Abstract
The N-terminal variable region of cardiac troponin T (TnT) is a regulatory structure that can be selectively removed during myocardial ischaemia reperfusion by μ-calpain proteolysis. Here we investigated the pathophysiological significance of this post-translational modification that removes amino acids 1–71 of cardiac TnT. Working heart preparations were employed to study rat acute myocardial infarction and transgenic mouse hearts over-expressing the N-terminal truncated cardiac TnT (cTnT-ND). Ex vivo myocardial infarction by ligation of the left anterior descending coronary artery induced heart failure and produced cTnT-ND not only in the infarct but also in remote zones, including the right ventricular free wall, indicating a whole organ response in the absence of systemic neurohumoral mechanisms. Left ventricular pressure overload in mouse working hearts produced increased cTnT-ND in both ventricles, suggesting a role of haemodynamic stress in triggering an acute whole organ proteolytic regulation. Transgenic mouse hearts in which the endogenous intact cardiac TnT was partially replaced by cTnT-ND showed lowered contractile velocity. When afterload increased from 55 mmHg to 90 mmHg, stroke volume decreased in the wild type but not in the transgenic mouse hearts. Correspondingly, the left ventricular rapid-ejection time of the transgenic mouse hearts was significantly longer than that of wild type hearts, especially at high afterload. The restricted deletion of the N-terminal variable region of cardiac troponin T demonstrates a novel mechanism by which the thin filament regulation adapts to sustain cardiac function under stress conditions.
We recently reported a restricted N-terminal truncation of cardiac troponin T (TnT) during myocardial ischaemia reperfusion, which selectively removes the N-terminal variable region (amino acids 1–71) but preserves the conserved regions of TnT (Zhang et al. 2006). Troponin T is a subunit of the troponin complex (Gordon et al. 2000) that mediates the Ca2+ regulation of muscle contraction (Tobacman, 1996). Through interactions with troponin C (TnC), troponin I (TnI), tropomyosin (Tm) and actin in the thin filament, TnT plays a coordinator role in the Ca2+-regulatory system of muscle. Three muscle type-specific TnT isoform genes and alternative RNA splicing express multiple TnT isoforms in cardiac, slow skeletal and fast skeletal muscles (Breitbart & Nadal-Ginard, 1986; Jin et al. 1992; Huang et al. 1999). The various TnT isoforms mainly differ in the N-terminal structures whereas their central and C-terminal regions are conserved (Jin et al. 2008).
The N-terminal region of TnT does not contain binding sites for any known thin filament proteins (Pearlstone & Smillie, 1982; Heeley et al. 1987). Deleting the N-terminal variable region does not diminish the regulatory activity of troponin (Pan et al. 1991; Fujita et al. 1992; Chandra et al. 1999). However, N-terminal alterations in TnT affect the overall protein conformation (Wang & Jin, 1998; Jin & Root, 2000) and binding to tropomyosin, TnI and TnC (Wang & Jin, 1998; Jin et al. 2000). Consistent with the conformational and functional effects, N-terminal alternatively spliced TnT isoforms convey changes in the activation of actomyosin ATPase (Gomes et al. 2002). Aberrant splicing of cardiac TnT (cTnT) in the N-terminal region was found in both hypertrophic and failing human hearts (Anderson et al. 1991) and animal models with dilated cardiomyopathy (Biesiadecki & Jin, 2002; Biesiadecki et al. 2002). Therefore, the N-terminal variable region of TnT is a modulatory structure that affects the Ca2+ regulation of muscle contraction.
Altered TnT isoform gene expression (Yu et al. 2007) and alternative RNA splicing (Anderson et al. 1995) occur during muscle adaptation to stress conditions. The half-life of troponin protein in myofibrils is 3–4 days (Martin, 1981) and therefore the transcriptional and RNA splicing mechanisms represent chronic regulations. In contrast, post-translational regulations provide a mechanism for rapid adaptation to acute stress conditions. Global ischaemia reperfusion in working heart preparations induced the production of the N-terminal amino acids 1–71 truncated cTnT (Zhang et al. 2006). Considering that the decreases in cardiac output in acute or chronic heart failure conditions would result in increased vasoconstriction as a systemic compensation (Zelis et al. 1968; Zelis & Flaim, 1982), pressure overload is a considerable stress factor in cardiac insufficiency. To investigate myocardial adaptation to energetic crisis against pressure load will help in the understanding of the pathophysiology of heart failure to improve treatment.
To examine the functional significance of the restricted N-terminal truncation of cTnT, we studied acute myocardial infarction in rat hearts and the function of transgenic mouse hearts over-expressing the N-terminal truncated cTnT (cTnT-ND). Ex vivo regional myocardial infarction induced heart failure and produced cTnT-ND as a whole organ response. Ex vivo left ventricular pressure overload also produced increased cTnT-ND in both ventricles. Compared with wild type control, transgenic mouse hearts in which the endogenous intact cTnT was partially replaced by cTnT-ND showed lowered ventricular contractile velocity but higher tolerance to increases in afterload through elongating the rapid ejection time. The data demonstrate a novel post-translational adaptation in the cardiac myofilament against workload.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committees and were conducted in accordance with the Guiding Principles in the Care and Use of Animals, as approved by the Council of the American Physiological Society.
Development of monoclonal antibodies against the N-terminal region of cardiac TnT
By immunization of Balb/c mice with an N-terminal peptide of human cTnT (amino acids 1–69) expressed in bacteria from cloned cDNA, monoclonal antibodies (mAbs) against the N-terminal variable region of cTnT were developed as previously described (Wang & Jin, 1998). The anti-cTnT antibody-producing hybridoma cell lines were introduced into 2,6,10,14-tetramethyl pentadecane (pristane, Sigma)-primed peritoneal cavity of Balb/c mice to produce mAb-enriched ascites fluids. The immunoglobulin isotypes were determined on the hybridoma cultural supernatant using a rat anti-mouse immunoglobulin isotyping kit from BD Biosciences according to the manufacturer's instructions.
SDS-polyacrylamide gel electrophoresis and Western blotting
Cardiac muscle was homogenized in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 2% SDS to extract the myofilament proteins. The samples were resolved by 14% Laemmli SDS-PAGE with an acrylamide: bisacrylamide ratio of 180: 1 using a Bio-Rad mini-gel system, and the resolved protein bands were transferred to a nitrocellulose membrane using a Bio-Rad semi-dry electrotransfer apparatus. The blotted nitrocellulose membrane was blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline (150 mm NaCl, 50 mm Tris-HCl, pH 7.5) and incubated with an anti-cTnT mAb CT3 (Jin et al. 2000) or an anti-cTnT N-terminal region mAb 3G7 (IgG1κ) diluted in Tris-buffered saline containing 0.1% BSA. The subsequent washes, incubation with alkaline phosphatase-labelled anti-mouse IgG second antibodies (Sigma), and 5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium substrate reaction were carried out as previously described (Biesiadecki et al. 2002).
