Abstract
Accurate measurements of arterial () currently require blood sampling because the end-tidal () of the expired gas often does not accurately reflect the mean alveolar and . Differences between and result from regional inhomogeneities in perfusion and gas exchange. We hypothesized that breathing via a sequential gas delivery circuit would reduce these inhomogeneities sufficiently to allow accurate prediction of from . We tested this hypothesis in five healthy middle-aged men by comparing their values with values at various combinations of (between 35 and 50 mmHg), (between 70 and 300 mmHg), and breathing frequencies (f; between 6 and 24 breaths min−1). Once each individual was in a steady state, was collected in duplicate by consecutive blood samples to assess its repeatability. The difference between and average was 0.5 ± 1.7 mmHg (P = 0.53; 95% CI −2.8, 3.8 mmHg) whereas the mean difference between the two measurements of was −0.1 ± 1.6 mmHg (95% CI −3.7, 2.6 mmHg). Repeated measures ANOVAs revealed no significant differences between and over the ranges of , f and target . We conclude that when breathing via a sequential gas delivery circuit, provides as accurate a measurement of as the actual analysis of arterial blood.
Accurate measurement of arterial () is important for the clinical assessment of patients and, in physiological studies, for the assessment of control of breathing and cerebral blood flow. Currently, the reference standard for measuring is analysis of arterial blood via direct arterial puncture. This invasive approach has a number of disadvantages for both the subject (discomfort and potential arterial wall damage) and investigator (restricted mobility of the catheter insertion site, cost, time delay for blood analysis, and limited temporal resolution of changes in ). As a result, investigators have long sought a suitable non-invasive method to measure .
Non-invasive methods of predicting from alveolar () consider the lung to be a tonometer in which CO2 equilibrates between alveolar gas and capillary blood. In reality, however, the lung is not a single homogeneous time-invariant gas exchange compartment. Rather, varies in different regions of the lung as a result of differences in ventilation-to-perfusion matching 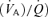 throughout the lung and, in each lung region, throughout the respiratory cycle (Dubois et al. 1952; Lenfant, 1967). The contribution to the of blood passing each alveolus reflects the average in that alveolus during the respiratory cycle (Jones et al. 1979; Robbins et al. 1990). , then, reflects the time- and flow-weighted averages of all alveolar ventilatory fluctuations in all
throughout the lung and, in each lung region, throughout the respiratory cycle (Dubois et al. 1952; Lenfant, 1967). The contribution to the of blood passing each alveolus reflects the average in that alveolus during the respiratory cycle (Jones et al. 1979; Robbins et al. 1990). , then, reflects the time- and flow-weighted averages of all alveolar ventilatory fluctuations in all 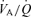 regions throughout the lung, i.e. the mean (Lenfant, 1967). As a result, the relation between the in the exhaled gas and the is so obscured that one cannot calculate the from the .
regions throughout the lung, i.e. the mean (Lenfant, 1967). As a result, the relation between the in the exhaled gas and the is so obscured that one cannot calculate the from the .
We reasoned that if the regional variations of in the lung could be reduced, then (a) the end-tidal () would accurately reflect the mean and (b) the would not be affected by the distribution of pulmonary blood flow. In other words, should equal mean , and, as mean is equal to (Jones et al. 1979; Robbins et al. 1990), should equal .
In our laboratory, we have experimented with a method of controlling by providing specific flows and concentrations of CO2 to a sequential gas delivery circuit (Slessarev et al. 2007). To the extent that minute ventilation 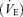 exceeds such gas flow, previously expired gas, stored in an expiratory gas reservoir, enters the lung (Somogyi et al. 2005). This gas, we hypothesized, reduces both the regional variations and respiratory fluctuations of towards a mean (see Fig. 2 in Prisman et al. 2007). We tested this hypothesis using the method of Slessarev et al. (2007) to prospectively target a series of values and measuring mean by analysing contemporaneously drawn arterial blood samples for . To test the robustness of the relation between and , we also varied the end-tidal values (PET,O2) and breathing frequencies (f) at each target .
exceeds such gas flow, previously expired gas, stored in an expiratory gas reservoir, enters the lung (Somogyi et al. 2005). This gas, we hypothesized, reduces both the regional variations and respiratory fluctuations of towards a mean (see Fig. 2 in Prisman et al. 2007). We tested this hypothesis using the method of Slessarev et al. (2007) to prospectively target a series of values and measuring mean by analysing contemporaneously drawn arterial blood samples for . To test the robustness of the relation between and , we also varied the end-tidal values (PET,O2) and breathing frequencies (f) at each target .
