Abstract
We determined the effects of acute intra-arterial vitamin C administration and chronic oral vitamin C supplementation on the capacity of the endothelium to release t-PA in overweight and obese adults. Net endothelial t-PA release was determined in vivo in response to intrabrachial infusions of bradykinin and sodium nitroprusside in 33 sedentary adults: 10 normal-weight (BMI: 23.4 ± 0.5 kg m−2; 7M/3F); and 23 overweight/obese (BMI: 31.2 ± 0.8 kg m−2; 15M/8F). In 10 normal weight and eight overweight/obese adults the dose–response curves to bradykinin and sodium nitroprusside were repeated with a coinfusion of the antioxidant vitamin C (24 mg min−1). Seventeen of the 23 overweight/obese adults completed a 3 month chronic oral vitamin C (500 mg day−1) supplementation intervention. Intra-arterial administration of vitamin C significantly potentiated t-PA release in overweight/obese adults. Net release of t-PA was ∼95% higher (P < 0.01) after (from −0.9 ± 1.1 to 94.6 ± 16.2 ng (100 ml tissue)−1 min−1) compared with before (from −0.8 ± 0.8 to 49.9 ± 7.7 ng (100 ml tissue)−1 min−1) vitamin C administration. Daily vitamin C supplementation significantly increased t-PA release in overweight/obese adults (from 0.2 ± 0.9 to 48.2 ± 6.5 ng (100 ml tissue)−1 min−1) before supplementation versus (0.3 ± 0.5 to 66.3 ± 8.7 ng (100 ml tissue)−1 min−1) after supplementation. These results indicate that the antioxidant vitamin C favourably affects the capacity of the endothelium to release t-PA in overweight/obese adults. Daily vitamin C supplementation represents an effective lifestyle intervention strategy for improving endothelial fibrinolytic regulation in this at-risk population.
Overweight and obesity are associated with increased cardiovascular morbidity and mortality resulting from accelerated progression of coronary atherosclerosis and elevated rates of atherothrombotic events (Must et al. 1999). Moreover, excess adiposity contributes to a greater risk of recurrent coronary events after an acute myocardial infarction (Rea et al. 2001). The precise mechanisms contributing to increased thrombotic tendency are not completely understood. We have previously demonstrated that the capacity of the endothelium to release tissue-type plasminogen activator (t-PA), the primary activator of endothelial fibrinolysis, is impaired in overweight and obese adults (Van Guilder et al. 2005). The ability of endothelial cells to release t-PA and activate plasminogen on the surface of a developing clot is a vital defence mechanism against thrombosis. Furthermore, disruption of endothelial t-PA release is predictive of future adverse cardiovascular events in patients with coronary artery disease (Robinson et al. 2007), and thus may be an underlying mechanism contributing to the pro-thrombotic state that is characteristic of obesity.
Regular physical activity reduces many of the cardiovascular health risks associated with overweight and obesity (Blair & Brodney, 1999). We and others have shown that a programme of regular aerobic exercise training can improve obesity-related endothelial dysfunction (Sciacqua et al. 2003; Van Guilder et al. 2005). Despite the well-known cardioprotective effects of regular physical activity, only ∼20% of adults in the United States habitually engage in the recommended amount of aerobic exercise required to improve cardiovascular health, while more than 37% are sedentary (Kruger et al. 2007). Clinical and epidemiological studies indicate that obese adults are the most sedentary with ∼45% not participating in any regular aerobic exercise programme and ∼35% participating irregularly (Kruger et al. 2007). Thus, although the beneficial effects of regular exercise are well known, many obese adults do not choose to, or cannot, exercise. From a public health perspective, it is important to identify other safe, easily managed lifestyle strategies aimed at reducing atherothrombotic risk in overweight and obese adults.
Oxidative stress plays a central role in the pathogenesis of cardiovascular disease and is thought to contribute to the accelerated rate of atherothrombosis in overweight and obese adults (Morrow, 2003). Evidence from experimental studies indicates that reactive oxygen species (ROS) (Shatos et al. 1990) and oxidized low-density lipoprotein (Zhang et al. 1998) can inhibit t-PA mRNA expression and synthesis, and impair the release of t-PA from cultured human endothelium. In addition to blunting the production of t-PA, oxidants may alter the structural characteristics of t-PA, which in turn may adversely affect endothelial fibrinolytic activity. For example, Feng & Hart (1995) demonstrated that the fibrin binding affinity of t-PA and the ability to activate plasminogen are markedly impaired in the presence of oxidant stress. Because obesity is associated with a pro-oxidative state (Morrow, 2003), antioxidant therapy may be an effective alternative strategy to exercise for improving endothelial fibrinolytic function in overweight and obese adults. Currently, it is unknown whether vitamin C favourably affects endothelial fibrinolytic regulation in this at-risk population.
Accordingly, the experimental aims of the present study were to determine the effects of acute intra-arterial vitamin C administration and chronic oral vitamin C supplementation on the capacity of the endothelium to release t-PA in overweight and obese adults.
Methods
Subjects
Thirty-three sedentary adults (22 male/11 female) were studied: 10 normal weight (BMI < 25 kg m−2; 7M/3F); and 23 overweight and obese (BMI ≥ 27.0 kg m−2; 15M/8F). All subjects were sedentary and had not participated in a regular aerobic exercise programme for at least 2 years prior to the start of the study. Subjects were excluded from the study if they presented a history or evidence of hepatic, renal, or haematological disease; peripheral vascular disease; stroke; diabetes (fasting plasma glucose > 7.0 mmol l−1; dyslipoproteinaemia (total cholesterol ≥ 6.0 mmol l−1, LDL-cholesterol ≥ 4.5 mmol l−1, triglycerides ≥ 2.5 mmol l−1; Fedder et al. 2002); and hypertension (BP ≥ 140/90 mmHg; Chobanian et al. 2003). All subjects were screened for clinical evidence of coronary artery disease with resting and maximal exercise electrocardiograms and blood pressure. None of the subjects smoked or were taking medication including vitamins. All of the women were at least 1 year postmenopausal (mean: 7 ± 1 years) and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. All experiments conformed to the Declaration of Helsinki.
Body composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto, Webb City, MO, USA). Percentage body fat was determined by dual energy X-ray absorptiometry (Lunar Radiation Corporation, Madison, WI, USA). Body mass index (BMI) was calculated as weight (kilograms) divided by height (metres) squared. Minimal waist circumference was measured according to standard techniques.
Treadmill exercise test
To assess aerobic fitness subjects performed incremental treadmill exercise using a modified Balke protocol. Maximal oxygen consumption (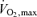 ) was measured using on-line computer-assisted open circuit spirometry. In addition, heart rate and rating of perceived exertion (RPE) were measured throughout exercise and total exercise time to exhaustion was recorded.
