Abstract
Short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were evaluated in the masseter muscles of 12 subjects and the cortical silent period (SP) in nine subjects. Motor evoked potentials (MEPs) were recorded from contralateral (cMM) and ipsilateral (iMM) masseters, activated at 10% of maximal voluntary contraction (MVC). Interstimulus intervals (ISIs) were 2 and 3 ms for SICI, 10 and 15 ms for ICF. TMS of the left masseteric cortex induced MEPs that were larger in the cMM than the iMM; stimulation of right masseteric cortex produced a similar asymmetry in response amplitude. SICI was only observed using a CS intensity of 70% AMT and was equal in both cMM and iMM. SICI was stronger at higher TS intensities, was abolished by muscle activation greater than 10% MVC, and was unaffected by coil orientation changes. Control experiments confirmed that SICI was not contaminated by any inhibitory peripheral reflexes. However, ICF could not be obtained because it was masked by bilateral reflex depression of masseter EMG caused by auditory input from the coil discharge. The SP was bilateral and symmetric; its duration ranged from 35 to 70 ms depending on TS intensity and coil orientation. We conclude that SICI is present in the cortical representation of masseter muscles. The similarity of SICI in cMM and iMM suggests either that a single pool of inhibitory interneurons controls ipsi- and contralateral corticotrigeminal projections or that inhibition is directed to bilaterally projecting corticotrigeminal fibres. Finally, the corticotrigeminal projection seems to be weakly influenced by inhibitory interneurons mediating the cortical SP.
The masticatory motor system plays a number of different roles in mastication, speech, swallowing and respiration, all of which require jaw muscles to perform motor tasks that differ in force produced, as well as rapidity, shape and precision of mouth movements. The basic rhythmic output to jaw muscles for normal chewing originates in a central pattern generator localized in the brainstem reticular formation (Nakamura & Katakura, 1995), whose activity is modulated by volition and afferent feedback. Although the masticatory motor cortex is known to initiate and to have an important role in the fine control and coordination of jaw movements (Murray et al. 1991; Lin et al. 1993), there have been relatively few studies of its intrinsic physiology in humans.
TMS studies have shown that stimulation of the corticotrigeminal projection from one hemisphere induces short latency motor evoked potentials (MEPs) in masseter and digastric muscles of both sides (Gooden et al. 1999; Nordstrom et al. 1999; Butler et al. 2001) that are consistent with the existence of direct monosynaptic excitatory connections to trigeminal motoneurons. However, a number of outstanding questions remain. The first is the symmetry of the cortical output to the left and right masseters. Guggisberg et al. (2001) found that the responses were symmetrical in agreement with anatomical studies (Kuypers, 1958; Iwatsubo et al. 1990). In contrast, all other reports suggest that the cortical projections are not symmetrical, but predominantly contralateral (Cruccu et al. 1989; Carr et al. 1994; Nordstrom et al. 1999; Butler et al. 2001; McMillan et al. 2001; Pearce et al. 2003). In addition, it is unknown whether the differences relate to hemispheric dominance. A second question is whether or not it is possible to observe short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) in masseteric cortex. SICI and ICF are due to activity in inhibitory and facilitatory interneuronal systems in cortex and can be studied using a paired pulse TMS in a conditioning–test protocol (Kujirai et al. 1993; Chen et al. 2008). When the interval between a subthreshold conditioning stimulus (CS) and a suprathreshold test stimulus (TS) is 1–5 ms, the test MEP is inhibited while it is facilitated (ICF) at ISIs of 7–20 ms. SICI and ICF were described for the first time and characterized in the hand motor cortex (Kujirai et al. 1993; Chen et al. 2008). They have recently been studied in the digastric muscles (Jaberzadeh et al. 2007), but never in masseter. One reason for this is that MEPs in masseter are difficult if not impossible to obtain in subjects at rest and can only be observed during background contraction, which is a condition that markedly reduces or abolishes these phenomena (Ridding et al. 1995).
There are several differences in reflex and cortical control of trigeminal and spinal muscles (ref. in Luschei & Goldberg, 1981), and thus data from the corticospinal system may not be simply extrapolated to the corticotrigeminal system. For example, the silent period in ongoing EMG activity following a TMS stimulus is thought in the corticospinal system to reflect suppression of motor cortical output after the stimulus. A recent paper by Jaberzadeh et al. (2008) described the properties of masseter cortical silent period (SP) and because of its short duration suggested that inhibitory mechanisms may be weak in the masseteric motor cortex. Indeed, Sowman et al. (2008) recently suggested that the masseter silent period may be mainly due to suppression of masseter motoneurones in the trigeminal nucleus rather than at the cortex.
The aim of the present study therefore was to examine the controversy about the asymmetry of TMS-induced masseter MEPs and to clarify whether it could relate to hemispheric dominance. Second, we assessed whether SICI and ICF operate in the control of masseter muscles and whether they produce similar effects on corticobulbar neurons projecting to ipsilateral versus contralateral masseter motoneuron pools. Finally, the SP which follows masseter MEPs was evaluated using different coil orientations to assess whether its short duration is related to the direction of TMS-induced current.
Methods
Subjects
Twelve healthy volunteers (9 males and 3 females; mean age 30.8 ± 1.0 years; range 26–36 years) participated in the study. None of the subjects had a history of neurological diseases and all participants had complete natural dentition. Prior to the study all subjects gave their written informed consent and the procedure, approved by the local ethics committee, was in accordance with the ethical standards established in the Declaration of Helsinki. No side-effects were noted in any of the individuals tested.
Participants were seated on a comfortable chair with their head and neck supported. They were instructed to activate masseter muscles at 10% of maximal voluntary contraction (MVC). This contraction level had to be maintained steady during recordings with the aid of visual feedback of the rectified and filtered EMG activity of the left and right masseter muscles. Auditory feedback of muscle EMG activity was also provided. To avoid fatigue, there were short breaks during each experimental block. The EMG activity level was determined offline by analysing the rectified EMG in a time window of 50 ms preceding the stimulus.
EMG recordings
TMS-evoked motor potentials (MEPs) were recorded from the left and right active masseter muscles using 9 mm diameter Ag–AgCl surface cup electrodes. The reference electrode was placed at the mandibular angle and the active electrode over the muscle belly, 1–2 cm frontally and cranially to the reference electrode. This electrode placement was demonstrated to be the optimal to avoid cross-talk responses from facial muscles (Guggisberg et al. 2001). The ground electrode was placed over the forehead. In experiment 9, MEPs were recorded from the active right first dorsal interosseous muscle (FDI). The active electrode was placed over the muscle belly, the reference electrode over the metacarpophalangeal joint of the index finger and the ground electrode over the forearm. Unrectified and rectified EMG activities were recorded (Digitimer D360 amplifier, Digitimer Ltd, Welwyn Garden City, UK) from both masseter and right FDI. EMG signal was amplified (×1000), filtered (bandwidth 3–3000 Hz) and sampled (5 kHz per channel) from 50 ms before to 100 ms after stimulus delivery, using a 1401 plus A/D converter (Cambridge Electronic Design, Cambridge, UK) and Signal 3.06 software on a computer.
TMS stimulation
Transcranial magnetic stimulation was performed using a figure-of-eight shaped coil with external loop diameter of 9 cm connected to two Magstim 200 stimulators through a Y connector or Bistim module (Magstim Co., Whitland, Dyfed, UK). All the experiments of TMS of the masseteric motor cortex, unless specified, were performed with the coil held tangentially to the skull over the left hemisphere with the handle pointing forwards and laterally and rotated 120 deg away from the midline. Thus, the current induced in the brain, which had a posteromedial direction (PM), paralleled approximately the assumed line of the central sulcus. The optimal spot for masseter activation was carefully searched in each subject into an area 4–10 cm lateral to the vertex and 0–4 cm frontal to the bi-auricular line. These area and coil orientation were previously found to be optimal for TMS to elicit in the contralateral and ipsilateral masseter muscle the largest MEP with the lowest stimulation intensity (McMillan et al. 1998; Guggisberg et al. 2001). The optimal coil position able to evoke the largest and stable MEPs in both masseters was marked on the scalp to ensure identical placement of the coil throughout the experiments. The stimulus intensity was given as a percentage of maximum stimulator output (% MSO). The active motor threshold (AMT) was defined as the minimum stimulus intensity capable of inducing MEPs greater than 100 μV peak to peak amplitude in at least 5 out of 10 consecutive trials performed during isometric contraction of the tested muscle at 10% of MVC. Frequency of TMS stimulation was 0.2 Hz.
Experimental procedures
Experiment 1. Single pulse TMS to left and right masticatory motor cortex
This experiment was aimed at testing the asymmetry of corticobulbar projections to masseter muscles. Eight right-handed subjects participated. The degree of left hemispheric dominance was ascertained using the Edinburgh Handedness Inventory (Oldfield, 1971). Single pulse TMS was delivered to both hemispheres over the optimal spot searched as described above. For each side of the brain stimulated, 20 MEPs were recorded from both contralateral and ipsilateral masseter muscle (cMM and iMM, respectively). The intensity of stimulation delivered to the left hemisphere was adjusted to elicit a MEP of 0.5 mV peak to peak amplitude in active cMM. The same value of intensity was used to stimulate the right hemisphere. Contralateral MEP (cMEP) and ipsilateral MEP (iMEP) amplitudes were then evaluated.
Experiment 2. Paired pulse TMS to the left masseteric motor area: effects of different CS intensity
To evaluate short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) the paired pulse protocol described by Kujirai et al. (1993) was used. All 12 subjects participated in this experiment. The protocol consists of a subthreshold CS followed by a suprathreshold TS. TMS was delivered to the left hemisphere and four conditioning pulse intensities were used (60, 70, 80 and 90% of AMT). TS intensity was adjusted to elicit in the active right masseter a MEP of 0.5 mV peak to peak amplitude, which is smaller than that (1 mV) usually used in other muscles (Kujirai et al. 1993). This is why it was difficult to get a 1 mV MEP in masseters in some subjects unless delivering high intensity stimulation, which was regarded as unpleasant by them. The 2 and 3 ms interstimulus interval (ISIs) for SICI and the 10 and 15 ms ISIs for ICF were examined in a randomized order. For each conditioning pulse intensity, 60 trials were recorded (12 trials for each of the four ISIs and 12 trials for the unconditioned MEP). In six subjects, time course of paired pulse TMS was assessed delivering a CS of 70% of AMT and testing 14 ISIs (1, 2, 3, 4, 5, 6, 8, 10, 15, 20, 25, 30, 40 and 50 ms). Twelve responses for each ISI and 12 responses for the test stimulus given alone were collected and averaged. Mean amplitude of conditioned MEPs was expressed as a percentage of the average test MEP. Onset latency and peak latency of the test MEP were also evaluated in both the ipsilateral and the contralateral side. More precisely, the onset latency of the ipsilateral MEP was evaluated only when the onset of the negative deflection from the isoelectric line was clearly detectable, i.e. not contaminated by the stimulus artifact or by the root compound muscle action potential (rCMAP) elicited by the direct stimulation of the trigeminal nerve root.
