Abstract
The present study investigated motor unit (MU) loss in a murine model of familial amyotrophic lateral sclerosis (ALS). The fast-twitch tibialis anterior (TA) and medial gastrocnemius (MG) muscles of transgenic SOD1G93A and SOD1WT mice were studied during the presymptomatic phase of disease progression at 60 days of age. Whole muscle maximum isometric twitch and tetanic forces were 80% lower (P < 0.01) in the TA muscles of SOD1G93A compared to SOD1WT mice. Enumeration of total MU numbers within TA muscles showed a 60% reduction (P < 0.01) within SOD1G93A mice (38 ± 7) compared with SOD1WT controls (95 ± 12); this was attributed to a lower proportion of the most forceful fast-fatigable (FF) MU in SOD1G93A mice, as seen by a significant (P < 0.01) leftward shift in the cumulative frequency histogram of single MU forces. Similar patterns of MU loss and corresponding decreases in isometric twitch force were observed in the MG. Immunocytochemical analyses of the entire cross-sectional area (CSA) of serial sections of TA muscles stained with anti-neural cell adhesion molecule (NCAM) and various monoclonal antibodies for myosin heavy chain (MHC) isoforms showed respective 65% (P < 0.01) and 28% (P < 0.05) decreases in the number of innervated IIB and IID/X muscle fibres in SOD1G93A, which paralleled the 60% decrease (P < 0.01) in the force generating capacity of individual fibres. The loss of fast MUs was partially compensated by activity-dependent fast-to-slower fibre type transitions, as determined by increases (P < 0.04) in the CSA and proportion of IIA fibres (from 4% to 14%) and IID/X fibres (from 31% to 39%), and decreases (P < 0.001) in the CSA and proportion of type IIB fibres (from 65% to 44%). We conclude that preferential loss of IIB fibres is incomplete at 60 days of age, and is consistent with a selective albeit gradual loss of FF MUs that is not fully compensated by sprouting of the remaining motoneurons that innervate type IIA or IID/X muscle fibres. Our findings indicate that disease progression in fast-twitch muscles of SOD1G93A mice involves parallel processes: (1) gradual selective motor axon die-back of the FF motor units that contain large type IIB muscle fibres, and of fatigue-intermediate motor units that innervate type IID/X muscle fibres, and (2) activity-dependent conversion of motor units to those innervated by smaller motor axons innervating type IIA fatigue-resistant muscle fibres.
Amyotrophic lateral sclerosis (ALS) is an adult onset neurodegenerative disease characterized by progressive and preferential loss of motoneurons (Cleveland, 1999). Of the 10% of ALS cases that are familial in origin, 20% have been linked to mutations in the cytosolic antioxidant enzyme, Cu/Zn superoxide dismutase (SOD1) gene (Rosen, 1993). Transgenic mice that over-express human mutant SOD1 with a glycine to alanine conversion at the 93rd codon (SOD1G93A) develop a stereotypic syndrome, including motoneuron loss from the lumbar spinal cord that coincides with the onset of clinical symptoms, such as the development of tremors and hindlimb weakness, at 90 days of age but not before (Gurney et al. 1994; Chiu et al. 1995). Therefore the period prior to 90 days has typically been referred to as presymptomatic. Anatomical studies have, however, reported axonal loss from ventral roots at 47 days of age (Fischer et al. 2004) and denervation of hindlimb muscles (Frey et al. 2000) within the 90 day presymptomatic period.
All studies except one employed electromyography (EMG) to investigate whether the number of functional motor units is normal or begins to decline before the onset of symptoms at 90 days of age. The EMG studies yielded contradictory findings. A linear loss of functional motor units from the medial gastrocnemius (MG) beginning at 47 days of age (Kennel et al. 1996) was inconsistent with the significant biphasic loss of gastrocnemius motor units that was reported much later in development (Azzouz et al. 1997). The only EMG study to also record muscle contractile force in ALS mice demonstrated that whole muscle force of the extensor digitorum longus (EDL) fell only after the onset of symptoms at 90 days of age (Derave et al. 2003).
Our recent study of motor units in four hindlimb muscles reported that the contractile force of fast-twitch but not the slow-twitch muscles has already begun to decline by 40 days of age and continues until 90 days of age, after which overt symptoms manifest: the decline in the muscle contractile force parallels a decline in the number of functional motor units (Hegedus et al. 2007). Importantly, there was a greater reduction in the mean contractile force of the fast-twitch medial gastrocnemius of the SOD1G93A mouse as compared to the reduction in numbers of functional motor units (Hegedus et al. 2007). This finding indicated that there was not the normal sprouting capacity of intact motor units to reinnervate denervated muscle fibres and thereby sustain muscle force. Indeed, Frey et al. (2000) reported motoneurons that innervate type IIB muscle fibres fail to sprout but motoneurons innervating type I and IIA muscle fibres retained sprouting competence (see also Pun et al. 2006). The greater reduction in muscle contractile force as compared with motor unit number (Hegedus et al. 2007) is also consistent with a preferential loss of the most forceful motor units that contain type IIB muscle fibres, as suggested by Frey et al. (2000). However Pun et al. (2006) reported that all type IIB muscle fibres were lost in the hindlimb muscles of the SOD1G93A mice by 55 days of age. If indeed this is the case, it would follow that only the motor units containing type I, type IIA and type IID/X muscle fibres would remain in the ∼60-day-old SOD1G93A mice and further that the contractile force of the remaining motor units would be greater than normal due to the effective axonal sprouting from the motoneurons that innervate the remaining muscle fibres.
Without whole muscle and single motor units force recordings it is not possible to discern whether individual motor unit forces and the number of muscle fibres per motor unit (innervation ratio; IR) increase. The IR and the motor unit forces should increase in parallel due to the collateral sprouting of motoneurons of the surviving motor units that contain the type I, IIA and IID/X muscle fibres. Normally motor units expand to a maximum of 5- to 8-fold by sprouting and compensate for loss of functional motor units, as in poliomyelitis (Tam et al. 2002; Tam & Gordon, 2003; Gordon et al. 2004). In ALS, the few human studies in which both EMG and force record motor units showing were made from singled that unitary EMG amplitudes increased but the motor unit forces did not (Milner-Brown et al. 1974a,b; Vogt & Nix, 1997).
We have undertaken to record motor unit contractile force in the fast-twitch tibialis anterior (TA) and medial gastrocnemius (MG) muscles of SOD1G93A transgenic mice and wild-type control mice at 60 days of age. This is the age when mice are asymptomatic (Chiu et al. 1995; Atkin et al. 2005) and when it has been reported that all type IIB muscle fibres are denervated (Frey et al. 2000; Pun et al. 2006). By combining force recordings from single isolated motor units with immunocytochemical analysis of innervated and denervated muscle fibres, we determined the extent and specificity of fibre type denervation, and whether any compensatory enlargement of motor units in the SOD1G93A transgenic mouse influences the distribution of motor unit forces and, in turn, the contractile force of whole muscles.
Methods
SOD1G93A and SOD1WT mice
All of the experiments were carried out in accordance with Canadian Council for Animal Care, and the University of Alberta Health Sciences Animal Policy and Welfare Committee approved all experimental procedures which includes minimizing the number of animals used and their suffering.