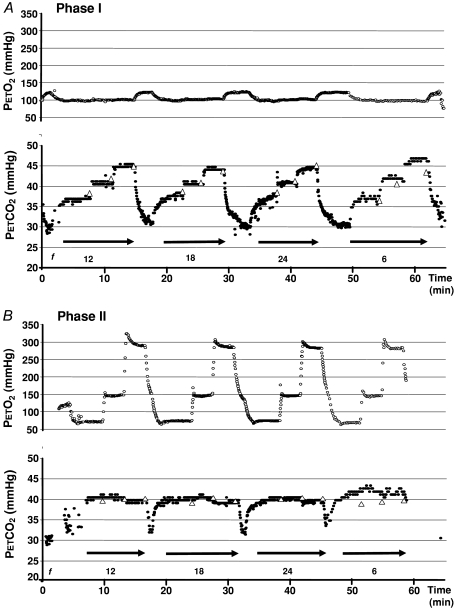
Figure 2. Example data.
A, PET,O2 and from one subject during Phase I of the protocol. Each end-tidal value is represented by a filled circle. Averages of two values at end of each 3 min test interval are represented by open triangles. Horizontal arrows designate the time during which f was maintained by breathing in synchrony with a metronome. Subjects were administered room air in the intervals between fixed f. Reductions in between tests can be partly attributed to a rebound hyperventilation after hypercarbia, as was previously reported by Wise et al. (2007) and partly to artifact as the face mask was flooded with air at high flows between tests. In Phase I, before each period of breathing at controlled f, subjects were encouraged to hyperventilate for about 1–2 min to below a of 35 mmHg so they could undergo a sharp step up to the new target . This was done to minimize the time to attain target end-tidal values and thereby shorten the duration of the protocol. measurements and arterial blood sampling were performed only after a steady state was achieved. The increases in PET,O2 between periods of fixed f were the result of hyperventilation on room air. B, PET,O2 and from one subject during Phase II of the protocol. Between periods of fixed f, subjects breathed room air without restriction.
Methods
Participants
The study was approved by the University Health Network Research Ethics Board. Informed written consent was obtained from five middle-aged male subjects. Their anthropomorphic and pulmonary function test data performed at the clinical pulmonary function laboratory at the Toronto General Hospital are presented in Table 1. Subjects were healthy except as noted in the caption to Table 1.
Table 1.
Subject anthropomorphic and pulmonary function data
| Subject | Age (years) | Weight (kg) | Height (cm) | VC (l,% predicted) | FEV1/VC (% predicted) | FRC (l,% predicted) | DLCO (% predicted) |
|---|---|---|---|---|---|---|---|
| 1 | 45 | 77 | 176 | 5.1 (113) | 77 (104) | 2.9 (78) | 30 (93) |
| 2* | 33 | 81 | 177 | 4.4 (90) | 73 (95) | 3.1 (81) | 29 (91) |
| 3§ | 49 | 89 | 174 | 5.0 (116) | 66 (90) | 3.5 (98) | 33 (103) |
| 4 | 59 | 81 | 177 | 6.0 (144) | 65 (92) | 4.6 (122) | 32 (90) |
| 5 | 53 | 76 | 175 | 4.7 (113) | 77 (107) | 3.2 (88) | 26.5 (88) |
History of mild asthma, occasional use of inhaled beta agonists, occasional smoker of cigarettes.
History of smoking of about one package of cigarettes per week for 20 years.
Setting and PET,O2
Measurements of O2 consumption 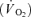 and CO2 production
and CO2 production 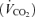 were used to target and PET,O2. Subjects were seated comfortably at room temperature breathing via a sequential gas delivery circuit and mask (Fig. 1 in Slessarev et al. 2007). Adhesive tape (Tegaderm, 3M Health Care, St Paul, MN, USA) was applied as necessary to prevent leaks between the face and the mask. Gas was sampled continuously from inside the mask.
were used to target and PET,O2. Subjects were seated comfortably at room temperature breathing via a sequential gas delivery circuit and mask (Fig. 1 in Slessarev et al. 2007). Adhesive tape (Tegaderm, 3M Health Care, St Paul, MN, USA) was applied as necessary to prevent leaks between the face and the mask. Gas was sampled continuously from inside the mask. 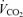 and
and 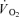 were determined for each subject as follows:
were determined for each subject as follows:
where FE,CO2 and FE,O2 are the fractional concentrations of CO2 and O2 of mixed expired gas (sampled from the expiratory reservoir) and 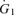 in this instance is the flow of air.