Experiment 3. Paired pulse TMS to the left masseteric motor area: effects of different levels of masseter activation on SICI
To evaluate whether the level of background EMG activity influences inhibitory interneurons mediating SICI, the effects induced by three levels of masseter contraction (10, 25 and 50% of MVC, respectively) on SICI were evaluated in five subjects. For each level of contraction, the intensity of the test stimulus was adjusted to induce a test MEP of 0.5 mV peak to peak amplitude, the intensity of CS used was 70% and 90% of AMT, and the ISIs were 2 and 3 ms. In each experimental condition, 36 pulses, 12 pulses for each ISI and 12 pulses of the TS alone, were delivered in a randomized order. Only contralateral MEPs were evaluated and the mean level of the rectified averaged EMG was assessed in a 50 ms epoch preceding the stimulus. For the lowest level of contraction (10% MVC) the AMT was defined as the minimum stimulus intensity required to produce MEPs greater than 100 μV peak to peak in at least 5 out of 10 consecutive stimuli. For 25 and 50% MVC, AMT was defined as the minimum stimulus intensity able to produce in the contralateral masseter at least 5 out of 10 MEPs with peak to peak amplitude greater than the 95% confidence interval of the prestimulus mean EMG activity (Mills & Nithi, 1997). To avoid fatigue a break was allowed when needed.
Experiment 4. Paired pulse TMS to the left masseteric motor area: effects of different TS intensity on SICI
To evaluate how the size of the test MEP influences the amount of masseter SICI, two TS intensities inducing a test MEP of 0.5 mV and of 1 mV peak to peak amplitude, respectively, were tested in five subjects. In both experimental conditions the CS intensity was 70% of AMT and the level of bilateral muscle contraction was 10% of MVC. SICI was evaluated in the contralateral muscle and a total of 36 MEPs (12 test MEPs and 12 conditioned MEPs at the 2 and 3 ms ISIs) were recorded in a randomized order.
Experiment 5. Paired pulse TMS to the left masseteric motor area: effects of coil orientation on SICI
To test whether the amount of SICI is affected by TMS-induced brain current direction, two different coil orientations were used in the same five subjects enrolled in experiments 3 and 4. SICI obtained with the standard coil position used in all the experiments (handle pointing forwards and laterally and rotated 120 deg away from the midsagittal line, inducing a PM current in the brain approximately parallel to the assumed line of the central sulcus) was compared with that obtained with the coil positioned as follow: handle pointed backward and laterally, rotated 30 deg away from the midsagittal line to induce a current with anteromedial direction (AM) and perpendicular to the presumed direction of central sulcus (Sakai et al. 1997). TS intensity was adjusted to obtain a 0.5 mV test MEP in the contralateral masseter muscle activated at 10% MVC. CS intensity was 70% of AMT. In each experimental condition (PM and AM current direction), a total of 36 MEPs were collected (12 unconditioned MEPs and 12 conditioned MEPs for both 2 and 3 ms ISIs).
Experiment 6. TMS versus TES of the left masseteric motor cortex
To lend support to the hypothesis that the inhibition of the conditioned MEP observed at SICI intervals is due to an interaction of CS and TS at cortical level, the effects induced on SICI by different types of conditioning and test stimuli (magnetic versus electric stimulation) were compared in one subject. Transcranial electrical stimulation (TES) was given with a high voltage electric stimulator D180A (Digitimer) supplying a maximum output of 750 V and using a pulse width of 50 μV. Anodal stimulation was delivered through 9 mm diameter Ag–AgCl cup electrodes fixed to the scalp with collodion; the cathode was placed at the vertex, the anode over the masseter motor spot previously detected with the single pulse TMS. Rate of stimulation was less then one stimulus every 5 s. Three paired pulse blocks were performed, each consisting of 36 stimuli (12 pulses each for the 2 and 3 ms ISIs and 12 pulses of test alone) delivered in a randomized order. In the first block both CS (70% of AMT) and TS (intensity able to evoke a 0.5 mV MEP in the active contralateral masseter) were magnetic; in the second block CS was a magnetic pulse and TS was electric (the intensity of electric stimulation was adjusted to elicit a 0.5 mV MEP in the active contralateral masseter); in the third block CS was an electric stimulus whose intensity was set at 70% of the electric AMT and TS was a magnetic pulse. Electric AMT was defined as the minimum electric stimulus intensity required to produce MEPs of amplitude greater than 100 μV peak to peak in at least 3 out of 6 consecutive stimuli in the active right masseter (10% MVC).
Experiment 7. SMU recordings
This experiment was aimed at evaluating the effects of both single and paired pulse TMS on the ongoing activity of masseter single motor units (SMUs). Eleven SMUs were recorded from the right masseter of six subjects via disposable concentric needle electrodes (SLE Diagnostic, type B0400/02). Subjects were instructed to voluntarily activate a SMU at a firing rate around 10 Hz. Great care was taken to keep the same SMU during recording and to this aim an audio-visual feedback was provided. Transcranial magnetic stimulation was delivered to the left masticatory motor area at a frequency of about 0.2 ± 10% Hz. Two different conditions were randomly intermixed: the TS given alone, and a paired pulse stimulation consisting of a subthreshold CS (70% AMT) followed by TS at an ISI of 3 ms. The intensity of TS was set at a value able to elicit at least one clear peak in the online poststimulus time histogram (PSTH), constructed plotting the probability of firing per stimulus in the ordinate against the latency in abscissa. For each condition 100 trials were collected to construct PSTH. A peak of at least four or more counts in two adjacent time bins (bin width 0.2 ms) after 100 stimuli was considered significant following the criteria of Day et al. (1989).
Experiment 8. Silent period in the masseteric motor cortex
The aim of this experiment was to evaluate how intracortical inhibitory neurons mediating the masseter cortical silent period (SP) are influenced by current direction and stimulus intensity. The SP was studied in nine subjects. Twelve single pulses were delivered at a frequency of 0.2 Hz. A TS intensity able to induce a MEP of 0.5 mV peak to peak in the contralateral masseter and the usual coil orientation (120 deg) were used. The duration of SP was measured off-line from the onset of the MEP to the resumption of voluntary EMG activity. Responses of both contralateral and ipsilateral masseter muscles were analysed. In five subjects SP duration was evaluated bilaterally in three different conditions (coil 120 deg – MEP 0.5 mV; coil 120 deg – MEP 1 mV; coil 30 deg – MEP 0.5 mV) in which coil orientation or pulse intensity was changed.
Control experiments
Experiment 9. Paired pulse TMS to the left hand motor area
All 12 subjects participated in this experiment. Paired pulse TMS was delivered to the cortical representation of the right FDI. The 2 and 3 ms ISIs were used to explore SICI and the 10 and 15 ms ISIs were used to examine ICF. The intensity of CS was 70% of AMT while TS intensity was adjusted to elicit a MEP of 0.5 mV peak to peak amplitude in the right FDI activated at 10% MVC. The two coil orientations reported in experiment 5 were used. A total number of 60 trials for each coil position were recorded (12 conditioned MEPs for each of the four ISIs tested plus 12 unconditioned MEPs).
Experiment 10. Effects induced by repetitive TMS at CS intensity and by loud clicks on voluntary masseter EMG activity
To exclude whether a CS-induced peripheral inhibition of voluntary masseter muscle EMG could be responsible for the inhibition of the conditioned masseter MEP observed at ICF intervals, in six subjects a repetitive TMS (rTMS), consisting of 300 pulses at frequency 1 Hz with an intensity of 60% of AMT, was given to the left masseteric motor cortex (real stimulation) and 3 cm above the vertex (sham stimulation). The effects induced by rTMS in unrectified and rectified averaged masseter EMG were evaluated bilaterally measuring onset latency, peak latency and duration of the evoked responses.
To verify whether the inhibition of the conditioned MEP observed at ICF intervals could be due to a specific acoustic inhibition of masseter EMG activity induced by the noise sent out by the discharging coil, the time courses of masseter EMG responses to real and sham rTMS (see above) were compared to those of responses induced by loud clicks. To this aim three subjects underwent recording of the jaw acoustic reflex (Deriu et al. 2005, 2007). Click stimuli (0.1 ms duration, 70 dB NHL intensity, 3 Hz frequency, n = 500) were delivered bilaterally through TDH-49P earphones (Telephonics, Huntington, NY, USA) during voluntary activation of masseter muscles at 30% MVC. The recording electrode position was the same as that used in TMS experiments. EMG activity was amplified (×5000), filtered (0.3–2000 Hz) and sampled (5 kHz) from 50 ms before to 100 ms after stimulus delivery. Onset and peak latency as well as duration of the p16 wave were measured bilaterally from the unrectified averages. Amplitudes of masseter EMG responses to rTMS (real and sham) and to click stimulation were not measured and compared because of the different experimental protocol, type and intensity of stimulation.
Experiment 11. Blink reflex
This experiment was aimed at evaluating whether a CS intensity of 70% AMT was able to activate the cutaneous branches of the supraorbital nerve. Five subjects participated. The TMS-induced blink reflex was used as an index of trigeminal activation. EMG responses were recorded from the resting left and right orbicularis oculi muscles (left OO and right OO, respectively), using Ag–AgCl surface cup electrodes. The recording electrode was placed in the middle of the lower lid, and the reference electrode was placed at the lateral angle of the eye. Twenty single pulses were delivered at rate lower than one every 10 s at the three different sites of stimulation: (i) left supraorbital nerve, positioning the coil over the supraorbital notch, (ii) left masseteric motor cortex, and (iii) 3 cm above the vertex to evaluate whether the noise produced by the discharging coil triggered an acoustic blink reflex.
Experiment 12. Effects of supraorbital nerve stimulation on masseter MEP
The effects induced by magnetic stimulation of the supraorbital nerve on the size of TMS-induced masseteric MEPs were studied using a conditioning–test protocol through two different stimulating coils. There were five subjects. The conditioning stimulus (70% of AMT) was delivered through a focal coil that had the intersection of the two wings placed over the left supraorbital foramen and the handle pointing laterally. A second coil was placed over the spot of the left masseteric motor cortex, with the optimal orientation (120 deg), to elicit a 0.5 mV MEP in contralateral masseter muscle. The conditioned MEP amplitude at ISIs of 2 and 3 ms was compared with the amplitude of the test MEP.
