Abstract
Pancreatic β-cells secrete insulin by Ca2+-dependent exocytosis of secretory granules. β-cell exocytosis involves SNARE (soluble NSF-attachment protein receptor) proteins similar to those controlling neurotransmitter release and depends on the close association of L-type Ca2+ channels and granules. In most cases, the secretory granules fuse individually but there is ultrastructural and biophysical evidence of multivesicular exocytosis. Estimates of the secretory rate in β-cells in intact islets indicate a release rate of ∼15 granules per β-cell per second, 100-fold higher than that observed in biochemical assays. Single-vesicle capacitance measurements reveal that the diameter of the fusion pore connecting the granule lumen with the exterior is ∼1.4 nm. This is considerably smaller than the size of insulin and membrane fusion is therefore not obligatorily associated with release of the cargo, a feature that may contribute to the different rates of secretion detected by the biochemical and biophysical measurements. However, small molecules like ATP and GABA, which are stored together with insulin in the granules, are small enough to be released via the narrow fusion pore, which accordingly functions as a molecular sieve. We finally consider the possibility that defective fusion pore expansion accounts for the decrease in insulin secretion observed in pathophysiological states including long-term exposure to lipids.
Insulin secretion in vivo follows a characteristic biphasic time course: an initial component, which develops rapidly but only lasts a few minutes (1st phase), is followed by a slowly developing but sustained component (2nd phase) (Curry et al. 1968). Type-2 diabetes is associated with complete loss of 1st phase secretion and strong reduction of 2nd phase release (Hosker et al. 1989). If we knew the cellular/molecular background of biphasic insulin secretion, then we might understand what goes wrong in type-2 diabetes.
The last 15 years have, thanks to the application of several high-resolution techniques like capacitance measurements (Neher, 1998), carbon fibre amperometry (Wightman et al. 1991; Chow et al. 1992) and single-vesicle imaging (TIRF and confocal) (Steyer et al. 1997), witnessed an explosion in our knowledge about exocytosis in the β-cell. Here we attempt to review some of these new data. We will first discuss the β-cell biology of biphasic insulin secretion. Second, we will describe the roles of some of the proteins that are part of the molecular machinery involved in insulin granule exocytosis. Third, we will evaluate the possibility that the release of biomolecules from β-cell granules is regulated in a size-dependent manner by the fusion pore. Fourth, we present some evidence indicating that insulin granules, in addition to fusing individually with the plasma membrane, can undergo compound exocytosis. Finally, we summarize the data to suggest that defects of the exocytotic process may be of pathophysiological significance and even contribute to the lowered insulin secretory capacity in diabetes.
The insulin granule
Insulin plays a central role in the fuel homeostasis of the entire body. It is secreted by the islets of Langerhans in the pancreas. Human insulin is synthesized as a 110 amino acid precursor (prepro-insulin) in the rough endoplasmic reticulum. Following removal of a 24-residue signal sequence and packaging in the Golgi complex, insulin is stored as pro-insulin in the immature secretory granules where conversion into its biologically active form is catalysed by the concerted activities of PC1-2 and the exoprotease CPH, to produce mature insulin and C-peptide (Goodge & Hutton, 2000). Once mature insulin has been formed, it can be stored for several days before being released or degraded by crinophagia (i.e. the intracellular destruction of the insulin granules by the lysosomes; Schnell et al. 1988). Interestingly and for reasons that remain obscure, the granules last to be generated are the most likely ones to undergo release when the β-cell is stimulated (Gold et al. 1982; Duncan et al. 2003).
Every β-cell contains 10 000–13 000 secretory granules (Dean, 1973; Olofsson et al. 2002; Straub et al. 2004). These have a diameter of ∼350 nm and often contain an electron-dense Zn2-insulin6 crystal, which is surrounded by a halo. A single insulin granule contains ∼1.6 amol (10−18 mol) of insulin (8 fg or 106 molecules of insulin) (Rorsman & Renström, 2003). In addition to insulin, the secretory granules contain another ∼50 polypeptides (Baillyes et al. 1992), some of which have biological functions like islet amyloid polypeptide (IAPP) and chromogranin A. The granules also store a number of low-molecular weight compounds like ATP, GABA, serotonin and glutamate and high concentrations of metal ions including Zn2+ and Ca2+ (Hutton et al. 1983). Ultrastructural studies have revealed that ∼600 granules are docked with the plasma membrane (Olofsson et al. 2002; Straub et al. 2004) and a further ∼1500 are situated ≤ 0.2 μm from the cell surface (Olofsson et al. 2002).
The β-cell is electrically excitable and changes in the membrane potential couple variations in plasma glucose concentration to stimulation or inhibition of insulin secretion (Ashcroft & Rorsman, 1989). This process is well understood and involves glucose metabolism, production of ATP, closure of KATP channels, membrane depolarization and Ca2+-dependent action potential firing. Insulin is then released by Ca2+-dependent exocytosis of the secretory granules. Once plasma glucose has been lowered by insulin-dependent uptake into the target organ, reversal of the process outlined above leads to cessation of insulin secretion. Thus, plasma glucose is under feedback control of insulin via changes in β-cell metabolism, KATP channel closure and electrical activity/secretion. The ability of glucose to elicit electrical activity is referred to as its ‘triggering’ action. In addition, glucose exerts an ‘amplifying’ action on secretion and that results in a greater secretory response for a given increase in cytosolic [Ca2+]i (Henquin, 2000). The molecular identity of the ‘amplifying’ signal remains to be established. The triggering and amplifying effects of glucose also operate in human β-cells (Henquin et al. 2006). Human β-cells are equipped with KATP channels which maintain a negative membrane potential at low glucose concentrations and are closed in response to glucose stimulation (Gromada et al. 1998). Interestingly, the complements of the voltage-gated ion channels are quite different in mouse and human β-cells (Braun et al. 2008).