Transgenic mice expressing a high copy number of the glycine to alanine base pair mutation at the 93rd codon of the cytosolic Cu/Zn superoxide dismutase (SOD1) gene (SOD1G93A B6JSL-TgN (SOD1-G93A)) were obtained from Jackson Laboratories, USA. The transgenic male SOD1G93A mice were crossed with non-transgenic B6JSL hybrid females, and the resulting progeny were identified using a standard PCR protocol for the human SOD1 (Rosen, 1993). This was performed on ear biopsy samples taken at the time of weaning (approximately 21 days of age). The mice were identified using ear punches, and kept in standard animal housing with free access to water and standard rodent chow. Non-transgenic wild-type littermates were used as age matched controls (SOD1WT). The SOD1G93A mice become symptomatic at approximately 90 days of age. Symptoms included fine shaking, tremors and spasticity in the hind-legs (Chiu et al. 1995). Complete paralysis of the hindlimbs occurs within 30–40 days of initial symptom onset. The SOD1WT mice have not been reported to develop ALS-like disease (Gurney et al. 1994).
Electrophysiological studies
Surgery
Mice were anaesthetized with an intraperitoneal (i.p.) injection of a cocktail made up of ketamine (100 mg ml−1) and acepromazine (10 mg ml−1) in sterile saline, at a dosage of 17.5 ml (kg body weight)−1. Supplemental anaesthesia was given i.p. as needed to abolish the hindpaw-pinch reflex. The body temperature of the mice was maintained using a heat lamp, and saline was administered subcutaneously at 45 min intervals. A laminectomy was done to isolate the L4 and L5 ventral roots. The TA or the MG muscle tendons were isolated bilaterally and tied with a 4.0 silk thread for attachment with 4.0 silk to a strain gauge (Kulite model KH-102). Two silver wire electrodes were sutured alongside the sciatic nerves for stimulation (Fig. 1A). Both hindlimbs were prepared in the event of any problems in root dissection or hindlimb immobilization. One hindlimb was immobilized by clamping the knee and the ankle, while being careful not to interfere with the blood supply to the muscles. A paraffin pool was fashioned to dissect and tease the ventral roots and elicit motor unit forces. After completion of electrophysiological recordings, the TA and MG muscles were removed and the anaesthetized mice were killed via cervical dislocation.
Figure 1. Schematic illustration of the experimental set-up for recording whole muscle and motor unit contractile force from mouse tibialis anterior (TA) or medial gastrocnemius (MG) muscles.
A, supra-maximal stimulation of the sciatic nerve through silver wire electrodes elicits whole muscle contractile twitch and tetanic forces; stimulation of teased ventral root filaments resulted in all-or-none motor unit contractile force. B, as the amplitude of the stimulation to the teased ventral roots increases, motor units with higher thresholds are progressively recruited, resulting in the incremental increase of the recorded muscle contractile force. Template subtraction was used to calculate the force of the recruited motor units, and the number of motor units was estimated by dividing the whole muscle twitch force by the average motor unit force. The complete time scale of the recorded twitch forces of the muscle and motor units is 100 ms.
Isometric recordings of muscle and motor unit contractile force and calculation of motor unit numbers
Evoked isometric forces were amplified and visualized on an oscilloscope and digitized using Axoscope software (v. 8.0, Axon Instruments, USA). Muscle length was adjusted for maximal isometric twitch force in response to stimulation of the sciatic nerve. Whole muscle twitch and tetanic forces were recorded in response to single and repetitive suprathreshold (twice threshold amplitude) stimulation of the sciatic nerve at 0.5 and 100 Hz, respectively (Fig. 1A). Ventral roots were teased into filaments (up to 5 filaments) to recruit motor units by incremental steps in order to obtain single motor unit twitch contractile forces and to estimate the number of motor units. We used the incremental twitch subtraction (ITS-MUNE) modification of the motor unit number estimation (MUNE) that was first described by McComas et al. (1971) for EMG measurements. The validity of this new ITS-MUNE method is described in detail by Major et al. (2007) and uses incremental stimulation of a peripheral nerve to recruit motor units and to record muscle twitch force. Other than stimulating ventral root filaments and not the peripheral nerve to recruit motor units, the ITS-MUNE method as described by Major et al. (2007) was used in this study to determine motor unit twitch forces and to calculate motor unit number. Briefly, stimuli of 100 μs duration were applied at a rate of 0.5 s−1 to each teased ventral root filament via an electrode array with the most distal electrode grounded as illustrated in Fig. 1. Starting with a stimulus voltage below threshold, the voltage was manually increased over a range from 0 to 10 V. The isometric twitch force was monitored on an oscilloscope for discrete increments in peak twitch force whilst digitizing the forces with Clampfit software (v. 8.0, Axon Instruments). With each force increment, the stimulus amplitude was held constant for > 10 stimuli at force levels below 50% and at least 15 stimuli at force levels above 50% of maximum twitch force. In light of changes in excitability that have been measured in ALS patients (using threshold tracking) (Tamura et al. 2006; Kanai et al. 2006) but have not yet been experimentally explored in animal models of ALS, we explored the implications using computer simulations to conclude that a minimum of 15 stimuli should be given at each stimulus level to reduce the alternation of stimulated motor axons (Hegedus et al. 2008). To further minimize the problem of alternation, the recruitment of motor units in varying combinations (such as 1 + 2, 1 + 2 + 3, 1 + 3) (Brown & Milner-Brown, 1976) that normally becomes more prevalent with recruitment of more than 66% of the motor unit pool (Major et al. 2007), up to five ventral root filaments were dissected for incremental stimulation.
The twitch force associated with ∼8–15 random increments in force was chosen by the computer for various stimulus voltage intensities; the motor unit twitch waveforms were obtained by subtraction; a histogram of the motor unit twitch samples was displayed (for details, see Major et al. 2007); and the motor unit number was calculated by the ratio of the twitch force of the muscle and the average motor unit force (Fig. 1B).
TA muscles were isolated and studied in four SOD1G93A and four SOD1WT mice at 60 days of age, a time point that was approximately a month before the onset of symptoms but when only 40% of motor units remain (Hegedus et al. 2007). To confirm that motor units were lost from fast-twitch hindlimb muscles, motor units were also enumerated in the fast-twitch antigravity MG muscles of an additional four SOD1G93A and four SOD1WT mice at 60 days of age.
Muscle fibre type determination for the TA muscle
Muscle collection and sectioning
The TA muscles from 60-day-old SOD1G93A (n = 4) and SOD1WT (n = 4) mice were carefully removed immediately after the force measurements were completed. Muscles were then embedded in cutting medium (Tissue-Tek Optimal Cutting Medium Compound, Miles Scientific, USA) and frozen in melting isopentane cooled in liquid nitrogen (−156°C). Samples were stored at −80°C until they were sectioned. Serial 12 μm thick sections were cut at −25°C and collected onto glass Superfrost Plus slides (Fisher Scientific, CA, USA), air dried for 1 h, and stored at −80°C until immunohistochemical staining.