Masseteric motor area localization by an image-guided TMS system
In one subject the position of masseteric motor cortex in the precentral gyrus surface was localized and compared with the well known position of the FDI cortical representation. In a preliminary session a brain magnetic resonance imaging (MRI) study was performed and the obtained data were transferred to a neuronavigational system (BrainSight Frameless system, Rogue Research Inc., Montreal, Canada). Landmarks on the subject's head were coregistered with landmarks on the structural MRI to allow tracking of the position of the TMS coil with respect to the underlying cortex through trackers attached to the TMS coil and to the subject. A figure-of-eight coil with the trackers was used to find, on the left hemisphere, both the optimal spot to evoke a MEP in both masseter muscles (120 deg coil orientation) and the optimal spot to elicit a MEP in the right FDI (30 deg coil orientation). These coordinates were projected and marked on the structural MRI. A tridimensional MRI reconstruction displaying the site of stimulation of the left precentral gyrus was then created.
Data analysis
Statistical analysis was performed with SPSS v. 13 software (SPSS Inc, Chicago, IL, USA). In the analysis performed with a repeated measures analyses of variance (ANOVA), compound symmetry was evaluated testing the sphericity with the Mauchly's test. The Greenhouse–Geisser correction was used to compensate for non-spherical data. In case of significant F-values, Student's paired t test was used for post hoc analysis applying the Bonferroni correction for multiple comparisons. A P-value < 0.05 was considered significant. Unless otherwise stated, values are expressed as means ± standard error of the mean.
In experiment 1 the asymmetry of corticobulbar projections (contralateral versus ipsilateral) was tested assessing the difference between the amplitudes of mean cMEP and iMEP elicited by stimulation of both hemispheres using Student's paired t test. Background prestimulus EMG activity was evaluated with two-way ANOVA comparing SIDE of stimulation (left and right hemisphere) and SITE of recording (iMM and cMM).
In experiment 2, latency differences between iMEP and cMEP and amplitude differences between conditioned and test MEP were evaluated. Onset latency, peak latency and mean amplitude of the MEP test and mean amplitude of the conditioned MEP at each ISI were measured in both the contra- and the ipsilateral sides. Onset and peak latencies of unconditioned MEPs recorded from both sides were compared using Student's paired t test. The effects of the differents CS intensities were analysed separately for the ipsilateral and contralateral masseter using two-way ANOVA with ISIs (2, 3, 10, 15 ms) and INTENSITY (60, 70, 80, 90% of AMT) as within subject factors. If a significant interaction was found, a two-way ANOVA was applied separately to analyse the effects of the four CS INTENSITIES on the SICI and ICF ISIs, respectively. Two-way ANOVA was applied to analyse the interaction between SIDEs (cMEP versus iMEP) and ISIs (2, 3, 10, 15 ms) only for the CS intensity of 70% AMT, which had been proved to be the most effective. Two-way ANOVA was also applied to test the effects of ISIs (Test, 1, 2, 3, 4, 5, 6, 8, 10, 15, 20, 25, 30, 40 and 50 ms) and SIDE of recording (cMEP versus iMEP) on the conditioned MEP time course.
In experiment 3 the effects of different levels of contraction on SICI were assessed with two-way ANOVA comparing three different LEVEL OF CONTRACTION (10, 25, 50% MVC) with ISIs (2 ms and 3 ms) for each of the two CS INTENSITIES used (70% and 90% AMT).
In experiments 4 and 5 the effects of TS intensity and coil orientation on SICI were tested with two-way ANOVA comparing, respectively, two TS INTENSITIES inducing a test MEP of 0.5 mV and 1 mV and two COIL ORIENTATIONS (120 deg and 30 deg) with ISIs (2 ms and 3 ms).
In experiment 5 the effect of coil orientation on the MEP onset latency was also evaluated with Student's paired t test.
In experiment 7 the effects of paired pulse TMS on the probability of firing of masseter SMU was evaluated. Onset latency, peak duration and number of counts in unconditioned and conditioned PSTH were compared separately using Student's paired t test.
In experiment 8 the duration of the masseter silent period was evaluated on both sides under different stimulus intensities and current directions. SP duration was compared between sides with Student's paired t test (n = 9 subjects). In five subjects two-way ANOVA was performed using SIDES (cSP versus iSP) and CONDITIONS (coil 120 deg – MEP 0.5 mV; coil 120 deg – MEP 1 mV; coil 30 deg – MEP 0.5 mV) as within subject factors.
In experiment 9 the amount of SICI and ICF observed in MM and FDI muscle with the same experimental conditions were compared. SICI and ICF in the FDI motor cortex were analysed with two-way ANOVA comparing ISIs (2, 3, 10, 15 ms) and COIL ORIENTATION (120 deg and 30 deg). A comparison among MUSCLES (FDI30deg, FDI120deg, cMM) and ISIs 2, 3, 10, 15 ms was then done using two-way ANOVA.
In experiment 10 the effects induced on the ongoing masseter EMG activity of both sides by real and sham subthreshold TMS and by loud click stimulation were compared. The mean prestimulus EMG activity was evaluated with repeated measures ANOVA comparing GROUPS (group 1 versus group 2), CONDITIONS (real TMS versus sham TMS) and SIDES (left MM versus right MM). In group 2, mean onset latency, mean peak latency and mean duration of the masseter EMG responses to both real and sham rTMS were compared with two-way ANOVA using CONDITIONS (real TMS versus sham TMS) and SIDES (left MM versus right MM) as within subject factors. The onset latency, peak latency and duration of the click-induced p16 wave were compared between sides using Student's paired t test. The difference among CONDITIONS (real TMS, sham TMS, click stimulation) was evaluated separately for the p16 onset latency, peak latency and duration by the one way ANOVA.
In experiment 12 the effects of supraorbital nerve stimulation on masseter MEPs were evaluated with two-way ANOVA using ISIs (2 and 3 ms) and SIDES (cMEP versus iMEP) as within subject factors.
Results
TMS of the masseteric motor cortex was followed by bilateral MEP in active masseter muscles. The contralateral response was observed in all 12 participants, the ipsilateral response in 11/12 subjects. The TS intensity used to elicit a MEP of 0.5 mV in the contralateral active masseter muscle was 58.5 ± 1.9% and the value of AMT was 38.2 ± 2.3% of the maximum stimulator output (MSO). The stimulus intensity used to evoke a 0.5 mV MEP test in the active FDI was 38.5 ± 2.4% and the AMT was 32.1 ± 1.9% of the MSO when the coil had the classical orientation used to stimulate the hand motor area (30 deg from the midsagittal line). With the coil orientation which was found to be optimal for the masseteric motor area (120 deg from midsagittal line), the MEP test in the active FDI was induced by a mean TS intensity of 49.2 ± 2.8% and the AMT was 43.9 ± 2.3% of the MSO.
Experiment 1. Single pulse TMS to left and right masticatory motor cortex
Masseter muscle responses evoked by TMS of the masticatory motor cortex were bilateral but asymmetric, with the contralateral response predominating. Single pulse TMS delivered to the left hemisphere of right handed subjects (mean laterality quotient equal to 87.3 ± 4.0) induced masseter MEPs whose amplitude was 0.506 ± 0.05 mV in the contralateral muscle and 0.330 ± 0.04 mV in the ipsilateral muscle. When TMS was applied to the right hemisphere using the same intensity of stimulation, the mean MEP amplitude was 0.463 ± 0.12 mV in the cMM and 0.293 ± 0.07 mV in the iMM. Student's paired t test showed a significant difference between the size of cMEP and iMEP evoked by left stimulation (P = 0.03) as well as by right stimulation (P = 0.02). By contrast, no differences were seen between the contralateral masseter responses and between the ipsilateral masseter responses to the stimulation of each hemisphere. Two-way ANOVA showed no significant side difference in the background EMG activity whose values were: 42.8 ± 2.8 μV (cMM) and 40.1 ± 4.3 μV (iMM) in the 50 ms preceding the left TMS, and 39.4 ± 5.4 μV (cMM) and 40.2 ± 3.5 μV (iMM) in the 50 ms preceding the right TMS.
