Abstract
Skeletal muscle has been identified as a secretory organ. We hypothesized that IL-6, a cytokine secreted from skeletal muscle during exercise, could induce production of other secreted factors in skeletal muscle. IL-6 was infused for 3 h into healthy young males (n = 7) and muscle biopsies obtained at time points 0, 3 and 6 h in these individuals and in resting controls. Affymetrix microarray analysis of gene expression changes in skeletal muscle biopsies identified a small set of genes changed by IL-6 infusion. RT-PCR validation confirmed that S100A8 and S100A9 mRNA were up-regulated 3-fold in skeletal muscle following IL-6 infusion compared to controls. Furthermore, S100A8 and S100A9 mRNA levels were up-regulated 5-fold in human skeletal muscle following cycle ergometer exercise for 3 h at ∼60% of 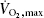 in young healthy males (n = 8). S100A8 and S100A9 form calprotectin, which is known as an acute phase reactant. Plasma calprotectin increased 5-fold following acute cycle ergometer exercise in humans, but not following IL-6 infusion. To identify the source of calprotectin, healthy males (n = 7) performed two-legged dynamic knee extensor exercise for 3 h with a work load of ∼50% of peak power output and arterial–femoral venous differences were obtained. Arterial plasma concentrations for calprotectin increased 2-fold compared to rest and there was a net release of calprotectin from the working muscle. In conclusion, IL-6 infusion and muscle contractions induce expression of S100A8 and S100A9 in skeletal muscle. However, IL-6 alone is not a sufficient stimulus to facilitate release of calprotectin from skeletal muscle.
in young healthy males (n = 8). S100A8 and S100A9 form calprotectin, which is known as an acute phase reactant. Plasma calprotectin increased 5-fold following acute cycle ergometer exercise in humans, but not following IL-6 infusion. To identify the source of calprotectin, healthy males (n = 7) performed two-legged dynamic knee extensor exercise for 3 h with a work load of ∼50% of peak power output and arterial–femoral venous differences were obtained. Arterial plasma concentrations for calprotectin increased 2-fold compared to rest and there was a net release of calprotectin from the working muscle. In conclusion, IL-6 infusion and muscle contractions induce expression of S100A8 and S100A9 in skeletal muscle. However, IL-6 alone is not a sufficient stimulus to facilitate release of calprotectin from skeletal muscle.
Recently, skeletal muscle has been identified as an endocrine organ, which expresses and releases cytokines and other small peptides – known as myokines – most prominently IL-6 (Pedersen et al. 2007). IL-6 is not only produced during muscle contraction (Steensberg et al. 2001), but also released from skeletal muscle, contributing markedly to the systemic circulation of IL-6 (Steensberg et al. 2000; Steensberg et al. 2002). As shown by Rosendal et al. (2005), local concentrations of IL-6 in skeletal muscle during exercise might be an order of magnitude higher than the plasma values. We have previously demonstrated that in human skeletal muscle in vivo, IL-6 is regulated in an autocrine positive manner (Keller et al. 2005). Furthermore, skeletal muscle expresses both the IL-6 receptor (IL-6R) and its coreceptor gp130 (Saito et al. 1992; Keller et al. 2003), which indicates that IL-6 autocrine or paracrine signalling may play an important role in the skeletal muscle response and adaptation to exercise. This is further corroborated by the observation that skeletal muscle STAT-3 signalling, a part of the IL-6 signalling cascade, is induced by exercise (Trenerry et al. 2007).
The systemic effects of IL-6 include enhanced lipolysis and oxidation of fatty acids, without any changes in plasma levels of catecholamines, glucagon or insulin (Van Hall et al. 2003). The observation that IL-6 regulates both fat and carbohydrate metabolism is corroborated by studies of IL-6 knock-out mice, as they have mature-onset obesity and impaired glucose tolerance (Wallenius et al. 2002). In support of a possible role of IL-6 in glucose metabolism, IL-6 stimulation of cultured human muscle cells was found to increase basal glucose uptake and metabolism (Carey et al. 2006; Glund et al. 2007).
As IL-6 is one of the most highly up-regulated genes in skeletal muscle in response to exercise and is able to work in both an autocrine and paracrine manner as described above, we rationalized that perhaps IL-6 would be able to induce expression of other secreted factors in skeletal muscle. Thus, we hypothesized that IL-6 infusion would lead to changes in gene expression in skeletal muscle and that some of the changed genes would represent novel secreted factors. We therefore undertook global gene expression analysis to identify which genes underwent a change in expression level in human skeletal muscle following IL-6 infusion. One of the identified genes was that for S100A8, a constituent of calprotectin, a dimer of S100A8 and S100A9. Calprotectin has been designated as an acute phase reactant (increased plasma concentration following inflammation) (Striz & Trebichavsky, 2004). Calprotectin has also been shown to increase in serum following exercise (Fagerhol et al. 2005; Mooren et al. 2006; Peake et al. 2007). We examined the skeletal muscle mRNA response of both S100A8 and S100A9 in response to both an IL-6 infusion and to acute exercise. We also examined whether or not IL-6 infusion or exercise led to increased calprotectin plasma levels and we determined whether or not there was a net release of calprotectin from skeletal muscle following acute exercise.
Methods
Human volunteers
The subjects were healthy untrained males, who all had a negative medical history and were in normal physical condition. The subjects did not use any medication and had not had any febrile illness in the 2 weeks preceding the study. All volunteers underwent a medical examination and a standard set of blood tests. Purpose and possible risks and discomforts of the study were explained to the participants before written consents were obtained. All study protocols were approved by the local Ethical Committee of Copenhagen and Frederiksberg Communities and were performed in accordance with the Declaration of Helsinki.
