Abstract
The inflation of an intravascular balloon positioned at the superior vena cava and right atrial junction (SVC-RAJ) reduces sodium or water intake induced by various experimental procedures (e.g. sodium depletion; hypovolaemia). In the present study we investigated if the stretch induced by a balloon at this site inhibits a rapid onset salt appetite, and if this procedure modifies the pattern of immunohistochemical labelling for Fos protein (Fos-ir) in the brain. Male Sprague–Dawley rats with SVC-RAJ balloons received a combined treatment of furosemide (Furo; 10 mg (kg bw)−1) plus a low dose of the angiotensin-converting enzyme inhibitor captopril (Cap; 5 mg (kg bw)−1). Balloon inflation greatly decreased the intake of 0.3 m NaCl for as long as the balloon was inflated. Balloon inflation over a 3 h period following Furo–Cap treatment decreased Fos-ir in the organum vasculosum of the lamina terminalis and the subfornical organ and increased Fos-ir in the lateral parabrachial nucleus and caudal ventrolateral medulla. The effect of balloon inflation was specific for sodium intake because it did not affect the drinking of diluted sweetened condensed milk. Balloon inflation and deflation also did not acutely change mean arterial pressure. These results suggest that activity in forebrain circumventricular organs and in hindbrain putative body fluid/cardiovascular regulatory regions is affected by loading low pressure mechanoreceptors at the SVC-RAJ, a manipulation that also attenuates salt appetite.
Disturbances of body fluid balance activate behavioural, hormonal and autonomic responses to correct such perturbations and restore water and sodium homeostasis. The brain receives both neural and hormonal signals that provide information about the status of body fluids. Low pressure cardiopulmonary receptors are one source of important afferent input controlling the activity of brain networks implicated in maintaining fluid balance. One set of cardiopulmonary receptors involved in the regulation of fluid homeostasis resides in the systemic circulation in the vicinity of the superior vena cava–right atrial junction (SVC-RAJ).
In rats, small intravascular balloons can be placed at the SVC-RAJ, and inflation of these caval–atrial balloons stimulates low pressure receptors without interfering with venous return to the heart (Kaufman, 1984). Stretch of the SVC-RAJ attenuates water drinking in response to 24 h water deprivation, subcutaneous injection of isoproterenol, peritoneal dialysis with hyperoncotic colloid, and ad libitum water intake (Kaufman, 1984). Inflation of balloons placed at the SVC-RAJ has also been shown to decrease sodium intake in two models of slowly developing salt appetite induced by overnight sodium depletion by peritoneal dialysis with hypertonic colloid or by daily injections of deoxycorticosterone acetate (DOCA; Toth et al. 1987). It is interesting to note that inflation of atrial balloons does not affect drinking in response to intravenous hypertonic saline in rats (Kaufman, 1984). This suggests that inflation of SVC-RAJ balloons does not interfere in a non-specific manner with fluid ingestion per se, but specifically inhibits behaviours related to extracellular fluid deficits or pharmacological treatments that mimic aspects of extracellular fluid depletion (Kaufman, 1984).
Several studies have used immunohistochemistry for Fos, the protein product of the proto-onco gene c-fos, to map changes in activity of this immediate early gene (Priestley, 1987) in response to isotonic volume expansion or stretch of the SVC-RAJ in conscious and anaesthetized animals. Isotonic volume expansion results in increased Fos immunoreactivity (Fos-ir) in the area postrema (AP), nucleus of the solitary tract (NTS), caudal ventrolateral medulla (CVLM), and the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei (Badoer et al. 1997; Randolph et al. 1998; Potts et al. 2000; Cunningham et al. 2002). Stretch of the SVC-RAJ in female rats increases Fos-ir in the PVN (Deng & Kaufman, 1998). In anaesthetized rats inflation of an atrial balloon increases Fos-ir in parvocellular neurons in the PVN and also inhibits renal sympathetic nerve activity (Pyner et al. 2002).
In the present study we investigated whether stretch of the SVC-RAJ inhibits an acutely generated salt appetite which has been shown to induce changes in brain Fos (Zardetto-Smith et al. 1993; Thunhorst et al. 1998). Salt appetite was produced by combined treatment with the diuretic furosemide (Furo) and the angiotensin-converting enzyme inhibitor captopril (Cap). In rats this treatment produces a significant intake of hypertonic saline solution (the operational definition of a salt appetite) within a short period of time (1–2 h; Fitts & Masson, 1989) due to increased formation of angiotensin II (ANG II) in the brain and acute hypovolaemia and hypotension (Thunhorst et al. 1994; Thunhorst & Fitts, 1994; Thunhorst & Johnson, 1994). Because of the nature of the brief time course for the production of Furo–Cap-induced salt appetite, the method is ideal for using Fos-ir to characterize brain nuclei in which c-fos expression is altered (Zardetto-Smith et al. 1993; Thunhorst et al. 1998) by experimental interventions (e.g. SVC-RAJ balloon inflation). In addition, brain regional Fos-ir was characterized in Furo–Cap treated animals under conditions with the atrial balloon inflated or deflated. In further experiments the specificity of the effects of SVC-RAJ balloon inflation was determined by demonstrating that the manipulation has no effect on the intake of a palatable milk solution in sated rats. Finally the effects of stretch of the SVC-RAJ on blood pressure and heart rate were investigated.
Methods
Animals
Male Sprague–Dawley rats weighing about 300 g were used. They were housed individually in hanging, stainless steel cages with free access to standard laboratory diet (Harlan Teklad Laboratory Diets), water and 0.3 m NaCl solution. Temperature was maintained at 23 ± 2°C and humidity at 55 ± 10%, on a 12: 12 light: dark cycle (onset at 06.00 h). All procedures were in accordance with the Animal Care and Use policies of the University of Iowa.
Drugs
Furosemide was administered s.c. at 10 mg (kg bw)−1. Captopril (Sigma, St Louis, MO, USA) was administered s.c. at 5 mg (kg bw)−1.
