Abstract
A rise in intracellular calcium levels ([Ca2+]i) is a key trigger for the lethal effects of the excitatory neurotransmitter glutamate in various central neurons, but a consensus has not been reached on the pathways that mediate glutamate-dependent increases of [Ca2+]i in retinal ganglion cells (RGCs). Using Ca2+ imaging techniques we demonstrated that, in the absence of external Mg2+, the Ca2+ signal evoked by glutamate was predominantly mediated by NMDA-type glutamate receptors (NMDA-Rs) in immunopanned RGCs isolated from neonatal or adult rats. Voltage-gated Ca2+ channels and AMPA/kainate-Rs contributed a smaller portion of the Ca2+ response at saturating concentrations of glutamate. Consistent with NMDA-R involvement, extracellular Mg2+ inhibited RGC glutamate responses, while glycine had a potentiating effect. With Mg2+ present externally, the effect of AMPA/kainate-R antagonists was enhanced and both NMDA- and AMPA/kainate-R antagonists greatly reduced the glutamate-induced increases of RGC [Ca2+]i. This finding indicates that the primary contribution of AMPA/kainate-Rs to RGC glutamatergic Ca2+ dynamics is through the depolarization-dependent relief of the Mg2+ block of NMDA-R channels. The effect of glutamate receptor antagonists on glutamatergic Ca2+ signals from RGCs in adult rat retinal wholemounts yielded results similar to those obtained using immunopanned RGCs. Additional experiments on isolated RGCs revealed that during a 1 h glutamate (10–1000 μm) exposure, 18–28% of RGCs exhibited delayed Ca2+ deregulation (DCD) and the RGCs that underwent DCD were positive for the death marker annexin V. RGCs with larger glutamate-evoked Ca2+ signals were more likely to undergo DCD, and NMDA-R blockade significantly reduced the occurrence of DCD. Identifying the mechanisms underlying RGC excitotoxicity aids in our understanding of the pathophysiology of retinal ischaemia, and this work establishes a major role for NMDA-R-mediated increases in [Ca2+]i in glutamate-related RGC death.
The synaptic release of the excitatory neurotransmitter glutamate onto retinal ganglion cell (RGC) dendrites is an essential part of the visual pathway (reviewed by Thoreson & Witkovsky, 1999; Yang, 2004), but excessive or prolonged exposure to glutamate has long been considered lethal to these retinal output neurons (Lucas & Newhouse, 1957; Olney, 1969; Sisk & Kuwabara, 1985). While a rise in intracellular calcium levels ([Ca2+]i) is a key factor in the initiation of glutamate-related (excitotoxic) death of neurons throughout the CNS (see Sattler & Tymianski, 2000; Khodorov, 2004), the dynamics of altered [Ca2+]i that occur in RGCs undergoing excitotoxic death have yet to be directly characterized.
N-Methyl-d-aspartate-type glutamate receptors (NMDA-Rs) are permeable to Ca2+ (Mayer & Westbrook, 1987; Ascher & Nowak, 1988) and, consistent with a role for NMDA-Rs in mediating excitotoxic RGC death, intravitreal injections of the selective agonist NMDA causes RGC loss in rodents in vivo (Siliprandi et al. 1992; Lam et al. 1999; Schlamp et al. 2001; Manabe et al. 2005; Nakazawa et al. 2005; Pernet et al. 2007; Reichstein et al. 2007). However, in addition to NMDA-Rs, rodent RGCs have also been shown to possess α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) and kainate-type ionotropic glutamate receptors, based on electrophysiological recordings (Aizenman et al. 1988; Taschenberger et al. 1995; Chen & Diamond, 2002) and single-cell molecular profiling using RT-PCR (Jakobs et al. 2007). Although AMPA- and kainate-Rs are generally less permeable to Ca2+ than NMDA-Rs, AMPA-Rs that lack the GluR2 subunit are permeable to this cation (Jonas & Burnashev, 1995; Dingledine et al. 1999) and therefore this receptor subclass may also directly contribute to the glutamatergic Ca2+ responses of RGCs (see Osswald et al. 2007). Additionally, the indirect activation of voltage-gated Ca2+ channels (VGCCs), occurring after membrane depolarization due to NMDA-R and/or AMPA/kainate-R stimulation, could add to increases in RGC [Ca2+]i (Ishida et al. 1991; Schubert & Akopian, 2004).
RGCs can be isolated into purified cultures using a Thy1 immunopanning technique (Barres et al. 1988; Meyer-Franke et al. 1995). This system should prove advantageous for assessing the relationships between glutamate, [Ca2+]i, and excitotoxicity in a central neuron as it eliminates the potential for the modulation of glutamatergic responses by neuroactive compounds released by other cells present in mixed cultures or intact tissue preparations. For example, treatment of mixed retinal cultures (in which RGCs make up < 1% of the cell population) with kainate causes an endogenous release of glutamate that contributes to the effect of kainate on RGCs (Sucher et al. 1991). However, investigations of excitotoxic death in purified RGC cultures have generated conflicting data that contradict previous in vivo studies (described above) linking NMDA-Rs to RGC death. Otori et al. (1998) reported that glutamate-induced Ca2+ influx and excitotoxicity occurs in immunopanned RGCs through activation of AMPA/kainate-Rs (due to similar pharmacology, AMPA- and kainate-Rs are often grouped together) rather than NMDA-Rs. In contrast, the findings of Ullian et al. (2004) suggest that RGCs in purified cultures, although expressing functional NMDA-Rs, are invulnerable to the excitotoxic actions of glutamate, NMDA or kainate. In light of these discrepancies, the goal of this work was to investigate the underlying pathways of Ca2+ entry that contribute to glutamatergic Ca2+ responses in RGCs and to investigate the relationship of [Ca2+]i to excitotoxic RGC death.
Methods
Purified RGC cultures
All procedures were performed in accordance with the Dalhousie University Committee for the Use of Laboratory Animals. Unless noted otherwise, chemicals were obtained from Sigma-Aldrich (Oakville, ON, Canada). Natural litters of Long–Evans rats (Charles River, Montreal, QC, Canada) were killed at age 7–8 days postnatal by over-exposure to halothane vapours and decapitation. Following enucleation, the retinas were dissected from the eyes in Hibernate-A medium (BrainBits, Springfield, IL, USA) with 2% B27 supplements (Invitrogen, Burlington, ON, USA) and 10 μg ml−1 gentamicin. The retinas were incubated for 30 min at 37°C in 10 ml Ca2+/Mg2+-free Dulbecco's phosphate-buffered saline (DPBS; Invitrogen) containing 165 units of papain (Worthington Biochemicals, Lakewood, NJ, USA), 1 mm l-cysteine, and 0.004% DNase, and then mechanically triturated in an enzyme inhibitor solution of DPBS (with Ca2+ and Mg2+) containing 1.5 mg ml−1 ovomucoid (Roche Diagnostics, Laval, QC, Canada), 1.5 mg ml−1 bovine serum albumin (BSA), and 0.004% DNase. The suspension was centrifuged (200 g for 11 min at 25°C) and then washed in DPBS containing higher concentrations (10 mg ml−1) of ovomucoid and BSA. After spinning down, the cells were resuspended in DPBS containing 0.2 mg ml−1 BSA and 5 μg ml−1 insulin prior to incubation on the panning plates. In some experiments, RGC cultures were instead generated from adult Long–Evans rats that were 6–15 weeks old (3–4 rats per culture). Following dissection, the adult rat retinas were dissociated using the same methodology as for neonatal retinas except that a 60 min incubation period in papain solution was applied prior to trituration due to the more extensive synaptic connections in these aged retinas.
Purified RGC cultures (produced from either neonatal or adult retinas) were generated from the dissociated cell suspensions, as in previous work (see Hartwick et al. 2004, 2005 for details), using a two-step Thy1.1-based immunopanning procedure that was described originally by Barres et al. (1988). The immunopanned RGCs were plated onto poly d-lysine/laminin-coated Biocoat glass coverslips (12 mm; BD Biosciences, Bedford, MA, USA) in four-well tissue culture plates at a density of 3.5 × 104 cells per well. The cells were cultured in 600 μl of serum-free culture medium consisting of Neurobasal-A with 2% B27 supplements, 1 mm glutamine, 50 ng ml−1 brain-derived neurotrophic factor (BDNF), 10 ng ml−1 ciliary neurotrophic factor (CNTF) (BDNF and CNTF were generously provided by Regeneron, Tarrytown, NY, USA), 5 μm forskolin, and 10 μg ml−1 gentamicin. Cultures were maintained at 37°C in a humidified 5% CO2–air atmosphere. For Ca2+ imaging, coverslips with plated cells were removed from the culture medium and incubated at 37°C for 30 min in a solution containing esterfied Ca2+ indicator dye (5 μm fura-2 AM or fura-4F AM, depending on the experiment; both from Invitrogen). The fura dyes were first dissolved in dimethyl sulfoxide (0.1% final concentration) and then solubilized in Hanks' balanced salt solution (HBSS) with 0.1% pluronic acid F-127 (Invitrogen).
Retinal wholemount preparation
Retinal wholemounts were prepared from adult Long–Evans rats aged 6–10 weeks and RGCs were loaded with Mr 10 000 dextran-conjugated fura Ca2+ indicator dye (10% wt/vol, dissolved in purified water; Invitrogen). The rats were killed by over-exposure to halothane followed by decapitation. The eyes were enucleated, the anterior segment removed, and the posterior eye-cups were immersed in Hibernate-A medium with 2% B27 supplements for the retina dissection. Each retina was carefully dissected and mounted on black filters (HABP 045; Millipore, Bedford, MA, USA) with the RGC layer uppermost. A small volume (∼0.5 μl) of the fura dextran was deposited into each retinal wholemount (passing through all the retinal layers) using a tapered 26-gauge needle fitted to a 10 μl syringe (Hamilton, Reno, NV, USA). To allow the dextran to be retrogradely transported to RGC somata, the wholemounts were then incubated in the dark at room temperature in Hibernate-A/B27 medium for 7–12 h prior to Ca2+ imaging. Further details on the technique of loading RGCs in retinal wholemounts with fura dextran are described in previous work (Baldridge, 1996; Hartwick et al. 2005).