in this instance is the flow of air. 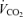 and
and 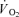 were measured every 45 s until three consecutive values differed by less than 10%. The last such readings were taken as the
were measured every 45 s until three consecutive values differed by less than 10%. The last such readings were taken as the 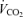 and
and 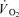 for calculating the target end-tidal values.
for calculating the target end-tidal values. 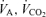 and
and 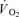 were then used to calculate the required gas flow to the circuit and its inspired fractional concentrations of CO2 and O2 to obtain the targeted and PET,O2 (see Slessarev et al. 2007 for details).
were then used to calculate the required gas flow to the circuit and its inspired fractional concentrations of CO2 and O2 to obtain the targeted and PET,O2 (see Slessarev et al. 2007 for details).
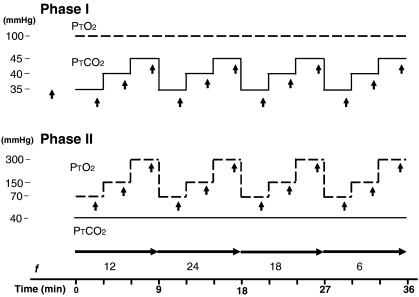
Figure 1. Experimental protocol.
Time course showing target end-tidal PO2 (PT,O2) and target end-tidal (PT,CO2). In Phase I, PT,O2 was kept constant and PT,CO2 was set to three different levels for each of four different frequencies (f). In Phase II, PT,CO2 was kept constant and PT,O2 was set to three different levels for each of four different f. Upward pointing arrowheads indicate time for duplicate arterial blood sampling. Horizontal arrows indicate period of constant f.
We used a custom made gas blender fitted with and sensors to blend gases in response to computer instructions (Respiract™, Thornhill Research Inc., Toronto, Canada). The rapid response CO2 sensor (Ir3107, Servomex Group Ltd, Sugar Land, TX, USA) is accurate within ±0.1% CO2 in the range of 0–10% CO2 and the O2 sensor (UFO 130, Teledyne Analytical Instruments, City of Industry, CA, USA) to within 1% with a resolution of 0.1%. Both sensors underwent a two-point calibration before every experiment and were configured to report and at BTPS. Expiratory flows were monitored continuously via a turbine (Universal Ventilation Meter, Vacu-Med, Ventura, CA, USA) placed in the expiratory limb of the circuit, and analysed for tidal volume (VT) and 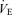 . All analog data were digitized, analysed and recorded on a computer after analog-to-digital conversion with a customized commercial data acquisition program (LabVIEW, National Instruments, Austin, TX, USA).
. All analog data were digitized, analysed and recorded on a computer after analog-to-digital conversion with a customized commercial data acquisition program (LabVIEW, National Instruments, Austin, TX, USA).
A 22-gauge catheter was inserted aseptically under local anaesthesia into a radial artery and maintained with a continuous slow flush of 0.9% saline solution. During the last minute of each 3 min target condition, two consecutive arterial blood samples (∼2 ml) were drawn over approximately 20 s into heparinized syringes. Blood was analysed within 20 min via a clinically maintained point-of-care blood gas analyser (RapidPoint® 405: Bayer HealthCare Diagnostics, Medfield, MA, USA). Analyser results on test samples are analysed daily and certified to be within 4% of reading for values less than 55 mmHg and within 6% of reading for values less than 150 mmHg and 10% for values greater than 150 mmHg. Quality control included automatic one-point calibrations every 30 min and two-point calibrations every fourth calibration. All blood gas values were reported at 37 °C.
Experimental protocol
To evaluate the relations between and , we targeted and measured during two experimental phases: (1) was held constant; and (2) was varied. In Phase I (Fig. 1), f was held constant at 6, 12, 18, or 24 breaths min−1 while was targeted to three of four partial pressures (35, 40, 45, 50 mmHg) for 3 min each, at each f, and PET,O2 was kept constant at 100 mmHg.
Phase II was similar to Phase I except that was held constant at 40 mmHg while PET,O2 was targeted to three different partial pressures (70, 150, 300 mmHg) at each of the four f. Breathing frequency was held constant and the target cycled (versus vice versa) to minimize the duration of hypercapnia, which some subjects found uncomfortable. The different values of f were applied in a randomized order in each subject and maintained by instructing the subject to breathe in time with a metronome. These tests resulted in comparisons of measured averaged over the last 60 s of each breathing period with the average from both arterial blood samples, for six different combinations of targeted and , at each of the four f, for a total of 24 comparisons per subject.