IL-6 infusion
Seven healthy untrained young men, age 27 ± 5 years, weight 81 ± 3 kg and BMI 24.5 ± 2 kg m−2 (means ± s.e.m.), participated in the study. On the day of the experiment, subjects arrived at the laboratory at 08.00 h following an overnight fast. Subjects rested in the supine position until 9 h after the start of infusion. Subjects were infused with recombinant human IL-6 (rhIL-6; Sandoz, Basel, Switzerland) for 3 h at a rate of 5 μg h−1 in a volume of 25 ml h−1. The rhIL-6 was administered in 20% human albumin (Statens Serum Institut, Copenhagen, Denmark) via an antecubital vein. On the following day, the subjects again reported to the laboratory after an overnight fast. Skeletal muscle biopsies from the vastus lateralis of musculus quadriceps muscle were obtained before (0 h), immediately after the rhIL-6 infusion (3 h) and at 6 h and 24 h after the start of infusion. To acquire the 24 h samples, the subjects reported to the laboratory the following day after an overnight fast. From the rhIL-6 infusion group we randomly chose three subjects for microarray analysis.
Bicycle exercise
Eight healthy untrained young men, age 25 ± 2 years, weight 82 ± 1 kg and BMI 24.6 ± 0.4 kg m−2 (means ± s.e.m.), participated in the study. The subjects performed an incremental maximal exercise test to determine 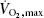 on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden) at least 5 days before the experimental day. On the experimental day, subjects arrived at 07.00 h, after an overnight fast. Furthermore, the participants were instructed to refrain from exercise for at least 48 h before the experiment. The subjects rested for approximately 10 min in the supine position after which a venous catheter was placed in an antecubital vein. Subsequently, the subjects performed 3 h of cycling at approximately 60% of
on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden) at least 5 days before the experimental day. On the experimental day, subjects arrived at 07.00 h, after an overnight fast. Furthermore, the participants were instructed to refrain from exercise for at least 48 h before the experiment. The subjects rested for approximately 10 min in the supine position after which a venous catheter was placed in an antecubital vein. Subsequently, the subjects performed 3 h of cycling at approximately 60% of 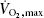 , followed by 6 h of recovery. Muscle biopsies were obtained from the vastus lateralis of musculus quadriceps muscle prior to exercise (0 h), immediately after exercise (3 h), and at 4.5, 6, 9 and 24 h. To acquire the 24 h samples, the subjects reported to the laboratory the following day after an overnight fast.
, followed by 6 h of recovery. Muscle biopsies were obtained from the vastus lateralis of musculus quadriceps muscle prior to exercise (0 h), immediately after exercise (3 h), and at 4.5, 6, 9 and 24 h. To acquire the 24 h samples, the subjects reported to the laboratory the following day after an overnight fast.
Two-legged knee extensor exercise
Seven healthy untrained young men, age 26 ± 1 years, weight 86 ± 9 kg and BMI 26.4 ± 2 kg m−2 (means ± s.e.m.), participated in the study. The maximal power output (Pmax) was determined during dynamic one-legged knee extensor exercise with the use of a modified ergometer, as described earlier (Hansen et al. 2005). On the experimental day, participants reported to the laboratory at 08.00 h after an overnight fast. Furthermore, the participants were instructed to refrain from exercise for at least 48 h before the experiment. The subjects remained supine for the next 3 h.
Under sterile conditions and after application of local anaesthesia (lidocaine, 20 mg ml−1, SAD, Denmark), indwelling catheters were placed in the femoral artery and vein using the guidewire (Seldinger) technique (Berneus et al. 1954). The femoral artery was cannulated ∼2 cm below the inguinal ligament and the catheter (20 G, Arrow, PA, USA) was advanced ∼10 cm in the proximal direction. The femoral venous catheter (18 G, Arrow) was inserted ∼2 cm below the inguinal ligament and advanced ∼5 cm in the distal direction. Of note, the distal orientation of the femoral venous catheter is crucial in order to avoid contamination of the blood with blood draining from the lower abdomen and the saphenous vein as discussed previously (Van Hall et al. 1999). The exercise bout consisted of 3 h of dynamic two-legged knee extensor exercise at 60 extensions min−1, with the workload per leg set to 50% of the individual and actual Pmax. Blood samples were obtained just before the exercise started and then after each hour of exercise. At each sample point the femoral arterial blood flow was measured with the ultrasound Doppler technique as previously validated (Radegran, 1997). An ultrasound Doppler was used (model CFM 800; Vingmed Sound, Horten, Norway) equipped with an annular phased array transducer probe (11.5 mm diameter; Vinmed Sound) operating at an imaging frequency of 7.5 MHz and variable Doppler frequencies of 4.0–6.0 MHz (high-pulsed repetition frequency mode, 4–36 kHz). The site for vessel diameter determination and blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and deep femoral branch. The femoral artery was isolated at a fixed perpendicular angle. The femoral artery was visualized with a fixed perpendicular angle, and the diameter was determined along the central path of the ultrasound beam. The blood velocity was measured in triplicate immediately prior to each blood sample.
Water was consumed ad libitum, whereas food was not permitted until the end of the recovery period. Muscle biopsies were taken from the vastus lateralis of musculus quadriceps muscle before the start of exercise, immediately after exercise, and 2 h post-exercise.
Resting controls
Seven healthy untrained young men, age 25 ± 3 years, weight 81 ± 10 kg and BMI 24.5 ± 2 kg m−2 (means ± s.e.m.), participated in the study. On the day of the experiment, subjects arrived at the laboratory at 08.00 h following an overnight fast. Subjects rested in the supine position for 9 h. Skeletal muscle biopsies from the vastus lateralis of musculus quadriceps muscle were obtained at 0 h, 3 h, 6 h and 24 h. To acquire the 24 h samples, the subjects reported to the laboratory the following day after an overnight fast.
Muscle biopsies
Muscle biopsies were obtained using the Bergström percutaneous needle method with suction (Bergstrom, 1975) from the vastus lateralis of musculus quadriceps muscle. Before each biopsy, local anaesthesia (lidocaine, 20 mg ml−1; SAD) was applied to the skin and fascia superficial to the biopsy site. A new incision site was made for each biopsy, and all incision sites were at minimum 3 cm apart. Visible connective tissue and blood contamination were removed before the biopsies were frozen in liquid nitrogen and subsequently stored at −80°C until further analysis.