Surgery
Inflatable balloons were made of Silastic tubing. One end of the tubing was sealed with silicone glue and allowed to dry. Then, a small portion close to this end was warmed, stretched, and expanded repeatedly by injecting saline into it. The section of the stretch-stressed tubing remains expandable when it is at body temperature and inflated with saline. Femoral catheters were made from pieces of polyethylene tubing (PE-50) ∼20 cm in length that were heat-welded to shorter pieces of PE-10, 3 cm in length.
The balloon was inserted under an Equithesin-like (0.30 ml per 100 g bw) anaesthetic (composed of 194.4 ml sodium pentobarbital (50 mg ml−1) and 42.5 g chloral hydrate per 1000 ml distilled water; 3 ml kg−1, i.p.; University of Iowa Hospital Pharmacy, Iowa City, IA, USA). An incision was made in the neck and the right jugular vein was exposed. A small incision was made in the jugular through which the inflatable balloon was inserted and positioned to terminate at the SVC-RAJ (Kaufman, 1984). In the rat, the left superior vena cava joins the inferior vena cava, so that placement of the balloon does not interfere with venous return from the head to the heart (Kaufman, 1984). The other end of the cannula was led subcutaneously to the nape of the neck, where it was exposed and sutured in place. The animals were allowed 4 days to recover from surgery before the experiments began. During the experiments, balloons were inflated by filling them with isotonic saline, and the open end of the Silastic tubing was closed with a stainless steel obturator. For this procedure, the animals were held by hand while a 1 ml syringe was attached to the Silastic balloon catheter.
For femoral artery catheter placement, rats were anaesthetized with the Equithesin-like anaesthetic (0.33 ml per 100 g bw). The PE-10 end of the catheter was inserted ∼3 cm into the abdominal aorta through the femoral artery. The PE-50 end of the catheter was tunneled subcutaneously and exposed at the back of the neck. It was filled with heparinized saline and plugged with a stainless steel obturator.
Procedures
Sodium and water intakes after fluid-depletion
One group of rats was used to study the effects of balloon inflation on water and sodium intake in response to treatment with Furo–Cap. All animals received two pretests with Furo–Cap treatment before experimental testing with balloon inflation. These pretests ensured that all animals were reliable responders to the depletion procedures. Testing was performed with the animals in their home cages. Water, saline and food were removed from the cages, and all rats were injected with Furo (10 mg (kg bw)−1, s.c.) and again 5 min later with a low dose of Cap (5 mg (kg bw)−1, s.c.). In the two pilot tests, water and 0.3 m NaCl were provided 1 h later in glass burettes (0.1 ml resolution) placed on the front of each cage and intakes were recorded for 2 h. In the experimental tests, the atrial balloon was inflated (i.e. using 0.1 ml of isotonic saline) in half of the animals 10 min after injections of Furo–Cap, and in the other half the balloon was not inflated. One hour later, water and saline were provided as above and intakes were measured at 15, 30, 45, 60, 90 and 120 min. Then, the balloons were deflated for those rats in which they had been inflated, and the intakes of water and 0.3 m NaCl were measured for another 15, 30 and 60 min. Two days later, the procedure was repeated with the treatment and balloon inflation conditions reversed for each rat. Thus, each animal served as its own control.
Fos-ir after fluid depletion
One group of rats was used to study the effects of balloon inflation on expression of c-fos in response to treatment with Furo–Cap. Testing was performed in the animals' home cages. Water, saline and food were removed from the cages, and all rats were injected with Furo (10 mg (kg bw)−1, s.c.) and again 5 min later with a low dose of Cap (5 mg (kg bw)−1, s.c.). All animals received one pilot test as above with the Furo–Cap treatment before an experimental test with the balloons inflated. In the experimental tests, the atrial balloon was inflated (0.1 ml) in half of the animals 10 min after injections of Furo–Cap, and in the other half the balloon was not inflated. The balloon remained inflated for 3 h. then both groups of animals were killed for subsequent immunohistochemistry.
Perfusion and Fos immunohistochemistry
At the designated time during testing, the rats were anaesthetized with a lethal dose of pentobarbital sodium (50–75 mg, 1–1.5 ml per rat). The heart was exposed, and the animal was perfused via the ascending aorta with ∼200 ml of 0.01 m phosphate buffered saline (PBS; pH 7.6) followed by ∼200 ml of 4% paraformaldehyde in 0.1 m PBS. The brain was removed and post-fixed in 4% paraformaldehyde for ∼4 h and then placed in 30% sucrose in 0.01 m PBS overnight. Sectioning was usually done the next day. Coronal sections (40 μm) were cut through the basal fore- and hind-brain on a freezing microtome. Table 1 lists the brain structures that were analysed in this study, their abbreviations and the mean areas of each structure analysed. At least every other section of the organum vasculosum of the lamina terminalis (OVLT) and subfornical organ (SFO), and every third section of other parts of the brain to be analysed were used for Fos-ir staining using the avidin–biotin–peroxidase technique. The primary antibody against Fos protein was raised from rabbits (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Tissue sections were incubated overnight in primary antibody (1: 4000 with 0.3% Triton X-100) at room temperature on a shaker, washed three times with PBS for 5 min each, incubated in goat anti-rabbit serum (secondary antibody – 1: 200) on a shaker for 1 h at room temperature and again washed three times with PBS for 5 min each. Amplification of the reaction was processed using the Vectastain ABC kit (Vector labs, ‘Elite’ ABC reagent, Burlingame, CA, USA). The sections were treated for ∼4 min in 2 mg ml−1 diaminobenzidine tetrahydrochloride (Sigma, St Louis, MO, USA) dissolved in 0.01 m PBS and 0.03% hydrogen peroxide. The chromagenic reaction was monitored microscopically. The sections were mounted on gelatinized slides, dried overnight, dehydrated in alcohol, and then placed under a coverslip with Depex.
Table 1.