Calcium imaging
Following fura dye loading, the isolated RGCs (on coverslips) or retinal wholemounts (on filters) were transferred to a microscope chamber that was constantly superfused with 100% oxygen-bubbled HBSS. The HBSS was warmed to 34–35°C and delivered by a peristaltic pump to the chamber at a flow rate of approximately 1 ml min−1. In most experiments, the HBSS (pH 7.4) was modified to be nominally free of magnesium ions (Mg2+) and contained 2.6 mm CaCl2 and 15 mm Hepes buffer. For experiments testing the effect of extracellular Mg2+, MgCl2 was substituted for some of the CaCl2 so that the final divalent concentrations were 0.8 mm Mg2+ and 1.8 mm Ca2+, unless specified otherwise. The fura-loaded RGCs were alternately stimulated with 340 and 380 nm light, with a period of excitation for each wavelength of 1000–1500 ms for the retinal wholemounts and 400 ms for the isolated cells. During treatments, image pairs were collected as often as every 5 s (10 s for retinal wholemounts) but to limit photodamage, images were collected less frequently (20–40 s) during intervening periods. Details on the microscope rig and related apparatus used for calcium imaging have been described in previous work (Hartwick et al. 2004; Hartwick et al. 2005). The fluorescence images (8-bit processing, 4 × 4 binning) were converted into ratiometric (340 nm/380 nm) data by imaging software (Imaging Workbench 2.2, Axon Instruments, Union City, CA, USA).
Glutamatergic Ca2+ dynamics were assessed in the isolated RGCs using two treatment paradigms. RGCs were either exposed to glutamate receptor agonists (glutamate, glycine, NMDA, kainate; all dissolved directly in HBSS on the day of the experiment; kainate was obtained from Tocris, Ellisville, MO, USA) for a relatively brief 30 s pulse, or were treated with glutamate/glycine for a prolonged 1 h period. RGCs in the 30 s treatment experiments were loaded with high affinity fura-2 AM (KD ∼224 nm) dye, while RGCs exposed to glutamate for 1 h were loaded with low affinity fura-4F AM (KD ∼770 nm) dye. For fura dextran-loaded RGCs in retinal wholemounts, the treatment period with glutamatergic agonists was 2 min and the glutamate transporter inhibitor dl-threo-β-benzyloxyaspartate (TBOA; Tocris) was added to the glutamate treatment solution. In both prolonged glutamate exposure experiments on isolated RGCs and experiments on retinal wholemounts, the imaging rig was modified in that a 0.6× coupling tube (HRP060; Diagnostic Instruments, Sterling Heights, MI, USA) was placed between the CCD camera and microscope to expand the field of view afforded by the 40× objective.
Imaging analysis
For studies on isolated RGCs, circular regions of interest were drawn around individual RGC somata, and the mean fura ratio within each region was monitored throughout the experiments. Background fluorescence was measured from a region on the coverslip devoid of RGCs and subtracted from each image. For the experiments in which isolated RGCs were exposed to glutamate for 30 s pulses, the fura-2 ratios (R) were converted to [Ca2+]i using the formula:
with a Kd for fura-2 of 224 nm, and where Fo/Fs is the ratio of fluorescence intensity at 380 nm excitation in Ca2+-free solution over the intensity in solution with saturated Ca2+ levels (Grynkiewicz et al. 1985; Kao, 1994). A sample (n = 5–15) of the isolated RGCs in a given imaging session were superfused first with Ca2+-free HBSS (10 mm Mg2+, 2 mm BAPTA, 10 μm ionomycin) to determine the minimum value for the fura-2 ratio (Rmin), and then with saturating Ca2+ solution (0.9% saline with 20 mm Ca2+, 10 μm ionomycin) to determine the maximum fura-2 ratio (Rmax). Mean values for Fo/Fs, Rmin and Rmax were calculated from these RGCs and used to convert the fura-2 ratios for other RGCs in the imaging session to [Ca2+]i. The variability in these values from different imaging sessions was relatively minor; for 10 consecutive calibration experiments, the mean Rmin was 0.26 ± 0.03 (s.d.) and the mean Rmax was 2.57 ± 0.12 (s.d.). Ca2+ influx was measured as the peak [Ca2+]i obtained following each treatment (with glutamate or related agonists) minus the average baseline [Ca2+]i measured prior to the first treatment. In trials assessing the effect of glutamate receptor antagonists and VGCC blockers, RGCs that had a negligible response to glutamate (peak [Ca2+]i < 150 nm) were excluded from analysis. In the prolonged glutamate exposure (1 h) experiments, the initial Ca2+ influx (Δfura-4F ratio) was measured as the peak fura-4F ratio occurring during the first 400 s of the glutamate treatment minus the average baseline ratio prior to treatment.
For studies on RGCs in retinal wholemounts, the mean fura ratio was monitored in RGC somata that exhibited fluorescence with 380 nm excitation. As in previous work (Hartwick et al. 2005), all experiments were performed on dextran-loaded cell bodies that were located in the GCL near labelled RGC axon bundles, and that were > 200 μm from the dextran injection site to ensure that imaged cells were RGCs. The response to each treatment was calculated as the Δfura ratio, the peak fura ratio minus baseline fura ratio. The baseline fura ratio for every cell was calculated as the average fura ratio measured in the three images prior to each treatment, and the peak ratio was the maximum ratio value obtained in the 400 s following each treatment. For illustration, fura ratio traces of RGCs from retinal wholemounts were slope-corrected for a gradual decrease in baseline ratio due to decreasing background fluorescence. All data analysis was performed on the non-baseline-corrected raw data. Data from either isolated RGCs or retinal wholemount preparations are presented as means ± s.d.
RGC death assay
After the 1 h glutamate exposure, RGCs were imaged for another 15 min to monitor for recovery. The superfusing HBSS was then stopped and 5 μl of fluorescently tagged annexin V (Alexa Fluor 488 annexin V; Invitrogen) was added to the microscope chamber (chamber volume ∼0.5 ml) to compare the Ca2+ imaging data with a marker of cell death. After 10 min incubation, the flow of HBSS was resumed for 5 min to wash. Images of annexin V fluorescence were captured with the CCD camera using an FITC filter set (XF100 set; excitation 475 nm, emission 535 nm; dichroic 505 nm; Omega Optical) and saved, through the imaging software program, with and without the circular regions of interest that had been drawn for Ca2+ imaging. Annexin V fluorescence was assessed using Photoshop 5.0 software (Adobe Systems, San Jose, CA, USA). Using the histogram function, a cell was deemed annexin V positive if the mean luminance minus 1 s.d. within its outlined region of interest was greater than background luminance in a section devoid of cells.
Results
Glutamatergic Ca2+ dynamics of neonatal rat RGCs
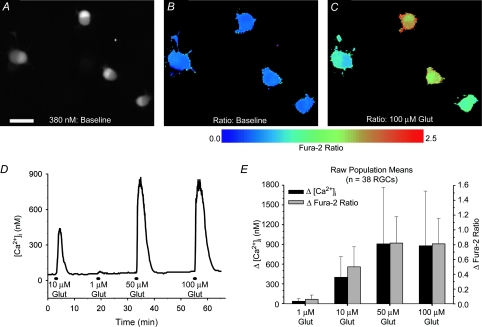
Ca2+ imaging experiments were performed on cultured RGCs 1–3 days after their isolation through Thy1-based immunopanning. Upon loading of fura-2 Ca2+ indicator dye, the isolated RGCs typically displayed a bi-lobed pattern of fluorescence, under either 340 or 380 nm excitation, presumably due to an asymmetrical nucleus position (Fig. 1A). To investigate the relationship of glutamate concentrations to Ca2+ responses, isolated RGCs (n = 38; pooled from 8 imaging experiments on RGCs from 3 separate cultures) were challenged with 30 s treatments of 1, 10, 50 and 100 μm glutamate (example trace in Fig. 1D). Glycine (10 μm), a coagonist with glutamate for NMDA-Rs (Johnson & Ascher, 1987), was included in all glutamate treatment solutions, and the superfusing HBSS was nominally Mg2+-free. The mean changes (peak response minus baseline) in fura-2 fluorescence ratios (340/380 nm; measured in RGC somata; Fig. 1B and C) induced by the different glutamate concentrations are shown in Fig. 1E. The ratiometric data from these experiments (and in subsequent fura-2 imaging experiments on the isolated cells) were also converted to absolute [Ca2+]i (see Methods for calibration details) to give an approximation of the magnitude of the rise in [Ca2+]i caused by the glutamate treatments (Fig. 1E). The responses to the four different glutamate concentrations were all significantly different from each other (P < 0.01, Friedman ANOVA, Tukey; using either Δfura-2 ratio or Δ[Ca2+]i data) with the exception of 50 versus 100 μm (P > 0.05), indicating that 50 μm glutamate was sufficient to evoke the maximum Ca2+ response. During the 1 μm glutamate treatments, there was negligible alteration in [Ca2+]i from baseline levels (rise of 35 nm ± 37 from a mean baseline [Ca2+]i of 70 nm ± 24), signifying that this concentration was below the threshold required to consistently elicit a response under our recording conditions. The rises in [Ca2+]i produced by the 10 μm glutamate exposures were, on average, roughly one-half (51.9% ± 25.2; within-cell analysis) of those elicited by the saturating 100 μm glutamate treatments.
Figure 1. Concentration–response relationship for glutamate-induced increases of [Ca2+]i in cultured RGCs isolated from neonatal rats.
A, fluorescence (380 nm excitation, 510 nm emission) of immunopanned RGCs loaded with the Ca2+ indicator dye fura-2 AM. Scale bar = 25 μm. B and C, pseudocolour images of fura-2 fluorescence ratios (340/380 nm) in same RGCs at baseline (B) and at peak response to 100 μm glutamate (C). D, example trace (fura-2 ratios converted to [Ca2+]i) from an isolated RGC exposed for 30 s to 1, 10, 50 and 100 μm glutamate (plus 10 μm glycine; extracellular Mg2+ absent). E, summary of mean (±1 s.d.; n = 38 RGCs) responses produced by the glutamate treatments. Both the Δ[Ca2+]i and the Δfura-2 ratios (peak response minus baseline) are shown.
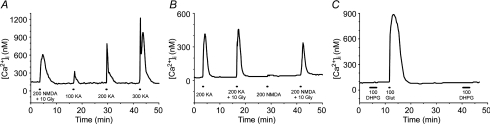
In addition to glutamate, increases in RGC [Ca2+]i also occurred following application of either NMDA or kainate, selective ligands for NMDA-Rs and AMPA/kainate-Rs, respectively (Fig. 2). In a direct comparison of the Ca2+ signal induced by 200 μm NMDA (with 10 μm glycine) to that by increasing concentrations of kainate in isolated RGCs (n = 33), the magnitude of NMDA-induced Ca2+ influx (363 nm ± 357) was similar (P > 0.05, q = 0.202, Friedman ANOVA, Tukey) to that due to 200 μm kainate (398 nm ± 499) but was significantly (P < 0.01) greater and smaller than the responses elicited by 100 μm (104 ± 189 nm) and 300 μm (907 ± 786 nm) kainate, respectively (example trace in Fig. 2A). As expected, RGC responses to NMDA were highly dependent on the presence of the NMDA-R coagonist glycine in the treatment solution (Fig. 2B). The RGC Ca2+ signals stimulated by 200 μm NMDA alone were significantly less (P < 0.001, Wilcoxon) than the signal produced by 200 μm NMDA plus 10 μm glycine (in n = 34 RGCs, the normalized NMDA-induced Δ[Ca2+]i was only 25.3 ± 11.9% of the same RGCs' response to NMDA plus glycine), but glycine did not significantly (P = 0.134, Wilcoxon) enhance kainate responses (n = 19 RGCs treated with 200 μm kainate alone and then 200 μm kainate plus 10 μm glycine). In addition to Ca2+ influx linked to ionotropic glutamate receptors, the stimulation of group I metabotropic glutamate receptors (mGluR) can produce a rise in [Ca2+]i in many CNS neurons (Linden et al. 1994; Guatteo et al. 1999). However, the selective group I mGluR agonist (S)-3,5-dihydroxyphenylglycine hydrate (DHPG) had no direct effect on the [Ca2+]i of the immunopanned RGCs (Fig. 2C; representative of n = 15 RGCs tested), and therefore we focused on ionotropic glutamate receptors for the remainder of the study.
