dextran 70 and TR-dextran 40, and was completed within a few seconds to minimize light-dye damage (Vink & Duling, 1996, 2000). After baseline recordings were made (20–30 min), bradykinin (BK, 10−5m; Sigma) or SNP (10−6m; Sigma) were randomly administered to the superfusate, and recordings of the same capillaries made, starting after 10 min. After 30 min, the vasodilator was washed out, the cremaster allowed to recover for 60 min, and baseline recordings again made. Finally, the other vasodilator was added to the superfusate and recordings repeated during this condition for 20–30 min. During baseline and administration of each vasodilator, proximal 1st or 2nd order arterioles were recorded as well for monitoring of arteriolar dilatation (as percentage of baseline) in response to BK and SNP.
Data analysis
Video images were digitized using a frame grabber (DT3152, PCI Local Bus) and image analysis software (Image-Pro Plus version 3.0, Media-Cybernetics, Silver Springs, PA, USA). Arteriolar diameter was measured from the distance between digital callipers positioned at the arteriolar wall. Capillary anatomical diameter and width of RBCs were measured from trans-illumination images by positioning digital callipers at the inside of the capillary wall and at the RBC border, respectively.
The glycocalyx RBC exclusion zone was determined by subtracting RBC width from capillary anatomic diameter and dividing the difference by two (Vink & Duling, 1996, 2000; Platts & Duling, 2004; Rubio-Gayosso et al. 2006). Glycocalyx exclusion zones for both dextrans were determined from epi-illumination images by measuring the width of the dye column with callipers, and subtracting this from the width of the capillary anatomic diameter, and dividing the difference by two (Vink & Duling, 1996, 2000; Platts & Duling, 2004; Rubio-Gayosso et al. 2006).
Capillary tube haematocrit (Hct, in percentage) was calculated from measurements of capillary anatomical diameter (Dc, in μm), flux of RBCs (F, in cells s−1), and velocity of RBCs (V, in μm s−1) in each capillary, using the formula: Hct = [F/(V·π/4·Dc2)] × MCV (Sarelius & Duling, 1982; Constantinescu et al. 2001), where MCV is the mean corpuscular volume of RBCs in mice (44 μm3) (Murdock et al. 2000). F was determined during slow-motion video playback from the time required for at least 50 RBCs to pass through a reference point inside the capillary. V was determined by measuring the length of a capillary segment and dividing it by the time required for RBCs to traverse this segment. The individual boundaries of RBCs could not be determined at high capillary velocities due of the limited video rate, and as a result capillaries with V > 500 μm s−1 were discarded from the analysis. Capillaries were grouped based on RBC velocity of < 50, 50–100, …, 450–500 μm s−1 (Vink & Duling, 1996), because of the often observed correlation between red blood cell velocity and capillary tube haematocrit (Klitzman & Duling, 1979; Sarelius & Duling, 1982; Damon & Duling, 1984; Desjardins & Duling, 1990).
Data are presented as means ± s.e.m. with n referring to the number of vessels or animals studied. In both animal groups, effects of BK or SNP were tested against baseline, or against the other vasodilator, using Student's paired t tests. Differences between C57Bl/6 and ApoE3-Leiden mice were tested using Student's two sample t tests. Results were considered statistically significant with P ≤ 0.05.
Results
Mean arterial pressure and heart rate were 60–70 mmHg and 300–400 beats min−1, respectively, during experiments and did not change during respective treatments. Systemic haematocrit was 42.2 ± 0.8% in C57Bl/6 mice and 40.7 ± 1.3% in ApoE3-Leiden mice.
Arteriolar responses
In control mice, BK and SNP increased arteriolar (n = 10) diameter to 125 ± 2% (P < 0.05) and 128 ± 2% (P < 0.05) of baseline (36.2 ± 1.5 μm), respectively. In ApoE3-Leiden mice, arteriolar (n = 10) diameter was increased from a baseline of 36.3 ± 1.1 μm to 116 ± 1% (P < 0.05) and 131 ± 2% (P < 0.05) during BK and SNP, respectively. Arteriolar dilatation in ApoE3-Leiden mice during BK was reduced compared to SNP in the same animals (P < 0.05) and compared to dilatation during BK in C57Bl/6 mice (P < 0.05).
Capillary responses
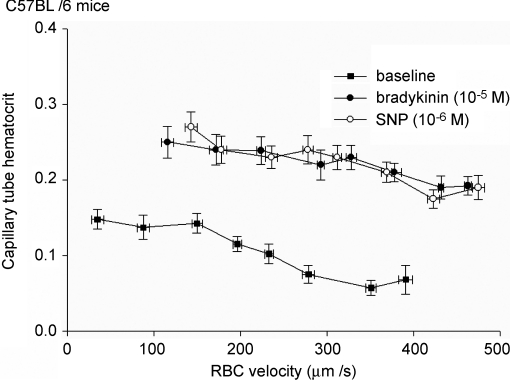
Control mice. Responses were measured in n = 71 capillaries of control C57Bl/6 mice. Capillary anatomic diameter was 4.9 ± 0.1 μm during baseline and did not change after vasodilator administration. RBC flux was 11.2 ± 0.7 cells s−1 during baseline, and 36.1 ± 2.2 cells s−1 (P < 0.05) and 35.4 ± 2.2 cells s−1 (P < 0.05) during BK and SNP, respectively, while RBC velocity increased from 291 ± 15 μm s−1 during baseline to 408 ± 20 μm s−1 (P < 0.05) during superfusion of BK and to 423 ± 16 μm s−1 during SNP (P < 0.05). Capillary tube haematocrit varied from 7.1 ± 1.7% at high RBC velocities to 14.8 ± 1.3% at low velocities during baseline conditions (Fig. 1); on average, capillary tube haematocrit was 8.7 ± 0.3%. Tube haematocrit increased during administration of BK (21.2 ± 1.2%; P < 0.05) and SNP (22.2 ± 0.9%; P < 0.05). During vasodilator administration, the relation between capillary tube haematocrit and RBC velocity was shifted in a parallel manner, resulting in a higher tube haematocrit at a given RBC velocity compared to baseline (Fig. 1). Capillary tube haematocrit and red blood cell velocity were not different after washout of the 1st vasodilator compared to before vasodilator administration.
