Abstract
Activity in both muscle spindle endings and cutaneous stretch receptors contributes to the sensation of joint movement. The present experiments assessed whether muscle pain and subcutaneous pain distort proprioception in humans. The ability to detect the direction of passive movements at the interphalangeal joint of the thumb was measured when pain was induced experimentally in four sites: the flexor pollicis longus (FPL), the subcutaneous tissue overlying this muscle, the flexor carpi radialis (FCR) muscle and the subcutaneous tissue distal to the metacarpophalangeal joint of thumb. Tests were conducted when pain was at a similar subjective intensity. There was no significant difference in the ability to detect flexion or extension under any painful or non-painful condition. The detection of movement was significantly impaired when pain was induced in the FPL muscle, but pain in the FCR, a nearby muscle that does not act on the thumb, had no effect. Subcutaneous pain also significantly impaired movement detection when initiated in skin overlying the thumb, but not in skin overlying the FPL muscle in the forearm. These findings suggest that while both muscle and skin pain can disturb the detection of the direction of movement, the impairment is site-specific and involves regions and tissues that have a proprioceptive role at the joint. Also, pain induced in FPL did not significantly increase the perceived size of the thumb. Proprioceptive mechanisms signalling perceived body size are less disturbed by a relevant muscle nociceptive input than those subserving movement detection. The results highlight the complex relationship between nociceptive inputs and their influence on proprioception and motor control.
Our proprioceptive ability to sense the position and movement of limb segments is a prerequisite to enable us to maintain balance, body orientation and coordination of movements. Muscle spindles are considered the most important peripheral receptor involved in the sense of position and movement (e.g. Goodwin et al. 1972; Roll & Vedel, 1982; Gandevia, 1985), although there is evidence to suggest skin (e.g. Edin & Johansson, 1995; Collins et al. 2005) and to a lesser extent joint receptors (Ferrell et al. 1987) also contribute (for review see McCloskey, 1978; Gandevia, 1996; Proske, 2006). Another potential contributor to the sensation of joint position and movement is input related to central motor commands. Recent evidence suggests that such efferent signals bias judgements of joint position (e.g. Saxton et al. 1995; Walsh et al. 2004) even when afferent signals are absent (Gandevia et al. 2006).
While the function of proprioceptive afferents during natural movements has been the subject of many investigations, it still remains unclear how the central processing of proprioceptive signals arising from these afferents changes during pain (Capra & Ro, 2000). Abnormal proprioception is often seen in people with musculoskeletal pain syndromes (e.g. Sainburg et al. 1993; Brumagne et al. 2000; Baker et al. 2002). For example, in patients with cervical pain, reproduction of joint position was impaired (Revel et al. 1991), and pain intensity and reproduction of joint position were improved with therapy (Rogers, 1997). These clinical observations have led to consistent reports that pain disturbs proprioception. However, while some clinical studies have demonstrated a link between proprioceptive impairment and pain, others have failed to do so (e.g. Skinner et al. 1984). In 220 patients with painful osteoarthritis at the knee there was little association between measures of knee position sense and measures of pain and disability (Bennell et al. 2003). Therefore, the clinical evidence remains inconsistent.
Studies of proprioception using experimentally induced pain also have inconsistent links with proprioceptive disturbance in healthy subjects. Some have shown that pain altered movement and posture (e.g. Arendt-Nielsen et al. 1996; Svensson et al. 1997; Blouin et al. 2003; Corbeil et al. 2004) and force matching (Weerakkody et al. 2003). However, at the ankle joint, movement detection thresholds were disturbed only when high-intensity pain was induced simultaneously in an agonist and its antagonist muscle (Matre et al. 2002). In contrast, position sense at the knee was not reduced by pain in the infrapatellar fat pad (Bennell et al. 2005).
If pain does disturb proprioception, there are multiple sites in the central nervous system where nociceptive inputs could alter proprioceptive processing of inputs from muscle, skin and joint. Stimulation of nociceptors may interfere with proprioception at such as convergent sites of afferent inputs in the dorsal horn (e.g. Capra & Ro, 2000), at subcortical somatosensory relay nuclei, and at the sensorimotor cortex (Le Pera et al. 2001; Martin et al. 2007).
The aim of this study was to investigate whether induction of pain from specific muscle and subcutaneous sites distorts proprioception in humans. The interphalangeal joint of the thumb was used as it is flexed by only one muscle, the flexor pollicis longus with its belly in the forearm. Furthermore, this muscle is absent or rudimentary in non-human primates (Straus, 1942) and is important for human manual dexterity. The muscle is easily accessed for injection. Both muscle and skin pain were investigated to uncover whether any disturbance of proprioception from nociceptor activity was general or specific in nature. Hypertonic saline was used to produce pain as this method is safe and generates controllable levels of pain (e.g. Kellgren, 1937; Graven-Nielsen et al. 1998). Therefore, proprioceptive acuity was assessed at the thumb interphalangeal joint when pain was induced experimentally in four sites: the flexor pollicis longus, the subcutaneous tissue overlying this muscle, a nearby muscle which does not act on the thumb, and the subcutaneous area around the joint itself. As pain may also distort the body image (e.g. Ramachandran, 1998; McCabe et al. 2004), including the perceived size of the digits (Gandevia & Phegan, 1999), this aspect of proprioception was also explored.
Methods
The ability to detect the direction of passive movements imposed at the interphalangeal joint of the thumb was measured under control conditions and when pain was generated at four different locations in healthy subjects: the flexor pollicis longus muscle (FPL), a subcutaneous site over the FPL, the flexor carpi radialis muscle (FCR) and a subcutaneous site on the dorsal aspect of the proximal phalanx of the thumb (Fig. 1). Saline solution was injected into a different site in four separate experiments conducted on different days, that were completed in a pseudorandom order. On each day, thresholds movement, which had a displacement and velocity such that the subject could correctly nominate the direction of movement on ∼60% of presentations, was determined. Any decrease in proprioceptive acuity would be expected to decrease the percentage of correct detections of movement direction, whereas an improvement in proprioceptive acuity would increase correct detections (Refshauge et al. 2003; Weerakkody et al. 2007).