β-cell biology of biphasic insulin secretion
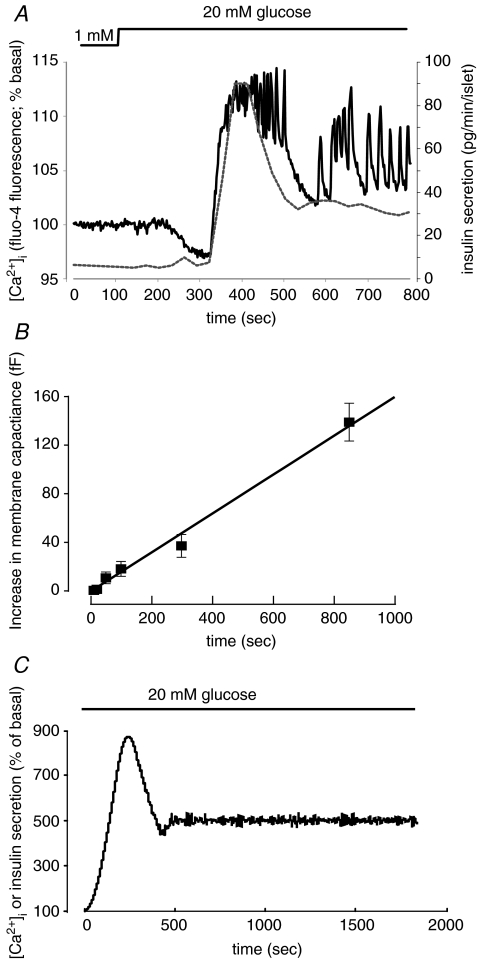
In response to a glucose challenge from 1 to 20 mm, insulin secretion from intact mouse islets increases 15-fold to a peak value of 90 pg islet−1 min−1. The latter value corresponds to 11 granules β-cell−1 min−1, assuming 1000 β-cells per islet and 8 fg of insulin per granule (Fig. 1A). Over the duration of 1st phase insulin secretion (4 min), a single β-cell can thus be estimated to release a total of ∼20 granules. Capacitance measurements on isolated β-cells have identified a pool of readily releasable granules (RRP) that are immediately available for release. Under basal conditions (no activation of protein kinases), the size of RRP has been estimated as ∼60 fF (Gromada et al. 1999). This equates to ∼20 granules using a conversion factor of ∼3 fF per granule (MacDonald et al. 2006; Braun et al. 2007). The close agreement of these numbers led to the proposal that 1st phase insulin secretion may reflect the release of RRP and that these represent a subset of the docked pool (Daniel et al. 1999; Rorsman & Renström, 2003). Indeed, ultrastructural analysis before and after a high-K+ depolarization (a condition believed to simulate 1st phase insulin secretion) leads to a slight reduction of the physically docked granules (Olofsson et al. 2002). More recently, TIRF imaging of isolated β-cells has provided direct evidence that the initial component of insulin secretion involves release of already docked granules (Ohara-Imaizumi et al. 2007). In this scenario, the end of 1st phase insulin secretion reflects the emptying of the RRP, and 2nd phase secretion involves the supply of new granules for release by ATP- and time-dependent granule priming, which thus becomes rate-limiting for exocytosis. It is possible that glucose-induced acceleration of granule priming underlies the amplifying effect of glucose on insulin secretion. Although there is consensus that membrane depolarization (evoked by high-K+ stimulation or voltage-clamp depolarizations) is associated with discharge of docked granules, there is an ongoing debate with regard to the role of the docked granules in glucose-induced 1st phase insulin release. Thus, a recent study reported that 1st phase secretion did not involve the docked granules but rather granules that approached the plasma membrane during stimulation only to be immediately released (‘restless newcomers’; Shibasaki et al. 2007).
Figure 1. [Ca2+]i and biphasic insulin secretion.
A, biphasic insulin secretion measured in ∼100 isolated islets (grey dotted line) and [Ca2+]i recorded from a single intact islet (black continuous trace). B, exocytosis measured in β-cells in situ. Note linear relationship between pulse length and response. Data from Göpel et al. (2004). C, mathematical simulation of [Ca2+]i and secretion. Summation of the responses in 400 islets obtained by assuming a 1st phase response of 800% of the basal value that occurred with a delay of 0–180 s and lasted 300 s followed by oscillations that had a variable amplitude of 200–400% of basal and a random period of 10–30 s.
It should be emphasized that apart from the insulin secretion data, all studies summarized above were based on experiments in isolated β-cells and it is not self-evident that such data can be extrapolated to the situation in situ. Indeed, when capacitance measurements are carried out on superficial β-cells in intact mouse islets, the evidence for a defined RRP is less convincing and exocytosis appears to proceed linearly with time/stimulus (Fig. 1B; Göpel et al. 2004). Using pancreatic slices and β-cells located in the islet centre, the relationship between stimulation and exocytosis was also found to be linear (Speier & Rupnik, 2003).
Changes in islet [Ca2+]i will report the summed behaviour of all superficial β-cells that in turn is due to β-cell electrical activity (Sánchez-Andrés et al. 1995). The similar and biphasic kinetics of β-cell electrical activity and insulin secretion in intact mouse islets was first noted more than 30 years ago (Meissner, 1976; Meissner & Atwater, 1976). The temporal correlation between glucose-evoked insulin secretion and changes in islet [Ca2+]i (Fig. 1A) is indeed suggestive of a direct link between the two processes. This may seem incompatible with the finding that no synchronization of [Ca2+]i across the pancreas was observed in simultaneous in vivo recordings of electrical activity in different islets (Valdeolmillos et al. 1996). However, theoretical analyses suggest that the fact that the single-islet response is biphasic might nevertheless suffice to explain the biphasic response observed systemically. As illustrated in Fig. 1C, summation of the secretory responses from 400 islets predicts a secretory response similar to that which can be measured in the perfused pancreas and groups of perifused islets.