Experiment 2. Paired pulse TMS to the left masseteric motor area: effects of different CS intensity
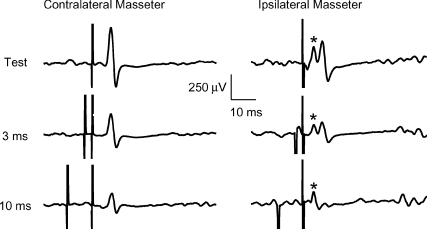
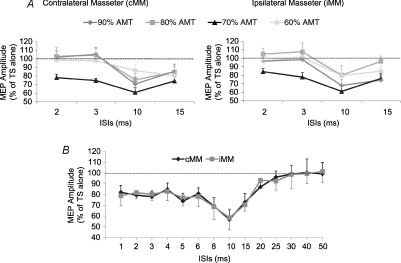
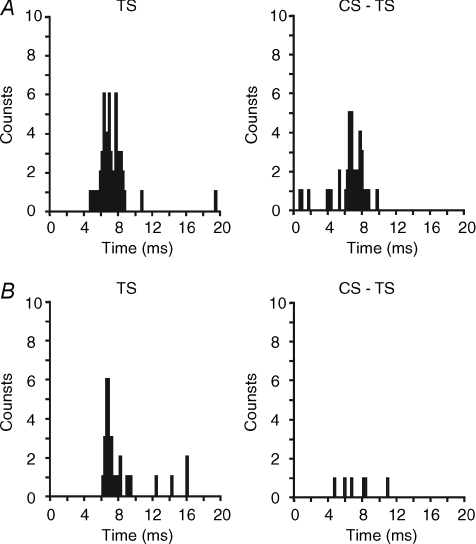
The onset latency of the contralateral MEP (6.0 ± 0.27 ms) was clearly detectable in all 12 subjects, while in the ipsilateral MEP it was measurable (5.9 ± 0.29 ms) only in 9/11 subjects because in two subjects it overlapped with the stimulus artifact and with the root compound muscle action potential (rCMAP) elicited by a direct activation of the ipsilateral trigeminal root at latency of 2.1 ± 0.02 ms (Fig. 1). Peak latency of the test MEP was 8.3 ± 0.26 ms for cMEP (12 subjects) and 8.2 ± 0.26 ms for iMEP (11 subjects). No significant differences were seen between the ipsilateral and contralateral side for latency onset (n = 9 subjects) and peak latency (n = 11 subjects). Results of the paired pulse protocol (Fig. 2A) showed that only with a CS of 70% AMT was the amplitude of the conditioned MEP significantly reduced in comparison with that of the unconditioned MEP at all ISIs tested, with a stronger effect at 10 and 15 ms ISIs. This finding was quite surprising because the 10 and 15 ms ISIs are those ISIs which provide evidence of ICF in all other cortical representations. In regard to the effects of different CS intensities on the contralateral and ipsilateral MEP, a two-factor ANOVA revealed an effect of ISIs (cMEP: F(1.8,19.8) = 12.1, P = 0.0005; iMEP: F(1.8,18) = 11.3, P = 0.0008) and a significant interaction between ISIs and CS INTENSITY (cMEP: F(12,132) = 2.7, P = 0.002; iMEP: F(12,120) = 2.3, P = 0.01). The two-way ANOVA performed only for SICI ISIs (2 and 3 ms) demonstrated a significant effect of CS INTENSITY for both cMM (F2.4,54.4 = 10, P = 0.0008) and iMM (F1.9,39.6 = 10.4, P < 0.0001). Post hoc analysis revealed significant differences among CS INTENSITIES both for cMM (70% versus 90%, P = 0.0003; 70% versus 80%, P = 0.0007; 70% versus 60%, P = 0.0001), and iMM (70% versus 90%, P = 0.02; 70% versus 80%, P = 0.004; 70% versus 60%, P = 0.003) (Fig. 2A). Two-way ANOVA on ICF ISIs (10 and 15 ms) showed a significant effect of CS INTENSITY on cMM (F3,69 = 4.3, P = 0.008) and iMM (F2,41.5 = 10, P = 0.0002). Also in this case the post hoc test provided evidence that the CS intensity of 70% AMT induced the strongest inhibitory effect, which was significantly different from that induced by a CS of 80% AMT (cMM: P = 0.02; iMM P = 0.004) and of 60% AMT (cMM: P = 0.02; iMM P = 0.02), but not from that induced by a CS of 90% AMT. With a CS of 70% AMT the mean peak to peak amplitude of the unconditioned cMEP was 0.525 ± 0.06 mV, while amplitudes of the conditioned cMEPs were 0.410 ± 0.04 mV, 0.392 ± 0.04 mV, 0.320 ± 0.03 mV and 0.389 ± 0.04 mV at ISIs of 2, 3, 10 and 15 ms, respectively. For the iMEP mean amplitude values were 0.339 ± 0.03 mV (test MEP), 0.286 ± 0.03 mV (2 ms ISI), 0.264 ± 0.03 mV (3 ms ISI), 0.207 ± 0.02 mV (10 ms ISI) and 0.259 ± 0.03 mV (15 ms ISI). Two-way ANOVA comparing SIDE and ISIs with a CS intensity of 70% AMT revealed no significant differences between SIDES and a significant effect of ISIs (F4,40 = 21, P < 0.0001). Post hoc analysis showed that MEP inhibition was significant at all four ISIs both for cMEP (2 ms: P = 0.007; 3 ms: P < 0.0001; 10 ms: P = 0.001; 15 ms: P = 0.007) and iMEP (2 ms: P = 0.01; 3 ms: P < 0.02; 10 ms: P = 0.004; 15 ms: P = 0.03). No significant differences were detected in the prestimulus EMG activity at each ISI and between sides. Figure 2B shows the time course of conditioned MEP using a CS of 70% AMT. Mean amplitude values (n = 6 subjects) of contralateral and ipsilateral masseter conditioned MEPs for each ISI tested (from 1 to 50 ms) are plotted in the ordinate. It was found that an early period of inhibition (from 1 to 6 ms) was followed by a late stronger inhibition at ICF ISIs (starting at 6 ms ISI, with a peak of maximal inhibition at 10 ms and lasting until 22 to 25 ms), without differences between sides. ANOVA revealed an effect of ISIs (F14,70 = 5.4, P < 0.0001).
Figure 1. Motor evoked potentials (MEPs) recorded from active contralateral (left panel) and ipsilateral (right panel) masseter muscle of a representative subject following TMS of the left masseteric motor cortex.
Top traces show unconditioned MEPs obtained delivering the test stimulus (TS) alone; the middle and bottom traces show conditioned MEPs obtained preceding the TS with a subthreshold conditioning stimulus (CS) delivered at 3 and 10 ms interstimulus intervals (ISIs). Each trace is the average of 12 single trials. TS intensity was adjusted to elicit a test MEP of 0.5 mV peak to peak amplitude in the active contralateral masseter; CS intensity was set at 70% of active motor threshold. In the right panel, the asterisk over the first peak indicate the root compound muscle action potential (rCMAP) secondary to the TMS-induced direct activation of the ipsilateral trigeminal root. Note that compared to the test MEP, the amplitude of conditioned MEPs is reduced both at the SICI ISI (3 ms) and at the ICF ISI (10 ms), while the rCMAP is not affected by paired pulse stimulation.
Figure 2. Mean amplitude of contralateral and ipsilateral masseter conditioned MEPs induced by paired pulse TMS of the left masseteric motor cortex.
A, effects of four different CS intensities (plotted with different colours and symbols: 90% AMT (♦), 80% AMT (▪), 70% AMT (s), 60% AMT (•)) were evaluated in 12 subjects. Note that at SICI ISIs (2 and 3 ms) a MEP inhibition was seen only with a CS of 70% AMT, while at ICF ISIs (10 and 15 ms) a clear inhibition rather than a facilitation was seen at all CS intensities, being 70% of AMT the CS intensity inducing the strongest effect. B, time course (from 1 ms to 50 ms) of the effect of a subthreshold CS of 70% of AMT on the size of masseter EMG responses evoked in both cMM (♦) and iMM (▪) by a suprathreshold TS. Averaged data from 6 subjects show an early period of inhibition (from 1 to 6 ms) followed by a late stronger inhibition at ICF ISIs, with a peak of maximal inhibition at 10 ms ISI lasting until 20–25 ms ISI. The abscissa indicates interstimulus intervals; the ordinate indicates conditioned MEP amplitude expressed as a percentage of the unconditioned MEP induced by the TS given alone, taken as 100% (dotted horizontal line). Error bars represent standard error of the mean.
Experiment 3. Paired pulse TMS to the left masseteric motor area: effects of different levels of masseter activation on SICI
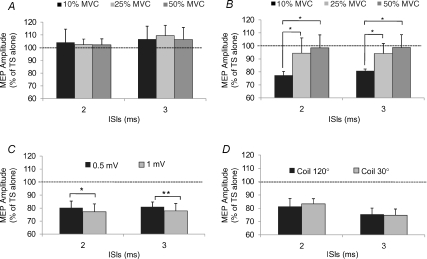
Mean TS intensities used to evoke a MEP test were, respectively, 61.5 ± 2.1%, 50 ± 2.5% and 39 ± 2.2% for the three levels of muscle contraction studied (10, 25 and 50% MVC). AMT values were 37 ± 1.9% (10%MVC), 30 ± 2.1% (25% MVC) and 25 ± 2.2% (50% MVC). Mean prestimulus EMG activities were 37.6 ± 4.9 μV (10% MVC), 63.5 ± 5.3 μV (25% MVC) and 108 ± 6.5 μV (50% MVC). Delivering a CS of 90% AMT, no SICI was seen at any level of contraction (Fig. 3A). On the contrary, when the CS intensity was 70% AMT a significant inhibition of the conditioned MEP was seen but only at 10% MVC (P = 0.01) (Fig. 3B). ANOVA showed an effect of CONTRACTION LEVEL (F2,18 = 4, P = 0.04) and an interaction between CONTRACTION LEVEL and ISIs (F2,18 = 4, P = 0.04). Post hoc analysis revealed that inhibition of the conditioned MEP observed at 10% MVC was significantly different from that observed at 25% MVC (P = 0.04) and at 50% MVC (P = 0.03).
Figure 3. Effects of different levels of masseter contraction (A and B), TS intensities (C) and coil orientation (D) on SICI assessed in the contralateral masseter muscle of 5 subjects.
Two CS intensities (A = 90% AMT; B = 70% AMT) and three levels of contraction (10% MVC, black column; 25% MVC, light grey column; 50% MVC, dark grey column) were evaluated. No significant inhibition at any level of contraction was seen using a CS of 90% AMT; on the contrary a CS of 70% AMT was effective in inducing a significant inhibition, but only at a contraction level of 10% MVC. SICI was significantly larger at all ISIs tested when the TS intensity was higher, while it was unaffected by changes of coil orientation. The ordinate indicates mean conditioned MEP amplitude expressed as a percentage of the test MEP amplitude, taken as 100% (dotted horizontal line) and abscissa reports interstimulus intervals (ISIs). Error bars represent standard error of the mean. Asterisks indicate significant differences (*P < 0.05;**P < 0.01).
Experiment 4. Paired pulse TMS to the left masseteric motor area: effects of different TS intensities on SICI
Results of experiment 4 demonstrate that the higher was the TS intensity, the larger was SICI (Fig. 3C). TS intensities used to obtain cMEPs of 0.5 and 1 mV were 59 ± 2.3% and 76 ± 2.6% of the MSO, respectively. ANOVA showed a significant effect of TS INTENSITY on SICI ISIs (F1,4 = 26.7, P = 0.007), which was confirmed by post hoc analysis (2 ms: P = 0.02; 3 ms: P = 0.003).
Experiment 5. Paired pulse TMS to the left masseteric motor area: effects of coil orientation on SICI
The results of experiment 5 demonstrated that changing the coil orientation from 120 deg to 30 deg had no effect on the amount of SICI (Fig. 3D). On the contrary, the cMEP onset latency was significantly affected (Student's paired t test: P = 0.007) by the position of the coil being shorter (5.7 ± 0.24 ms) when the coil was kept at 120 deg with respect to that observed (6.5 ± 0.26 ms) when the coil was held at 30 deg.
Experiment 6. TMS versus TES of the left masseteric motor cortex
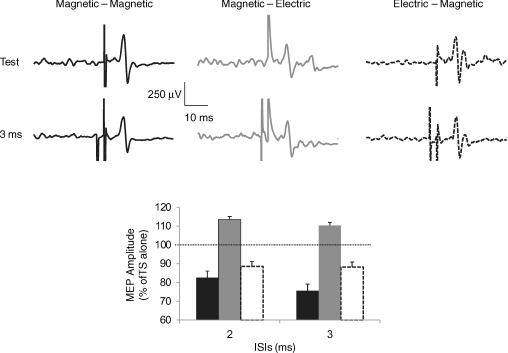
The results of this experiment, performed in a single subject to evaluate the effects of electric or magnetic shocks used as TS or CS, are illustrated in Fig. 4. In the first block (left panel) both pulses were magnetic. In this condition a clear suppression of the conditioned cMEP amplitude was evident at 2 and 3 ms ISIs, with a strongest effect at 3 ms. In the second block (middle panel) the same magnetic CS did not induce any cMEP suppression but a slight facilitation of the anodal muscle response evoked by an electric TS. In the third block (right panel), the electric CS was able to induce a small inhibition of the muscle response to a magnetic TS. Mean onset latencies of contralateral test MEP induced by magnetic TS were 6.4 ± 0.05 ms and 6.5 ± 0.09 ms (first and third block), respectively, while the mean onset latency of the test cMEP elicited by an electric TS was 5.0 ± 0.04 ms.