Figure 1. BK and SNP increase capillary tube haematocrit and RBC velocity in control mice.
Capillary tube haematocrit decreases with increasing RBC velocity, during baseline and in the presence of vasodilator. Vasodilator administration is associated with an increased capillary tube haematocrit for any given RBC velocity.
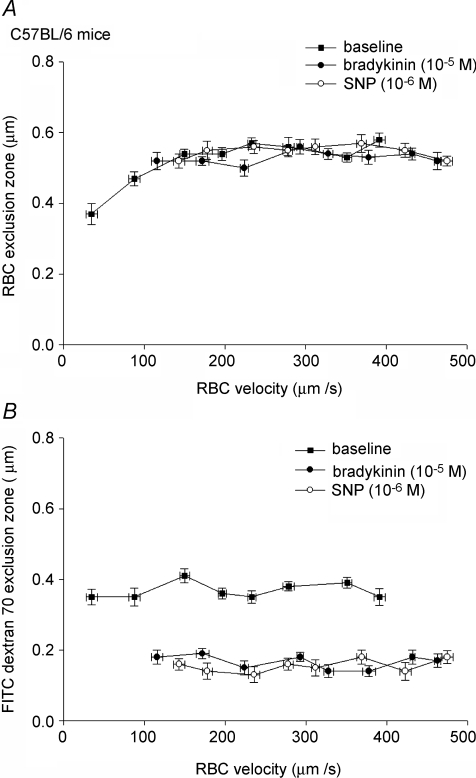
In C57Bl/6 mice, exclusion of RBCs from the endothelium was 0.55 ± 0.02 μm during baseline conditions, and was not changed after administration of BK (0.55 ± 0.01 μm) or SNP (0.57 ± 0.01 μm). In contrast, the FITC-dextran 70 exclusion zone, which was 0.37 ± 0.01 μm during baseline, was significantly reduced during superfusion of BK (0.17 ± 0.01 μm; P < 0.05) and SNP (0.15 ± 0.01 μm; P < 0.05). The exclusion zone for FITC-dextran 70 after washout of the 1st vasodilator and before application of the 2nd was 0.38 ± 0.01 μm, and not different from baseline values. These discrepant effects of BK and SNP on the exclusion of RBCs versus that of the dye are further exemplified in Fig. 2, which shows the exclusion zone for RBCs (top panel) and FITC-dextran 70 (bottom panel) for different RBC velocities during baseline and vasodilator administration. Exclusion zones appeared independent of RBC velocity in the range of 100–500 μm s−1. Below 100 μm s−1, red blood cell exclusion seemed to be impaired (Fig. 2, top panel). The exclusion zone of TR-dextran 40 was 0.07 ± 0.02 μm during baseline conditions, and not changed after administration of BK (0.01 ± 0.02 μm) or SNP (0.03 ± 0.01 μm).
Figure 2. Exclusion of FITC-dextran 70, not RBCs, decreases during BK and SNP superfusion in control mice.
Top panel, RBC exclusion zone, used as measure of glycocalyx thickness, decreases at RBCs velocities < 100 μm s−1, and is not affected by BK and SNP. Bottom panel, FITC-dextran 70 exclusion zone is independent of RBC velocity, and decreases during vasodilator administration.
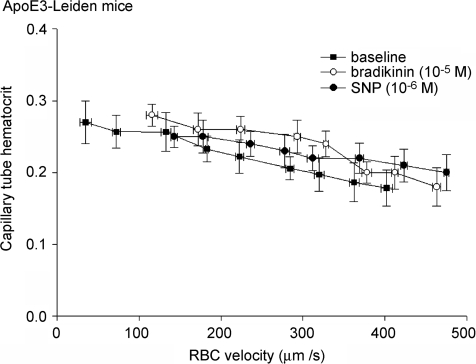
Hyperlipidemic mice. In ApoE3-Leiden mice, the majority of capillaries (n = 55) were found to present subendothelial lipid deposits and to have impaired glycocalyx exclusion properties (see below). The other capillaries (n = 10) were without signs of lipid deposits and demonstrated normal (i.e. comparable to control mice) exclusion of RBCs and FITC-dextran 70 from the vessel wall. As we were interested in the effect of vasodilator modulation during a condition of glycocalyx degradation, only those capillaries with subendothelial lipid deposits are presented here. Anatomical diameter of these capillaries was 5.1 ± 0.1 μm during baseline and not different during vasodilator administration. RBC flux was higher (P < 0.05) than in control mice, 23.1 ± 1.7 cells s−1 during baseline, and 41.3 ± 1.2 cells s−1 (P < 0.05 versus baseline) and 39.4 ± 2.0 cells s−1 (P < 0.05) during BK and SNP, respectively, while RBC velocity increased from 225 ± 16 μm s−1 during baseline to 382 ± 18 μm s−1 (P < 0.05) during superfusion of BK and to 364 ± 17 μm s−1 during SNP (P < 0.05). Figure 3 indicates that for a given RBC velocity, capillary tube haematocrit did not change after vasodilator administration. Thus, average capillary tube haematocrit was 23 ± 0.8% during baseline conditions (P < 0.05 versus C57Bl/6), and not affected during administration of BK (24.3 ± 1.1%) and SNP (22.7 ± 1.4%).