) was measured using on-line computer-assisted open circuit spirometry. In addition, heart rate and rating of perceived exertion (RPE) were measured throughout exercise and total exercise time to exhaustion was recorded.
Metabolic measurements
Fasting plasma lipid and lipoprotein, glucose and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the General Clinical Research Center. Insulin resistance was estimated using the homeostasis model assessment (HOMA-IR) derived from fasting glucose and insulin concentrations (Matthews et al. 1985). Plasma concentrations of vitamin C (ascorbic acid) were determined by high performance liquid chromatography using established methods (Frei et al. 1989).
Intra-arterial fibrinolytic protocol
All measurements were performed in a temperature controlled room between 07.00 and 10.00 h after a 12 h overnight fast as previously described by our laboratory (Van Guilder et al. 2005). Briefly, an intravenous catheter was placed in a deep antecubital vein of the non-dominant arm. Thereafter, a 5 cm, 20-gauge catheter was introduced into the brachial artery of the same arm under local anaesthesia (1% lidocaine). Forearm blood flow (FBF) was measured using strain-gauge venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA, USA) and presented as ml (100 ml forearm volume)−1 min−1. Following the measurement of resting blood flow for 5 min, bradykinin was infused intra-arterially at rates of 12.5, 25 and 50 ng (100 ml tissue)−1 min−1 and sodium nitroprusside at 1.0, 2.0 and 4.0 μg (100 ml tissue)−1 min−1 for 5 min at each dose as previously described (Van Guilder et al. 2005). To avoid an order effect, the sequence of drug administration was randomized. Forearm volume was determined by the water displacement method.
Net endothelial release of t-PA antigen and PAI-1 antigen in response to bradykinin and sodium nitroprusside was calculated according to Jern et al. (1997) using the following equation:
where Cv and Ca represent the concentration in the vein and artery, respectively. For both t-PA and PAI-1, a positive difference indicated a net release and a negative difference, net uptake. Arterial and venous blood samples were collected simultaneously at the beginning and the end of each drug dose to determine t-PA and PAI-1 antigen concentrations. All samples were collected into tubes containing 0.45 m sodium citrate buffer, pH 4.3 (Stabilyte, Biopool AB, Sweden), aliquoted and stored for analysis. Plasma concentrations of t-PA and PAI-1 antigen were determined by enzyme immunoassay. Haematocrit was measured in triplicate using the standard microhaematocrit technique and corrected for trapped plasma volume within the trapped erythrocytes. The total amount of t-PA antigen released across the forearm in response to bradykinin was calculated as the total area under each curve above baseline using a trapezoidal model. In order to avoid confounding effects from potential infection/inflammation associated fibrinolytic changes, all subjects were free of recent infection/inflammation (< 2 weeks) as determined by questionnaire.
Acute vitamin C administration
The acute effects of intra-arterial vitamin C on the capacity of the endothelium to release t-PA was determined in 10 normal weight and 8 of the 23 (7M/1F) obese adults. After allowing sufficient time (∼20 min) for FBF and plasma fibrinolytic concentrations to return to baseline following the initial infusions of bradykinin and sodium nitroprusside described above, vitamin C (24 mg min−1) was infused at a constant rate. This dose of vitamin C has been shown to increase the local forearm plasma concentration to levels that protect plasma from free-radical mediated lipid peroxidation (Frei et al. 1989) and to improve endothelial function in obese adults (Perticone et al. 2001). After 20 min, the acute vitamin C infusion was maintained at the same rate whilst the bradykinin and sodium nitroprusside dose–response curves were repeated in the same order as performed earlier. Net endothelial release rates of t-PA antigen and PAI-1 antigen were determined at time 0, after 20 min vitamin C infusion, and after each dose of bradykinin and sodium nitroprusside.
Vitamin C supplementation
Seventeen of the 23 obese adults (10 males/7 females) participated in a 3 month oral vitamin C supplementation intervention. Each morning, subjects consumed one 500 mg tablet of timed-release vitamin C by mouth, except on the morning of a testing session. Subjects did not take any other vitamins during the course of the study. Subjects returned to the General Clinical Research Center every 2 weeks to have their body weight measured and meet with a study research assistant to discuss any problems he/she may be experiencing. Adherence to the vitamin C supplementation study was documented every 2 weeks by pill counts and measurement of plasma vitamin C concentrations at baseline, 6 and 12 weeks.
Statistical analysis
Differences in subject baseline characteristics and area under the curve data were determined by between-groups analysis of variance (ANOVA). Group differences in FBF and endothelial t-PA and PAI-1 antigen release in response to bradykinin and sodium nitroprusside were determined by repeated measures ANOVA. When indicated by a significant F-value, a post hoc test using the Newman–Keuls method was performed to identify differences amongst the groups. Relations between variables of interest were assessed by Pearson's correlation coefficient and linear regression analysis. Changes in basal and agonist-stimulated endothelial t-PA and PAI-1 antigen release in response to both acute and chronic vitamin C administration were determined by repeated measures ANOVA. All data are expressed as means ± s.e.m. Statistical significance was set a priori at P < 0.05.
Results
Subjects
Table 1 presents selected subject characteristics. By design, all anthropometric characteristics were higher (P < 0.01) in overweight/obese adults compared with normal weight controls. There were no differences in age, blood pressure, 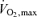 , plasma lipid and lipoprotein concentrations, and baseline plasma levels of vitamin C between the groups. Although within clinically normal ranges, plasma glucose and insulin concentrations, as well as HOMA-IR were higher (P < 0.01) in the overweight/obese compared with normal weight subjects.
, plasma lipid and lipoprotein concentrations, and baseline plasma levels of vitamin C between the groups. Although within clinically normal ranges, plasma glucose and insulin concentrations, as well as HOMA-IR were higher (P < 0.01) in the overweight/obese compared with normal weight subjects.
Table 1.
