Abstract
Ageing is associated with impaired endothelium-derived nitric oxide (NO) function in human microvessels. We investigated the impact of cardiorespiratory fitness and exercise training on physiological and pharmacological NO-mediated microvascular responses in older subjects. NO-mediated vasodilatation was examined in young, older sedentary and older fit subjects who had two microdialysis fibres embedded into the skin on the ventral aspect of the forearm and laser Doppler probes placed over these sites. Both sites were then heated to 42°C, with Ringer solution infused in one probe and N-nitro-l-arginine methyl ester (l-NAME) through the second. In another study, three doses of ACh were infused in the presence or absence of l-NAME in similar subjects. The older sedentary subjects then undertook exercise training, with repeat studies at 12 and 24 weeks. The NO component of the heat-induced rise in cutaneous vascular conductance (CVC) was diminished in the older sedentary subjects after 30 min of prolonged heating at 42°C (26.9 ± 3.9%CVCmax), compared to older fit (46.2 ± 7.0%CVCmax, P < 0.05) and young subjects (41.2 ± 5.2%CVCmax, P < 0.05), whereas exercise training in the older sedentary group enhanced NO-vasodilator function in response to incremental heating (P < 0.05). Similarly, the NO contribution to ACh responses was impaired in the older sedentary versus older fit subjects (low dose 3.2 ± 1.3 versus 6.6 ± 1.3%CVCmax; mid dose 11.4 ± 2.4 versus 21.6 ± 4.5%CVCmax; high dose 35.2 ± 6.0 versus 52.6 ± 7.9%CVCmax, P < 0.05) and training reversed this (12 weeks: 13.7 ± 3.6, 28.9 ± 5.3, 56.1 ± 3.9%CVCmax, P < 0.05). These findings indicate that maintaining a high level of fitness, or undertaking exercise training, prevents age-related decline in indices of physiological and pharmacological microvascular NO-mediated vasodilator function. Since higher levels of NO confer anti-atherogenic benefit, this study has potential implications for the prevention of microvascular dysfunction in humans.
Interest in cutaneous microvascular nitric oxide (NO) function has increased in recent years as studies have consistently shown it to be a mediator of vasodilator responses to a variety of stimuli (Kellogg et al. 1998, 1999, 2003; Shastry et al. 1998; Minson et al. 2001; Holowatz et al. 2003; Stewart et al. 2007). In large arteries, NO-related arterial wall function may reflect aggregate atherogenic risk (Vita & Keaney, 2002; Green et al. 2004, 2008) and it was recently proposed that skin vasodilator function may similarly reflect generalized microvascular function and, thereby, represent a useful translational model for investigating preclinical microvascular disease status (Holowatz et al. 2007b). This viewpoint is supported by recent evidence indicating that cutaneous microvessel dysfunction is correlated with coronary endothelial dysfunction (Shamim-Uzzaman et al. 2002; Bonetti et al. 2004; Khan et al. 2008) and cardiovascular risk factors such as hypercholesterolaemia (Khan et al. 1999), hypertension (Carberry et al. 1992; Rizzoni et al. 2003) and type II diabetes (Sokolnicki et al. 2007). Nonetheless, microvascular physiology differs from that of larger arteries and it is important to independently assess the response of microvessels to various risk factors and interventions.
The impact of ageing on skin microvascular function has been well researched and reviewed (Holowatz et al. 2007a). Studies that have employed the optimal microdialysis techniques (Cracowski et al. 2006) to elicit NO blockade have demonstrated that NO function is attenuated in older sedentary subjects in response to physiological stimuli such as localized heating (Minson et al. 2002). Other evidence indicates that NO plays a role in the vasodilator responses to pharmacological agonists such as acetylcholine (ACh) (Boutsiouki et al. 2004; Holowatz et al. 2005; Kellogg et al. 2005). These studies suggest stimulus specificity, in that the impact of ageing on NO vasodilator function apparently differs according to whether physiological and pharmacological NO-dependent stimuli are administered. No previous studies have investigated the impact of ageing on both physiological and pharmacological microvascular function using NO blockade in humans.
Even less well studied is the impact of cardiopulmonary fitness and exercise training on microvascular NO function. A few studies have used either local heating (Franzoni et al. 2004; Colberg et al. 2005), or ACh stimulation (Wang, 2005), but these have not employed NO blockade techniques to directly assess differences in the contribution of NO to physiological or pharmacological stimulation in subjects of different fitness levels, or in older sedentary subjects who undertake an exercise training program. In addition, such studies have typically utilized approaches such as iontophoresis, which has well accepted limitations in experiments of repeated measures and cross-sectional design, including the confounding effects associated with using an electrical current for drug administration into the skin, the resistance of the vehicle used to deliver ACh, and individual variations of the electrical resistance characteristics of the skin barrier (Cracowski et al. 2006). In the present study we therefore investigated the impact of cardiopulmonary fitness, and exercise training, on the contribution of NO to both pharmacological and physiological skin blood flow (SkBF) responses in older subjects using the approaches of microdialysis and laser Doppler flowmetry. We hypothesized that ageing would be associated with impairment in microvascular NO-mediated responses and that chronic exercise and short-term exercise training would ameliorate this dysfunction. Research into the microvascular effects of ageing and exercise training are important, given the central role of NO in prevention of atherosclerosis and the increasing prevalence of microvascular disease in the Western world.
Methods
Ethical approval
All study procedures undertaken within this investigation were approved by the Ethics Committee of Liverpool John Moores University and conformed to that guidelines outlined by the Declaration of Helsinki. All subjects gave written informed consent.
Subject characteristics
We recruited 21 recreationally active young (Y) subjects (11 M, 10 F; age 27 ± 1 years; 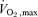 49 ± 2 ml kg−1 min−1), 30 older sedentary (OS) individuals (14 M, 16 F; 60 ± 1 years; 28 ± 1 ml kg−1 min−1) and 20 older fit (OF) subjects (11 M 9 F; 58 ± 1 years; 45 ± 2 ml kg−1 min−1). These subjects were randomly assigned to participate in either the physiological (n = 12 Y, 18 OS, 12 OF) or pharmacological (n = 12 Y, 16 OS, 12 OF) microvascular substudies, described below. Three Y, 4 OS and 4 OF subjects volunteered to participate in both protocols. The details of each group are included in Table 1. Healthy controls were defined as individuals undertaking less than 3 h of regular exercise per week at a recreational level, whilst the older sedentary group reported undertaking no regular exercise. All participants were screened for cardiac abnormalities and cardiovascular disease prior to entering the study. Subjects were specifically questioned regarding any history of hypercholesterolaemia, hypertension, insulin resistance and diabetes. Any such subjects were excluded. Those who smoked or had a family history of premature coronary disease or were on medications of any type were also excluded, as were individuals taking vitamin or any other supplements.