The number of docked granules is sufficient for several hours of glucose-induced insulin secretion. However, granule exocytosis must eventually be balanced by the supply of new granules towards the plasma membrane. Directed movements of secretory granules occur along a microtubule network (Ivarsson et al. 2004). Like other secretory cells (Vitale et al. 1995), the β-cells are equipped with a dense actin web below the plasma membrane that controls the docking of the secretory granules (Fig. 2). Disruption of the actin network with latrunculin or cytochalasin B leads to strong stimulation of insulin secretion in rodent and human islets (Orci et al. 1972; Jewell et al. 2008). The importance of reorganization of the actin network is highlighted by recent experiments in NCAM-deficient mice (Esni et al. 1999). Insulin secretion from these mice at high glucose concentrations (30 mm) is reduced by > 50% relative to wild-type islets. This defect is associated with impaired reorganization of the actin network. Disruption of the actin network by pre-treatment of the islets with cytochalasin B resulted in strong stimulation of insulin secretion evoked by 30 mm glucose in NCAM−/− islets and abolished the difference between wild-type and knockout islets (C. S. Olofsson, P. Rorsman & H. Semb, unpublished observations). These data raise the possibility that remodelling of the cytoskeleton contributes to the amplifying action of glucose on insulin secretion.
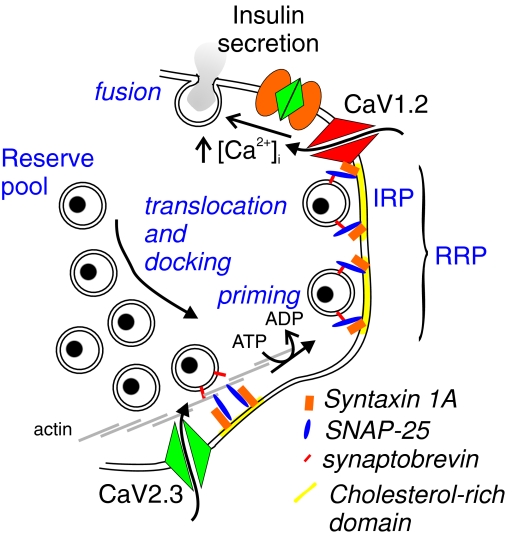
Figure 2. Insulin secretion.
In mouse β-cells, exocytosis is principally dependent on Ca2+ influx through Cav1.2 L-type Ca2+ channels. A subset of the release-competent granules (readily releasable pool; RRP) is believed to be situated in the immediate vicinity of the Ca2+ channels and is referred to as the immediately releasable pool (IRP). Only a few per cent of the total granule number exist in RRP/IRP and the remainder belongs to a reserve pool. These granules must undergo a number of priming reactions and/or physical translocation to dock with the plasma membrane and attain release competence. The existence of cholesterol-rich membrane areas, to which the exocytotic proteins syntaxin 1A, SNAP-25 and synaptobrevin/VAMP-2 concentrate, as well as an actin web between the plasma membrane and the majority of secretory granules is indicated.
Molecular control of β-cell exocytosis
Exocytosis in the β-cell mostly occurs in response to an elevation of cytosolic [Ca2+]i and bears strong resemblance to the release of neurotransmitters in the synapse (Fig. 2). Indeed, many of the SNARE proteins (soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor) critical to synaptic vesicle release are also expressed in the β-cell and presumably fulfil the same function(s) (Lang, 1999; Gerber & Sudhof, 2002). This includes the t-SNAREs syntaxin 1 and SNAP-25 as well as the v-SNARE synaptobrevin/VAMP-2.
Syntaxin 1 and SNAP-25 aggregate into ∼400 complexes in mouse β-cells (Vikman et al. 2006). The latter number is similar to the number of docked granules in the β-cell as estimated by ultrastructural studies (Olofsson et al. 2002). It is also close to the number of voltage-gated L-type Ca2+ channels in a single β-cell as estimated by non-stationary fluctuation analysis (Barg et al. 2001). The syntaxin 1/SNAP-25 clusters concentrate in cholesterol-rich membrane regions (membrane rafts) (Fig. 2). Removal of cholesterol from the plasma membrane using methyl-β-cyclodextrin (MBCD) reduces insulin secretion and inhibits exocytosis as monitored by TIRF microscopy (Ohara-Imaizumi et al. 2004) and capacitance measurements (Vikman & Eliasson, 2005). The effects of MBCD treatment on secretion are associated with the translocation of SNAP-25 from the plasma membrane to the cytosol (Vikman & Eliasson, 2005).
Immunoneutralization of SNAP-25 leads to inhibition of rapid exocytosis (i.e. that which can be evoked by a single 500 ms depolarization) via a direct effect that cannot be accounted for by inhibition of the Ca2+ current (Vikman et al. 2006). In chromaffin cells, PKA- and PKC-dependent phosphorylation/dephosphorylation of SNAP-25 at Thr-138 (Nagy et al. 2004) and Ser-187 (Nagy et al. 2002), respectively, plays a central role in the modulation of the secretory capacity. Recent data indicate that overexpression of a mutant form of SNAP-25 lacking the last nine residues (i.e. SNAP-251-197) leads to ablation of the cAMP-induced enhancement of β-cell exocytosis (Eliasson et al. 2005). Phosphorylation of SNAP-25 by PKC has also been found to stimulate exocytosis in insulin-secreting cell lines (Shu et al. 2008). In addition, high concentrations of cAMP (> 10 μm) enhance rapid exocytosis through a PKA-independent mechanism (Eliasson et al. 2003) that culminates in activation of the low-affinity cAMP sensor cAMP-GEFII via RIM2 (Seino & Shibasaki, 2005; Shibasaki et al. 2007). Exactly how cAMP-GEFII enhances exocytosis remains unknown but the effect is also lost upon overexpression of the truncated form of SNAP-25 (Eliasson et al. 2005). Thus, SNAP-25 appears to play an integral part in the adjustment of β-cell exocytosis over a wide range of cAMP concentrations. Inhibitory agonists like somatostatin inhibit exocytosis by G-protein-dependent activation of the protein phosphatase calcineurin (Renström et al. 1996). It is tempting to speculate that this is mediated by dephosphorylation of SNAP-25.
SNAP-25 is also involved in the priming of new granules for release. The blind-drunk mouse (+/Bdr) has a mutation in SNAP-25b (Jeans et al. 2007), which is coexpressed in mouse β-cells with SNAP-25a. Islets from +/Bdr mice exhibit reduced glucose-stimulated insulin secretion whereas K+-stimulated release was unaffected. As discussed above, the high K+ stimulation protocol is believed to principally evoke exocytosis of RRP granules (Rorsman & Renström, 2003). Indeed, in capacitance measurements, exocytosis evoked by brief stimulations was unaffected in +/Bdr β-cells, whereas that observed during protracted and repetitive stimulation was strongly affected. In silico analysis of protein interactions indicates that the Bdr mutation stabilizes the SNARE complex, an effect that can be envisaged to result in slower disassembly of the complex following exocytosis. This might interfere with the supply of new granules to the release sites. This idea is suggested by analogy to the effects upon intracellular application of antibodies against NSF (Vikman et al. 2003), a protein with a well-established role in the disassembly of the SNARE complex (Barnard et al. 1997).