Selected subject characteristics
| Normal weight | Overweight/obese | |
|---|---|---|
| n | 10 | 23 |
| Age (years) | 53 ± 1 | 54 ± 2 |
| Sex (M/F) | 7/3 | 15/8 |
| Body mass (kg) | 71.2 ± 1.7 | 93.5 ± 3.6* |
| BMI (kg m−2) | 23.4 ± 0.5 | 31.2 ± 0.8* |
| Body fat (%) | 25.7 ± 2.8 | 36.9 ± 1.5* |
| Waist circumference (cm) | 82.9 ± 1.8 | 103.5 ± 2.8* |
| Systolic BP (mmHg) | 116 ± 4 | 122 ± 2 |
| Diastolic BP (mmHg) | 76 ± 3 | 80 ± 1 |
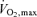 (l min−1) (l min−1) |
2.6 ± 0.2 | 2.7 ± 0.2 |
| Total cholesterol (mmol l−1) | 5.0 ± 0.2 | 5.1 ± 0.2 |
| LDL-cholesterol (mmol l−1) | 3.2 ± 0.2 | 3.3 ± 0.2 |
| HDL-cholesterol (mmol l−1) | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Triglycerides (mmol l−1) | 1.1 ± 0.1 | 1.5 ± 0.2 |
| Glucose (mmol l−1) | 4.8 ± 0.1 | 5.3 ± 0.1* |
| Insulin (pmol l−1) | 25.2 ± 2.5 | 44.3 ± 5.0* |
| HOMA-IR | 0.9 ± 0.1 | 1.8 ± 0.2* |
| Plasma vitamin C (mg dl−1) | 1.3 ± 0.1 | 1.3 ± 0.1 |
Values are means ± s.e.m. BMI: body mass index; BP: blood pressure; LDL: low-density lipoprotein; HDL: high-density lipoprotein; HOMA-IR: homeostasis model of insulin resistance. *P < 0.05 versus normal weight.
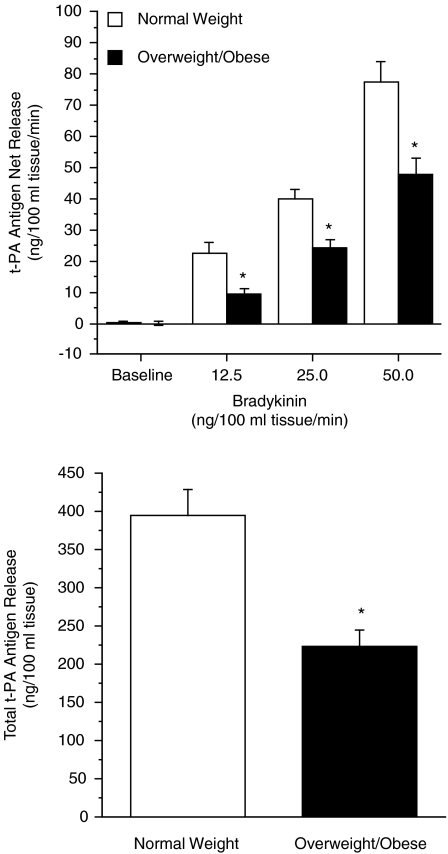
There were no significant differences in resting plasma arterial or venous concentrations of t-PA antigen (Table 2) or PAI-1 antigen (Table 3) between the normal weight and overweight/obese groups. Basal endothelial t-PA release was not significantly different between the groups. However, the capacity of the endothelium to release t-PA in response to bradykinin was markedly blunted in the obese compared with normal weight subjects (Fig. 1). Net release of t-PA antigen was ∼45% less (P < 0.01) in the obese (from 0.3 ± 0.7 to 45.7 ± 5.4 ng (100 ml tissue)−1 min−1) compared with normal weight subjects (from 0.2 ± 0.5 to 74.4 ± 7.9 ng (100 ml tissue)−1 min−1). Consequently, the total amount of t-PA release (area under the bradykinin curve) was significantly lower (70%) in the obese (207 ± 25 ng (100 ml tissue)−1) than normal weight adults (351 ± 38 ng (100 ml tissue)−1); Fig. 1. There was no consistent or significant effect of sodium nitroprusside on t-PA release in either the normal weight (from −0.2 ± 1.2 to 6.5 ± 9.9 ng (100 ml tissue)−1 min−1) or obese (from −0.6 ± 0.7 to 1.8 ± 3.8 ng (100 ml tissue)−1 min−1) subjects. Neither bradykinin nor sodium nitroprusside evoked significant changes in PAI-1 antigen release in either group (data not shown).
Table 2.
Plasma arterial and venous concentrations of t-PA antigen (ng ml−1) at baseline and in response to bradykinin (ng (100 ml tissue)−1 min−1) and sodium nitroprusside (μg (100 ml tissue)−1 min−1) in the normal weight and overweight/obese adults
| Bradykinin | Sodium nitroprusside | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12.5 | 25.0 | 50.0 | Baseline | 1.0 | 2.0 | 4.0 | |
| Cross-sectional Study | ||||||||
| Normal weight | ||||||||
| Venous concentration | 9.3 ± 1.0 | 12.7 ± 0.9* | 14.7 ± 0.9* | 19.2 ± 1.0* | 8.6 ± 1.1 | 8.8 ± 1.1 | 8.8 ± 0.8 | 9.1 ± 1.2 |
| Arterial concentration | 8.9 ± 0.9 | 9.1 ± 0.9 | 9.9 ± 0.9 | 10.5 ± 0.8 | 8.8 ± 1.0 | 8.4 ± 1.0 | 8.5 ± 0.9 | 9.5 ± 1.3 |
| Overweight/obese | ||||||||
| Venous concentration | 10.1 ± 0.8 | 13.0 ± 1.0 | 14.1 ± 1.1* | 17.6 ± 1.4* | 9.9 ± 1.2 | 10.3 ± 1.1 | 11.2 ± 1.4 | 11.3 ± 1.8 |
| Arterial concentration | 9.9 ± 0.8 | 10.7 ± 0.9 | 11.0 ± 0.9 | 11.5 ± 0.9 | 10.1 ± 1.2 | 10.5 ± 1.1 | 11.0 ± 1.5 | 11.0 ± 1.8 |
| Intra-arterial vitamin C | ||||||||
| Saline | ||||||||
| Normal weight | ||||||||
| Venous concentration | 10.1 ± 1.8 | 13.3 ± 1.2 | 15.6 ± 1.1* | 19.6 ± 0.9* | 9.4 ± 1.7 | 9.6 ± 1.7 | 9.6 ± 1.1 | 9.7 ± 1.9 |
| Arterial concentration | 9.0 ± 1.5 | 9.5 ± 1.4 | 10.7 ± 1.2 | 10.6 ± 1.3 | 9.3 ± 1.5 | 9.0 ± 1.4 | 8.7 ± 1.3 | 10.5 ± 2.0 |
| Overweight/obese | ||||||||
| Venous concentration | 9.7 ± 1.6 | 11.6 ± 1.7 | 13.8 ± 1.9 | 16.9 ± 2.3* | 9.1 ± 1.6 | 9.7 ± 1.6 | 10.2 ± 1.7 | 9.6 ± 1.6 |
| Arterial concentration | 10.0 ± 1.6 | 10.2 ± 1.5 | 11.1 ± 1.6 | 11.8 ± 1.7 | 9.5 ± 1.6 | 9.9 ± 1.6 | 10.0 ± 1.5 | 9.9 ± 1.6 |
| Vitamin C | ||||||||
| Normal weight | ||||||||
