Abstract
We have shown previously that a maternal junk food diet during pregnancy and lactation plays a role in predisposing offspring to obesity. Here we show that rat offspring born to mothers fed the same junk food diet rich in fat, sugar and salt develop exacerbated adiposity accompanied by raised circulating glucose, insulin, triglyceride and/or cholesterol by the end of adolescence (10 weeks postpartum) compared with offspring also given free access to junk food from weaning but whose mothers were exclusively fed a balanced chow diet in pregnancy and lactation. Results also showed that offspring from mothers fed the junk food diet in pregnancy and lactation, and which were then switched to a balanced chow diet from weaning, exhibited increased perirenal fat pad mass relative to body weight and adipocyte hypertrophy compared with offspring which were never exposed to the junk food diet. This study shows that the increased adiposity was more enhanced in female than male offspring and gene expression analyses showed raised insulin-like growth factor-1 (IGF-1), insulin receptor substrate (IRS)-1, vascular endothelial growth factor (VEGF)-A, peroxisome proliferator-activated receptor-γ (PPARγ), leptin, adiponectin, adipsin, lipoprotein lipase (LPL), Glut 1, Glut 3, but not Glut 4 mRNA expression in females fed the junk food diet throughout the study compared with females never given access to junk food. Changes in gene expression were not as marked in male offspring with only IRS-1, VEGF-A, Glut 4 and LPL being up-regulated in those fed the junk food diet throughout the study compared with males never given access to junk food. This study therefore shows that a maternal junk food diet promotes adiposity in offspring and the earlier onset of hyperglycemia, hyperinsulinemia and/or hyperlipidemia. Male and female offspring also display a different metabolic, cellular and molecular response to junk-food-diet-induced adiposity.
Obesity and related disorders are on the rise in many countries worldwide. The rate is higher in women than men and populations are affected at an increasingly earlier age (WHO, 2003). The large scale increase in obesity over the past few decades is generally attributed to a change in diet combined with a more sedentary lifestyle. People consume increasing proportions of ‘away-from-home’ foods with nearly half of the food budget being spent in restaurants in the USA (FDA, 2004). Manufactured foods are often industrially processed, contain high levels of fat, sugar and salt to increase palatability and sales, and despite being dense in energy, they can be less nutritious in terms of vitamins and essential nutrients than wholesome ‘homemade’ foods; therefore, they are often qualified as ‘junk food’. The widespread and easy access to junk food is generally implicated in the obesity rise both in children and adults but little is known about the influence of such a maternal junk food diet during pregnancy and lactation on the offspring's development and growth. We have developed an animal model to examine this issue in rats using energy-dense palatable processed foods rich in fat, sugar and salt, designed for human consumption. We have demonstrated that offspring exposed to such a maternal junk food diet during their fetal and suckling lives developed exacerbated overeating and overweight gain by the end of adolescence (Bayol et al. 2007). We have also shown that weanling pups born to mothers fed the junk food diet in pregnancy and lactation exhibited increased adiposity characterized by adipocyte hypertrophy accompanied by accumulation of lipids in skeletal muscle; arguably an early sign of metabolic disruption (Bayol et al. 2005). In the present study, we aim at further characterizing the longer term influence of a maternal junk food diet on adiposity in both male and female offspring at the end of adolescence (10 weeks postpartum) and examine the expression of several genes involved in adipocyte growth and function to bring some insight into the molecular mechanisms involved.
The importance of white adipose tissue for the safe storage of fat is clearly illustrated in a transgenic mouse study showing that mice which do not produce adipose tissue accumulate lipids in other organs such as liver and skeletal muscle and develop insulin resistance and type 2 diabetes shortly after birth (Moitra et al. 1998; Friedman, 2002). However, increased adiposity is also implicated in the metabolic syndrome and among all fat depots, visceral adiposity plays an essential role in the development of insulin resistance and type 2 diabetes (Gabriely & Barzilai, 2003).
White adipocytes are undetectable in embryonic rodents and pre-adipocytes can differentiate into mature lipid-filled adipocytes throughout life into old age (Ailhaud et al. 1992). Adipocyte proliferation and differentiation is controlled by a number of factors. Insulin-like growth factor-1 (IGF-1) promotes pre-adipocyte proliferation while their differentiation is modulated by the insulin receptor substrate (IRS)-1 downstream of the insulin (IR) and IGF-1 (IGF-1R) receptors (Holly et al. 2006). IRS-1 links to the phosphatidyl inositol kinase 3 (PI-3 kinase) pathway and induces transcription factors such as peroxisome proliferator-activated receptor-γ (PPARγ) to promote adipocyte differentiation and lipid synthesis (Miki et al. 2001). Angiogenesis is also essential for adipocyte differentiation and the vascular endothelial growth factor (VEGF) plays a key role in regulating this process (Nishimura et al. 2007). As well as promoting pre-adipocyte proliferation, IGF-1 can also alter the secretion of adipokines such as adiponectin and leptin into the bloodstream. Adiponectin acts via the AMP kinase on skeletal muscle to promote insulin sensitivity and its expression is increased by IGF-1 and PPARγ (Berger, 2005; Holly et al. 2006). On the contrary, IGF-1 reduces leptin expression and secretion into the bloodstream (Boni-Schnetzler et al. 1996; Bianda et al. 1997). Leptin is a well characterized regulator of appetite and energy balance. It acts as a ‘sensor’ of adipose tissue mass and inhibits feeding while increasing thermogenesis (Jequier, 2002). The serine protease adipsin is also secreted by adipocytes into the bloodstream. Its exact function is unclear but its expression is increased following glucose infusion accompanied by increased fat mass (Flier et al. 1987) and it may be involved in triglyceride storage (Van Harmelen et al. 1999).