Left anterior descending coronary artery ligation and reperfusion in isolated working rat hearts
Langendorff–Neely working heart preparations were obtained from 450–500 g male Sprague–Dawley rats as previously described (Barbato et al. 2005). Briefly, sodium heparin (300 IU, i.p.) was given 1 h before anaesthetizing the rat with sodium pentobarbital (50 mg kg−1 body weight, i.p.). The thoracic cavity was opened by a transverse incision to rapidly isolate the heart. The heart was placed in Krebs–Henseleit buffer at room temperature and cannulated through the aorta to establish retrograde perfusion at 80 mmHg. During a 15 min period of perfusion, the left atrium was cannulated and coronary effluent was collected via a cannula inserted into pulmonary artery. The working mode was initiated by switching to anterograde perfusion at 15 mmHg preload and 70 mmHg afterload. The aortic pressure was measured using an MLT844 pressure transducer (Capto, Horten, Norway). A 27-gauge needle was used to puncture the apex of the left ventricle and a 1.4 French Millar catheter (model SPR 671; Millar Instruments, Houston, TX, USA) was inserted along the path into the left ventricle. Intraventricular placement of the catheter was confirmed by the systolic and diastolic pressure values detected. Heart rate, intraventricular pressure and the maximum rate of left ventricular pressure development (±dP/dt) were recorded as analog signals amplified by an ML 110 Bridge Amplifier (AD Instruments, Colorado Springs, CO, USA) and sampled at 1000 Hz using a Powerlab/16 SP digital data archiving system (AD Instruments) and stored on computer disk for subsequent analysis.
A suture was placed around the left anterior descending (LAD) coronary artery to produce regional ischaemia and reperfusion as described previously (Ferdinandy et al. 1995). After 30 min stabilization in the working mode, LAD was ligated together with a short piece of PE-50 tubing to reduce tissue damage. LAD occlusion was confirmed by decreases in left ventricular pressure and stroke volume. After 20 min ischaemia, reperfusion was initiated by cutting the suture. Cardiac function was monitored during 40 min of reperfusion.
After the functional measurements, all hearts were rapidly dissected and muscle samples stored at −80°C for Western blot examination of cTnT modification in the ischaemic site, peripheral tissue and remote zones including the septum, right ventricle and atria.
Transgenic mice expressing the N-terminal truncated cardiac TnT in the heart
As previously described (Biesiadecki et al. 2002), transgenic mice were constructed on a C57BL/6 background using a cloned mouse cardiac α-myosin heavy chain (α-MHC) gene promoter (Subramaniam et al. 1993) (generously provided by Dr Jeffrey Robbins, University of Cincinnati) to direct a heart-specific, postnatal expression of cDNA encoding the N-terminal truncated cTnT (Zhang et al. 2006). The microinjection and embryo implantation were performed at a service facility at Case Western Reserve University. Genotyping and segregation of the transgene allele confirmed the establishment of two transgenic mouse founder lines. The expression of cTnT-ND lacking amino acids 1–71 in the transgenic mouse cardiac muscle was verified by Western blots as described above. The incorporation of the exogenous TnT into the cardiac muscle thin filaments of the transgenic mice was verified by Western blots on Triton X-100-washed cardiac myofibrils prepared as previously described (Zhang et al. 2006). The relative amounts of intact and N-terminal truncated cTnT in the transgenic mouse cardiac muscle were determined by densitometric quantification of the Western blots. Wild type C57BL/6 mice of similar age were used as controls. Mice of both sexes were used for functional characterization. All transgenic mouse hearts used in the functional studies were examined by Western blotting to confirm the expression of cTnT-ND and replacement of intact cTnT.
Histological examination
After killing, hearts from the transgenic and wild-type mice were rapidly excised and relaxed in PBS containing 50 mm KCl. The hearts were fixed in 3.7% formaldehyde in PBS containing 50 mm KCl for paraffin sectioning and were stained with haematoxylin–eosin at a service facility. The sections were examined under a Zeiss Axiovert 100 microscope.
Isolated working mouse heart preparations and functional measurement
Cardiac function of transgenic and wild type mice was measured in isolated working heart preparations (Barbato et al. 2005). Thirty minutes after intraperitoneal injection of 100 units of heparin, the mice were anaesthetized with pentobarbital sodium (100 mg kg−1 body weight, i.p.) and the heart was rapidly isolated. Similar to the rat protocol described above, several modifications are described in the following.
A modified 18-gauge needle 6 mm long with thinned wall to reduce the outside diameter was used as the aortic cannula. A pin with a pointed end was placed inside the needle as a guide to facilitate cannulation. This approach significantly improved the success rate of our mouse working heart preparation to near 100%. A modified 16-gauge needle was used to cannulate the pulmonary vein for antegrade perfusion through the left atria. Bevelled PE-50 tubing was used to cannulate the pulmonary artery to collect coronary flow.
After all cannulations were established, the heart was switched to working mode by opening the left atrial perfusion. In all experiment, the hearts were perfused with non-recycled Krebs–Hensileit buffer aerated with 95% O2–5% CO2 at 37°C to avoid the effects of metabolic chemicals and hormones. The buffer contents were as follows: 118 mm NaCl, 4.7 mm KCl, 2.25 mm CaCl2, 2.25 mm MgSO4, 1.2 mm KH2PO4, 0.32 mm EGTA, 25 mm NaHCO3, 15 mm d-glucose and 2 mm sodium pyruvate, pH 7.4 adjusted at 37°C. The high concentration of d-glucose (Gauthier et al. 1998) and sodium pyruvate (Wang et al. 2002) effectively prevented contractile cycling (cyclic fluctuations) caused by metabolic substrate insufficiency in ex vivo working mouse heart (Sutherland et al. 2003) to allow the hearts to be functionally stable in working mode for over 2 h.
A 30-gauge needle was used to puncture the left ventricle wall from the apex to make a path for the insertion of a 1.2 French pressure–volume (P–V) catheter (Model 898B, Scisense, London, Ontario, Canada, calibrated for pressure and volume at 37°C). Aortic pressure was measured using an MLT844 pressure transducer (Capto, Horten, Norway). An air bubble was placed in the compliance chamber to mimic in vivo arterial compliance. The size of the bubble affected the aortic pressure trace and the shape of the left ventricular P–V loop. Based on conditions established by previous studies, we kept the bubble size at 0.5 ml in all experiments for isolated working mouse heart, which resembled similar P–V loop as that recorded in vivo (How et al. 2005).
Heart rate was controlled at 480 beats min−1 by using an isolated stimulator (A365, World Precision Instrument) with two platinum microelectrodes attached to the right atrium. Cardiac outputs were measured by the actual aortic flow and coronary effluent (measured as pulmonary artery flow) recorded in real time by calibrated drop counting using a pair of copper electrodes (one was attached to an iron clip to produce a different potential from the other) feeding to computer software (Chart 5, AD Instruments) via a Powerlab/16 SP digital data archiving system (AD Instruments). Baseline function of the isolated working hearts was measured for the aortic pressure, intraventricular pressure, the maximum rate of left ventricular pressure development (±dP/dtmax) and left ventricular volume. Stroke volume (μl (mg heart tissue)−1) was calculated from the sum of aortic flow and coronary effluent, normalized to heart rate. Time to peak pressure (TP), time to 50% peak pressure (TP50) and the time for 75% relaxation (RT75) were measured from the left ventricular pressure traces.
Cardiac function at various preload and afterload pressures
During stabilization of the mouse working heart preparations and the measurement of baseline functions, the preload pressure was 10 mmHg and the afterload was at 55 mmHg (Barbato et al. 2005; Huang et al. 2008). To evaluate the effect of cTnT-ND on cardiac function against pressure overloading, ventricular performance was measured while increasing the afterload from 55 mmHg to 90 mmHg or to up to 145 mmHg. A pressure of 20 mmHg was used for a high preload condition to compare with the 10 mmHg base line condition. The hearts were allowed to stabilize for 5 min after switching to each preload or afterload condition before functional measurements were performed.