| Venous concentration | 8.5 ± 1.5 | 14.2 ± 1.0* | 16.3 ± 1.6* | 18.8 ± 1.3* | 7.2 ± 2.3 | 6.3 ± 1.5 | 9.0 ± 2.9 | 9.0 ± 2.5 |
| Arterial concentration | 9.2 ± 1.8 | 9.6 ± 1.6 | 10.6 ± 1.3 | 9.3 ± 1.3 | 8.8 ± 2.1 | 7.5 ± 1.6 | 6.6 ± 1.4 | 7.7 ± 2.3 |
| Overweight/obese | ||||||||
| Venous concentration | 9.0 ± 1.6 | 13.7 ± 1.5 | 14.6 ± 1.7* | 19.9 ± 2.8* | 5.3 ± 4.7 | 6.2 ± 5.1 | 7.4 ± 5.8 | 6.7 ± 6.0 |
| Arterial concentration | 9.0 ± 1.5 | 10.0 ± 1.5 | 10.2 ± 1.5 | 11.1 ± 1.7 | 6.7 ± 6.0 | 6.7 ± 5.6 | 6.5 ± 5.8 | 6.5 ± 5.8 |
| Vitamin C Intervention | ||||||||
| Before supplementation | ||||||||
| Venous concentration | 10.5 ± 0.9 | 13.8 ± 1.1 | 14.7 ± 1.2* | 18.6 ± 1.6* | 10.4 ± 1.4 | 10.6 ± 1.3 | 11.6 ± 1.7 | 11.9 ± 2.3 |
| Arterial concentration | 10.3 ± 0.9 | 11.2 ± 1.0 | 11.3 ± 0.9 | 11.9 ± 0.9 | 10.5 ± 1.4 | 10.9 ± 1.8 | 11.3 ± 1.9 | 11.5 ± 2.3 |
| After supplementation | ||||||||
| Venous concentration | 13.1 ± 1.1 | 16.5 ± 1.4 | 18.0 ± 1.5* | 20.7 ± 1.4* | 12.8 ± 1.7 | 13.8 ± 3.0 | 13.2 ± 1.6 | 11.8 ± 1.2 |
| Arterial concentration | 13.2 ± 1.2 | 13.4 ± 1.2 | 13.3 ± 1.0 | 13.5 ± 1.0 | 12.3 ± 1.4 | 12.3 ± 1.5 | 12.6 ± 1.5 | 12.0 ± 1.2 |
P < 0.05 versus baseline same group.
Table 3.
Plasma arterial and venous concentrations of PAI-1 antigen (ng ml−1) at baseline in response to bradykinin (ng (100 ml tissue)−1 min−1) and sodium nitroprusside (μg (100 ml tissue)−1 min−1) in the normal weight and overweight/obese adults
| Bradykinin | Sodium nitroprusside | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12.5 | 25.0 | 50.0 | Baseline | 1.0 | 2.0 | 4.0 | |
| Cross-sectional Study | ||||||||
| Normal weight | ||||||||
| Venous concentration | 10.2 ± 2.1 | 11.5 ± 2.4 | 10.4 ± 2.3 | 11.2 ± 2.2 | 9.7 ± 1.9 | 10.2 ± 1.9 | 10.1 ± 1.9 | 9.9 ± 1.9 |
| Arterial concentration | 10.7 ± 2.2 | 10.9 ± 2.3 | 10.6 ± 2.2 | 10.2 ± 2.2 | 10.0 ± 2.0 | 9.8 ± 1.9 | 10.4 ± 2.1 | 9.7 ± 2.0 |
| Overweight/obese | ||||||||
| Venous concentration | 15.2 ± 2.7 | 15.8 ± 2.7 | 15.3 ± 2.7 | 15.4 ± 2.6 | 13.7 ± 2.3 | 14.0 ± 2.3 | 14.1 ± 2.3 | 14.1 ± 2.4 |
| Arterial concentration | 17.8 ± 3.0 | 15.4 ± 2.7 | 14.9 ± 2.8 | 15.1 ± 2.7 | 15.1 ± 2.5 | 13.8 ± 2.2 | 13.8 ± 2.3 | 14.2 ± 2.5 |
| Intra-arterial vitamin C | ||||||||
| Saline | ||||||||
| Normal weight | ||||||||
| Venous concentration | 10.0 ± 2.5 | 9.4 ± 2.4 | 9.5 ± 2.4 | 9.7 ± 2.2 | 8.7 ± 2.1 | 10.2 ± 2.5 | 10.1 ± 2.3 | 9.3 ± 2.1 |
| Arterial concentration | 10.0 ± 2.2 | 10.4 ± 2.5 | 10.0 ± 2.3 | 9.7 ± 2.3 | 9.1 ± 2.2 | 9.2 ± 2.3 | 8.8 ± 2.2 | 8.7 ± 2.2 |
| Overweight/obese | ||||||||
| Venous concentration | 14.6 ± 4.3 | 15.0 ± 4.2 | 15.3 ± 4.3 | 14.8 ± 4.2 | 12.2 ± 2.9 | 12.5 ± 3.1 | 12.7 ± 2.9 | 12.1 ± 2.7 |
| Arterial concentration | 16.1 ± 4.6 | 15.4 ± 4.3 | 14.5 ± 4.3 | 14.9 ± 4.3 | 13.2 ± 3.2 | 13.1 ± 3.2 | 13.1 ± 3.5 | 13.2 ± 3.4 |
| Vitamin C | ||||||||
| Normal weight | ||||||||
| Venous concentration | 7.2 ± 1.7 | 8.6 ± 2.3 | 8.4 ± 2.3 | 8.0 ± 2.0 | 7.2 ± 2.3 | 6.3 ± 1.5 | 9.0 ± 2.9 | 9.0 ± 2.5 |
| Arterial concentration | 8.8 ± 2.2 | 9.2 ± 2.3 | 8.7 ± 2.2 | 8.0 ± 2.0 | 8.8 ± 2.1 | 7.5 ± 1.6 | 6.6 ± 1.4 | 7.7 ± 2.3 |
| Overweight/obese | ||||||||
| Venous concentration | 8.6 ± 1.4 | 8.8 ± 1.5 | 8.6 ± 1.5 | 9.2 ± 1.4 | 5.3 ± 4.7 | 6.2 ± 5.1 | 7.4 ± 5.8 | 6.7 ± 6.0 |
| Arterial concentration | 10.1 ± 1.5 | 10.1 ± 2.0 | 9.5 ± 1.7 | 8.8 ± 1.5 | 6.7 ± 6.0 | 6.7 ± 5.6 | 6.5 ± 5.8 | 6.5 ± 5.8 |
| Vitamin C intervention | ||||||||
| Before supplementation | ||||||||
| Venous concentration | 18.1 ± 3.5 | 18.5 ± 3.6 | 17.7 ± 3.7 | 18.0 ± 3.4 | 14.6 ± 3.0 | 14.9 ± 2.9 | 14.6 ± 3.0 | 15.2 ± 3.2 |
| Arterial concentration | 21.6 ± 3.8 | 18.2 ± 3.6 | 17.8 ± 3.7 | 17.7 ± 3.5 | 15.9 ± 3.3 | 14.3 ± 2.8 | 14.3 ± 3.0 | 15.1 ± 3.3 |
| After supplementation | ||||||||
| Venous concentration | 18.6 ± 4.1 | 18.5 ± 3.5 | 17.8 ± 3.5 | 17.9 ± 3.2 | 19.3 ± 4.9 | 18.2 ± 3.9 | 17.6 ± 4.0 | 17.6 ± 3.9 |
| Arterial concentration | 18.5 ± 4.5 | 17.4 ± 3.9 | 15.3 ± 3.7 | 16.0 ± 3.4 | 20.2 ± 5.2 | 18.7 ± 4.2 | 17.8 ± 4.1 | 16.2 ± 3.7 |
Figure 1. Net release and total amount of tissue-type plasminogen activator (t-PA) antigen released (area under the curve) across the forearm in response to bradykinin in normal weight and overweight/obese adults.