Figure 3. Lack of effect of BK and SNP on capillary tube haematocrit in hyperlipidemic mice.
In capillaries with lipid deposits of ApoE3-Leiden mice, capillary tube haematocrit decreases with increasing RBC velocity, during baseline and in the presence of vasodilator. Vasodilator administration does not affect capillary tube haematocrit for any given RBC velocity.
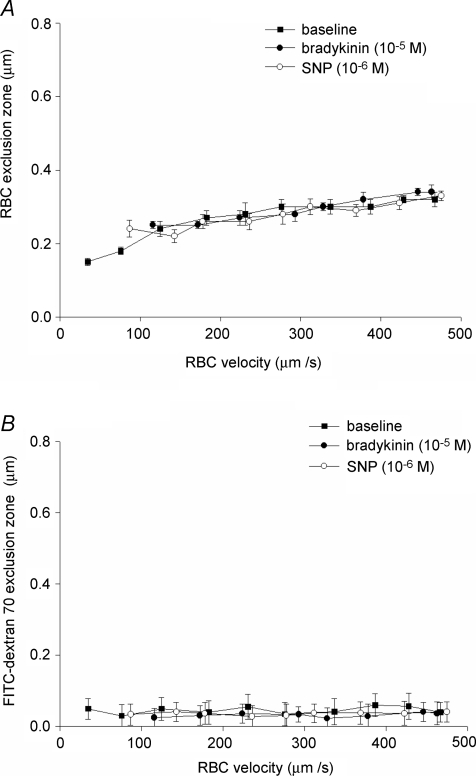
In the capillaries with subendothelial lipid deposits, the exclusion zone of RBCs from the endothelium increased from 0.15 ± 0.02 μm at velocities less than 50 μm s−1 to 0.33 ± 0.02 μm at velocities > 450 μm s−1 (Fig. 4, top panel), illustrating a reduction in glycocalyx exclusion over the entire range of RBC velocities compared to the control C57Bl/6 mice (Fig. 2, upper panel). On average, RBC exclusion was 0.30 ± 0.02 μm during baseline conditions (P < 0.05 versus C57Bl/6), and not changed after administration of BK or SNP. The FITC-dextran 70 exclusion zone was only 0.05 ± 0.02 μm during baseline (P < 0.05 versus C57Bl/6), not dependent on RBC velocity (Fig. 4, bottom panel) and not changed during superfusion of BK (0.10 ± 0.02 μm) and SNP (0.04 ± 0.02 μm) (Fig. 4, bottom panel). The exclusion zone of TR-dextran 40 was 0.02 ± 0.03 μm during baseline conditions, and not changed after administration of BK (0.04 ± 0.02 μm) or SNP (0.01 ± 0.02 μm).
Figure 4. Glycocalyx exclusion is not changed during vasodilator superfusion in hyperlipidemic mice.
Top panel, RBC exclusion zone; bottom panel, FITC-dextran 70 exclusion zone. In capillaries with lipid deposits in ApoE3-Leiden mice, RBC and FITC-dextran 70 exclusion zones were profoundly reduced during baseline compared to control mice (Fig. 2), yet not affected by administration of BK and SNP.
Discussion
The endothelial glycocalyx has been indicated to significantly reduce perfused capillary volume under control conditions by excluding flowing plasma and RBCs from a relatively thick region near the luminal endothelial surface. The present study shows that bradykinin (BK) and sodium nitroprusside (SNP) increase capillary tube haematocrit about 2.5-fold in cremaster capillaries of healthy C57Bl/6 mice. This increase was paralleled by an increased accessibility of the glycocalyx domain for FITC-labelled 70 kDa dextrans without a change in RBC exclusion. BK and SNP had no effect on tube haematocrit or glycocalyx barrier properties in capillaries of hyperlipidemic ApoE3-Leiden mice that showed signs of glycocalyx degradation. Our data suggest that vasodilator substances can increase functionally perfused capillary volume by modulation of glycocalyx exclusion.