Calculation of functional parameters
Ejection phases of the cardiac cycle were determined from aortic pressure, left ventricular pressure and aortic pressure dP/dt traces. In the Langendorff–Neely ex vivo working heart system, the aortic pressure curve demonstrates a notch in the systolic phase indicating the opening of the aortic valve and another notch in the diastolic phase indicating the closure of the aortic valve. Although these notches are not sharp enough to determine the exact time of the aortic valve opening and closure, the corresponding reversing points of the aortic pressure development (dP/dt) curve accurately reflect the time points of aortic valve opening and closure. The duration between the two time points in each ventricular pumping cycle represents the total ejection time. The rapid ejection time is determined as the duration from the aortic valve opening to the peak of ventricle pressure and the reduced ejection time is determined as that from the peak of ventricular pressure to the closure of the aortic valve.
Stroke work (ml mmHg (g heart tissue)−1) was calculated as stroke volume × mean aortic pressure (diastolic pressure + one-third of pulse pressure). Tension–time index (TTI) of the cardiac cycle was originally defined as the area under the systolic portion of the aortic pressure curve calculated from the left ventricular mean systolic pressure and duration (Braunwald et al. 1957). As described by Neely et al. (1967), we calculated TTI in the present study as the aortic pressure–time integral from the end of diastole to the closing of the aortic valve. Neely and co-workers reported that myocardial oxygen consumption was linearly related to the aortic pressure–time integral in rat isolated working heart (Neely et al. 1967). Accordingly, we used aortic pressure–time integral as an indicator of oxygen consumption in the mouse working heart experiments to calculate pressure work efficiency by dividing stroke work with TTI.
Data analysis
Densitometry analysis of Western blots was performed using the NIH Image 1.61 software on images digitized at 600 dpi. All functional analyses were performed in a blinded setting. Quantitative data were documented as mean ± s.e.m. The statistical significance of differences between the mean values was analysed by two-tail unpaired Student's t test unless noted in the table or figure legends.
Results
Regional myocardial ischaemia–reperfusion injury induced whole organ production of cTnT-ND
Ex vivo myocardial regional infarction in rat working hearts produced detectable myocardial injuries. Functional measurements showed that 20 min LAD ligation resulted in significant decreases in left ventricular maximum pressure (LVPmax), ±dP/dtmax of left ventricular pressure development and stoke volume (SV) as compared with the 90 min perfusion control (Table 1). The decreased cardiac function did not recover after 40 min of reperfusion. The failing of ventricular pumping function due to regional ischaemia–reperfusion injury was further indicated by the increases in LVPmin.
Table 1.
Function of isolated rat working hearts
| Baseline (n = 3) | Ischaemia (n = 3) | Ischaemia–reperfusion (n = 3) | |
|---|---|---|---|
| Intrinsic heart rate (beats min−1) | 224.4 ± 6.9 | 218.6 ± 13.4 | 213.7 ± 2.0 |
| LVPmax (mmHg) | 128.23 ± 5.08 | 112.93 ± 6.25* | 115.8 ± 1.626* |
| LVPmin (mmHg) | 4.57 ± 0.46 | 6.53 ± 0.146* | 5.98 ± 0.65 |
| SV (μl (mg heart tissue)−1) | 0.076 ± 0.005 | 0.052 ± 0.003* | 0.063 ± 0.01* |
| +dP/dtmax (mmHg s−1) | 3756 ± 124 | 3140 ± 203* | 3266 ± 76* |
| −dP/dtmax (mmHg s−1) | 2947 ± 170 | 2287 ± 55.22** | 2265 ± 135* |
Nine adult rats were randomly separated into three groups for working heart studies. One group of the hearts was continuously worked for 90 min to measure the baseline function. The second group was used to produce 20 min regional ischaemia by LAD ligation after 30 min stabilization at the base line conditions. The third group was given 40 min reperfusion after the 20 min LAD ligation. The results showed that cardiac function was significantly decreased after LAD ligation. The cardiac function remained suppressed after reperfusion.
P < 0.05,
P < 0.01 versus the baseline controls.
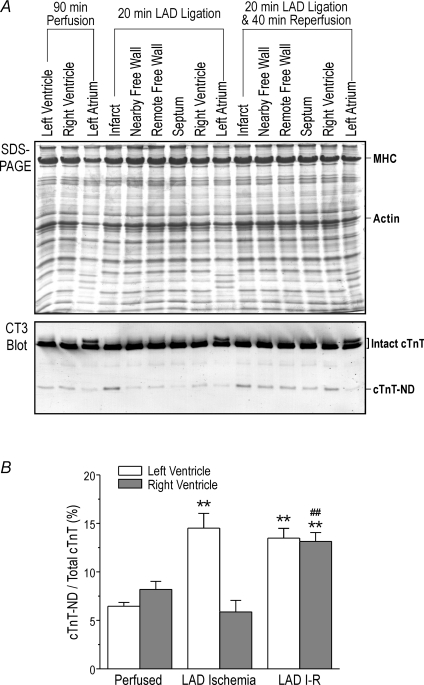
Western blots detected increased amounts of a cTnT fragment in the ischaemic region produced by LAD ligation in rat working hearts in comparison with the perfusion control (Fig. 1). Only the infarct zone showed a significant amount of the cTnT fragment in those cases in which the whole heart function was less affected. However, significant amounts of the truncated cTnT was present in the infarct as well as remote zones including the remaining left ventricular free wall, septum and right ventricle when the infarction produced significant decreases in cardiac function (Fig. 1B). A higher level of the cTnT fragment was found in vivo in the non-infarct zone of ischaemia–reperfused pig hearts (data not shown). The results demonstrate a whole organ response to myocardial energetic crisis in isolated working hearts in the absence of neurohumoral regulation.
Figure 1. Induction of N-terminal truncated cardiac TnT by regional ischaemia reperfusion.
Regional ischaemia reperfusion was produced in isolated rat working hearts by reversible ligation of the left anterior descending (LAD) coronary artery. A, the SDS-PAGE and Western blots using anti-TnT mAb CT3 on total myocardial protein extracts from perfused control, ischaemia and ischaemia–reperfusion groups demonstrated that the ischaemic area had significant production of cTnT-ND. In the ischaemia-reperfused hearts, cTnT-ND was also produced in remote zones of the myocardia, indicating an ex vivo whole organ response. B, quantification of the Western blots showed that the levels of cTnT-ND versus total cTnT increased in the left ventricular ischaemic area after LAD ligation and in both left and right ventricles after LAD ischaemia–reperfusion (I-R). n = 3 in each group. **P < 0.01 versus perfused controls; ##P < 0.01 versus right ventricle after LAD ischaemia.