Immunohistochemistry
Monoclonal anti-myosin heavy chain (MHC) antibodies were harvested in culture from hybridoma clones (i.e. BA-D5; SC-71; BF-F3) that were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Monoclonal anti-MHCIIx (clone BF-35) (also known as MHCIId) was a generous gift from Professor Schiaffino (University of Padova, Italy). Immunostaining was completed according to previously published procedures (Putman et al. 2003; Martins et al. 2006). Briefly, before treatment with antibodies, the sections were washed in phosphate buffered saline (PBS) with 0.1% (v:v) Tween-20 (PBS-T), with PBS, and then incubated for 15 min in 3% H2O2 in methanol. Sections were stained for either the MHCI isoform (clone BA-D5), MHCIIa isoform (clone SC-71), all MHC isoforms except MHCIIx (clone BF-35), neural cell adhesion molecule (NCAM) (Chemicon International Ltd, Hampshire, UK) or dystrophin (clone DYS2) (Novocastra Laboratories Ltd, Newcastle, UK) as follows. Sections were incubated at room temperature for 1 h in a blocking solution (BS-1: 1% bovine serum albumin, 10% horse serum in PBS-T (pH 7.4) containing Avidin-D Blocking Reagent (Vector Laboratories Inc., Burlingame, CA, USA). Sections that were stained for MHCIIb (clone BF-F3) or NCAM were blocked at room temperature for 1 h in a similar solution, with the exception that goat serum was substituted for horse serum (BS-2). The primary antibodies were diluted in the appropriate blocking solutions that also contained Biotin Blocking Reagent (Vector Laboratories Inc.), overlaid onto sections and incubated overnight at 4°C. The dilutions of the primary antibodies were: BA-D5, 1: 400 in BS-1; SC-71, 1: 100 in BS-1; BF-35, 1: 10 000 in BS-1; BF-F3, 1: 400 in BS-2; DYS2, 1: 10 in BS-1; NCAM 1: 400 in BS-2. Sections were washed as before and either biotinylated horse anti-mouse IgG (1: 200, Vector Laboratories Inc., for BA-D5, SC-71, BF-35 and DYS2), biotinylated goat anti-rabbit IgG (1: 400; Vector Laboratories Inc., for NCAM) or biotinylated goat anti-mouse IgM (1: 200, Vector Laboratories Inc.; for BF-F3) was applied for 1 h at room temperature. Sections were then washed and incubated with Vectastain ABC reagent (avidin–biotin horseradish peroxidase (HRP) complex; Vector Laboratories Inc.) for 1 h and washed. Immunoreactivity was detected by reaction with diaminobenzidine, H2O2 and NiCl2 in 50 mm Tris-HCl (pH 7.4) (Vector Laboratories Inc.). The reaction was stopped by rinsing the sections with distilled H2O. Sections were then dehydrated in ethanol, cleared in xylene and mounted using Entellan (Darmstadt, Germany).
Immunohistochemical analyses
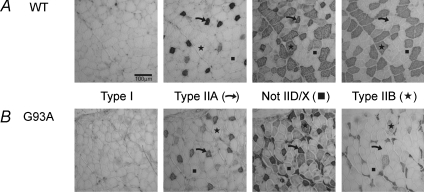
Muscle fibre cross sectional areas (CSA) and fibre types were quantified over the entire cross section of four SOD1G93A and four SOD1WT TA muscles obtained from 60-day-old mice. This comprehensive morphological analysis encompassed a total of 13 924 muscle fibres. Pictures of the entire TA cross sections were taken with a Leitz Diaplan microscope (Ernst Leitz Wetzlar GmbH, Germany) fitted with a Pro Series high performance charge-coupled device camera (Media Cybernetics, USA), and analysed using a custom designed analytical imaging program (Putman et al. 2000). Fibre types were identified by the staining for various MHC isoforms; type I fibres stained for MHCI, type IIA for MHCIIa and type IIB fibres for MHCIIb (Fig. 2). Type IID/X fibres were identified as those that remained unstained by clone BF-35. These same fibres were also immuno-negative with all other anti-MHC antibodies.
Figure 2. Immunohistochemical staining for type I, IIA, not IID/X and IIB fibres on 12 μm thick cross-sections of TA muscles from SOD1WT (A) and SOD1G93A (B) mice.
Staining was completed using anti-myosin heavy chain (MHC) monoclonal antibodies: type I fibres (anti-MHCI, clone BA-D5); type IIA fibres (anti-MHCIIa, clone SC-71); type IID/X fibres are unstained (anti-MHC not IIX (also known as not IID), clone BF-35); type IIB fibres (anti-MHCIIb, clone BF-F3). The scale bar is 100 μm.
Statistics
The data are presented as arithmetic means ± standard errors of the mean (s.e.m.) except for the frequency distributions where the data are presented as the arithmetic means ± standard deviations (s.d.). Statistical significance between groups was assessed using Student's t test in SigmaPlot (v. 8.0; Systat Software Inc., San Jose, CA, USA). Differences were considered statistically significant at P < 0.05. Distributions were tested for normality using a one-sided Kolmogrov–Smirnov test. For non-normally distributed data, significant differences were identified using the Mann–Whitney U test (SPSS v. 14.0; SPSS Inc., Chicago, IL, USA).
Results
Contractile forces of muscles and their motor units
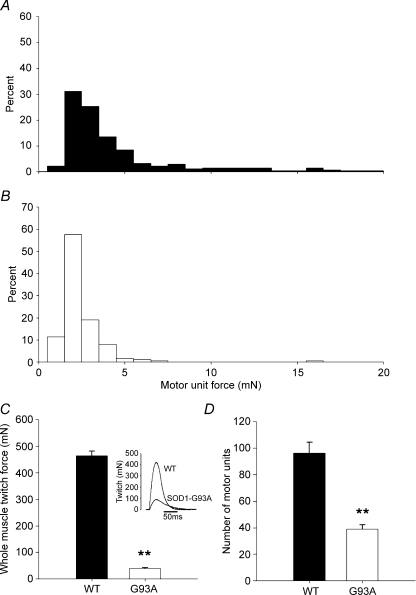
At 60 days of age, the maximum isometric twitch and tetanic contractile forces developed by TA muscles in response to supra-maximal stimulation of the sciatic nerve were ∼80% lower in SOD1G93A mice as compared to the SOD1WT mice (P < 0.01; Fig. 3A and B). In order to determine why forces were reduced, we isolated ventral root filaments to record isometric twitch forces in single motor units and used ITS-MUNE to estimate the number of motor units. Recordings were made in 33 ± 10 and 65 ± 6 TA motor units in the SOD1G93A and SOD1WT mice, respectively. The number of motor units in the TA muscles of four SOD1G93A mice at 60 days (38 ± 6.7) was significantly less (P < 0.01) than in the TA muscles of four SOD1WT mice (95 ± 12.4) (Fig. 3C). This is a 60% reduction in the number of motor units within TA muscles of SOD1G93A mice, as compared to the SOD1WT mice.
Figure 3. There was a significant decline in the whole muscle twitch (A), the tetanic contractile forces (B) and the number of intact motor units (C) in the SOD1G93A mouse TA muscles as compared to control SOD1WT muscles.
A, representative tracing of whole muscle twitch force indicating a decline in twitch force and a concomitant increase in the half-relaxation time of the muscle. The whole muscle twitch and tetanic forces decline in parallel (A and B) to approximately a fifth of the control SOD1WT mouse TA muscle values. Motor unit loss in TA muscles of SOD1G93A mice paralleled the reductions in whole muscle twitch and tetanic force production and accounted for the higher proportion of smaller less forceful motor units. Data are presented as means ± s.e.m. and statistical significance is indicated as ** for P < 0.01.