Figure 2. NMDA- and kainate-induced Ca2+ influx in isolated RGCs.
A, example fura-2 ratio trace for an RGC treated with 200 μm NMDA plus 10 μm glycine, and then 100, 200 and 300 μm kainate. B, glycine (10 μm) significantly enhanced the response evoked by 200 μm NMDA, but not 200 μm kainate. C, in contrast to glutamate, NMDA or kainate, the metabotropic glutamate receptor agonist DHPG (100 μm) had no direct effect on RGC [Ca2+]i. Mg2+-free HBSS was used as the extracellular solution.
Mechanisms of glutamate-induced Ca2+ influx in isolated RGCs
The experiments with the glutamatergic agonists NMDA and kainate confirmed that there are at least two distinct pathways through which glutamate could potentially influence RGC [Ca2+]i. Glutamate is the natural endogenous ligand for glutamate receptors, and its action can differ from that of its related agonists. In particular, the activation of AMPA-Rs by glutamate is accompanied by rapid desensitization, but this desensitization is markedly reduced if AMPA-Rs are instead stimulated with kainate (Dingledine et al. 1999). Therefore, the magnitude of kainate-induced Ca2+ influx may be exaggerated, as compared to that mediated by glutamate stimulation of AMPA/kainate-Rs.
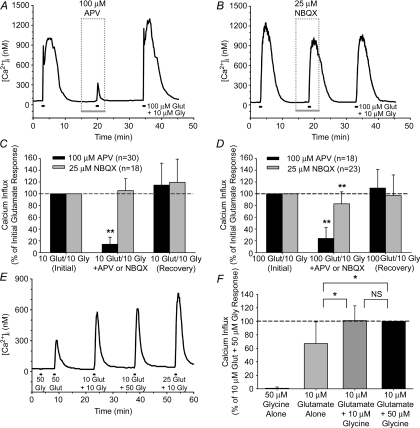
To test the relative contribution of NMDA-R and AMPA/kainate-R-mediated pathways, we assessed the effect of the respective antagonists d(−)-2-amino-5-phosphonopentanoic acid (APV) and 2,3-dihydro-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX) on the Ca2+ signal produced by a 30 s treatment of 10 and 100 μm glutamate (plus 10 μm glycine). These concentrations were chosen because, as shown in Fig. 1, they elicited the near-threshold (10 μm) and maximum (100 μm) Ca2+ responses in the isolated RGCs in Mg2+-free conditions. NBQX was used as the AMPA/kainate-R antagonist because it was observed in initial experiments that the related quinoxalines 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) quenched fura-2 fluorescence and absorbed light preferentially at 340 nm relative to 380 nm. Unlike DNQX and CNQX at the same concentration, 25 μm NBQX did not artificially alter the ratiometric data (see online Supplemental material).
The NMDA-R antagonist APV (100 μm) greatly (P < 0.01, Friedman ANOVA, Tukey; as compared to both initial and recovery responses with APV absent) reduced the [Ca2+]i elevation that occurred with either 10 or 100 μm glutamate treatment (Fig. 3A, C and D). The AMPA/kainate-R antagonist NBQX (25 μm) did not affect (P = 0.418, Friedman ANOVA) RGC responses to 10 μm glutamate, but it did have a small yet significant (P < 0.01, Friedman ANOVA, Tukey) effect on 100 μm glutamate responses (Fig. 3B, C and D). These results indicate that glutamate-induced Ca2+ influx occurs predominantly through NMDA-R activation, although at higher glutamate concentrations there is a small AMPA/kainate-R contribution. Glycine was included in the glutamate treatment solutions because it is a coagonist with glutamate at NMDA-Rs (Johnson & Ascher, 1987), and there is evidence that endogenous retinal levels of glycine or d-serine sufficiently activate the glycine binding site of RGC NMDA-Rs in vivo (Hama et al. 2006). While glycine had no effect on RGC [Ca2+]i on its own, the addition of glycine to the glutamate solutions significantly (P < 0.05, Friedman ANOVA, Tukey) enhanced RGC responses (Fig. 3E and F), and this is consistent with NMDA-Rs mediating the glutamate-related rise in RGC [Ca2+]i.
Figure 3. Effect of glutamate (Glut) receptor antagonists and glycine (Gly) on glutamate-induced Ca2+ influx in isolated RGCs.
A and B, example fura-2 ratio traces illustrating effects of NMDA-R antagonist APV (A) and AMPA/kainate-R antagonist NBQX (B) on Ca2+ signals evoked by 100 μm Glut. C and D, mean data (+1 s.d.) showing effect of antagonists on Ca2+ influx due to 10 μm (C) and 100 μm (D) Glut treatments (with 10 μm Gly; extracellular Mg2+ absent). Ca2+ influx in these experiments was calculated as the change in [Ca2+]i, following conversion of the fura-2 ratios through calibration experiments, and the data was normalized to initial RGC glutamate responses (dashed line). E and F, example fura-2 ratio trace (E) and mean normalized data (+1 s.d.) (F) summarizing effect of 50 μm Gly alone, 10 μm Glut alone, 10 μm Glut plus 10 μm Gly, and 10 μm Glut plus 50 μm Gly on RGCs (n = 14). Gly alone did not affect [Ca2+]i, but potentiated RGC responses to Glut. *P < 0.05, **P < 0.01, Friedman ANOVA, Tukey, within-cell comparisons on normalized data.
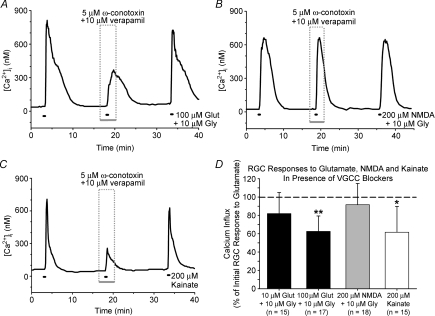
We next sought to assess the contribution of VGCCs to glutamate-evoked changes in [Ca2+]i, as Ca2+ could also pass through these channels following the RGC membrane depolarization induced by glutamate treatment. We first attempted to inhibit all VGCC types with the non-selective blocker cadmium, but this divalent cation interfered with the fura-2 dye (data not shown). Instead, RGCs were stimulated with glutamate, NMDA or kainate in the presence of 5 μm ω-conotoxin GVIA plus 10 μm verapamil, concentrations that have been shown to inhibit N- and L- type (CaV2.2 and CaV1 of more recent nomenclature) VGCCs, respectively, in isolated cells (Freedman et al. 1984; Boland et al. 1994). Verapamil was used rather than the L-type VGCC blocker nifedipine, because the latter compound absorbed asymmetrically at the 340 and 380 nm excitation wavelengths (see Supplemental material for illustration of the effect of asymmetrical fluorescence quenching on fura-2 ratiometric Ca2+ imaging). It has previously been shown that 10 μm verapamil significantly reduced the overall change in intracellular Ca2+ in isolated rat retinas exposed to NMDA (Melena & Osborne, 2001).
The VGCC blocking cocktail of verapamil and ω-conotoxin GVIA significantly (P < 0.05, Friedman ANOVA, Tukey) reduced the Ca2+ influx induced by either 100 μm glutamate or 200 μm kainate, but did not (P > 0.05) alter RGC responses to 10 μm glutamate or 200 μm NMDA (Fig. 4). These results suggest that VGCCs are not involved in Ca2+ signals produced by a near-threshold glutamate dose (10 μm), but these channels do contribute significantly to changes in [Ca2+]i evoked by a saturating glutamate concentration (100 μm) in the isolated RGCs. A concentration of 200 μm was used for both NMDA and kainate, as these concentrations had been shown to elicit roughly equivalent Ca2+ signals (see Fig. 2A). In accord, the mean magnitude of Ca2+ responses evoked by 200 μm kainate was not significantly different (P = 0.91, t test) from the magnitude of responses produced by 200 μm NMDA (with 10 μm glycine) treatment. These results indicate a greater role for VGCCs in AMPA/kainate-R-mediated Ca2+ signals relative to comparable NMDA-R-drive responses.
Figure 4. Effect of voltage-gated Ca2+ channel (VGCC) inhibition on glutamatergic RGC Ca2+ influx.
A–C, representative fura-2 ratio traces for RGCs treated with 100 μm glutamate (A), 200 μm NMDA (B) and 200 μm kainate (C) in the presence or absence of 5 μm ω-conotoxin GVIA and 10 μm verapamil. D, mean data (+1 s.d.) showing effects of VGCC blocking cocktail on Ca2+ influx induced by 10 μm and 100 μm glutamate, 200 μm NMDA and 200 μm kainate (extracellular Mg2+ absent; 10 μm glycine added to glutamate and NMDA solutions). Ca2+ influx was the change in [Ca2+]i due to treatment, and was normalized to the initial glutamate, NMDA or kainate responses (dashed line). *P < 0.05, **P < 0.01, Friedman ANOVA, Tukey, compared to initial and recovery responses.