Statistics
All data are expressed as the mean ±s.d. unless otherwise noted. To assess the variability of between the two blood samples for each subject during each experimental condition, we calculated an intraclass correlation coefficient (ICC); values approaching 1.0 indicate a strong degree of agreement between the two assessments, while values near 0 indicate little or no relation between the measured pairs. To assess the differences between targeted values and measured values and values, a series of repeated measures ANOVAs was performed to determine whether these differences were significant, and whether experimental phase or f significantly affected the magnitudes of the differences. Subject identifiers were included as a random effect in these models to account for the relatedness of observations from the same subject. The analyses were performed using the SAS System v.9.1 software package. Statistical significance was set at the P = 0.05 level. Bland–Altman analysis (Bland & Altman, 1986) was used to calculate the limits of agreement between and average . We also calculated the repeatability coefficient (as defined by the British Standards Institution), referred to by Bland & Altman (1986), which refers to the 95% confidence intervals of differences between two repeated measures of the same quantity by the same method.
Results
All subjects completed the protocol without difficulty. Figure 2 shows the results obtained during a typical experiment. With subjects synchronizing f to a metronome (at constant 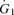 ), VT was inversely related to f (1.96 ± 0.36, 1.19 ± 0.28, 0.98 ± 0.2 and 0.85 ± 0.19 l at f = 6, 12, 18 and 24 breaths min−1, respectively; P < 0.01).
), VT was inversely related to f (1.96 ± 0.36, 1.19 ± 0.28, 0.98 ± 0.2 and 0.85 ± 0.19 l at f = 6, 12, 18 and 24 breaths min−1, respectively; P < 0.01).
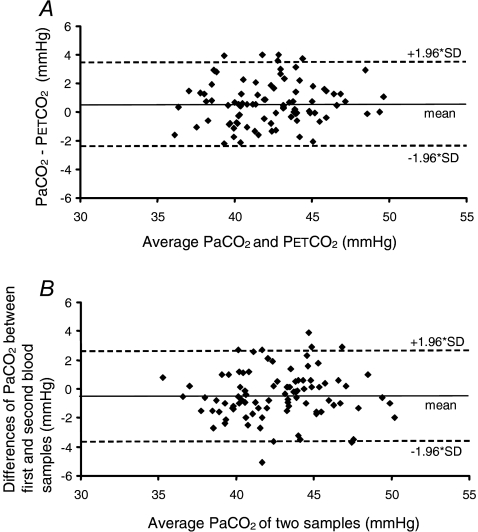
For all f and PET,O2 values, the difference between and average was 0.5 ± 1.7 mmHg (P = 0.53; 95% CI −2.8, 3.8 mmHg) (Fig. 3A). There were no significant differences between and average regardless of , f, or target (repeated measures ANOVAs). Differences between target and actual and between target and are presented in Table 2. The only significant difference between target and occurred at f = 6 breaths min−1.
Figure 3. Bland–Altman plots.
The limits of agreement between and (A) and the repeatability coefficient for repeat measurements (B).
Table 2.
Summary of differences between target end-tidal (), end-tidal () and arterial () , reported as mean ±s.d. (95% CI) and categorized by breathing frequency and experimental phase
| Breaths min−1 | 6 | 12 | 18 | 24 | (12, 18, 24) |
|---|---|---|---|---|---|
| Phase I: constant, varied | |||||
| – | 2.38 ± 0.99* | 0.98 ± 1.43 | 1.20 ± 1.55 | 0.62 ± 1.67 | 0.93 ± 1.53 |
| (1.83, 2.93) | (0.19, 1.77) | (0.34, 2.06) | (−0.30, 1.54) | (0.47, 1.39) | |
| – | 1.86 ± 2.52 | 1.61 ± 2.19 | 2.10 ± 2.10 | 1.44 ± 2.33 | 1.72 ± 2.17 |
| (0.46, 3.25) | (0.40, 2.82) | (0.94, 3.26) | (0.15, 2.73) | (1.06, 2.37) | |
| Phase II: varied, constant | |||||
| – | 2.11 ± 1.93* | 0.94 ± 1.71 | 0.75 ± 1.64 | 0.83 ± 1.75 | 0.84 ± 1.66 |
| (1.04, 3.18) | (−0.01, 1.89) | (−0.16, 1.66) | (−0.14, 1.79) | (0.34, 1.34) | |
| – | 1.24 ± 2.49 | 1.48 ± 2.04 | 1.14 ± 2.41 | 1.27 ± 2.64 | 1.30 ± 2.33 |
| (−0.14, 2.61) | (0.35, 2.61) | (−0.19, 2.48) | (−0.20, 2.73) | (0.60, 2.00) | |
| Combined Phases | |||||
| – | 2.24 ± 1.51* | 0.96 ± 1.55 | 0.97 ± 1.59 | 0.72 ± 1.68 | 0.89 ± 1.59 |
| (1.68, 2.81) | (0.39, 1.54) | (0.38, 1.57) | (0.10, 1.35) | (0.55, 1.22) | |
| – | 1.55 ± 2.48 | 1.55 ± 2.08 | 1.62 ± 2.27 | 1.36 ± 2.45 | 1.51 ± 2.25 |
| (0.62, 2.47) | (0.77, 2.32) | (0.77, 2.47) | (0.44, 2.27) | (1.04, 2.00) | |
Data show differences in mmHg.