Figure 4. Contralateral masseter MEPs obtained in a single subject using transcranial electric and magnetic stimuli combined in a paired pulse protocol.
Panels at the top report mean EMG traces: each trace is the average of 12 single trials. In the left panel (black traces) both pulses were magnetic; in the middle panel (dark grey traces) the CS was magnetic and the TS was an anodal stimulus; in the right panel (black broken traces) the CS was electric and the TS was a magnetic stimulus. In each panel, top traces show unconditioned MEPs obtained delivering the TS alone; bottom traces show conditioned MEPs obtained preceding the TS with a subthreshold CS delivered at 3 ms interstimulus interval (ISI). The graph reports mean amplitudes of conditioned cMEPs obtained at 2 and 3 ms ISIs (columns are plotted with same colours as those used in the panels above). Ordinate indicates mean conditioned MEP amplitude expressed as a percentage of the test MEP amplitude, taken as 100% (dotted horizontal line) and abscissa reports ISIs. Error bars represent standard error of the mean. Note that the amplitude of the conditioned MEPs was suppressed only when the TS was magnetic, independently of the kind of CS used. On the contrary, no MEP suppression was induced when the TS was electric; in this case a slight facilitation was rather seen.
Experiment 7. SMU recording
Eleven single motor units were recorded from contralateral MM of six subjects. Mean TS and CS intensities used were 55.6 ± 4.9% and 27.3 ± 2.4% of MSO, respectively. Only 8 of 11 SMUs responded to TS alone, which was able to evoke a single peak in the PSTH of seven SMU and two peaks in the PSTH of one SMU. Student's t test showed that the mean onset latency of PSTH peaks induced by TS alone (6.8 ± 0.4 ms) was not significantly different from that of peaks following paired pulse TMS (6.9 ± 0.5 ms). On the contrary, a significant reduction of the total number of counts (single pulse TMS: 34.3 ± 3.2 counts; paired pulse TMS: 28.4 ± 2.7 counts; P = 0.04) and of peak duration (single pulse TMS: 2.0 ± 0.1 ms; paired TMS: 1.7 ± 0.2 ms; P = 0.01) was observed after paired pulse stimulation (Fig. 5).
Figure 5. Effects of single and paired transcranial magnetic stimulation of the left masseteric motor cortex on the firing of single motor units (SMU) recorded from the contralateral masseter muscle.
The figure reports responses of 2 SMUs to the test stimulus alone (TS) and to the paired pulse stimulation (CS-TS) at an interstimulus interval of 3 ms. PSTHs (bin width 0.2 ms) were constructed cumulating SMU responses to 100 consecutive stimulations and plotting the probability of firing per stimulus (the ordinate) against the latency (the abscissa). Time zero refers to the time of TS delivery. Responses to TS alone consisted of a single peak in the PSTH. Paired pulse stimulation induced a reduction of the count number in unit A and a clear suppression of unit B response.
Experiment 8. Silent Period in the masseteric motor cortex
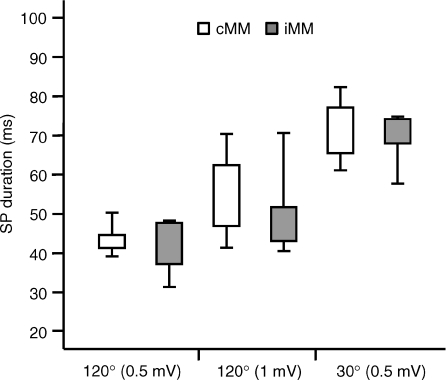
In nine subjects mean SP duration ranged from 20 to 73.5 ms (36.5 ± 7.3 ms) in the cMM and from 18 to 70.5 (34.2 ± 7.0 ms) in the iMM, with no significant differences between sides (P > 0.05). In five subjects SP duration was further evaluated in three different conditions in which coil orientation or pulse intensity were changed (Fig. 6). In the first condition (coil 120 deg – MEP 0.5 mV) the AMT was 39 ± 2.3%, TS intensity was 59 ± 2.3% (151% AMT) and SP duration was 41.7 ± 3.2 ms for iSP and 43.8 ± 1.4 ms for cSP. In the second condition (coil 120 deg – MEP 1 mV) AMT was as above, TS intensity was 76 ± 2.6% (195% AMT) and SP duration was 51.1 ± 5.3 ms (iSP) and 54.3 ± 5.3 ms (cSP). In the third condition (coil 30 deg – MEP 0.5 mV) AMT was 57 ± 4.6%, TS intensity was 72 ± 4.7% (126% AMT) and SP duration was 69.5 ± 3.1 ms for iSP and 71.6 ± 3.8 ms for cSP. Two-way ANOVA (SIDES × CONDITIONS) showed a significant effect of CONDITIONS (F2,8 = 14.7, P = 0.002).
Figure 6. Cortical silent period in masseteric motor cortex.
The box plots show mean SP duration measured in the contralateral (cMM, white boxes) and ipsilateral (iMM, grey boxes) masseter muscle of 5 subjects who were studied in 3 different conditions (coil 120 deg – MEP 0.5 mV; coil 120 deg – MEP 1 mV; coil 30 deg – MEP 0.5 mV) in which coil orientation or test pulse intensity was changed. SP duration was in all conditions not significantly different between sides. With the optimal coil orientation for MM (120 deg), SP duration increased with increasing the test pulse intensity. Changing the coil orientation from 120 deg to 30 deg, a further increase of SP duration was observed. Note that the pulse intensity used to obtain a 0.5 mV MEP with the coil orientated at 30 deg was comparable to the intensity able to evoke a 1 mV MEP with the coil orientated at 120 deg. Error bars represent standard error of the mean.
Experiment 9. Paired pulse TMS to the left hand motor area
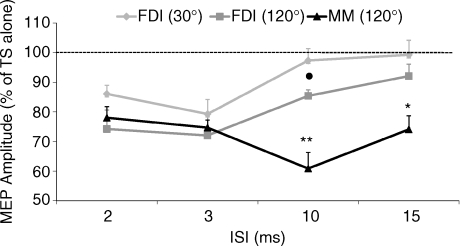
All 12 subjects participated in this experiment, which was aimed at comparing the SICI and ICF evidenced for the first time in the cortical representation of masseter muscles to those, well known and widely described, in the cortical representation of the FDI. Two coil orientations were used to induce motor responses in the active FDI: the classical orientation used to stimulate the hand motor area (30 deg from the midsagital line) and the optimal one for the masseter motor area (120 deg). In this experiment two-way ANOVA revealed a significant effect of ISIs (F4,44 = 17.7, P < 0.0001). A post hoc test showed a significant inhibition of FDI conditioned MEPs at 2 and 3 ms ISIs when the coil orientation was 30 deg (2 ms: P = 0.002; 3 ms: P < 0.01) and at 2, 3 and 10 ms ISIs when the coil was orientated at 120 deg (2 ms: P = 0.02; 3 ms: P < 0.03; 10 ms: P = 0.003). The comparison of MUSCLE responses (FDI30deg, FDI120deg, MM120deg) at the different ISIs (2, 3, 10, 15 ms), with two-way ANOVA, showed a significant effect of ISIs (F4,44 = 21.8, P < 0.0001) and an interaction between ISIs and MUSCLE (F8,88 = 5.5, P < 0.0001). Post hoc analysis showed that masseter MEP inhibition at 10 and 15 ms ISIs was significantly different from that of the FDI30deg (10 ms: P = 0.01; 15 ms: P = 0.01) and from that of FDI120deg (10 ms: P = 0.002; 15 ms: P = 0.04). Furthermore, post hoc analysis revealed a significant difference between FDI30deg and FDI120deg at 10 ms ISI (P = 0.003). By contrast no differences were seen between FDI and masseter at the SICI intervals (Fig. 7).
Figure 7. Comparison of SICI and ICF assessed in the cortical representation of masseter (MM) and of first dorsal interosseus (FDI) muscles.
The same TMS paired pulse protocol was applied to the left hemisphere of 12 subjects and EMG responses were recorded from contralateral muscles, both activated at 10% MVC. TS intensity was adjusted to elicit a test MEP of 0.5 mV peak to peak and CS intensity was equal to 70% of AMT. Two coil orientations were used to induce motor responses in the active FDI: coil handle pointing backwards and rotated 30 deg from the midline (FDI30deg, bright grey) and coil handle pointing forwards and rotated 120 deg from the midline (FDI120deg, dark grey). This last coil orientation was also used to induce masseter MEPs (MM120deg, black line). Ordinate indicates mean conditioned MEP amplitude expressed as a percentage of the test MEP amplitude, taken as 100% (dotted horizontal line) and abscissa reports ISIs. Error bars represent standard error of the mean. Note that SICI observed at the 2 and 3 ms ISI is not significantly different in the two muscles. On the contrary, MEP inhibition observed in MM at 10 and 15 ms ISI is significantly different (*P < 0.05; **P < 0.01) from that observed in FDI muscle with both coil orientations used. Moreover, a significant difference between FDI30deg and FDI120deg at 10 ms ISI was observed (•P < 0.05).
Experiment 10. Effects induced by repetitive TMS at CS intensity and by loud clicks on voluntary masseter EMG activity
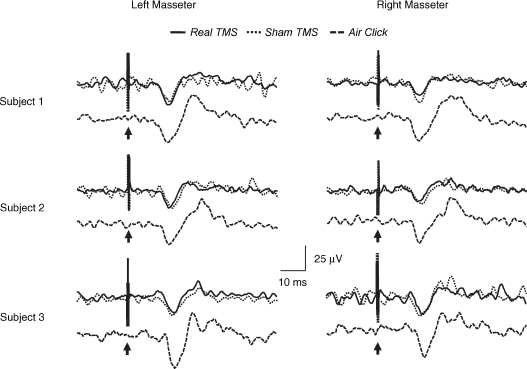
Real and sham rTMS at 60% AMT intensity induced bilaterally in the averaged unrectified EMG of 3/6 subjects (group 1) clear superimposable positive waves which were followed by an inconsistent negative wave (Fig. 8). Mean onset latency of the positive wave was 12.7 ± 0.4 ms (left MM) and 13.0 ± 0.2 ms (right MM) during real rTMS and 12.7 ± 0.2 ms (left MM) and 12.8 ± 0.3 ms (right MM) during sham stimulation. Mean peak latency of the positive wave was 16.5 ± 0.3 ms (left MM) and 16.7 ± 0.2 ms (right MM) during real rTMS and 16.8 ± 0.1 ms (left MM) and 16.7 ± 0.2 ms (right MM) during sham stimulation. The duration of the positive wave ranged from 10.5 to 13.5 ms following real rTMS and from 11.2 to 12.9 ms following sham rTMS. Two-way ANOVA comparing CONDITIONS (real TMS versus sham TMS) and SIDES (left MM versus right MM) showed no significant differences in onset latencies, peak latencies and duration. The remaining three subjects (group 2) showed negligible or no EMG responses to both real and sham TMS (not shown). In the six subjects who undewent rTMS, mean prestimulus EMG activity was 38.6 ± 1.4 μV (left MM) and 38.8 ± 1.1 μV (right MM) during real stimulation and 38.3 ± 1.0 μV (left MM) and 38.5 ± 0.9 μV (right MM) during sham stimulation. Two-way ANOVA comparing CONDITIONS (real TMS versus sham TMS) and SIDES (left MM versus right MM) showed no significant differences. Similarly no significant differences in mean prestimulus EMG were found comparing GROUPS (group 1 versus group 2), CONDITIONS (real TMS versus sham TMS) and SIDES (left MM versus right MM). As described by Colebatch & Rothwell (2004), such waveforms elicited in the unrectified EMG averages represent short periods of suppression of ongoing activity that may not be visible in rectified EMG averages.