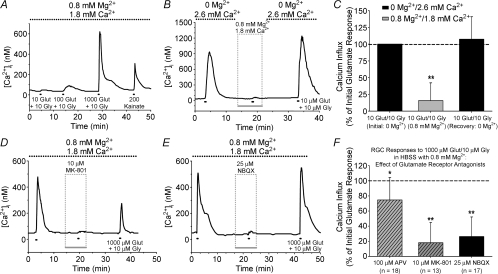
The superfusing HBSS used in all Ca2+ imaging experiments presented so far was nominally Mg2+-free. Mg2+ is known to exert a voltage-dependent block of the NMDA-R channel pore (Mayer et al. 1984; Nowak et al. 1984). In the presence of 0.8 mm Mg2+, the magnitude of the Ca2+ signal induced by various glutamate concentrations was blunted as compared to recordings in Mg2+-free HBSS, with 10 and 100 μm glutamate often being below threshold (example trace in Fig. 5A; compare to Fig. 1D). To directly test the effect of extracellular Mg2+, isolated RGCs (n = 12) were exposed to consecutive glutamate treatments (10 μm; with 10 μm glycine) while the superfusing solution alternated between Mg2+-free HBSS and HBSS containing 0.8 mm Mg2+ (Fig. 5B). Glutamate-induced Ca2+ influx was significantly diminished (P < 0.01, Friedman ANOVA, Tukey) with extracellular Mg2+ present as compared to initial and recovery responses in Mg2+-free HBSS (Fig. 5C). In these experiments, the concentration of Ca2+ was reduced in the Mg2+-containing HBSS in order to keep the overall divalent cation levels constant. To control for the possibility that it was the decreased Ca2+, rather than the presence of Mg2+, that caused the reduction in the glutamate-induced Ca2+ responses, we performed additional control experiments in which only the Ca2+ concentration of the external solution was varied. RGC responses to 10 μm glutamate (with glycine) in Mg2+-free HBSS containing 1.8 mm Ca2+ were 95 ± 36% (n = 49; within-cell analysis) of those obtained after switching the cells to Mg2+-free HBSS containing 2.6 mm Ca2+, and were not significantly different (P > 0.05, Wilcoxon). Thus, the reduction in the RGC glutamate responses illustrated in Fig. 5A–C was directly due to the addition of extracellular Mg2+.
Figure 5. Effect of extracellular Mg2+ on glutamate-induced Ca2+ influx in isolated RGCs.
A, fura-2 ratio trace for an RGC, in HBSS containing 0.8 mm Mg2+, exposed to 10, 100 and 1000 μm glutamate (plus 10 μm glycine). Responses to lower glutamate concentrations (≤ 100 μm) were attenuated with Mg2+ present, but note that RGC still responded to 200 μm kainate (indicating AMPA/kainate-Rs were not inhibited by the extracellular Mg2+). B and C, representative trace (B) and mean normalized Ca2+ influx (change in [Ca2+]i +1 s.d.) (C) for RGCs (n = 12) treated with 10 μm glutamate in the absence or presence of 0.8 mm Mg2+. D and E, fura-2 ratio traces illustrating effect of NMDA-R antagonist MK-801 (10 μm) (D) and AMPA/kainate-R antagonist NBQX (25 μm) (E) on RGC Ca2+ influx evoked by 1000 μm glutamate (plus 10 μm glycine) in HBSS containing 0.8 mm Mg2+. F, mean data (+1 s.d.) for effect of NMDA-R antagonists (hatched bars) and AMPA/kainate-R antagonist (filled bar) on glutamate-induced Ca2+ influx (normalized to initial response; dashed line). *P < 0.05, **P < 0.01, Friedman ANOVA, Tukey, compared to initial and recovery responses.
The effects of glutamate receptor antagonists were then re-assessed on RGCs challenged with 1000 μm glutamate with 0.8 mm Mg2+ present. Only RGCs exhibiting a Δ[Ca2+]i of at least 150 nm (roughly equivalent to a Δfura-2 ratio of 0.2) due to glutamate treatment were included in analysis. Under these conditions, the AMPA-kainate-R antagonist NBQX (25 μm) reduced (P < 0.01, Friedman ANOVA, Tukey) the glutamate-evoked Ca2+ signals (Fig. 5E and F). The NMDA-R antagonist APV, at 100 μm, had a small but significant (P < 0.05) effect (Fig. 5F). However, the effect of APV was greater at increasing concentrations (data not shown), suggesting that this competitive antagonist was being out-competed by the high (1000 μm) glutamate concentration. To confirm this hypothesis, we tested the non-competitive NMDA-R channel blocker MK-801 (10 μm), and found that this antagonist exhibited a strong inhibitory effect (P < 0.01; Fig. 5D and F). Therefore, in the presence of external Mg2+, antagonism of either NMDA-Rs or AMPA/kainate-Rs resulted in near abolition of glutamate-induced Ca2+ influx.
Glutamate-induced Ca2+ influx in adult rat RGCs: isolated cells and retinal wholemounts
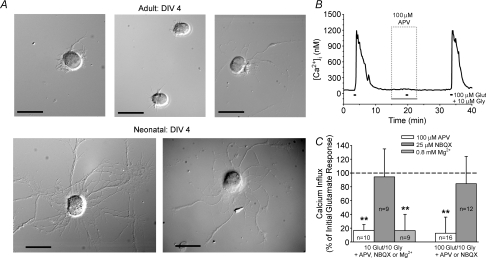
All preceding experiments were performed on purified RGC cultures generated from neonatal rats (age 7–8 postnatal days). At this age, RGC dendrites are just beginning to form functional glutamatergic synapses with retinal bipolar cells (Bansal et al. 2000; Wong et al. 2000), raising the possibility that RGC glutamate receptor expression in neonates differs from adults. Embryonic or early postnatal animals have traditionally been used for neuronal cultures, as the neurons at younger ages have not yet formed extensive synaptic connections and are generally thought to be less susceptible to damage during dissociation. To test whether the mechanisms underlying glutamate-induced Ca2+ influx change as rats mature, purified RGC cultures were generated from adult rats (age 6–15 weeks) using the Thy1 immunopanning technique.
The yield of immunopanned RGCs extracted from adult retinas ranged between 10 000 and 17 000 RGCs per retina, as compared to 25 000–40 000 RGCs per retina for neonatal rats. This reduction is not due to developmental RGC loss as the total number of RGCs at age 7–8 days is roughly the same as in adult rats (Potts et al. 1982), but is likely to be due to the loss of more cells to damage during dissociation. In contrast to immunopanned RGCs from neonatal rats, RGCs isolated from adult rat retinas displayed little neurite outgrowth in culture (Fig. 6A). Despite this morphological difference, glutamate-induced Ca2+ influx (with glycine present and extracellular Mg2+ absent) was primarily mediated through NMDA-R activation in cultured adult RGCs as in neonatal RGCs. The NMDA-R antagonist APV (100 μm) significantly reduced (P < 0.01, Friedman ANOVA, Tukey, compared to both initial and recovery responses) the Ca2+ influx induced by 10 μm or 100 μm glutamate, while the AMPA/kainate-R antagonist NBQX had negligible (P > 0.05) effect (Fig. 6B and C). Also similar to the neonatal RGCs, the Ca2+ responses of isolated adult RGCs exposed to 10 μm glutamate were significantly (P < 0.01) reduced with 0.8 mm Mg2+ present (Fig. 6C; experimental protocol same as shown in Fig. 5B)
Figure 6. Glutamate-induced Ca2+ influx in immunopanned RGCs generated from adult rats.
A, DIC images of RGCs isolated from adult (6 weeks old) and neonatal (8 days old) rats after 4 days in vitro (DIV). In contrast to neonatal RGCs, adult RGCs exhibited little to no neurite outgrowth even after 4 days in culture. Scale bars = 25 μm. B, representative trace illustrating effect of NMDA-R antagonist APV on adult RGC responses to 100 μm glutamate (with 10 μm glycine; extracellular Mg2+ absent). C, mean data (change in [Ca2+]i +1 s.d.) showing effect of glutamate receptor antagonists and extracellular Mg2+ on Ca2+ responses evoked by 10 and 100 μm glutamate in RGCs isolated from adult rats (normalized to initial response; dashed line). **P < 0.01, Friedman ANOVA, Tukey, compared to initial and recovery responses.
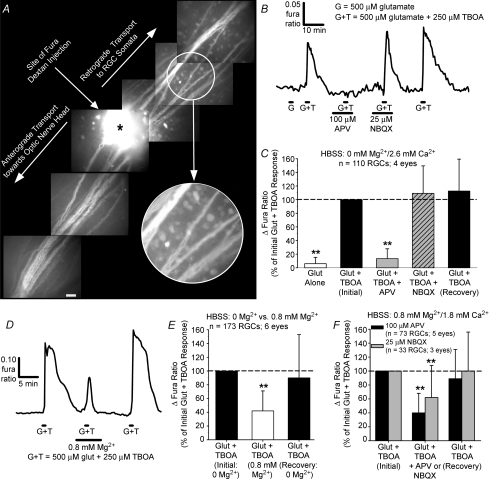
To determine whether the mechanisms underlying glutamatergic Ca2+ dynamics were altered by the dissociation procedure, we next tested the relative effectiveness of glutamate receptor antagonists on RGCs in retinal wholemounts prepared from adult rats. RGCs were retrogradely loaded with dextran-conjugated fura Ca2+ indicator dye (Fig. 7A) following injection of the dye into the filter-mounted retinas. Due to intercell differences in dye loading inherent with this technique (see Hartwick et al. 2005) and due to changing background fluorescence (attributed to the washing out of excess fura dextran that remained on the retinal surface from the injection), a valid calibration of the ratiometic data to absolute [Ca2+]i was not possible and Ca2+ responses were measured as the raw changes in fura fluorescence ratios. As demonstrated in previous work (Hartwick et al. 2005), micromolar concentrations of glutamate were ineffective at stimulating an RGC Ca2+ response in this preparation. This is due to the efficiency of retinal glutamate uptake that quickly removes glutamate, but not NMDA or kainate, from the extracellular milieu. At 500 μm, glutamate did elicit a detectable Ca2+ signal when coapplied with the glutamate transporter inhibitor TBOA (Fig. 7B and C). In Mg2+-free conditions, the RGC responses to glutamate plus TBOA were significantly (P < 0.01, Friedman ANOVA, Tukey) reduced by APV but not (P > 0.05) by NBQX (Fig. 7B and C). Upon perfusion of the retinas with 0.8 mm Mg2+, RGC Ca2+ responses were again smaller (P < 0.01) relative to the responses elicited from the same RGCs with extracellular Mg2+ absent (Fig. 7D and E). With 0.8 mm Mg2+ present in the external solution, the RGC Ca2+ responses evoked by glutamate plus TBOA were significantly (P < 0.01) inhibited by either APV and NBQX (Fig. 7F). Therefore, as in isolated RGCs, NMDA-Rs had a predominant role in mediating the glutamate-induced rise in RGC Ca2+ levels in this intact retina preparation in Mg2+-free conditions, while the activation of both NMDA-Rs and AMPA/kainate-Rs facilitates RGC Ca2+ responses with extracellular Mg2+ present (see Discussion for further explanation).
Figure 7. Glutamatergic Ca2+ dynamics of RGCs in adult rat retinal wholemounts.