Ex vivo one sided high afterload induced the production of cTnT-ND in both ventricles
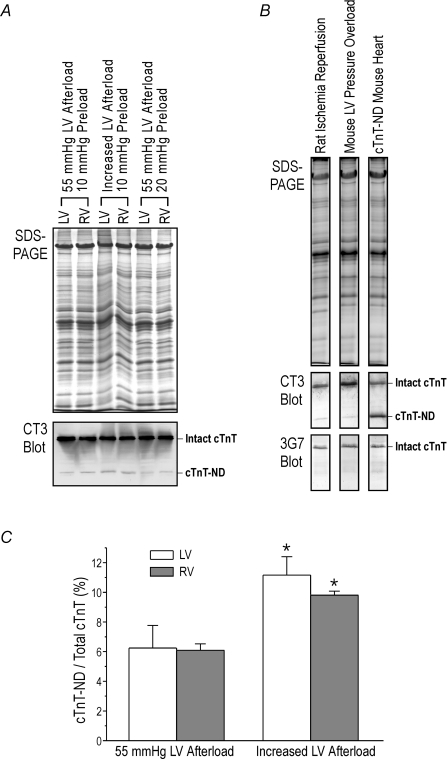
After applying pressure overload to the left ventricle (120–145 mmHg, an afterload higher than the physiological blood pressure of mouse), the mouse working hearts were soon failing to maintain cardiac output against the high afterload and the aortic pressure produced by left ventricular pumping was eventually decreased to approximately 90 mmHg with much reduced cardiac output. This outcome indicates a ventricular failure condition induced by pressure overload. In comparison to the control hearts working for 60 min at 55 mmHg afterload, 40 min working under the pressure overload-induced failing condition produced the truncated cTnT in the ventricular muscle (Fig. 2A). Similar to that seen in the myocardial infarction-induced heart failure (Fig. 1), Western blots detected the cTnT fragment in both left and right ventricles of the mouse hearts worked under left ventricular pressure overload, further demonstrating an ex vivo whole organ response to energetic crisis (Fig. 2A). In contrast, volume overload under 20 mmHg preload did not produce cTnT-ND (Fig. 2A).
Figure 2. Induction of cTnT-ND by pressure overload.
A, the SDS-PAGE and mAb CT3 Western blot showed that in working heart preparations from wild type C57B/L6 mice, high left ventricular pressure load (120–145 mmHg afterload at 10 mmHg preload for 40 min) induced the production of cTnT-ND in both left and right ventricles as compared with the controls at 55 mmHg afterload and 10 mmHg preload or 55 mmHg afterload and 20 mmHg preload. B, Western blots using mAb 3G7 against the N-terminal region of cTnT showed that the cTnT fragments produced in rat and mouse hearts recognized by mAb CT3 lacks the N-terminal epitope, same as the cTnT-ND protein expressed in the transgenic mouse heart. C, densitometry quantification of the Western blots showed that left ventricular (LV) pressure overload induced increased levels of cTnT-ND in both LV and right ventricle (RV) as compared with the 55 mmHg afterload controls (*P < 0.05; n = 3 in each group).
The cTnT fragment found in mouse working hearts under pressure overload as well as in ischaemia–reperfused rat hearts was confirmed as the product of restricted N-terminal truncation (Zhang et al. 2006) by Western blotting using mAb 3G7 specific to the N-terminal segment of cTnT. Figure 2B shows that the cTnT fragment was recognized by mAb CT3 recognizing the central region but not mAb 3G7 against the N-terminal region, the same as the cTnT fragment lacking amino acids 1–71 (cTnT-ND) expressed in transgenic mouse heart.
We have previously reported the production of cTnT-ND in ex vivo rat working hearts treated with global myocardial ischaemia–reperfusion (Zhang et al. 2006). The production of cTnT-ND in regional ischaemia–reperfusion-injured failing rat working hearts (Fig. 1B), and in pressure-overloaded mouse working hearts (Fig. 2C) suggests that haemodynamic stresses and/or energetic crisis may induce the restricted proteolytic modification of cTnT.
Transgenic mouse lines over-expressing cTnT-ND in the heart
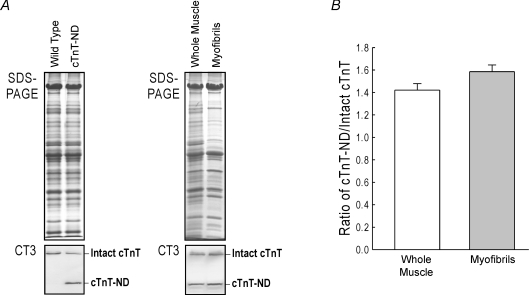
To develop an integrated experimental system for the study of the pathophysiological role of cTnT-ND in cardiac function and adaptation to haemodynamic stresses in energetic crisis, we constructed transgenic mice over-expressing cTnT-ND in adult cardiac muscle. Western blots on total protein extracts and isolated myofibrils from the transgenic mouse hearts showed that the exogenous cTnT-ND was successfully expressed in the transgenic mouse cardiac muscle and effectively incorporated into the myofilaments (Fig. 3A). Densitometric analysis confirmed the high level expression and proportional myofibril incorporation of cTnT-ND to replace most of the endogenous intact cTnT (Fig. 3B).
Figure 3. Transgenic mouse hearts over-expressing cTnT-ND to replace the endogenous intact cardiac TnT.
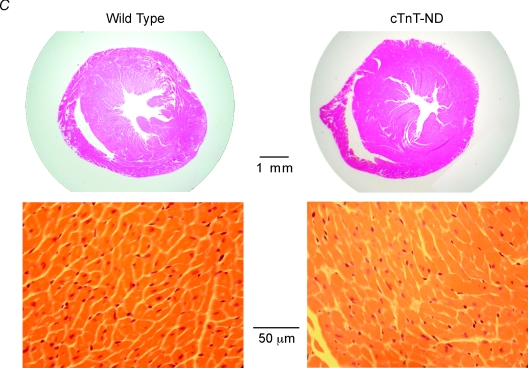
A, SDS-PAGE and Western blot using mAb CT3 demonstrated the high level expression of cTnT-ND in the transgenic mouse hearts. B, densitometry analysis showed a proportional incorporation of cTnT-ND into the myofibrils of the transgenic mouse hearts. C, H&E staining of ventricular cross sections showed no apparent hypertrophy or myocardial degeneration in the cTnT-ND transgenic mouse hearts.
The cTnT-ND transgenic mice showed apparently normal baseline life activities. Two transgenic founder lines were established for functional studies. Histological examination did not see myocardial hypertrophy or degeneration (Fig. 3C). The postnatally up-regulated expression of the exogenous cTnT-ND and cardiac phenotype of the two transgenic lines are similar, excluding any effect of the chromosomal integration sites. Therefore, both lines were used in the functional characterization.
Modification of myocardial contractility and cardiac function by cTnT-ND in transgenic mouse hearts
Cardiac function was studied ex vivo in working heart preparations from cTnT-ND transgenic mice and wild type littermates. At baseline preload and afterload (10 mmHg and 55 mmHg, respectively), cTnT-ND hearts showed lower contractile velocity (+dP/dtmax) than that of wild type hearts (Table 2) while other parameters, including relaxation velocity (−dP/dtmax) and stroke volume, were not changed. When the hearts were tested under higher afterload (90 mmHg) for responses to pressure overload stress, contractile and relaxation velocities as well as LVPmax increased significantly in both cTnT-ND and WT hearts as compared with that at 55 mmHg afterload. When afterload increased from 55 mmHg to 90 mmHg, stroke volume decreased significantly in WT hearts but did not change in cTnT-ND hearts (Table 2 and Fig. 4), demonstrating a unique tolerance to pressure overload.
Table 2.
