Abstract
Fructose consumption has increased dramatically but little is known about mechanisms regulating the intestinal fructose transporter GLUT5 in vivo. In neonatal rats, GLUT5 can be induced only by luminal fructose and only after 14 days of age, unless the gut is primed with dexamethasone prior to fructose perfusion. To elucidate the mechanisms underlying dexamethasone modulation of GLUT5 development, we first identified the receptor mediating its effects then determined whether those effects were genomic. The glucocorticoid receptor (GR) antagonist RU486 dose-dependently prevented the dexamethasone-mediated effects on body weight, intestinal arginase2 (a known GR-regulated gene) and GLUT5. In contrast, an antagonist of the mineralocorticoid receptor as well as agonists of progesterone (PR) and pregnane-X (PXR) receptors did not block the effects of dexamethasone. These receptor antagonists and agonists had no effect on the intestinal glucose transporter SGLT1. Translocation of the GR into the enterocyte nucleus occurred only in dexamethasone-injected pups perfused with fructose, was accompanied by marked increases in brush border GLUT5 abundance, and was blocked by RU486. A priming duration of ∼24 h is optimal for induction but actinomycin D injection before dexamethasone priming prevented dexamethasone from allowing luminal fructose to induce GLUT5. Actinomycin D had no effect on dexamethasone-independent fructose-induced increases in glucose-6-phosphatase mRNA abundance, suggesting that it did not prevent fructose-induction of GLUT5, but instead prevented dexamethasone-induced synthesis of an intermediate required by fructose for GLUT5 regulation. In suckling rats < 14 days old, developmental regulation of transporters may involve cross-talk between hormonal signals modulating intestinal maturation and nutrient signals regulating specific transporters.
Worldwide consumption of fructose in the form of high-fructose corn syrup has skyrocketed in recent years, with estimated increases in the USA ranging from 2000 (Gross et al. 2004) to 4000% (Putnam et al. 2002). This marked increase has been linked to the obesity epidemic, including childhood obesity (Ludwig et al. 2001). Consumption of soft drinks has increased 500% in the past 50 years. Soft drinks like carbonated sodas and artificially flavoured fruit juices generally use high-fructose corn syrup as sweeteners, and are now the leading source of added sugars in young children's diets (Ludwig et al. 2001). Despite the importance of fructose to human health, relatively little is known about the regulation of fructose absorption in the small intestine, particularly in neonates.
Fructose absorption from the intestinal lumen is mediated by the fructose transporter GLUT5 (Slc2A5) (Burant et al. 1992). GLUT5 is a facilitative transport system specific for fructose and localized in the apical membrane of intestinal epithelial cells (Manolescu et al. 2007). The sodium-dependent glucose transporter SGLT1 (Slc5A1) is also localized in the apical membrane and is responsible for most glucose transport (Gromova et al. 2006; Wright et al. 2007) whereas GLUT2 (Slc2A2) is the primary glucose and fructose transporter in the basolateral membrane (Boudry et al. 2007) but is postulated to translocate transiently to the apical membrane at high glucose or high fructose loads (Gouyon et al. 2003). GLUT5 is normally expressed at low baseline levels throughout the suckling (0–14 days of age) and weaning (14–28 days) stages in neonatal rats. GLUT5 expression and activity, however, are dramatically enhanced by precocious introduction of fructose into the intestinal lumen before weaning is completed, but only after ∼14 days of age, indicating sharply defined developmental limits in regulation. In differentiated cells lining the intestinal villi of 20-day-old pups, marked increases in GLUT5 mRNA abundance occur within a few hours of fructose consumption. The increase is actinomycin D sensitive, indicating that the fructose effect on GLUT5 at 20 days of age is direct and genomic (Jiang & Ferraris, 2001).
In a previous study, we found by microarray one subset of fructose-responsive genes in intestines of 20-day-old pups whose GLUT5 can be directly modulated by its substrate fructose (Cui et al. 2004), and another subset of fructose-responsive genes in 10-day-old pups whose GLUT5 cannot be up-regulated by fructose. We then also determined which of the fructose-responsive genes in 10-day-old pups changed in expression in 20-day-old pups, i.e. fructose-responsive genes whose expression changed with age (Douard et al. 2008). We found by gene-clustering analysis that a large number of age-sensitive and fructose-responsive genes were modulated by glucocorticoids. We then found that injection of 10-day-old pups with a synthetic glucocorticoid, dexamethasone (Dex), allows luminal fructose to modulate intestinal GLUT5 expression and activity in these pups. This exciting discovery suggests that marked differences in GLUT5 regulation exist between the suckling 10-day-old and weaning 20-day-old stages, and that Dex treatment mimics the effect of age on intestinal GLUT5 expression.