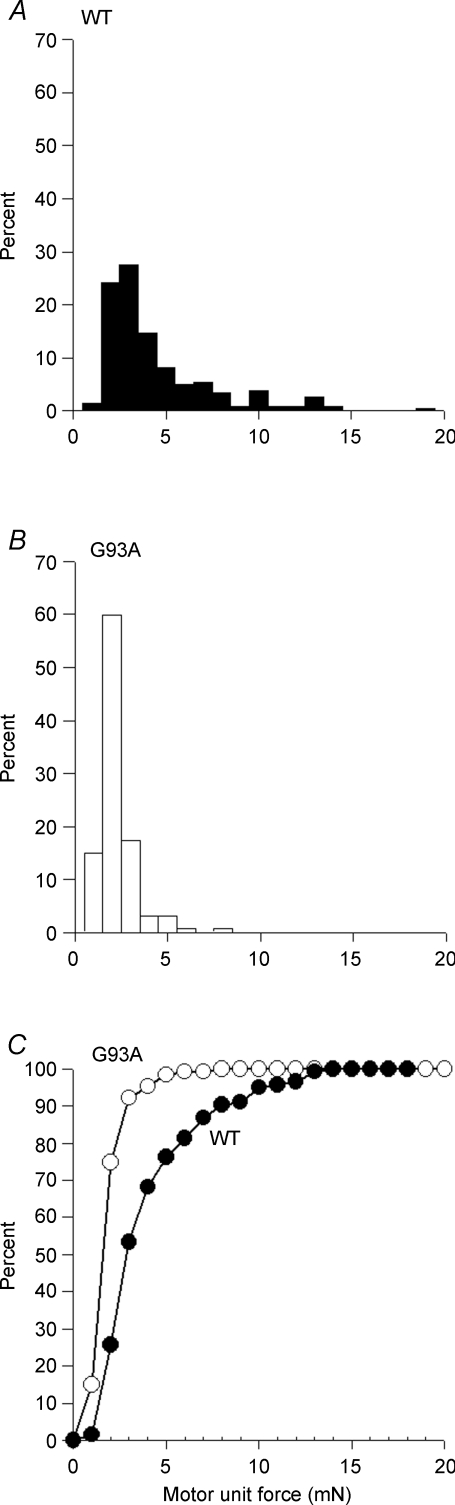
The frequency distributions of motor unit twitch forces generated within each of the TA muscles in SOD1G93A or SOD1WT mice were not significantly different. The unit forces were therefore combined for direct comparison of the distributions for the SOD1G93A and SOD1WT mouse muscles. The typically right skewed distribution of twitch forces in the muscles of the SOD1WT mice (Fig. 4A) was less pronounced in the SOD1G93A mouse TA (Fig. 4B), with 90% of the motor units developing twitch forces between 0.5 and 4 mN as compared to 70% in the control SOD1WT mouse TA muscles (Fig. 4A and B). The mean range of motor unit forces in the TA muscles of SOD1G93A mice (3.6 ± 1.4 mN) was significantly less than observed in TA muscles of SOD1WT mice (13 ± 2 mN, P < 0.001). The maximum motor unit force was only 8.1 mN in the SOD1G93A mouse TA muscle, compared with 18.9 mN in TA muscles of the SOD1WT mouse (Fig. 4A and B). The reduced proportion of the most forceful motor units in the SOD1G93A mouse TA muscles and the consequent significant reduction in range are further illustrated in the cumulative frequency histograms (Fig. 4C). The significant (P < 0.01) leftward shift of the cumulative frequency histogram in the SOD1G93A mice indicates a disproportionate decline in both the number and the proportion of the most forceful motor units and an increase in contractile force of the smaller motor units.
Figure 4. Changes in the frequency distributions of motor unit twitch forces in SOD1G93A mouse TA muscles, as compared to age matched SOD1WT control mouse TA muscles.
A, in the SOD1WT the distribution was skewed toward large motor units; the minimum motor unit force was 0.5 mN and the maximum was 18.4 mN. Approximately one-third of all motor units produced forces above 4.0 mN. B, in the SOD1G93A mouse muscles, the average motor unit force was half that of the SOD1WT mouse TA muscles, and only one-tenth of the motor units produced forces above 4.0 mN; the maximum motor unit force was only 8.1 mN. C, the increase in the proportion of less forceful motor units is best illustrated by a cumulative frequency histogram. There was a significant (P < 0.01) leftward shift of motor unit forces, demonstrating loss of the most forceful motor units and an increase in size of the smaller motor units.
Innervation ratio and force per fibre
Denervated muscle fibres in the TA muscles in the SOD1G93A mouse were identified by NCAM immunoreactivity to distinguish them from the innervated NCAM negative fibres (Fig. 5A and B) (Gordon et al. 2008). Angulated, NCAM positive fibres were smaller and contained NCAM within the muscle fibres, compared with the more normal-looking NCAM positive fibres where NCAM was concentrated at the cell membrane (Fig. 5B). Angulation is indicative of long-term denervation (Cullen et al. 1992) and it was interesting that these fibres contained NCAM within the fibres and that these were more frequently encountered within the superficial regions of TA muscles of SOD1G93A mice (Gordon et al. 2008). In contrast, within the deeper regions of the muscle, NCAM positive muscle fibres displayed hexagonal profiles, indicating that they had only recently become denervated. A comparison of typical whole muscle cross-sections immunostained for type IIB fibres (Fig. 5C and D) clearly shows that TA muscles of the SOD1G93A mouse were smaller than those of SOD1WT mice. The mean TA CSA in SOD1G93A transgenic mice (1.4 ± 0.93 mm2) was only ∼40% of the CSA of SOD1WT transgenic mouse TA muscles (3.4 ± 0.12 mm2); there was also an obvious decrease in the proportion of type IIB muscle fibres and corresponding increases in the proportions of type IID/X and IIA fibres.
Figure 5. Representative cross-sections of TA muscles in 60-day-old SOD1WT and SOD1G93A mice that have been stained for neural cell adhesion molecule (NCAM) to identify NCAM positive, denervated muscle fibres.
In SOD1WT mouse TA muscles (A), the fibres did not express NCAM, indicating that they were all normally innervated. In the SOD1G93A mouse TA muscles (B), numerous muscle fibres expressed NCAM. The number of NCAM negative muscle fibres that were innervated by motor axons is summarized in Fig. 6. Representative photomicrographs of TA muscles from SOD1WT (C) and SOD1G93A (D) mice are immunostained with anti-MHCIIb monoclonal antibody (clone BF-F3), which labels type IIB fibres. There was considerable whole muscle and type IIB fibre atrophy of the muscles of SOD1G93A mice compared with SOD1WT. There was also overt loss of type IIB fibres, particularly from the superficial regions of TA muscles from SOD1G93A mice. The scale bar in A is 100 μm; the scal bar in D is 1 mm.
The total number of innervated muscle fibres in the TA of SOD1G93A mice was reduced by ∼40% (Fig. 6A). This was less than the ∼60% decrease in motor unit number (Fig. 3C) and in the force per muscle fibre (motor unit force divided by number of muscle fibres) (Fig. 6C), and much less than the ∼80% reduction in whole muscle twitch and tetanic forces (Fig. 3A and B), presumably associated with the loss of the most forceful motor units that contained the largest muscle fibres. The average measure of the innervation ratio (IR) obtained from the ratio of the number of innervated muscle fibres to the number of motor units (Fig. 6B) tended toward higher levels in SOD1G93A compared with SOD1WT mice, increasing by 44%.
Figure 6. The number of innervated muscle fibres (A), muscle fibres per motor unit (B) and force produced per muscle fibre (C).
The average number of muscle fibres per motor unit (innervation ratio; IR) in the SOD1G93A mouse TA muscle tended to increase by approximately 44%, compared to age-matched control SOD1WT. Despite the relatively static IR, the force produced by each motor unit declined (Fig. 4), indicating that the contractile force producing capacity of each motor unit was reduced. Data are presented as means ± s.e.m. and statistical significance is indicated as ** for P < 0.01.
Muscle fibre size
The force per muscle fibre is dependent on fibre CSA. There being a wide regional variation in CSA of muscle fibres within TA muscles of SOD1G93A and SOD1WT mice, with the larger type IIB and IID/X muscle fibres distributed in the more superficial regions and the type IIA fibres distributed within the deep portion of the muscle (Fig. 5C and D), we measured the CSA of all the muscle fibres in each of the muscles studied. The distribution of CSAs within TA muscles of SOD1WT mice was skewed toward the right (Fig. 7A), compared with SOD1G93A mice (Fig. 7D). Consequently the average fibre CSA was significantly lower (P < 0.001) in the TA muscles of SOD1G93A mice (mean ± s.d.: 1089 ± 581 μm2) as compared to those of SOD1WT mice (1551 ± 856 μm2).