Brain structures and their mean areas quantified for Fos-ir in rats with noninflated and inflated SVC-RAJ balloons
| Brain structure | Abbreviations* | Non-inflated | Inflated | ||
|---|---|---|---|---|---|
| mm2 | s.e.m | mm2 | s.e.m. | ||
| Organum vasculosum of the lamina terminalis | OVLT | 0.084 | 0.007 | 0.075 | 0.008 |
| Subfornical organ | SFO | 0.238 | 0.019 | 0.254 | 0.021 |
| Supraoptic nucleus | SON | 0.041 | 0.003 | 0.055 | 0.005 |
| Magnocellular paraventricular nucleus | mPVN | 0.068 | 0.003 | 0.062 | 0.003 |
| Parvocellular paraventricular nucleus | pPVN | 0.153 | 0.007 | 0.123 | 0.011 |
| Area postrema | AP | 0.170 | 0.012 | 0.178 | 0.015 |
| Lateral parabrachial nucleus | LPBN | 0.080 | 0.003 | 0.086 | 0.007 |
| Medial nucleus of the solitary tract | mNTS | 0.157 | 0.001 | 0.175 | 0.008 |
| Caudal ventrolateral medulla | CVLM | 0.022 | 0.003 | 0.035 | 0.003 |
Abbreviations used in the text and figures. All values are group means and the standard error of the mean (s.e.m.) of the areas expressed in mm2 under both conditions for each site. There is no overall significant difference between the areas analysed for each of the two conditions.
Quantification
Cells expressing positive nuclear Fos-ir were counted by hand in matched, representative sections of the tissue. A picture was taken of each section, and using the ImageJ program (National Institutes of Health) each section of the brain that was counted was outlined and the area measured. The area was divided by the number of Fos-ir positive cells it contained, and the results were expressed as Fos positive cells per square millimetre (mm2).
Dessert test
A separate group of rats was used to assess the effects of balloon inflation on ingestion of sweetened condensed milk, i.e. a dessert test (Johnson & Schwob, 1975) as a measure of the specificity of the effects of balloon inflation on ingestive behaviours. Rats with ad libitum access to food and water were offered 25% sweetened condensed milk (Carnation brand mixed with tap water, 1: 3) for 2 h daily. The milk was offered as a choice with tap water with both fluids provided in burettes attached to the front of each cage. The milk intakes stabilized after 5–7 days. For testing, the milk was offered while one-half of the group had their balloons inflated (0.1 ml inflation volume) and the other half did not. Water and milk intakes were recorded every 30 min for 2 h. The next day the balloon inflation conditions were reversed and the test was repeated.
Blood pressure and heart rate recording
One group of rats was used to assess the effects of balloon inflation on blood pressure and heart rate in conscious, freely moving animals. These rats were first implanted with atrial balloons and then with femoral arterial catheters a week later. Recordings of arterial blood pressure and heart rate occurred the next day. Catheters were connected to a pressure transducer (Maxxim Medical, Athens, TX, USA) coupled to a multichannel recorder through a custom-designed amplifier (University of Iowa, Iowa City, IA, USA). The analog input was converted into a digital signal using a PowerLab data acquisition system (ADInstruments, Mountain View, CA, USA). This program permits sampling of haemodynamic data directly onto a computer. Mean arterial pressure was derived electronically using a low-pass filter. Heart rate was determined by measuring the number of heartbeats triggered from the arterial pressure pulse. The animals were brought to a room separate from the colony and were held by hand while the catheter lines were connected to a pressure transducer. The animals were placed in a cage and allowed to adapt before acquisition of baseline measurements. Then, the atrial balloon was inflated using 0.05 ml volume followed 20 min later by inflation using 0.1 ml volume for 20 min. Finally, the balloon was deflated and additional measurements were obtained for the last 20 min.
Verification of balloon placement
At the completion of each experiment, the rats were overdosed with sodium pentobarbital. When they were completely unresponsive, an autopsy was performed to determine the position of the atrial balloon. Animals in which the balloon was not correctly positioned at the SVC-RAJ were eliminated from the study.
Statistical analysis
The data were analysed by analysis of variance (ANOVA). For behavioural data, balloon inflation condition and time were used as within-subject factors. For Fos-ir data the results for each nucleus were analysed separately with the balloon inflation condition as the between-subject factor. Fisher's LSD t tests were used for comparisons when the overall F was significant. The results are reported as means ±s.e.m. Differences were considered significant at P < 0.05.
Results
Water and sodium intakes after fluid depletion
Intakes were analysed as rates (i.e. intakes in ml per 15 min). Inflation of the balloon at the SVC-RAJ significantly reduced saline ingestion beginning with the first measurement, and significantly reduced saline ingestion for as long as the balloon was inflated compared to the non-inflated control condition (Time–Treatment interaction, F8,40= 6.09, P < 0.05). Upon deflation of the balloon, saline ingestion significantly increased such that rats drank more saline in the 15 min following balloon deflation than in the previous 1 h and 45 min (i.e. since the first measurement). Water intake was not altered by balloon inflation compared to the non-inflated control condition (F1,5= 2.07, n.s.), and there was no interaction effect of Time × Treatment (F8,40= 0.37, n.s.). The cumulative intakes of 0.3 m NaCl and water are presented in Fig. 1. In the two pretests with Furo–Cap, the amounts of 0.3 m NaCl and water intake were, respectively, 2.5 ± 0.8 and 6.8 ± 1.0 ml per 2 h for the first test and 5.5 ± 1.2 and 7.8 ± 1.2 ml per 2 h for the second test.
Figure 1. Cumulative intake of 0.3 m NaCl (top) and water (bottom) after fluid depletion induced by combined subcutaneous injections with furosemide and captopril (time 0) in rats with and without inflation of a small balloon at the junction of the superior vena cava–right atrium.
The animals were given access to the fluids 1 h after the furosemide plus captopril injections (i.e. time point 0). Balloons were deflated at the 120 min time point. Results are expressed as means ±s.e.m., n = 6. *Significantly different from the non-inflated control condition.
The effect of SVC-RAJ balloon inflation on c-fos expression after fluid depletion
Inflation of the balloon in animals depleted by Furo–Cap treatment decreased Fos-ir levels in the OVLT (F1,10= 18.25; P < 0.01), and SFO (F1,10= 9.33; P < 0.05) compared to Fos-ir levels in the non-inflated condition. Balloon inflation after Furo–Cap treatment increased Fos-ir levels in the lateral parabrachial nucleus (LPBN) (F1,10= 5.44; P < 0.05), and CVLM (F1,10= 17.23; P < 0.01) compared to levels in the non-inflated condition. Balloon inflation did not affect Fos-ir levels in the SON, magnocellular PVN (mPVN), parvocellular PVN (pPVN), AP, and medial NTS (mNTS) (all F1,10 values ≤ 2.14; n.s.), Figs 2 and 3.