Values are means ± s.e.m.; *P < 0.05 versus normal weight.
Acute intra-arterial vitamin C administration
Infusion of vitamin C significantly increased forearm plasma vitamin C concentrations to similar levels (range: 13.0–18.2 mg dl−1) in both the normal weight and obese subjects. FBF responses to bradykinin and sodium nitroprusside were not significantly affected by vitamin C administration in either group (Table 4). There was no change in baseline net release rates of t-PA antigen to vitamin C in either group. In the normal weight adults, co-infusion of vitamin C did not significantly affect the t-PA response to bradykinin (Fig. 2). Net release rates of t-PA were similar after (from −0.7 ± 0.9 to 95.0 ± 16.2 ng (100 ml tissue)−1 min−1) versus before (from 0.2 ± 0.2 to 88.6 ± 11.9 ng (100 ml tissue)−1 min−1) the administration of vitamin C. In contrast to the normal weight subjects, net endothelial release of t-PA was ∼90% higher (P < 0.01) after (from −0.9 ± 1.1 to 94.6 ± 16.2 ng (100 ml tissue)−1 min−1) compared with before (from −0.8 ± 0.8 to 49.9 ± 7.7 ng (100 ml tissue)−1 min−1) the administration of vitamin C in obese adults (Fig. 2). Moreover, the total amount of t-PA antigen released in response to bradykinin significantly increased from 206 ± 32 to 385 ± 59 ng (100 ml tissue)−1 with vitamin C (Fig. 2). There was no significant effect of vitamin C on the t-PA response to sodium nitroprusside or on the PAI-1 antigen response to either bradykinin or sodium nitroprusside in either group (data not shown).
Table 4.
Forearm Blood Flow (ml (100 ml tissue)−1 min−1) responses to bradykinin (ng (100 ml tissue)−1 min−1) and sodium nitroprusside (μg (100 ml tissue)−1 min−1) in the normal weight and overweight/obese adults
| Bradykinin | Sodium nitroprusside | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12.5 | 25.0 | 50.0 | Baseline | 1.0 | 2.0 | 4.0 | |
| Cross-sectional study | ||||||||
| Normal weight | 4.6 ± 0.3 | 13.7 ± 0.8 | 15.6 ± 0.6 | 17.6 ± 0.9 | 4.7 ± 0.3 | 13.5 ± 0.4 | 15.3 ± 0.4 | 16.9 ± 0.4 |
| Overweight/obese | 4.2 ± 0.2 | 10.3 ± 0.7* | 11.8 ± 0.7* | 13.5 ± 0.9* | 4.0 ± 0.4 | 12.2 ± 0.5 | 14.3 ± 0.5 | 16.5 ± 0.5 |
| Intra-arterial vitamin C | ||||||||
| Normal weight | ||||||||
| Saline | 4.5 ± 0.3 | 13.1 ± 0.8 | 14.9 ± 0.7 | 17.1 ± 0.7 | 3.9 ± 0.4 | 12.7 ± 0.4 | 15.5 ± 1.2 | 17.3 ± 1.0 |
| Vitamin C | 4.1 ± 0.2 | 13.5 ± 0.5 | 15.6 ± 0.7 | 17.5 ± 0.7 | 3.8 ± 0.3 | 13.3 ± 1.8 | 15.5 ± 1.3 | 17.7 ± 1.1 |
| Overweight/obese | ||||||||
| Saline | 4.4 ± 0.4 | 10.7 ± 1.1 | 12.9 ± 0.9 | 15.8 ± 1.1 | 4.1 ± 0.2 | 12.3 ± 0.8 | 14.1 ± 0.9 | 16.8 ± 0.7 |
| Vitamin C | 4.3 ± 0.4 | 11.2 ± 0.8 | 13.1 ± 1.1 | 16.6 ± 1.0 | 4.3 ± 0.2 | 13.5 ± 1.0 | 15.7 ± 0.7 | 17.4 ± 0.4 |
| Vitamin C intervention | ||||||||
| Before supplementation | 4.2 ± 0.3 | 10.3 ± 0.7 | 11.4 ± 0.9 | 12.8 ± 0.9 | 4.0 ± 0.3 | 11.0 ± 0.6 | 13.5 ± 0.4 | 15.5 ± 0.6 |
| After supplementation | 4.1 ± 0.2 | 11.6 ± 0.8 | 13.2 ± 0.8 | 15.4 ± 0.8† | 4.1 ± 0.3 | 11.4 ± 0.6 | 13.3 ± 0.8 | 14.5 ± 0.8 |
P < 0.05 versus normal weight
P < 0.05 versus before supplementation.
Figure 2. Net release and total amount of tissue-type plasminogen activator (t-PA) antigen released (area under the curve) across the forearm in response to bradykinin before and after intra-arterial vitamin C administration in normal weight and overweight/obese adults.
Values are means ± s.e.m. *P < 0.05 versus saline.