49 ± 2 ml kg−1 min−1), 30 older sedentary (OS) individuals (14 M, 16 F; 60 ± 1 years; 28 ± 1 ml kg−1 min−1) and 20 older fit (OF) subjects (11 M 9 F; 58 ± 1 years; 45 ± 2 ml kg−1 min−1). These subjects were randomly assigned to participate in either the physiological (n = 12 Y, 18 OS, 12 OF) or pharmacological (n = 12 Y, 16 OS, 12 OF) microvascular substudies, described below. Three Y, 4 OS and 4 OF subjects volunteered to participate in both protocols. The details of each group are included in Table 1. Healthy controls were defined as individuals undertaking less than 3 h of regular exercise per week at a recreational level, whilst the older sedentary group reported undertaking no regular exercise. All participants were screened for cardiac abnormalities and cardiovascular disease prior to entering the study. Subjects were specifically questioned regarding any history of hypercholesterolaemia, hypertension, insulin resistance and diabetes. Any such subjects were excluded. Those who smoked or had a family history of premature coronary disease or were on medications of any type were also excluded, as were individuals taking vitamin or any other supplements.
Table 1.
Subjects characteristics at baseline
| Young | Sedentary older | Fit older | ||||
|---|---|---|---|---|---|---|
| ACh (n = 12) | LH (n = 12) | ACh (n = 18) | LH (n = 18) | ACh (n = 16) | LH (n = 16) | |
| Age (years) | 26±1 | 27±1 | 59±1† | 60±1†‡ | 58±2 | 58±1 |
| Body weight (kg) | 68±2 | 69±3 | 85±4†‡ | 83±4†‡ | 65±2 | 69±3 |
| BMI | 23±1 | 23±1 | 29±1†‡ | 29±1†‡ | 23±1 | 24±1 |
| DEXA | ||||||
| Fat mass (kg) | 15±2 | 13±1 | 26±2†‡ | 28±2†‡ | 14±2 | 15±2 |
| Lean body mass (kg) | 52±3 | 53±3 | 57±3 | 54±4 | 49±2 | 52±3 |
| % body fat | 21±3 | 20±2 | 31±2†‡ | 34±2†‡ | 21±2 | 22±2 |
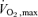 (ml kg−1 min−1) (ml kg−1 min−1) |
50±3 | 48±3 | 28±1†‡ | 28±1†‡ | 46±3 | 44±2 |
| Resting blood pressure (mmHg) | ||||||
| Systolic | 109±3 | 105±3 | 126±3† | 123±5† | 124±4† | 115±5 |
| Diastolic | 66±3 | 60±1 | 72±1 | 70±2† | 70±3 | 67±3 |
Values are means±s.e.m.,
P < 0.01 versus young subjects,
P < 0.01 versus older fit subjects. LH, local heating protocol; ACh, acetylcholine protocol.
Subjects attended the testing facility at the Research Institute for Sport and Exercise Sciences on several occasions. In all cases, they were fasted for 8 h prior to being assessed and were asked to refrain from exercise or consuming caffeine or alcohol for 24 h prior to their attendance. All premenopausal females were tested during the early follicular phase of their menstrual cycle. The study protocols are provided in Fig. 1. Upon arrival, subjects were instrumented (as below) and cannulation for microdialysis probe insertion was undertaken (∼15 min). Following a ∼90 min equilibration period, under euthermic conditions (22.4 ± 0.1°C, relative humidity 31.4 ± 0.2%), one of two experimental protocols was undertaken.
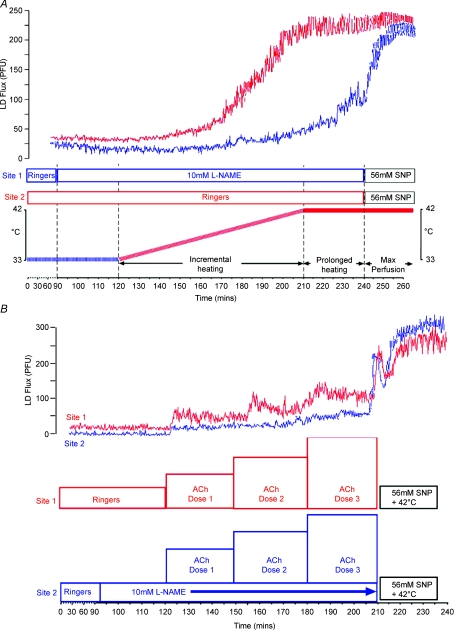
Figure 1. Exercise protocols and representative data.
A, experimental protocol indicating the time lines associated with Ringer solution and l-NAME infusion at the microdialysis membrane sites and periods of incremental local heating and prolonged local heating at 42°C. The upper panel presents simultaneously derived data from an individual at the heating sites perfused with Ringer solution (upper trace) and l-NAME (lower trace). B, experimental protocol indicating the time lines associated with incremental doses of acetylcholine (ACh) in the absence or presence of l-NAME infusion at the microdialysis membrane sites. The upper panel presents simultaneously derived data from an individual at the site perfused with ACh alone (upper trace) and ACh in the presence of l-NAME (lower trace).
Protocol 1. Physiological NO-mediated vasodilatation
Following the equilibration period, the skin surrounding both microdialysis probes was gradually heated, using local heating disks (Perimed 455, Stockholm, Sweden), from 33 to 42°C at a rate of 0.5°C per 5 min (90 min). Thereafter, both sites were continuously heated at 42°C for a further 30 min. This slow ramping protocol was used to minimize the impact of heating on axon reflexes, which are less NO-mediated than slow heating component responses (Minson et al. 2001; Houghton et al. 2006). Ringer solution was infused throughout the protocol in one probe and N-nitro-l-arginine methyl ester (l-NAME; 10 mm, 5 μl min−1, Merck Biosciences, Germany) infused through the second, from 30 min prior to heating onset (Fig. 1A). Sodium nitroprusside (SNP, 56 mm, Mayne Pharma, Warwickshire, UK) was infused at the end of the protocol for 30 min (Minson et al. 2002; Cracowski et al. 2006).
Protocol 2. Pharmacological NO-mediated vasodilatation
In this substudy (Fig. 1B), pharmacological NO-mediated microvascular function was assessed by infusing three incremental doses of ACh (0.15, 1.5, 15 mm, Miochol-E, Novartis, Stein, Switzerland) through one microdialysis probe for 30 min per dose. Identical doses of ACh were simultaneously infused through a second probe, in the presence of an l-NAME co-infusion (10 mm), which began 30 min prior to the initial ACh dose. As above, sodium nitroprusside (56 mm) combined with heating at 42°C was used to induce maximal skin perfusion at the end of the protocol. Those subjects who participated in both of the above protocols had studies performed on separate days, at the same time of day, with identical preparation.