The t-SNARE syntaxin 1 has multiple roles in the exocytotic process. Intracellular application of antibodies directed against the N-terminal part of syntaxin 1 inhibits β-cell exocytosis. This effect appeared to be secondary to strong inhibition of the voltage-gated Ca2+ current (Vikman et al. 2006). Syntaxin 1 changes from a closed to an open conformation during granule priming in a process mediated by munc-18 (Yang et al. 2000; Dulubova et al. 2007). Whilst playing an important role in the docking of the granules with the plasma membrane, syntaxin 1 does not appear to be required for granule priming and 2nd phase insulin secretion is unaffected in islets from syntaxin-deficient mice (Ohara-Imaizumi et al. 2007). Intriguingly, syntaxin 1 also modulates the activity of ATP-regulated K+ channels as well as voltage-gated delayed rectifying K+ channels in insulin-secreting β-cells (Leung et al. 2007) and its function may accordingly not be limited to the regulation of exocytosis.
Mouse β-cells also express syntaxin 4. Islets from heterozygous syntaxin 4+/− mice exhibit reduced 1st and 2nd phase secretion (Spurlin & Thurmond, 2006). Syntaxin 4 exists in an open and a closed state. In its closed state, syntaxin 4 tethers F-actin to the plasma membrane and thereby prevents granule docking. Glucose-induced reorganization of the actin network liberates syntaxin 4 (it enters an ‘open’ state) and allows the granules to traffic to the plasma membrane and interact with syntaxin 4 (Jewell et al. 2008).
The CAPS (Ca2+-dependent activator protein for secretion) proteins (Olsen et al. 2003; Speidel et al. 2008) and munc-13 (Kang et al. 2006) are likewise important for granule priming. Whereas munc13–1 mediates vesicle priming by activating syntaxin and promoting SNARE complex formation, the role of the CAPS proteins in insulin secretion is not fully understood. However, the similarities between munc13–1 and CAPS deficiency suggest that the proteins co-operate in the priming process. In addition, CAPS proteins influence granule stability and ablation leads to increased crinophagia (Speidel et al. 2008).
Another protein influencing granule docking in β-cells is granuphilin (Wang et al. 1999). Ablation of granuphilin leads to a reduction of the number of docked granules but nevertheless stimulates insulin secretion (Torii et al. 2002). Conversely, overexpression of granuphilin leads to suppression of insulin secretion and there is evidence that up-regulation of granuphilin could mediate the inhibitory effects of long-term exposure to lipids on insulin secretion (Kato et al. 2006). Granuphilin mediates its effects on exocytosis via interaction with the syntaxin 1/munc18-1 complex (Fukuda et al. 2005).
MicroRNAs (miRs) are small non-coding RNAs that can regulate the translation of specific target proteins (Gauthier & Wollheim, 2006). Overexpression of miRs 375, 96 and 124 results in inhibition of glucose-induced insulin secretion (Poy et al. 2004; Lovis et al. 2008). These effects were associated with changes in the expression of exocytotic proteins including SNAP-25 (Lovis et al. 2008). It will be interesting to study the phenotype of mice lacking these miRs.
Ca2+ sensor of β-cell exocytosis
Insulin secretion is a Ca2+-dependent process. Experiments using photorelease of caged Ca2+ in conjunction with capacitance measurements indicate that exocytosis triggered by a step increase in [Ca2+]i occurs with a delay of ∼10 ms, is half-maximal at 17 μm and exhibits high co-operativity as suggested by a Hill coefficient > 5 (Barg et al. 2001). Regulation of exocytosis in β-cells by a low-affinity Ca2+ sensor is in agreement with the finding that exocytosis evoked by voltage-clamp depolarizations in mouse β-cells echoes the Ca2+ current and stops almost immediately upon repolarization and closure of the Ca2+ channels (Ammala et al. 1993; Barg et al. 2001). There is evidence that in mouse β-cells L-type Ca2+ channels and the secretory granules assemble into a tight complex which ensures that the exocytotic machinery is exposed to the very high [Ca2+]i in the immediate vicinity of the Ca2+ channels (Wiser et al. 1999). However, exocytosis in β-cells also proceeds at low [Ca2+]i and at rates inconsistent with the idea that exocytosis is regulated solely by a low-affinity Ca2+ sensor. Indeed, β-cells have been shown to contain a highly Ca2+-sensitive pool of granules (HCSP) (Barg & Rorsman, 2004; Yang & Gillis, 2004). Exocytosis of HCSP granules occurs at [Ca2+]i levels close to the resting level and is enhanced by PKA and PKC activators. It is tempting to attribute exocytosis at widely different [Ca2+]i to the involvement of distinct Ca2+ sensors.
Synaptotagmins represent the obvious candidate for Ca2+ sensors of membrane fusion in β-cells. There are 16 isoforms of synaptotagmins: synaptotagmins 5, 7 and 9 have been detected in primary β-cells (Iezzi et al. 2004, 2005; Gauthier et al. 2007; Grise et al. 2007; Gustavsson et al. 2008). Synaptotagmin-7 knockout mice exhibit impaired glucose tolerance and lowered basal as well as glucose-induced insulin levels (Gustavsson et al. 2008). Insulin secretion in vitro was likewise reduced by ∼50%. In neurones, synaptotagmin-9 is coupled to fast neurotransmitter release (Xu et al. 2007). In rat islets (Iezzi et al. 2005), down-regulation of synaptotagmin-9 by siRNAs reduces glucose- and tolbutamide-induced insulin release (i.e. when secretion is evoked during the brief and localized Ca2+ entry associated with individual action potentials) but had less effect when the apparent Ca2+ sensitivity of exocytosis was increased by elevation of intracellular cAMP (cf. Yang & Gillis, 2004). Collectively, these observations indicate that synaptotagmin-9 constitutes the low-affinity Ca2+ sensor of exocytosis also in β-cells. If this turns out to be the case, then either synaptotagmin 5 or 7 may represent the high-affinity Ca2+ sensor(s).