Figure 8. Effects of subthreshold TMS and of loud click stimulation on masseter voluntary EMG activity.
Recordings from 3 subjects who exhibited a large inhibition (> 40%) of the masseter conditioned MEP at 10 ms interstimulus interval are reported. TMS (300 pulses, 1 Hz frequency, intensity of 60% AMT) delivered to the left masseteric motor cortex (real TMS, continuous black line) or 3 cm above the vertex (sham TMS, dotted black line) produced superimposable responses in the averaged unrectified EMG activity of both masseter muscles, consisting of a positive wave followed by an inconsistent negative wave. For each subject averaged unrectified responses to bilateral click stimulation (500 stimuli, 3Hz frequency, intensity of 70 dB NHL), named as the jaw acoustic reflex, are also reported (broken black line) below responses to real and sham rTMS. Time course and shape of masseter EMG responses observed in the three different conditions are parallel. Arrows indicate the time of stimulus delivery.
Interestingly, on the basis of responses to paired pulse TMS at 10 ms ISI and with a CS intensity of 60% AMT (see experiment 2), subjects of group 1 were found to show a strong inhibition (bigger than 40%) of the conditioned MEP. On the contrary, subjects of group 2 exhibited a weak inhibition (less than 10%) of the conditioned masseter MEP, in the same experimental conditions.
The three subjects showing a large inhibition of the conditioned MEP as well as a clear EMG response to real and sham rTMS (group 1) underwent to recording of the jaw acoustic reflex. Loud click stimulation induced bilaterally in the mean unrectified masseter EMG a clear p16 wave followed by a less clear n21 wave (Fig. 8) which corresponded to an inhibitory deflection lasting 10–12 ms in the rectified EMG (not shown). The p16 wave had onset latency of 12.9 ± 0.7 ms (left MM) and 13.1 ± 0.6 ms (right MM), peak latency of 16.7 ± 0.3 ms (left MM) and 16.6 ± 0.3 ms (right MM) and duration ranging from 10.2 ms to 13.5 ms. Student's paired t test showed no significant differences in latencies and duration between sides.
Onset latency, peak latency and duration of the acoustic p16 wave and of the rTMS-induced positive wave were separately compared by one-way ANOVA, which did not show any significant difference among the three conditions (real TMS, sham TMS, click stimulation).
Experiment 11. Blink reflex
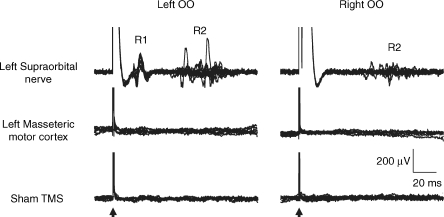
CS intensity of 70% AMT was able to evoke in all five subjects studied a normal blink reflex, with an ipsilateral R1 and a bilateral R2 components, only when the stimulation was delivered directly over the supraorbital notch. By contrast no blink reflex was seen when the same stimulus was applied to the skull over the left masseteric motor cortex or when a sham stimulation (coil held 3 cm above the vertex) was given (see Fig. 9).
Figure 9. Responses of orbicularis muscles (OO) of a representative subject to subthreshold magnetic stimulation delivered to different stimulation sites.
Twenty single EMG traces recorded from the left and right OO are superimposed. Note that magnetic stimulation at an intensity of 70% AMT was able to induce a normal blink reflex, with an ipsilateral R1 and a bilateral R2 components, only when the stimulation was delivered over the supraorbital notch. By contrast, no response was elicited by magnetic stimulation delivered either over the left masseteric motor cortex or 3 cm above the vertex (sham TMS).
Experiment 12. Effects of supraorbital nerve stimulation on masseter MEP
Subthreshold TMS (intensity of 70% AMT) delivered over the supraorbital notch, which was proved to be able to activate cutaneous trigeminal branches of the supraorbital nerve (see experiment 11), was not able to suppress masseter motor potentials evoked by a suprathreshold TMS delivered to the masseteric motor area at 2 and 3 ms intervals (ANOVA: P > 0.05).
Masseteric motor area localization by an image-guided TMS system
Figure 10 shows the tridimensional brain MRI reconstruction of one subject displaying both the masseter and FDI spots. It can be observed that representations of the two muscles in the precentral gyrus are very close, with the masseter spot localized anterior and lateral to the FDI spot.
Figure 10. Masseteric motor area localization by a Image-Guided TMS system.
A tridimensional brain MRI reconstruction of one subject is shown. Masseter and FDI spots are displayed with grey dots. Both representations on the precentral gyrus appear to be very close and the masseter spot is localized forwards and laterally with respect to the FDI spot.
Discussion
The data in this study confirm the bilateral, predominantly contralateral nature of the corticobulbar projection to the masseter muscles. We also show that there is no asymmetry in the output of left and right hemisphere. The single motor unit data are consistent with previous reports of the difficulty in recruiting multiple I-wave activity with a single TMS stimulus. The new data demonstrate that it is possible to detect SICI in the masseter motor area even though the SP was minimal. The interpretation of conditioning–test intervals appropriate to ICF is complicated by the presence of a short latency inhibition in masseter caused by an acoustic reflex from the noise of the TMS coil discharge.
Corticobulbar projections to masseter excited by single pulse TMS over motor cortex
In humans, anatomical studies suggest that corticobulbar projections to the trigeminal motor nucleus are bilateral and symmetric (Kuypers, 1958; Iwatsubo et al. 1990). However, TMS studies are contradictory. Guggisberg et al. (2001) reported no significant differences in amplitude of MEPs evoked in ipsilateral and contralateral masseter MEPs whereas others found that MEPs were larger in contralateral than ipsilateral muscle (Cruccu et al. 1989; Carr et al. 1994; Nordstrom et al. 1999; Butler et al. 2001; McMillan et al. 2001; Nordstrom, 2007). The present data are consistent with the latter reports, with a mean asymmetry in amplitude of 36%, which is comparable to the 39% reported by Nordstrom et al. (1999) and Butler et al. (2001). The implication is that a substantial proportion of the projections to ipsilateral and contralateral muscles come from separate populations of corticobulbar fibres. Remaining inputs may be provided by bilaterally projecting branches of shared fibres. This would account for the synchronization between motor unit discharge in right and left masseters reported by Carr et al. (1994) during tonic voluntary contraction.
In spite of the asymmetry of the ipsilateral versus contralateral MEP, we found that there is no asymmetry in the output of left and right hemisphere, at least in the right handed subjects that we examined. A lack of contralateral dominance has been demonstrated also during chewing, using a transcranial Doppler ultrasound technique (Ono et al. 2007). The lack of hemispheric dominance for masseter muscles is not surprising if one considers that mastication and most of motor acts involving masseters are not movements on one side and both masticatory muscles work even during unilateral motor tasks. Therefore, a bilateral output of movement to control muscle activity may be considered necessary although inputs of oral sensation in controlling a bolus has dominance on the working side.
The differences in MEP asymmetry between our results and those of Guggisberg et al. (2001) probably relates to the fact that they used an intensity of stimulation of about 80% of MSO to obtain a mean target MEP of 1 mV, while we adjusted the intensity of stimulation (∼58% MSO) to elicit a 0.5 mV MEP in the contralateral masseter. It is possible that the (unilateral) projections to ipsilateral muscle are less excitable than those to contralateral muscle. At high intensities such as those used by Guggisberg et al. (2001), pure ipsilateral projections would be recruited as well, reducing the amplitude difference between the two sides. This hypothesis is consistent with SMU studies (Nordstrom et al. 1999; Pearce et al. 2003) showing that masseter low threshold motoneurons receive monosynaptic short-latency excitatory inputs principally from the contralateral hemisphere, while stimulation of the ipsilateral motor cortex, at the same intensity, excites only a small number of low threshold motor units.
The MEP onset latencies (6.0 ms) obtained in the present study are in the same range as those reported previously (Cruccu et al. 1989: 5.9 ms; Macaluso et al. 1990: 6.2 ms; Nordstrom et al. 1999: 6.6 ms; Butler et al. 2001: 7.0 ms; Guggisberg et al. 2001: 5.9 ms). Like Guggisberg et al. (2001), we found that the lowest threshold responses occurred with a coil orientation approximately parallel to the central sulcus (120 deg), inducing current in a postero-medial direction. Rotating the coil to 30 deg, to induce a postero-anterior (PA) current approximately perpendicular to the line of the sulcus, increased the latency of the response by 0.8 ms. A similar change in latency is observed in hand muscles after stimulation over the hand area of the motor cortex. Direct recordings of descending corticospinal volleys has shown that this is probably due to the fact that stimulating parallel to the central sulcus tends to recruit D waves at lower intensity relative to threshold than the PA direction, which favours I1 waves. Whether a similar explanation holds for the masseter cortex is unclear. In the one subject who was examined in the present study, the MEP latency was 1.5 ms shorter (5.0 versus 6.5 ms) when transcranial anodal electrical stimulation was used. Since TES is thought to recruit preferentially D waves at threshold, we suspect that the majority of TMS responses were initiated by I1 wave activity in the corticospinal system in this subject, even with a 120 deg coil orientation. Guggisberg et al. (2001) in contrast found equal latencies for MEPs evoked by anodal and magnetic stimulation. However, they used higher TMS intensities than employed here, which are known to recruit d-wave activity more readily than at low intensities (Werhahn et al. 1994; Di Lazzaro et al. 2004).