Figure 2. Positive cells per section in fluid-deplete rats with (n = 4) or without (n = 8) inflation of a small balloon at the junction of the superior vena cava–right atrium.
In the top are the forebrain areas: organum vasculosum of lamina terminalis (OVLT), subfornical organ (SFO), supraoptic nucleus (SON), and portions of the magnocellular (mPVN) and parvocellular (pPVN) paraventricular nucleus (PVN) of the hypothalamus. In the bottom are the hindbrain areas: lateral parabrachial nucleus (LPBN), area postrema (AP), medial nucleus of the solitary tract (mNTS) and caudal ventrolateral medulla (CVLM). Results are expressed as means ±s.e.m. All counts have been standardized to 1 mm2. The means for each structure under both conditions is presented in Table 1. ANOVA on brain sites and conditions indicated no overall significant difference in size of area between groups. *Significantly different from non-inflated control condition.
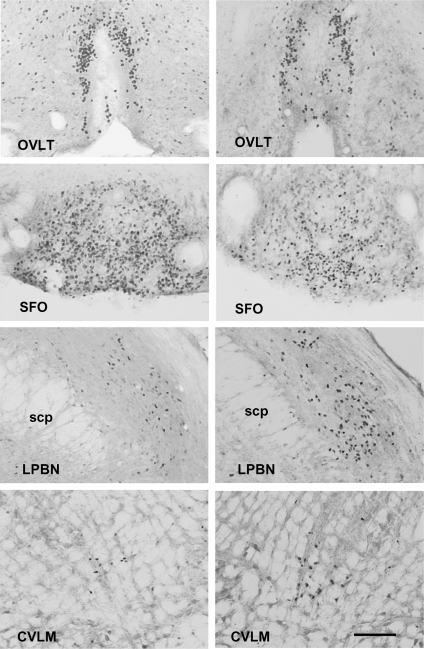
Figure 3. Representative photomicrographs of coronal sections demonstrating Fos-ir in fluid-deplete rats with balloon inflation at the junction of the superior vena cava–right atrium for 3 h (right) or without balloon inflation (left).
OVLT, organum vasculosum of lamina terminalis; SFO, subfornical organ; LPBN, lateral parabrachial nucleus; CVLM, caudal ventrolateral medulla. Black deposits indicate Fos-ir cell nuclei. The estimated scale bar shown in the bottom right is 200 μm, applicable to all photomicrographs, and based on the atlas by Paxinos & Watson (1998). Abbreviations: scp = superior cerebellar peduncle.
Dessert test
Daily intakes of 25% sweetened condensed milk stabilized after 5–7 days of training to about 25–30 ml per 2 h of access. The amount of water consumed during these 2 h access periods was trivial, usually < 0.2 ml.
Intakes were analysed as rates, i.e. ml per 30 min. Inflation of the balloon at the SVC-RAJ did not affect drinking of 25% sweetened condensed milk (F1,5= 2.25; n.s.). Cumulative intakes are presented in Fig. 4. Water intake was not affected by balloon inflation compared to the non-inflated control condition (0.3 ± 0.1 versus 0.2 ± 0.1 ml per 2 h), respectively.
Figure 4. Cumulative intake of 25% sweetened condensed milk with or without inflation of a small balloon at the junction of the superior vena cava–right atrium.
Results are expressed as means ±s.e.m., n = 6.
Blood pressure and heart rate recordings in conscious rats
Inflation of the balloon in conscious, freely moving animals with either 0.05 ml or 0.1 ml of isotonic saline did not significantly change mean arterial pressure of the rats compared to baseline measures obtained before balloon inflation or to measures obtained during the recovery period when the balloon was deflated (Table 2). There was a significant increase in heart rate during inflation of the balloon with 0.1 ml of saline (Treatment effect, F3,9= 7.69, P < 0.05). After deflation of the balloon, heart rate returned to baseline levels.
Table 2.
Blood pressure and heart rate responses in conscious freely moving rats with or without inflation of a balloon at the junction of the superior vena cava-right atrium
| Conditions | MAP (mmHg) | HR (bpm) |
|---|---|---|
| Baseline | 114 ± 3 | 398 ± 19 |
| Balloon inflation (0.05 ml) | 110 ± 4 | 421 ± 7 |
| Balloon inflation (0.1 ml) | 110 ± 4 | 478 ± 18* |
| Recovery (after balloon deflation) | 111 ± 3 | 401 ± 11 |
Results are expressed as means ±s.e.m. Mean arterial pressure (MAP) did not change over the 20 min measurement periods after balloon inflation and after balloon deflation. Heart rate (HR) increased during balloon inflation at the greater volume. n = 4.
Significantly different from all other conditions.
Discussion
The present work shows that inflation of a balloon placed at the SVC-RAJ inhibits salt appetite and affects Fos protein levels in brain areas that control cardiovascular and body fluid homeostasis. Stretching of the SVC-RAJ inhibited ingestion of hypertonic NaCl solutions after a treatment that has been shown to produce acute fluid depletion accompanied by mild hypotension, but did not affect the ingestion of water or a highly palatable solution of 25% sweetened condensed milk. Activation of SVC-RAJ mechanoreceptors in rats treated with Furo–Cap decreased Fos-ir in the OVLT and SFO, and increased Fos-ir in the LPBN and CVLM compared to controls. Stretch of the SVC-RAJ under baseline conditions did not affect mean arterial blood pressure and had only small effects on heart rate over 20 min observation periods following balloon inflations and deflation.