We undertook assessments using both physiological (heating) and pharmacological (ACh) NO-mediated responses in the skin because the biochemical pathways were likely to differ between these stimuli. Previous studies in humans using microdialysis approaches indicate that NO is the primary mediator of vasodilatation that occurs during the secondary plateau phase of a slow local heating protocol (Kellogg et al. 1999; Minson et al. 2001). A recent study excluded neuronal NO synthase as a mediator of local heating induced skin vasodilatation, indicating that eNOS may be largely responsible (Kellogg et al. 2008). Conversely, some (Boutsiouki et al. 2004; Holowatz et al. 2005; Kellogg et al. 2005), but not all (Stewart et al. 2007), studies suggest that NO plays a more limited role in the mediation of cutaneous vasodilatation to ACh, and it is suggested that other mediators such as prostanoids, EDHF and sensory nerves may co-mediate ACh responses at some doses. We constructed dose–response curves in the presence of l-NAME to quantify the contribution of NO to ACh responses at each dose and to determine whether fitness or training alter this contribution.
Exercise training programme
Following assessments at study entry, 16 out of the 30 older sedentary subjects agreed to participate in a longitudinal exercise training study, initially involving three sessions of exercise per week at an intensity of 30% heart rate reserve (HRR) performed for 30 min per visit (treadmill walking and cycling). HRR was calculated using the following formula: ((Max HR – Resting HR) × intensity) + Resting HR. The resting and maximal heart rate measures were derived from a maximal exercise test (see below) undertaken prior to and at the end of the initial and repeat 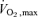 assessments. Two sessions were supervised by an exercise physiologist in a dedicated training facility, with the other session performed at home or in regional gymnasia. Compliance with the home-based component was assessed by regional site visits and regular telephone check-ups. After the initial 6 weeks, the frequency of exercise increased to five sessions per week. Repeat assessments were performed following 12 weeks of this regimen, after which the exercise intensity increased to 60% HRR. Further assessments were undertaken at 24 weeks. Eight subjects undertook repeated physiological heating protocols at 12 and 24 weeks; the pharmacological protocol was repeated in the other eight subjects. A further nine subjects acted as time controls, five undergoing repeated physiological and four pharmacological testing at baseline and after 24 weeks.
assessments. Two sessions were supervised by an exercise physiologist in a dedicated training facility, with the other session performed at home or in regional gymnasia. Compliance with the home-based component was assessed by regional site visits and regular telephone check-ups. After the initial 6 weeks, the frequency of exercise increased to five sessions per week. Repeat assessments were performed following 12 weeks of this regimen, after which the exercise intensity increased to 60% HRR. Further assessments were undertaken at 24 weeks. Eight subjects undertook repeated physiological heating protocols at 12 and 24 weeks; the pharmacological protocol was repeated in the other eight subjects. A further nine subjects acted as time controls, five undergoing repeated physiological and four pharmacological testing at baseline and after 24 weeks.
Experimental measures
Microdialysis fibre instrumentation and assessment of forearm skin blood flow
After being seated comfortably in a custom-designed bed, the left arm was supinated and supported for insertion of microdialysis fibres and consequent measurement of laser-Doppler flux. The insertion sites were marked on the skin and cold packs were applied. Two microdialysis fibres (Linear 30, CMA Microdialysis Ltd, Stockholm, Sweden), containing 10 mm long 6 kDa membranes, were inserted by first placing a 21-gauge needle subdermally for threading and placement. The needles were then removed and the embedded fibres perfused with Ringer solution at a rate of 5 μl min−1 using a microinfusion pump (Model 11 plus, Harvard Apparatus, MA, USA). To obtain an index of skin blood flow, cutaneous red cell flux was measured by placing integrated laser-Doppler probes, each consisting of a seven-laser array (Model 413, Periflux 5001 System, Perimed AB, Sweden), above each microdialysis fibre. The laser-Doppler probe signals were continuously monitored via an online software chart recorder (PSW, Perimed, Sweden). At each designated study time point (5 min intervals), SkBF was assessed by averaging laser-Doppler flux (LDF), measured in perfusion units (PU), over a stable 2 min period. These data were subsequently converted to cutaneous vascular conductance (CVC), calculated as LDF/MAP (PU mmHg−1), where mean arterial pressure (MAP) was derived from contemporaneous automated blood pressure measures in the contralateral arm. Values were then expressed relative to the maximal CVC achieved during infusion of 56 mm SNP at 42°C, as %CVCmax, the preferred method of data expression adopted in the literature (Cracowski et al. 2006).
Assessment of physical fitness
Exercise testing was undertaken on a treadmill ergometer (Pulsar 4.0, h/p/cosmos, Nussdorf-Traunstein, Germany), with initial workload set at 4 km h−1 at 5% gradient and step-wise increments in speed and grade every 3 min until volitional exhaustion. Heart rate and rhythm were continuously recorded by 12-lead ECG and blood pressure was measured during the last 30 s of each 3 min stage. All tests performed in older subjects were medically supervised. The volume of oxygen consumed during exercise was directly calculated from minute ventilation, measured using a pneumotach and simultaneous breath-by-breath analysis of expired gas fractions (Medgraphics CPX/D and Ultima CardiO2 systems, St Paul, MN, USA). Gas analysers and flow probes were calibrated before each test. Oxygen consumption was recorded during the final 40 s of each stage of the test and expressed relative to body weight (ml kg−1 min−1). Maximal oxygen consumption was calculated as the highest consecutive 10 s period of gas exchange data occurring in the last minute before volitional exhaustion, which generally occurred due to leg fatigue or breathlessness.
Data analysis
All data are expressed as %CVC and analysis was performed on this normalized data (Cracowski et al. 2006). Changes in %CVCmax responses during local heating were assessed within groups using two-way ANOVA, with repeated measures performed on data collected prior to the local heating (baseline), during incremental and prolonged heating data points. This analysis was performed for data collected from the Ringer solution and l-NAME sites and also on data representing the contribution of NO to %CVC responses, which was calculated by subtracting l-NAME %CVC data from Ringer solution %CVC data at equivalent heating time points. Student's t test for unpaired data was also performed on baseline, incremental heating and prolonged heating data points. Changes in %CVCmax during ACh administration were assessed within groups using two-way ANOVA, with repeated measures performed on data collected at baseline (baseline) and at each of the three doses of ACh. This analysis was performed for data collected from the ACh and ACh + l-NAME sites. The contribution of NO to %CVC responses was also calculated by subtracting ACh + l-NAME %CVC data from ACh alone %CVC data at equivalent time points. Student's t test for unpaired data was also performed on area under the dose–response curves, calculated for each individual. Significance was set at the 5% probability level. Results were recorded as mean values ± standard error of the mean.
Results
Subject characteristics for the cross-sectional and longitudinal exercise training designs are presented in Tables 1 and 2, respectively. Regarding the training study, 1633 of the total number of 1728 training sessions were attended (94.5%) and subjects were closely monitored such that they maintained their prescribed HR during the supervised sessions.
Table 2.