Of the single motor units that we recorded in masseter, 88% responded to single pulse contralateral TMS with a single narrow peak of excitation (mean latency 6.8 ms, duration 2 ms) in the PSTH. Our findings are in agreement with previous data (Nordstrom et al. 1999; Pearce et al. 2003) from authors who also reported that almost all contralateral SMU responded to TMS with a single peak (latency 7.0 ms, duration 1.5 ms). Given the arguments above, this may have been produced by D/I1 or I1/I2 wave activity in the corticobulbar pathway. The relative lack of multiple descending inputs is unexpected. It is possible that they would have been recruited at higher stimulus intensities, but these are difficult to explore while maintaining isolated recordings from single units. Nevertheless, multiple peaks are readily observed in motor unit recordings from hand muscles at similar relative intensities of stimulation (e.g. Day et al. 1989). The fact that they are missing in masseter may be one reason why it is difficult to obtain any masseter MEP responses when subjects are relaxed. Resting motoneurones are thought to require several corticospinal EPSPs in order to depolarize them sufficiently to reach discharge threshold. If only one or two volleys are produced by each TMS pulse, then recruitment may be limited, and the resting threshold elevated.
Short interval intracortical inhibition
This is the first report of SICI in the masseter motor cortex. The amount of SICI was the same in ipsilateral and contralateral masseter suggesting either that the majority of MEPs on the two sides are driven by input from branched corticobulbar fibres and/or that unilaterally projecting fibres to contra- or ipsilateral masseter are controlled by the same pool of inhibitory interneurons as previously suggested for tongue muscles (Muellbacher et al. 2001) and for anterior digastric muscles (Jaberzadeh et al. 2007).
As in the hand representation, SICI is likely to be due to interactions between CS and TS within the cortex. There was no SICI in the single subject in whom we used transcranial electrical stimulation to evoke the test MEP. TES at such intensities produces primarily d-activation of the cortical output neurons, which is not suppressed by intracortical SICI. Although the data obtained in only one subject should be interpreted with due caution, they suggest that the inhibition of the conditioned MEP is of cortical origin. This hypothesis is strengthened by the finding that there was no evidence in a larger number of subjects for any peripheral reflex suppression at short latency from the CS (see below).
Because it was not possible to obtain MEPs at rest in masseter, we had to evaluate SICI during active muscle contraction. In addition, the intensity of the TS was set at a level able to elicit a mean MEP test of 0.5 mV because some subjects experienced higher intensities required to elicit larger MEPs as uncomfortable. These two factors make it difficult to compare the amount of SICI in masseter with that in other muscles since the latter are conventionally studied at rest. SICI is reduced by muscular activity (Ridding et al. 1995) and it is usually bigger when the test size is ≥ 1mV (Sanger et al. 2001; Roshan et al. 2003). Nevertheless, when we compared directly the amount of SICI in contralateral active masseter with SICI in active FDI (see experiment 9) using a CS of 70% AMT, we found that SICI was very similar in the two muscles. This confirms a previous assumption that SICI is independent of the excitability of corticospinal projections (Chen et al. 1998).
It is less clear why we saw SICI only at 70% AMT and not at other CS intensities. One possibility is that voluntary activation reduces the threshold for short interval intracortical facilitation (SICF, or I-wave facilitation), which occurs around the same ISIs as SICI. The usual threshold for SICF at rest is around 90–100%, but if it fell to 80% AMT during activation then it could potentially occlude SICI, at least at certain ISIs. Against this suggestion are data from a study in hand muscles in which SICI was evaluated during active contraction: SICI increased as CS increased from 85 to 95% AMT (Hanajima et al. 1998). Further work is required to resolve this issue.
As described in hand muscles (Sanger et al. 2001; Roshan et al. 2003), the amount of SICI increased at higher intensities of TS. This is thought to be due to the fact that SICI primarily inhibits late I3 waves rather than I1 activity. Since I3 waves are recruited more strongly as the TS is increased, inhibition becomes more prominent. The effect of the level of background contraction on the amount of SICI has not to our knowledge been investigated in hand muscles. However, the present data show that in masseter, increasing contraction levels to 25% MVC can reduce or abolish SICI. Presumably this is due to a facilitation of excitatory interneurons or a disfacilitation of inhibitory interneurons (Di Lazzaro et al. 1998; Mills & Kimiskidis, 1996) by increasing levels of volitional input.
Changing the coil orientation from parallel (120 deg) to perpendicular (30 deg) to the central sulcus had no effect on the amount of SICI (Fig. 3D). In the hand motor cortex, changing the orientation of the CS also has no effect on SICI, which has led to the idea that the interneurons mediating SICI do not have a preferential orientation with respect to the surface of the cortex (Ziemann & Rothwell, 2000). The same may therefore be true for the masseter motor area. It should be noted, though, that in the studies on the hand area, two coils were used, allowing the orientation of the CS to be changed without affecting the orientation of the TS (Ziemann et al. 1996). In the present experiments, we used just a single coil, and therefore changed orientation of both CS and TS. In the hand area, a TS orientation of 30 deg is more likely to recruit I3 waves than the 120 deg angle (Sakai et al. 1997). If the same were true in the masseter cortex, then we might have expected slightly more SICI at 30 deg than at 120 deg. The fact that this was not the case may reflect differences in I3 recruitment in masseter and hand areas of cortex, or lack of power in the present experiments due to the relatively small number of subjects used in this part of the experiment.
Single motor unit study
Responses of the same SMU to paired pulse stimulation (3 ms ISI and CS of 70% AMT) consisted of a single peak in the PSTH which had the same latency as the test peak but a significantly shorter duration and a smaller number of counts. With the exception of one SMU, we were not able to elicit the consecutive peaks corresponding to recruitment of later I waves, which are usually more affected by SICI. This may account for the relatively small reduction in the number of counts that we observed, which corresponded to an average suppression of 83%. Higher stimulus intensities such as used in the main surface EMG experiments may have recruited a larger number of later peaks and resulted in the much greater suppression (∼60–70%) of MEPs. As noted above, the coil orientation used in our study (parallel to the central sulcus) was also non-optimal for evoking late I wave activation (Sakai et al. 1997; Hanajima et al. 1998a).
Intracortical facilitation
The finding of a significant suppression of MEPs at ISI = 10 ms at all intensities of CS was unexpected, since, as far as we know, such marked depression of the conditioned MEP at ICF ISIs has never been described before. Since it is well known that background voluntary contraction markedly reduces or abolishes ICF (Ridding et al. 1995; Paradiso et al. 2005) and that the optimal CS intensity to induce ICF is 90% AMT (Kujirai et al. 1993; Zieman et al. 1996), we expected not to find any significant ICF in the masseteric motor cortex in our experimental conditions, i.e muscle contraction at 10% MVC and CS of 70% AMT. Both these conditions can also account for the absence of ICF in the hand motor cortex, when the coil orientation was optimal to stimulate the hand motor area (i.e. 30 deg, antero-medial current direction). However, when the coil orientation was optimal to stimulate the masticatory area (120 deg, postero-medial current direction) a significant inhibition of the conditioned MEP was observed in the FDI at 10 ms ISI. This may be due to a prolongation of SICI-induced suppression of the conditioned MEP, which has been reported to continue up to 20 ms ISI when a posteriorly directed current is used (Hanajima et al. 1998). However, this seems unlikely because it occurred with CS intensities (60% AMT) well below those required to produce SICI (Ziemann & Rothwell, 2000). We therefore think it more likely that another factor contributes to this effect.
Control experiments showed that subthreshold TMS of the masseteric motor cortex leads to a positive–negative wave in the average unrectified EMG with a duration of about 12 ms. Indeed, the same waveform occurred when CS was discharged away from the scalp, consistent with an auditory reflex suppression. As described by Colebatch & Rothwell (2004) such responses correspond to a short period of inhibition in the underlying EMG activity that is often not visible in rectified EMG records. It is equivalent to the jaw-acoustic reflex which was originally described by Meier-Ewert et al. (1974) as a bilateral silent period in the active masseter EMG. More recently, this reflex has been characterized in surface as well as single unit EMG recordings (Deriu et al. 2005, 2007) as a bilateral and symmetric p16 or p16/n21 wave in the averaged unrectified masseter EMG corresponding to an inhibitory deflection in the mean rectified EMG. SMU recordings demonstrated the inhibitory nature of the response, consisting of a 3–8 ms interruption of unit firing (Deriu et al. 2005).
The close parallelism between the jaw acoustic reflex and the inhibitory response induced in masseter EMG by both real and sham TMS (see Fig. 8) strongly supports the hypothesis that this last response is the result of an acoustic stimulation produced by the noise sent out by the discharging coil. Interestingly, the subjects with clear EMG suppression following real and sham subthreshold TMS also showed a large inhibition of the conditioned MEP, whereas subjects with negligible or no EMG responses to subthreshold TMS had very little MEP inhibition. This is therefore further support for the acoustic origin of MEP suppression observed at ICF intervals.
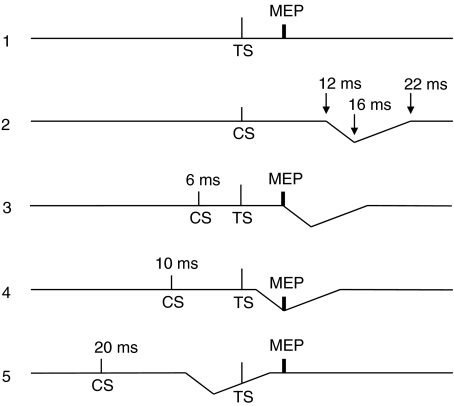
We speculate that the acoustic reflex interacts with the TS in an ICF paradigm as indicated by the model in Fig. 11 with suppression of MEPs at ISI = 10 ms and 15 ms. It is interesting to note that suppression was most powerful with CS = 70% AMT, rather that at 80 or 90% AMT as might have been expected if the depth of the acoustic reflex suppression was related to the loudness of the CS discharge. This may be because CS at 80 or 90% AMT also evoked concurrent ICF that counteracted the expected increase in reflex inhibition.
Figure 11. Model explaining the inhibition of the conditioned MEP observed at ICF interstimulus intervals (10 and 15 ms).
TS is the magnetic test stimulus, which was able to produce in active masseter muscles a test MEP (trace 1). CS is the magnetic conditioning stimulus, which was unable to induce in active masseter muscles a MEP, but rather induced bilateral EMG suppression (represented by the downward deflection of the trace) with onset and time course indicated in trace 2. When both CS and TS are given (traces 3–5) no interference of the acoustically induced silent period with the MEP occurs at ISIs < 6 ms, while this interference occurs at ISIs > 6 ms. In particular maximal interference occurs at 10 ms ISI as MEP falls exactly at the time of maximal peak of inhibition (10 ms ISI + 6 ms MEP latency = 16 ms) of masseter EMG (trace 4). This model fits with the time course illustrated in Fig. 2B.