A, montage of fluorescence micrographs (380 nm excitation, 510 emission) showing retrograde loading of RGC somata following injection of fura dextran into the retinal wholemount at the site denoted by an asterisk. Scale bar = 50 μm. The encircled area is shown at higher magnification to highlight individual dye-loaded RGC somata. B, representative trace illustrating that glutamate is more effective at evoking a detectable Ca2+ signal when coapplied with the glutamate transporter inhibitor TBOA, and the glutamate plus TBOA response is blocked by APV but not affected by NBQX with extracellular Mg2+ absent. C, mean normalized data (Δfura ratio +1 s.d.) summarizing effect of glutamate receptor antagonists on RGC responses in Mg2+-free conditions. D and E, example trace (D) and mean normalized data (E) showing inhibitory effect of extracellular Mg2+ (0.8 mm) on RGC responses to glutamate plus TBOA in this intact retina preparation. F, mean normalized data summarizing effect of glutamate receptor antagonists on glutamatergic RGC responses with 0.8 mm extracellular Mg2+ present. **P < 0.01, Friedman ANOVA, Tukey, compared to initial and recovery responses to glutamate plus TBOA.
RGC calcium dynamics and deregulation during prolonged glutamate exposure
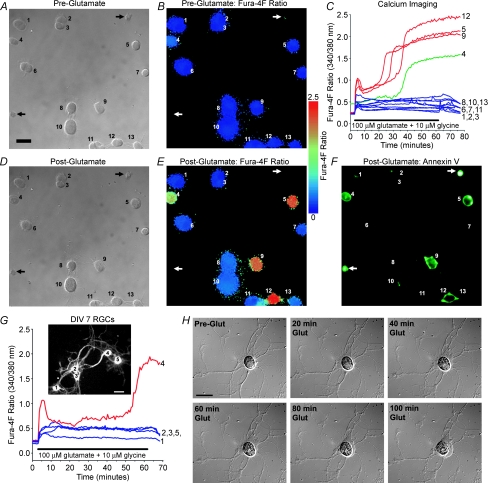
RGC Ca2+ dynamics were next monitored during a more prolonged glutamate exposure of 1 h. For these experiments, immunopanned RGCs from neonatal rats were again used, and all experiments were performed in Mg2+-free HBSS unless otherwise specified. In other central neurons, it has been reported that the high affinity Ca2+ indicator fura-2 can underestimate the rise in [Ca2+]i associated with glutamate excitotoxicity due to dye saturation (Hyrc et al. 1997; Stout & Reynolds, 1999). To minimize this possibility, the low-affinity Ca2+ indicator dye fura-4F (KD ∼770 nm) was instead employed. The overall design of these experiments is illustrated in Fig. 8. The fura-4F ratio was monitored in RGCs treated for 1 h with different concentrations of glutamate plus 10 μm glycine. Upon glutamate exposure, RGCs generally exhibited an abrupt rise in the fura-4F ratio, which was then maintained at a relatively stable level (Fig. 8C). Cells that maintained this [Ca2+]i homeostasis throughout the 1 h exposure showed recovery towards baseline levels during the ensuing 15 min wash-out period. However, certain cells exhibited a latent loss in Ca2+ homeostasis that was characterized by a large and irreversible rise in the fura-4F ratio. Cells that underwent this delayed Ca2+ deregulation (DCD), previously described in other central neurons (Manev et al. 1989; Randall & Thayer, 1992; Tymianski et al. 1993; Rajdev & Reynolds, 1994), showed no recovery during the wash-out period, with the fura-4F ratio remaining elevated (Fig. 8E). Calibration of fura-4F ratios to [Ca2+]i was not performed because the irreversible rise in [Ca2+]i that occurred during DCD exceeded the dynamic range of the fura-4F indicator dye.
Figure 8. Excitotoxic death of isolated RGCs is associated with delayed calcium deregulation (DCD).
A and B, DIC image (A) and pseudocolour image (B) of the fura-4F ratio (340/380 nm) of example RGCs, isolated from neonatal rats (first day in vitro [DIV]), prior to glutamate exposure. C, the fura-4F ratio was monitored in these RGCs while they were continuously superfused with 1000 μm glutamate (plus 10 μm glycine; in Mg2+-free HBSS) for 1 h, followed by 15 min wash-out. Of the 13 RGCs imaged, 9 cells maintained homeostatic Ca2+ levels throughout glutamate treatment and recovered towards baseline levels during wash-out (blue traces). DCD was evident in 3 cells (denoted by numbers 5, 9 and 12), and was characterized by a large increase in the fura ratio with no recovery (red traces). RGCs with elevated baseline fura ratios (> 0.3), such as cell 4 (green trace), were excluded from analysis. D–F, DIC image (D), pseudocolour image of the fura-4F ratio (E) and annexin V fluorescence (F) in these same cells after glutamate exposure and wash-out. The DCD-exhibiting RGCs (cells 5, 9 and 12) and the RGC with elevated baseline [Ca2+]i (cell 4) were stained by the death marker annexin V. Note that the cells denoted by the arrows also were annexin V-positive, but these unviable cells did not load the fura dye (absent from ratiometric images). G, fura-4F ratio traces for five RGCs on DIV 7 that were treated with 100 μm glutamate (plus 10 μm glycine; in Mg2+-free HBSS) for 1 h. Inset: fura fluorescence image (380 nm excitation) of the corresponding five RGCs from which the fura traces were recorded, with enhanced contrast for neurite visualization. H, DIC images of a glutamate-treated RGC (DIV 7), illustrating the morphological changes that occur in an RGC undergoing excitotoxic death (see Supplemental Movie 1).
At the end of some of these experiments, the cells were incubated with fluorescently tagged annexin V that had been added to the microscope chamber. During apoptosis, the lipid phosphatidylserine is translocated from the cytoplasmic side of the plasma membrane to its outer surface (Koopman et al. 1994), and therefore annexin V fluorescence can be used to identify apoptotic cells. Necrotic cells can also be stained by annexin V, as this marker can enter lysed cells and stain phosphatidylserine on the inner membrane surface. Annexin V fluorescence was therefore used as a general marker for cell death, rather than to distinguish apoptosis from necrosis. As evident in Fig. 8F, the three cells that underwent Ca2+ deregulation (denoted by numbers 5, 9 and 12) were stained by annexin V at the conclusion of the experiment.
To be consistent with the studies employing 30 s glutamate treatments (Figs 1–5), the effect of a 1 h exposure to glutamate in the presence and absence of glutamate receptor blockers (Figs 9 and 10) was tested on RGCs that had been cultured for 1–3 days. Cells from short-term cultures were chosen for experiments throughout this work because the purified RGCs are likely to receive much less glutamatergic stimulation in culture as compared to in vivo, and it was not known whether glutamate receptor expression and/or Ca2+ buffering is consequently altered in RGCs during long-term culture. However, a concern with the use of semiacute cultures is that RGCs that had been injured during the dissociation process may not have yet been completely eliminated from the cultures. The Ca2+ imaging technique provided two separate measures of cell viability to preclude unhealthy cells from influencing the results regarding the neurotoxicity of glutamate. First, similar to the commonly used cell viability marker calcein AM, the fura dye contains an AM ester that must be enzymatically cleaved by endogenous esterases in order for cells to exhibit fura fluorescence. Thus, unviable cells could not be imaged and were automatically excluded from these experiments (see two example annexin V-positive cells, denoted by arrows in Fig. 8A, B and D–F, which showed negligible fura fluorescence). Second, RGCs that exhibited elevated baseline fura ratios (such as annexin V-positive cell 4 in Fig. 8A, B and D–F, denoted by green trace in Fig. 8C) were deemed unhealthy and excluded from analysis (a criterion of baseline fura-4F ratio > 0.3 was used to exclude cells). Furthermore, to determine whether DCD occurrence was unique to acute RGC cultures, a group of RGCs (n = 34) that had been cultured for 7 days were treated with 100 μm glutamate for 1 h. DCD was observed in 14.7% (5 of 34) of these RGCs (see example in Fig. 8G), confirming that this phenomenon also occurs in RGCs cultured for 1 week. The morphological changes exhibited by a representative DIV 7 RGC undergoing excitotoxic death is illustrated in Fig. 8H and in Supplemental Movie 1.
Figure 9. Relationship of glutamate concentration and Ca2+ influx to DCD incidence in isolated RGCs.
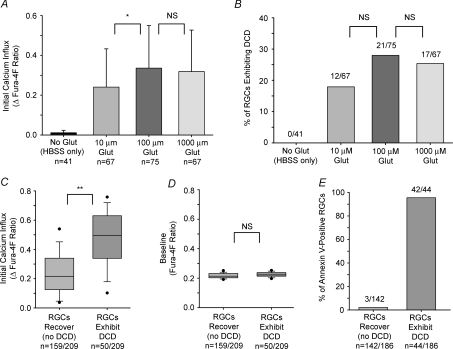
A, mean Ca2+ influx (peak fura-4F ratio over first 400 s of glutamate exposure minus baseline ratio; +1 s.d.) induced by 10, 100 and 1000 μm glutamate (plus 10 μm glycine; in Mg2+-free HBSS) during the 1 h glutamate exposure. B, the proportion of cells exhibiting DCD in each treatment group. A group of RGCs were maintained in HBSS for 1 h (glutamate absent) as controls. C and D, box-plots of pooled data for all RGCs treated with glutamate (10–1000 μm; n = 209) illustrating that Ca2+ responses were significantly related to initial Ca2+ influx (C), but not to baseline Ca2+ levels (D). The upper and lower boundaries of the box indicate the 75th and 25th percentiles, the line within the box marks the median, the whiskers above and below the box indicate the 90th and 10th percentiles, while the points represent outlying data. E, of the 209 RGCs treated with glutamate, 186 of these RGCs were examined for annexin V fluorescence at the conclusion of the experiment. Annexin V staining paralleled the imaging data, confirming that DCD was associated with cellular death. *P < 0.05, **P < 0.01, Kruskal–Wallis ANOVA, Dunn's post hoc test for Ca2+ influx data (comparing the three glutamate-treated groups); Chi-square for DCD incidence data (comparing the three glutamate-treated groups); Mann–Whitney test for comparison of box-plot data.
Figure 10. Effect of glutamate receptor blockade on Ca2+ influx and DCD occurrence in isolated RGCs.