Function of working heart preparations from cTnT-ND transgenic and wild type mice
| 55 mmHg afterload | 90 mmHg afterload | |||
|---|---|---|---|---|
| WT (n = 5) | cTnT-ND (n = 4) | WT (n = 5) | cTnT-ND (n = 4) | |
| Heart weight/body weight (mg g−1) | 5.89 ± 0.46 | 5.82 ± 0.38 | — | — |
| Heart rate (beats min−1) | 480 | 480 | 480 | 480 |
| LVPmax (mmHg) | 72.48 ± 1.78 | 73.02 ± 1.21 | 103.12 ± 1.27‡ | 105.21 ± 1.91‡ |
| LVPmin (mmHg) | 2.02 ± 0.56 | 1.87 ± 0.46 | 1.78 ± 0.84 | 2.04 ± 0.82 |
| +dP/dtmax (mmHg s−1) | 3847 ± 128 | 3479 ± 104* | 5024 ± 224‡ | 4796 ± 281‡ |
| –dP/dtmax (mmHg s−1) | 3311 ± 151 | 3331 ± 122 | 5268 ± 231‡ | 5123 ± 376‡ |
| TP50 (ms) | 19.5 ± 0.9 | 20.1 ± 0.8 | 20.6 ± 0.6 | 21 ± 0.4 |
| TP (ms) | 43.3 ± 4.6 | 43.8 ± 2.9 | 38.5 ± 1.0 | 42 ± 2.6 |
| TR75 (ms) | 44.5 ± 3.5 | 44.2 ± 1.7 | 50 ± 0.2 | 47 ± 1.4 |
| Stroke volume (μl mg−1) | 0.096 ± 0.010 | 0.107 ± 0.006 | 0.074 ± 0.010‡ | 0.102 ± 0.007† |
The functional measurements show that cTnT-ND hearts decreased +dP/dtmax at 55 mmHg afterload in comparison with the wild type (WT) hearts. When afterload increased from 55 mmHg to 90 mmHg, LVPmax and ±dP/dtmax increased in both WT and cTnT-ND hearts. Whereas stroke volume decreased significantly in WT hearts when the afterload was increased, cTnT-ND hearts sustained the stroke volume, demonstrating a tolerance to pressure overload.
P < 0.05 versus WT control;
P < 0.05 versus WT control at 90 mmHg afterload by one-tailed t test;
P < 0.01 versus that at 55 mmHg afterload by paired Student's t test.
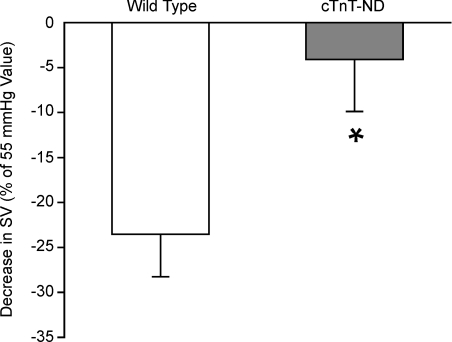
Figure 4. Tolerance of cTnT-ND transgenic mouse hearts to increased pressure load.
When the afterload increased from 55 mmHg to 90 mmHg in mouse working experiments, the stroke volume (SV) of wild type (WT) hearts decreased nearly 25% whereas cTnT-ND transgenic mouse hearts had only a minimum decrease from the baseline level, showing a significant tolerance to the pressure overload. n = 5 for WT and n = 4 for cTnT-ND. *P < 0.05 versus wild type.
Elongated ejection time resulted in the increased cardiac output in the cTnT-ND heart
One of the advantages of the ex vivo working heart approach is that we can precisely control heart rate, preload and afterload pressures and directly measure left ventricular output. When the heart rate, preload and afterload were set identical in the functional comparisons between wild type and cTnT-ND transgenic mouse hearts, the left ventricular pressure showed no difference and the contractile velocity was not higher but lower in the cTnT-ND hearts (Table 2). Therefore, the higher stroke volume of the cTnT-ND hearts compared with the wild type controls at 90 mmHg afterload, as directly measured by the aortic and coronary outflow volume, cannot be attributed to an increase in myocardial contractility. To investigate the mechanism by which the cTnT-ND hearts were able to better sustain stroke volume against increased pressure load, we analysed the time parameters of the cardiac cycle.
In comparison to the wild type hearts, the contraction and relaxation time parameters of the transgenic mouse left ventricle showed no significant difference in the time to develop peak pressure (TP), time to develop 50% peak pressure (TP50), and time to reach 75% relaxation from peak pressure (TR75) at 55 mmHg or 90 mmHg afterload (Table 2). The time to develop peak and 50% peak LVP do not reflect the maximum +dP/dt that was decreased in the cTnT-ND hearts (Table 2). Therefore, these contractile and relaxation time parameters are non-informative for explanation of the increased stroke volume in cTnT-ND hearts.
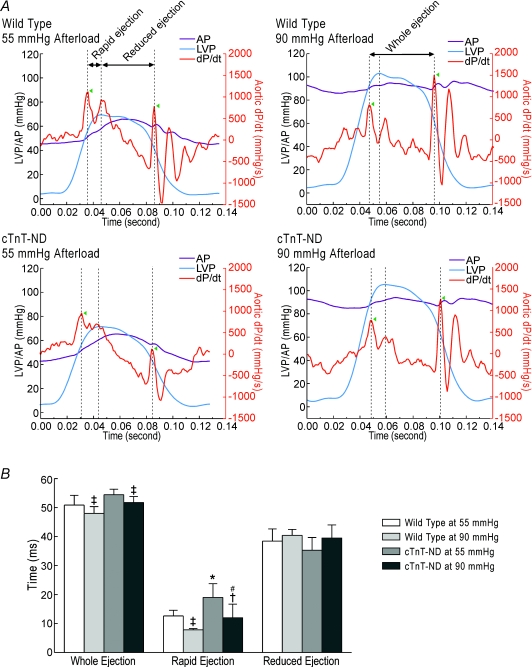
The stroke volume in each pumping cycle is produced only during the ejection phase when the ventricular pressure is higher than the aortic pressure. Therefore, the ejection time is a key factor to determine cardiac function. Figure 5A shows the determination of the time points for the opening and closing of the aorta valve from the aortic development pressure (dP/dt) curve. The total ejection time measured from the opening to closing of the aortic valve was similar between cTnT-ND and wild type hearts (Fig. 5B). The rapid-ejection phase from the opening of the aortic valve to the peak of left ventricle pressure is much more important in sustaining ventricular output than the reduced-ejection phase from the peak of left ventricle pressure to the closing of aortic valve. The results in Fig. 5 show that the rapid-ejection phase was significantly elongated in cTnT-ND hearts as compared with that of the wild type hearts. The elongation of the rapid ejection phase was proportionally more at 90 mmHg afterload than that at 55 mmHg afterload. Therefore, the elongated time of ventricular rapid ejection might be responsible for the cTnT-ND hearts being able to sustain cardiac output against pressure overload. The data suggest that the proteolytic removal of the N-terminal variable region of cTnT alters the function of cardiac muscle thin filaments and kinetics of myocardial contraction to elongate the time of ventricular rapid ejection and increase stroke volume.
Figure 5. Elongated ventricular ejection time in cTnT-ND hearts.