P < 0.02.
A duplicate analysis of was performed to establish the repeatability coefficient of the reference standard measure as the benchmark for declaring and interchangeable. The ICC between blood measurements was 0.94 (95% CI: 0.91, 0.96, P < 0.01) and the mean difference between two consecutive measurements was −0.1 ± 1.6 mmHg (mean absolute difference 1.4 ± 1.1 mmHg).
The repeatability coefficient for was −3.7, 2.5 (P = 0.47) (Fig. 3B), practically identical to that between and average (s.d. 1.7 versus 1.6 mmHg, respectively). This was true even when the data were analysed separately for f of 6 (0.70 ± 1.94, 95% CI: −0.03, 1.42; P = 0.15) and for f of 12–24 breaths min−1 (−0.62 ± 1.49, 95% CI: −0.93, −0.31; P = 0.76). In other words, measurement of was interchangeable with measurement of .
Discussion
This is the first study to show consistent and close agreement between and over a wide range of , and f. The designation of agreement is taken from the approach advocated by Bland & Altman (1986) for assessing the interchangeability of measures of a physiological quantity – in this case, . The accepted reference standard is analysis of invasively acquired arterial blood. Therefore, as part of this study, we tested the repeatability of of duplicate consecutively drawn blood samples during steady state conditions of . The mean difference between blood samples was −0.1 mmHg, indicating no systematic bias. The repeatability coefficient reflects the range of differences between readings for 95% of duplicate readings, and this variability reflects the sum of machine (±1.6 mmHg), handling and actual blood variability (Lenfant, 1967). Because the limits of agreement between and were the same as between any two readings of the reference standard, we can designate them as being interchangeable under these experimental conditions. The methodology we used therefore extends the ability to perform physiological research when the , as the independent variable, must be both controlled and measured accurately.
The need for a new method of using to assess may be questioned since has been deemed to be an acceptable estimate of at rest (Jones et al. 1979; Robbins et al. 1990). However, closer scrutiny identifies multiple exceptions and uncertainties in this relation. In subjects breathing room air, consistently underestimates at rest (Robbins et al. 1990) and overestimates it during exercise (Matell, 1963; Jones et al. 1979; Williams & Babb, 1997; Benallal et al. 2002). Various factors affect the difference between and ; these include VT (Jones et al. 1979), age (Miller & Tenney, 1956; Holland et al. 1968), the presence of obstructive lung disease (Liu et al. 1995; Prause et al. 1997), and gravity (Barr, 1963). Most of these factors are not taken into account in the calculation of with the above-mentioned methods, resulting in uncertainty. We therefore investigated if the sequential gas delivery method of attaining a target (which also does not take any of these conditions into account) establishes conditions in the lung that ensure that equals mean (as measured by ).
A previously reported method, dynamic end-tidal forcing (DEF), targets end-tidal values using an integral-proportional feedback loop to make breath-by-breath corrections to inspired gases, but it does not actually target . In studies of the control of breathing, Robbins et al. (1990) allowed subjects to breathe freely in response to targeted changes in over the range of 40–50 mmHg using DEF and compared the in exhaled gas to measured . At rest, significantly underestimated with a large s.d. (mean ±s.d., −1.35 ± 2.64 mmHg). St Croix et al. (1995) used DEF in older subjects with a protocol (but not a method of targeting ) similar to ours; they found that the difference between and varied with the fractional concentration of inspired CO2. Despite studying subjects with greater alveolar deadspace than younger subjects (Robbins et al. 1990), they also found that significantly overestimated (+2.9 ± 1.7 mmHg) and explained this finding by noting that DEF targets regardless of its effect on mixed venous and mean . They concluded that caution must be exercised when inferring the degree of stimulation (i.e. the ) at the chemoreceptors based on measurements of . This is particularly true under hypercapnic conditions because -to- differences ‘… were often very large, indicating that end-tidal forcing is not useful for deriving individual values’ of . In contrast, we observed that targeted , and were independent of both the inspired fractional concentration of CO2 and VT (repeated measures ANOVAs), except at f = 6 breaths min−1, a finding discussed in greater detail below.