Purification and extraction of mRNA
Total RNA was extracted from the skeletal muscle tissue with TriZol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Briefly, 20–30 mg wet weight of skeletal muscle was dissolved in 1 ml TriZol and homogenized for the microarray samples, using a Brinkman Polytron (version PT 2100 on setting 26) or a Qiagen Tissuelyser Retsch MM300 (3 min at 30 Hz). The aqueous phase was transferred to a fresh tube, 100 μl of isoamyl alcohol–chloroform was added and it was vigorously shaken. After 2–3 min of rest, the samples were spun at 20 000 g for 15 min at 4°C, and the upper aqueous phase was transferred to a new tube. The aqueous phase was mixed with 0.5 ml of isopropanol, placed in the freezer for 1 h and centrifuged at 20 000 g for 15 min at 4°C. The liquid phase was then aspirated, and the pellet washed with 0.5 ml of 75% ethanol, followed by centrifugation for 10 min at 12 000 g. The ethanol was aspirated and the remaining pellets were briefly air dried and redissolved in 15 μl of diethyl pyrocarbonate-treated water on ice and stored at −80°C
Real time RT-PCR
Reverse transcription (RT) reactions were performed using random hexamers on 2 μg RNA using an RT kit (Applied Biosystems, Foster City, CA, USA) in a reaction volume of 100 μl. The resulting cDNA product was stored at −20°C until further analysis. Primers used were predeveloped TaqMan Gene Expression Assays no. Hs00195814_m1 (HDAC4), Hs00206843_m1 (HDAC9), Hs00271535_m1 (MEF2A) Hs00243297_m1 (UCP3), Hs00372607_m1 (PP2MC), Hs00188025_m1 (FKBP5), Hs00374263_m1 (S100A8), Hs01903958_s1 (MALAT-1), and Hs00374431_m1 (USP2) (Applied Biosystems). 18S rRNA was amplified using predeveloped assay reagents (Applied Biosystems). All mRNA levels were determined by real time RT-PCR using an ABI Prism 7900 sequence detector (Applied Biosystems). Briefly, diluted RT product (template) was mixed with 2× TaqMan Universal Master Mix and the predeveloped TaqMan assay reagent. The total volume was adjusted to 10 μl with RNAse free water, followed by amplification on an ABI Prism 7900 sequence detector (Applied Biosystems). In addition to the samples of interest, standardized dilutions of pooled cDNA from all samples were amplified. The relative contents of the genes were quantified on the basis of the standard curve method using the standardized control dilutions for the standard curve. Samples were run in triplicate and all samples were run together allowing relative comparison between all conditions. 18S rRNA was used to normalize mRNA expression values. 18S rRNA values were not significantly different between time points in any of the studies.
Statistics
All mRNA data were log-transformed in order to obtain a normal distribution and hence mRNA results are presented as geometric means with geometric standard errors of the mean. Statistical analyses were carried out employing a mixed model analysis, with a random subject-specific component introduced that allowed adjustment for interindividual variation, followed by post hoc Student's t test with Bonferroni's correction to identify differences between groups at specific time points. The fit of the mixed model was evaluated by testing the residuals for normality and by inspection of the residual plots. Calprotectin net release data were analysed using a non-parametric ANOVA (Kruskal–Wallis) with Dunn's multiple comparison as post hoc tests (all time points compared with the 0 h time point) and hence the results are displayed as medians with interquartile range. All statistics were performed using SAS 9.1.2 (SAS Institute Inc., Cary, NC, USA). A P-value < 0.05 was considered significant.
Microarray analysis
Total RNA was further purified with the RNeasy kit (Qiagen, Albertslund, Denmark), and synthesized to double-strand cDNA using Superscript Choice System (Invitrogen, Carlsbad, CA, USA) with an oligo-dT primer containing a T7 RNA polymerase promoter (GenSet, Evry, France). The cDNA was used as a template for an in vitro transcription reaction to synthesize biotin-labelled antisense cRNA (BioArray High Yield RNA Transcript Labeling Kit; Enzo Diagnostics, Farmingdale, NY, USA). After fragmentation at 94°C for 35 min in fragmentation buffer (40 mm Tris, 30 mm magnesium acetate, 10 mm potassium acetate), the labelled cRNA was hybridized for 16 h to Affymetrix HG-U133 Plus 2.0 (Affymetrix Inc., Santa Clara, CA, USA). The HG-U133 Plus 2.0 array uses 25-mer oligonucleotide probes and contains ∼54 000 probe sets covering ∼47 400 transcripts and ∼38 500 well-characterized genes. After hybridization the array was washed and stained with streptavidin phycoerythrin solution using the Fluidics Station 450 (Affymetrix Inc.). Finally the arrays were scanned using the GeneChip Scanner 3000 (Affymetrix Inc.) to obtain non-normalized expression levels.
Affymetrix CEL-files were normalized using gcRMA (Wu et al. 2004) available in the Bioconductor package version 1.7 (http://www.bioconductor.org) for the statistical software package R version 2.21 (R Development Core Team, 2005). The normalized data were imported into the freeware application DNA-chip Analyser (dChip) available at http://www.dchip.org (Li & Hung, 2001). We used dChip to exclude all probes having fold change < 2 (3 h and 6 h compared to 0 h) and we further excluded all non-specific probes (_x suffixes). The expression values from the remaining probes were log10 transformed and analysed for significant changes in gene expression using a one-way repeated measures ANOVA (PROC MIXED, SAS 9.1.2, SAS Institute). In this study we present significantly (P < 0.05) changed known genes (EST and hypothetical proteins excluded). For a complete list of significantly changed known genes, including probe i.d., GenBank accession numbers, and fold change, see the online supplemental material. Figures and calculations of fold change were performed by dChip. We selected a subset of eight genes from the genes with significantly changed mRNA expression in the microarray analysis and measured gene expression using RT-PCR as described above. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) (Edgar et al. 2002) and are accessible through GEO Series accession number GSE10685.