Figure 7. The percentage of innervated muscle fibres plotted as distributions of fibre cross-sectional areas (CSAs) in the SOD1WT (A–C) and SOD1G93A mouse (D–E) TA muscles.
In B and E, the number of each type of innervated fibre is plotted as a percentage of the total number of innervated fibres. In C and F, the number of innervated muscle fibres of each type is plotted as a percentage of the total muscle CSA (i.e. fibre area density). In A and D, mean ± s.d. of the muscle fibre CSAs are shown. In B, C, E and F, the percentage values indicate the relative proportions of type IIA, IID/X and IIB muscle fibres and the inverted triangles show the mean CSA for each muscle fibre type. A, in the SOD1WT mouse TA muscle, the distribution of fibre CSAs was skewed towards the right, with a high proportion of muscle fibres having large CSA. B, the skewed distribution was accounted for by the differences in the proportion of the different muscle fibre types; the greatest proportion of muscle fibres was the large type IIB in the SOD1WT mouse TA muscle. C, when the number of muscle fibres of each type is expressed as a percentage of whole muscle CSA, it becomes clear that 80% of the muscle CSA is occupied by the large type IIB muscle fibres in the SOD1WT mouse TA muscle. D, the distribution of CSA in the SOD1G93A mouse TA muscle was also skewed, but the range of CSA and the average CSA were both smaller than in age matched SOD1WT mice. E, the different distribution was due to both a change in the proportion of muscle fibre types with substantially fewer type IIB fibres and a decrease in the average CSA of the type IIB muscle fibres. In addition to the decline in the average CSA of type IIB fibres, the type IIA fibres were larger, resulting in smaller differences between the mean CSA of type IIA, IID/X and IIB fibres. F, the proportions of innervated muscle fibres expressed as a percentage of the whole muscle CSA (fibre area density) was the same as the proportions of innervated muscle fibres expressed as a percentage of the total number of innervated muscle fibres.
More detailed analyses of all muscle fibre subtypes revealed that the relative CSAs and proportions of muscle fibres were substantially altered in SOD1G93A transgenic mice compared with SOD1WT mice (Fig. 7B and E). The type IIB fibres displayed the largest CSAs and were most abundant (65%) in SOD1WT TA muscles (Fig. 7B) (Hamalainen & Pette, 1993). In the SOD1G93A mouse muscles (Fig. 7E) on the other hand, the proportion and CSA of type IIB fibres were significantly reduced (P < 0.001). The same parameters were both significantly elevated in the population of transitional type IID/X fibres (P < 0.05) and in the fast-oxidative type IIA fibres (P < 0.05) (Fig. 7B and E).
When the number of innervated muscle fibres of each type was plotted as a percentage of the total muscle CSA (i.e. fibre area density), we found that the greater proportion of the muscle CSA was comprised of the large type IIB muscle fibres (80%; Fig. 7C) as compared to 65% with respect to the total number of innervated muscle fibres of a given type (Fig. 7B). In the SOD1G93A mice, on the other hand, the proportions of the fibre types was the same whether or not the proportion of innervated muscle fibres was plotted as a percentage of the total number of innervated muscle fibres or the whole muscle CSA.
The median CSA and mean numbers of innervated fibres in TA muscles of SOD1G93A and SOD1WT transgenic mice are shown in Fig. 8 for each fibre subtype. Large differences in the sizes of the various subtypes (i.e. IIA < IID/X < IIB) within TA muscles of 60-day-old SOD1WT transgenic mice were almost completely lost in the SOD1G93A TA muscles. The most dramatic change was that the CSAs of type IID/X and IIB fibres were similar in SOD1G93A TA muscles (Fig. 8A). The median size of type IIB fibres in SOD1G93A mice was significantly lower than in SOD1WT mice; on the other hand the median CSAs of the IID/X and IIA fibres were significantly greater in the TA muscles of SOD1G93A mice as compared to SOD1WT mice. The relatively small number of hybrid muscle fibre types in SOD1G93A TA muscles had a median CSA that was intermediate between that of type IID/X and IIB fibres seen within TA muscles of SOD1WT mice.
Figure 8. Median cross-sectional area (A) and mean number (B) of innervated fibres of TA muscles that were classified on the basis of MHC isoform immunohistochemistry.
A, the median CSA of type IIB muscle fibres was significantly smaller in the TA muscles of SOD1G93A mice compared with those of SOD1WT mice. Conversely, type IIA and IID/X fibres were significantly larger. Significant differences were determined with a non-parametric test, as fibre cross-sectional area data were not normally distributed (see Fig. 6). B, the numbers (mean ± s.e.m.) of innervated type IIB and type IID/X fibres were reduced by 61% and 28% in SOD1G93A mouse TA, respectively. The number of type IIA muscle fibres in SOD1G93A mouse TA increased by almost 2-fold, as compared to SOD1WT mouse TA muscle. There were no innervated muscle fibres that coexpressed two or more MHC isoforms in the SOD1WT TA muscle, while a small proportion of muscle fibres coexpressed two different MHC isoforms in the SOD1G93A mouse TA muscle. Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01.
The reduced proportion of type IIB fibres in the TA muscles of the SOD1G93A mice (Fig. 7B and E) translated to a 65% reduction in the number of innervated type IIB muscle fibres (Fig. 8B), and a 28% decrease in the number of IID/X fibres (Fig. 8B). The number of type IIA muscle fibres increased by approximately twofold. These changes indicated denervation of a substantial portion of type IIB fibres in SOD1G93A mouse TA muscles, as well as activity-dependent conversion of fast fatigable fibres (FF = IIB) and fast fatigue intermediate fibres (FI = IID/X) into slower fatigue resistant (FR) type IIA fibres. The coexpression of two different MHC isoforms within a small population of muscle fibres was also consistent with a fast-to-slower fibre type conversion within TA muscles of SOD1G93A mice (Putman et al. 2000). Using the present methods our analyses included type IIA/IIB, type IIA/D(X) and type IIB/D(X) hybrid fibres (Putman et al. 2000; Martins et al. 2006).
Medial gastrocnemius muscle
The percentage distributions of motor unit twitch forces in MG muscles from 60-day-old SOD1G93A and SOD1WT transgenic mice are compared in Fig. 9. As observed in the TA muscles, both the range and average motor unit forces were reduced in the SOD1G93A mice (Fig. 9A and B). The twitch forces of MG muscles from SOD1G93A mice were significantly smaller (Fig. 9C), as in the TA muscles, and there was a corresponding reduction in the number of intact motor units (Fig. 9D). These similarities between the decline in muscle and motor unit twitch forces in the TA and MG muscles in SOD1G93A mice also indicates that the preferential loss of the most forceful FF motor units and their composite type IIB muscle fibres accounts for the decline in muscle and motor unit forces within MG muscles of SOD1G93A mice.
Figure 9. Motor unit force declined in medial gastrocnemius (MG) muscles, in parallel with a decline in whole muscle twitch force and a loss of functional motor units.
A, in normal 60-day-old SOD1WT mouse MG muscles the distribution of motor unit forces was skewed towards the left, with a high proportion of less forceful motor units. B, the distribution of motor units in the SOD1G93A mouse MG muscle was also skewed to the left, but there was only one motor unit that had a force greater than ∼6 mN (C). In parallel with the loss of the most forceful motor units from the MG, the whole muscle twitch force declined by ∼80%. A representative force tracing is seen in the inset. D, similar to the TA muscle (Fig. 3C), the number of intact motor units in the MG also declined by ∼60% in 60-day-old SOD1G93A mice.