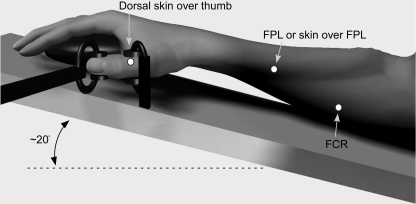
Figure 1. Experimental arrangement used to impose thumb movements and location of hypertonic saline injection sites.
Pain was initiated at 4 locations (dots in figure): the flexor pollicis longus muscle (FPL), a subcutaneous site over FPL (same spot), flexor carpi radialis muscle (FCR), and a subcutaneous site on the dorso-medial aspect of the thumb proximal phalanx. The forearm and hand were supported on a padded splint, which was tilted upward with the forearm at an angle of ∼20 deg.
Subjects
A total of 12 healthy adult subjects participated (9 male, 3 female; aged 22–50 years), with most involved in more than one study. Nine subjects participated in all four experiments, while of the remaining three subjects, one participated in three experiments, and two in two experiments. The total number of subjects in each experiment was: 11 (FPL), 12 (subcutaneous FPL), 10 (FCR) and 10 (subcutaneous thumb). All experiments conformed to the Declaration of Helsinki. Written consent to participate was obtained and the studies were approved by the local Human Research Ethics Committee.
Experimental set-up
The right forearm and hand were supported on a padded splint, which was tilted upward with the forearm at an angle of ∼20 deg to the horizontal so that the thumb remained in the horizontal plane (Fig. 1). The forearm was positioned in pronation with the palm of the hand resting on a fixed mould, shaped as a computer mouse. The metacarpophalangeal joint of the thumb was positioned in the middle of its physiological range (∼45 deg flexion). This posture provided a comfortable rest position for the hand.
Flexion and extension movements were imposed about the interphalangeal joint of the thumb (Fig. 1). The distal phalanx of the thumb was coupled to a linear servomotor under positional feedback, driven by a variable ramp generator. The distal phalanx of the thumb was coupled by a small padded clamp (∼1.5 cm2 in contact area) over the sides of the digit, designed to minimize the disturbance of the skin on the dorsal and palmar surfaces of the thumb. For each subject the clamp was placed 1.5 cm distal to the axis of rotation of the joint. The proximal phalanx of the thumb was stabilized by a similar clamp so that movement was confined to the thumb interphalangeal joint. A barrier was positioned over the hand so that subjects could not see the hand or the apparatus. Measures of actual angular displacement and geometric calculation were used to calibrate the equipment prior to data collection. Subjects were reminded to relax their hand and arm muscles throughout the study.
Experimental pain stimulus
Sterile hypertonic saline (5%) was infused to produce pain felt in the deep tissue. To initiate pain in the FPL, in the skin overlying the FPL, and in the FCR, a bolus injection with 0.2 ml saline was delivered over ∼5 s. For injections delivered over the proximal phalanx of the thumb the initial volume was 0.1 ml. Once the subject reported that the initial pain had reached a plateau, an infusion was carried out by a computer-controlled syringe pump (Graseby, 3100). A tube was connected from the syringe to a catheter (24G, 19 mm) which was inserted before control measurements and which remained for the rest of the recordings (Fig. 2A). A steady infusion rate of 85–170 μl min−1 was given until the trials were completed. The infusion rate was adjusted to maintain the pain at a level of ∼4–6 on a 10 point scale (with 0 being no pain and 10 being maximal pain). Subjects were asked to rate the perceived pain on a visual analog scale by turning a dial marked in steps from 0 to 10 over 300 deg of rotation. The dial was the moving arm of a potentiometer and its output was recorded by computer. The pain intensity reflected the combined effects of the local pain and any referred pain sensations. The peak and average pain rating over the period were measured.
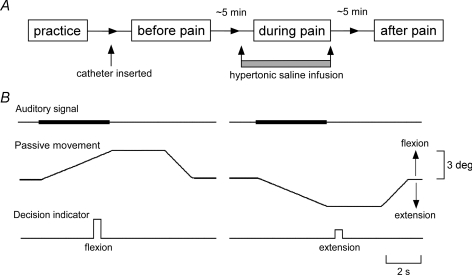
Figure 2. Time course of the experimental protocol and the waveforms and timing of test movements, auditory signals and decision indicators.
A, after preliminary practice a set of test movements was performed before pain (control period), during pain and after pain. The catheter was inserted after the practice period. B, a standard auditory signal was present during the test passive movements (with or without vibration). The finger was then held at its full excursion for 3 s before being returned to the neutral position. Subjects gave responses of either ‘flexion’, ‘extension’ or ‘not sure’.
During the infusion, subjects were instructed to indicate the distribution and region of the pain on small-scale body maps of the lower arm and hand. The average area of the pain distribution was later calculated by digitization and pixel counting. During the pain, subjects were asked at regular intervals between the test movements to indicate the level of pain and to illustrate any changes in its distribution. To check the quality of the experimental pain, subjects completed an adapted short McGill pain questionnaire immediately after the test.
Ten subjects were also asked to estimate the size of their digits by selection of a simple two-dimensional outline or ‘template’ of the digit which best matched its ‘size’ (Gandevia & Phegan, 1999). Templates of thumbs were randomly arranged on sheets. The templates consisted of single-line drawings of the body part, with a range of magnification of the templates of 3.6-fold. Subjects were asked to select the template which best matched the perceived size of the thumb. Subjects were asked to respond within ∼15 s. Eight estimates were made before, during and after pain was introduced into the FPL and skin overlying the FPL. The perceived size of the index finger was also measured using similar sets of templates.
Subjects were always asked whether they felt ‘pins and needles’ (paraesthesiae) during the experiment. Such sensations could be caused if the infusion was being given close to cutaneous nerves or pressure was placed inadvertently on superficial nerves because of the subject's posture. As paraesthesiae could hinder a proprioceptive task, the two subjects who reported paraesthesiae during the test session were re-tested at least a week later, and their original results were discarded.