A, left ventricular pressure (LVP), aortic pressure (AP) and aortic dP/dt traces simultaneously collected in working heart preparations from wild type and cTnT-ND mice at 55 mmHg and 90 mmHg afterloads are shown together using the Origin software. In each cardiac cycle, the opening and closing of aorta valve were identified by the reversing points of aortic dP/dt trace (indicated by the arrowheads). The duration between the opening and closing points represents the ventricular ejection time in which the rapid ejection phase is defined as the duration from the opening of aortic valve to the peak of left ventricular pressure and the reduced ejection phase is from the peak of left ventricular pressure to the closing of aortic valve. The representative traces showed that cTnT-ND hearts had a longer ventricular rapid ejection time in comparison with that of the wild type hearts. B, the ejection time data are summarized to show that when afterload was increased from 55 mmHg to 90 mmHg, the ejection time was shortened in both wild type and cTnT-ND hearts. However, the reduction of rapid ejection phase in cTnT-ND hearts was significantly less than that in WT hearts, demonstrating a tolerance to pressure overload. n = 5 for WT and n = 4 for cTnT-ND. Statistical significance was analysed by paired two-tailed Student's t tests except for noted. *P < 0.05 versus WT control; †P < 0.05 versus 55 mmHg afterload; ‡P < 0.01 versus 55 mmHg afterload; #P < 0.05 versus WT control (by unpaired one-tailed Student's t test).
Effect of cTnT-ND on cardiac efficiency
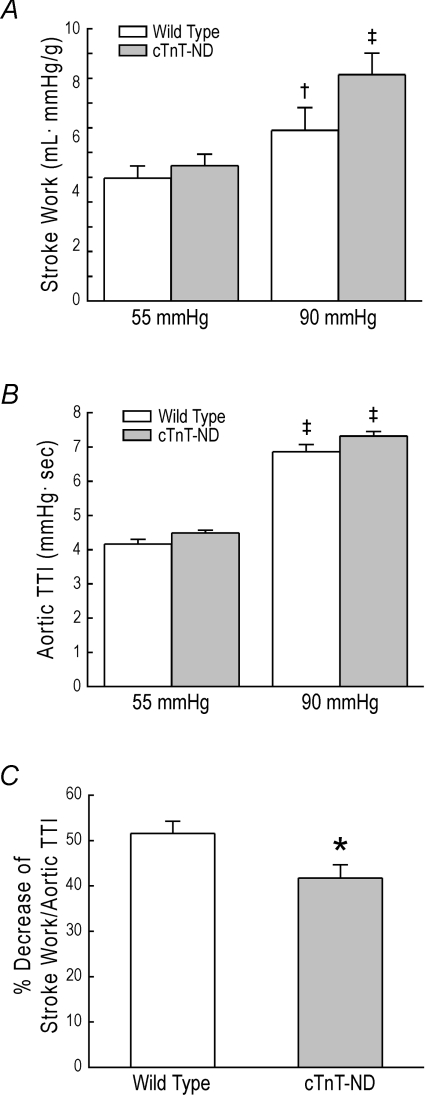
To evaluate the pathophysiological value of the production of TnT-ND in myocardial adaptation to energetic crisis, cardiac stroke work was calculated from left ventricular output and the aortic pressure integral (Fig. 6A) and analysed against the aortic TTI, which is an indicator of oxygen consumption (Fig. 6B). When the afterload was increased from 55 mmHg to 90 mmHg, stroke work increased in both WT and cTnT-ND hearts; cTnT-ND hearts showed a trend of greater increase than that of WT hearts while aortic TTI was increased similarly in both groups. The higher pumping efficiency of cTnT-ND hearts against pressure overload was further demonstrated by a significant reduction of the high afterload-produced decrease in stroke work normalized to aortic TTI in cTnT-ND heart compared with that in WT hearts (Fig. 6C). The results suggest that the presence of cTnT-ND in the cardiac muscle improves pumping efficiency of the heart.
Figure 6. Effect of cTnT-ND on myocardial efficiency.
A, when the afterload was increased from 55 mmHg to 90 mmHg, stroke work significantly increased in both wild type and cTnT-ND hearts. Although statistic significance was not established (P = 0.063 in unpaired one-tailed Student's t test), cTnT-ND hearts showed a trend of greater increase in stroke work than that of wild type hearts. B, aortic TTI as an indicator of oxygen consumption was increased when the afterload was increased with no significant difference between wild type and cTnT-ND hearts. C, cardiac efficiency calculated as stroke work versus aortic TTI showed that the decrease resulted from increasing afterload from 55 to 90 mmHg was significantly less in cTnT-ND heart than that in wild type hearts. n = 5 for wild type and n = 4 for cTnT-ND. *P < 0.05 versus wild type; †P < 0.05 versus 55 mmHg afterload; ‡P < 0.01 versus 55 mmHg afterload (by paired Student's t test).
Discussion
Post-translational modification of myofilament proteins provides rapid responses for myocardial adaptation to physiological and pathological stresses. Two major post-translational mechanisms of myofilament protein regulation are phosphorylation (Sumandea et al. 2004; Maughan, 2005) and proteolysis (Murphy et al. 2000; Communal et al. 2002; Zhang et al. 2006). While the proteolytic C-terminal truncation of cardiac TnI resulted in myocardial stunning (Murphy et al. 2000), a restricted deletion of the N-terminal extension of cardiac TnI found during haemodynamic adaptation (Yu et al. 2001) enhances the relaxation of cardiac muscle (Barbato et al. 2005). We previously reported the restricted proteolytic N-terminal truncation of cTnT by μ-calpain to selectively remove the entire variable region but preserve the conserved regions (Zhang et al. 2006). In the present study, we investigated the functional significance of this post-translational modification of cTnT and the results provided demonstrate an adaptation mechanism other than deterioration in the myocardium under stress conditions. The following discussions focus on the significance of our findings.
Pressure overload and energetic crisis induces restricted N-terminal truncation of cardiac TnT
Haemodynamic response to decreased cardiac output results in vasoconstriction that increases the ventricular afterload (Zelis et al. 1968; Zelis & Flaim, 1982). Therefore, pressure overload is a major stress in heart failure conditions such as in acute myocardial infarction. To dissect the complex neurohumoral adaptations in heart failure, the isolated working heart models allow us to examine the direct response of myocardium to pressure overload. A previous report described a cTnT fragment in ventricular muscle of rat with pulmonary hypertension (Kogler et al. 2003). Another study found a decreased level of intact cTnT in rat heart under right ventricular pressure overload (Schott et al. 2005). These observations suggest that chronic haemodynamic stress may result in adaptive modification of thin filament proteins. Although the nature of the cTnT fragments was not characterized in the Kogler study, its gel mobility resembles that of cTnT-ND and supports our hypothesis that haemodynamic stress, particularly pressure overload, induces cTnT N-terminal truncation as a post-translational mechanism to modify myocardial function in adaptations to physiological and pathological conditions. Although relatively low levels of cTnT-ND were seen in our acute ex vivo ischaemia and pressure overloaded models, they demonstrate an endogenous mechanism that produces restricted proteolytic modification of cTnT. We have previously demonstrated a much higher level of cTnT-ND produced by ischaemia–reperfusion treatment of isolated cardiomyocytes (Zhang et al. 2006), indicating the potency of this proteolytic regulation in cardiac muscle cells.
cTnT-ND plays a role in myocardial adaptation to stress conditions
Hearts from 10-month-old cTnT-ND transgenic mice showed no hypertrophy (Table 2), indicating that cTnT-ND is not simply a structural destruction in ischaemia–reperfusion injury. Functional characterization of the cTnT-ND transgenic mouse hearts only detected a decreased contractile velocity whereas the other baseline parameters were normal, supporting the non-destructive nature of cTnT-ND. On the other hand, cTnT-ND hearts showed a tolerance to pressure overload by having elongated rapid ejection time to sustain stroke volume (Figs 4 and 5), supporting a novel observation that the production of cTnT-ND is a compensatory regulation for functional adaptation in myocardial energetic crisis.