Discussion
Here we provide the first evidence of preferential loss of the most forceful motor units containing type IIB muscle fibres in the SOD1G93A transgenic mouse that is coupled with fibre type transition from fast-fatigable motor units containing type IIB muscle fibres toward motor units containing fast fatigue-intermediate type IID/X fibres and fast fatigue-resistant type IIA muscle fibres. Nonetheless, there remained a substantial number of innervated type IIB muscle fibres and a trend toward increased number of muscle fibres in the remaining motor units. This is in apparent contradiction to the findings of Caroni and colleagues, who reported the abrupt denervation of all IIB fibres by postnatal day 55 (Pun et al. 2006). Our findings of early and selective loss of the most forceful motor units in presymptomatic mice (Fig. 4) are consistent with anatomical data of preferential die-back of the largest motor axons (Fischer et al. 2004), and, to some extent, the consequent denervation of the most forceful type IIB muscle fibres (Frey et al. 2000). This selective loss accounted for the disproportionate decline in whole muscle contractile force in large fast-twitch muscles as compared to the decline in motor unit numbers (Figs 3 and 9) that was also previously observed at 40 and 80 days of age (Hegedus et al. 2007). The combined anatomical and electrophysiological approach allowed us to show that the shift towards smaller motor unit forces (Fig. 4) was due to the selective denervation of the largest type IIB muscle fibres (Fig. 8) and an increased proportion of less forceful, type IID/X and type IIA muscle fibres (Fig. 7).
Reduced motor unit and muscle forces
The force of motor units in the presymptomatic SOD1G93A transgenic mice was reduced (Fig. 4) rather than increased as might be expected if there were effective axonal sprouting of FI, FR and S motoneurons to compensate for the partial denervation of the fast-twitch muscles. Early studies of ALS patients had observed that the force of the surviving motor units did not increase as might be expected for effective axonal sprouting and from the accompanying increased EMG potential amplitudes (Milner-Brown et al. 1974a; Vogt & Nix, 1997). Indeed, the progressive clumping of muscle fibre types that ensues from reduced numbers of innervated motor units in healthy cats (Rafuse & Gordon, 1996) may be sufficient for surface EMG recordings to display increased EMG amplitudes in this and other studies of transgenic mouse models of ALS where motor unit forces were not recorded (Kennel et al. 1996; Azzouz et al. 1997; Shefner et al. 1999).
In those muscles of the transgenic SOD1G93A mice, as in the TA and the MG muscles studied here, the majority of the muscle fibres are type IID/X and IIB at whose neuromuscular junctions the chemorepellant semaphorin 3 A is expressed in the terminal Schwann cells (De Winter et al. 2006) and where effective axonal sprouting and muscle reinnervation does not occur in the SODG93A mouse (Frey et al. 2000). In the present study, the absence of type I muscle fibres and the small component of type IIA muscle fibres (Fig. 7) are likely to account for the inability to discern a significant increase in innervation ratio (Fig. 6) due to early compensatory axonal sprouting of motoneurons that innervate type IIA muscle fibres in the 60-day-old SOD1G93A mouse TA muscle.
Selective vulnerability and changes within fast-twitch type II muscle fibre subtypes
There is already significant loss of motor units in fast-twitch muscles of the SODG93A mouse at 40 days of age (Hegedus et al. 2007) and a loss of ∼60% of motor units at 60 days of age (present study). In this study of fast-twitch TA and MG muscles we did not observe the complete denervation of the type IIB muscle fibres in the fast-twitch TA muscles of the ankle flexor muscle compartment in the SODG93A transgenic mouse that was reported by Pun et al. (2006). Direct quantification of the number of both innervated and denervated muscle fibres in our study, using NCAM immunoreactrivity to discern NCAM positive denervated muscle fibres clearly showed that ∼55% of the type IIB muscle fibres within TA muscles were innervated at 60 days of age (Fig. 8). The recent study of Pun et al. (2006) used an indirect method of silver chloride cholinesterase staining to describe a selective and complete denervation of type IIB muscle fibres within the ankle flexors (TA) and extensors (MG and LG) of the SODG93A mouse between postnatal days 48 and 55. In this study we also observed a 28% reduction in the number of innervated type IID/X muscle fibres and an increase in the number of innervated type IIA muscle fibres at 60 days of age. Thus while our data confirm that the fast IIB fibre subtype population is selectively vulnerable early in disease progression, their loss is neither abrupt nor complete at postnatal day 60. Moreover a subset of fast type IID/X fibres also begins to display selective vulnerability by postnatal day 60, while a significant proportion of innervated type IID/X and IIB fibres underwent compensatory transformation into fast fatigue-resistant type IIA muscle fibres. This most likely resulted from increased recruitment of the surviving motor units innervating type IID/X and IIB fibres (see below). Importantly this transition may have also conferred similar adaptive changes upon their respective innervating α-motoneurons that prolonged survival of the affected α-motoneurons. This would explain the trend toward an increase in the IR at 60 days (Fig. 6B), the gradual motor unit loss throughout the life span of the SODG93A mouse (Hegedus et al. 2007), and that clinical signs manifest when the motoneurons innervating the type IIA and I muscle fibres finally succumb during the late symptomatic phase of disease (Schaefer et al. 2005; Hegedus et al. 2007).
Previous studies by others (Frey et al. 2000; Pun et al. 2006) report that denervation of type IIB fibres is complete within fast-twitch muscles of SODG93A mice by postnatal day 60. Their findings might relate to the use of paraformaldehyde fixation, followed by partial antigenic recovery using trypsin digestion. These treatments, respectively, reduce or abolish staining with MHC monoclonal antibodies and increase cross-reactivity of isoform-specific MHC monoclonal antibodies with all other MHC isoforms (personal observation). Alternatively, the use of an indirect method to determine muscle fibre denervation coupled with the use of monoclonal antibody RT-D9 (specific to IID/X and IIB fibres; Schiaffino et al. 1989) to identify type IIA fibres, could readily account for an overestimation of the proportion of innervated fibres within this fibre population, at the expense of identifying innervated type IIB (and IID/X) fibres (Pun et al. 2006).
Analysis of neuromuscular junctions in double transgenic G93A SOD1/yellow fluorescent protein (YFP) transgenic mice revealed that, in contrast to neuromuscular junctions in symptomatic and end-stage animals where axon terminals were found to be in various stages of fragmentation, the terminal nerve branch morphology and the postsynaptic acetylcholine receptor appearance before 77 days of age showed little if any alterations (Schaefer et al. 2005). This analysis indicates that the loss of functional motor units in the 60-day-old mouse that we observed is likely to be due to the withdrawal of all the terminals at the neuromuscular junctions of the type IIB muscle fibres and die-back of the axons. This die-back precedes the significant decline in motoneuron numbers detected only by the onset of symptomatic disease (Gurney et al. 1994).
Muscle fibre conversion
Increased neuromuscular activity of the surviving ∼40% functional motor units in the SOD1G93A mouse probably further accounts for reduced proportions of the large type IIB muscle fibres and increased proportions of the smaller type IID/X and IIA fibres, as those type IIB fibres that sustain their innervation would be stimulated to undergo gradual phenotypic transformation to type IID/X (FI) and IIA (FR) muscle fibres (Figs 7 and 8). This is especially evident by the rightward shift in the cross-sectional areas of IID/X and IIA fibres. Daily recruitment of all motor units by chronic low frequency electrical stimulation (Delp & Pette, 1994; Putman et al. 2000, 2004a) or functional overload by synergist ablation (Dunn & Michel, 1997) results in fibre type conversions that generally progress from the large IIB fibres to IID/X and eventually to IIA fibres. Such fibre type transitions occur in conjunction with slowing of isometric contractile properties and corresponding metabolic conversion to fatigue resistant, oxidative fibres in normal cats (Gordon et al. 1997), rats (Putman et al. 2004a) and mice (Simoneau & Pette, 1988). In rat muscles, fibre type conversion was evident relatively quickly with reduction in the CSA of the innervated type IIB fibres after 12 days (Delp & Pette, 1994). Thus the increased neuromuscular activity of fewer functional motor units in the 60-day-old SOD1G93A mouse muscles can explain the progressive conversion of IIB fibres to type IID/X and to type IIA fibres (Fig. 7). The activity-dependent fibre type conversion in SOD1G93A mouse muscles may also be linked to the increased motoneuron activity that is associated with membrane hyperexcitability (Kuo et al. 2004).