White adipose tissue stores energy in the form of triglycerides. These triglycerides either originate from the etherification of free fatty acids following hydrolysis of dietary fats by lipoprotein lipase (LPL) (Mead et al. 2002) or the conversion of glucose via the pentose and malate cycles (Flatt, 1970). Dietary triglycerides are transported from the digestive system into the bloodstream following packaging into chylomicrons. In white adipose tissue, LPL catalyses the hydrolysis of chylomicrons leading to the release of non-esterified free fatty acids which are then taken up by adipocytes and are re-esterified for storage as triglycerides (Mead et al. 2002). The chylomicron remnants are then transported to the liver where they contribute to the formation of cholesterol (Mead et al. 2002). The transfer of glucose into adipocytes is mostly directed by the insulin-dependant glucose transporter (Glut) 4 but Glut 1 and Glut 3 are also involved (Hainault et al. 1991; Trayhurn et al. 2006). All of these factors have been shown to play a role in adipose tissue growth and/or in the molecular control of glucose and lipid homeostasis. However, changes in the rate of transcription of these factors in the context of early life exposure to a junk food diet have not been characterized.
Therefore, the overall aim of the present study is to examine the cellular and molecular response of the perirenal fat pad, namely a major visceral fat pad present both in males and females, from rat offspring exposed to a junk food diet at various stages of growth from conception till the end of adolescence. This is to determine whether a maternal junk food diet in pregnancy and lactation can influence adiposity in the offspring. This study also aims at highlighting sex differences in the offspring's response to junk-food-diet-induced adiposity.
Methods
Ethical considerations
All animal work was approved by the Royal Veterinary College Ethics and Welfare committee and was carried out under Home Office licence to comply with the UK Animals (Scientific Procedures) Act 1986.
Animals
The animals used in this study were the same as some used previously, therefore a detailed account of the experimental procedure has been published elsewhere (Bayol et al. 2007). Briefly, 24 virgin female Wistar rats purchased from Charles River (Margate, Kent, UK) were mated with Wistar males in wire-bottomed cages. On the day a copulation plug was found they were isolated and assigned to one of four nutritional groups as shown in Table 1. At birth, litters which contained between 10–16 pups were kept in the study while outsized litters were discarded in order to standardize litter sizes. At weaning (21 days postpartum) three males and three females from each litter were housed in groups of three such that the male littermates were separated from the females, and were allowed to grow until 10 weeks of age. Therefore, 144 animals were analysed in total, giving 36 animals in each nutritional group consisiting of 18 males and 18 females in each group.
Table 1.
Experimental design: type of diet given during gestation, lactation and post-weaning up to 10 weeks of age and the corresponding group names
| Group name | Gestation | Lactation | Post-weaning |
|---|---|---|---|
| CCC | Chow | Chow | Chow |
| CCJ | Chow | Chow | Junk food |
| JJC | Junk food | Junk food | Chow |
| JJJ | Junk food | Junk food | Junk food |
The experiment was split into three growth phases, namely, gestation, lactation and post-weaning, during which the animals were fed either a control (C) or a junk food (J) diet. The control diet consisted of standard rodent chow RM3 given ad libitum (SDS Ltd, Betchworth, Surrey, UK) while the junk food diet consisted of RM3 plus eight types of palatable processed foods designed for human consumption all given ad libitum. The processed food items included biscuits, chocolate, doughnuts, muffins, potato crisps, sweets and cheese; detailed information about the nutritional value and ingredients of all foods used in the model has been published elsewhere (Bayol et al. 2007). At the end of the study (10 weeks postnatal), food was removed 2 h prior to kill to stabilize blood glucose and other metabolites and the animals were culled by rising concentration of CO2.
Serum biochemistry
Blood samples were collected immediately after kill following section of the aorta. Glucose levels were immediately measured from whole blood using the One Touch Ultra glucose meter (LifeScan, Buckinghamshire, UK). After collection, the blood samples were left to clot on ice for at least 15 min before centrifugation. Serum samples were stored at −80°C and were subsequently analysed for insulin, triglyceride and cholesterol content by the Diagnostics Laboratory Services at the Royal Veterinary College, London, UK.
Histological analysis
After kill, the perirenal fat depots were dissected out and weighed. Half of each depot was fixed in buffered formalin (BDH, UK) and stored at room temperature until processing for wax embedding, while the other half was flash frozen in liquid nitrogen and stored at −80°C for RNA extraction. The formalin-fixed fat pads were processed for histological analysis as previously described (Bayol et al. 2005). The sections were graphically analysed using the Kontron image analysis software (Zeiss, Germany) to determine adipocyte areas and numbers. These measurements were taken in five different microscopic frames chosen randomly such that approximately 160–460 cells were measured from each sample depending on adipocyte size and density.
Total RNA purification
Adipose tissue was homogenized in Tri Reagent (Sigma, UK) followed by chloroform extraction and ethanol precipitation. Precipitated RNA was then loaded onto Qiagen RNeasy columns (Qiagen, Crawley, UK) for DNase treatment and further purification. Total RNA was eluted with Sigma Pure water (Sigma, UK) before spectrophotometric measurement of concentration and purity using the Nanodrop N-1000 system (Nanodrop Technologies, Wilmington, DE, USA). Total RNA integrity was verified by formaldehyde gel electrophoresis ensuring that the 18S and 28S ribosomal RNA bands were intact under UV light.
Reverse transcription and real-time PCR
The protocol used for the reverse transcription and real-time PCR has been published elsewhere (Bayol et al. 2005). The reverse transcription and subsequent real-time PCR were carried out simultaneously on all samples using the same master-mix to prevent variability in the reverse transcription and amplification efficiency between samples.
One microgram of total RNA from each sample was reverse transcribed in a 20 μl reaction volume using the Quantitect Reverse Transcription kit (Qiagen) according to manufacturers' instructions.