Glucocorticoid-modulated activities are classically explained by non-genomic and genomic mechanisms. Non-genomic actions are relatively rapid (latency time < 1 h) and are mediated by distinct plasma membrane/cytosolic receptors (Tasker et al. 2006). In contrast, the genomic mechanism involves binding of the glucocorticoid to cytosolic steroid receptors that subsequently translocate to the nucleus in a ligand-dependent manner, and then the hormone–receptor complex either by itself or with other transcription factors interacts with genomic DNA at a glucocorticoid response element to initiate transcription (Lu & Cidlowski, 2006). This response to the transcriptional effects of glucocorticoids requires a latency time ranging from several hours to days. Both non-genomic and genomic actions have been previously described in the intestine (Harvey et al. 2002; Boivin et al. 2007). Glucocorticoids mediate a plethora of effects, but one classical effect involves precocious maturation of the gastrointestinal tract. For example, they cause an early appearance of certain hydrolytic enzymes and nutrient transporters in the enterocytes of many species (Galand, 1989; Lebenthal & Lebenthal, 1999; Agbemafle et al. 2005). However, the molecular mechanisms underlying the glucocorticoid-induced maturation of absorptive functions are not known. Moreover, the interaction of endogenous signals like steroids with environmentally derived signals like enteral nutrients has never been investigated. This is especially true in the transition period between late gestation and suckling stages when enteral nutrients enter the lumen for the first time, or between suckling and weaning stages when the diet shifts from homogeneous (like milk) to heterogeneous nutrient sources. These transition periods involving dietary shifts may parallel changes in hormone signals.
The small intestine of suckling rats has long been considered an excellent model of that in the pre-term human newborn (Solomon et al. 2001). Since glucocorticoid analogues are used to induce lung maturation in prematurely born infants, then the effects of these analogues on intestinal maturation can best be studied in suckling rats. Because GLUT5 is regulated by both glucocorticoids and its substrate fructose, it is therefore an excellent model to study the interaction between dietary and hormonal signals during early development of the intestine.
Dex, like the endogenous hormone cortisol, binds the glucocorticoid (GR), mineralocorticoid (MR) and pregnane-X (PXR) receptors which we have shown to be expressed in the neonatal rat intestine. In this study, we first determined the identity of the receptor involved in mediating the glucocorticoid-allowed, fructose-induced precocious development of GLUT5. After showing that the precocious fructose enhancement of GLUT5 by Dex is GR-mediated, we tracked by immunocytochemistry the translocation of the GR following Dex administration and fructose perfusion. Finally, we examined whether the effect of Dex on GLUT5 is genomic or non-genomic, and whether it requires upstream intermediates that in turn mediate the effect of fructose on GLUT5.
Methods
Animals and treatment
All the procedures conducted in this study were approved by the Institutional Animal Care and Use Committee, UMDNJ-New Jersey Medical School. Pregnant female Sprague–Dawley rats were purchased from Taconic (Germantown, NY, USA), housed in the research animal facility under a 12 h light: 12 h dark photoperiod and controlled temperatures (22–24°C), and fed a commercial diet ad libitum (Purina Mills, Richmond, IN, USA). After birth (day 0), the rat pups were kept with their dams until 10 days of age when these were removed randomly and used for experiments described below.
Experimental design
In our recent microarray study (Douard et al. 2008) comparing the fructose-perfused intestines of 10- and 20-day-old pups, we found along with GLUT5 a large number of fructose-responsive, age-sensitive genes that were regulated by glucocorticoids. Precocious administration of Dex to 10-day-old suckling pups renders GLUT5 fructose-responsive. Four sets of experiments were done to elucidate the mechanisms underlying the effect of Dex on GLUT5 regulation by fructose.