Behaviourally, the results from simulated blood volume expansion upon inflation of an atrial balloon is inhibition of either water or saline intake (Kaufman, 1984; Toth et al. 1987). Prior experiments have shown that right atrial stretch decreases sodium appetite that appears with a slow onset several hours following peritoneal dialysis with hyperoncotic colloid or after 2 or 3 days of daily treatments with DOCA (Toth et al. 1987). The behavioural procedure used here is an acute model of experimentally induced sodium appetite that produces significant ingestion of water and hypertonic saline beginning within 1–2 h after treatment (Fitts & Masson, 1989). The results show that stretch of the SVC-RAJ inhibited the ingestion of 0.3 m NaCl solution induced by Furo–Cap treatment for as long as the balloon was inflated. With deflation of the balloon, the rats quickly consumed 0.3 m NaCl solution. There was behavioural specificity for the inhibition of salt ingestion demonstrated as an intrinsic component of the Furo–Cap test as water intake was minimally affected by atrial stretch.
Further evidence of the selective nature of the inhibitory effects on salt appetite arising from receptors in the region of the SVC-RAJ is also provided by the results of the dessert test. That is, inflation of a balloon at the SVC-RAJ did not alter intake of a highly palatable 25% solution of sweetened condensed milk. The consumption of milk involves motor patterns that are very similar, if not identical, to those required for the consumption of 0.3 m NaCl. Whereas, the behaviours of water intake (thirst) and 0.3 m saline intake (salt appetite) are motivated by proximal neural and humoral stimuli such as ANG II, aldosterone, and/or reduced input from low- and high-pressure baroreceptors, the consumption of sweetened condensed milk in non-food and non-water deprived animals is presumably driven only by the palatable qualities of the liquid ingesta. The observations that balloon inflation did not interfere with milk ingestion in the so-called ‘dessert test’ bolsters the argument of the specificity of SVC-RAJ stretch-induced inhibitory effects on Furo–Cap-induced salt appetite. In other words, it is doubtful that balloon inflation causes some type of general malaise that might non-specifically disrupt ingestive behaviour.
Furo–Cap treatment increases Fos protein in the OVLT, SFO, SON, mPVN, pPVN, AP and LPBN (Thunhorst et al. 1998). The appearance of a sufficient amount of Fos protein in a given cell or brain region to permit detection of significant differences by immunocytochemistry cannot be taken as an unequivocal indication that there has been a change in neuronal discharge frequency nor that such changes are a quantitative expression of such activity in a particular neuron or brain region. Given the current understanding of the function of neuronal Fos, such changes are likely to reflect changes associated with intracellular signalling.
In previous work we demonstrated that both the increased Fos-ir in the OVLT, SFO, SON and PVN and the generated salt appetite response are dependent on ANG II, as the Fos expression and saline intake are eliminated by administering very high doses of captopril or an angiotensin type 1 receptor antagonist during depletion (Thunhorst et al. 1998). Furo–Cap treatment also increases Fos-ir in the NTS, but in this case the Fos expression is not reduced by high doses of captopril during fluid depletion indicating that neural signals related to a reduction of blood pressure and volume might be involved in Fos-ir expression in the NTS under these circumstances (Thunhorst et al. 1998).
In the present study, the OVLT, SFO, SON, mPVN, pPVN, AP and LPBN had substantial levels of Fos-ir activity after combined Furo–Cap treatment. When the atrial balloon was inflated during Furo–Cap treatment, Fos-ir levels decreased in the OVLT and SFO. The OVLT and SFO are rich in ANG II receptors (Song et al. 1992). Circulating ANG II produces a strong expression of Fos-ir in circumventricular organs (McKinley et al. 1992). Since Fos-ir and the production of sodium and water intake during Furo–Cap treatment are dependent on the actions of the endocrine renin–angiotensin system on these nuclei (Thunhorst et al. 1994; Thunhorst & Johnson, 1994; Thunhorst et al. 1998), it is possible that the decreased Fos protein in these structures after balloon inflation is due to decreased activity of the circulating renin–angiotensin system. However, Kaufman (1987) showed that the plasma renin activity is not altered by balloon inflation in normovolaemic or hypovolaemic rats. In this light, it seems likely that some other mechanism is responsible for decreased Fos-ir in the SFO and OVLT following balloon inflation found in the present study. Nevertheless given the difference in methods between Kaufman (1987) and the present studies, a reduction of renin–angiotensin levels after SVC-RAJ balloon inflation in conjunction with Furo–Cap treatment needs further examination.
Inflation of an atrial balloon releases atrial natriuretic peptide (ANP; Meikle & Kaufman, 1988), a hormone that is known to reduce water and sodium intake (Antunes-Rodrigues et al. 1985; Fitts et al. 1985). Increased ANP levels could act at the OVLT and SFO to decrease levels of neuronal activation and subsequently fluid ingestion. Another possibility is that an ascending neural pathway with inputs deriving from systemic visceral afferents produces a decrease in neuronal activity in the circumventricular organs of the lamina terminalis. The SFO receives afferent input from the NTS (Ciriello et al. 1996; Zardetto-Smith & Gray, 1987).
In the caudal brainstem, the AP and the NTS did not show changes in Fos-ir after inflation of the SVC-RAJ balloon. The AP, like the SFO and OVLT, is a sensory circumventricular organ which putatively is accessed by circulating humoral factors related to fluid balance and cardiovascular regulation (Johnson & Gross, 1993). The NTS is firmly established as the primary central site receiving cardiopulmonary, baroreceptor and visceral neural afferent input (Norgren, 1981). Studies by Kantzides, Badoer and colleagues (Kantzides et al. 2005; Kantzides & Badoer, 2006) have shown that inflation of a SVC-RAJ balloon increases Fos expression in the NTS by approximately 2–2.5 times in comparison to the non-inflated condition. Because Furo–Cap treatment causes both hypovolaemia and hypotension (Thunhorst & Johnson, 1994, 2001), it might be expected that during balloon inflation activation of afferents from mechanoreceptors in the region of the SVC-RAJ would result in a decrease of elevated NTS Fos-ir. However, in the present study this was not the case; there was no significant overall difference in Fos-ir in the NTS between the inflated versus the non-inflated conditions in Furo–Cap treated rats. This finding may reflect the possibility that different pools of neurons within the defined boundaries of the mNTS are activated or inhibited under each of the two conditions studied (e.g. in this case hypovolaemia/hypotension alone versus hypovolaemia/hypotension + SVC-RAJ stretch). The idea of differential recruitment of neuronal pools in the NTS as the intensity or pattern of pressure/volume baroreceptor input changes is supported by a study by Hines et al. (1994). In this case increasing atrial stretch by injections of graded volumes of saline produced graded inhibition of renal sympathetic nerve activity, but no consistent change in discharge rate of a given NTS neuron. Rather, additional NTS neurons appeared to be recruited as receptor loading increased. Since the Fos-ir method provides only a single static measure of putative activity in a region (i.e. a ‘snap-shot’ of activity), it is impossible to determine whether different subpopulations of mNTS neurons are being recruited, while others are being suppressed simultaneously.