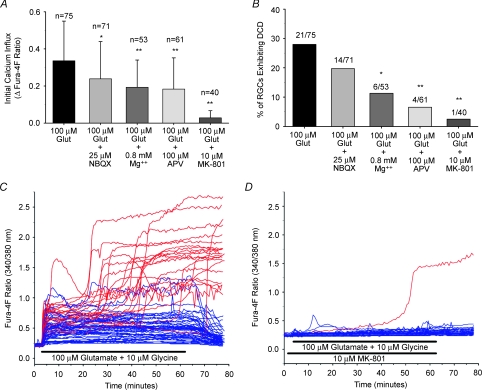
A, mean Ca2+ influx (peak fura-4F ratio over first 400 s of glutamate exposure minus baseline ratio; +1 s.d.) induced by 100 μm glutamate (plus 10 μm glycine) in the presence or absence of AMPA/kainate-R (NBQX) and NMDA-R (Mg2+, APV and MK-801) blockers. The data for 100 μm glutamate (blockers absent) is re-plotted from data shown in Fig. 9. B, the proportion of cells exhibiting DCD in each treatment group. C and D, fura-4F ratio traces of all RGCs (n = 75) treated with 100 μm glutamate plus 10 μm glycine for 1 h (with 15 min wash-out) alone (C), and in the presence of the non-competitive NMDA-R antagonist MK-801 (n = 40) (D). RGCs exhibiting DCD are denoted by red traces while RGCs surviving the glutamate insult are denoted by blue traces. *P < 0.05, **P < 0.01, as compared to 100 μm glutamate-treated group; Kruskal–Wallis ANOVA, Dunn's post hoc test for Ca2+ influx data; Chi-square for DCD incidence data.
For the data shown in Figs 9 and 10, all results are based on the pooled data from at least three different immunopanned RGC cultures. Ca2+ influx in these experiments was calculated as the Δfura-4F ratio, the peak ratio during the first 400 s of glutamate exposure minus the mean baseline ratio before glutamate treatment. The time frame of 400 s was utilized in order to compare the Ca2+ levels, prior to the onset of DCD, of those cells that deregulated versus those that did not (no cells underwent deregulation during the first 400 s). Also, the peak fura ratio (not including the increase associated with DCD) generally occurred during this initial response before decaying to a stable level. There was no significant difference in the mean initial Ca2+ influx between cells treated with either 100 μm or 1000 μm glutamate, while the influx induced by 10 μm glutamate was slightly less (P < 0.05, Q = 3.001, Kruskal–Wallis ANOVA, Dunn's) than that for 100 μm (Fig. 9A). These results are consistent with the findings in Fig. 1 that showed that 100 μm glutamate was sufficient to evoke maximum Ca2+ responses in isolated RGCs, and verifies that the earlier findings were not due to saturation of the high-affinity fura-2 dye. DCD occurred in 18–28% of the RGCs exposed to 10, 100 and 1000 μm glutamate for 1 h (Fig. 9B), and there was no significant difference (P = 0.351, Chi-square) in the proportion of cells undergoing DCD during treatment with the different glutamate concentrations. As a control, DCD was not observed in any RGCs (n = 41) that were maintained in HBSS (no glutamate) for 1 h.
Pooling the data together for the three glutamate concentrations, the incidence of DCD was related to the magnitude of the glutamate-induced Ca2+ responses, as RGCs with larger Ca2+ responses were most likely to deregulate (Fig. 9C). This susceptibility was not linked to baseline Ca2+ levels (Fig. 9D), indicating that DCD-exhibiting cells were not already injured prior to glutamate exposure. The presence of annexin V fluorescence was determined at the conclusion of the experiment for 186 of the RGCs treated with glutamate (Fig. 9E). The vast majority of deregulating RGCs in this group were stained by annexin V (42 of 44 cells), while RGCs that recovered from glutamate treatment usually did not exhibit annexin V fluorescence (139 of 142 cells were annexin V-negative). Therefore, these results indicate that the RGCs that underwent DCD were indeed dead or dying.
To assess the effect of glutamate receptor inhibition on DCD occurrence, RGCs were exposed for 1 h to 100 μm glutamate (plus 10 μm glycine; in Mg2+-free HBSS unless noted otherwise) with AMPA/kainate-R and NMDA-R blockers present. Each of the tested agents, NBQX (25 μm; AMPA/kainate-R antagonist), extracellular Mg2+ (0.8 mm; NMDA-R channel blocker), APV (100 μm; NMDA-R antagonist) and MK-801 (10 μm; NMDA-R antagonist), significantly (P < 0.05, Kruskal–Wallis ANOVA, as compared to group treated only with 100 μm glutamate) reduced the initial Ca2+ influx (Fig. 10A). RGCs treated with compounds targeting NMDA-Rs exhibited a significant reduction (P < 0.01, Chi-square, as compared to group treated with 100 μm glutamate alone) in DCD occurrence (Fig. 10B). Consistent with the observed relationship of calcium influx to DCD incidence (Fig. 9C), there was a general trend suggesting that greater reductions in glutamate-induced calcium influx were associated with fewer cells exhibiting DCD. APV (100 μm) was not as effective at blocking glutamate-induced Ca2+ signals as in the brief (30 s) glutamate pulse experiments (see Fig. 3), but this is likely to be because APV is a competitive antagonist and competes with glutamate throughout the 1 h exposure period. The non-competitive antagonist MK-801 provided near-complete protection, and its effect on glutamate-induced Ca2+ influx and DCD incidence is perhaps best illustrated by examining the optical recordings for all RGCs (n = 40) treated with 100 μm glutamate in the presence of MK-801 (Fig. 10D) as compared to traces for RGCs (n = 75) treated with 100 μm glutamate alone (Fig. 10C). In agreement with the previous experiments employing 30 s glutamate treatments (Fig. 3), blockade of NMDA-Rs with MK-801 nearly abolished Ca2+ influx, and significantly prevented the incidence of Ca2+ deregulation and ensuing cell death.
Discussion
In this work, we showed that RGC excitotoxicity was characterized by delayed Ca2+ deregulation, and that RGCs exhibiting larger glutamate-evoked Ca2+ responses were more likely to undergo DCD. Glutamate-induced Ca2+ influx occurred predominantly through NMDA-R activation in RGCs isolated from neonatal or adult rats and in RGCs from retinal wholemounts. Antagonism of NMDA-Rs significantly reduced the occurrence of DCD and protected RGCs from excitotoxic death.
Glutamate-induced RGC calcium influx is mediated primarily by NMDA-Rs
An increase in RGC [Ca2+]i occurred following stimulation of either NMDA-Rs or AMPA/kainate-Rs with their respective selective agonists, NMDA and kainate, confirming that glutamate could potentially alter RGC Ca2+ dynamics through either pathway. In Mg2+-free external solution, the NMDA-R antagonist APV abolished Ca2+ responses induced by a low glutamate concentration (10 μm) in RGCs isolated from neonatal rats, and blocked the majority of that due to a saturating glutamate concentration (100 μm). The AMPA/kainate-R antagonist NBQX had a small but significant effect on only RGC responses to 100 μm glutamate. As compared to NMDA-Rs, AMPA/kainate-Rs have a lower affinity for glutamate and desensitize quickly (Dingledine et al. 1999). These characteristics offer a mechanistic explanation for the findings of the present study: at near-threshold glutamate concentrations, AMPA/kainate-Rs are not stimulated and the response is entirely NMDA-R-mediated; at higher glutamate concentrations, AMPA/kainate-Rs are activated but desensitize quickly and contribute only a minor portion of the overall Ca2+ signal.
Consistent with a role for NMDA-Rs, the changes in RGC [Ca2+]i produced by the glutamate treatments were reduced by the addition of Mg2+ to the superfusing solution and they were potentiated with the coapplication of glycine. With extracellular Mg2+ present, a similar reduction in the glutamate-induced Ca2+ responses was observed following antagonism of either NMDA-Rs or AMPA/kainate-Rs (using MK-801 and NBQX, respectively). Thus, while the effect of NMDA-R blockade on RGC glutamate responses was comparable regardless of external Mg2+ levels, the inhibitory effect of NBQX was more pronounced when the bathing solution contained Mg2+. The most parsimonious explanation for these data is that AMPA/kainate-R activation is primarily required to depolarize RGCs and relieve the NMDA-R Mg2+-block, rather than directly contributing to the rise in [Ca2+]i. Therefore, stimulation of both NMDA-Rs and AMPA/kainate-Rs is necessary for robust glutamate-related RGC Ca2+ responses with external Mg2+ present but, as shown by the experiments with extracellular Mg2+ absent, the Ca2+ signal itself is mostly mediated through NMDA-R activation.
Ca2+ can flow directly through glutamate receptor-associated channels or through VGCCs following neuronal depolarization. By blocking L- and N-type (CaV1 and CaV2.2) VGCCs, we found that influx through these channels contributes one-third, on average, of the RGC Ca2+ signal due to a saturating (100 μm) concentration of glutamate. In contrast, the Ca2+ responses induced by 10 μm glutamate were not significantly affected by the VGCC blocking cocktail, suggesting that the Ca2+ signals evoked by this concentration of glutamate (shown to be near-threshold under our recording conditions) were predominantly mediated by direct flux through NMDA-R channels. It is possible that VGCC involvement was underestimated, as only L- and N-type VGCCs were targeted, but these channels have been shown to mediate the majority of the Ca2+ current in rat RGCs (Karschin & Lipton, 1989; Schmid & Guenther, 1999). In addition, while the average magnitude of the RGC Ca2+ responses to 200 μm NMDA was not statistically different from the responses evoked by 200 μm kainate, the VGCC blockers significantly affected the kainate-driven but not the NMDA-driven Ca2+ signals. These results imply that at this concentration, NMDA stimulates considerable Ca2+ flux through NMDA-R channels without depolarizing the neuron to the threshold potential for VGCC activation. Although our data do not rule out a contribution for Ca2+-permeable AMPA/kainate-Rs, they do indicate that 200 μm kainate depolarized the RGCs more than 200 μm NMDA (presumably due to proportionally greater inward current through AMPA/kainate-R channels) and that VGCC-mediated Ca2+ flux comprises a significant component of the kainate-induced Ca2+ responses of the isolated RGCs.
A potential concern in using RGC cultures is that dissociation alters the glutamate receptor expression from that in vivo. There is evidence, based on electrophysiological recordings and immunohistochemical analysis, that AMPA/kainate-Rs reside within synaptic clefts while NMDA-Rs on rat RGCs are instead located extrasynaptically (Chen & Diamond, 2002; Zhang & Diamond, 2006), although other work has suggested that extrasynaptic NMDA-Rs are a feature of ON RGCs rather than OFF RGCs (Sagdullaev et al. 2006). As RGCs in purified cultures develop few synapses (Ullian et al. 2001), AMPA/kainate-Rs may be underrepresented in immunopanned RGCs. Ca2+ imaging of RGCs in retinal wholemounts addressed this issue. Consistent with previous work demonstrating the efficiency of glutamate uptake in this intact retina preparation (Hartwick et al. 2005), 500 μm glutamate alone had no effect on RGCs but it induced a detectable Ca2+ signal with the coapplication of the glutamate transporter inhibitor TBOA. The bath application of glutamate plus TBOA is meant to mimic the rise in extracellular glutamate levels that occurs when glutamate transporter function is disrupted in conditions such as ischaemia/hypoxia (for review, see Camacho & Massieu, 2006). Under Mg2+-free conditions, the RGC responses to glutamate plus TBOA were blocked by NMDA-R but not AMPA/kainate-R antagonism, in agreement with the results obtained using the cultured RGCs. With extracellular Mg2+ present, the glutamate-related Ca2+ signals were reduced and there was increased inhibition of the responses by NBQX, findings that were again similar to those observed using immunopanned RGCs from neonatal rats. These data, along with comparable data obtained from isolated RGCs panned from adult rat retinas, argue against developmental differences in the mechanisms underlying glutamatergic Ca2+ dynamics in 7- to 8-day-old rats relative to those in adult rats.