Standard protocol for movement detection
Flexion and extension movements of ∼1–3 deg were imposed around the thumb interphalangeal joint from an initial position of ∼45 deg flexion. Each movement was held at its full excursion for 3 s (Fig. 2B). Subjects were allowed to nominate the direction of movement during the movement itself or during the subsequent 3 s hold period. However, a response was recorded as a ‘Not sure’ if it was given after the thumb began to return to the initial position. For each subject the velocity of the imposed movements was selected as that at which the subject detected the direction of ∼60% of the control movements. This threshold for detection of the direction of applied movement was found through preliminary trials. The ∼60% detection level was chosen as it was a mid value, so any increase or decrease in detection rates could be observed (Refshauge et al. 2003). The threshold velocities ranged from 0.3 to 1.0 deg s−1. The velocity of the reset movement back to the starting position remained constant (1.25 deg s−1). Subjects received the same instructions before each session. Subjects detected a movement but nominated the wrong direction (false positive) on average only 3% of trials.
A set of test movements consisted of 10 extension and 10 flexion movements in random order. Subjects nominated the direction of movement using a pad with three buttons labelled ‘flexion’, ‘extension’ and ‘not sure’. They were instructed to signal the direction as soon as they were sure and to press ‘not sure’ if unsure of the direction of movement.
After a preliminary practice period, the set of test movements was performed 3 times: before pain (control period), during pain and after pain (Fig. 2A). Measurements were made after the catheter was positioned. Measurements during pain started once subjects reached a plateau pain level that was higher than a level of 2. The final measurements began 5 min after pain decreased to a level of 0.
Data analysis
Initial analyses were performed to determine if the peak pain intensity and distribution of pain varied across the various sites at which hypertonic saline had been injected. For each independent variable, we used one-way repeated measures ANOVAs (four sites – FPL, FCR, subcutaneous FPL and subcutaneous thumb). However, for the perceived thumb size only two sites were tested (FPL and subcutaneous FPL).
A two-way repeated measures ANOVA was used to assess the effects and interactions of pain according to the site (FPL, FCR, subcutaneous FPL and subcutaneous thumb) and time (before, during and after pain). This was done for the nine subjects that participated in all four experiments. In addition, one-way repeated measures ANOVAs (three conditions – before, during and after pain) were used for each dependent variable at each injection site for the full set of data (n = 10–12 subjects). The appropriate main effects were decomposed a posteriori using a HSD Tukey test. Regression analyses were done to assess the relationships of peak pain intensity and pain area with the change in the number of correct detections (before pain compared to during pain). All statistical analyses were performed using Statistica 6.1 (Statsoft, OK, USA) and statistical significance was set at P < 0.05.
Results
Subjects were asked to nominate the direction of passive movements applied at the thumb interphalangeal joint when they could do so with certainty. Pain impaired movement detection, and this effect depended on the site at which pain was initiated, rather than whether it was intramuscular or subcutaneous in origin.
Experimentally induced pain
The characteristics of intramuscular and subcutaneous pain were distinct in most subjects. Infusion of hypertonic saline into the muscle induced a deep local pain. The most common pain descriptor was ‘aching’. This occurred for infusions into both the FPL (80% of subjects) and FCR (90%). In contrast, subcutaneous pain was described as mostly ‘sharp’ for the skin overlying the FPL (90% of subjects), and as ‘sharp’ (30%) or ‘cramping’ (40%) when initiated over the thumb.
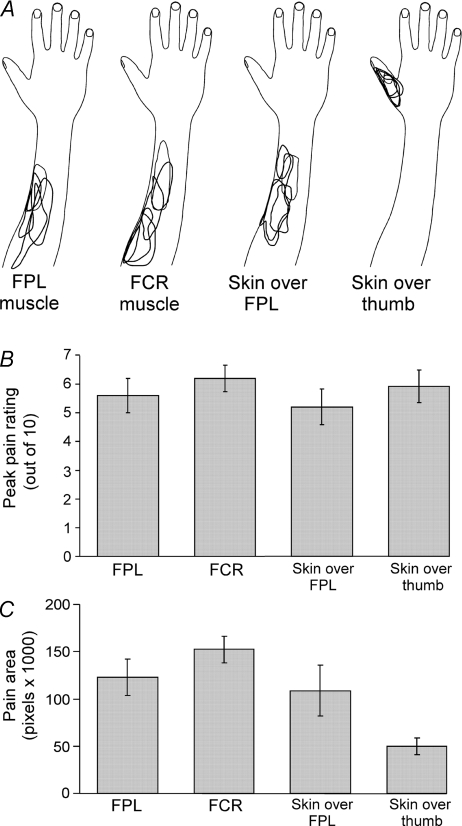
Soon after the bolus injection of hypertonic saline, pain increased rapidly and once the rating of pain began to plateau the infusion was started. Pain then remained relatively stable. For the groups of subjects, peak pain ratings showed no significant difference in pain intensity between the four sites (P = 0.11; Fig. 3B). The mean pain rating experienced for the duration of measurements was also not significantly different (P = 0.25). The pain distribution drawing in Fig. 3A illustrates the general trend for most subjects. Infusion of hypertonic saline into the FPL and FCR produced pain around the injection site and often the pain spread distally toward the wrist and even the hand. Infusion into skin overlying the FPL produced a similar area of pain but it radiated less distally. For infusion into skin overlying the thumb, the pain was localized at the thumb. These patterns were confirmed when the perceived area of the distribution of the pain was calculated for the group of subjects (Fig. 3C). The pain in the FCR was significantly larger in area than in the skin overlying the thumb (P < 0.007; Fig. 3C). However, regression analyses showed no significant relationship between the change in the number of correct detections and pain area (r2 = 0.04) or peak pain intensity (r2 = 0.012).
Figure 3. Pain ratings and area of pain distribution in response to hypertonic saline infusions.