Plasma measurements of calprotectin and IL-6
Plasma IL-6 concentration was measured using a high-sensitivity ELISA kit (no. HS600B; R&D Systems, Minneapolis, MN, USA), which detects total IL-6 independent of binding to soluble receptors, with sensitivity of ∼0.04 pg ml−1 and intra- and interassay coefficients of variation of < 8%. Plasma calprotectin was measured using an ELISA kit (HK325; Hycult biotechnology, Uden, the Netherlands) which detects the S100A8–S100A9 dimer but not the monomers, with a sensitivity of ∼1.5 ng ml−1, an intra-assay coefficient of variation of < 6% and an interassay coefficient of variation of < 15%.
Results
Gene expression profiling of human skeletal muscle tissue in response to IL-6 infusion
Muscle biopsies were obtained at baseline as well as immediately after and 3 h after an infusion of rhIL-6 for 3 h (n = 7). We randomly selected three persons for gene expression profiling using Affymetrix microarrays. We identified 50 genes, whose expression was significantly changed more than 2-fold in skeletal muscle tissue at the 3 h or 6 h time points compared with the 0 h time point (Fig. 1). Focusing on genes potentially involved in inflammation and metabolism, we selected eight genes out of these and performed quantitative real-time PCR analysis of the gene expression levels for these genes in seven subjects who received rhIL-6 infusion and seven controls (Table 1). Overall we found an effect of time or interaction between time and treatment in 6 out of 8 samples, hence validating the microarray findings in Fig. 1. However, we found that only three genes, FKBP5, S100A8 and USP2, were significantly changed by rhIL-6 infusion compared to controls. As there was only a trend towards difference between rhIL-6 infusion and controls for HDAC4 and PP2MC (interaction of time × treatment, P = 0.0541 and P = 0.0764, respectively) (Table 1), care should be taken not to overinterpret the microarray results without validation against control subjects. S100A8 forms a dimer with S100A9 named calprotectin, which is an acute phase reactant and present in plasma (Striz & Trebichavsky, 2004). As we originally hypothesized that IL-6 infusion would lead to an increase in secreted factors, we decided to focus on the S100A8 result and calprotectin as a putative skeletal muscle secreted factor.
Figure 1. Gene expression profiling of the response of human skeletal muscle to IL-6 infusion.
Hierarchical clustering of the expression pattern of significantly (P < 0.05) changed genes over time (not compared with a control group) in human skeletal muscle following an IL-6 infusion from 0 h to 3 h, with more than a 2-fold increase or decrease, as described in Methods. Red indicates up-regulation and green indicates down-regulation.
Table 1.
Validation of IL-6 responsive genes in human skeletal muscle
| Gene | Treatment | 0 h (a.u.) | 3 h (a.u.) | 6 h (a.u.) | Main effect |
|---|---|---|---|---|---|
| FKBP5 | IL-6 | 2.18 (0.79–3.57)# | 3.22 (1.83–4.61) | 3.46 (2.07–4.85) | Time, P < 0.0001 |
| Con | 0.64 (−0.75–2.03) | 2.68 (1.29–4.07)*** | 3.61 (2.22–5.00)*** | Time × Treat, P < 0.01 | |
| HDAC4 | IL-6 | 1.47 (0.85–2.08) | 2.11 (1.50–2.73) | 3.24 (2.62–3.85)* | Time, P < 0.01 |
| Con | 1.54 (0.93–2.16) | 1.68 (1.06–2.29) | 1.99 (1.37–2.01) | ||
| HDAC9 | IL-6 | 2.92 (2.28–3.57) | 1.25 (0.61–1.90) | 1.23 (0.59–1.88) | Time, P < 0.0001 |
| Con | 3.09 (2.44–3.73) | 2.18 (1.53–2.82) | 1.36 (0.71–2.00)** | ||
| MEF2A | IL-6 | 1.54 (0.87–2.20) | 1.55 (0.89–2.22) | 1.75 (1.09–2.42) | |
| Con | 1.88 (1.22–2.55) | 1.72 (1.06–2.39) | 1.77 (1.11–2.44) | ||
| PPM2C | IL-6 | 3.20 (2.29–4.11) | 1.47 (0.55–2.38)* | 1.57 (0.66–2.48)* | Time, P < 0.0001 |
| Con | 5.11 (4.19–6.01) | 3.34 (2.42–4.24) | 2.05 (1.14–2.96)*** | Treat, P < 0.05 | |
| S100A8 | IL-6 | 1.30 (0.30–2.30) | 4.14 (3.14–5.14)***### | 2.60 (1.59–3.60) | Time, P < 0.01,Treat,P < 0.01 |
| Con | 1.76 (0.75–2.76) | 1.10 (0.09–2.10) | 1.37 (0.37–2.37) | Time × Treat, P < 0.01 | |
| UCP3 | IL-6 | 1.22 (0.62–1.82) | 1.51 (0.91–2.11) | 1.99 (1.39–2.59) | |
| Con | 1.63 (1.03–2.23) | 1.71 (1.11–2.31) | 1.63 (1.03–2.23) | ||
| USP2 | IL-6 | 2.94 (2.08–3.80) | 2.19 (1.32–3.05) | 2.34 (1.48–3.20) | Time × Treat, P < 0.05 |
| Con | 2.03 (1.16–2.89) | 2.80 (1.94–3.66) | 2.24 (1.38–3.10) |
RT-PCR results of selected genes on human subjects treated with IL-6 versus controls (con). FKBP5: FK506 binding protein 5; HDAC4: histone deacetylase 4; HDAC9: histone deacetylase 9; PPM2C: protein phosphatase 2C, magnesium-dependent, catalytic subunit; S100A8: S100 calcium binding protein A8 (calgranulin A); UCP3: uncoupling protein 3; USP2: ubiquitin specific protease 2. All values are shown as means with 95% confidence intervals in arbitrary units (a.u.).