Cortical silent period
Our results demonstrated that the masseter cortical silent period is bilateral and symmetrical. However, in comparison with hand muscles, the duration is short. Thus, with the optimal coil orientation for masseter muscles (120 deg) and a stimulus intensity (151% AMT) able to induce a 0.5 mV MEP, its duration was in the order of about 40 ms, without differences between sides. In agreement with previous work in masseter (Cruccu et al. 1989; Jaberzadeh et al. 2008) as well as in hand muscles (Valls-Solé et al. 1992; Wassermann et al. 1996) SP duration increased with increasing pulse intensity (54.3 ms for a 1 mV MEP evoked at 195% AMT). These values are much shorter than that reported in hand muscles (100–200 ms) at comparable intensities above threshold, and are in agreement with the idea that the duration of the SP depends on the strength of the corticospinal projection (Ziemann et al. 1993; Werhahn et al. 1995). A similar short duration of masseter SP was previously reported by Cruccu et al. (1989) using a circular coil over the vertex and more recently by Jaberzadeh et al. (2008) using a focal coil. However, in the latter study, the duration of the SP was longer than ours (102 ± 4 ms versus 54.3 ± 5.3 ms for a MEP of ∼1 mV) even though the pulse intensity was similar (70–79% versus 76 ± 2.6% MSO). This is probably due to the different coil orientations used in the two studies (45 deg versus 120 deg); indeed there was a significant increase of SP duration (up to 65–88 ms) in the present study when the coil orientation was changed from 120 deg to 30 deg. Previous observations in hand muscles (Orth & Rothwell, 2004) also found that SP duration depends on coil orientation, but in those muscles the change in MEP amplitude at different orientations matched the change in SP duration, with the effect that the ratio of SP duration/MEP amplitude remained approximately constant, at least for stimulus intensities above 130% AMT. In masseter the stimulus intensity was 126% AMT at 30 deg whilst it was 150% AMT at 120 deg, so that we might have expected the SP/MEP ratio to have been constant, but this was not the case. Even though we adjusted the stimulus intensity so that the MEP amplitude was the same (∼0.5 mV) for the two orientations, the SP was longer at 30 deg than at 120 deg. Why this should occur is unclear. It may be that the range of intensities over which the ratio SP/MEP is constant is different in the masseter from in the hand muscles. Nevertheless, like the optimal orientation for eliciting MEPs, it demonstrates that the relative orientation of excitable elements in the masseter cortex differs from that in the hand area.
Cruccu et al. (1989) suggested that the masseter silent period induced by TMS may be due to excitation of corticofugal inhibitory connections and to reflex activation of inhibitory inputs. Indeed a recent study (Sowman et al. 2008) has shown that the jaw jerk in relaxed masseter is suppressed after a TMS pulse with the same time course as the silent period in contracting muscles. This would therefore be compatible with a brainstem origin of the silent period. However, a contribution from cortical mechanisms cannot be excluded. Changes in the level of muscle contraction do not influence its duration and it is observed following a subthreshold TMS intensity (70–80% AMT), which is not thought to be able to modify brainstem excitability (Jaberzadeh et al. 2008).
Masseteric motor area localization by a image-guided TMS system
In agreement with Penfield & Boldrey (1937) the image guided study found that the excitable projections to masseter and intrinsic hand muscle partially overlap in the precentral gyrus with the masseter localized anterior and lateral with respect to the FDI. A similar finding was obtained for FDI and digastric muscles using a TMS mapping technique (Gooden et al. 1999).
Conclusions
In conclusion our study confirms the functional asymmetry of the corticobulbar projection to masseter muscles with contralateral predominance and demonstrates that inhibitory interneurons mediating SICI can be tested as in the cortical representation of hand muscles. It was not possible to assess the activity of facilitatory interneurons mediating ICF because it was masked by a marked acoustic reflex depression of masseter EMG. Finally, we confirm that SP in masseter is much shorter than in hand muscles, suggesting that the inhibitory circuits involved in the SP are less excitable or numerous than in hand motor cortex. Evaluation of intracortical circuits in masseter motor cortex may provide further physiological insight into pathologies affecting the trigeminal motor system.
Acknowledgments
The authors are very greateful to Mr Richard Symonds and Mr Peter Owbridge for their valuable technical assistance. Dr Enzo Ortu and Dr Elena Giaconi were supported by grants from the Regione Autonoma della Sardegna (Master and Back Program).
References
- Butler SL, Miles TS, Thompson PD, Nordstrom MA. Task dependent control of human masseter muscles from ipsilateral and contralateral motor cortex. Exp Brain Res. 2001;137:65–70. doi: 10.1007/s002210000626. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol. 1994;475:217–227. doi: 10.1113/jphysiol.1994.sp020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell J, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC. Motor unit excitability changes mediating vesitbulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol. 2004;115:2567–2573. doi: 10.1016/j.clinph.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Berardelli A, Inghilleri M, Manfredi M. Functional organisation of the trigeminal motor system in man. A neurophysiological study. Brain. 1989;112:1333–1350. doi: 10.1093/brain/112.5.1333. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F, Ortu E, Capobianco S, Giaconi E, Melis F, Aiello E, Rothwell JC, Tolu E. Origin of sound-evoked EMG responses in human masseter muscles. J Physiol. 2007;580:195–209. doi: 10.1113/jphysiol.2006.123240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F, Tolu E, Rothwell JC. A sound-evoked vestibulomasseteric reflex in healthy humans. J Neurophysiol. 2005;93:2739–2751. doi: 10.1152/jn.01005.2004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooden BR, Ridding MC, Miles TS, Nordstrom MA, Thompson PD. Bilateral cortical control of the human anterior digastric muscles. Exp Brain Res. 1999;129:582–591. doi: 10.1007/s002210050928. [DOI] [PubMed] [Google Scholar]
- Guggisberg AG, Dubach P, Hess CW, Wuthrich C, Mathis J. Motor evoked potentials from masseter muscle induced by transcranial magnetic stimulation of the pyramidal tract: the importance of coil orientation. Clin Neurophysiol. 2001;112:2312–2319. doi: 10.1016/s1388-2457(01)00677-0. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- Jaberzadeh S, Pearce SL, Miles TS, Turker KS, Nordstrom MA. Intracortical inhibition in the human trigeminal motor system. Clin Neurophysiol. 2007;118:1785–1793. doi: 10.1016/j.clinph.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jaberzadeh S, Sakuma S, Zoghi M, Miles TS, Nordstrom MA. Focal transcranial magnetic stimulation of motor cortex evokes bilateral and symmetrical silent periods in human masseter muscles. Clin Neurophysiol. 2008;119:693–703. doi: 10.1016/j.clinph.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM. Corticobulbar connexions to the pons and lower brain-stem in man: an anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Lin LD, Murray GM, Sessle BJ. The effect of bilateral cold block of the primate face primary somatosensory cortex on the performance of trained tongue-protrusion task and biting tasks. J Neurophysiol. 1993;70:985–996. doi: 10.1152/jn.1993.70.3.985. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: mastication and voluntary biting. In: Mountcastle VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda: American Physiological Society; 1981. pp. 1237–1274. [Google Scholar]
- Macaluso GM, Pavesi G, Bonanini M, Mancia D, Gennari PU. Motor-evoked potentials in masseter muscle by electrical and magnetic stimulation in intact alert man. Arch Oral Biol. 1990;35:623–628. doi: 10.1016/0003-9969(90)90028-9. [DOI] [PubMed] [Google Scholar]
- McMillan AS, Graven-Nielsen T, Romaniello A, Svensson P. Motor potentials evoked by transcranial magnetic stimulation during isometric and dynamic masseter muscle contraction in humans. Arch Oral Biol. 2001;46:381–386. doi: 10.1016/s0003-9969(00)00129-1. [DOI] [PubMed] [Google Scholar]
- McMillan AS, Watson C, Walshaw D. Transcranial magnetic-stimulation mapping of the cortical topography of the human masseter muscle. Arch Oral Biol. 1998;43:925–931. doi: 10.1016/s0003-9969(98)00081-8. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert K, Gleitsmann K, Reiter F. Acoustic jaw reflex in man: its relationship to other brainstem and microreflexes. Electroencephalogr Clin Neurophysiol. 1974;36:629–637. doi: 10.1016/0013-4694(74)90229-6. [DOI] [PubMed] [Google Scholar]
- Mills KR, Kimiskidis V. Cortical and spinal mechanisms of facilitation to brain stimulation. Muscle Nerve. 1996;19:953–958. doi: 10.1002/(SICI)1097-4598(199608)19:8<953::AID-MUS3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20:570–576. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Boroojerdi B, Ziemann U, Hallett M. Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol. 2001;18:550–558. doi: 10.1097/00004691-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Murray GM, Lin LD, Moustafa EM, Sessle BJ. Effects of reversible inactivation by cooling of the primate face motor cortex on the performance of a trained tongue-protrusion task and a trained biting task. J Neurophysiol. 1991;65:511–530. doi: 10.1152/jn.1991.65.3.511. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res. 1995;23:1–19. [PubMed] [Google Scholar]
- Nordstrom MA. Insights into the bilateral cortical control of human masticatory muscles revealed by transcranial magnetic stimulation. Arch Oral Biol. 2007;52:338–342. doi: 10.1016/j.archoralbio.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS, Gooden BR, Butler SL, Ridding MC, Thompson PD. Motor cortical control of human masticatory muscles. Prog Brain Res. 1999;123:203–214. doi: 10.1016/s0079-6123(08)62857-5. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;1:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono T, Hasegawa Y, Hori K, Nokubi T, Hamasaki T. Task-induced activation and hemispheric dominance in cerebral circulation during gum chewing. J Neurol. 2007;254:1427–1432. doi: 10.1007/s00415-007-0570-3. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Paradiso OG, Cunic DI, Gunraj AC, Chen R. Representation of facial muscles in human motor cortex. J Physiol. 2005;567:323–336. doi: 10.1113/jphysiol.2005.088542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce SL, Miles TS, Thompson PD, Nordstrom MA. Responses of single motor units in human masseter to transcranial magnetic stimulation of either hemisphere. J Physiol. 2003;549:583–596. doi: 10.1113/jphysiol.2002.035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowman PF, Flavel SC, McShane CL, Miles TS, Nordstrom MA. Transcranial magnetic stimulation reduces masseter motoneuron pool excitability throughout the cortical silent period. Clin Neurophysiol. 2008;119:1119–1129. doi: 10.1016/j.clinph.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Classen J, Benecke R. The silent period induced by transcranial magnetic stimulation in muscles supplied by cranial nerves: normal data and changes in patients. J Neurol Neurosurg Psychiatry. 1995;59:586–596. doi: 10.1136/jnnp.59.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JKY, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelényi A, Hömberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between cortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]