RGC excitotoxicity is accompanied by delayed calcium deregulation
During prolonged exposure (1 h) to glutamate (10–1000 μm), approximately one-quarter (18–28%) of the immunopanned RGCs exhibited a latent loss of calcium homeostasis. This phenomenon, termed DCD, has been described in a number of other central neurons, including cerebellar granule (Manev et al. 1989), hippocampal (Randall & Thayer, 1992), spinal (Tymianski et al. 1993) and cortical (Rajdev & Reynolds, 1994) neurons, but this work represents its first characterization in a retinal neuron. RGCs undergoing DCD did not exhibit any recovery during the 15 min wash-out period, while RGCs that maintained a plateau [Ca2+]i throughout glutamate treatment recovered towards baseline levels. As there was no significant difference in the baseline fura-4F ratios between RGCs that developed DCD and those that did not, and RGCs that were maintained in only HBSS (no glutamate added) did not exhibit DCD, it is highly unlikely that the occurrence of DCD and subsequent cell death was not directly related to glutamate treatment. Annexin V staining at the culmination of the imaging experiments resulted in essentially exclusive labelling of RGCs that had underwent DCD, consistent with previous work showing DCD precedes, or at least coincides with, excitotoxic neuronal death (Tymianski et al. 1993).
Interestingly, the number of RGCs exhibiting a latent loss of Ca2+ homeostasis during the prolonged glutamate exposure was not appreciably different for the three glutamate concentrations (10, 100, 1000 μm) tested. Even at 1000 μm, only about one-quarter of the RGCs deregulated after 1 h, showing a similar steady loss of cells as the 1 h treatment with 10 or 100 μm glutamate. This finding suggests that it is extended exposure periods, and not brief contact with high concentrations, that triggers glutamate's toxic effects on RGCs. By inhibiting NMDA-R activation with either Mg2+, the competitive antagonist APV or the non-competitive antagonist MK-801, RGC Ca2+ responses were reduced and the occurrence of DCD significantly decreased. The AMPA/kainate-R antagonist NBQX had a small effect on RGC Ca2+ responses, but its effect in preventing DCD was not statistically significant. Therefore, NMDA-R activation played a major role in DCD and glutamate-related RGC death.
The nature of the secondary [Ca2+]i increase during DCD in RGCs was not assessed in the current work and represents a topic for future study. The mechanisms underlying DCD in other central neurons have been reported to involve either a reduction in the functional capacity of Ca2+ efflux mechanisms or a latent activation of plasma membrane ion channels (or a combination of both scenarios; for review, see Chinopoulos & Adam-Vizi, 2006). In support of reduced Ca2+ efflux, it has been demonstrated that glutamate-induced DCD in cerebellar granule neurons involves the calpain-mediated proteolysis of Na+/Ca2+ exchangers, which renders the neurons incapable of extruding Ca2+ in sufficient amounts to maintain homeostasis (Bano et al. 2005). In agreement with the secondary Ca2+ influx hypothesis, there is other evidence that excitotoxicity-related DCD in hippocampal and cortical neurons is due to the activation of a Ca2+-permeable channel that can be blocked by gadolinium, lanthanum or 2-aminoethoxydiphenyl borate (Chinopoulos et al. 2004; Deshpande et al. 2007). The identity of this putative channel remains unknown, but traditionally studied Ca2+-permeable channels such as glutamate receptors and VGCCs have been ruled out (Manev et al. 1989; Randall & Thayer, 1992; Deshpande et al. 2007).
There was considerable interneuronal variability in the magnitude of the glutamate-evoked Ca2+ signals in immunopanned RGCs, and this variability was evident with either the 30 s or 1 h glutamate treatment protocols (and with the use of either fura-2 or fura-4F indicator dyes). While the dissociation procedure may have contributed to this variability, studies assessing the accumulation of the cation channel permeant agmatine in mammalian retinas exposed to glutamatergic agonists indicate that RGCs are indeed a heterogeneous group of neurons with a broad spectrum of responses to excitatory input (Marc, 1999a,b; Marc & Jones, 2002; Sun et al. 2003). Furthermore, single-cell PCR analysis of mouse RGCs indicates that, while virtually all of these neurons express AMPA-, kainate- and NMDA-Rs, there is considerable intercell variability in the expression of the different subunits for these glutamate receptors (Jakobs et al. 2007), thereby providing a potential molecular basis for the heterogeneity of the glutamatergic Ca2+ responses. The magnitude of an individual RGC's glutamate-induced Ca2+ signal was linked to its susceptibility to excitotoxicity, as RGCs that displayed larger Ca2+ responses were more likely to undergo DCD. This finding is in general agreement with the ‘Ca2+ load’ hypothesis, which suggests that excitotoxic neuronal death correlates with the rise in [Ca2+]i (Hartley et al. 1993; Eimerl & Schramm, 1994; Lu et al. 1996). A competing, yet not mutually exclusive, proposal is that the route of Ca2+ entry (‘source specificity’ hypothesis) is a greater death determinant than the overall [Ca2+]i change, with Ca2+ influx through NMDA-Rs being more lethal than influx through VGCCs or AMPA/kainate-Rs (Tymianski et al. 1993; Sattler et al. 1998). As NMDA-Rs were the major contributor to RGC Ca2+ responses, blocking NMDA-R activation dramatically reduced Ca2+ influx and essentially eliminated the occurrence of DCD. Therefore, the results of these experiments are potentially compatible with either theory, and further research is necessary to test the ‘Ca2+ load’ versus ‘source specificity’ hypotheses in RGCs.
Many of the experiments in the current work were performed using a superfusing solution that contained no Mg2+, an experimental paradigm that is consistent with previous studies that have investigated DCD in other central neurons (see below). With Mg2+ in the external bath, a rise in [Ca2+]i could still be elicited from the isolated RGCs and the RGCs in the intact retina preparations, albeit to a lesser extent than when Mg2+ was absent. Similarly, the addition of Mg2+ to the extracellular milieu reduced, but did not completely protect against, the occurrence of DCD in the cultured RGCs. However, it is not necessarily a valid assumption that the experiments in Mg2+-containing HBSS serve as a better indicator for RGC susceptibility to excitotoxicity in the living eye. While it is unlikely that Mg2+-free conditions occur in the retina in vivo, the effectiveness of Mg2+ in blocking RGC NMDA-Rs is likely to be more pronounced in the purified cultures and the retinal wholemounts due to the voltage dependency of this inhibition. With synaptic glutamatergic input eliminated (the chromophore-regenerating retinal pigment epithelium was removed in the retinal wholemounts), the RGCs in these two in vitro preparations would be expected to be more hyperpolarized than RGCs in vivo and thus more affected by Mg2+. The depolarization of RGCs due to AMPA/kainate-R activation, shown here to play a role in facilitating NMDA-R-mediated Ca2+ influx when Mg2+ is present, would be mediated in vivo through the recurrent bursts of synaptically released glutamate generated in retinal responses to visual stimuli. That RGC NMDA-R activation can occur under physiological conditions is supported by a number of studies that have shown that intravitreal injections of NMDA in live rodents results in RGC death (examples include Siliprandi et al. 1992; Lam et al. 1999; Schlamp et al. 2001; Manabe et al. 2005; Nakazawa et al. 2005; Pernet et al. 2007; Reichstein et al. 2007).
Based on experiments using RGCs in purified cultures and in retinal wholemounts, Ullian et al. (2004) reported that these retinal neurons are invulnerable to the excitotoxic actions of glutamate. Our data instead indicate that excitotoxic death can occur in at least a subpopulation of RGCs, and this death is associated with a similar dysregulation of [Ca2+]i (DCD) that has been observed in other central neurons. The susceptibility of RGCs to excitotoxicity observed in our study is in agreement with the many in vivo studies of NMDA-R-mediated RGC death (examples listed in preceding paragraph). One reason for the differences in our findings may be related to the external solution used in the glutamate exposure experiments. In the present study, the immunopanned RGCs were transferred to HBSS for glutamate treatments, rather than adding glutamate to Neurobasal/B27 culture medium as in Ullian et al. (2004). Besides Mg2+, this culture medium contains vitamins, minerals, and antioxidants that are designed to enhance neuronal survival (Brewer et al. 1993). In particular, oxidative stress caused by free radical formation (such as peroxynitrate generated from nitric oxide) has been implicated as a downstream effector of NMDA-R-mediated excitotoxicity (Dawson et al. 1991; Dugan et al. 1995; Reynolds & Hastings, 1995). The antioxidants in Neurobasal/B27 have been shown to be neuroprotective in models of glutamate-induced neuronal death (Perry et al. 2004), indicating that this culture medium may confound studies of excitotoxicity.
While our results dispute the assertion that RGCs are completely immune to the excitotoxic effects of glutamate, Ullian et al. (2004) provided strong evidence that RGCs are much less vulnerable to excitotoxic death than retinal amacrine cells and hippocampal neurons. In the current study, the percentage of RGCs undergoing DCD (< 30%) during the 1 h exposure to glutamate was considerably less than the approximate 60–85% rate of DCD occurrence that has been observed in cultures of spinal (Tymianski et al. 1993), cortical (Chinopoulos et al. 2004), and cerebellar granule (Bano et al. 2005) neurons using similar glutamate treatment protocols and Ca2+ imaging protocols (including the use of Mg2+-free external solution). Why RGCs are less susceptible to glutamate excitotoxicity, as compared to other types of neurons, remains an important question. Interestingly, Ullian et al. (2004) found that RGCs exhibited significantly smaller NMDA-evoked currents relative to hippocampal neurons. It is possible that the total number of NMDA-Rs or the subunit composition of the expressed NMDA-Rs is different in RGCs, relative to more vulnerable neuronal types. For example, NMDA-Rs that contain NR2A or NR2B subunits, as compared to those with NR2C or NR2D, show a higher sensitivity to Mg2+ block (Kuner & Schoepfer, 1996), while NMDA-Rs containing the NR3A subunit exhibit reduced whole-cell currents and Ca2+ permeability (Dingledine et al. 1999). Each of these subunits has been identified on rodent RGCs (Sucher et al. 2003; Ullian et al. 2004; Jakobs et al. 2007) but the relative expression of different NMDA-R subunit combinations has yet to be clarified for RGCs. Alternatively, there are also data supporting the existence of endogenous neuroprotective mechanisms in RGCs that are activated downstream of NMDA-R-associated Ca2+ influx and that could serve to prevent DCD. An increase of [Ca2+]i in salamander RGCs has been shown to disrupt the organization of actin filaments in the cell membrane, and this actin remodelling may serve as a feedback mechanism to decrease Ca2+ influx and protect RGCs from excitotoxicity by reducing the risk of Ca2+ overload (Cristofanilli & Akopian, 2006; Cristofanilli et al. 2007). Similarly, it has been demonstrated that an increase in the expression of calcium/calmodulin-dependent protein kinase (CaMKIIαB) is stimulated in rat RGCs following glutamate treatment, and this signalling pathway is part of an intrinsic survival response that protects RGCs from excitotoxic death (Fan et al. 2007).