Changes in characteristics with training in older sedentary individuals
| Entry | 12 weeks | 24 weeks | ||||
|---|---|---|---|---|---|---|
| LH (n = 8) | ACh (n = 8) | LH (n = 8) | ACh (n = 8) | LH (n = 8) | ACh (n = 8) | |
| Body weight (kg) | 88±7 | 82±4 | 88±7 | 81±4 | 87±7 | 78±3* |
| BMI | 30±2 | 30±2 | 30±2 | 29±1 | 29±1 | 28±1* |
| DEXA | ||||||
| Fat mass (kg) | 29±2 | 29±3 | 29±2 | 28±3 | 28±2 | 26±3* |
| Lean body mass (kg) | 57±5 | 51±4 | 57±5 | 51±4 | 57±6 | 51±3 |
| % body fat | 33±2 | 35±4 | 33 + 2 | 34±3* | 32±2 | 32±3‡ |
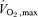 (ml kg−1 min−1) (ml kg−1 min−1) |
26±2 | 27±3 | 28±1* | 29±2* | 30±2†‡ | 34±3†‡ |
| Resting blood pressure (mmHg) | ||||||
| Systolic | 130±6 | 121±6 | 121±4 | 118±5 | 120±6 | 118±4 |
| Diastolic | 72±2 | 69±2 | 68±2 | 66±2 | 69±3 | 64±3 |
Values are means ± s.e.m.
P < 0.05 versus Entry,
P < 0.05 versus 12 weeks,
P < 0.01 versus Entry. LH, local heating protocol; ACh, acetylcholine protocol.
Comparisons between young (Y), old sedentary (OS) and older fit (OF) subjects
Physiological responses: effect of NO inhibition on %CVCmax responses to heating
Following the onset of local heating, %CVCmax rose steadily and significantly in all groups at both the microdialysis site perfused with Ringer solution and the microdialysis site perfused with l-NAME (P < 0.001, 1-way ANOVA) and peak measures differed significantly from those at baseline in all groups (P < 0.001, paired t tests all groups) at both sites (Fig. 2). l-NAME significantly decreased %CVC responses to heating in all groups (P < 0.001). These differences were in fact highly significant (P < 0.001) and remained so after correction for multiple comparisons (Bonferroni).
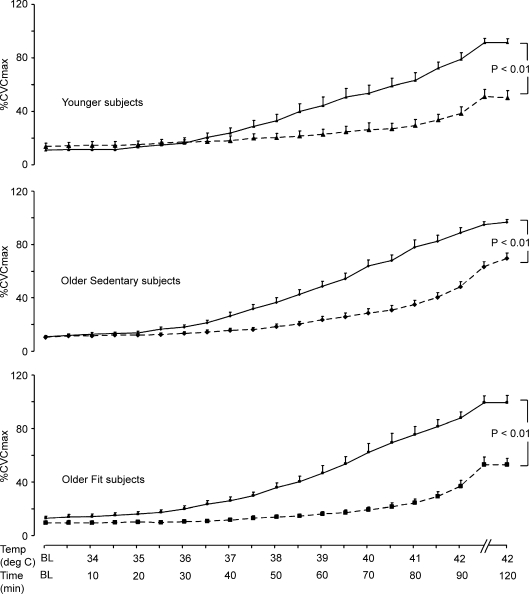
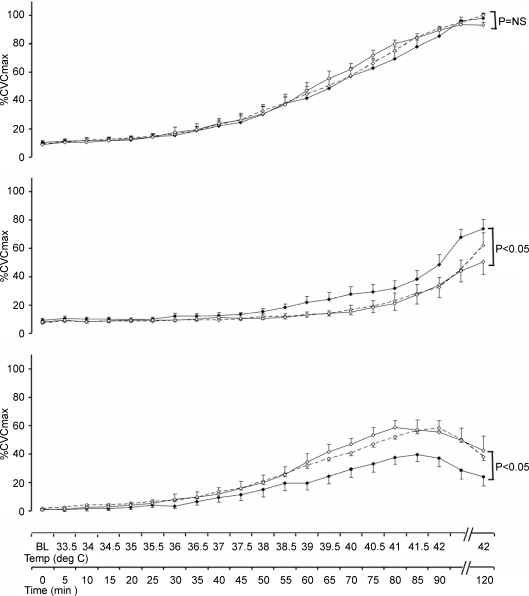
Figure 2. Time course of response to heating in the presence and absence of nitric oxide blockade.
Cutaneous vascular conductance, normalized to intraindividual maximal flow responses (%CVCmax), in the Ringer solution (continuous lines) and l-NAME (dashed lines) infusion sites in young (upper panel ▴), older sedentary (middle panel ♦) and older fit subjects (lower panel ▪) in response to incremental heating to 42°C (0–90 min) and prolonged local heating at 42°C (90–120 min). At both the Ringer solution and l-NAME sites, data did not differ prior to local heating, but rose steadily and significantly in all groups at the end of the incremental and prolonged phases (P < 0.001, all groups). Significant differences existed between the Ringer solution and l-NAME sites for all groups by 2-way ANOVA (P < 0.001).
To compare the impact of l-NAME on heating responses between groups, the contribution of NO to SkBF responses was calculated for each subject by subtracting l-NAME %CVC data from Ringer solution %CVC data at baseline, in response to peak heating at 42°C and prolonged heating (30 min) at 42°C (Fig. 3A). Analysis of these data revealed significant time by group interactions between Y and OS subjects (P < 0.005), and between OS and OF subjects (P < 0.05), when baseline, peak and prolonged heating data points were compared. There was also a main effect for group difference between the OS and OF subjects (P < 0.05). Conversely, OF and Y groups did not differ. These differences between OS and OF subjects in the NO contribution to the heating responses were not the product of differences in the Ringer solution site responses (see Fig. 2), since both groups achieved similar %CVCmax at the end of the protocol.
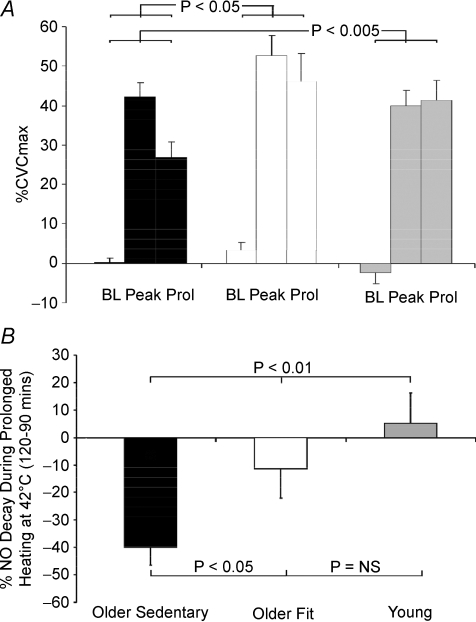
Figure 3. Comparison of responses at baseline, peak and prolonged heating.