A characteristic of the sequential gas delivery circuit is that the difference between  and
and 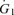 is made up with rebreathed gas from the expiratory reservoir. The higher in the previously exhaled gas entering high
is made up with rebreathed gas from the expiratory reservoir. The higher in the previously exhaled gas entering high 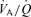 lung regions raises their values towards those of better perfused regions (Swenson et al. 1994; Brogan et al. 2004). This raises the in alveoli with high
lung regions raises their values towards those of better perfused regions (Swenson et al. 1994; Brogan et al. 2004). This raises the in alveoli with high 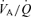 towards the of those with better matched
towards the of those with better matched 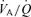 , resulting in a more homogeneous . In addition, larger VT and previously exhaled gas also reduces the intrabreath fluctuations in a given alveolus without changing the net equilibrium (see Somogyi et al. 2005). As a result, the distribution of throughout the lung and over the course of a breath cycle approaches the mean . An important consequence of having very similar values in all alveoli is that the is no longer dependent on the regional distribution of blood flow. We propose this as the most likely explanation for our observation that for f between 12 and 24 breaths min−1, the mean – i.e. – and targeted are identical.
, resulting in a more homogeneous . In addition, larger VT and previously exhaled gas also reduces the intrabreath fluctuations in a given alveolus without changing the net equilibrium (see Somogyi et al. 2005). As a result, the distribution of throughout the lung and over the course of a breath cycle approaches the mean . An important consequence of having very similar values in all alveoli is that the is no longer dependent on the regional distribution of blood flow. We propose this as the most likely explanation for our observation that for f between 12 and 24 breaths min−1, the mean – i.e. – and targeted are identical.
The same proposed mechanism accounts for the discrepancy between and at f = 6 breaths min−1. Consider that the amplitude of fluctuations of varies inversely with inspiratory duration and f and directly with VT; the effect of the latter is the most influential (Jones et al. 1979). At f = 6 breaths min−1, VT values were maximal, resulting in larger fluctuations of in the alveoli. The end-inspiratory falls because the lower f allows a greater accumulation of ‘fresh’ gas in the inspiratory reservoir during prolonged exhalation. In addition, prolonged dwell time in the alveoli allows the exhaled to equilibrate more completely with the in mixed venous blood (Dubois et al. 1952; Matell, 1963). Nevertheless, as already noted, the mean is maintained equal to the targeted .
We included changes in as part of the protocol to examine the effect of on the gradient between and . In our study, PET,O2 had no effect on or the difference between and . Larson & Severinghaus (1962) reported that changing from air to O2 breathing increased the mean end-tidal to arterial gradient by 1.5 mmHg. They suggested that O2 breathing may divert much of the pulmonary flow from non-dependent relatively poorly perfused parts of the lung to dependent areas of the lung, thereby increasing the alveolar dead space. If this effect was present in our study, it was too small to be observed, despite our many tests. It is also possible that the rebreathing of exhaled gases, hypercapnia, or both, may have masked this effect of increased alveolar dead space.
Limitations
Our subject sample was limited to five middle-aged male subjects at rest and therefore cannot be used to predict the response of a population. Our aim was limited to testing whether respiratory rate, changes in and affects the prediction of from when breathing with a sequential gas delivery system. Like St Croix et al. (1995) who carried out a very similar protocol using DEF, we assumed that pooling multiple data from each subject does not preclude the independence of our observations and approximates a larger sample size. All of our subjects were male; this is also consistent with other reported studies. Our subjects had a wide age range, and two had mild obstructive lung disease (Table 1), but there were no intersubject differences detected in the results. While we did not test elderly subjects, the rationale of our method with respect to distribution of previously exhaled gas to areas of alveolar deadspace predicts that the gradient between and will be less in subjects with somewhat larger anatomic and physiological deadspace but near-normal, age-adjusted ventilatory capacities. Although there is no theoretical reason that precludes using the method in subjects exercising moderately in a steady state, we have not yet attempted this.
Safety
In addition to standard physiological monitors such as end-tidal gas monitoring, electrocardiography and pulse oximetry, our main safety feature is that all source gases contain at least 10% O2 so that it is not possible to provide a hypoxic mixture if one or more of the gas sources suddenly fail.