The present results showed that balloon inflation increases Fos-ir in the LPBN during Furo–Cap treatment. Balloon inflation reduces water intake caused by isoproterenol and ablation of the ventrolateral portion of the LPBN abolished this reduction (Ohman & Johnson, 1995). Evidence indicates that the LPBN participates in the inhibition of thirst and sodium appetite by a serotonergic mechanism (Menani & Johnson, 1995; Menani et al. 1996, 1998, 2002; De Gobbi et al. 2000). Both cholecystokinin (Menani & Johnson, 1998; De Gobbi et al. 2001) and corticotropin releasing factor (De Castro e Silva et al. 2006) have also been shown to act in the LPBN to inhibit sodium intake. Therefore, increased Fos expression in the LPBN during balloon inflation might reflect increased activity of mechanisms that inhibit sodium appetite.
The results of the present study indicated that, in the hindbrain, the CVLM had increased Fos-ir after balloon inflation in Furo–Cap treated animals. The CVLM has been implicated as an area inhibiting sympathetic neural activity in response to increased baroreceptor input (Ciriello et al. 1986; Reis et al. 1988). Atrial balloon inflation reduces renal sympathetic nerve activity (Pyner et al. 2002). Systemic intravenous plasma volume expansion in rats and rabbits increases Fos-ir in the CVLM (Badoer et al. 1997; Shafton et al. 1999; Potts et al. 2000; Cunningham et al. 2002). However, in contrast no significant effect on CVLM Fos-ir was observed after SVC-RAJ balloon inflation in untreated rats (Kantzides et al. 2005). In comparison to the null effect of SVC-RAJ balloon stretch observed by Kantzides et al. (2005), the increased CVLM Fos-ir in the present study may have been due to the presence of Furo–Cap induced hypovolaemia and hypotension. A future study investigating the effects of balloon inflation on CVLM Fos-ir in both vehicle and Furo–Cap treated groups will be necessary to elucidate the nature of the effects of SVC-RAJ stretch in hypovolaemic/hypotensive states.
CVLM activation may also influence the activity of neurons within the SFO and OVLT. The CVLM sends projections to the SFO, OVLT and the median preoptic nucleus (Babic et al. 2004). The SFO has bidirectional connections to the midbrain raphe system, and the majority of cells in the raphe projecting to the SFO are serotonergic (Lind, 1986). There is a serotonergic pathway from the mNTS to the LPBN (Lança & van der Kooy, 1985). Activation of this pathway has been proposed to inhibit sodium ingestion (Menani & Johnson, 1995; Menani et al. 1996; Menani et al. 1998; Franchini et al. 2002). Injections of a retrograde marker into portions of the raphe system also label cells in the LPBN (Lind, 1986).
In the hypothalamus, balloon inflation did not change Fos-ir levels in the SON, mPVN or pPVN during Furo–Cap treatment. Electrophysiological studies show that oxytocinergic neurosecretory cells of the SON are not activated by right atrial stretch (Grindstaff et al. 2000). On the other hand, atrial balloon inflation decreases activity of vasopressin neurons (Grindstaff et al. 2000). SVC-RAJ stretch during Furo–Cap treatment did not affect Fos-ir expression in either the mPVN or pPVN portions of the hypothalamus. The PVN is a complex structure that exhibits a mixture of electrophysiological and neurochemical properties (Stern, 2001). The mPVN is associated with the hormonal control of the posterior pituitary (Poulain & Wakerley, 1982). The pPVN has roles related to cardiovascular regulation through its influences on sympathetic nerve activity (Badoer et al. 1997; Haselton et al. 1994; Pyner et al. 2002) and through projections to the neurohemal zone of the median eminence to influence the anterior pituitary hormones (e.g. corticotropin-releasing hormone and adrenocorticotropic hormone; Swanson et al. 1983).
Two previous studies (Deng & Kaufman, 1998; Pyner et al. 2002) using balloons to stretch the SVC-RAJ of otherwise untreated rats found increased Fos-ir in the PVN. In the present study we did not find changes in Fos-ir in either the mPVN or the pPVN with SVC-RAJ balloon inflation. In previous work (Thunhorst et al. 1998) using the same Furo–Cap treatment employed here, we found a significant increase in PVN Fos-ir in comparison to vehicle treated control rats. The failure to find a change in Fos-ir in Furo–Cap treated rats with SVC-RAJ balloon inflation versus those with no balloon-induced stretch may reflect a ceiling effect for c-fos expression. Alternatively, as discussed above for the NTS, different pools of neurons within components of the PVN may differentially express Fos under the two conditions so there is no net observable difference when the entire mPVN or pPVN is considered.
There were no changes in arterial blood pressure over the 20 min observation periods following 0.05 and 0.10 ml volume balloon inflations followed by balloon deflation, and except for the 0.10 ml inflation condition, there was no significant change in heart rate. These results are in agreement with previous studies in both anaesthetized (Kaufman et al. 1981) and unanaesthetized (Kantzides et al. 2005) rats showing increased heart rates without changes in arterial pressure upon expansion of a SVC-RAJ balloon. Although our functional and immunocytochemical studies were carried out for longer periods than our periodic 20 min cardiovascular recordings, the collective evidence suggests that changes in saline intake or brain Fos-ir with balloon inflation were probably not due to changes in blood pressure, although it is impossible to rule out that some alteration in blood pressure may have occurred outside the window of our study of cardiovascular parameters.