Vitamin C supplementation study
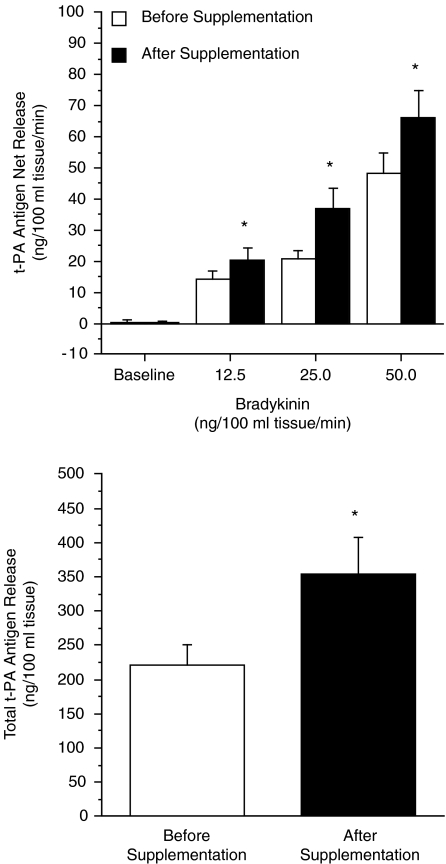
All 17 overweight and obese adults completed the 3 month vitamin C supplementation programme. There were no significant changes in anthropometric, haemodynamic or metabolic characteristics following the vitamin C intervention (Table 5). Vitamin C supplementation significantly increased (∼35%) plasma concentrations of vitamin C at 6 and 12 weeks (Table 5). FBF responses to bradykinin were not significantly different after compared with before vitamin C supplementation (Table 4). There was no significant affect of the vitamin C intervention on the FBF response to sodium nitroprusside (data not shown). Vitamin supplementation did not affect resting plasma arterial and venous concentrations of t-PA antigen (Table 2) and PAI-1 antigen (Table 3). Basal net release rates of t-PA antigen were not significantly different after the vitamin C supplementation programme. However, endothelial t-PA release in response to bradykinin was markedly higher (P < 0.05) after (from 0.3 ± 0.5 to 66.3 ± 8.7 ng (100 ml tissue)−1 min−1) compared with before (from 0.2 ± 0.9 to 48.2 ± 6.5 ng (100 ml tissue)−1 min−1) vitamin C supplementation. The total amount of t-PA antigen released to bradykinin increased by ∼60% (P < 0.05) after vitamin C supplementation (355 ± 54 versus 221 ± 29 ng (100 ml tissue)−1), Fig. 3. There was no significant effect of vitamin C supplementation on the t-PA response to sodium nitroprusside or on the PAI-1 antigen response to either bradykinin or sodium nitroprusside (data not shown).
Table 5.
Selected subject characteristics of the vitamin C supplementation study
| Before supplementation | After supplementation | |
|---|---|---|
| n | 17 | 17 |
| Body mass (kg) | 94.1 ± 4.2 | 94.1 ± 4.3 |
| BMI (kg m−2) | 31.7 ± 1.0 | 31.6 ± 1.0 |
| Body fat (%) | 37.6 ± 1.5 | 37.4 ± 1.6 |
| Waist circumference (cm) | 104.1 ± 3.4 | 103.2 ± 3.8 |
| Systolic BP (mmHg) | 124 ± 2 | 122 ± 3 |
| Diastolic BP (mmHg) | 80 ± 2 | 77 ± 2 |
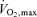 (l min−1) (l min−1) |
2.5 ± 0.2 | 2.5 ± 0.2 |
| Total cholesterol (mmol l−1) | 5.1 ± 0.2 | 5.0 ± 0.1 |
| LDL-cholesterol (mmol l−1) | 3.3 ± 0.2 | 3.2 ± 0.1 |
| HDL-cholesterol (mmol l−1) | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Triglycerides (mmol l−1) | 1.5 ± 0.2 | 1.3 ± 0.2 |
| Glucose (mmol l−1) | 5.3 ± 0.1 | 5.3 ± 0.1 |
| Insulin (pmol l−1) | 49.0 ± 6.2 | 50.5 ± 7.2 |
| HOMA-IR | 1.9 ± 0.3 | 2.0 ± 0.3 |
| Plasma vitamin C (mg dl−1) | 1.2 ± 0.1 | 1.6 ± 0.1* |
Values are mean ± s.e.m. BMI: body mass index; BP: blood pressure; LDL: low-density lipoprotein; HDL: high-density lipoprotein; HOMA-IR: homeostasis model of insulin resistance.
P < 0.05 versus before supplementation.
Figure 3. Net release and total amount of tissue-type plasminogen activator (t-PA) antigen released (area under the curve) across the forearm in response to bradykinin before and after 3 months of vitamin C supplementation in overweight/obese adults.
Values are means ± s.e.m.; *P < 0.05 versus before supplementation.
Discussion
The primary new findings of the present study are as follows. Firstly, in contrast to normal weight subjects, acute intra-arterial vitamin C administration significantly enhanced the capacity of the endothelium to release t-PA in overweight and obese adults. Secondly, daily oral vitamin C supplementation, independent of changes in body composition or habitual physical activity, can restore the ability of the endothelium to release t-PA in overweight and obese adults. Taken together, these results indicate that vitamin C favourably affects endothelial fibrinolytic regulation in this population. Moreover, chronic vitamin C supplementation represents a safe and easily managed lifestyle strategy for improving fibrinolytic potential, which may help to reduce the atherothrombotic risk associated with excess adiposity.
Intra-arterial administration of vitamin C at pharmacological doses has been shown to improve endothelial function, particularly endothelial vasodilator function, in a variety of conditions associated with oxidative stress including hypertension (Taddei et al. 1998), diabetes (Timimi et al. 1998), and obesity (Perticone et al. 2001). For example, Perticone et al. (2001) demonstrated that intra-arterial infusion of vitamin C (24 mg min−1) improves acetylcholine-mediated vasodilatation in middle-aged overweight and obese adults, supporting the concept that oxidative stress contributes to adiposity-related vasodilator dysfunction. The results of the present study extend these findings by demonstrating, for the first time, that intra-arterial vitamin C infusion potentiates endothelial t-PA release in overweight/obese adults. Indeed, the rate and total amount of t-PA antigen released in response to bradykinin with concomitant vitamin C administration increased markedly (∼95% and 90%, respectively) in overweight/obese adults to levels similar to that observed in normal weight controls. These results suggest that oxidative stress is a major contributor to endothelial fibrinolytic dysfunction in overweight and obese individuals. Interestingly, contrary to the study of Perticone and colleagues we did not observe a significant increase in bradykinin-mediated vasodilatation in our overweight/obese subjects. The reasons for this incongruity in the acute effects of intra-arterial vitamin C with acetylcholine and bradykinin on FBF in overweight/obese adults are not clear. It is possible that the favourable effects of vitamin C on endothelial vasodilatation are independent of vasodilatation mediated by endothelial derived hypopolarizing factor, a major contributor to bradykinin, but not acetylcholine.