Why do capacitance measurements report higher rates of exocytosis than insulin secretion assays?
The rate of exocytosis during depolarizations to 0 mV reported by capacitance measurements in β-cells in intact islets is as high as 40 fF s−1 (Göpel et al. 2004). The latter value equates to 15 granules per second using a conversion factor of ∼3 fF per granule. This is two orders of magnitude faster than the maximum rate of glucose-induced insulin secretion (0.16 granules s−1; see Fig. 1). Even if allowance is made for the fact that exocytosis at the physiological membrane potential of −20 mV is only 30% of that at zero millivolts (Göpel et al. 2004), it is clear that a significant difference persists that cannot be accounted for by other factors (like the absence or presence of cAMP).
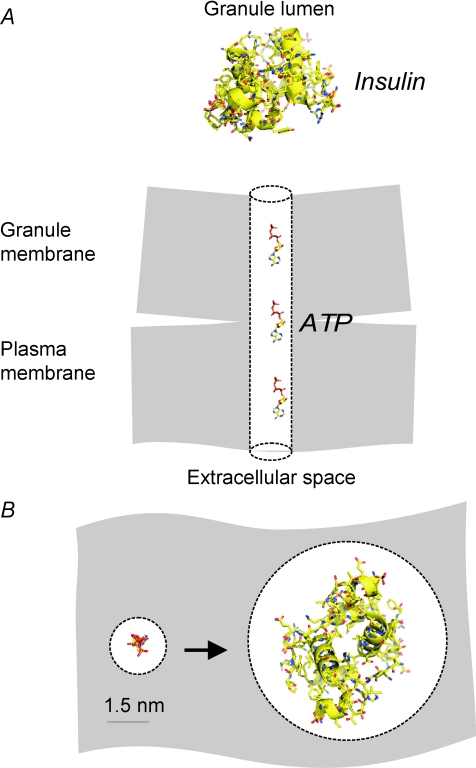
On-cell capacitance measurements allow determination of the conductance of the fusion pore, the structure connecting the granule lumen to the extracellular space (Fig. 3). Increases in cell capacitance become detectable when the conductance exceeds ∼200 pS. The latter value predicts a diameter of a cylindrical pore extending the width of the plasma membrane of < 1.5 nm (MacDonald et al. 2006). For comparison, insulin is ∼3 nm × 3 nm × 4 nm (Barg et al. 2002). Thus, a capacitance increase can be detected even when the fusion pore has not expanded sufficiently to allow the exit of insulin and membrane fusion is accordingly not obligatorily associated with insulin secretion. This might provide an explanation for the surprising finding that the rates of exocytosis monitored by capacitance measurements are generally much higher than those predicted from hormone release measurements.
Figure 3. The fusion pore as a molecular sieve.
Molecular dimensions of the fusion pore and space-filling models of insulin and ATP drawn to scale. A, cross-sectional view and B, the fusion pore viewed from the extracellular space into the granule lumen when the pore has expanded sufficiently to accommodate ATP (left) and insulin (right). Note that whereas insulin will not fit in a fusion pore with a diameter of only ∼1.5 nm, this is sufficient for ATP.
Exocytosis studied by ATP release measurements
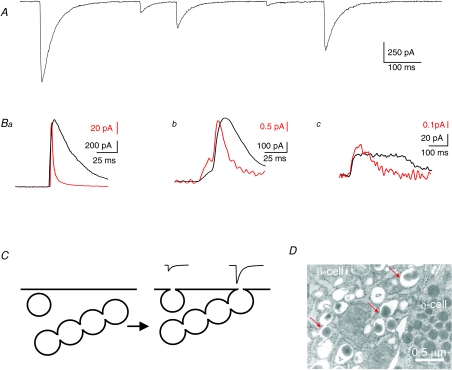
It is noteworthy that although the correlation between capacitance and insulin release measurements is weak at the quantitative level, a much better correlation is observed between capacitance and the release of low-molecular weight granule constituents like ATP (dimensions 1.6 nm × 1.1 nm × 0.5 nm; Fig. 3) (Braun et al. 2007). In cells that have been engineered to express ATP-sensitive P2X2 receptors at high density, exocytotic release of ATP will give rise to transient inward currents that are similar to those that can be recorded by amperometry (Fig. 4A and B). However, this novel technique has the advantage that the measurements can be performed over the entire cell surface whereas amperometry only covers 20–30% of the cell (Braun et al. 2007). In addition, there is no need to preload the cell with serotonin, which is required for amperometric recordings of exocytosis in the β-cell (Bokvist et al. 2000) and that may interfere with the measurements of insulin release (Zawalich et al. 2001). An average capacitance increase of 3.4 fF per exocytotic event was observed under conditions that would minimize the number of simultaneous exocytotic events (intracellular Ca2+ clamped at 0.2 μm) (Braun et al. 2007). This value is close to that expected for exocytosis of a secretory granule (MacDonald et al. 2006). It is of interest that although the amplitude distribution of the events could be reasonably described by a single Gaussian at low Ca2+, a long tail of very large events became observable when the cells were dialysed with a buffer containing 2–20 μm [Ca2+]i. These events were associated with a release of ATP at amounts corresponding to ∼10 ‘normal’ secretory granules and large stepwise capacitance increases (∼20 fF). Furthermore, they remained observable when the measurements were made in isolated outside-out patches that contain only a few per cent of the total cell surface area, making it unlikely that they can be explained by superimpositions of several unitary events. It is possible that global elevation of [Ca2+]i leads to fusion of several (5–10) granules inside the cell and that such multivesicular complexes are then released as one unit (Fig. 4C). There is ultrastructural evidence suggestive of several secretory granules prefusing within the cell and that these complexes may then undergo compound exocytosis (Fig. 4D).
Figure 4. Measurements of exocytotic ATP release suggest compound exocytosis.