It has been over 50 years since Lucas & Newhouse (1957) first documented the lethal effects of glutamate with the observation that excessive doses of this excitatory amino acid destroyed inner retinal neurons, including RGCs. Glutamate excitotoxicity has been implicated in the pathogenesis of a number of CNS neurodegenerative disorders (Choi, 1988; Lipton & Rosenberg, 1994), and in the RGC death that occurs during retinal ischaemia associated with central and branch retinal artery and vein occlusions (for review, see Osborne et al. 2004). The identification of mechanisms distinguishing the excitotoxic pathway from physiological glutamatergic signalling could aid in the development of effective yet safe neuroprotective therapies.
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes for Health Research (CIHR) to W.H.B. A.T.E.H. was an E. A. Baker Fellow, cosponsored by CIHR and the Canadian National Institute for the Blind, and a W. C. Ezell Fellow, sponsored by Bausch & Lomb through the American Optometric Foundation. We thank Tim Maillet for technical assistance.
Supplementary material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.154609/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol. 2008.154609
References
- Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci. 1996;16:5060–5072. doi: 10.1523/JNEUROSCI.16-16-05060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuve R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Boland LM, Morrill JA, Bean BP. ω-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J Neurosci. 1994;14:5011–5027. doi: 10.1523/JNEUROSCI.14-08-05011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37:11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Gerencser AA, Doczi J, Fiskum G, Adam-Vizi V. Inhibition of glutamate-induced delayed calcium deregulation by 2-APB and La3+ in cultured cortical neurones. J Neurochem. 2004;91:471–483. doi: 10.1111/j.1471-4159.2004.02732.x. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Akopian A. Calcium channel and glutamate receptor activities regulate actin organization in salamander retinal neurons. J Physiol. 2006;575:543–554. doi: 10.1113/jphysiol.2006.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Mizuno F, Akopian A. Disruption of actin cytoskeleton causes internalization of Cav1.2 (α1D), L-type calcium channels in salamander retinal neurons. Mol Vis. 2007;13:1496–1507. [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Limbrick DD, Sombati S, DeLorenzo RJ. Activation of a novel injury-induced calcium-permeable channel that plays a key role in causing extended neuronal depolarization and initiating neuronal death in excitotoxic neuronal injury. J Pharmacol Exp Ther. 2007;322:443–452. doi: 10.1124/jpet.107.123182. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S, Schramm M. The quantity of calcium that appears to induce neuronal death. J Neurochem. 1994;62:1223–1226. doi: 10.1046/j.1471-4159.1994.62031223.x. [DOI] [PubMed] [Google Scholar]
- Fan W, Li X, Cooper NG. CaMKIIaB mediates a survival response in retinal ganglion cells subjected to a glutamate stimulus. Invest Ophthalmol Vis Sci. 2007;48:3854–3863. doi: 10.1167/iovs.06-1382. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Dawson G, Villereal ML, Miller RJ. Identification and characterization of voltage-sensitive calcium channels in neuronal clonal cell lines. J Neurosci. 1984;4:1453–1467. doi: 10.1523/JNEUROSCI.04-06-01453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J Neurophysiol. 1999;82:1974–1981. doi: 10.1152/jn.1999.82.4.1974. [DOI] [PubMed] [Google Scholar]
- Hama Y, Katsuki H, Tochikawa Y, Suminaka C, Kume T, Akaike A. Contribution of endogenous glycine site NMDA agonists to excitotoxic retinal damage in vivo. Neurosci Res. 2006;56:279–285. doi: 10.1016/j.neures.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Kurth MC, Bjerkness L, Weiss JH, Choi DW. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J Neurosci. 1993;13:1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick AT, Lalonde MR, Barnes S, Baldridge WH. Adenosine A1-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:3740–3748. doi: 10.1167/iovs.04-0214. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Zhang X, Chauhan BC, Baldridge WH. Functional assessment of glutamate clearance mechanisms in a chronic rat glaucoma model using retinal ganglion cell calcium imaging. J Neurochem. 2005;94:794–807. doi: 10.1111/j.1471-4159.2005.03214.x. [DOI] [PubMed] [Google Scholar]
- Hyrc K, Handran SD, Rothman SM, Goldberg MP. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. J Neurosci. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Bindokas VP, Nuccitelli R. Calcium ion levels in resting and depolarized goldfish retinal ganglion cell somata and growth cones. J Neurophysiol. 1991;65:968–979. doi: 10.1152/jn.1991.65.4.968. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Ben Y, Masland RH. Expression of mRNA for glutamate receptor subunits distinguishes the major classes of retinal neurons, but is less specific for individual cell types. Mol Vis. 2007;13:933–948. [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Karschin A, Lipton SA. Calcium channels in solitary retinal ganglion cells from post-natal rat. J Physiol. 1989;418:379–396. doi: 10.1113/jphysiol.1989.sp017847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. Glutamate-induced deregulation of calcium homeostasis and mitochondrial dysfunction in mammalian central neurones. Prog Biophys Mol Biol. 2004;86:279–351. doi: 10.1016/j.pbiomolbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-D-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999;40:2391–2397. [PubMed] [Google Scholar]
- Linden DJ, Smeyne M, Connor JA. Trans-ACPD, a metabotropic receptor agonist, produces calcium mobilization and an inward current in cultured cerebellar Purkinje neurons. J Neurophysiol. 1994;71:1992–1998. doi: 10.1152/jn.1994.71.5.1992. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lu YM, Yin HZ, Chiang J, Weiss JH. Ca2+-permeable AMPA/kainate and NMDA channels: high rate of Ca2+ influx underlies potent induction of injury. J Neurosci. 1996;16:5457–5465. doi: 10.1523/JNEUROSCI.16-17-05457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. Arch Opthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005;46:4747–4753. doi: 10.1167/iovs.05-0128. [DOI] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- Marc RE. Kainate activation of horizontal, bipolar, amacrine, and ganglion cells in the rabbit retina. J Comp Neurol. 1999a;407:65–76. [PubMed] [Google Scholar]
- Marc RE. Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. J Comp Neurol. 1999b;407:47–64. doi: 10.1002/(sici)1096-9861(19990428)407:1<47::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427. doi: 10.1523/JNEUROSCI.22-02-00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Melena J, Osborne NN. Voltage-dependent calcium channels in the rat retina: involvement in NMDA-stimulated influx of calcium. Exp Eye Res. 2001;72:292–401. doi: 10.1006/exer.2000.0968. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Endo S, Takahashi H, Mori N, Tamai M. N-Methyl-D-aspartic acid suppresses Akt activity through protein phosphatase in retinal ganglion cells. Mol Vis. 2005;11:1173–1182. [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olney JW. Glutamate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969;28:455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Osswald IK, Galan A, Bowie D. Light triggers expression of philanthotoxin-insensitive Ca2+-permeable AMPA receptors in the developing rat retina. J Physiol. 2007;582:95–111. doi: 10.1113/jphysiol.2007.127894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998;39:972–981. [PubMed] [Google Scholar]
- Pernet V, Bourgeois P, Polo AD. A role for polyamines in retinal ganglion cell excitotoxic death. J Neurochem. 2007;103:1481–1490. doi: 10.1111/j.1471-4159.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Litzburg A, Gelbard HA. Antioxidants are required during the early critical period, but not later, for neuronal survival. J Neurosci Res. 2004;78:485–492. doi: 10.1002/jnr.20272. [DOI] [PubMed] [Google Scholar]
- Potts RA, Dreher B, Bennett MR. The loss of ganglion cells in the developing retina of the rat. Brain Res. 1982;255:481–486. doi: 10.1016/0165-3806(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Reynolds IJ. Glutamate-induced intracellular calcium changes and neurotoxicity in cortical neurons in vitro: effect of chemical ischemia. Neuroscience. 1994;62:667–679. doi: 10.1016/0306-4522(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992;12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichstein D, Ren L, Filippopoulos T, Mittag T, Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13–21. doi: 10.1016/j.exer.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory input. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sattler R, Charlton MP, Hafner M, Tymianski M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem. 1998;71:2349–2364. doi: 10.1046/j.1471-4159.1998.71062349.x. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001;7:192–201. [PubMed] [Google Scholar]
- Schmid S, Guenther E. Voltage-activated calcium currents in rat retinal ganglion cells in situ: changes during prenatal and postnatal development. J Neurosci. 1999;19:3486–3494. doi: 10.1523/JNEUROSCI.19-09-03486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Akopian A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience. 2004;125:583–590. doi: 10.1016/j.neuroscience.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Siliprandi R, Canella R, Carmignoto G, Schiavo N, Zanellato A, Zanoni R, Vantini G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8:567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223:250–258. doi: 10.1007/BF02153655. [DOI] [PubMed] [Google Scholar]
- Stout AK, Reynolds IJ. High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neuroscience. 1999;89:91–100. doi: 10.1016/s0306-4522(98)00441-2. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Aizenman E, Lipton SA. N-methyl-D-aspartate antagonists prevent kainate neurotoxicity in rat retinal ganglion cells in vitro. J Neurosci. 1991;11:966–971. doi: 10.1523/JNEUROSCI.11-04-00966.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Kohler K, Tenneti L, Wong HK, Grunder T, Fauser S, Wheeler-Schilling T, Nakanishi N, Lipton SA, Guenther E. N-methyl-D-aspartate receptor subunit NR3A in the retina: developmental expression, cellular localization, and functional aspects. Invest Ophthalmol Vis Sci. 2003;44:4451–4456. doi: 10.1167/iovs.02-1259. [DOI] [PubMed] [Google Scholar]
- Sun D, Rait JL, Kalloniatis M. Inner retinal neurons display differential responses to N-methyl-D-aspartate receptor activation. J Comp Neurol. 2003;465:38–56. doi: 10.1002/cne.10830. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Engert F, Grantyn R. Synaptic current kinetics in a solely AMPA-receptor-operated glutamatergic synapse formed by rat retinal ganglion neurons. J Neurophysiol. 1995;74:1123–1136. doi: 10.1152/jn.1995.74.3.1123. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J Neurosci. 2000;20:351–360. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004;73:127–150. doi: 10.1016/j.pneurobio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.