Glycocalyx degradation in hyperlipidemic mice
To test whether vasodilator effects on capillary haemodynamics and intravascular tracer distribution pertained to properties of the glycocalyx, we chose the high-fat fed Apo3-Leiden mouse as a model for atherogenic glycocalyx degradation. Our rationale for this was that we wanted to avoid transient effects and time-consuming infusions that are intrinsic to the use of glycocalyx-degrading enzymes (Desjardins & Duling, 1990; Henry & Duling, 1999; VanTeeffelen et al. 2005b, 2007), while we had previously demonstrated the potency of glycocalyx degradation by an atherogenic stimulus, in this case a bolus infusion of oxidized LDL (Constantinescu et al. 2001). The majority of capillaries in the hyperlipidemic mice indeed demonstrated a greatly reduced RBC exclusion under baseline conditions (Fig. 4), and the exclusion zone of ∼0.30 μm is very comparable with the reported RBC exclusion zone of 0.30–0.35 μm after the bolus of oxidized LDL (Constantinescu et al. 2001). In addition, the decrease in RBC exclusion after oxidized LDL was paralleled by an increase in capillary tube haematocrit towards 30–35% of baseline and an increase in RBC flux of 150–200% without a change in RBC velocity (Constantinescu et al. 2001), and this is very similar to the current haemodynamic observations in ApoE3-Leiden capillaries. Of note, the ApoE3-Leiden mice had not exclusively signs of glycocalyx degradation, and the chronic atherogenic conditions unquestionably impacted on many additional pathways related to vascular homeostasis as well as rheology. Thus, these animals develop severe hyperlipidemia and atherosclerosis after 3 months on a high-fat–high-cholesterol diet (van Vlijmen et al. 1994), while our own results indicate impairment of vascular barrier properties by the presence of subendothelial lipid deposits and endothelial dysfunction by the impaired arteriolar dilatation to BK (present data) and to reactive hyperaemia (VanTeeffelen et al. 2005a). In addition, the hyperlipidemic conditions per se might, by an increased plasma viscosity, have influenced microcirculatory perfusion characteristics in the ApoE3-Leiden mice.
Vasodilator effects on capillary perfusion
Marked increases in capillary tube haematocrit and red blood cell velocity during muscle contractions and adenosine were originally reported by Klitzman & Duling (1979). It was hypothesized that the changes in capillary tube haematocrit were related to metabolic and vasoactive modulation of the contribution of a 1.2-μm-thick slow-moving plasma layer to functional capillary perfusion (Klitzman & Duling, 1979). Ten years later it was indicated that this layer was represented by the endothelial glycocalyx, since enzymatic treatment of glycocalyx structures with heparinase was associated with a doubling in baseline capillary haematocrit (Desjardins & Duling, 1990).
In line with reports in the literature (Klitzman & Duling, 1979; Sarelius & Duling, 1982; Desjardins & Duling, 1987, 1990), average capillary tube haematocrit in healthy capillaries was low under control conditions, about 20% of systemic haematocrit. Capillary tube haematocrit, the fractional volume of a capillary occupied by RBCs, was derived from capillary anatomical diameter and the velocity and flux of RBCs in the capillary, measurements that all have potential errors associated with them. Nevertheless, this estimate has been used in many previous studies and the possible error associated with the calculation is regarded to be small: Sarelius & Duling (1982) estimated the standard deviation of the haematocrit determination to be ±17% (Sarelius & Duling, 1982). Furthermore, RBCs could not individually be distinguished in the present study at velocities greater than 500 μm s−1 (see Methods). This was, however, the case in only ∼5% of the capillaries and neglecting these data in the analysis did therefore not significantly impact on the results.
Under control conditions, capillary tube haematocrit increased with decreasing red blood cell velocity (Vrbc) (Fig. 1), and the decrease in RBC exclusion at velocities less than 200 μm s−1 (Fig. 2, top) seems to underlie this at least for the lower velocity range. This behaviour can be accounted for by glycocalyx deformation by expansion of the RBCs at low velocities, as was experimentally shown by Vink & Duling (1996), and argues against a shear-dependent compression of the glycocalyx (Pries et al. 1997). An increased red blood cell exclusion with increasing velocity was also predicted by the model of Secomb et al. (2001) on the motion of red blood cells in a glycocalyx-lined capillary using the lubrication theory (Secomb et al. 2001). This inverse relation between red blood cell velocity and capillary tube haematocrit seems at first in contrast to the early studies from Duling and co-workers in which a positive relation between red blood cell velocity (and arteriolar diameter) on the one hand and capillary tube haematocrit on the other hand was observed (Klitzman & Duling, 1979; Sarelius & Duling, 1982; Damon & Duling, 1984; Desjardins & Duling, 1990). In those studies, changes in red blood cell velocity evoked by addition of oxygen or adenosine in the superfusate were associated with concomitant changes in capillary tube haematocrit, suggesting a role for haemodynamic effects in RBC occupation of the capillary. Similarly, BK and SNP superfusion evoked arteriolar dilatations and increases in red blood cell velocity and capillary tube haematocrit in the current experiments. However, our data show that the relation between capillary tube haematocrit and Vrbc was actually shifted in a more or less parallel manner during vasodilator administration (Fig. 1). In the hyperlipidemic mice, a similar shift was observed during baseline conditions already (Fig. 3), which agrees with the observation of Desjardins & Duling (1990) and Constantinescu et al. (2001) that heparinase or oxidized-LDL treatment doubled baseline capillary tube haematocrit in the face of a constant red blood cell velocity (Desjardins & Duling, 1990; Constantinescu et al. 2001). In a recent study in which capillary haemodynamics in rat spinotrapezius muscle were studied during recovery from muscle contractions, a relation between changes in red blood cell velocity and capillary tube haematocrit was absent as well (Ferreira et al. 2006), illustrating that also under these physiologically relevant conditions capillary tube haematocrit is a parameter that can change independently from a change in red blood cell velocity. Further arguing against a simple haemodynamic effect of the vasodilators on capillary tube haematocrit is the observation that administration of BK or SNP in the ApoE3-Leiden animals increased red blood cell velocity but did not affect the relation between capillary tube haematocrit and red blood cell velocity (Fig. 3). In line herewith, Desjardins & Duling (1990) showed that glycocalyx treatment with heparinase obliterated the relationship between red blood cell velocity and capillary tube haematocrit in response to oxygen or adenosine supplementation to the superfusate. These findings altogether indicate the existence of an inverse relation between capillary tube haematocrit and Vrbc; this relation seems relevant for different ‘states’ of the glycocalyx (i.e. baseline, vasodilator, degradation).