In summary, the present studies indicate that activation of inhibitory mechanisms induced by stretch of the SVC-RAJ reduces a rapid onset form of sodium appetite. The SVC-RAJ manipulation that inhibits the rapid onset salt appetite was accompanied by increased c-fos expression (as assessed by Fos-ir) in the LPBN and the CVLM, while activity was reduced in the SFO and OVLT. Together the results suggest that stretch of the SVC-RAJ may generate inhibitory humoral and/or neural afferent signals that act on the brain to directly or indirectly reduce c-fos expression in brain areas such as the SFO and OVLT. SVC-RAJ stretch also activates both the LPBN, which has been implicated in the inhibition of salt appetite and thirst, and the CVLM which influences sympathetic tone.
Acknowledgments
This research was supported by National Institutes of Health Grants AG 025465 and MH 59239 to RLT, and HL 14388, HL 57472 and DK 66086 to AKJ.
References
- Antunes-Rodrigues J, McCann SM, Rogers LC, Samson WK. Atrial natriuretic factor inhibits dehydration- and angiotensin II-induced water intake in the conscious, unrestrained rat. Proc Natl Acad Sci U S A. 1985;82:8720–8723. doi: 10.1073/pnas.82.24.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic T, Roder S, Ciriello J. Direct projections from caudal ventrolateral medullary depressor sites to the subfornical organ. Brain Res. 2004;1003:113–121. doi: 10.1016/j.brainres.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Badoer E, McKinlay D, Trigg L, McGrath BP. Distribution of activated neurons in the rabbit brain following a volume load. Neuroscience. 1997;81:1065–1077. doi: 10.1016/s0306-4522(97)00232-7. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Caverson MM, Polosa C. Function of the ventrolateral medulla in the control of the circulation. Brain Res. 1986;396:359–391. doi: 10.1016/0165-0173(86)90005-6. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Rosas-Arellano MP, Solano-Flores LP. Direct projections to subfornical organ from catecholaminergic neurons in the caudal nucleus of the solitary tract. Brain Res. 1996;726:227–232. [PubMed] [Google Scholar]
- Cunningham JT, Bruno SB, Higgs KA, Sullivan MJ. Intrapericardial procaine affects volume expansion-induced fos immunoreactivity in unanesthetized rats. Exp Neurol. 2002;174:181–192. doi: 10.1006/exnr.2002.7863. [DOI] [PubMed] [Google Scholar]
- De Castro e Silva E, Fregoneze JB, Johnson AK. Corticotropin-releasing hormone in the lateral parabrachial nucleus inhibits sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1136–R1141. doi: 10.1152/ajpregu.00075.2003. [DOI] [PubMed] [Google Scholar]
- De Gobbi JIF, De Luca LA, Jr, Johnson AK, Menani JV. Interaction of serotonin and cholecystokinin in the lateral parabrachial nucleus to control sodium intake. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1301–R1307. doi: 10.1152/ajpregu.2001.280.5.R1301. [DOI] [PubMed] [Google Scholar]
- De Gobbi JIF, De Luca LA, Jr, Menani JV. Serotonergic mechanisms of the lateral parabrachial nucleus on DOCA-induced sodium intake. Brain Res. 2000;880:131–138. doi: 10.1016/s0006-8993(00)02784-0. [DOI] [PubMed] [Google Scholar]
- Deng Y, Kaufman S. Pregnancy-induced changes in central response to atrial distension mimicked by progesterone metabolite. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1875–R1877. doi: 10.1152/ajpregu.1998.275.6.R1875. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Masson DB. Forebrain sites of action for drinking and salt appetite to angiotensin or captopril. Behav Neurosci. 1989;103:865–872. doi: 10.1037/h0092457. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Thunhorst RL, Simpson JB. Diuresis and reduction of salt appetite by lateral ventricular infusions of atriopeptin II. Brain Res. 1985;348:118–124. doi: 10.1016/0006-8993(85)90367-1. [DOI] [PubMed] [Google Scholar]
- Franchini LF, Johnson AK, de Olmos J, Vivas L. Sodium appetite and Fos activation in serotonergic neurons. Am J Physiol Regul Integr Comp Physiol. 2002;282:R235–R243. doi: 10.1152/ajpregu.00766.2000. [DOI] [PubMed] [Google Scholar]
- Grindstaff RR, Grindstaff RJ, Cunningham JT. Effects of right atrial distension on the activity of magnocellular neurons in the supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1605–R1615. doi: 10.1152/ajpregu.2000.278.6.R1605. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst. 1994;50:1–11. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circ Res. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Schwob JE. Cephalic angiotensin receptors mediating drinking to systemic angiotensin II. Pharmacol Biochem Behav. 1975;3:1077–1084. doi: 10.1016/0091-3057(75)90020-9. [DOI] [PubMed] [Google Scholar]
- Kantzides A, Badoer E. Activation of NADPH-diaphorase-positive projections to the rostral ventrolateral medulla following cardiac mechanoreceptor stimulation in the conscious rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1626–R1638. doi: 10.1152/ajpregu.00532.2005. [DOI] [PubMed] [Google Scholar]
- Kantzides A, Owens NC, De Matteo R, Badoer E. Right atrial stretch activates neurons in autonomic brain regions that project to the rostral ventrolateral medulla in the rat. Neuroscience. 2005;133:775–786. doi: 10.1016/j.neuroscience.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Kaufman S. Role of right atrial receptors in the control of drinking in the rat. J Physiol. 1984;349:389–396. doi: 10.1113/jphysiol.1984.sp015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. Influence of right atrial stretch on plasma renin activity in the conscious rat. Can J Physiol Pharmacol. 1987;65:257–259. doi: 10.1139/y87-045. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Mackay B, Kappagoda CT. Effect of stretching the superior vena cava on heart rate in rats. Am J Physiol Heart Circ Physiol. 1981;241:H248–H254. doi: 10.1152/ajpheart.1981.241.2.H248. [DOI] [PubMed] [Google Scholar]
- Lança AJ, van der Kooy D. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience. 1985;14:1117–1126. doi: 10.1016/0306-4522(85)90281-7. [DOI] [PubMed] [Google Scholar]