A, NO contribution data at baseline, peak heating (42°C) and prolonged heating (30 min at 42°C) for each of the three subjects groups. Contribution of NO to SkBF responses in young, older sedentary and older fit subjects was calculated for each subject at each data point by subtracting l-NAME %CVC data from Ringer solution %CVC data at the same time point and skin temperature. A significant difference (2-way ANOVA) was evident between the OF and OS groups (P < 0.05) and the OS and Y groups (P < 0.005), whereas no difference existed between the OF and Y groups. B, the data from panel A presented as the change in NO contribution between the attainment of peak heating (90 min) and following 30 min prolonged heating at 42°C. The NO contribution to %CVC at 42°C declined significantly in the OS group (P < 0.001), but not in either the OF or the Y groups and significant differences existed between the groups (OS versus OF, P < 0.05; OS versus Y, P < 0.01; OF versus Y, NS).
Notably, the impact of 30 min of continuous heating at 42°C significantly differed between groups, with a large decrease from NO contribution at peak heating evident in the OS group relative to the Y (P < 0.01) and OF (P < 0.05) groups (Fig. 3B). There was no significant decline in the NO contribution during prolonged heating at 42°C in the Y subjects, or between the Y and OF groups. Indeed, the differences between the groups in terms of NO contribution described above appear to relate principally to the prolonged heating data, since the NO contribution declined in the OS subjects, but was preserved in Y subjects and intermediate in the OF group (Fig. 3B).
Pharmacological responses: effect of NO inhibition on %CVCmax responses to acetylcholine
Significant increases in %CVC were evident in response to each dose of ACh (P < 0.001, Fig. 4A) in all three groups. The change in %CVC in response to ACh was greater in the OF and Y, compared to the OS subjects (P < 0.05; Fig. 4A). l-NAME significantly attenuated %CVC at the low (P < 0.05), moderate (P < 0.001) and high (P < 0.001) ACh doses in all groups, but to a similar degree between groups (Fig. 4B). The contribution of NO to Ach-mediated vasodilatation was impaired in the OS compared to Y (P < 0.05) and OF subjects (P < 0.05) (Fig. 4C). It should be noted that Miochol-E, the formulation of ACh we used in the present experiment, contains a substantial amount of mannitol and we cannot exclude the possibility that this may have contributed to the vasodilator response. Nonetheless, the l-NAME effect is specific for NO contribution to ACh responses, whether the NO stimulus is derived from mannitol or ACh per se.
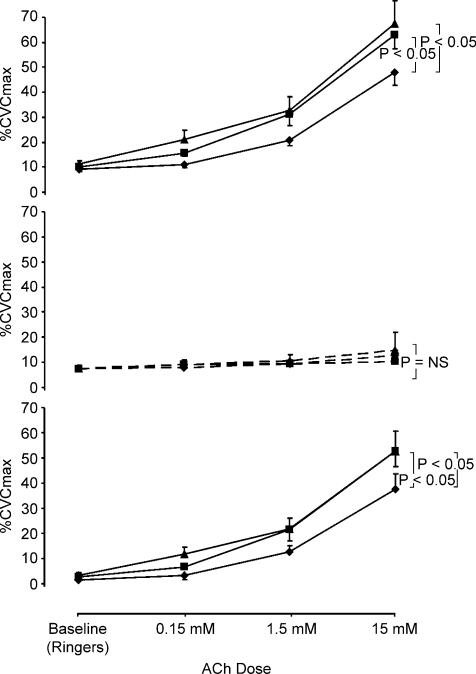
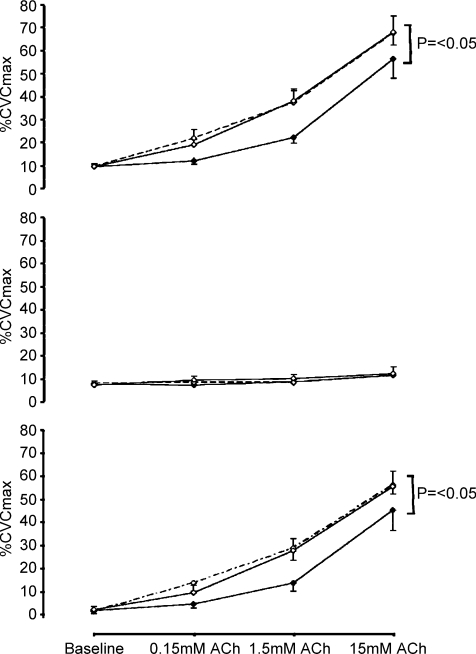
Figure 4. Dose–response curves to acetylcholine in the absence and presence of nitric oxide blockade.
Cutaneous vascular conductance, normalized to intraindividual maximal flow responses (%CVCmax), in the acetylcholine infusion site (upper panel; continuous lines) and simultaneously infused site which received simultaneous and identical ACh doses in the presence of l-NAME (middle panel; dashed lines). Older sedentary (♦), older fit (▪) and young subjects (▴) are represented in each panel. ACh infusion increased %CVC responses significantly more in the OF and Y (P < 0.05) than in the OS group. The lower composite panel reflects the contribution of NO to skin blood flow responses, calculated for each subject at each data point by subtracting l-NAME %CVC data from Ringer solution %CVC data at the same time-point. The NO contribution to ACh was greater in both the OF and Y (P < 0.05) than the OS group.
Effect of exercise training in older sedentary (OS) subjects
Subjects who participated in the physiological heating assessments showed no change between baseline, 12 and 24 week exercise training measures of systolic blood pressure, body mass, BMI, DEXA fat mass, DEXA lean mass, or DEXA percentage body fat (Table 2). However 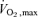 significantly increased at 24 weeks compared to baseline (P < 0.01) and 12 weeks (P < 0.05).
significantly increased at 24 weeks compared to baseline (P < 0.01) and 12 weeks (P < 0.05).
Similarly, those individuals randomised to participate in the ACh pharmacological studies showed no change between baseline, 12 and 24 week exercise training measures of blood pressure or DEXA lean mass. Decreases in body mass (P < 0.05), BMI (P < 0.05), DEXA fat mass (P < 0.05) and percentage body fat (P < 0.01) were observed at 24 weeks, and percentage body fat differed from baseline at 12 weeks (P < 0.05). Significant increases in 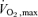 values were demonstrated at 24 weeks compared to both baseline (P < 0.01) and 12 weeks (P < 0.05).
values were demonstrated at 24 weeks compared to both baseline (P < 0.01) and 12 weeks (P < 0.05).