P < 0.05 versus 0 h time point,
P < 0.01 versus 0 h time point,
P < 0.001 versus 0 h time point.
P < 0.05 versus control at the same time point,
P < 0.001 versus control at the same time point.
IL-6 infusion increases S100A8 and S100A9 mRNA levels in human skeletal muscle tissue
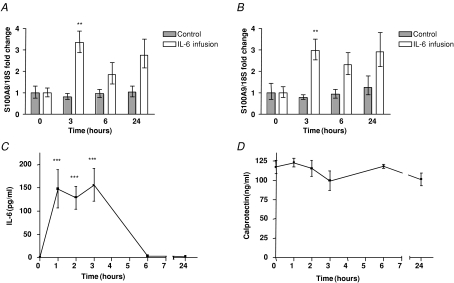
We found that both S100A8 and S100A9 mRNA levels increased approximately 3-fold in skeletal muscle tissue following 3 h of rhIL-6 infusion (n = 7) compared to controls (n = 7) (Fig. 2A and B). Plasma levels of IL-6 increased more than 100-fold during rhIL-6 infusion (Fig. 2C), but no increase in plasma calprotectin was observed (Fig. 2D).
Figure 2. IL-6 infusion increase S100A8 and S100A9 mRNA levels in human skeletal muscle tissue, but not plasma calprotectin levels.
A and B, S100A8 (A) or S100A9 (B) mRNA levels in muscle biopsies during a 3 h infusion of recombinant IL-6 (from 0 h to 3 h) in humans (n = 7) or controls (n = 7) as described in Methods. Data are presented as geometric means ± standard error normalized to the 0 h time point. **P < 0.01 versus controls (post hoc t tests, Bonferroni corrected). C and D, IL-6 plasma (C) or calprotectin plasma (D) levels in venous blood samples during a 3 h infusion of recombinant IL-6 (from 0 h to 3 h) in humans (n = 7). Data are presented as means ± standard error. **P < 0.01 versus 0 h (post hoc t tests, Bonferroni corrected).
Acute exercise increases S100A8 and S100A9 mRNA levels in human skeletal muscle tissue
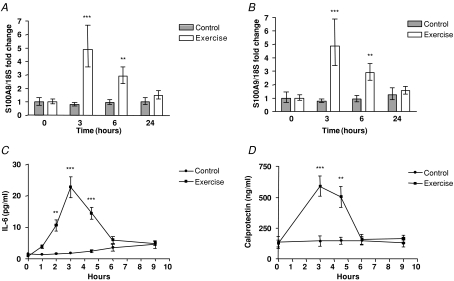
Given that working skeletal muscle produces IL-6, we investigated S100A8 and S100A9 mRNA levels in muscle biopsies in response to 3 h of cycle ergometer exercise in humans (n = 8) and in resting controls (n = 7). Both S100A8 and S100A9 mRNA levels increased approximately 5-fold in skeletal muscle tissue following bicycle exercise (Fig. 3A and B). Plasma levels of IL-6 increased approximately 30-fold in response to exercise (Fig. 3C) and plasma calprotectin increased approximately 5-fold in response to exercise (Fig. 3D).
Figure 3. Acute bicycle exercise increase S100A8 and S100A9 mRNA levels in human skeletal muscle tissue as well as plasma IL-6 and calprotectin levels.
A and B, S100A8 (A) or S100A9 (B) mRNA levels in muscle biopsies immediately before (0 h), immediately after (3 h), 3 h post (6 h) or 21 h post (24 h) 3 h of bicycle exercise in humans (n = 8) or resting controls (n = 7). Data are presented as geometric means ± standard error normalized to the 0 h time point. C and D, IL-6 plasma (C) or calprotectin plasma (D) levels immediately before (0 h), immediately after (3 h), 3 h post (6 h) or 21 h post (24 h) 3 h of bicycle exercise in humans (n = 8) or controls (n = 7). Data are presented as means ± standard error. **P < 0.01 versus controls, ***P < 0.001 versus controls (post hoc t tests, Bonferroni corrected).
Calprotectin is released from skeletal muscle tissue following exercise
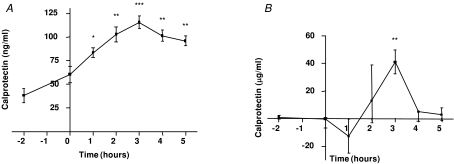
To identify the source of calprotectin, arterial and femoral venous plasma calprotectin were measured before and during two-legged knee extensor exercise. Arterial concentrations of calprotectin increased progressively throughout two-legged knee extensor exercise, reaching a maximal increase of approximately 2-fold at the end of the exercise bout (Fig. 4A). When arterial–venous differences of plasma calprotectin across the exercising limb were measured and plasma flow in the leg was taken into consideration, we were able to measure net flux of calprotectin and we observed an approximately 40 μg min−1 net leg release (P < 0.01) of calprotectin after 3 h of exercise (Fig. 4B), thereby providing indirect evidence that calprotectin is released from skeletal muscle.
Figure 4. Calprotectin is released from skeletal muscle tissue following exercise.
A and B, calprotectin plasma levels in arterial blood (A) or calprotectin net release (B) (Fick's principle: plasma flow multiplied with arterial–femoral venous differences) before and after 3 h of two-legged knee extensor exercise endurance exercise (from 0 h to 3 h) (n = 7). Data are presented as means ± standard error for calprotectin plasma levels and as medians with interquartile ranges for calprotectin net release levels. *P < 0.05 versus 0 h, **P < 0.01 versus 0 h, ***P < 0.001 versus 0 h.
Discussion
The main novel findings of this study were that (1) both IL-6 infusion and endurance exercise induce mRNA expression of both S100A8 and S100A9 in skeletal muscle tissue, (2) endurance exercise, but not IL-6 infusion, induces an increase in plasma calprotectin, and (3) calprotectin is released from the working muscle.