A, overlapped sample of five subjects, drawings of pain distribution during infusion of hypertonic saline into the flexor pollicis longus muscle (FPL), flexor carpi radialis muscle (FCR), skin over FPL, and the dorso-medial skin over the thumb. B, peak pain ratings for each location (mean ± s.e.m.; n = 10): FPL, FCR, skin over FPL, and the dorso-medial skin over the thumb. C, area of distribution for each location where hypertonic saline was infused (mean ± s.e.m.): FPL, FCR, skin over the FPL, and the dorso-medial skin over the thumb.
Effects of pain on detection of movement at the interphalangeal joint of the thumb
The effects on detection thresholds were similar for movements into flexion and extension under any painful or non-painful condition. There was an overall significant difference in the number of correct detections subjects made at different times (before, during and after pain; P < 0.001). However, the effect of pain was significantly greater at some locations compared to others as there was a significant interaction between time and pain location (P = 0.029). Therefore, the effect of pain was analysed at locations separately. While several studies suggest that females are more sensitive to painful stimuli than males (e.g. Berkley, 1997; Dao & LeResche, 2000), there was no significant difference in the peak pain perceived or the number of correct detections at different times (before, during and after pain) between male and female subjects (P = 0.2).
Intramuscular pain
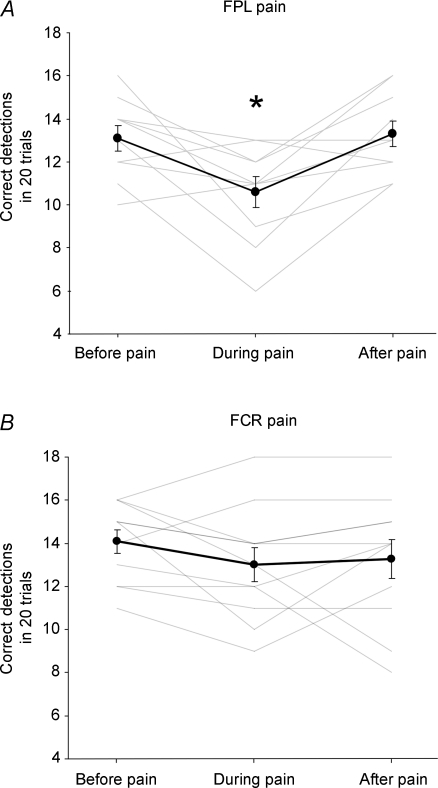
Detection of passively applied flexion and extension movements at the interphalangeal proximal joint of the thumb decreased when pain was induced in FPL (Fig. 4A). Detection was significantly lower during pain when compared to the periods before and after pain (P = 0.005 and P = 0.002, respectively). Before administration of hypertonic saline into the FPL muscle, on average subjects made 12.8 ± 0.6 out of 20 correct detections, while during pain subjects made 10.1 ± 0.8 correct detections, a decrease of 2.7 ± 0.8 detections. Detection did not differ when measured before and after the infusion (P = 0.3). In contrast, FCR pain had no effect on movement detection at the thumb interphalangeal joint (Fig. 4B). There was no difference in correct detections before, during or after the saline was infused into the FCR (P = 0.34).
Figure 4. Effect of muscle pain on detection of direction of passive movements applied to the interphalangeal joint of the thumb.
Proprioceptive acuity measured as the number of correct movement detections (mean ± s.e.m.) before, during and after hypertonic saline was infused into the flexor pollicis longus muscle (FPL; A) and flexor carpi radialis muscle (FCR; B). The group average (dark lines) and individual data (pale lines) are shown. There was a significant (*) decrease in detection when pain was induced in FPL.
Subcutaneous pain
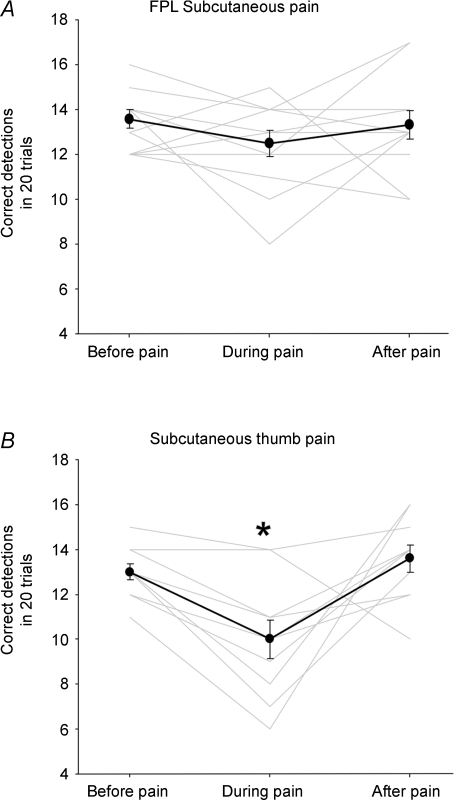
Pain induced in the skin overlying the FPL had no clear effect on movement detection (Fig. 5A). There was no significant difference in correct detections before, during or after the saline was infused into the skin overlying the FPL (P = 0.3). In contrast, the detection of movements at the thumb was significantly impaired when pain was induced in the skin overlying the thumb compared to before and after pain (Fig. 5B; P < 0.01 and P = 0.003, respectively). Before administration of hypertonic saline into the skin overlying the thumb, on average subjects got 13.0 ± 0.4 out of 20 correct detections, while during pain subjects made 10 ± 0.8 correct detections, a decrease of 3.0 ± 0.6 detections.
Figure 5. Effect of subcutaneous pain on detection of direction of passive movements applied to the interphalangeal joint of the thumb.
Proprioceptive acuity measured as the number of correct movement detections (mean ± s.e.m.) before, during and after hypertonic saline was infused into the skin over the flexor pollicis longus muscle (FPL) (A) and the dorso-medial skin over the thumb (B). The group average (dark lines) and individual data (pale lines) are shown. There was a significant (*) decrease in detection when pain was induced in the dorso-medial skin over the thumb.