All primers used for the real-time PCR and accession numbers for the mRNAs studied are listed in Table 2. The primers were designed using the Primer-3 Web-Software (Whitehead Institute for Biomedical Research, MA, USA) and synthesized by MWG-Biotech (Germany). The real-time PCR was based on SyBR green detection (Qiagen) and was performed using the Chromo-4 thermal cycler (MJ Research Inc., MA, USA; now owned by Bio-Rad, Herts, UK) with 2 μl cDNA product from the reverse transcription reaction following manufacturers' instructions. The relative concentrations of the target amplicons were calculated by the thermal cycler's software (Bio-Rad) from a standard curve created with duplicate serial dilutions of standard DNA (target sequence of interest). The standard curve was also used to verify the linearity of amplification of each transcript and r > 0.99 in all cases. The relative concentrations of target sequences in each run were expressed as numbers of copies and were normalized to 1 μg of total RNA as previously reported (Hameed et al. 2003; Bayol et al. 2004, 2005). All PCR products were checked for specificity and purity from a melting curve profile performed by the thermal cycler software created at the end of each run. Homology of the PCR products with the mRNAs of interest was further checked for size by agarose gel electrophoresis.
Table 2.
Primers used in the real time PCR in the 5′ to 3′ direction
| Target RNA | Forward | Reverse | Target length | Access number |
|---|---|---|---|---|
| IGF-1 | gcttgctcacctttaccag | aagtgtacttccttctgagtct | 300 | M17335 |
| IGF-1R | catgcaggagtgtccatcag | ctcgccggatgttaataagc | 194 | NM_052807 |
| IR | atctcctgggattcatgctg | tactgggtccagggtttgag | 196 | M29014 |
| IRS-1 | tcttggaatgtggaactgagg | tccagaaccttctatggcact | 162 | NM_012969 |
| VEGF-A | caatgatgaagccctggagt | tttcttgcgctttcgttttt | 211 | NM_031836 |
| PPARγ | ccctggcaaagcatttgtat | actggcacccttgaaaaatg | 222 | AB011365 |
| Leptin | tgacaccaaaaccctcatca | tagactgccagggtctggtc | 159 | NM_013076 |
| Leptin receptor | aacctgtgaggatgagtgtcagagt | ccttgctcttcatcagtttcca | 92 | AF287268 |
| Adiponectin | acccaaggaaacttgtgcag | catctcctgggtcaccctta | 155 | NM_144744 |
| Adipsin | cctacatggcttcagtgcaa | ccgggtgaagcactacactt | 204 | M92059 |
| Glut 1 | ctttgtgtctgccgtgctta | cacatacatgggcacaaagc | 124 | NM_138827 |
| Glut 3 | cgagagtccaaggttcttgc | tcctggatctcctggatcac | 105 | NM_017102 |
| Glut 4 | cttgggttgtggcagtgag | aggaccagtgtcccagtcac | 217 | D28561 |
| LPL | cttcaaccacagcagcaaaa | ggcccgatacaaccagtcta | 148 | NM_012598 |
Statistical analyses
All statistical analyses were performed as previously described (Bayol et al. 2007) using the SPSS 14.0 for Windows software (SPSS Inc., Chicago, IL, USA). Briefly, data were analysed by hierarchical two-way ANOVA following graphical verification of normal distribution and homogeneity of variances. Results for leptin and adipsin mRNA expression were not normally distributed and were therefore log transformed prior to analysis. The fixed factors were defined as dietary ‘group’ and ‘sex’ while ‘mother’ was defined as random factor. The models were set as ‘group’, ‘sex’, ‘mother (group)’ and ‘group × sex’ such that the statistical analyses were performed on individual offspring while taking the litter effect into account, namely, that some individuals came from the same mothers. For all variables measured (biochemistry, histology and real time-PCR parameters) the two-way ANOVA indicated interactions between ‘group’ and ‘sex’ (‘group × sex’, P < 0.05, n = 36 animals in each group), therefore the data files were split into sexes (n = 18 for each group) and the one-way ANOVA test was used to further analyse differences within the male population independently from the females. When the one-way ANOVA indicated statistical differences among the four dietary groups (P < 0.05), either the Tukey honestly significantly different (HSD) or the Games–Howell post hoc test was performed depending on whether equal variances should be assumed as indicated by the Levene's test for homogeneity of variances (P > 0.05 or P < 0.05, respectively). Results were considered statistically significant when P < 0.05.
Two-tailed Pearson's correlation analyses were also performed on some occasions to determine possible correlations between the various parameters measured. Correlations were considered as ‘strong’ when Pearson's 0.50 < r < 1.0.
Results
Serum biochemistry
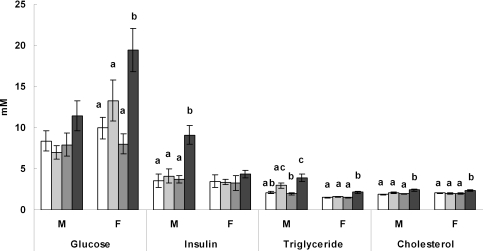
Results in Fig. 1 show that circulating glucose and insulin were differently affected in male and female offspring fed the junk food diet throughout the study (JJJ group). Namely, glucose levels were comparable among male offspring but were raised in females from the JJJ group compared with all other groups (P < 0.05 in all cases). In contrast, circulating insulin levels were raised in male offspring from the JJJ group compared with all other nutritional groups (P < 0.01 in all cases) but were unaffected in females. Levels of glucose and insulin were comparable between the CCC, CCJ and JJC groups in both male and female offspring, thus these parameters were only affected in offspring fed the junk food diet throughout the study. Results in Fig. 1 also show that circulating triglycerides were higher in the JJJ group compared with both the CCC and JJC groups (P < 0.01) but not with the CCJ group in males and were higher in the JJJ group compared with all other groups in females (P < 0.01 in all cases). Cholesterol levels were higher in both male and female offspring from the JJJ group compared with all other nutritional groups (P < 0.01 in all cases).
Figure 1. Serum biochemistry.
Circulating glucose, insulin, triglyceride and cholesterol levels in male (M) and female (F) rat offspring from the various dietary groups. Keys: open bar: CCC group, chow throughout; lightly shaded bar: CCJ group, chow in pregnancy and lactation followed by junk food diet after weaning; darkly shaded bar: JJC group, junk food diet in pregnancy and lactation followed by chow after weaning; black bar: JJJ group, junk food diet throughout. Results are mean ± s.e.m. Different letters indicate statistical differences (P < 0.05) by hierarchical two-way ANOVA followed by post hoc analyses with n = 18 males and n = 18 females in each nutritional group.