Asymptomatic disease despite motor unit loss
The lack of overt symptoms in the 60-day-old SOD1G93A mouse is surprising considering the loss of ∼80% of isometric contractile force of the TA and MG muscles (Figs 3 and 9). Muscle-specific tests have, however, detected early manifestation of preferential motor unit loss (Wooley et al. 2005). At 52 days of age, the maximum running speed was reduced in SOD1G93A mice (Veldink et al. 2003). In normal rodents, motor units in the TA muscle are progressively recruited with increased treadmill speeds (Roy et al. 1991), and therefore a decrease in the ability to sustain running speed is consistent with a loss of TA motor units, and in particular those that innervate the fastest type IIB fibres.
Model of muscle denervation in ALS
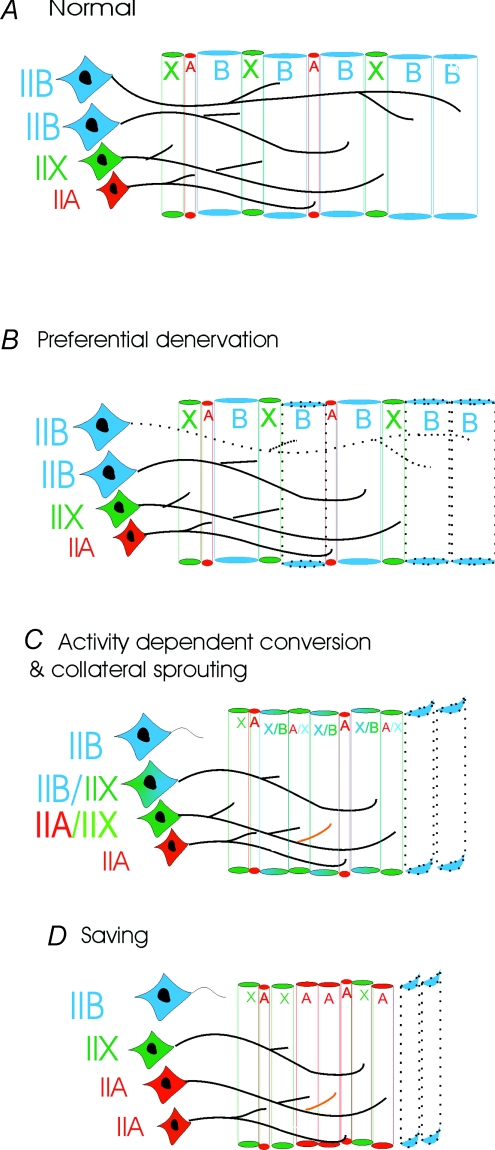
From observations in the SOD1G93A mouse model of ALS we now postulate a hypothesis to account for the observed dissociation of motor unit force and calculated innervation ratio that may also explain the reduced compensatory capacity of remaining motor units in both human and mouse forms of ALS (Fig. 10). We suggest that selective motor axon die-back in the SOD1G93A mice occurs by two parallel processes: (1) preferential denervation of the FF motor units that contain large type IIB muscle fibres, and (2) activity-dependent conversion of motor units to those innervated by smaller motoneurons and containing more fatigue-resistant muscle fibres. As the sprouting competence of the motor axons innervating the type IIB fibres is low (Frey et al. 2000), significant functional motor unit enlargement does not happen, and few fibres are converted due to reinnervation by collateral axonal sprouting (Fig. 10C). Instead, fibre type conversion occurs due to increased recruitment of the remaining innervated FF motor units containing the type IIB muscle fibres and the intrinsic hyperexcitability of the motoneurons (Kuo et al. 2004). As muscle fibres convert to type IIA, effective sprouting enlarges functional motor units with observed increased motor unit action potentials (Kennel et al. 1996) and enlarged terminal fields of innervation by identified nerves (Schaefer et al. 2005) in symptomatic SODG93A mice, particularly in late-stage disease. Reduced specific force of the muscle fibres may not, however, translate into increased motor unit forces.
Figure 10. A possible mechanism to describe neuromuscular change in presymptomatic SOD1G93A mouse TA muscles.
A, normal; in the age-matched control SOD1WT mouse TA muscles fibres are innervated by intact motor axons, and there is no coexpression of myosin heavy chains. B, preferential denervation; in early presymptomatic stages there is withdrawal of axon branches preferentially from the largest motor units, which innervate type IIB muscle fibres. C, activity-dependent conversion and collateral sprouting; not all of the motoneurons that innervate type IIB fibres die back, and those motor units that remain intact increase their activity to compensate for the selective and progressive loss of functional motor units. As a result of increased activity, the remaining muscle fibres change to slower, more oxidative phenotypes via coexpression of myosin heavy chains. This conversion is first evidenced by a decrease in CSA of the muscle fibres. There is also some collateral axonal sprouting from the intact motor units that innervate type IIA and IID/X muscle fibres, resulting in coexpression of muscle fibre myosin heavy chains. D, saving; collateral sprouting and activity-dependent conversion result in a progressive increase in the number of type IIA and IIX muscle fibres within motor units that become progressively enlarged by sprouting. The lower force production of the smaller muscle fibres in these surviving motor units does not compensate for the ongoing die-back and loss of the more forceful large motor units containing type IIB muscle fibres. Denervated muscle fibres that are not reinnervated become angulated and atrophy. Eventually, the proportion of type IIB muscle fibres becomes very low. As the type IIB muscle fibres produce the greatest amounts of force, the average force producing capacity of the remaining muscle fibres that are innervated by the remaining type IIA and type IIX motoneurons is reduced.
Human ALS disease
It remains to be seen whether preferential denervation and conversion cause changes in the muscle fibre proportions in ALS patients that account for dissociation between motor unit forces and increased size, as measured by EMG (Milner-Brown et al. 1974a; Venkatesh et al. 1995; Vogt & Nix, 1997). Single muscle fibre data indicating no change in the specific force of muscle fibres from human patients indicate dissociation may result from the same mechanisms as we have suggested for the SOD1G93A mice (Krivickas et al. 2002). Human muscles do not, however, contain type IIB fibres but rather express a small complement of FI type IID/X fibres (i.e. up to 10%) and larger proportions of FR type IIA (e.g. ∼45%) and S type I fibres (e.g. ∼45%) (Putman et al. 2004b). Thus attempts to evaluate preferential loss of large fast IIB fibres (Pun et al. 2006) will likely prove to be of no diagnostic value. In human patients conversion of many FR type IIA fibres to S type I fibres is likely to precede the onset of clinical signs and symptoms, which will undoubtedly coincide with significant loss of a substantial proportion of the remaining type IIA fibres. This is consistent with studies mapping motor endplates and axons in human ALS patients, which revealed that motoneurons exhibit a reduced ability to increase their IR, as compared to patients with other conditions of partial denervation (Coers et al. 1973).
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Council of Canada (NSERC) and the Alberta Heritage Foundation for Medical Research (AHFMR). J.H. received studentships from NSERC and AHFMR. T.G. is an AHFMR Senior Investigator and C.T.P. is an AHFMR Senior Scholar. The authors thank Drs A. J. McComas, D. J. Bennett and Kelvin Jones who provided helpful comments on the manuscript.