Effects of pain on perceived thumb size
For studies involving pain induced by injections into FPL and the skin overlying it, we examined another aspect of proprioception, the perceived size of the thumb. During pain in the FPL and skin overlying the FPL the perceived size of the thumb increased 3.6 ± 2.0% and 1.8% ± 1.8%, respectively. Despite these small increases, there was no significant change in the perceived size of the thumb or of the index finger (P = 0.17 and P = 0.32, respectively).
Discussion
The present study was done to determine whether muscle and subcutaneous pain distort a component of proprioception, namely the sensation of joint movement, which is considered to be predominantly signalled by muscle spindles (e.g. Matthews, 1988; Gandevia, 1996; Kandel et al. 2000; Proske et al. 2000), although inputs from cutaneous receptors are increasingly being recognized as making a contribution based on their encoding of joint position (e.g. Burke et al. 1988; Edin & Abbs, 1991; Edin, 1992; Edin & Johansson, 1995; Collins & Prochazka, 1996; Collins et al. 2005; Aimonetti et al. 2007). When pain was initiated in the FPL muscle, but not an adjacent muscle in the forearm (FCR), detection of passive movements at the interphalangeal joint of the thumb was impaired. Subcutaneous pain also impaired movement detection when initiated in skin overlying the thumb, but not in skin overlying the FPL muscle in the forearm. Hence, the major new finding is that while both muscle and skin pain can disturb proprioception, the impairment is site-specific and involves regions and tissues that are likely to have a proprioceptive role at the joint.
It is unlikely that disturbances in proprioception from pain in the FPL and the skin overlying the thumb were simply due to attention being diverted in response to pain, as the intensity of the pain generated at all four sites of infusion of hypertonic saline was similar. Though pain in the FCR and skin overlying the FPL was strong, it did not impair proprioceptive acuity. Also, regression analysis showed no relationship between the change in movement detection and peak pain or pain area. Therefore, it is likely that the location at which the pain was induced was critical for the proprioceptive impairment. This further suggests that only pain induced in ‘proprioceptively relevant’ areas acts on central processes involved in movement detection. As the effects of hypertonic saline were specific to the location of infusion, it was considered unnecessary to test non-noxious isotonic saline.
It is unlikely that proprioceptive afferents were activated directly by the injection of hypertonic saline. This procedure preferentially excites small-diameter fibres with minimal effect on the discharge of muscle spindle afferents or large-diameter cutaneous afferents (Paintal, 1960; Iggo, 1961; Thunberg et al. 2002). It is likely that activity in group III and IV muscle afferents is responsible for muscle pain following hypertonic saline injection as blockade of group IV muscle afferent fibres by lignocaine reduced muscle pain evoked by hypertonic saline, while a tourniquet block of large-diameter afferents had no effect (Andersen et al. 2000).
One possibility for the reduction in proprioceptive acuity from pain in the FPL is that pain modulates indirectly the proprioceptive input from muscle spindles via a reflex action on fusimotor neurons which then alter the discharge of muscle spindle endings. Studies in anaesthetized cats suggest activity in group III and IV muscle afferents can affect the muscle spindle system (e.g. Appelberg et al. 1983; Johansson et al. 1993; Djupsjobacka et al. 1995; Capra & Ro, 2000). However, for this mechanism to operate in conscious human subjects who are relaxed, it requires that there is a significant resting fusimotor drive which can be inhibited. There is much evidence that background fusimotor drive is low in the relaxed state in humans such that strong cutaneomuscular reflexes are difficult to evoke (e.g. Burke et al. 1979; Gandevia et al. 1994). However, it has been suggested that human fusimotor fibres respond to cognitive demand (Ribot et al. 1986), which appears to alter muscle spindle discharge in the absence of electromyographic activity (Burke et al. 1980; Ribot et al. 1986; Ribot-Ciscar et al. 2000). If fusimotor drive exerted a different effect on fusimotor neurons innervating FPL and its antagonist, detection of movement into flexion or extension would be affected differentially. However, no bias in detection was observed in any of our studies. Furthermore, the cutaneous pain over the thumb and over the FPL does not impair similarly, yet the cutaneous afferents are from the same dermatome. Thus, the available data do not favour a reflex change in spindle behaviour from a decline in fusimotor drive as the mechanism responsible for reduced movement detection.
The present study does not reveal the site at which proprioceptive processing is disturbed by the regionally specific nociceptive inputs. However, a central mechanism, with nociceptors interacting with central pathways conveying proprioceptive inputs, is likely. Low-threshold mechanoreceptors, cutaneous nociceptive afferents, group II, III and IV muscle afferents and joint afferents converge onto spinal interneurons (e.g. Schomburg, 1990). Convergence between nociceptive and non-nociceptive inputs also occurs at many levels including at the dorsal horn (Hoheisel & Mense, 1990) and at spinothalamic tract cells (Craig & Kniffki, 1985). Thus, while detection of passive movement probably reflects convergence of cutaneous and muscle inputs (e.g. Collins et al. 2000, 2005), nociceptor activity may impair processing of this proprioceptive information either before or after convergence of all relevant inputs. A reduction in tactile sensitivity, including to input from cutaneous slowly adpting type II receptors which contribute to proprioception, has been demonstrated with heat or cold pain evoked in the same area or dermatome as the tactile stimulus (e.g. Apkarian et al. 1994; Bolanowski et al. 2000). Thus, the interaction of pain with cutaneous sensation may occur separately from the effects of pain on proprioception.
Nociceptive inputs could disturb proprioceptive processing at the main relays for the proprioceptive inputs from the upper limb. The dorsal column nuclei (DCN) have access to both proprioceptive and nociceptive inputs. There is little direct evidence for convergence of muscle afferent input and nociceptive inputs. However, convergence between cutaneous and nociceptive signals has been documented (e.g. Cliffer et al. 1992; Berkley & Hubscher, 1995) and activation of nociceptors can in some conditions reduce DCN responses to non-noxious cutaneous stimuli (Costa-Garcia & Nunez, 2004). Convergence between nociceptive and innocuous inputs also occurs in the main sensory nucleus of the thalamus as shown anatomically and physiologically in non-human primates and through cell recordings in humans (Kenshalo et al. 1980; Chung et al. 1986; Ralston & Ralston, 1994; Lee et al. 1999). Again, studies on specific proprioceptive inputs are limited, but in the cat, wide dynamic range thalamic neurons with receptive fields in the knee joint or hind limb muscle also responded to intra-articular injection of potassium, bradykinin and capsaicin (Hutchison et al. 1994). However, the degree of interaction between noxious and innocuous inputs in the thalamus remains debated (Apkarian et al. 2000).