Other potential applications
This method of targeting end-tidal gases with a sequential gas delivery circuit (Slessarev et al. 2007) is suitable for studies of the control of breathing because and can be controlled independently, and independent of 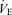 . Such precise control of can be also used to provide continuous assessment of the stimuli to CO2- and O2-responsive vascular beds in the brain (Vesely et al. 2001; Mikulis et al. 2005; Prisman et al. 2008) and eye (Gilmore et al. 2004; Venkataraman et al. 2005; Gilmore et al. 2007). While the method of Slessarev et al. (2007) has not yet been used to study coronary, renal and other vascular beds, it should be possible with the same sequential gas delivery system.
. Such precise control of can be also used to provide continuous assessment of the stimuli to CO2- and O2-responsive vascular beds in the brain (Vesely et al. 2001; Mikulis et al. 2005; Prisman et al. 2008) and eye (Gilmore et al. 2004; Venkataraman et al. 2005; Gilmore et al. 2007). While the method of Slessarev et al. (2007) has not yet been used to study coronary, renal and other vascular beds, it should be possible with the same sequential gas delivery system.
Conclusion
It is now possible to target , in addition to , using a sequential gas delivery system. For respiratory rates greater than 12 breaths min−1, the resulting will provide as accurate a measurement of as the analysis of an arterial blood sample.
Acknowledgments
We would like to thank Drs. Andrew Subudhi and Steve Iscoe for critically reviewing the manuscript prior to submission for publication.
Conflict of interest
J.D., L.F., S.I. and J.F. have made contributions to the intellectual property related to the development of the automated gas blender and the methodology of targeting end-tidal gases. Patent applications have been filed according the IP policies of the University Health Network (UHN) and the University of Toronto. All rights to the patent have been assigned to TSI, a company formed under the auspices of the Business Development Office of the UHN. J.D., L.F., and J.F. are minor share holders in TSI.
References
- Barr PO. Pulmonary gas exchange in man as affected by prolonged gravitational stress. Acta Psychiatr Scand Suppl. 1963;207:1–46. doi: 10.1111/j.1748-1716.1963.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Benallal H, Denis C, Prieur F, Busso T. Modeling of end-tidal and arterial PCO2 gradient: comparison with experimental data. Med Sci Sports Exerc. 2002;34:622–629. doi: 10.1097/00005768-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;(i):307–310. [PubMed] [Google Scholar]
- Brogan TV, Robertson HT, Lamm WJ, Souders JE, Swenson ER. Carbon dioxide added late in inspiration reduces ventilation-perfusion heterogeneity without causing respiratory acidosis. J Appl Physiol. 2004;96:1894–1898. doi: 10.1152/japplphysiol.00160.2003. [DOI] [PubMed] [Google Scholar]
- Dubois AB, Britt AG, Fenn WO. Alveolar CO2 during the respiratory cycle. J Appl Physiol. 1952;4:535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- Gilmore ED, Hudson C, Nrusimhadevara RK, Harvey PT, Mandelcorn M, Lam WC, Devenyi RG. Retinal arteriolar diameter, blood velocity, and blood flow response to an isocapnic hyperoxic provocation in early sight-threatening diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:1744–1750. doi: 10.1167/iovs.06-1016. [DOI] [PubMed] [Google Scholar]
- Gilmore ED, Hudson C, Venkataraman ST, Preiss D, Fisher J. Comparison of different hyperoxic paradigms to induce vasoconstriction: implications for the investigation of retinal vascular reactivity. Invest Ophthalmol Vis Sci. 2004;45:3207–3212. doi: 10.1167/iovs.03-1223. [DOI] [PubMed] [Google Scholar]
- Holland J, Milic-Emili J, Macklem PT, Bates DV. Regional distribution of pulmonary ventilation and perfusion in elderly subjects. J Clin Invest. 1968;47:81–92. doi: 10.1172/JCI105717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol. 1979;47:954–960. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- Larson CP, Jr, Severinghaus JW. Postural variations in dead space and CO2 gradients breathing air and O2. J Appl Physiol. 1962;17:417–420. doi: 10.1152/jappl.1962.17.3.417. [DOI] [PubMed] [Google Scholar]