Agonist-induced increases in capillary tube haematocrit: underlying mechanisms
Differences in mean red blood cell velocity compared to mean plasma velocity cause a reduction in tube haematocrit compared to systemic haematocrit (Goldsmith et al. 1989; Lipowsky, 2005). The lower limit attributed to this ‘single vessel Fåhraeus effect’ has been postulated to be a 50% reduction in capillary tube haematocrit compared to systemic haematocrit (Goldsmith et al. 1989), while a reduction beyond this value might be explained by the presence of an immobilized plasma volume represented by the glycocalyx (Klitzman & Duling, 1979; Desjardins & Duling, 1990; Vink & Duling, 1996; Secomb et al. 1998), or to be caused by microvascular network events, such as phase separation of RBCs and plasma at upstream bifurcations, or intercapillary heterogeneity of blood flow (‘network Fåhraeus effect’; Pries et al. 1986; Goldsmith et al. 1989). The network Fåhraeus effect can be altered according to perfusion heterogeneity, where less heterogeneity would lead to higher capillary tube haematocrits. A reduction in heterogeneity of capillary red blood cell velocity with vasodilatation or increased flow (Tyml & Cheng, 1995) has not consistently been observed, however (Damon & Duling, 1985, 1987), and with respect to our own results, the increase in red blood cell velocity during SNP or bradykinin administration did not seem to coincide with a robust reduction in perfusion heterogeneity in both control and hyperlipidemic animals, since the standard error of red blood cell velocity increased during vasodilator administration compared to baseline as well (see Results).
Therefore, we like to consider also the possibility that increases in glycocalyx accessibility for flowing plasma are underlying observed increases in capillary tube haematocrit towards 50% of systemic haematocrit. In the hyperlipidemic mice, the ratio between capillary tube haematocrit (23%) and systemic haematocrit (41%) was close to 0.5, suggesting that in these capillaries the entire anatomical volume of the capillary was available for perfusion, and that plasma retardation by the glycocalyx was irrelevant. Indeed, there was barely any exclusion of 70 kDa dextrans in these capillaries (Fig. 4, bottom panel), while the 0.30-μm-thick exclusion zone of RBCs might represent the lower limit for RBCs approaching the endothelium and reflect a plasma lubrication layer that is required for unimpeded motion of RBCs in capillaries (Vink & Duling, 1996; Secomb et al. 1998).
An important question is to which extent the effective glycocalyx thickness needs to be diminished to obtain values of capillary tube haematocrit that are half of the systemic values during vasodilator administration in the control animals. On the basis of a simple two-compartment model with RBCs and plasma flowing in the central core of the capillary and an additional immobilized plasma layer near the vessel wall representing the glycocalyx (Klitzman & Duling, 1979; Desjardins & Duling, 1990), an increase in perfused diameter of 1.8 μm in a capillary with an anatomical diameter of 4.9 μm would explain the 2.5-fold increase in capillary tube haematocrit with BK and SNP, agreeing with a reduction in blood-excluding glycocalyx thickness of 0.9 μm from baseline to vasodilator conditions. This value is comparable with the original estimations of the slow-moving plasma layer by Duling and co-workers (Klitzman & Duling, 1979; Desjardins & Duling, 1990), but significantly larger than the exclusion zones in the current and previous studies as well as microparticle image velocimetry (microPIV) measures, all indicating dimensions of the glycocalyx in cremaster vessels that range from ∼0.2–0.6 μm (Vink & Duling, 1996; Henry & Duling, 1999; Smith et al. 2003; Platts & Duling, 2004; Rubio-Gayosso et al. 2006; Weinbaum et al. 2007). This disparity calls for a prudent interpretation of the vasodilator-associated increases in capillary tube haematocrit on the basis of a change in effective glycocalyx thickness alone. On the other hand, estimations to explain the discrepancy between experimental estimates of apparent viscosity or flow resistance in microvessels in vitro versus in vivo by Pries and co-workers have generally yielded values of > 1 μm for effective glycocalyx thickness (Pries et al. 1997; Pries & Secomb, 2005), while in isolated small mesenteric arteries the exclusion zone for FITC-labelled 150 kDa dextrans was estimated to be in the order of a couple of microns (van Haaren et al. 2003). The basis for this discrepancy is unknown at the moment and inaccuracies in both the experimental measurements (see below) and the theoretical models used might underlie this difference.