The higher pumping efficiency of the cTnT-ND hearts indicated by the smaller reduction in stroke work normalized to aortic TTI in cTnT-ND hearts than that in WT hearts when the afterload was increased (Fig. 6C) demonstrates the compensatory value of TnT-ND in myocardial adaptation to energetic crisis. The cTnT-ND cardiac muscle showed slower contractile velocity than wild type control. A previous study showed that switching from the faster α-myosin to the slower β-myosin in mouse heart produced better energy efficiency in cardiac performance (Hoyer et al. 2007). Similarly, expression of slower myosin isoforms also increased the energy efficiency of skeletal muscle contraction (Reggiani et al. 1997). Our finding that the restricted N-terminal truncation of TnT results in slower contractile velocity demonstrated that modification of TnT structure and function could alter myosin cross-bridge kinetics and affect muscle energetic efficiency.
The N-terminal modification of cTnT specifically elongates the rapid ejection phase and maintains the total ejection time unchanged (Fig. 5B). Together with unchanged relaxation velocity, ventricular filling time would not be shortened. While the decreased contractile velocity due to cTnT-ND could contribute to the depressed function in myocardial ischaemia–reperfusion, it may provide a protective mechanism against Ca2+ overload-induced contractures (Piper et al. 2003).
N-terminal modulation of the function of cardiac TnT
Expression of alternatively spliced cTnT isoforms with changes in the N-terminal variable region has been found in human and animal models of heart failure (Anderson et al. 1995; Biesiadecki & Jin, 2002; Biesiadecki et al. 2002). Demonstrating a post-translational regulation of TnT structure and function, the μ-calpain-catalysed restrictive N-terminal truncation removes the entire N-terminal variable region (Zhang et al. 2006). cTnT-ND preserves the conserved regions and is retained in the cardiac myofibrils and therefore will alter the thin filament regulation.
We previously showed that cTnT-ND has an increased affinity to tropomyosin (Zhang et al. 2006). In reconstituted myofibrils, a similar N-terminal-deleted cTnT resulted in decreased force development (Chandra et al. 1999). Reconstituted troponin complex containing an N-terminal truncated fast TnT conferred a decrease in the maximum activation of actomyosin-S1 MgATPase (Pan et al. 1991). Based on the model that the N-terminal variable region of TnT is a modulatory structure (Wang & Jin, 1998; Jin & Root, 2000) that enhances the maximum level of Ca2+-activated ATPase (Pan et al. 1991) and force (Chandra et al. 1999), the present study suggests that proteolytic deletion of the entire N-terminal variable region of cTnT is a rapid mechanism to modulate the kinetics of cross-bridge cycling in adaptation to physiological and pathological stress conditions.
Stress-induced production of cTnT-ND is a whole organ response
The proteolytic deletion of the N-terminal variable region of cTnT is produced by μ-calpain cleavage (Zhang et al. 2006). The data that pressure overload, but not volume overload (Fig. 2A), induces the N-terminal truncation of cTnT suggest that mechanical stresses could trigger biochemical modifications in the myofilaments. When isolated working heart preparations were used to produce acute heart failure by regional ischaemia–reperfusion to generate left side heart failure, cTnT-ND occurred not only in the infarct area or restricted to the left ventricle but also in the right ventricle (Fig. 1). Similarly, when the working heart was treated with one sided ventricular pressure overload, cTnT-ND was produced in both ventricles (Fig. 2). Therefore, the pressure overload-induced production of cTnT-ND is a whole organ response.
The ventricular myocardium is an electrophysiological syncytium that performs synchronized contraction that undergoes near-uniform membrane action potential changes during the cardiac cycle (De Bakker & Van Rijen, 2006). The duration of the cardiac action potential is influenced by the mechanical loading conditions of the heart, a process termed ‘contraction–excitation feedback’ (Laboratory, 1982). Elevation of left ventricular peak systolic pressure was found to shorten the monophasic action potential in the heart (Coulshed et al. 1992). Therefore, this mechanism is worth further investigation for its role in the production of cTnT-ND in the responses of both ventricles to one sided pressure overload.
Conclusion
The present study demonstrated a post-translational regulation of cardiac myofilament function as a novel mechanism to compensate for heart function under stress conditions with decreased energetic supply and/or increased workload. In contrast to simple protein degradation, the restricted proteolysis of cTnT that selectively removes the N-terminal variable region and preserves the essential structure of TnT confers a rapid mechanism to modify the kinetics of myocardial contraction and increase energetic efficiency as a whole organ adaptation to sustain cardiac function in congestive heart failure.
Acknowledgments
We thank Dr Jeffrey Robbins for providing the mouse cardiac α-MHC gene promoter. This study was supported by grants from the National Institutes of Health HL-078773 and AR-048816 and the Arnold and Ann Berlin Cardiovascular Care Research Fund to J.-P. Jin. Han-Zhong Feng is a PhD candidate of Aerospace Physiology at Fourth Military Medical University, Xi'an, China. Brandon Biesiadecki participated in this study under the support of an American Heart Association Ohio Valley Affiliate Postdoctoral Fellowship when this research group work at Case Western Reserve University, Cleveland, Ohio.