Histology
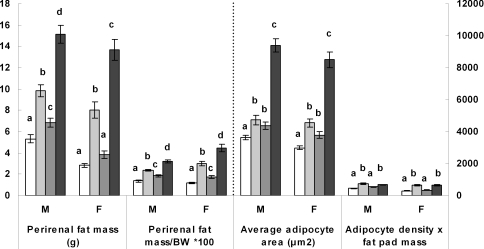
Figure 2 shows that both male and female offspring from the JJJ group displayed increased perirenal fat pad mass compared with offspring from all other groups and this was also true when the fat pad mass was expressed relative to body weight (P < 0.001 in all cases). The increased perirenal fat pad mass relative to body weight in the JJJ group compared with the CCC group was greater in female (260%) than in male (133%) offspring. Male and female offspring given free access to junk food after weaning alone, namely the CCJ group, also exhibited increased perirenal fat pad mass as well as increased fat mass relative to body weight compared with the CCC group (P < 0.001 for both sexes) but these adiposity parameters were lower in this group compared with the JJJ group (P < 0.001 for both sexes). The fat mass relative to body weight was 33% and 46% higher in the male and female offspring from the JJJ group, respectively, compared with the CCJ group, showing that female offspring accumulated more fat than males when mothers were fed junk food during gestation and lactation. Sex differences were also observed in offspring fed junk food after weaning alone, namely, when comparing the CCJ group with the CCC group, with perirenal fat mass relative to body weight being increased by 75% and 147% in male and female offspring, respectively. It is also important to note that offspring fed a maternal junk food diet in pregnancy and lactation but switched to rodent chow alone after weaning, namely the JJC group, also exhibited increased adiposity relative to body mass compared with offspring never exposed to the junk food diet, namely, the CCC group (P < 0.05 for both sexes); this increase was 37% and 43% for males and females, respectively.
Figure 2. Histological data.
Perirenal fat pad mass, perirenal fat pad mass relative to body weight (BW), average adipocyte area and (adipocyte area × fat pad mass) in male (M) and female (F) offspring from the various dietary groups. Keys: open bar: CCC group, chow throughout; lightly shaded bar: CCJ group, chow in pregnancy and lactation followed by junk food diet after weaning; darkly shaded bar: JJC group, junk food diet in pregnancy and lactation followed by chow after weaning; black bar: JJJ group, junk food diet throughout. Results are mean ± s.e.m. Different letters indicate statistical differences (P < 0.05) by hierarchical two-way ANOVA followed by post hoc analyses with n = 18 males and n = 18 females in each nutritional group.
To further examine the cellular nature of the increased adipose tissue mass, we measured the average adipocyte area and estimated the number of mature adipocytes in each fat pad by multiplying adipocyte density by fat pad mass as previously described (Bayol et al. 2005). Results in Fig. 2 show that the average adipocyte area was increased in both male and female offspring from the JJJ group compared with all other groups and this increase was 158% and 183% for males and females, respectively, compared with the CCC group (P < 0.001 for both sexes). The average adipocyte area was also increased in the CCJ and JJC groups compared with the CCC group (P < 0.05 for both) and was comparable between the CCJ and JJC groups. The estimated number of mature adipocytes in each fat pad was comparable in both male and female offspring fed the junk food diet after weaning, namely the CCJ and JJJ groups, and was increased in these two groups compared with those fed chow alone after weaning namely CCC and JJC groups (P < 0.05 in all cases). The CCJ and JJC groups had comparable numbers of mature adipocytes.
Taken together these results indicate an increased adiposity, namely either increased perirenal fat mass per se or relative to body weight, in offspring fed the junk food diet throughout the study compared with all other offspring including those fed junk food after weaning alone. There was also increased adiposity relative to body weight in offspring exposed to the maternal junk food diet in gestation and lactation compared with those from mothers fed the balanced chow diet throughout the study.
Gene expression analysis
Adipocyte growth and differentiation
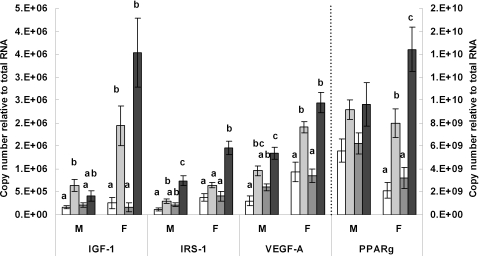
In male offspring, IGF-1 mRNA was expressed at higher levels in the CCJ group than in the CCC and JJC groups (P = 0.010 and P = 0.027, respectively) but levels in the JJJ group were comparable to all other groups indicating that male offspring fed the junk food diet throughout the study did not exhibit higher IGF-1 levels in their perirenal fat pads (Fig. 3). Female offspring weaned on junk food, namely the CCJ and JJJ groups exhibited a marked increase in IGF-1 mRNA levels compared with those weaned on chow alone, namely the CCC and JJC groups (P < 0.01 in all cases) but differences between the JJJ and CCJ groups did not reach statistical significance (P = 0.28) (Fig. 3). An attempt was made to measure the mRNA expression of IGF-1R and IR but levels were so low that accurate quantification was not possible using the protocol described.
Figure 3. Transcriptional analysis of genes that regulate adipocyte proliferation, differentiation and growth.
Real-time PCR analysis of IGF-1, IRS-1, VEGF-A and PPARγ mRNA expression in male (M) and female (F) offspring from the various dietary groups. Keys: open bar: CCC group, chow throughout; lightly shaded bar: CCJ group, chow in pregnancy and lactation followed by junk food diet after weaning; darkly shaded bar: JJC group, junk food diet in pregnancy and lactation followed by chow after weaning; black bar: JJJ group, junk food diet throughout. Results are mean ± s.e.m. Different letters indicate statistical differences (P < 0.05) by hierarchical two-way ANOVA followed by post hoc analyses with n = 18 males and n = 18 females in each nutritional group.