Vasodilator effects on glycocalyx exclusion properties: possible mechanisms
Consistent with previous measurements of anionic dextran distribution in rodent capillaries (Vink & Duling, 1996; Henry & Duling, 1999; Vink et al. 2000; Platts & Duling, 2004; Rubio-Gayosso et al. 2006), we observed a significant intravascular zone to which FITC-labelled 70 kDa dextrans had no access during control conditions (Fig. 2). The exclusion zone for the dextrans was 0.35–0.40 μm, which agrees with previous studies showing glycocalyx exclusion of FITC-labelled 70 kDa dextrans to be about 50% of RBC exclusion (Vink & Duling, 1996; Henry & Duling, 1999; Platts & Duling, 2004; Rubio-Gayosso et al. 2006). The larger dextran distribution volume compared to the RBCs seems to indicate that the dextrans partly permeate into the glycocalyx domain during resting conditions (VanTeeffelen et al. 2007), and as a result the endothelium–dextran gap probably provides an underestimation of true blood-excluding glycocalyx dimensions. Interestingly, the endothelium–dextran gap was found to be ∼1 μm when RBCs were absent in the capillary (Vink et al. 2003), and we like to consider therefore the possibility that the current measurements of both endothelium–RBC and endothelium–dextran dye gap are underestimating the effective solute-excluding glycocalyx volume because of passing RBCs partly compressing the glycocalyx. In the case where there is ample time for recovery of glycocalyx dimensions in between successive red blood cells, i.e. at the low RBC fluxes that are occurring during baseline, the integrated measurement of the endothelium–RBC or –dye gap across the length of the observed capillary will then result in an underestimation of the effective blood-excluding glycocalyx thickness. Recovery of glycocalyx thickness in response to an almost complete glycocalyx compression by passing leucocytes was estimated to follow a single exponential with a time characteristic (τ) of 0.38 s (Vink & Duling, 1996; Weinbaum et al. 2003), but recovery after partial compression by passing red blood cells might be faster. Effective glycocalyx thickness is then a result of true physical glycocalyx thickness, recovery time and red blood cell flux. For example, in the case of a 1.5-μm-thick glycocalyx, a τ of 0.19 s will be sufficient to get to an effective thickness of the layer of 0.9 μm when red blood cells are passing every 0.1 s (i.e. a RBC flux of 10 s−1); in the case of a true thickness of 1.0 μm, a τ of 0.09 is needed for RBCs passing every 0.1 s.
During BK and SNP administration, the exclusion of dextran was impaired by ∼0.15 μm while RBC exclusion was not altered (Fig. 2, top). The disparity in the effect of a certain stimulus on macromolecule exclusion versus that on RBC exclusion has been noticed in previous studies from Duling and co-workers also (Henry & Duling, 1999; Platts & Duling, 2004; Rubio-Gayosso et al. 2006). Thus, increases in FITC-labelled dextran accessibility without changes in RBC exclusion were also observed in cremaster vessels during ischaemia-reperfusion and hyaluronidase treatment. In the case of adenosine, Platts & Duling (2004) showed a profound reduction in FITC-dextran 70 exclusion at the micromolar concentration of adenosine, while a small reduction in RBC exclusion appeared at a 100-fold higher dose only (Platts & Duling, 2004). An increased dextran accessibility in the face of an unchanged RBC exclusion has been interpreted as a manifestation of a more open or porous structure of the glycocalyx (Henry & Duling, 1999; Platts & Duling, 2004; Rubio-Gayosso et al. 2006). Glycocalyx composition and dimensions are the ultimate result of continuous biosynthesis of polysaccharide structures and association with blood-borne proteins on one hand and shedding or release of components on the other hand, while the actual dynamic state of the glycocalyx depends on the intrinsic properties of its constituents, their interactions and the local microenvironment, such as cation content and pH (Mulivor & Lipowsky, 2004; Tarbell & Pahakis, 2006; Weinbaum et al. 2007). Any change in production, shedding and local conditions may therefore affect the permeation of 70 kDa dextrans into the glycocalyx domain.
Our results suggest a role for NO in the impairment of glycocalyx barrier properties by bradykinin and SNP. There is evidence that NO is capable of degrading heparan sulphates (Vilar et al. 1997), but this process seems too slow to explain the observed dynamics of changes in capillary tube haematocrit and glycocalyx exclusion. The pilot studies of Duling showed moment-to-moment variations in capillary tube haematocrit to occur in the face of a constant discharge haematocrit, suggesting that glycocalyx exclusion properties may change very rapidly (Desjardins & Duling, 1987). Platts & Duling (2004) found glycocalyx exclusion to be impaired at their first observation point, which occurred 6 min after adenosine administration had started (Platts & Duling, 2004), while our previous study in dog hearts suggests that adenosine-induced glycocalyx modulation may occur almost instantaneously (VanTeeffelen et al. 2005b), at least in the time frame of maximal coronary hyperaemia in response to a bolus of adenosine (∼15 s). As an alternative for degradation of polysaccharide structures by NO, we propose a change in charge density of the glycocalyx as a possible mechanism by which NO can rapidly change its properties. Our group previously demonstrated in small arteries of the rat mesentery that the glycocalyx can move from a collapsed to an extended state as ionic strength of the superfusate decreases (van Haaren et al. 2005), and we envision that NO by scavenging of superoxide anions within the glycocalyx (e.g. produced by glycocalyx-bound xanthine oxidase (Rubio-Gayosso et al. 2006)) might induce a similar effect. In addition, rapid reactions of NO effecting nitrosylation of albumin and other proteins (Stamler et al. 1992) within the glycocalyx might alter their binding affinity for glycocalyx polysaccharide structures, thereby changing the structural barrier properties of the glycocalyx (Adamson & Clough, 1992; VanTeeffelen et al. 2007). Altogether, these NO-mediated changes may convert the glycocalyx towards a more porous compartment with increased hydraulic and tracer conductivity without shedding of its glycosaminoglycan components. Theoretical models of red cell motion in a single file (Damiano, 1998; Secomb et al. 1998) have indeed shown that with a constant layer thickness an increase in hydraulic conductivity of the glycocalyx can take place without significant changes in RBC shape and that this causes capillary tube haematocrit to increase. Of note, vasodilator administration in the control mice resulted in capillary tube haematocrits that were very close to those during baseline conditions in the hyperlipidemic mice, suggesting that in the presence of the vasodilators the whole anatomic volume of the capillary was available for perfusion, and that the entire glycocalyx domain was accessed by plasma flow. Dextran exclusion was, however, only modestly reduced to ∼40% of baseline, and a zone of ∼0.15 μm thickness from which these molecules seemed to be excluded, remained (Fig. 2), illustrating that measures of 70 kDa dextran distribution cannot be simply interpreted as correct measures of plasma distribution. Although an increase in hydraulic conductivity of the endothelial cell layer by bradykinin and SNP is expected to occur as well, this alone seems not sufficient to explain the increase in dextran permeation into the glycocalyx, because in the absence of a reduction in reflection coefficient of the dextran, a sole increase in hydraulic conductivity will result in a dilution of this fluorescent tracer within the glycocalyx. In contrast, we actually observed an increase in 70 kDa dextran concentration in the glycocalyx compartment during vasodilator administration, demonstrating that the evoked impairment of tracer barrier properties surpassed the presumed reduction in hydraulic resistance.