Studies which have examined cortical responses to combined painful and non-painful stimuli suggest that the cortex is a likely site of interaction. During tonic muscle pain induced by intramuscular injection of ascorbic acid, subjects reported poor proprioceptive ability, and mid-latency peaks in sensory evoked potentials (SEPs) to peripheral nerve stimulation were reduced whereas early peaks were preserved (Rossi et al. 1998, 2003). However, painful cutaneous stimulation reduced one of the early components of sensory magnetic fields (SEFs) evoked by subsequent non-noxious cutaneous stimuli, which suggests gating at the primary somatosensory cortex (SI) or thalamus (Tran et al. 2003).
There are multiple cortical areas at which interactions between proprioception and pain could take place. There are nociceptive inputs, as well as inputs from all other sensory modalities, to the primary (SI) and to the secondary (SII) somatosensory areas (e.g. Matsumoto et al. 1987; Kenshalo et al. 1988; Stevens et al. 1993; Tran et al. 2003). In humans, imaging studies suggest that the network of areas activated by innocuous and noxious stimuli overlap although additional areas are activated by the painful stimuli (Niddam et al. 2002; Ferretti et al. 2004). In addition, there are distinct as well as overlapping areas activated by skin and muscle pain (Schreckenberger et al. 2005; Henderson et al. 2007). SII has been suggested as an area in which noxious and non-noxious inputs may be integrated although there is a separate area in SII which responds only to painful stimuli (Frot et al. 2001; Ferretti et al. 2004; Torquati et al. 2005). Finally, even the primary motor cortex may be a site at which nociceptive inputs interact with proprioception. While it has long been known that it receives projections from muscle, skin and joint afferents (e.g. Lemon & Porter, 1976; Strick & Preston, 1982; Weiller et al. 1996), recent evidence suggests that this region contributes to sensations of limb movement (Naito & Ehrsson, 2001) and that its excitability is reduced by muscle nociceptive inputs (Le Pera et al. 2001; Martin et al. 2007).
To move the thumb accurately requires not only knowledge of the relative muscle lengths and joint angles, but also information about the dimensions of the body segments. Pain could also disturb this perception. Despite the impairment in movement detection, we found that pain in FPL did not significantly increase the perceived size of the thumb. However, an increase in perceived size in the thumb occurred when small-diameter afferents in the thumb were activated by painful cooling (Gandevia & Phegan, 1999). The reasons for this difference are unclear, but our results suggest that mechanisms signalling perceived body size are less disturbed by a muscle nociceptive input than those subserving movement detection.
It is difficult to see a proprioceptive advantage conveyed by the changes in detection of movement reported here. It may be an unfortunate by-product of the central projection pathways of nociceptive and non-nociceptive afferents. The decrease in the ability to detect small movements would represent a reduction in the resolution of the proprioceptive system. However, it is unclear how this would influence detection and production of larger movements. Whatever the functional explanation, the results provide a reminder that while specialized inputs from skin, joint and muscle afferents generate useful proprioceptive signals, small-diameter inputs from the same tissue can interfere centrally with the signalling.
In conclusion, while both muscle and skin pain can impair detection of joint movement, the site from which the pain originates is a crucial determinant of whether proprioception will be disturbed. Impairment is site-specific to receptors that have a proprioceptive role at the joint. The findings have clinical relevance for understanding proprioceptive impairment at a joint. This may arise secondary to pathology in specific proximal muscles. Our results highlight the complex relation between the central actions of nociceptive inputs and their influence on proprioception and presumably also on motor control.
Acknowledgments
The work was supported by the National Health and Medical Research Council (NHMRC) of Australia. We are grateful to Professor Uwe Proske and Dr Lorimer Moseley for comments on the manuscript, and to Dr Peter Nickolls who assisted with the studies.