References
- Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 1995;76:681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 1991;69:1226–1233. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- Barbato JC, Huang QQ, Hossain MM, Bond M, Jin JP. Proteolytic N-terminal truncation of cardiac troponin I enhances ventricular diastolic function. J Biol Chem. 2005;280:6602–6609. doi: 10.1074/jbc.M408525200. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, Elder BD, Yu ZB, Jin JP. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem. 2002;277:50275–50285. doi: 10.1074/jbc.M206369200. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, Jin JP. Exon skipping in cardiac troponin T of turkeys with inherited dilated cardiomyopathy. J Biol Chem. 2002;277:18459–18468. doi: 10.1074/jbc.M200788200. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Welch GH, Jr, Sarnoff SJ. Hemodynamic effects of quantitatively varied experimental mitral regurgitation. Circ Res. 1957;5:539–545. doi: 10.1161/01.res.5.5.539. [DOI] [PubMed] [Google Scholar]
- Breitbart RE, Nadal-Ginard B. Complete nucleotide sequence of the fast skeletal troponin T gene. Alternatively spliced exons exhibit unusual interspecies divergence. J Mol Biol. 1986;188:313–324. doi: 10.1016/0022-2836(86)90157-9. [DOI] [PubMed] [Google Scholar]
- Chandra M, Montgomery DE, Kim JJ, Solaro RJ. The N-terminal region of troponin T is essential for the maximal activation of rat cardiac myofilaments. J Mol Cell Cardiol. 1999;31:867–880. doi: 10.1006/jmcc.1999.0928. [DOI] [PubMed] [Google Scholar]
- Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:6252–6256. doi: 10.1073/pnas.092022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulshed DS, Cowan JC, Drinkhill MJ, Hainsworth R. The effects of ventricular end-diastolic and systolic pressures on action potential and duration in anaesthetized dogs. J Physiol. 1992;457:75–91. doi: 10.1113/jphysiol.1992.sp019365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bakker JM, Van Rijen HM. Continuous and discontinuous propagation in heart muscle. J Cardiovasc Electrophysiol. 2006;17:567–573. doi: 10.1111/j.1540-8167.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Szilvassy Z, Droy-Lefaix MT, Tarrade T, Koltai M. KATP channel modulation in working rat hearts with coronary occlusion: effects of cromakalim, cicletanine, and glibenclamide. Cardiovasc Res. 1995;30:781–787. [PubMed] [Google Scholar]
- Fujita S, Maeda K, Maeda Y. Expression in Escherichia coli and a functional study of a β-troponin T 25 kDa fragment of rabbit skeletal muscle. J Biochem. 1992;112:306–308. doi: 10.1093/oxfordjournals.jbchem.a123896. [DOI] [PubMed] [Google Scholar]
- Gauthier NS, Matherne GP, Morrison RR, Headrick JP. Determination of function in the isolated working mouse heart: issues in experimental design. J Mol Cell Cardiol. 1998;30:453–461. doi: 10.1006/jmcc.1997.0610. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Guzman G, Zhao J, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem. 2002;277:35341–35349. doi: 10.1074/jbc.M204118200. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Heeley DH, Golosinska K, Smillie LB. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem. 1987;262:9971–9978. [PubMed] [Google Scholar]
- How OJ, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS. Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2979–H2985. doi: 10.1152/ajpheart.00084.2005. [DOI] [PubMed] [Google Scholar]
- Hoyer K, Krenz M, Robbins J, Ingwall JS. Shifts in the myosin heavy chain isozymes in the mouse heart result in increased energy efficiency. J Mol Cell Cardiol. 2007;42:214–221. doi: 10.1016/j.yjmcc.2006.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QQ, Chen A, Jin JP. Genomic sequence and structural organization of mouse slow skeletal muscle troponin T gene. Gene. 1999;229:1–10. doi: 10.1016/s0378-1119(99)00051-7. [DOI] [PubMed] [Google Scholar]
- Huang QQ, Feng HZ, Du Liu JJ, Stull LB, Moravec C, Huang X, Jin JP. Co-expression of skeletal and cardiac troponin T decreases mouse cardiac function. Am J Physiol Cell Physiol. 2008;294:C213–C222. doi: 10.1152/ajpcell.00146.2007. [DOI] [PubMed] [Google Scholar]
- Jin JP, Chen A, Ogut O, Huang QQ. Conformational modulation of slow skeletal muscle troponin T by an NH2-terminal metal-binding extension. Am J Physiol Cell Physiol. 2000;279:C1067–C1077. doi: 10.1152/ajpcell.2000.279.4.C1067. [DOI] [PubMed] [Google Scholar]
- Jin JP, Huang QQ, Yeh HI, Lin JJ. Complete nucleotide sequence and structural organization of rat cardiac troponin T gene. A single gene generates embryonic and adult isoforms via developmentally regulated alternative splicing. J Mol Biol. 1992;227:1269–1276. doi: 10.1016/0022-2836(92)90540-z. [DOI] [PubMed] [Google Scholar]
- Jin JP, Root DD. Modulation of troponin T molecular conformation and flexibility by metal ion binding to the NH2-terminal variable region. Biochemistry. 2000;39:11702–11713. doi: 10.1021/bi9927437. [DOI] [PubMed] [Google Scholar]
- Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation and functional adaptations of troponin and calponin. Crit Rev Eukaryot Gene Expr. 2008;18:93–124. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- Kogler H, Hartmann O, Leineweber K, Nguyen VP, Schott P, Brodde OE, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res. 2003;93:230–237. doi: 10.1161/01.RES.0000085042.89656.C7. [DOI] [PubMed] [Google Scholar]
- Laboratory MJ. Contraction-excitation feedback in myocardium. Physiological basis and clinical relevance. Circ Res. 1982;50:757–766. doi: 10.1161/01.res.50.6.757. [DOI] [PubMed] [Google Scholar]
- Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem. 1981;256:964–968. [PubMed] [Google Scholar]
- Maughan DW. Kinetics and energetics of the crossbridge cycle. Heart Fail Rev. 2005;10:175–185. doi: 10.1007/s10741-005-5248-2. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science. 2000;287:488–491. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967;212:804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Pan BS, Gordon AM, Potter JD. Deletion of the first 45 NH2-terminal residues of rabbit skeletal troponin T strengthens binding of troponin to immobilized tropomyosin. J Biol Chem. 1991;266:12432–12438. [PubMed] [Google Scholar]
- Pearlstone JR, Smillie LB. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem. 1982;257:10587–10592. [PubMed] [Google Scholar]
- Piper HM, Meuter K, Schafer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644–S648. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- Reggiani C, Potma EJ, Bottinelli R, Canepari M, Pellegrino MA, Stienen GJ. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol. 1997;502:449–460. doi: 10.1111/j.1469-7793.1997.449bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott P, Singer SS, Kogler H, Neddermeier D, Leineweber K, Brodde OE, Regitz-Zagrosek V, Schmidt B, Dihazi H, Hasenfuss G. Pressure overload and neurohumoral activation differentially affect the myocardial proteome. Proteomics. 2005;5:1372–1381. doi: 10.1002/pmic.200401005. [DOI] [PubMed] [Google Scholar]
- Subramaniam A, Gulick J, Neumann J, Knotts S, Robbins J. Transgenic analysis of the thyroid-responsive elements in the α-cardiac myosin heavy chain gene promoter. J Biol Chem. 1993;268:4331–4336. [PubMed] [Google Scholar]
- Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- Sutherland FJ, Baker KE, Shattock MJ, Hearse DJ. Responses to ischaemia and reperfusion in the mouse isolated perfused heart and the phenomenon of contractile cycling. Clin Exp Pharmacol Physiol. 2003;30:879–884. doi: 10.1046/j.1440-1681.2003.03926.x. [DOI] [PubMed] [Google Scholar]
- Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- Wang J, Jin JP. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry. 1998;37:14519–14528. doi: 10.1021/bi9812322. [DOI] [PubMed] [Google Scholar]
- Wang QD, Tokuno S, Valen G, Sjoquist PO, Thoren P. Cyclic fluctuations in the cardiac performance of the isolated Langendorff-perfused mouse heart: pyruvate abolishes the fluctuations and has an anti-ischaemic effect. Acta Physiol Scand. 2002;175:279–287. doi: 10.1046/j.1365-201X.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol. 2007;292:C1192–C1203. doi: 10.1152/ajpcell.00462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZB, Zhang LF, Jin JP. A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity. J Biol Chem. 2001;276:15753–15760. doi: 10.1074/jbc.M011048200. [DOI] [PubMed] [Google Scholar]
- Zelis R, Flaim SF. Alterations in vasomotor tone in congestive heart failure. Prog Cardiovasc Dis. 1982;24:437–459. doi: 10.1016/0033-0620(82)90012-3. [DOI] [PubMed] [Google Scholar]
- Zelis R, Mason DT, Braunwald E. A comparison of the effects of vasodilator stimuli on peripheral resistance vessels in normal subjects and in patients with congestive heart failure. J Clin Invest. 1968;47:960–970. doi: 10.1172/JCI105788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry. 2006;45:11681–11694. doi: 10.1021/bi060273s. [DOI] [PMC free article] [PubMed] [Google Scholar]