Effects of training on responses to heating and acetylcholine
Exercise training in the OS subjects enhanced NO-vasodilator function during local heating at 12 weeks and 24 weeks (2-way ANOVA, baseline versus 12 versus 24 weeks for heating points from 40 to 42°C, P < 0.05; Fig. 5C). Further analysis indicated significant differences between baseline and 12 week data for these peak heating time points (P < 0.05, 2-way ANOVA), which remained significant after Bonferoni corrections were performed. Specifically, differences were evident between baseline and 12 week data at 40.5°C (33.2 ± 5.8 versus 46.9 ± 3.1%CVCmax; P = 0.05), 41°C (37.6 ± 5.1 versus 52.3 ± 3.4%CVCmax; P < 0.05), 41.5°C (39.6 ± 4.9 versus 56.1 ± 5.3%CVCmax; P < 0.05) and 42°C (37.1 ± 6.0 versus 58.1 ± 6.2%CVCmax; P < 0.05). Similarly, baseline and 24 week heating data differed significantly (2-way ANOVA, for heating points from 40 to 42°C, P < 0.05) after Bonferoni correction. Differences were evident at 40°C (29.2 ± 5.7 versus 46.9 ± 4.1%CVCmax; P < 0.05), 40.5°C (33.2 ± 5.8 versus 53.6 ± 4.9%CVCmax; P < 0.05) and 41°C (37.6 ± 5.1 versus 58.7 ± 4.7%CVCmax; P < 0.05). No differences existed between data at 12 and 24 weeks. The significant differences that existed between OS, OF and Y subjects at baseline, were abolished by exercise training.
Figure 5. Effects of exercise training on heating responses: Impact of nitric oxide blockade.
The impact of 12 (◊ dashed lines) and 24 weeks (◊ continuous line) of exercise training in older sedentary subjects, compared to data collected at entry to the study (♦ continuous line), on the NO contribution to %CVC responses (lower panel), calculated for each subject at each data point by subtracting l-NAME %CVC data (middle panel) from Ringer solution %CVC data (upper panel) at the same time points and skin temperatures. Whilst training did not modify %CVC at the Ringer solution site, the impact of l-NAME and the contribution of NO to %CVC responses were significantly greater after training (P < 0.05) at both 12 and 24 weeks. This abolished the initial difference that existed between the old sedentary and older fit subjects (see Fig. 2).
Exercise training in the OS subjects enhanced the NO component of ACh-mediated vasodilator function at 12 and 24 weeks, relative to entry data (P < 0.05, 2-way ANOVA baseline versus 12 and 24 weeks). No differences were evident between the 12 and 24 week data (Fig. 6C). Further analysis indicated significant differences between baseline and 12 week data (P < 0.05, 2-way ANOVA). Post hoc t tests indicated differences predominantly at the mid dose (low dose 4.4 ± 1.6 versus 13.7 ± 3.6, P = 0.07; mid dose 13.5 ± 3.2 versus 28.9 ± 5.3, P < 0.05, high dose 45.1 ± 8.9 versus 56.1 ± 3.9, P = 0.2). The area under the dose–response curve reinforced this finding, with differences evident between baseline and 12 weeks (P < 0.05). Baseline and 24 week training data differed at the mid dose of ACh (27.6 ± 5.4, P < 0.05), but did not achieve significance at the low (9.6 ± 3.1, P = 0.08) or highest doses (55.5 ± 6.8%, P = 0.3). Whereas significant differences existed between OS and OF subjects (P < 0.05), and OS and Y subjects (P < 0.05) at baseline, exercise training in the OS group abolished these differences between subjects.
Figure 6. Effects of exercise training on acetylcholine responses: impact of nitric oxide blockade.
The impact of 12 (◊ dashed lines) and 24 weeks (◊ continuous line) of exercise training in older sedentary subjects, compared to data collected at entry to the study (♦ continuous line), on the NO contribution to %CVC responses (lower panel), calculated for each subject at each data point by subtracting l-NAME %CVC data (middle panel) from Ringer solution %CVC data (upper panel) at equivalent doses of ACh. Training significantly increased %CVC in response to ACh at both 12 and 24 weeks (P < 0.05, 2-way ANOVA baseline versus 12 versus 24 weeks) and the contribution of NO to the ACh response was significantly greater after training (P < 0.05). This abolished the initial difference that existed between the old sedentary and older fit subjects (see Fig. 4).
In the cohort of subjects who did not undertake exercise training, but acted as longitudinal time controls, there were no significant differences in the NO contribution to %CVC responses to local heating (peak values at 42°C pre: 41.2 ± 8.8 versus post: 37.8 ± 7.5%CVCmax; prolonged heating at 42°C: pre: 29.6 ± 11.2 versus post: 28.1 ± 9.7%CVC). Similarly no differences existed between the NO contribution to ACh responses at the low (pre: −1.7 ± 3.6 versus post: 0.7 ± 2.4%CVCmax, NS), intermediate (pre: 3.6 ± 1.2 versus post: 3.1 ± 1.7%CVC, NS) or high (pre: 14.7 ± 6.4 versus post: 15.5 ± 2.7%CVC, NS) doses.
Maximal cutaneous vascular responses
No significant differences were observed following 30 min of 56 mm SNP infusion combined with local heating to 42°C, between the control and l-NAME sites, within each group (Y 3.3 ± 0.1 versus 3.0 ± 0.2, OS 3.0 ± 0.2 versus 2.7 ± 0.2, OF 3.0 ± 0.2 versus 2.9 ± 0.1 units, NS for all comparisons, paired t tests). Furthermore no differences were observed between groups, or within OS subjects, following exercise training, in response to either local heating (Baseline: 2.6 ± 0.4, 12 weeks: 2.5 ± 0.3, 24 weeks: 2.5 ± 0.3 units, P = 0.8) or ACh infusion (Baseline: 3.1 ± 0.4, 12 weeks: 3.0 ± 0.2, 24 weeks: 2.6 ± 0.2 units, P = 0.2). These data indicate that the differences we observed in %CVC data are not due to differences in the maximal CVC data between groups or after training. The similarity between maximal CVC responses elicited between groups and before and after training also suggest that differences we observed in the NO contribution to heating and ACh reflect changes in the function of the microvasculature, without underlying changes in vasodilator capacity.
Discussion
The present study indicates that the contribution of NO to both heating- and ACh-mediated vasodilatation is impaired in the older sedentary subjects relative to older fit and younger individuals. Furthermore, exercise training in unfit older subjects normalized both physiological and pharmacological NO-mediated microvascular function. These results strongly suggest that exercise prevents age-related decline in microvascular NO-mediated vasodilator function in healthy humans.
It is well established that NO bioavailability in conduit and resistance vessels can be enhanced by exercise training, particularly in populations who initially exhibit impaired NO function (Green et al. 2004). The findings of the current study extend previous observations that exercise retards age-related endothelial dysfunction in large arteries (DeSouza et al. 2000; Taddei et al. 2000) to the microvasculature, since both cross-sectional and longitudinal training data indicated that exercise ameliorated age effects on both physiological and pharmacological NO-mediated microvessel function. Furthermore, our data indicate that exercise training enhances microvascular function within 12 weeks, with little additional adaptation present following a further period of training.