To our knowledge, one other study has investigated the influence of vitamin C administration on local endothelial fibrinolytic capacity. Pellegrini et al. (2004) demonstrated that acute intra-arterial vitamin C (25 mg min−1) had no affect on endothelial t-PA release in cigarette smokers. The benign influence of vitamin C on endothelial fibrinolytic capacity in cigarette smokers compared with the beneficial effects observed in overweight and obese adults in the present study is intriguing. One potential reason for this discrepancy is likely to be differential effects of cigarette smoking and increased adiposity on endothelial function. Although cigarette smoking and increased adiposity are associated with oxidative stress (Barua et al. 2003; Morrow, 2003), the atherogenic impact of smoking on the endothelium may be more acute and severe. For example, tobacco smoke exerts proatherogenic endothelial effects through chronic activation of pro-inflammatory cytokines (Orosz et al. 2007), increased platelet adhesion and aggregation (Rival et al. 1987), and smooth muscle cell migration and proliferation, as well as promoting monocyte adhesion and transendothelial migration into the subintimal space (Powell, 1998). In this regard, persistent endothelial cell injury in cigarette smokers may cause chronic stimulation of t-PA release to defend against areas of arterial denudation, atheroma and thrombus deposition. Consequently, impaired release of t-PA in smokers may reflect the subsequent depletion of the intracellular storage pool (Newby et al. 2001). This is thought to be an underlying factor in the augmented risk of sudden cardiac death attributed to acute thombotic events in cigarette smokers (Burke et al. 1997). With respect to the potential beneficial effects of vitamin C, the results of the study conducted by Pellegrini et al. (2004) and the present investigation highlight the importance of defining the context in which reduced endothelial release of t-PA is observed.
To corroborate and extend our findings demonstrating the salutary effects of intra-arterial administration of vitamin C on endothelial t-PA release, we performed a follow-up vitamin C intervention trial in overweight/obese adults. The results of our intervention study provide compelling evidence that chronic oral vitamin C supplementation improves the capacity of the endothelium to release t-PA in this at-risk population. The total amount of t-PA antigen released in response to bradykinin increased by ∼60% after 3 months of vitamin C supplementation. Interestingly, the increase in t-PA release in the overweight/obese adults following vitamin C supplementation was similar to that observed in our acute intra-arterial vitamin C study. Of note, this improvement was not associated with changes in body mass, adiposity, arterial blood pressure, plasma cholesterol or maximal aerobic capacity, indicating a primary modulating effect of chronic vitamin C supplementation on endothelial regulation of t-PA release. From a clinical perspective, it is important to emphasize that the improvements observed in endothelial t-PA release were accomplished with a moderate dose of vitamin C (500 mg) that resulted in a significant (∼35%) increase in plasma vitamin C levels. Moreover, the enhancement in endothelial fibrinolytic function observed with chronic vitamin C supplementation was remarkably similar to levels we have previously reported in overweight and obese subjects who participated in a regular aerobic exercise training programme (Van Guilder et al. 2005). Indeed, the total amount of t-PA antigen released in response to bradykinin after vitamin C supplementation (355 ± 54 ng (100 ml tissue)−1) was similar to that observed after exercise training (320 ± 31 ng (100 ml tissue)−1). Considering that many overweight and obese adults do not choose to exercise (Morrato et al. 2007), oral vitamin C supplementation may represent a safe alternative lifestyle intervention for improving endothelial fibrinolytic function and thereby reducing their risk of atherothrombotic vascular disease.
The mechanisms by which vitamin C favourably affects endothelial release of t-PA are not completely understood. Vitamin C plays an important role in protecting endothelial cells from oxidative damage by acting as a reducing agent and antioxidant, free radical scavenger and enzyme cofactor (May, 2000; Padayatty et al. 2003). In fact, vitamin C completely abolishes lipid oxidative damage induced by peroxyl radicals and spares other endogenous antioxidants (Frei et al. 1989). Furthermore, in vitro studies have demonstrated that vitamin C effectively inhibits oxidative modification of LDL (Siow et al. 1998), and scavenges superoxide (Jackson et al. 1998). Therefore, the antioxidant properties of vitamin C are essential for enhancing the survival and function of endothelial cells exposed to oxidative stress (Montecinos et al. 2007). Vitamin C may exert favourable effects on endothelial fibrinolytic regulation by preventing endothelial damage induced by oxidants and through cell-specific antioxidant mechanisms that increase t-PA transcription, synthesis and release (Yoshino et al. 2002).
It is worth noting that although we observed significant group- and intervention-related differences in endothelial t-PA release, basal plasma arterial and venous concentrations of t-PA antigen and PAI-1 antigen were not significantly different between the normal weight and overweight/obese groups, nor were they affected by vitamin C supplementation. The lack of a significant adiposity-related difference in plasma t-PA and PAI-1 antigen concentrations are in contrast with previous studies (Landin et al. 1990; Rosito et al. 2004). This inconsistency may be due to the overall good clinical health of our overweight/obese adults. Indeed, all subjects were carefully screened for cardiometabolic abnormalities that usually accompany increased adiposity and are associated with elevated plasma t-PA and PAI-1 concentrations, such as dyslipidaemia, hyperinsulinaemia, diabetes and coronary artery disease. Importantly, these findings, viewed together, provide further evidence that circulating steady-state plasma concentrations of t-PA antigen and PAI-1 antigen do not reflect the ability of the endothelium to rapidly and locally release t-PA, and, in certain instances, can provide a misleading assessment of endogenous fibrinolytic potential.
There are a number of experimental considerations regarding the present study. Firstly, with respect to the oral vitamin C intervention, we recognize that our study design is somewhat weakened by the lack of a placebo control group. However, the lack of change in anthropometric, haemodynamic or metabolic variables as well as lifestyle behaviour during the intervention suggests that the vitamin C-induced increase in endothelial t-PA release was a primary effect of the intervention. Nevertheless, we cannot dismiss the possibility of study bias and over-estimation of treatment effects. Secondly, from a clinical perspective, epidemiological studies investigating the benefits of dietary intake and/or supplementation with antioxidant vitamins on cardiovascular disease risk have been largely disappointing (Kushi et al. 1996; Losonczy et al. 1996). However, it should be noted that findings from these studies are difficult to interpret for a variety of reasons, the most important of which are flaws in experimental design, the inclusion of healthy and diseased patients with differences in medication use, extent and severity of oxidative stress and disease, and the lack of hard cardiovascular endpoints including myocardial infarction and stroke (Frei, 1999; Carr et al. 2000; Frei, 2003; Morrow, 2003; Padayatty et al. 2003). Nevertheless, the results of the present study are encouraging, and are consistent with other clinical studies supporting a positive role of vitamin C on endothelial function in at-risk populations free of overt cardiovascular disease.