Figure 3 also shows that IRS-1 levels were increased in both male and female offspring exposed to the junk food diet throughout the study (JJJ) compared with all other groups (P < 0.01 for all cases). In male offspring, IRS-1 levels were higher in the CCJ than in the CCC group (P = 0.03) but this difference did not reach statistical significance in females (P = 0.08). IRS-1 mRNA expression was comparable between the CCC and JJC groups in both male and female offspring.
There was a marked up-regulation of VEGF-A mRNA in female offspring weaned on junk food, namely the CCJ and JJJ groups, compared with those weaned on chow alone, namely the CCC and JJC groups (P < 0.01 in all cases), but levels between the CCJ and JJJ groups and between the CCC and JJC were comparable (Fig. 3). In males, changes in VEGF-A expression were not as marked as in females. Male offspring from the JJJ group expressed higher levels of VEGF-A than those weaned on chow alone, namely the CCC and CCJ groups (P < 0.01). The difference between the CCJ and JJJ group and between the CCC and JJC groups did not reach statistical significance (P = 0.122 and P = 0.054, respectively).
Variation in PPARγ mRNA expression did not reach statistical significance in male offspring (P = 0.07). In females, PPARγ was expressed at markedly higher levels in those weaned on junk food (CCJ and JJJ groups), compared with those weaned on chow alone, namely the CCC and JJC groups (P < 0.05 for all cases). Levels were also higher in the JJJ group compared with the CCJ group (P = 0.04) but they were comparable between the CCC and JJC groups (Fig. 3).
Overall, results in Fig. 3 show that the changes in the expression of genes involved in adipocyte proliferation and differentiation were more marked in female than male offspring weaned on junk food which is consistent with the greater increase in perirenal fat mass per body weight in females. The differences between the JJJ and CCJ group did not always fall within statistical significance.
Adipokines and adipsin
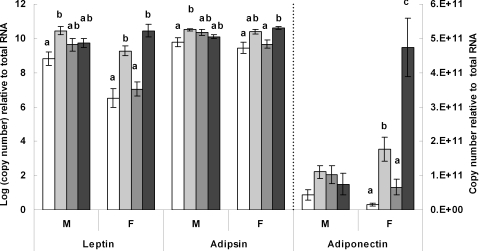
Results in Fig. 4 show that leptin mRNA expression was higher in male offspring from the CCJ group compared with the CCC group (P < 0.01) but was comparable in all other groups indicating that the maternal junk food diet did not have long-term effects on leptin expression in the perirenal fat pad of male offspring. In females, leptin was expressed at higher levels in those weaned on the junk food diet, namely, the CCJ and JJJ groups compared with those weaned on chow alone, namely the CCC and JJC groups (P < 0.01, in all cases). Leptin levels were comparable in the CCC and JJC groups as well as in the CCJ and JJJ groups suggesting that the maternal diet had little effect on long-term leptin expression.
Figure 4. Transcriptional analysis of adipokines and adipsin.
Real-time PCR analysis of leptin, adipsin and adiponectin mRNA expression in male (M) and female (F) offspring from the various dietary groups. Keys: open bar: CCC group, chow throughout; lightly shaded bar: CCJ group, chow in pregnancy and lactation followed by junk food diet after weaning; darkly shaded bar: JJC group, junk food diet in pregnancy and lactation followed by chow after weaning; black bar: JJJ group, junk food diet throughout. Results are mean ± s.e.m. Different letters indicate statistical differences (P < 0.05) by hierarchical two-way ANOVA followed by post hoc analyses with n = 18 males and n = 18 females in each nutritional group.
Figure 4 also shows that adipsin mRNA expression was higher in the CCJ group compared with the CCC group (P < 0.05) but was comparable among all other groups in male offspring. In females, adipsin levels were higher in the JJJ group compared with both the CCC and JJC groups (P < 0.05) but were comparable between the JJJ and CCJ groups.
Figure 4 shows that adiponectin mRNA expression was unaffected in male offspring. In females, levels were dramatically increased in the JJJ group compared with all other groups (P < 0.05 in all cases). Adiponectin was also increased in the CCJ group compared with both the CCC and JJC groups (P < 0.05) but there were no differences between the CCC and JJC groups. The expression pattern of adiponectin appeared similar to that of PPARγ, therefore Pearson's correlation analyses were performed. Results showed a strong correlation between PPARγ and adiponectin mRNA expression (r = 0.661, P < 0.01).
Glucose and fatty acid uptake
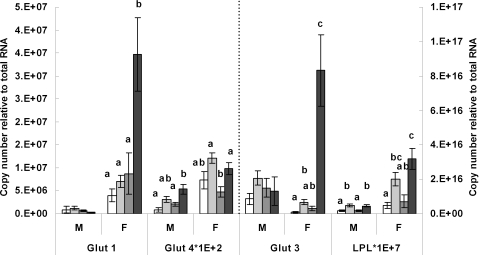
Figure 5 shows no change in Glut 1 mRNA levels in male offspring but a very dramatic increase in females fed the junk food diet throughout the study (JJJ group) when compared with all other groups (P < 0.05, for all cases). A similar pattern of expression was observed for Glut 3. Levels of Glut 3 mRNA were comparable among male offspring but there was a very marked up-regulation in the JJJ females compared with all other groups (P < 0.01). Glut 3 mRNA expression was also raised in the CCJ females compared with the CCC (P = 0.01) but not with the JJC groups (P = 0.31). There was a strong correlation between Glut 1 and Glut 3 mRNA expression (r = 0.607, P < 0.01). The overall expression pattern of Glut 4 was different from that of Glut 1 and Glut 3. Male offspring from the JJJ group expressed more Glut 4 mRNA than those in the CCC and JJC groups (P < 0.05) but levels were comparable to the CCJ group (Fig. 5). In females, Glut 4 mRNA was comparable in the CCC, CCJ and JJJ groups but were lower in the JJC group compared with both the CCJ and JJJ groups (P = 0.05).
Figure 5. Transcriptional analysis of genes that regulate glucose and lipid transport.