Whilst previous studies have concluded that SkBF in trained subjects is higher at any given level of internal temperature than in sedentary or less trained individuals (Johnson, 1998), and that exercise training improves skin blood flow in exercising older men (Thomas et al. 1999), studies of the impact of training on NO-mediated microvascular responses to local heating are scant and have produced conflicting findings (Franzoni et al. 2004; Colberg et al. 2005). These studies used plasma or interstitial measures of NO, but did not block NO production using an antagonist. The present study is the first to specifically quantify the NO contribution to impaired microvascular function using both cross-sectional and longitudinal exercise comparisons and our data are internally consistent in that both fitness and training reversed age related NO-mediated microvascular impairment. Of interest, we did not observe impaired responses to heating stimuli at the Ringer solution control sites among Y, OS and OF subjects (Fig. 2). This is in contrast with some (Richardson, 1989; Weiss et al. 1992; Evans et al. 1993; Rooke et al. 1994; Martin et al. 1995; Minson et al. 2002; Franzoni et al. 2004), but not all (Munce & Kenney, 2003; Colberg et al. 2005), previous studies regarding the impact of age and training on local heating induced skin blood flow responses. Nonetheless, our blockade studies clearly indicate that the contribution of NO to the local heating response did significantly differ between groups (Fig. 3A). In particular, NO function differed as the peak local heating stimulus was prolonged (Fig. 3B). Since the heating response was maintained in older sedentary subjects, despite a clear decline in the NO contribution to this response, we suggest the existence of compensatory mechanisms which preserve heat-induced vasodilator responses in the face of impaired NO microvascular function with age. The presence of redundancy in vasomotor control is well established in other arterial beds (Joyner & Wilkins, 2007). We speculate that redundancy amongst the many vasoactive pathways in the skin may subserve a thermoregulatory role, in that protection may be afforded where one or more pathways are impaired. It is possible that in an older cohort of subjects than those studied here, redundant or compensatory mechanisms may not be as effective and this may explain some previous findings of impaired skin blood flow responses in elderly subjects (Richardson, 1989; Weiss et al. 1992; Evans et al. 1993; Rooke et al. 1994; Martin et al. 1995; Minson et al. 2002).
Previous studies which have assessed the impact of exercise on ACh responses have all relied upon plasma measures of NO (Wang, 2005) or cross-sectional comparisons between fit and unfit individuals (Kvernmo et al. 1998; Boegli et al. 2003; Lenasi & Strucl, 2004). All have utilized iontophoresis of acetylcholine (Kvernmo et al. 1998; Boegli et al. 2003; Lenasi & Strucl, 2004; Wang, 2005), an approach with well established limitations compared to microdialysis delivery (Cracowski et al. 2006). Our findings, utilizing specific NO blockade in the presence of ACh dose–response curves, indicate that fitness and exercise training enhance the NO component of skin blood flow responses to ACh.
A noteworthy observation relating to our pharmacological experiments involves the degree to which l-NAME blocked the ACh response. At the highest dose of ACh we used, the average degree of blockade with l-NAME was around 75 ± 4%. This compared to 34 ± 6% and 58 ± 4% with the low and intermediate doses. It has recently been suggested that NO plays a limited role in the mediation of cutaneous vasodilatation to ACh, with perhaps as little as 30% contribution to the overall response (Boutsiouki et al. 2004; Holowatz et al. 2005; Kellogg et al. 2005). The magnitude of this contribution of NO to ACh responses in the skin approximates that ascribed to NO in the forearm following intrabrachial ACh infusion (∼50%) (Cockcroft et al. 1994; Newby et al. 1997). However, a recent study undertaken by Stewart et al. (2007) demonstrated a similar magnitude of effect of NOS inhibition to that observed in the current study. It seems likely that differences in doses of ACh and NO blockade might explain the disparity between reports regarding the NO dependency of ACh responses in the skin. Our dose–response curve data do suggest that differences exist in the proportional contribution of NO to ACh-mediated dilatation as ACh doses increase. Further studies may be required to specifically address this issue, but it is generally accepted that a large part of the ACh response is NO dependent, whilst other mediators such as prostanoids, endothelium derived hyperpolarizing factor (EDHF), and sensory nerves may also contribute (Boutsiouki et al. 2004; Holowatz et al. 2005; Kellogg et al. 2005; Stewart et al. 2007).
The mechanisms responsible for impaired microvascular NO-mediated dilatation, and training-mediated improvement in this pathway, remain unclear, although several explanations exist. Some previous evidence suggests that supplementation with the NO precursor, l-arginine, can reverse age-related NO dysfunction or that arginase might impair NO bioavailability in aged skin microvessels (Holowatz et al. 2006b). Recent evidence published in this journal suggests that, in rat skeletal muscle arterioles, cofactors for NO such as tetrahydrobiopterin (BH4) play a key role in the age-related decrease in NO microvascular function (Delp et al. 2008; Heffernan et al. 2008) and there are some previous data suggesting that BH4 supplementation restores human conduit vessel flow-mediated dilatation in older individuals (Eskurza et al. 2005). Our novel finding of exhaustion of NO function on prolonged exposure to a heating stimulus might be taken as supporting an explanation involving substrate or cofactor depletion. An alternative, though not mutually exclusive, explanation relates to age-related increase in oxidative stress. Studies have suggested that administration of antioxidants reverses age-related impairment in endothelial function, indicating that enhanced NO ‘quenching’ might be responsible for decreased bioavailability with age and that exercise prevents NO inactivation (Eskurza et al. 2004; Holowatz et al. 2006a). Whilst acute exercise may represent a pro-oxidant stimulus, exercise training can reduce the production of radical species (Leeuwenburgh & Heinecke, 2001) and enhance antioxidant defences (Sen, 1995), which may explain the preserved NO function in the fit and trained older subjects we studied. Furthermore, it is possible that increases in NO production may also have occurred through up-regulated endothelial cell gene expression in response to repeated exposure of shear and/or circumferential wall stress with each exercise training session. In any case, our observation that both heating-induced and ACh-mediated NO function were impaired in older sedentary subjects, and that both were enhanced by exercise and fitness, indicates that non-specific or generalized effects on NO-mediated microvascular function are apparent.
The strengths of this study include its combination of cross-sectional and longitudinal study designs, the use of the direct microdialysis infusion technique, the assessment of NO contribution to SkBF responses using specific NO blockade and the novel examination of both physiological and pharmacological NO-mediated pathways. However, there are also several limitations. As intended, the older fit subjects we recruited possessed a significantly higher level of cardiovascular fitness than their sedentary counterparts and were well matched for age and sex. Predictably, however, the sedentary subjects exhibited significantly higher BMI, body weight and percentage body fat than the fit cohort (Table 1). However, the possibility that the differences we observed between fit and sedentary subjects in terms of the NO contribution to SkBF are attributable to the difference in fat mass, rather than cardiopulmonary fitness, is diminished by the observation of enhanced NO function following exercise training in the unfit group. Similarly, baseline differences in BP between groups are unlikely to explain the effect of training on NO function. In addition, we performed correlations between adiposity (DEXA fat mass) and the highest dose of ACh and also the peak heating responses between the young and old subjects. Similar analysis was performed between systolic and diastolic BP and these key outcome variables. There were no significant correlations between subjects or for changes in body fat, SBP or DBP and ACh (r = −0.131, P = 0.419; r = −0.125, P = 0.441; r = −0.12, P = 0.462) or peak heating responses (r = 0.002, P = 0.988; r = −0.180, P = 0.254; r = −0.202, P = 0.199) between subjects. These findings enhance confidence that the differences we observed are not due to exercise-related differences or changes in body fatness or BP. Finally, as stated in the Methods, subjects were specifically questioned about any diagnoses of hypercholesterolaemia, hypertension, insulin resistance syndrome and diabetes and no subject was taking any medications. In addition, all subjects were asymptomatic and healthy and completed a 12-lead ECG stress test to maximal exertion on entry to the study under clinical supervision. We are therefore confident that these potential confounding influences on vascular function did not impact substantively on the results of the present study. Nonetheless, we acknowledge that there may have been occult or subclinical differences in lipid or blood glucose levels between the older groups we recruited.