Gene expression changes in skeletal muscle in response to IL-6 infusion
The microarray analysis revealed that 50 genes were significantly changed more than 2-fold in human skeletal muscle tissue in response to an IL-6 infusion. Validation of this result by RT-PCR of eight genes showed a significant change in expression in six cases. The discrepancy between the microarray study and the RT-PCR validation may be attributable to a larger variation between subjects in the RT-PCR study than in the microarray study. The strength of the study is the repeated measures design, which provides more statistical power than if samples were pooled before performing the hybridization (Zhang & Gant, 2005). Thus we are convinced that the microarray results can largely be considered correct. However, the two-way ANOVA of RT-PCR data found a difference between the IL-6 infusion group and the control group for only three genes out of eight. However, two genes, HDAC4 and PPM2C, had a trend towards interaction (P < 0.1). This indicates that care should be taken not to overinterpret results from the IL-6 infusion microarray study.
We were surprised about the low number of genes changed in skeletal muscle tissue following an IL-6 infusion that mimics plasma levels of IL-6 seen in subjects following a marathon run (Fischer, 2006) or patients that had survived sepsis (Herrmann et al. 2000). IL-6 acts through its binding to the IL-6R, which is found in a membrane bound form and in a soluble form (Saito et al. 1992). After IL-6 is bound, the IL-6R combines with gp130 and conveys its actions through the JAK/STAT signalling pathway and subsequently through SOCS-1 and SOCS-3 (Murray, 2007). Furthermore, binding of IL-6 to its receptor leads to an activation of the MAPK and PI3K pathways (Heinrich et al. 2003). Thus, IL-6 is able to activate a variety of signalling pathways, all known to promote changes in gene expression levels. Maybe the systemic levels of IL-6 are not important or high enough for gene regulation in skeletal muscle tissue, whereas the high local IL-6 levels seen following exercise (Rosendal et al. 2005) might induce expression of more genes. An in vitro microarray study in fibroblasts found that only 57 genes changed their expression levels following IL-6 stimulation, indicating that perhaps IL-6 mostly exerts post-transcriptional effects leading to altered signalling pathways (Weigert et al. 2006) rather than changes in gene expression.
Calprotectin mRNA induction by IL-6 infusion and endurance exercise
S100A8 belongs to a family of more than 20 different proteins, characterized by their calcium binding S100 domain (Donato, 2003). S100A8 forms a heterodimer with S100A9, another member of the S100 protein family, and translocates to membranes and intermediate filaments in a calcium-dependent manner (Rammes et al. 1997; Hermani et al. 2006). The S100A8–S100A9 complex, calprotectin, is secreted by a largely unknown mechanism from neutrophils and monocytes when they are activated (Rammes et al. 1997; Boussac & Garin, 2000) or as a result of cell disruption or cell death (Voganatsi et al. 2001). Calprotectin was originally found in neutrophils, where it constitutes up to 60% of total cytosol protein (Berntzen & Fagerhol, 1990). However, calprotectin is also present in other cells like monocytes and acute phase macrophages, but not in mature tissue bound macrophages or different epithelial tissues (Striz & Trebichavsky, 2004).
To our knowledge, this is the first study showing an induction of S100A8 and S100A9 mRNA expression levels in skeletal muscle tissue by IL-6 and exercise. The tight correlation between S100A8 and S100A9 mRNA induction we observe in skeletal muscle tissue, both following IL-6 infusion and exercise, is remarkable. However, this observation is in contrast to induction of S100A8 mRNA expression by growth factors in fibroblasts (Rahimi et al. 2005) or by inflammatory mediators in monocytes (Xu & Geczy, 2000), where only S100A8, but not S100A9, is induced, suggesting that another signalling pathway is responsible for induction of S100A8 and S100A9 in skeletal muscle tissue. Furthermore, we see a higher induction of S100A8 and S100A9 mRNA levels in skeletal muscle tissue following exercise than IL-6 infusion, perhaps due to the large difference between systemic concentrations and local concentrations of IL-6 present in skeletal muscle tissue during exercise (Rosendal et al. 2005).
Calprotectin has been found to be released from monocytes following activation of protein kinase C (PKC) (Rammes et al. 1997), independently of the PKCβ isoform, whereas PKCα was shown to induce calprotectin in keratinocytes (Cataisson et al. 2005). However, another study found no induction of S100A8 and S100A9 protein following PKC activation (Koike et al. 1992). IL-6 has been shown to activate PKCδ in several cell types (Jain et al. 1999), while exercise was found to activate PKCζ and PKCλ (Perrini et al. 2004) as well as atypical PKC (aPKC) (Rose et al. 2004). Interestingly, aPKC activation by exercise was found to be increased in well-trained individuals compared with controls (Roglans et al. 2002) and the exercise induced plasma calprotectin increase was found to be increased in well-trained individuals (Mooren et al. 2006), perhaps suggesting an involvement of aPKC in regulation of S100A8 and S100A9 in skeletal muscle tissue.
Our observation that IL-6 does not induce release of calprotectin from muscle, but only induces an increase in the mRNA expression levels of its constituents, suggests that calprotectin synthesis and release are governed by two separate signalling pathways. Differences in PKC isoform activation between a singular IL-6 signal and exercise, which activates both IL-6 signalling pathways and a host of other signalling pathways, may be one explanation. Alternatively, the local IL-6 concentration in skeletal muscle during IL-6 infusion, compared with exercise, might be an order of magnitude too low for calprotectin release induction or another signal induced by exercise is required for calprotectin release.