Real-time PCR analysis of Glut 1, Glut 3, Glut 4 and LPL mRNA expression in male (M) and female (F) offspring from the various dietary groups. Keys: open bar: CCC group, chow throughout; lightly shaded bar: CCJ group, chow in pregnancy and lactation followed by junk food diet after weaning; darkly shaded bar: JJC group, junk food diet in pregnancy and lactation followed by chow after weaning; black bar: JJJ group, junk food diet throughout. Results are mean ± s.e.m. Different letters indicate statistical differences (P < 0.05) by hierarchical two-way ANOVA followed by post hoc analyses with n = 18 males and n = 18 females in each nutritional group.
Figure 5 also shows that LPL mRNA levels were higher in males weaned on the junk food diet compared with those weaned on chow alone (P < 0.05) and levels were comparable between the CCC and JJC groups as well as between the CCJ and JJJ groups. In females, LPL mRNA levels were higher in the JJJ group compared with the CCC and JJC groups (P < 0.05 for both) but were comparable between the CCJ and JJC groups and between the CCC and JJC groups. LPL mRNA expression was higher in the CCJ group than in the CCC group (P < 0.01).
Discussion
This study was aimed at examining the influence of a maternal junk food diet in pregnancy and lactation on circulating glucose, insulin, triglyceride and cholesterol as well as the associated cellular and molecular adaptations in the perirenal fat pads of the offspring at the end of adolescence (10 weeks postpartum). The perirenal fat pad was chosen because it is a major visceral fat pad present in both male and female rats which is involved in the development of insulin resistance and type 2 diabetes (Gabriely et al. 2002) and therefore plays a role in regulating whole-body glucose homeostasis.
The study was designed to perform three layers of investigation. Firstly, to determine the role of the maternal diet in promoting an earlier onset of symptoms associated with junk-food-diet-induced obesity in offspring. This was achieved by comparing offspring fed the junk food diet in pregnancy, lactation and after weaning (JJJ group) with those fed the junk food only after weaning (CCJ group). Secondly, this study enabled an examination of the irreversible effects of a maternal junk food diet in pregnancy and lactation in offspring which were switched to the balanced chow diet after weaning. Therefore comparisons between the JJC and CCC groups helped determine signs of early life programming of adiposity through a maternal junk food diet. Finally, the study was designed to determine how male and female offspring responded to the junk food diet and thereby determining possible sex differences.
Junk food diet and adiposity: the role of the maternal diet
This study shows that a maternal junk food diet in pregnancy and lactation promoted increased adiposity in offspring given free access to junk food after weaning. This is illustrated by the finding that the perirenal fat pad mass relative to body weight was greater in both male and female offspring fed the junk food diet throughout the study compared with those fed the junk food diet after weaning alone; this increased adiposity is in line with the excessive weight gain previously reported in the same animals (Bayol et al. 2007). This study also shows that a maternal junk food diet in pregnancy and lactation could also induce a long-term or irreversible increase in adiposity in offspring which were fed chow alone after weaning, as perirenal fat pad mass relative to body weight was increased in the JJC group compared with the CCC group. In a previous study we have shown that weanling pups exposed to the same junk food diet during pregnancy and lactation exhibited increased perirenal fat pad mass at weaning (3 weeks of age) (Bayol et al. 2005). The present study shows that rehabilitation to a chow diet for 7 weeks post-weaning did not reduce adiposity to control levels. Therefore, a maternal junk food diet in pregnancy and lactation induced a lasting increase in adiposity into adulthood when compared with offspring which were never given access to junk food. In all groups where increased adiposity was observed, it was characterized by an increase in average adipocyte area and, as can be expected, this adipocyte hypertrophy was more pronounced in offspring fed the junk food diet throughout the study. However, it is interesting to note that the adipocyte hypertrophy previously reported at weaning in pups exposed to a maternal junk food in pregnancy and lactation (Bayol et al. 2005) persisted up to the end of adolescence in the JJC group compared with the CCC group despite rehabilitation to a chow diet for 7 weeks from weaning.
The number of mature, lipid-filled adipocytes was estimated by multiplying adipocyte density by fat pad mass as previously described (Bayol et al. 2005). Results showed that the number of mature adipocytes was increased in offspring fed the junk food diet after weaning (namely the CCJ and JJJ groups) but not in those fed the junk food in their fetal and suckling lives alone, namely the JJC group. We have reported in a previous study that despite a marked increase in perirenal fat pad mass, the number of mature adipocytes was not changed in weanling pups from mothers fed the junk food diet in pregnancy and lactation (Bayol et al. 2005). Therefore, the present study suggests that the formation of new mature adipocytes may have been more active after weaning. It is important to point out that the histological technique used in this study does not allow the detection of very small adipocytes and pre-adipocytes, thus only mature, lipid-filled adipocytes were counted.
Although both male and female offspring fed the junk food diet throughout the study exhibited increased perirenal fat mass relative to body weight compared with all other groups, this increase was higher in female than in male offspring being 260% and 133%, respectively, compared with the CCC group. This shows that female offspring were prone to greater junk-food-diet-induced adiposity than male littermates and this is also in line with the greater body mass increase in females as previously reported in the same animals (Bayol et al. 2007).
Gene expression and changes in adipose tissue cellularity
In this section, gene expression analyses are discussed in the context of histological observations. It is therefore important to point out that histological data reflect the result of cumulative phenotypic changes which may have occurred throughout the life of the animals while the gene expression data can be more transient and thus more likely to respond to a more immediate stimulus such as the food eaten by the animals around the time of kill.