In summary, we observed decreased NO-mediated vasodilator function in older sedentary, but healthy, volunteers compared to younger subjects and age-matched older individuals who maintained a high level of cardiopulmonary fitness. Exercise training in older sedentary subjects reverses this impairment in microvascular NO function in vivo. These findings indicate that the age-related decline in microvascular NO-mediated vasodilator function can be ameliorated by exercise. Since higher levels of NO confer antiatherogenic benefit, this study has potentially important implications for the prevention of microvascular dysfunction in humans.
Acknowledgments
We thank Dr Stefanie Bracknell for medical supervision during all 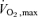 treadmill tests and for supervising Prof Green. This study was supported by a grant from the British Heart Foundation (FS/05/117/19971).
treadmill tests and for supervising Prof Green. This study was supported by a grant from the British Heart Foundation (FS/05/117/19971).
References
- Boegli Y, Gremion G, Golay S, Kubli S, Liaudet L, Leyvraz PF, Waeber B, Feihl F. Endurance training enhances vasodilation induced by nitric oxide in human skin. J Invest Dermatol. 2003;121:1197–1204. doi: 10.1046/j.1523-1747.2003.12518.x. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am College Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension. 1992;20:349–355. doi: 10.1161/01.hyp.20.3.349. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Chowienczyk PJ, Brett SE, Ritter JM. Effect of NG-monomethyl-L-arginine on kinin-induced vasodilation in the human forearm. Br J Clin Pharmacol. 1994;38:307–310. doi: 10.1111/j.1365-2125.1994.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg SR, Parson HK, Nunnold T, Holton DR, Swain DP, Vinik AI. Change in cutaneous perfusion following 10 weeks of aerobic training in Type 2 diabetes. J Diabetes Complications. 2005;19:276–283. doi: 10.1016/j.jdiacomp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger C, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores the age-related decile in endothelium-dependent vasodilation. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Rendell M, Bartek J, Connor S, Bamisedun O, Dovgan D, Giitter M. Thermally-induced cutaneous vasodilatation in aging. J Gerontol. 1993;48:M53–M57. doi: 10.1093/geronj/48.2.m53. [DOI] [PubMed] [Google Scholar]
- Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quinones-Galvan A. Effects of age and physical fitness on microcirculatory function. Clin Sci (Lond) 2004;106:329–335. doi: 10.1042/CS20030229. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana AJ, O'Driscoll G, Taylor R. Effects of exercise training on vascular endothelial nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008 doi: 10.1152/japplphysiol.01028.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan KS, Vieira VJ, Valentine RJ. Microvascular function and ageing: L-arginine, tetrahydrobiopterin and the search for the fountain of vascular youth. J Physiol. 2008;586:2041–2042. doi: 10.1113/jphysiol.2008.151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol. 2006a;291:H2965–H2970. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006b;574:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev. 2007a;35:119–125. doi: 10.1097/jes.0b013e3180a02f85. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. The cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2007b doi: 10.1152/japplphysiol.00858.2007. in press. [DOI] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM. Physical training and the control of skin blood flow. Med Sci Sports Exerc. 1998;30:382–386. doi: 10.1097/00005768-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol. 2007;583:855–860. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol. 2008;586:847–857. doi: 10.1113/jphysiol.2007.144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vasc Med. 1999;4:233–238. doi: 10.1177/1358836X9900400405. [DOI] [PubMed] [Google Scholar]
- Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008 doi: 10.1042/CS20070431. in press. [DOI] [PubMed] [Google Scholar]
- Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol Occup Physiol. 1998;79:30–36. doi: 10.1007/s004210050469. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8:829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- Lenasi H, Strucl M. Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc. 2004;36:606–612. doi: 10.1249/01.mss.0000121948.86377.51. [DOI] [PubMed] [Google Scholar]
- Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol. 1995;79:297–301. doi: 10.1152/jappl.1995.79.1.297. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Munce TA, Kenney WL. Age-specific modification of local cutaneous vasodilation by capsaicin-sensitive primary afferents. J Appl Physiol. 2003;95:1016–1024. doi: 10.1152/japplphysiol.00934.2002. [DOI] [PubMed] [Google Scholar]
- Newby DE, Boon NA, Webb DJ. Comparison of forearm vasodilatation to substance P and acetylcholine: contribution of nitric oxide. Clin Sci. 1997;92:133–138. doi: 10.1042/cs0920133. [DOI] [PubMed] [Google Scholar]
- Richardson D. Effects of age on cutaneous circulatory response to direct heat on the forearm. J Gerontol. 1989;44:M189–M194. doi: 10.1093/geronj/44.6.m189. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- Rooke GA, Savage MV, Brengelmann GL. Maximal skin blood flow is decreased in elderly men. J Appl Physiol. 1994;77:11–14. doi: 10.1152/jappl.1994.77.1.11. [DOI] [PubMed] [Google Scholar]
- Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- Shamim-Uzzaman QA, Pfenninger D, Kehrer C, Chakrabarti A, Kacirotti N, Rubenfire M, Brook R, Rajagopalan S. Altered cutaneous microvascular responses to reactive hyperaemia in coronary artery disease: a comparative study with conduit vessel responses. Clin Sci (Lond) 2002;103:267–273. doi: 10.1042/cs1030267. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;292:E314–E318. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H2161–H2167. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Pierzga JM, Kenney WL. Aerobic training and cutaneous vasodilation in young and older men. J Appl Physiol. 1999;86:1676–1686. doi: 10.1152/jappl.1999.86.5.1676. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? (Editorial) Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Wang JS. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. Eur J Appl Physiol. 2005;93:429–434. doi: 10.1007/s00421-004-1176-4. [DOI] [PubMed] [Google Scholar]
- Weiss M, Milman B, Rosen B, Eisenstein Z, Zimlichman R. Analysis of the diminished skin perfusion in elderly people by laser Doppler flowmetry. Age Ageing. 1992;21:237–241. doi: 10.1093/ageing/21.4.237. [DOI] [PubMed] [Google Scholar]