Calprotectin is released from the working muscle
Calprotectin has previously been shown to be increased in serum or plasma following exercise (Fagerhol et al. 2005; Mooren et al. 2006; Peake et al. 2007). Fagerhol et al. (2005) found an increase of between 3.4-fold ( test) and 96.3-fold (marathon run) in plasma calprotectin following acute exercise of varying intensity and length, with the most strenuous and prolonged exercise giving the highest response. Mooren et al. (2006), however, only found a 7-fold increase in calprotectin following a marathon run. Interestingly, Mooren et al. (2006) also found that more strenuous exercise produced a faster calprotectin increase, and that well-trained individuals had a 1.5-fold higher increase in calprotectin following a marathon run, whereas the increase in white blood cells did not differ between well-trained and moderately trained individuals. As calprotectin levels in neutrophils are not increased by exercise (Fagerhol et al. 2005), this strongly suggests that a source other than neutrophils is responsible for the increase in calprotectin levels seen during exercise.
test) and 96.3-fold (marathon run) in plasma calprotectin following acute exercise of varying intensity and length, with the most strenuous and prolonged exercise giving the highest response. Mooren et al. (2006), however, only found a 7-fold increase in calprotectin following a marathon run. Interestingly, Mooren et al. (2006) also found that more strenuous exercise produced a faster calprotectin increase, and that well-trained individuals had a 1.5-fold higher increase in calprotectin following a marathon run, whereas the increase in white blood cells did not differ between well-trained and moderately trained individuals. As calprotectin levels in neutrophils are not increased by exercise (Fagerhol et al. 2005), this strongly suggests that a source other than neutrophils is responsible for the increase in calprotectin levels seen during exercise.
When comparing the release of calprotectin from the contracting muscle with the amount accumulated in the body (Fig. 4), it is clear that the release markedly surpassed the rate of accumulation. The increase in arterial plasma calprotectin concentration during the last 2 h of exercise was 32.1 ng ml−1 (12.1–52.1 ng ml−1, 95% confidence interval (CI)). Assuming that the calprotectin produced is diluted in the extracellular space (∼12 l), the total amount of calprotectin accumulated during the last 2 h of exercise was about 385.2 μg (145.2–625.2 μg, 95% CI) or 3.21 μg min−1 (1.21–5.21 μg min−1, 95% CI). This implies that the highest net release of calprotectin from the working leg was approximately 13-fold higher than the rate of calprotectin accumulation (3 h median; 41.2 μg min−1, Fig. 4). This suggests that skeletal muscle may be the main source behind the increase in plasma calprotectin seen during exercise. However the exact cellular source of calprotectin cannot be identified from the indirect evidence of skeletal muscle calprotectin release conferred above. Thus we cannot rule out that exercise-induced activation of, for example, neutrophils or macrophages embedded in muscle tissue may contribute to the observed calprotectin release.
The physiological role of calprotectin released during exercise
After secretion, calprotectin interacts with endothelial heperan sulphate proteoglycans, and it has been suggested that the complex plays an important role in extravasation of leucocytes (Robinson et al. 2002). Calprotectin can also act in a cytokine manner, as an extracellular ligand for cell surface receptors by binding to the receptor for advanced glycation end products (RAGE), leading to activation of cellular pathways involving the p38 or p44/42 MAP kinases, cdc42/Rac and NFκB signalling components (Hermani et al. 2006). The close association between activated inflammatory cells and release of S100A8/S100A9 has made it a marker of the activity in different autoimmune diseases, like rheumatoid arthritis and inflammatory bowel diseases (Striz & Trebichavsky, 2004; Hermani et al. 2006). Furthermore, an increase in serum calprotectin was seen in type 1 diabetics (Bouma et al. 2004).
Calprotectin has also been found to be able to induce apoptosis in a variety of tumour cell lines (Yui et al. 1995), including colon cancer cell lines (Ghavami et al. 2004), and to be able to inhibit matrix metalloproteinases (Isaksen & Fagerhol, 2001), indicating a possible role for calprotectin in cancer protection. Although speculative, skeletal muscle-derived calprotectin might be involved in mediating the protective effect of regular exercise against colon cancer and breast cancer (Thune & Furberg, 2001).
Prolonged heavy exertion has been shown to have a negative impact on the immune system (Nieman & Pedersen, 1999). This ‘open window’ of altered immunity (which can last from 3 to 72 h) provides an increased risk of obtaining a subclinical or clinical viral or bacterial infection (Nieman & Pedersen, 1999). Interestingly, prolonged heavy exertion has been shown to increase calprotectin levels for days (Fagerhol et al. 2005) and patients suffering from hypercalprotectinaemia have recurrent infections as well as increased systemic inflammation (Sampson et al. 2002), suggesting a possible role for calprotectin in exercise immunology.
Conclusion
IL-6 infusion changed the expression of only a small subset of genes in skeletal muscle tissue, among others S100A8, a subunit of calprotectin. The mRNA levels of both subunits of calprotectin, S100A8 and S100A9, were found to increase in skeletal muscle tissue during both IL-6 infusion and exercise. However, IL-6 infusion had no effect on plasma calprotectin levels, whereas calprotectin increased during endurance exercise and was found to cause a net release of calprotectin from the working leg. The clinical significance of muscle-released calprotectin remains to be identified. However, calprotectin has the potential to mediate both beneficial and detrimental health effects of exercise, such as on the one hand offering protection against some cancers and on the other being a player in exercise-induced immune impairment following heavy exertion.
Acknowledgments
The authors are grateful for the excellent technical assistance of Hanne Villumsen, Ruth Rousing and Bettina Mentz. The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (no. 02-512-55). This study was further supported by the Danish Medical Research Council, the Commission of the European Union (contract no. LSHM-CT-2004-005272 EXGENESIS), and by grants from Gangstedfonden and Direktør Emil Hertz og Hustru Inger Hertz Fond. The Copenhagen Muscle Research Centre is supported by grants from the Capital Region of Denmark and the University of Copenhagen.