References
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol. 2007;580:649–658. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OK, Graven-Nielsen T, Matre D, Arendt-Nielsen L, Schomburg ED. Interaction between cutaneous and muscle afferent activity in polysynaptic reflex pathways: a human experimental study. Pain. 2000;84:29–36. doi: 10.1016/S0304-3959(99)00174-8. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T, Bruggemann J, Airapetian LR. Segregation of nociceptive and non-nociceptive networks in the squirrel monkey somatosensory thalamus. J Neurophysiol. 2000;84:484–494. doi: 10.1152/jn.2000.84.1.484. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Stea RA, Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosensory Motor Res. 1994;11:259–267. doi: 10.3109/08990229409051393. [DOI] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64:231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- Baker V, Bennell K, Stillman B, Cowan S, Crossley K. Abnormal knee joint position sense in individuals with patellofemoral pain syndrome. J Orthopaedic Res. 2002;20:208–214. doi: 10.1016/S0736-0266(01)00106-1. [DOI] [PubMed] [Google Scholar]
- Bennell KL, Hinman RS, Metcalf BR, Crossley KM, Buchbinder R, Smith M, McColl G. Relationship of knee joint proprioception to pain and disability in individuals with knee osteoarthritis. J Orthopaedic Res. 2003;21:792–797. doi: 10.1016/S0736-0266(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Bennell K, Wee E, Crossley K, Stillman B, Hodges P. Effects of experimentally-induced anterior knee pain on knee joint position sense in healthy individuals. J Orthopaedic Res. 2005;23:46–53. doi: 10.1016/j.orthres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nature Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Corbeil P, Teasdale N. Postural stability is altered by the stimulation of pain but not warm receptors in humans. BMC Musculoskeletal Disorders. 2003;4:23. doi: 10.1186/1471-2474-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanowski SJ, Maxfield LM, Gescheider GA, Apkarian AV. The effects of stimulus location on the gating of touch by heat- and cold-induced pain. Somatosensory Motor Res. 2000;17:195–204. doi: 10.1080/08990220050020607. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, McKeon B, Westerman RA. Induced changes in the thresholds for voluntary activation of human spindle endings. J Physiol. 1980;302:171–181. doi: 10.1113/jphysiol.1980.sp013236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Skuse NF, Stuart DG. The regularity of muscle spindle discharge in man. J Physiol. 1979;291:277–290. doi: 10.1113/jphysiol.1979.sp012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra NF, Ro JY. Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain. 2000;86:151–162. doi: 10.1016/s0304-3959(00)00231-1. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Surmeier DJ, Sorkin LS, Kim J, Willis WD. Response characteristics of neurons in the ventral posterior lateral nucleus of the monkey thalamus. J Neurophysiol. 1986;56:370–390. doi: 10.1152/jn.1986.56.2.370. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: basic characterization and antidromic activation from the thalamus. J Neurophysiol. 1992;68:818–832. doi: 10.1152/jn.1992.68.3.818. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Sensory integration in the perception of movements at the human metacarpophalangeal joint. J Physiol. 2000;529:505–515. doi: 10.1111/j.1469-7793.2000.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Corbeil P, Blouin JS, Teasdale N. Effects of intensity and locus of painful stimulation on postural stability. Pain. 2004;108:43–50. doi: 10.1016/j.pain.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Costa-Garcia M, Nunez A. Nociceptive stimuli induce changes in somatosensory responses of rat dorsal column nuclei neurons. Brain Res. 2004;1025:169–176. doi: 10.1016/j.brainres.2004.07.082. [DOI] [PubMed] [Google Scholar]
- Craig AD, Kniffki KD. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol. 1985;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao TT, LeResche L. Gender differences in pain. J Orofacial Pain. 2000;14:169–184. discussion 184–195. [PubMed] [Google Scholar]
- Djupsjobacka M, Johansson H, Bergenheim M, Sjolander P. Influences on the gamma-muscle-spindle system from contralateral muscle afferents stimulated by KCl and lactic acid. Neuroscience Res. 1995;21:301–309. doi: 10.1016/0168-0102(94)00864-c. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCloskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol. 1987;386:63–71. doi: 10.1113/jphysiol.1987.sp016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A, Del Gratta C, Babiloni C, Caulo M, Arienzo D, Tartaro A, Rossini PM, Romani GL. Functional topography of the secondary somatosensory cortex for nonpainful and painful stimulation of median and tibial nerve: an fMRI study. Neuroimage. 2004;23:1217–1225. doi: 10.1016/j.neuroimage.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia-Larrea L, Guenot M, Mauguiere F. Responses of the supra-sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra-cerebral recordings. Pain. 2001;94:65–73. doi: 10.1016/S0304-3959(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Illusory movements produced by electrical stimulation of low-threshold muscle afferents from the hand. Brain. 1985;108:965–981. doi: 10.1093/brain/108.4.965. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor commands. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Exercise, section 12, Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. [Google Scholar]
- Gandevia SC, Phegan CM. Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. J Physiol. 1999;514:609–616. doi: 10.1111/j.1469-7793.1999.609ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol. 2006;571:703–710. doi: 10.1113/jphysiol.2005.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Wilson L, Cordo PJ, Burke D. Fusimotor reflexes in relaxed forearm muscles produced by cutaneous afferents from the human hand. J Physiol. 1994;479:499–508. doi: 10.1113/jphysiol.1994.sp020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972;175:1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Fenger-Gron LS, Svensson P, Steengaard-Pedersen K, Arendt-Nielsen L, Staehelin Jensen T. Quantification of deep and superficial sensibility in saline-induced muscle pain – a psychophysical study. Somatosensory Motor Res. 1998;15:46–53. doi: 10.1080/08990229870943. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S. Response behaviour of cat dorsal horn neurones receiving input from skeletal muscle and other deep somatic tissues. J Physiol. 1990;426:265–280. doi: 10.1113/jphysiol.1990.sp018137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Luhn MA, Schmidt RF. Responses of lateral thalamic neurons to algesic chemical stimulation of the cat knee joint. Exp Brain Res. 1994;101:452–464. doi: 10.1007/BF00227338. [DOI] [PubMed] [Google Scholar]
- Iggo A. Non-myelinated afferent fibres from mammalian skeletal muscle. J Physiol. 1961;155:52–53. [Google Scholar]
- Johansson H, Djupsjobacka M, Sjolander P. Influences on the gamma-muscle spindle system from muscle afferents stimulated by KCl and lactic acid. Neuroscience Res. 1993;16:49–57. doi: 10.1016/0168-0102(93)90008-e. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. 4th edn. New York: McGraw-Hill; 2000. [Google Scholar]