In conclusion, the results of the present study indicate that antioxidant therapy, specifically vitamin C, favourably affects the capacity of the endothelium to release t-PA in overweight and obese adults. Importantly, chronic daily vitamin C supplementation represents a safe and effective lifestyle intervention strategy for improving endothelial fibrinolytic regulation in overweight and obese adults who do not engage in regular aerobic exercise. Improved endothelial fibrinolytic function may alleviate some of the cardiovascular risk and thrombotic burden in this at-risk population.
Acknowledgments
We would like to thank all of the subjects who participated in the study as well as Yoli Casas and Rebecca Keith for their technical assistance. This study was supported by National Institute of Health Awards HL068030, DK062061, HL076434, and RR00051 and American Diabetes Association Clinical Research Award.
References
- Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–2347. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S646–S662. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- Carr AC, Zhu BZ, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and α-tocopherol (vitamin E) Circ Res. 2000;87:349–354. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Fedder DO, Koro CE, L'Italien GJ. New National Cholesterol Education Program III guidelines for primary prevention lipid-lowering drug therapy: projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation. 2002;105:152–156. doi: 10.1161/hc0202.101971. [DOI] [PubMed] [Google Scholar]
- Feng YH, Hart G. In vitro oxidative damage to tissue-type plasminogen activator: a selective modification of the biological functions. Cardiovasc Res. 1995;30:255–261. [PubMed] [Google Scholar]
- Frei B. On the role of vitamin C and other antioxidants in atherogenesis and vascular dysfunction. Proc Soc Exp Biol Med. 1999;222:196–204. doi: 10.1046/j.1525-1373.1999.d01-136.x. [DOI] [PubMed] [Google Scholar]
- Frei B. To C or not to C, that is the question! J Am Coll Cardiol. 2003;42:253–255. doi: 10.1016/s0735-1097(03)00574-6. [DOI] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Jern C, Seeman-Lodding H, Biber B, Winso O, Jern S. An experimental multiple-organ model for the study of regional net release/uptake rates of tissue-type plasminogen activator in the intact pig. Thromb Haemost. 1997;78:1150–1156. [PubMed] [Google Scholar]
- Kruger J, Yore MM, Kohl HW., 3rd Leisure-time physical activity patterns by weight control status: 1999–2002 NHANES. Med Sci Sports Exerc. 2007;39:788–795. doi: 10.1249/mss.0b013e3180333efc. [DOI] [PubMed] [Google Scholar]
- Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- Landin K, Stigendal L, Eriksson E, Krotkiewski M, Risberg B, Tengborn L, Smith U. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator-1. Metabolism. 1990;39:1044–1048. doi: 10.1016/0026-0495(90)90164-8. [DOI] [PubMed] [Google Scholar]
- Losonczy KG, Harris TB, Havlik RJ. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr. 1996;64:190–196. doi: 10.1093/ajcn/64.2.190. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Montecinos V, Guzman P, Barra V, Villagran M, Munoz-Montesino C, Sotomayor K, Escobar E, Godoy A, Mardones L, Sotomayor P, Guzman C, Vasquez O, Gallardo V, van Zundert B, Bono MR, Onate SA, Bustamante M, Carcamo JG, Rivas CI, Vera JC. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J Biol Chem. 2007;282:15506–15515. doi: 10.1074/jbc.M608361200. [DOI] [PubMed] [Google Scholar]
- Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- Morrow JD. Is oxidant stress a connection between obesity and atherosclerosis? Arterioscler Thromb Vasc Biol. 2003;23:368–370. doi: 10.1161/01.ATV.0000063107.86298.FD. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Newby DE, McLeod AL, Uren NG, Flint L, Ludlam CA, Webb DJ, Fox KA, Boon NA. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001;103:1936–1941. doi: 10.1161/01.cir.103.15.1936. [DOI] [PubMed] [Google Scholar]
- Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol. 2007;292:H130–H139. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Pellegrini MP, Newby DE, Johnston NR, Maxwell S, Webb DJ. Vitamin C has no effect on endothelium-dependent vasomotion and acute endogenous fibrinolysis in healthy smokers. J Cardiovasc Pharmacol. 2004;44:117–124. doi: 10.1097/00005344-200407000-00016. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3:21–28. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- Rea TD, Heckbert SR, Kaplan RC, Psaty BM, Smith NL, Lemaitre RN, Lin D. Body mass index and the risk of recurrent coronary events following acute myocardial infarction. Am J Cardiol. 2001;88:467–472. doi: 10.1016/s0002-9149(01)01720-9. [DOI] [PubMed] [Google Scholar]
- Rival J, Riddle JM, Stein PD. Effects of chronic smoking on platelet function. Thromb Res. 1987;45:75–85. doi: 10.1016/0049-3848(87)90258-1. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2007;27:1651–1656. doi: 10.1161/ATVBAHA.107.143248. [DOI] [PubMed] [Google Scholar]
- Rosito GA, D'Agostino RB, Massaro J, Lipinska I, Mittleman MA, Sutherland P, Wilson PW, Levy D, Muller JE, Tofler GH. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91:683–689. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- Sciacqua A, Candigliota M, Ceravolo R, Scozzafava A, Sinopoli F, Corsonello A, Sesti G, Perticone F. Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care. 2003;26:1673–1678. doi: 10.2337/diacare.26.6.1673. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Doherty JM, Stump DC, Thompson EA, Collen D. Oxygen radicals generated during anoxia followed by reoxygenation reduce the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in human endothelial cell culture. J Biol Chem. 1990;265:20443–20448. [PubMed] [Google Scholar]
- Siow RC, Sato H, Leake DS, Pearson JD, Bannai S, Mann GE. Vitamin C protects human arterial smooth muscle cells against atherogenic lipoproteins: effects of antioxidant vitamins C and E on oxidized LDL-induced adaptive increases in cystine transport and glutathione. Arterioscler Thromb Vasc Biol. 1998;18:1662–1670. doi: 10.1161/01.atv.18.10.1662. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1998;31:552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab. 2005;289:E807–E813. doi: 10.1152/ajpendo.00072.2005. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Suzuki K, Urano T, Aoki K, Takada Y, Kazui T, Takada A. Enhanced secretion of tissue plasminogen activator by simultaneous use of retinoic acid and ascorbic acid from tissue cultured gastroepiploic artery. Life Sci. 2002;70:1461–1470. doi: 10.1016/s0024-3205(01)01513-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ren S, Sun D, Shen GX. Influence of glycation on LDL-induced generation of fibrinolytic regulators in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1140–1148. doi: 10.1161/01.atv.18.7.1140. [DOI] [PubMed] [Google Scholar]