Adipocyte differentiation and growth is orchestrated by a number of factors and the transcription of some of these has been examined in this study. IGF-1 has been shown to promote pre-adipocyte proliferation (Holly et al. 2006) and in the present study, mRNA levels were markedly up-regulated in all females fed the junk food diet after weaning, while IGF-1 levels were not as dramatically affected in males. A greater increase in IGF-1 transcription may indicate greater pre-adipocyte proliferation in females fed the junk food diet after weaning than in males, which is in line with the greater percentage adiposity observed in females. However, greater pre-adipocyte proliferation cannot be confirmed by the histological analysis method used since it does not allow the detection of pre-adipocytes. Nevertheless, greater pre-adipocyte proliferation would indicate a greater potential for further adipose tissue mass increase in females than males beyond the growth stage examined and into later stages of adulthood. IRS-1 and VEGF-A have both been implicated in adipocyte differentiation (Holly et al. 2006; Nishimura et al. 2007) and results showed increased levels of both factors in male and female offspring exposed to the junk food diet throughout the study (JJJ group) compared with both groups of offspring weaned on chow alone, namely the CCC and JJC groups, while these factors were expressed at either lower (IRS-1) or comparable (VEGF-A) levels in the CCJ group. A greater overall increase in IRS-1 and VEGF-A in the JJJ group may indicate greater adipocyte differentiation in this group and this is in line with the greater adiposity observed in the JJJ group over all other groups. PPARγ is a key regulator of lipid synthesis in adipocytes (Miki et al. 2001) and results showed a marked increase in this factor in females weaned on the junk food diet but not in males. Again, this is in line with a greater percentage of increased adiposity in females. PPARγ has been shown to regulate the expression of adiponectin (Berger, 2005) and the present study shows a strong correlation between the expression profiles of PPARγ and adiponectin which supports a link between the expression of these two factors. Leptin is secreted by adipose tissue into the bloodstream and acts on the brain to reduce appetite; thus, it is sometimes regarded as a ‘sensor’ of fat mass (Jequier, 2002). We have previously reported an examination of the feeding behaviour of the animals used in this study and results showed that both male and female offspring weaned on junk food consumed more energy than those weaned on chow (Bayol et al. 2007). The present study shows that this hyperphagia was also accompanied by increased perirenal fat pad mass in those same offspring. In light of this, one may expect a greater expression of leptin in the white adipose of both male and female offspring weaned on the junk food diet in an attempt to reduce appetite and fat mass but this greater expression was only observed in female offspring and leptin transcription was not markedly affected in males weaned on junk food. Given that leptin is involved in controlling adipose tissue mass and that the percentage increase in fat mass was greater in females than males, this may explain the greater changes in leptin mRNA expression in females; however, the present data do not show a clear link between fat mass, appetite and leptin mRNA expression, particularly in males. Another study has highlighted sex differences in central leptin sensitivity with leptin having a longer term effect on appetite suppression in females, while in males, insulin was more potent at reducing appetite (Clegg et al. 2003). In light of this, the present study shows that male and female offspring fed the junk food diet throughout the study also displayed sex differences in terms of insulin secretion and leptin transcription with males having higher circulating insulin levels, while in females, leptin transcription is increased. These results reinforce the concept that males and females may utilize a different molecular machinery to control appetite and fat mass. However, despite increased circulating insulin in males and leptin expression in females, both male and female offspring in the JJJ group exhibited exacerbated hyperphagia compared with all other groups (Bayol et al. 2007). Therefore, their respective molecular response to the junk food diet was not sufficient to prevent the overeating. Adipsin is also secreted by adipose tissue but its exact function is not clear. Its expression is increased following glucose infusion (Flier et al. 1987) and it may also be involved in triglyceride storage (Van Harmelen et al. 1999). In the present study, despite the marked changes in adiposity among the four nutritional groups examined, there were overall very little changes in adipsin expression, therefore it is difficult to discuss what its biological function might be.
Taken overall, the gene expression profiles of genes that regulate adipocyte growth and differentiation as well as adipokines showed some differences between male and female littermates. Results indicate that the perirenal fat pads of females fed the junk food diet throughout the study were more transcriptionally active than those of males which is consistent with a greater increase in adipose tissue mass in females than males. It is also interesting to note that PPARγ, adiponectin and IRS-1 were sometimes expressed at higher levels in offspring fed the junk food diet throughout the study (JJJ group) when compared with offspring fed junk food after weaning alone (CCJ group). This shows that the maternal junk food diet could have a long-term influence on the mRNA expression of some genes involved in adipocyte growth and differentiation, and in light of results published in a previous study using the same animals (Bayol et al. 2007), this may be caused by the increased appetite and energy intake in the JJJ group.
Sex differences in the perirenal fat pad adaptation to junk-food-diet-induced changes in circulating glucose, insulin and lipids
Serum glucose and insulin profiles were differently affected in male and female offspring fed the junk food diet throughout the study. Males from the JJJ group exhibited normal glycaemia and raised insulinemia while their female littermates exhibited hyperglycaemia and normal insulinemia, showing sex differences in the metabolic response to junk food feeding. Sex differences were not as marked with regards to serum lipids profile. Circulating triglycerides and cholesterol were higher in both male and female offspring fed the junk food diet throughout the study compared with offspring from any other groups with the exception of male triglyceride levels being comparable between the JJJ and CCJ groups. Taken overall, increased glucose, insulin, triglyceride and/or cholesterol in the JJJ offspring show that a maternal junk food diet promoted an earlier onset of junk-food-diet-induced metabolic disruption in offspring.
The marked increase in circulating glucose in females from the JJJ group compared with all other groups was accompanied by a dramatic up-regulation of Glut 1 and Glut 3 mRNA expression in perirenal adipose tissue while Glut 4 expression was relatively little affected. Unlike Glut 1 and Glut 3, Glut 4 actions are largely regulated at the post-transcriptional level; Glut 4 is normally sequestered in the cytoplasm and its translocation to the plasma membrane is controlled by insulin (Hainault et al. 1991; Trayhurn et al. 2006). This may explain why so little variation in Glut 4 mRNA level were observed in the present study. However, this study shows that increased Glut 1 and Glut 3 expression in adipose tissue appears to constitute an important mechanism for the uptake of excess circulating glucose into adipocytes in female offspring. It is interesting to note that the male offspring from the JJJ group, which did not exhibit raised circulating glucose levels, did not show raised Glut 1 and Glut 3 mRNA either. In contrast, males which exhibited raised circulating insulin levels, showed increased insulin-dependant Glut 4 mRNA expression compared with both the CCC and JJC group but these changes did not reach statistical significance when compared with the CCJ group. Overall, the data show that glycaemia and insulinemia were differently affected in male and female offspring fed the junk food diet throughout the study and this was accompanied by sex differences in the expression profile of glucose transporters in the perirenal fat pads, namely, Glut 1 and Glut 3 mRNAs were more actively expressed in females and Glut 4 in males.