Glycocalyx modulation and regulation of capillary perfusion
From our present data and the aforementioned data from Duling and co-workers, we would like to propose the concept in which vasoactive substances can ‘recruit’ capillary volume for perfusion by increasing accessibility of the glycocalyx for flowing plasma, as reflected by the increased dextran accessibility, without necessarily changing glycocalyx dimensions (Van Teeffelen et al. 2007). Acting in parallel to relaxation of resistance vessels, this may enable coordination of substrate delivery (flow) and exchange (surface area and permeability) (Van Teeffelen et al. 2007). The potency of adenosine to ‘recruit’ glycocalyx volume in the coronary vascular bed was recently determined in canine hearts (VanTeeffelen et al. 2005b). We compared the increase in coronary conductance during adenosine-induced hyperaemia to the increase in conductance during reactive hyperaemia and found that under control conditions adenosine increased conductance up to 40% more than reactive hyperaemia (VanTeeffelen et al. 2005b). The difference was, however, virtually abolished upon enzymatic treatment of the glycocalyx, which resulted in an increase in reactive hyperaemia without changing adenosine hyperaemia (VanTeeffelen et al. 2005b). Together with the lack of effect of BK and SNP on capillary tube haematocrit and glycocalyx exclusion in the hyperlipidemic mice in the present study, the latter finding in the canine heart also suggests that the capability for agonist-induced modulation of the glycocalyx is substantially lost in the case of glycocalyx perturbation. Glycocalyx perturbation, as has been demonstrated during hyperglycaemic and atherogenic conditions in patients and animal models (Constantinescu et al. 2001; Nieuwdorp et al. 2006; Van Teeffelen et al. 2007), may accordingly be associated with an impairment of the ability of vasoactive substances to recruit glycocalyx volume, and together with a loss of shear-mediated vasodilatation (VanTeeffelen et al. 2007), contribute to a deterioration of perfusion regulation.
Conclusion
In conclusion, the present data show that both bradykinin and sodium nitroprusside administration onto cremaster muscles of healthy C57Bl/6 mice robustly increase capillary tube haematocrit as well as the accessibility of the glycocalyx domain for FITC-dextran 70. In contrast, vasodilator modulation of tube haemotocrit and glycocalyx exclusion is abolished in capillaries with signs of glycocalyx degradation (hyperlipidemic mice). Our data suggest that vasodilator substances can increase functionally perfused capillary volume by effectively reducing glycocalyx exclusion. Modulation of blood-excluding glycocalyx volume in capillaries by vasodilator substances in parallel to relaxation of resistance vessels may enable coordination of substrate delivery and exchange.
Acknowledgments
The authors would like to thank Professor Dr L. M. Havekes for granting permission to use ApoE3-Leiden mice in the current experiments. This work was supported by the Netherlands Organization for Scientific Research (NWO Grant 902–16-192) and the Netherlands Heart Foundation (NHS Grant 2005T073 and 2003B181).