Supplementary material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.153551/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2008.153551
References
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Laboratory Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Berneus B, Carlsten A, Holmgren A, Seldinger SI. Percutaneous catheterization of peripheral arteries as a method for blood sampling. Scand J Clin Laboratory Invest. 1954;6:217–221. [PubMed] [Google Scholar]
- Berntzen HB, Fagerhol MK. L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Laboratory Invest. 1990;50:769–774. doi: 10.1080/00365519009091071. [DOI] [PubMed] [Google Scholar]
- Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–1986. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- Boussac M, Garin J. Calcium-dependent secretion in human neutrophils: a proteomic approach. Electrophoresis. 2000;21:665–672. doi: 10.1002/(SICI)1522-2683(20000201)21:3<665::AID-ELPS665>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Cataisson C, Pearson AJ, Torgerson S, Nedospasov SA, Yuspa SH. Protein kinase Cα-mediated chemotaxis of neutrophils requires NF-κB activity but is independent of TNFα signaling in mouse skin in vivo. J Immunol. 2005;174:1686–1692. doi: 10.4049/jimmunol.174.3.1686. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerhol MK, Nielsen HG, Vetlesen A, Sandvik K, Lyberg T. Increase in plasma calprotectin during long-distance running. Scand J Clin Laboratory Invest. 2005;65:211–220. doi: 10.1080/00365510510013587. [DOI] [PubMed] [Google Scholar]
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–175. doi: 10.1189/jlb.0903435. [DOI] [PubMed] [Google Scholar]
- Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56:1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK. Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol. 2005;98:93–99. doi: 10.1152/japplphysiol.00163.2004. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermani A, De SB, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-κB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–197. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Herrmann W, Ecker D, Quast S, Klieden M, Rose S, Marzi I. Comparison of procalcitonin, sCD14 and interleukin-6 values in septic patients. Clin Chem Laboratory Med. 2000;38:41–46. doi: 10.1515/CCLM.2000.007. [DOI] [PubMed] [Google Scholar]
- Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–292. doi: 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Zhang T, Kee WH, Li W, Cao X. Protein kinase C d associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem. 1999;274:24392–24400. doi: 10.1074/jbc.274.34.24392. [DOI] [PubMed] [Google Scholar]
- Keller P, Keller C, Carey AL, Jauffred S, Fischer CP, Steensberg A, Pedersen BK. Interleukin-6 production by contracting human skeletal muscle: autocrine regulation by IL-6. Biochem Biophys Res Commun. 2003;310:550–554. doi: 10.1016/j.bbrc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, Hidalgo J, Pedersen BK. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J. 2005;19:1181–1183. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- Koike T, Harada N, Yoshida T, Morikawa M. Regulation of myeloid-specific calcium binding protein synthesis by cytosolic protein kinase C. J Biochem. 1992;112:624–630. doi: 10.1093/oxfordjournals.jbchem.a123950. [DOI] [PubMed] [Google Scholar]
- Li C, Hung WW. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-8-research0032. RESEARCH0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren FC, Lechtermann A, Fobker M, Brandt B, Sorg C, Volker K, Nacken W. The response of the novel pro-inflammatory molecules S100A8/A9 to exercise. Int J Sports Med. 2006;27:751–758. doi: 10.1055/s-2005-872909. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Pedersen BK. Exercise and immune function. Recent developments. Sports Med. 1999;27:73–80. doi: 10.2165/00007256-199927020-00001. [DOI] [PubMed] [Google Scholar]
- Peake J, Peiffer JJ, Abbiss CR, Nosaka K, Okutsu M, Laursen PB, Suzuki K. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur J Appl Physiol. 2007;102:391–401. doi: 10.1007/s00421-007-0598-1. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21–24. doi: 10.2337/diabetes.53.1.21. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Rahimi F, Hsu K, Endoh Y, Geczy CL. FGF-2, IL-1β and TGF-β regulate fibroblast expression of S100A8. FEBS J. 2005;272:2811–2827. doi: 10.1111/j.1742-4658.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277:3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- Roglans N, Sanguino E, Peris C, Alegret M, Vazquez M, Adzet T, Diaz C, Hernandez G, Laguna JC, Sanchez RM. Atorvastatin treatment induced peroxisome proliferator-activated receptor a expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. J Pharmacol Exp Ther. 2002;302:232–239. doi: 10.1124/jpet.302.1.232. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Michell BJ, Kemp BE, Hargreaves M. Effect of exercise on protein kinase C activity and localization in human skeletal muscle. J Physiol. 2004;561:861–870. doi: 10.1113/jphysiol.2004.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2005;98:477–481. doi: 10.1152/japplphysiol.00130.2004. [DOI] [PubMed] [Google Scholar]
- Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol. 1992;148:4066–4071. [PubMed] [Google Scholar]
- Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, Kovar IZ, Beattie JH, Wolska-Kusnierz B, Saito Y, Roth J. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002;360:1742–1745. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, van Schjerling PHG, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striz I, Trebichavsky I. Calprotectin – a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–253. [PubMed] [Google Scholar]
- Thune I, Furberg AS. Physical activity and cancer risk: dose–response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33:S530–S550. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol. 2007;102:1483–1489. doi: 10.1152/japplphysiol.01147.2006. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Gonzalez-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise: methodological considerations. Proc Nutr Soc. 1999;58:899–912. doi: 10.1017/s0029665199001202. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70:130–134. [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schauble M, Haring HU, Schleicher ED. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem. 2006;281:7060–7067. doi: 10.1074/jbc.M509782200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA, Robert G, Francisco MM, Forrest S. A Model Based Background Adjustment for Oligonucleotide Expression Arrays. (May 2004). Johns Hopkins University, Dept. of Biostatistics Working Papers. Working Paper 1 [Google Scholar]
- Xu K, Geczy CL. IFN-γ and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. J Immunol. 2000;164:4916–4923. doi: 10.4049/jimmunol.164.9.4916. [DOI] [PubMed] [Google Scholar]
- Yui S, Mikami M, Yamazaki M. Induction of apoptotic cell death in mouse lymphoma and human leukemia cell lines by a calcium-binding protein complex, calprotectin, derived from inflammatory peritoneal exudate cells. J Leukoc Biol. 1995;58:650–658. doi: 10.1002/jlb.58.6.650. [DOI] [PubMed] [Google Scholar]
- Zhang SD, Gant TW. Effect of pooling samples on the efficiency of comparative studies using microarrays. Bioinformatics. 2005;21:4378–4383. doi: 10.1093/bioinformatics/bti717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.