A, membrane currents recorded from a β-cell engineered by adenoviral infection to express ATP-sensitive P2X2 receptors. Note large amplitude variation. B, parallel recordings of ATP release (using P2X2 receptors; black) and 5-HT (using carbon fibre amperometry; red). The three examples are interpreted to represent full fusion (a), opening of the fusion pore (giving rise to the initial pedestal; Chow et al. 1992) (b), stand alone pedestal during a release event aborted prior to full fusion (c). Some of the events detected by the P2X2Rs appear more long-lived than the amperometric responses. This may reflect slow unbinding of ATP from the P2X2Rs but this feature is not consistently observed. C, schematic diagram of single-vesicle and compound exocytosis. Under certain conditions (e.g. global elevation of [Ca2+]i) granules can prefuse inside the β-cells (left). Following elevation of [Ca2+]i to exocytotic levels, the single granules as well as the multivesicular structures undergo exocytosis and are connected via a single fusion pore thus accounting for the small- and large-amplitude events illustrated above (right). D, electron micrograph showing a multivesicular structure within a β-cell (red arrows). A δ-cell is shown to the right.
Kiss-and-run exocytosis
Careful analysis of the ATP-evoked current transients reveals that although many of them activated very quickly, others initially developed with a slow time course until a rapid further increase was observed (Fig. 4B). It is tempting to attribute the initial component to the gradual expansion of the fusion pore until the granule membrane collapses into the plasma membrane. Interestingly, a fairly large number (30%) of stand alone slow events was observed (MacDonald et al. 2006) that may represent exocytotic events aborted before full fusion (kiss-and-run) (Fig. 4A). In addition to ATP, the inhibitory neurotransmitter GABA may also be released via the fusion pore (Braun et al. 2004, 2007; Wendt et al. 2004). Parallel recordings of GABA release with amperometric detection of serotonin suggest that at least a subset of the insulin granules store GABA (Braun et al. 2007). In mouse and rat islets GABA functions as a paracrine inhibitor of glucagon secretion (Wendt et al. 2004; Bailey et al. 2007).
The notion that the fusion pore may function as a sieve is corroborated by several recent reports indicating that as little as < 25% of the exocytotic events (identified as an increase in granule pH and pHluorin fluorescence; Obermüller et al. 2005) are associated with emptying of the granule content (monitored, for example, as the disappearance of NPY-Venus; Tsuboi & Rutter, 2003). In the remaining 75% of the events, the fusion only opens transiently (kiss-and-run exocytosis).
Pathophysiology of β-cell exocytosis: implications for type-2 diabetes
Interestingly, long-term exposure to glucose appears to increase the fraction of kiss-and-run events at the expense of full fusion and following 48 h exposure to high glucose, only 5% of the release events proceed to full fusion (Tsuboi et al. 2006). We have observed a similar effect in islets subjected to long-term exposure to the lipids and palmitate, a condition that leads to a strong reduction of insulin secretion. In adrenal chromaffin cells, the likelihood of a granule undergoing full fusion increases with [Ca2+]i (Elhamdani et al. 2006). It is tempting to speculate that granules undergoing kiss-and-run exocytosis are the ones situated far away from the Ca2+ channels (Fig. 5). Indeed, in mice fed a high-fat diet for 15 weeks and that exhibit impaired glucose-induced insulin secretion, a rapid component of exocytosis believed to reflect granules in close proximity to the Ca2+ channels is selectively inhibited (S. Collins, A. Toye, D. Gauguier & P. Rorsman, unpublished observations). The latter effect was reminiscent of that observed when β-cells are dialysed with an excess of recombinant synprint peptide, the part of the L-type Ca2+ channel that is associated with the exocytotic core complex (Wiser et al. 1999). The effects of high glucose and/or lipids were selective for the β-cells and glucagon secretion was not suppressed and if anything it was enhanced (Collins et al. 2008). Insulin secretion is also reduced following removal of plasma membrane cholesterol (J. Vikman & L. Eliasson, unpublished observations). This highlights the high sensitivity of β-cell exocytosis to changes in the lipid environment of the plasma membrane. Taken together, these observations suggest that insulin secretion can be regulated at the level of fusion pore and that its failure to expand contributes to insulin secretion defects under pathophysiological states like hyperglycaemia and hyperlipidaemia. It is now important to determine which factors determine the expansion of the fusion pore and whether they are defective in diabetic β-cells and thereby contribute to the insufficient insulin secretion that is a hallmark of this disorder.
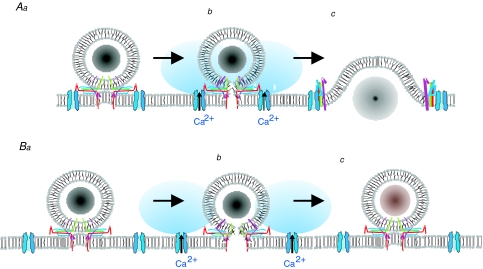
Figure 5. Kiss-and-run/full fusion and Ca2+ channels.
Full fusion takes place when the granules and Ca2+ channels are situated in close proximity to each other so that the exocytotic machinery is exposed to the high Ca2+ concentrations that occur close to the inner mouth of the Ca2+ channel (A). Cholesterol depletion or other procedures that lead to the disruption of the Ca2+ channel–granule complex will result in the exocytotic machinery being exposed to [Ca2+]i too low to trigger full fusion thus favouring transient (kiss-and-run) exocytosis (B). In A and B, a–c correspond to docked granules, the opening of the fusion pore and full fusion/kiss-and-run exocytosis, respectively.
The impairment of insulin secretion in islets from type-2 diabetic donors correlates with strong (> 70%) reduction of the mRNA levels for key exocytotic proteins like synaptotagmin 5, syntaxin 1, SNAP-25 and VAMP-2 (Ostenson et al. 2006). Lower levels of these proteins can be speculated to result in the assembly of fewer Ca2+ channel/secretory granule complexes with resultant favouring of kiss-and-run exocytosis. It is pertinent that the diabetic islets used in the above study were refractory not only to stimulation with glucose but also to glibenclamide. This observation raises the interesting possibility that some forms of type-2 diabetes involve defective secretory machinery and that it does not solely result from impaired regulation of the KATP channels. It is not known whether type-2 diabetes is associated with altered levels of any miRs but, given that several of them influence both the levels of the exocytotic proteins (Lovis et al. 2008) and exocytosis itself (Poy et al. 2004), this is an intriguing possibility that should be explored.