Despite increased circulating triglycerides and cholesterol in the offspring fed the junk food diet throughout the study, levels of LPL mRNAs were increased in all male and female offspring weaned on the junk food diet compared with offspring never exposed to junk food. This may indicate an actively increased fatty acid uptake into the perirenal fat depot in all offspring weaned on the junk food diet, which is in line with the greater perirenal fat pad mass in the CCJ and JJJ groups. In a previous study, we showed that all offspring weaned on the junk food diet increased their dietary fat intake compared with those weaned on chow with an exacerbated fat intake in the JJJ offspring compared with the CCJ group (Bayol et al. 2007). The present study shows that this increased dietary fat intake was accompanied by increased circulating triglycerides and cholesterol in the JJJ group but not in the CCJ group; therefore it appears that the peripheral fatty acid uptake was sufficient to maintain normal lipidemia in the CCJ group but not in the JJJ group. Results show that the up-regulation of LPL transcription in the perirenal fat pad was comparable between the CCJ and JJJ group, therefore there were no signs of an attempt to further increase fatty acid uptake via increased LPL transcription in the perirenal fat pads of the JJJ offspring.
Conclusions
The present study shows that junk-food-diet-induced adiposity and associated metabolic disruptions were increased in adult offspring whose mothers had been fed a junk food diet in pregnancy and lactation when compared with offspring only fed the junk food diet after weaning. This study also shows that a maternal junk food diet in pregnancy and lactation can irreversibly promote adiposity in offspring through lasting adipocyte hypertrophy and increased perirenal fat pad mass relative to body weight, even when those offspring were fed exclusively a balanced chow diet after weaning.
The increased adiposity was more enhanced in female than male offspring and this was accompanied by an overall greater transcriptional activity for factors that regulate adipocyte growth and function in the perirenal fat pad of females. The changes in gene expression were also consistent with increased adipocyte proliferation and differentiation as well as increased glucose and lipid uptake from the bloodstream in female offspring fed the junk food throughout the study.
This study further emphasizes the importance of a balanced maternal diet in pregnancy and lactation for the prevention of diet-induced adiposity and associated metabolic disruptions in offspring.
Acknowledgments
The authors wish to thank members of staff of the Biological Services Unit of the Royal Veterinary College for their help and advice with the animal work. They also wish to thank Samantha Farrington for her help with the animal work and specimen collection as well as Helen Smith for her help with the image analysis. The authors also wish to thank Aviva Petrie for her help and advice with the statistical analysis. This work was funded by the Wellcome Trust.
References
- Ailhaud G, Grimaldi P, Negrel R. A molecular view of adipose tissue. Int J Obes Relat Metab Disord. 1992;16:S17–S21. [PubMed] [Google Scholar]
- Bayol S, Jones D, Goldspink G, Stickland NC. The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br J Nutr. 2004;91:331–339. doi: 10.1079/BJN20031070. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol. 2005;567:951–961. doi: 10.1113/jphysiol.2005.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JP. Role of PPARγ, transcriptional cofactors, and adiponectin in the regulation of nutrient metabolism, adipogenesis and insulin action: view from the chair. Int J Obes (Lond) 2005;29:S3–S4. doi: 10.1038/sj.ijo.0802904. [DOI] [PubMed] [Google Scholar]
- Bianda T, Hussain MA, Glatz Y, Bouillon R, Froesch ER, Schmid C. Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med. 1997;241:143–150. doi: 10.1046/j.1365-2796.1997.94101000.x. [DOI] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Gosteli-Peter MA, Moritz W, Froesch ER, Zapf J. Reduced ob mRNA in hypophysectomised rats is not restored by growth hormone (GH), but further suppressed by exogenously administered insulin-like growth factor (IGF) I. Biochem Biophys Res Commun. 1996;225:296–301. doi: 10.1006/bbrc.1996.1169. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- FDA. FDA Talk Paper: 2004 FDA accomplishments. Food and Drug Administration. 2004 http://www.fda.gov/bbs/topics/ANSWERS/2005/ANS01346.html.
- Flatt JP. Conversion of carbohydrate to fat in adipose tissue: an energy-yielding and, therefore, self-limiting process. J Lipid Res. 1970;11:131–143. [PubMed] [Google Scholar]
- Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- Friedman J. Fat in all the wrong places. Nature. 2002;415:268–269. doi: 10.1038/415268a. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Barzilai N. Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep. 2003;3:201–206. doi: 10.1007/s11892-003-0064-3. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Hainault I, Guerre-Millo M, Guichard C, Lavau M. Differential regulation of adipose tissue glucose transporters in genetic obesity (fatty rat). Selective increase in the adipose cell/muscle glucose transporter (GLUT 4) expression. J Clin Invest. 1991;87:1127–1131. doi: 10.1172/JCI115077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly J, Sabin M, Perks C, Shield J. Adipogenesis and IGF-1. Metab Syndr Relat Disord. 2006;4:43–50. doi: 10.1089/met.2006.4.43. [DOI] [PubMed] [Google Scholar]
- Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, Terauchi Y, Kamon J, Kaburagi Y, Matsui J, Akanuma Y, Nagai R, Kimura S, Tobe K, Kadowaki T. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21:2521–2532. doi: 10.1128/MCB.21.7.2521-2532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines – energy regulation from the human perspective. J Nutr. 2006;136:1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem. 1999;274:18243–18251. doi: 10.1074/jbc.274.26.18243. [DOI] [PubMed] [Google Scholar]
- WHO. Obesity and overweight. World Health Organization; 2003. http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/print.html. [Google Scholar]