- Lind RW. Bi-directional, chemically specified neural connections between the subfornical organ and the midbrain raphe system. Brain Res. 1986;384:250–261. doi: 10.1016/0006-8993(86)91161-3. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Badoer E, Oldfield BJ. Intravenous angiotensin II induces Fos-immunoreactivity in circumventricular organs of the lamina terminalis. Brain Res. 1992;594:295–300. doi: 10.1016/0006-8993(92)91138-5. [DOI] [PubMed] [Google Scholar]
- Meikle AD, Kaufman S. Stretch-induced reduction in atrial content of natriuretic factor is locally mediated. Am J Physiol Regul Integr Comp Physiol. 1988;254:R284–R288. doi: 10.1152/ajpregu.1988.254.2.R284. [DOI] [PubMed] [Google Scholar]
- Menani JV, Barbosa SP, De Luca LA, Jr, De Gobbi JI, Johnson AK. Serotonergic mechanisms of the lateral parabrachial nucleus and cholinergic-induced sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2002;282:R837–R841. doi: 10.1152/ajpregu.00311.2001. [DOI] [PubMed] [Google Scholar]
- Menani JV, De Luca LA, Jr, Johnson AK. Lateral parabrachial nucleus serotonergic mechanisms and salt appetite induced by sodium depletion. Am J Physiol Regul Integr Comp Physiol. 1998;274:R555–R560. doi: 10.1152/ajpregu.1998.274.2.r555. [DOI] [PubMed] [Google Scholar]
- Menani JV, Johnson AK. Lateral parabrachial serotonergic mechanisms: angiotensin-induced pressor and drinking responses. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1044–R1049. doi: 10.1152/ajpregu.1995.269.5.R1044. [DOI] [PubMed] [Google Scholar]
- Menani JV, Johnson AK. Cholecystokinin actions in the parabrachial nucleus: effects on thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1431–R1437. doi: 10.1152/ajpregu.1998.275.5.r1431. [DOI] [PubMed] [Google Scholar]
- Menani JV, Thunhorst RL, Johnson AK. Lateral parabrachial nucleus and serotonergic mechanisms in the control of salt appetite in rats. Am J Physiol Regul Integr Comp Physiol. 1996;270:R162–R168. doi: 10.1152/ajpregu.1996.270.1.R162. [DOI] [PubMed] [Google Scholar]
- Norgren R. The central organization of the gustatory and visceral afferent systems in the nucleus of the solitary tract. In: Katsuki Y, Norgren R, Sato M, editors. Brain Mechanisms of Sensation. New York: John Wiley & Sons; 1981. pp. 143–160. [Google Scholar]
- Ohman LE, Johnson AK. Role of lateral parabrachial nucleus in the inhibition of water intake produced by right atrial stretch. Brain Res. 1995;695:275–278. doi: 10.1016/0006-8993(95)00929-k. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson Ch. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Potts PD, Ludbrook J, Gillman-Gaspari TA, Horiuchi J, Dampney RA. Activation of brain neurons following central hypervolaemia and hypovolaemia: contribution of baroreceptor and non-baroreceptor inputs. Neuroscience. 2000;95:499–511. doi: 10.1016/s0306-4522(99)00426-1. [DOI] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Priestley JV. Immunocytochemical Techniques for the Localization of Neurochemically Characterized Nerve Pathways. In: Turner AJ, Bachelard HS, editors. Neurochemistry, A Practical Approach. Oxford, Washington: IRL Press; 1987. pp. 65–112. [Google Scholar]
- Pyner S, Deering J, Coote JH. Right atrial stretch induces renal nerve inhibition and c-fos expression in parvocellular neurones of the paraventricular nucleus in rats. Exp Physiol. 2002;87:25–32. doi: 10.1113/eph8702279. [DOI] [PubMed] [Google Scholar]
- Randolph RR, Li Q, Curtis KS, Sullivan MJ, Cunningham JT. Fos expression following isotonic volume expansion of the unanesthetized male rat. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1345–R1352. doi: 10.1152/ajpregu.1998.274.5.R1345. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Morrison S, Ruggiero DA. The C1 area of the brainstem in tonic and reflex control of blood pressure. State of the art lecture. Hypertension. 1988;11:I8–I13. doi: 10.1161/01.hyp.11.2_pt_2.i8. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, McGrath B, Badoer E. Volume expansion does not activate neuronal projections from the NTS or depressor VLM to the RVLM. Am J Physiol Regul Integr Comp Physiol. 1999;277:R39–R46. doi: 10.1152/ajpregu.1999.277.1.R39. [DOI] [PubMed] [Google Scholar]
- Song K, Allen AM, Paxions G, Mendelsohn FA. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol. 1992;316:467–484. doi: 10.1002/cne.903160407. [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Fitts DA. Peripheral angiotensin causes salt appetite in rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R171–R177. doi: 10.1152/ajpregu.1994.267.1.R171. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Johnson AK. Renin-angiotensin, arterial blood pressure, and salt appetite in rats. Am J Physiol Regul Integr Comp Physiol. 1994;266:R458–R465. doi: 10.1152/ajpregu.1994.266.2.R458. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Johnson AK. Effects of hypotension and fluid depletion on central angiotensin-induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1726–R1733. doi: 10.1152/ajpregu.2001.281.5.R1726. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Morris M, Johnson AK. Endocrine changes associated with a rapidly developing sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1168–R1173. doi: 10.1152/ajpregu.1994.267.5.R1168. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Xu Z, Cicha MZ, Zardetto-Smith AM, Johnson AK. Fos expression in rat brain during depletion-induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1807–R1814. doi: 10.1152/ajpregu.1998.274.6.r1807. [DOI] [PubMed] [Google Scholar]
- Toth E, Stelfox J, Kaufman S. Cardiac control of salt appetite. Am J Physiol Regul Integr Comp Physiol. 1987;252:R925–R929. doi: 10.1152/ajpregu.1987.252.5.R925. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Gray TS. A direct neural projection from the nucleus of the solitary tract to the subfornical organ in the rat. Neurosci Lett. 1987;80:163–166. doi: 10.1016/0304-3940(87)90647-1. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann N Y Acad Sci. 1993;689:161–176. doi: 10.1111/j.1749-6632.1993.tb55545.x. [DOI] [PubMed] [Google Scholar]