References
- Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992;445:473–486. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol. 2001;280:H1051–H1057. doi: 10.1152/ajpheart.2001.280.3.H1051. [DOI] [PubMed] [Google Scholar]
- Damiano ER. The effect of the endothelial-cell glycocalyx on the motion of red blood cells through capillaries. Microvasc Res. 1998;55:77–91. doi: 10.1006/mvre.1997.2052. [DOI] [PubMed] [Google Scholar]
- Damon DH, Duling BR. Distribution of capillary blood flow in the microcirculation of the hamster: an in vivo study using epifluorescent microscopy. Microvasc Res. 1984;27:81–95. doi: 10.1016/0026-2862(84)90043-8. [DOI] [PubMed] [Google Scholar]
- Damon DH, Duling BR. Evidence that capillary perfusion heterogeneity is not controlled in striated muscle. Am J Physiol Heart Circ Physiol. 1985;249:H386–H392. doi: 10.1152/ajpheart.1985.249.2.H386. [DOI] [PubMed] [Google Scholar]
- Damon DH, Duling BR. Are physiological changes in capillary tube hematocrit related to alterations in capillary perfusion heterogeneity? Int J Microcirc Clin Exp. 1987;6:309–319. [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Microvessel hematocrit: measurement and implications for capillary oxygen transport. Am J Physiol Heart Circ Physiol. 1987;252:H494–H503. doi: 10.1152/ajpheart.1987.252.3.H494. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol Heart Circ Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, Padilla DJ, Musch TI, Poole DC. Temporal profile of rat skeletal muscle capillary haemodynamics during recovery from contractions. J Physiol. 2006;573:787–797. doi: 10.1113/jphysiol.2006.104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HL, Cokelet GR, Gaehtgens P. Robin Fahraeus: evolution of his concepts in cardiovascular physiology. Am J Physiol Heart Circ Physiol. 1989;257:H1005–H1015. doi: 10.1152/ajpheart.1989.257.3.H1005. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol Heart Circ Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol. 1979;237:H481–H490. doi: 10.1152/ajpheart.1979.237.4.H481. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- Murdock RC, Reynolds C, Sarelius IH, Waugh RE. Adaptation and survival of surface-deprived red blood cells in mice. Am J Physiol Cell Physiol. 2000;279:C970–C980. doi: 10.1152/ajpcell.2000.279.4.C970. [DOI] [PubMed] [Google Scholar]
- Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- Platts SH, Duling BR. Adenosine A3 receptor activation modulates the capillary endothelial glycocalyx. Circ Res. 2004;94:77–82. doi: 10.1161/01.RES.0000108262.35847.60. [DOI] [PubMed] [Google Scholar]
- Platts SH, Linden J, Duling BR. Rapid modification of the glycocalyx caused by ischemia-reperfusion is inhibited by adenosine A2A receptor activation. Am J Physiol Heart Circ Physiol. 2003;284:H2360–H2367. doi: 10.1152/ajpheart.00899.2002. [DOI] [PubMed] [Google Scholar]
- Pries AR, Ley K, Gaehtgens P. Generalization of the Fahraeus principle for microvessel networks. Am J Physiol Heart Circ Physiol. 1986;251:H1324–H1332. doi: 10.1152/ajpheart.1986.251.6.H1324. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW. Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol. 2005;289:H2657–H2664. doi: 10.1152/ajpheart.00297.2005. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P. Microvascular blood flow resistance: role of endothelial surface layer. Am J Physiol Heart Circ Physiol. 1997;273:H2272–H2279. doi: 10.1152/ajpheart.1997.273.5.H2272. [DOI] [PubMed] [Google Scholar]
- Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- Sarelius IH, Duling BR. Direct measurement of microvessel hematocrit, red cell flux, velocity, and transit time. Am J Physiol Heart Circ Physiol. 1982;243:H1018–H1026. doi: 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- Secomb TW, Hsu R, Pries AR. A model for red blood cell motion in glycocalyx-lined capillaries. Am J Physiol Heart Circ Physiol. 1998;274:H1016–H1022. doi: 10.1152/ajpheart.1998.274.3.H1016. [DOI] [PubMed] [Google Scholar]
- Secomb TW, Hsu R, Pries AR. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am J Physiol Heart Circ Physiol. 2001;281:H629–H636. doi: 10.1152/ajpheart.2001.281.2.H629. [DOI] [PubMed] [Google Scholar]
- Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Tyml K, Cheng L. Heterogeneity of red blood cell velocity in skeletal muscle decreases with increased flow. Microcirculation. 1995;2:181–193. doi: 10.3109/10739689509146766. [DOI] [PubMed] [Google Scholar]
- van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285:H2848–H2856. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- van Haaren PM, VanBavel E, Vink H, Spaan JA. Charge modification of the endothelial surface layer modulates the permeability barrier of isolated rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2005;289:H2503–H2507. doi: 10.1152/ajpheart.00587.2005. [DOI] [PubMed] [Google Scholar]
- Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17:101–105. doi: 10.1016/j.tcm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Brands J, Jansen C, Spaan JA, Vink H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension. 2007;50:261–267. doi: 10.1161/HYPERTENSIONAHA.107.089250. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Constantinescu AA, Vink H, Spaan JA. Hypercholesterolemia impairs reactive hyperemic vasodilation of 2A but not 3A arterioles in mouse cremaster muscle. Am J Physiol Heart Circ Physiol. 2005a;289:H447–H454. doi: 10.1152/ajpheart.01298.2004. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Dekker S, Fokkema DS, Siebes M, Vink H, Spaan JA. Hyaluronidase treatment of coronary glycocalyx increases reactive hyperemia but not adenosine hyperemia in dog hearts. Am J Physiol Heart Circ Physiol. 2005b;289:H2508–H2513. doi: 10.1152/ajpheart.00446.2005. [DOI] [PubMed] [Google Scholar]
- van Vlijmen BJ, van den Maagdenberg AM, Gijbels MJ, van der Boom H, HogenEsch H, Frants RR, Hofker MH, Havekes LM. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J Clin Invest. 1994;93:1403–1410. doi: 10.1172/JCI117117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar RE, Ghael D, Li M, Bhagat DD, Arrigo LM, Cowman MK, Dweck HS, Rosenfeld L. Nitric oxide degradation of heparin and heparan sulphate. Biochem J. 1997;324:473–479. doi: 10.1042/bj3240473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- Vink H, Stace TM, Damiano ER. High resolution 3D intravital fluorescence microscopy reveals partial exclusion of anionic tracers within a 1 micron thick capillary endothelial cell glycocalyx. FASEB J. 2003;17:A70–A70. [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]