Acknowledgments
This work was supported by the Wellcome Trust (P.R.), The European Union (Network of Excellence Biosim and the Integrated Project Eurodia) and The Swedish Research Council (L.E.). F.A. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
References
- Ammala C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Ravier MA, Rutter GA. Glucose-dependent regulation of γ-aminobutyric acid (GABAA) receptor expression in mouse pancreatic islet α-cells. Diabetes. 2007;56:320–327. doi: 10.2337/db06-0712. [DOI] [PubMed] [Google Scholar]
- Baillyes EM, Guest PC, Hutton JC. Insulin synthesis. In: Ashcroft FM, Ashcroft SJH, editors. Insulin: Molecular Biology to Pathology. Oxford: IRL Press; 1992. pp. 64–96. [Google Scholar]
- Barg S, Ma X, Eliasson L, Galvanovskis J, Göpel SO, Obermüller S, Platzer J, Renström E, Trus M, Atlas D, Striessnig J, Rorsman P. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renström E, Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Barg S, Rorsman P. Insulin secretion: a high-affinity Ca2+ sensor after all? J Gen Physiol. 2004;124:623–625. doi: 10.1085/jgp.200409206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard RJ, Morgan A, Burgoyne RD. Stimulation of NSF ATPase activity by α-SNAP is required for SNARE complex disassembly and exocytosis. J Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K, Holmqvist M, Gromada J, Rorsman P. Compound exocytosis in voltage-clamped mouse pancreatic β-cells revealed by carbon fibre amperometry. Pflugers Arch. 2000;439:634–645. doi: 10.1007/s004249900211. [DOI] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic β-cells. Electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- Braun M, Wendt A, Birnir B, Broman J, Eliasson L, Galvanovskis J, Gromada J, Mulder H, Rorsman P. Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic β-cells. J Gen Physiol. 2004;123:191–204. doi: 10.1085/jgp.200308966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Wendt A, Karanauskaite J, Galvanovskis J, Clark A, Macdonald PE, Rorsman P. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic β cells. J Gen Physiol. 2007;129:221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Collins SC, Salehi A, Eliasson L, Olofsson CS, Rorsman P. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia. 2008 doi: 10.1007/s00125-008-1082-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48:1686–1690. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- Dean PM. Ultrastructural morphometry of the pancreatic β-cell. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Gaisano H, Vikman J. Reduced stimulation by cAMP in insulin-secreting cells overexpressing truncated SNAP-25. Diabetologia. 2005;48:A172. [Google Scholar]
- Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esni F, Taljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol. 1999;144:325–337. doi: 10.1083/jcb.144.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Imai A, Nashida T, Shimomura H. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18-2-dependent manner. J Biol Chem. 2005;280:39175–39184. doi: 10.1074/jbc.M505759200. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Duhamel DL, Iezzi M, Theander S, Saltel F, Fukuda M, Wehrle-Haller B, Wollheim CB. Synaptotagmin VII splice variants α, β, and δ are expressed in pancreatic β-cells and regulate insulin exocytosis. FASEB J. 2007;22:194–206. doi: 10.1096/fj.07-8333com. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Wollheim CB. MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nat Med. 2006;12:36–38. doi: 10.1038/nm0106-36. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Sudhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51:S3–S11. doi: 10.2337/diabetes.51.2007.s3. [DOI] [PubMed] [Google Scholar]
- Gold G, Gishizky ML, Grodsky GM. Evidence that glucose ‘marks’ beta cells resulting in preferential release of newly synthesized insulin. Science. 1982;218:56–58. doi: 10.1126/science.6181562. [DOI] [PubMed] [Google Scholar]
- Goodge KA, Hutton JC. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic β-cell. Semin Cell Dev Biol. 2000;11:235–242. doi: 10.1006/scdb.2000.0172. [DOI] [PubMed] [Google Scholar]
- Göpel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, Salehi A, Rorsman P. Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J Physiol. 2004;556:711–726. doi: 10.1113/jphysiol.2003.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grise F, Taib N, Monterrat C, Lagree V, Lang J. Distinct roles of the C2A and the C2B domain of the vesicular Ca2+ sensor synaptotagmin 9 in endocrine β-cells. Biochem J. 2007;403:483–492. doi: 10.1042/BJ20061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7–36) amide stimulates exocytosis in human pancreatic β-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- Gromada J, Høy M, Renström E, Bokvist K, Eliasson L, Göpel S, Rorsman P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J Physiol. 1999;518:745–759. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Sudhof TC, Han W. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of gliclazide therapy. Metabolism. 1989;38:767–772. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- Hutton JC, Penn EJ, Peshavaria M. Low-molecular weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Eliasson L, Fukuda M, Wollheim CB. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett. 2005;579:5241–5246. doi: 10.1016/j.febslet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci. 2004;117:3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- Ivarsson R, Obermüller S, Rutter GA, Galvanovskis J, Renström E. Temperature-sensitive random insulin granule diffusion is a prerequisite for recruiting granules for release. Traffic. 2004;5:750–762. doi: 10.1111/j.1600-0854.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- Jeans AF, Oliver PL, Johnson R, Capogna M, Vikman J, Molnar Z, Babbs A, Partridge CJ, Salehi A, Bengtsson M, Eliasson L, Rorsman P, Davies KE. A dominant mutation in Snap25 causes impaired vesicle trafficking, sensorimotor gating, and ataxia in the blind-drunk mouse. Proc Natl Acad Sci U S A. 2007;104:2431–2436. doi: 10.1073/pnas.0610222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous actin regulates insulin exocytosis through direct interaction with syntaxin 4. J Biol Chem. 2008;283:10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, He Z, Xu P, Fan J, Betz A, Brose N, Xu T. Munc13-1 is required for the sustained release of insulin from pancreatic β cells. Cell Metab. 2006;3:463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Kato T, Shimano H, Yamamoto T, Yokoo T, Endo Y, Ishikawa M, Matsuzaka T, Nakagawa Y, Kumadaki S, Yahagi N, Takahashi A, Sone H, Suzuki H, Toyoshima H, Hasty AH, Takahashi S, Gomi H, Izumi T, Yamada N. Granuphilin is activated by SREBP-1c and involved in impaired insulin secretion in diabetic mice. Cell Metab. 2006;4:143–154. doi: 10.1016/j.cmet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259:3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- Leung YM, Kwan EP, Ng B, Kang Y, Gaisano HY. SNAREing voltage-gated K+ and ATP-sensitive K+ channels: tuning β-cell excitability with syntaxin-1A and other exocytotic proteins. Endocr Rev. 2007;28:653–663. doi: 10.1210/er.2007-0010. [DOI] [PubMed] [Google Scholar]
- Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the machinery of exocytosis of insulin-secreting cells by microRNAs. Biol Chem. 2008;389:305–312. doi: 10.1515/BC.2008.026. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic β cells. Cell Metab. 2006;4:283–290. doi: 10.1016/j.cmet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Meissner HP. Electrical characteristics of the β-cells in pancreatic islets. J Physiol (Paris) 1976;72:757–767. [PubMed] [Google Scholar]
- Meissner HP, Atwater IJ. The kinetics of electrical activity of β cells in response to a ‘square wave’ stimulation with glucose or glibenclamide. Horm Metab Res. 1976;8:11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Nagy G, Matti U, Nehring RB, Binz T, Rettig J, Neher E, Sorensen JB. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci. 2002;22:9278–9286. doi: 10.1523/JNEUROSCI.22-21-09278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sorensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Obermüller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, Matsushima S, Kawakami H, Watanabe T, Akagawa K, Nagamatsu S. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Kumakura K, Nakamichi Y, Nagamatsu S. Site of docking and fusion of insulin secretory granules in live MIN6 beta cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy. J Biol Chem. 2004;279:8403–8408. doi: 10.1074/jbc.M308954200. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Göpel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, Efanov AM, Meister B, Thams P, Yang SN, Rorsman P, Berggren PO, Gromada J. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic β cells. Proc Natl Acad Sci U S A. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Gabbay KH, Malaisse WJ. Pancreatic β-cell web: its possible role in insulin secretion. Science. 1972;175:1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Renström E, Ding WG, Bokvist K, Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting β cells by activation of calcineurin. Neuron. 1996;17:513–522. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Renström E. Insulin granule dynamics in pancreatic β cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrés JV, Gomis A, Valdeolmillos M. The electrical activity of mouse pancreatic β-cells recorded in vivo shows glucose-dependent oscillations. J Physiol. 1995;486:223–228. doi: 10.1113/jphysiol.1995.sp020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell AH, Swenne I, Borg LA. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988;252:9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki JI, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Liu X, Yang Y, Takahashi M, Gillis KD. Phosphorylation of SNAP-25 at Ser187 mediates enhancement of exocytosis by a phorbol ester in INS-1 cells. J Neurosci. 2008;28:21–30. doi: 10.1523/JNEUROSCI.2352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel D, Salehi A, Obermueller S, Lundquist I, Brose N, Renström E, Rorsman P. CAPS1 and CAPS2 regulate stability and recruitment of insulin granules in mouse pancreatic β cells. Cell Metab. 2008;7:57–67. doi: 10.1016/j.cmet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic β-cells. Pflugers Arch. 2003;446:553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic β-cells. Mol Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- Straub SG, Shanmugam G, Sharp GW. Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the β-cells of rat pancreatic islets. Diabetes. 2004;53:3179–3183. doi: 10.2337/diabetes.53.12.3179. [DOI] [PubMed] [Google Scholar]
- Torii S, Zhao S, Yi Z, Takeuchi T, Izumi T. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol Cell Biol. 2002;22:5518–5526. doi: 10.1128/MCB.22.15.5518-5526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Ravier MA, Parton LE, Rutter GA. Sustained exposure to high glucose concentrations modifies glucose signaling and the mechanics of secretory vesicle fusion in primary rat pancreatic β-cells. Diabetes. 2006;55:1057–1065. doi: 10.2337/diabetes.55.04.06.db05-1577. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Rutter GA. Insulin secretion by ‘kiss-and-run’ exocytosis in clonal pancreatic islet β-cells. Biochem Soc Trans. 2003;31:833–836. doi: 10.1042/bst0310833. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M, Gomis A, Sánchez-Andrés JV. In vivo synchronous membrane potential oscillations in mouse pancreatic β-cells: lack of co-ordination between islets. J Physiol. 1996;493:9–18. doi: 10.1113/jphysiol.1996.sp021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman J, Eliasson L. Depletion of cholesterol using a low concentration of methyl-betacyclodextrin reduces the exocytotic response in single mouse pancreatic B-cells. Diabetologia. 2005;48:A66. [Google Scholar]
- Vikman J, Ma X, Hockerman GH, Rorsman P, Eliasson L. Antibody inhibition of synaptosomal protein of 25 kDa (SNAP-25) and syntaxin 1 reduces rapid exocytosis in insulin-secreting cells. J Mol Endocrinol. 2006;36:503–515. doi: 10.1677/jme.1.01978. [DOI] [PubMed] [Google Scholar]
- Vikman J, Ma X, Tagaya M, Eliasson L. Requirement for N-ethylmaleimide-sensitive factor for exocytosis of insulin-containing secretory granules in pancreatic β-cells. Biochem Soc Trans. 2003;31:842–847. doi: 10.1042/bst0310842. [DOI] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Yokota H, Izumi T. Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic β cells. J Biol Chem. 1999;274:28542–28548. doi: 10.1074/jbc.274.40.28542. [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser O, Trus M, Hernández A, Renström E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gillis KD. A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol. 2004;124:641–651. doi: 10.1085/jgp.200409081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Tesz GJ, Zawalich KC. Are 5-hydroxytryptamine-preloaded β-cells an appropriate physiologic model system for establishing that insulin stimulates insulin secretion? J Biol Chem. 2001;276:37120–37123. doi: 10.1074/jbc.M105008200. [DOI] [PubMed] [Google Scholar]