References
- Atkin JD, Scott RL, West JM, Lopes E, Quah AK, Cheema SS. Properties of slow- and fast-twitch muscle fibres in a mouse model of amyotrophic lateral sclerosis. Neuromuscul Disord. 2005;15:377–388. doi: 10.1016/j.nmd.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Leclerc N, Gurney M, Warter JM, Poindron P, Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:45–51. doi: 10.1002/(sici)1097-4598(199701)20:1<45::aid-mus6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Brown WF, Milner-Brown HS. Some electrical properties of motor units and their effects on the methods of estimating motor unit numbers. J Neurol Neurosurg Psychiatry. 1976;39:249–257. doi: 10.1136/jnnp.39.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24:515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Coers C, Telerman-Toppet N, Gerard JM. Terminal innervation ratio in neuromuscular disease. II. Disorders of lower motor neuron, peripheral nerve, and muscle. Arch Neurol. 1973;29:215–222. doi: 10.1001/archneur.1973.00490280027003. [DOI] [PubMed] [Google Scholar]
- Cullen MJ, Johnson MA, Astaglia FL. Pathological reactions of skeletal muscle. In: Mastaglia FL, editor. Skeletal Muscle Pathology. Edinburgh: Churchill Livingstone; 1992. pp. 123–184. [Google Scholar]
- De Winter F, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, van Muiswinkel FL, Verhaagen J. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32:102–117. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Delp MD, Pette D. Morphological changes during fiber type transitions in low-frequency-stimulated rat fast-twitch muscle. Cell Tissue Res. 1994;277:363–371. doi: 10.1007/BF00327784. [DOI] [PubMed] [Google Scholar]
- Derave W, Van Den Bosch L, Lemmens G, Eijnde BO, Robberecht W, Hespel P. Skeletal muscle properties in a transgenic mouse model for amyotrophic lateral sclerosis: effects of creatine treatment. Neurobiol Dis. 2003;13:264–272. doi: 10.1016/s0969-9961(03)00041-x. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Michel RN. Coordinated expression of myosin heavy chain isoforms and metabolic enzymes within overloaded rat muscle fibers. Am J Physiol Cell Physiol. 1997;273:C371–C383. doi: 10.1152/ajpcell.1997.273.2.C371. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. 2004;26:174–185. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- Gordon T, Ly V, Hegedus J, Tyreman N. Early detection of denervated muscle fibers in the G93A mouse model of amyotrophic lateral sclerosis. Neurol Res. 2008 doi: 10.1179/174313208X332977. in press. [DOI] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Rafuse VF, Munson JB. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats.1. Muscle and motor unit properties. J Neurophysiol. 1997;77:2585–2604. doi: 10.1152/jn.1997.77.5.2585. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hamalainen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993;41:733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Jones KE, Gordon T. Development and use of the ITS-MUNE method in mice. In: Bromberg MB, editor. Motor Unit Number Estimation and DGEMG – Supplement to Clinical Neurophysiology Series. Elsevier; 2008. [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kanai K, Kuwabara S, Misawa S, Tamura N, Ogawara K, Nakata M, Sawai S, Hattori T, Bostock H. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain. 2006;129:953–962. doi: 10.1093/brain/awl024. [DOI] [PubMed] [Google Scholar]
- Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Yang JI, Kim SK, Frontera WR. Skeletal muscle fiber function and rate of disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:636–643. doi: 10.1002/mus.10257. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults AN, Fu R, Bar PR, Anelli R, Heckman CJ, Kroese AB. Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol. 2004;91:571–575. doi: 10.1152/jn.00665.2003. [DOI] [PubMed] [Google Scholar]
- Major LA, Hegedus J, Weber DJ, Gordon T, Jones KE. Method for counting motor units in mice and validation using a mathematical model. J Neurophysiol. 2007;97:1846–1856. doi: 10.1152/jn.00904.2006. [DOI] [PubMed] [Google Scholar]
- Martins KJ, Gordon T, Pette D, Dixon WT, Foxcroft GR, Maclean IM, Putman CT. Effect of satellite cell ablation on low-frequency-stimulated fast-to-slow fibre-type transitions in rat skeletal muscle. J Physiol. 2006;572:281–294. doi: 10.1113/jphysiol.2005.103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Sica RE, Campbell MJ, Upton AR. Functional compensation in partially denervated muscles. J Neurol Neurosurg Psychiatry. 1971;34:453–460. doi: 10.1136/jnnp.34.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Contractile and electrical properties of human motor units in neuropathies and motor neurone disease. J Neurol Neurosurg Psychiatry. 1974a;37:670–676. doi: 10.1136/jnnp.37.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Pattern of recruiting human motor units in neuropathies and motor neurone disease. J Neurol Neurosurg Psychiatry. 1974b;37:665–669. doi: 10.1136/jnnp.37.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Putman CT, Dixon WT, Pearcey JA, Maclean IM, Jendral MJ, Kiricsi M, Murdoch GK, Pette D. Chronic low-frequency stimulation upregulates uncoupling protein-3 in transforming rat fast-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004a;287:R1419–R1426. doi: 10.1152/ajpregu.00421.2004. [DOI] [PubMed] [Google Scholar]
- Putman CT, Dusterhoft S, Pette D. Satellite cell proliferation in low frequency-stimulated fast muscle of hypothyroid rat. Am J Physiol Cell Physiol. 2000;279:C682–C690. doi: 10.1152/ajpcell.2000.279.3.C682. [DOI] [PubMed] [Google Scholar]
- Putman CT, Kiricsi M, Pearcey J, Maclean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Xu X, Gillies E, Maclean IM, Bell GJ. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol. 2004b;92:376–384. doi: 10.1007/s00421-004-1104-7. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanisms and significance of fiber type grouping in reinnervated muscles. J Neurophysiol. 1996;75:282–297. doi: 10.1152/jn.1996.75.1.282. [DOI] [PubMed] [Google Scholar]
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, Siwek DF, Upton-Rice M, Brown RH., Jr Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurol. 1999;53:1239–1246. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Pette D. Specific effects of low-frequency stimulation upon energy metabolism in tibialis anterior muscles of mouse, rat, guinea pig and rabbit. Reprod Nutr Dev. 1988;28:781–784. doi: 10.1051/rnd:19880512. [DOI] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Gordon T. Effect of exercise on stability of chronically enlarged motor units. Muscle Nerve. 2002;25:359–369. doi: 10.1002/mus.10057. [DOI] [PubMed] [Google Scholar]
- Tam SL, Gordon T. Mechanisms controlling axonal sprouting at neuromuscular junction. J Neurocytol. 2003;32:961–974. doi: 10.1023/B:NEUR.0000020635.41233.0f. [DOI] [PubMed] [Google Scholar]
- Tamura N, Kuwabara S, Misawa S, Kanai K, Nakata M, Sawai S, Hattori T. Increased nodal persistent Na+ currents in human neuropathy and motor neuron disease estimated by latent addition. Clin Neurophysiol. 2006;117:2451–2458. doi: 10.1016/j.clinph.2006.07.309. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, Van Den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Shefner JM, Logigian EL. Does muscle reinnervation produce electromechanical dissociation in amyotrophic lateral sclerosis? Muscle Nerve. 1995;18:1335–1337. doi: 10.1002/mus.880181119. [DOI] [PubMed] [Google Scholar]
- Vogt T, Nix WA. Functional properties of motor units in motor neuron diseases and neuropathies. Electroencephalogr Clin Neurophysiol. 1997;105:328–332. doi: 10.1016/s0924-980x(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1 (G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]