- Kellgren J. Observations on referred pain arising from muscle. Clin Sci. 1937;3:175–190. [Google Scholar]
- Kenshalo DR, Jr, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res. 1988;454:378–382. doi: 10.1016/0006-8993(88)90841-4. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Jr, Giesler GJ, Jr, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol. 1980;43:1594–1614. doi: 10.1152/jn.1980.43.6.1594. [DOI] [PubMed] [Google Scholar]
- Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol. 2001;112:1633–1641. doi: 10.1016/s1388-2457(01)00631-9. [DOI] [PubMed] [Google Scholar]
- Lee J, Dougherty PM, Antezana D, Lenz FA. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comparative Neurol. 1999;410:541–555. doi: 10.1002/(sici)1096-9861(19990809)410:4<541::aid-cne3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976;194:313–339. doi: 10.1098/rspb.1976.0082. [DOI] [PubMed] [Google Scholar]
- McCabe CS, Haigh RC, Shenker NG, Lewis J, Blake DR. Phantoms in rheumatology. Novartis Found Symp. 2004;260:154–174. doi: 10.1002/0470867639.ch11. discussion 174–178, 277–279. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiol Rev. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Martin PG, Weerakkody NS, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2007;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matre D, Arendt-Neilsen L, Knardahl S. Effects of localization and intensity of experimental muscle pain on ankle joint proprioception. Eur J Pain. 2002;6:245–260. doi: 10.1053/eujp.2002.0332. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Sato T, Yahata F, Suzuki TA. Physiological properties of tooth pulp-driven neurons in the first somatosensory cortex (SI) of the cat. Pain. 1987;31:249–262. doi: 10.1016/0304-3959(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharmacol. 1988;66:430–438. doi: 10.1139/y88-073. [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH. Kinesthetic illusion of wrist movement activates motor-related areas. Neuroreport. 2001;12:3805–3809. doi: 10.1097/00001756-200112040-00041. [DOI] [PubMed] [Google Scholar]
- Niddam DM, Yeh TC, Wu YT, Lee PL, Ho LT, Arendt-Nielsen L, Chen AC, Hsieh JC. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage. 2002;17:1437–1450. doi: 10.1006/nimg.2002.1270. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve. 2006;34:545–558. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60:85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Ralston HJ, 3rd, Ralston DD. Medial lemniscal and spinal projections to the macaque thalamus: an electron microscopic study of differing GABAergic circuitry serving thalamic somatosensory mechanisms. J Neuroscience. 1994;14:2485–2502. doi: 10.1523/JNEUROSCI.14-05-02485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS. Consciousness and body image: lessons from phantom limbs, Capgras syndrome and pain asymbolia. Philos Trans R Soc Lond B Biol Sci. 1998;353:1851–1859. doi: 10.1098/rstb.1998.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Collins DF, Gandevia SC. The detection of human finger movement is not facilitated by input from receptors in adjacent digits. J Physiol. 2003;551:371–377. doi: 10.1113/jphysiol.2003.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M, Andre-Deshays C, Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil. 1991;72:288–291. [PubMed] [Google Scholar]
- Ribot E, Roll JP, Vedel JP. Efferent discharges recorded from single skeletomotor and fusimotor fibres in man. J Physiol. 1986;375:251–268. doi: 10.1113/jphysiol.1986.sp016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol. 2000;523:271–282. doi: 10.1111/j.1469-7793.2000.t01-1-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG. The effects of spinal manipulation on cervical kinesthesia in patients with chronic neck pain: a pilot study. J Manipulative Physiol Ther. 1997;20:80–85. [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Rossi A, Decchi B, Groccia V, Della Volpe R, Spidalieri R. Interactions between nociceptive and non-nociceptive afferent projections to cerebral cortex in humans. Neurosci Lett. 1998;248:155–158. doi: 10.1016/s0304-3940(98)00354-1. [DOI] [PubMed] [Google Scholar]
- Rossi S, della Volpe R, Ginanneschi F, Ulivelli M, Bartalini S, Spidalieri R, Rossi A. Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and motor ‘defensive’ strategies. Clin Neurophysiol. 2003;114:1351–1358. doi: 10.1016/s1388-2457(03)00073-7. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton JM, Clarkson PM, James R, Miles M, Westerfer M, Clark S, Donnelly AE. Neuromuscular dysfunction following eccentric exercise. Med Sci Sports Exerc. 1995;27:1185–1193. [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- Skinner HB, Barrack RL, Cook SD, Haddad RJ., Jr Joint position sense in total knee arthroplasty. J Orthopaedic Res. 1984;1:276–283. doi: 10.1002/jor.1100010307. [DOI] [PubMed] [Google Scholar]
- Stevens RT, London SM, Apkarian AV. Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res. 1993;631:241–246. doi: 10.1016/0006-8993(93)91541-y. [DOI] [PubMed] [Google Scholar]
- Straus WL. Rudimentary digits in primates. Q Rev Biol. 1942;17:228–243. [Google Scholar]
- Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol. 1982;48:150–159. doi: 10.1152/jn.1982.48.1.150. [DOI] [PubMed] [Google Scholar]
- Svensson P, Houe L, Arendt-Nielsen L. Bilateral experimental muscle pain changes electromyographic activity of human jaw-closing muscles during mastication. Exp Brain Res. 1997;116:182–185. doi: 10.1007/pl00005738. [DOI] [PubMed] [Google Scholar]
- Thunberg J, Ljubisavljevic M, Djupsjobacka M, Johansson H. Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp Brain Res. 2002;142:319–326. doi: 10.1007/s00221-001-0941-4. [DOI] [PubMed] [Google Scholar]
- Torquati K, Pizzella V, Babiloni C, Del Gratta C, Della Penna S, Ferretti A, Franciotti R, Rossini PM, Romani GL. Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: an MEG study using fMRI constraints. Neuroimage. 2005;26:48–56. doi: 10.1016/j.neuroimage.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Tran TD, Hoshiyama M, Inui K, Kakigi R. Electrical-induced pain diminishes somatosensory evoked magnetic cortical fields. Clin Neurophysiol. 2003;114:1704–1714. doi: 10.1016/s1388-2457(03)00151-2. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Hesse CW, Morgan DL, Proske U. Human forearm position sense after fatigue of elbow flexor muscles. J Physiol. 2004;558:705–715. doi: 10.1113/jphysiol.2004.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Mahns DA, Taylor JL, Gandevia SC. Impairment of human proprioception by high-frequency cutaneous vibration. J Physiol. 2007;581:971–980. doi: 10.1113/jphysiol.2006.126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Percival P, Canny BJ, Morgan DL, Proske U. Force matching at the elbow joint is disturbed by muscle soreness. Somatosens Mot Res. 2003;20:27–32. doi: 10.1080/0899022031000083816. [DOI] [PubMed] [Google Scholar]
- Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, Muller S, Diener HC, Thilmann AF. Brain representation of active and passive movements. Neuroimage. 1996;4:105–110. doi: 10.1006/nimg.1996.0034. [DOI] [PubMed] [Google Scholar]