- Lenfant C. Time-dependent variations of pulmonary gas exchange in normal man at rest. J Appl Physiol. 1967;22:675–684. doi: 10.1152/jappl.1967.22.4.675. [DOI] [PubMed] [Google Scholar]
- Liu Z, Vargas F, Stansbury D, Sasse SA, Light RW. Comparison of the end-tidal arterial PCO2 gradient during exercise in normal subjects and in patients with severe COPD. Chest. 1995;107:1218–1224. doi: 10.1378/chest.107.5.1218. [DOI] [PubMed] [Google Scholar]
- Matell G. Time-courses of changes in ventilation and arterial gas tensions in man induced by moderate exercise. Acta Physiol Scand. 1963;58:1–53. [Google Scholar]
- Mikulis DJ, Krolczyk G, Desal H, Logan W, Deveber G, Dirks P, Tymianski M, Crawley A, Vesely A, Kassner A, Preiss D, Somogyi R, Fisher JA. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg. 2005;103:347–355. doi: 10.3171/jns.2005.103.2.0347. [DOI] [PubMed] [Google Scholar]
- Miller RM, Tenney SM. Dead space ventilation in old age. J Appl Physiol. 1956;9:321–327. doi: 10.1152/jappl.1956.9.3.321. [DOI] [PubMed] [Google Scholar]
- Prause G, Hetz H, Lauda P, Pojer H, Smolle-Juettner F, Smolle J. A comparison of the end-tidal-CO2 documented by capnometry and the arterial pCO2 in emergency patients. Resuscitation. 1997;35:145–148. doi: 10.1016/s0300-9572(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Prisman E, Slessarev M, Azami T, Nayot D, Milosevic M, Fisher J. Modified oxygen mask to induce target levels of hyperoxia and hypercarbia during radiotherapy: a more effective alternative to carbogen. Int J Radiat Biol. 2007;83:457–462. doi: 10.1080/09553000701370894. [DOI] [PubMed] [Google Scholar]
- Prisman E, Slessarev M, Han J, Poublanc J, Mardimae A, Crawley A, Fisher J, Mikulis D. Comparison of the effects of independently-controlled end-tidal PCO2 and PO2 on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imaging. 2008;27:185–191. doi: 10.1002/jmri.21102. [DOI] [PubMed] [Google Scholar]
- Robbins PA, Conway J, Cunningham DA, Khamnei S, Paterson DJ. A comparison of indirect methods for continuous estimation of arterial PCO2 in men. J Appl Physiol. 1990;68:1727–1731. doi: 10.1152/jappl.1990.68.4.1727. [DOI] [PubMed] [Google Scholar]
- Slessarev M, Han J, Mardimae A, Prisman E, Preiss D, Volgyesi G, Ansel C, Duffin J, Fisher JA. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi RB, Vesely AE, Preiss D, Prisman E, Volgyesi G, Azami T, Iscoe S, Fisher JA, Sasano H. Precise control of end-tidal carbon dioxide levels using sequential rebreathing circuits. Anaesth Intensive Care. 2005;33:726–732. doi: 10.1177/0310057X0503300604. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Cunningham DA, Kowalchuk JM, McConnell AK, Kirby AS, Scheuermann BW, Petrella RJ, Paterson DH. Estimation of arterial PCO2 in the elderly. J Appl Physiol. 1995;79:2086–2093. doi: 10.1152/jappl.1995.79.6.2086. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Robertson HT, Hlastala MP. Effects of inspired carbon dioxide on ventilation-perfusion matching in normoxia, hypoxia, and hyperoxia. Am J Respir Crit Care Med. 1994;149:1563–1569. doi: 10.1164/ajrccm.149.6.8004314. [DOI] [PubMed] [Google Scholar]
- Venkataraman ST, Hudson C, Fisher JA, Flanagan JG. The impact of hypercapnia on retinal capillary blood flow assessed by scanning laser Doppler flowmetry. Microvasc Res. 2005;69:149–155. doi: 10.1016/j.mvr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Vesely A, Sasano H, Volgyesi G, Somogyi R, Tesler J, Fedorko L, Grynspan J, Crawley A, Fisher JA, Mikulis D. MRI mapping of cerebrovascular reactivity using square wave changes in end-tidal PCO2. Magn Reson Med. 2001;45:1011–1013. doi: 10.1002/mrm.1134. [DOI] [PubMed] [Google Scholar]
- Williams JS, Babb TG. Differences between estimates and measured PaCO2 during rest and exercise in older subjects. J Appl Physiol. 1997;83:312–316. doi: 10.1152/jappl.1997.83.1.312. [DOI] [PubMed] [Google Scholar]
- Wise RG, Pattinson KT, Bulte DP, Chiarelli PA, Mayhew SD, Balanos GM, O'Connor DF, Pragnell TR, Robbins PA, Tracey I, Jezzard P. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2007;27:1521–1532. doi: 10.1038/sj.jcbfm.9600465. [DOI] [PubMed] [Google Scholar]