Experiment 1: Identity of the steroid receptor mediating precocious maturation of GLUT5 regulation
Experiment 1A examined the role of the GR. Two groups of five pups each were injected subcutaneously at 7 days of age with RU486 or mifeprestone (RU, a specific GR antagonist). Two doses were used: 2 μg (g body weight (BW))−1, equivalent to the human dose or RU1x, and 20 μg (g BW)−1 (RU10x) by injecting 2 and 20 μg μl−1, respectively (volume of injection was 1 μl (g BW)−1). At 8 and 9 days of age, these pups received RU1x and Dex or RU10x and Dex (see Fig. 1A for a schematic diagram of the design). The dose of Dex used was 0.1 μg (g BW)−1, using a solution of 0.1 μg μl−1. A negative control group of five pups was injected subcutaneously with vehicle (5% DMSO in PBS, using a volume equal to those used in the experimental groups) at 7, 8 and 9 days of age. The positive control was given vehicle at 7 days then Dex at 8 and 9 days old. After the injections when pups were 10 days of age, the intestines were perfused for 4 h with 100 mm fructose to stimulate GLUT5. This concentration of sugar is physiological as previously described and has been shown to be optimal and consistent in inducing synthesis of GLUT5 mRNA and protein (Ferraris et al. 1990; Jiang et al. 2001; Cui et al. 2005). After perfusion, the jejunum was flushed and tissues isolated for mRNA extraction and immunocytochemistry or everted for sugar uptake measurements. For all the pups, BWs were recorded with each injection and at 10 days old. Then they were used to measure daily growth rates. At the conclusion of each study, rat pups were killed using an intraperitoneal injection of Euthasol (Virbac AH, Inc., TX, USA; 0.2 ml (kg BW)−1).
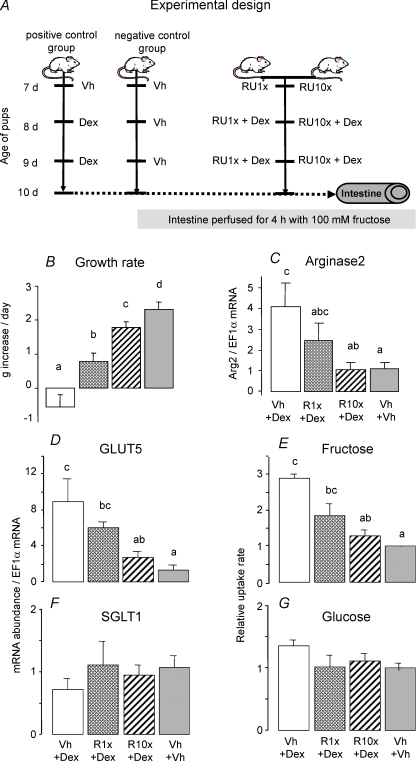
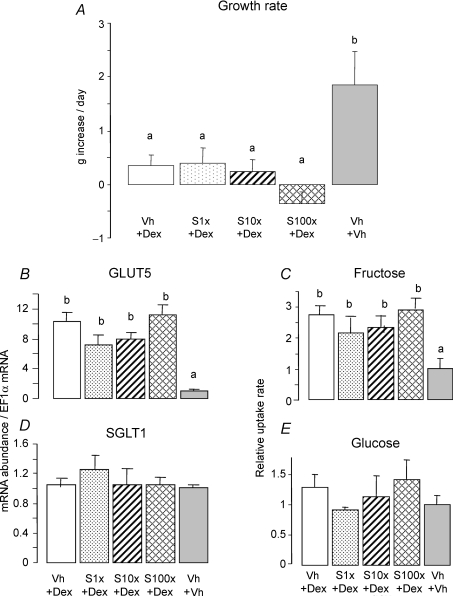
Figure 1. RU486 blockade of the effect of dexamethasone (Dex) on fructose-induced increases in GLUT5 expression and activity.
A, rats received an injection of RU486 (RU1x: 2 μg g−1; RU10x: 20 μg (g body weight (BW))−1) at 7 days of age then a daily injection of Dex and RU (Dex: 0.1 μg (g BW)−1) at 8 and 9 days old. RU1x approximates the dosage typically provided to humans. The rats of the positive control group received vehicle (Vh, 5% DMSO in PBS) injection at 7 days old and then a daily injection of Dex (0.1 μg (g BW)−1) at 8 and 9 days old. Dex dose is about 25% of that given to humans but is more than sufficient to stimulate GLUT5 (Douard et al. 2008). In the negative control group, the pups received three successive injections of vehicle at 7, 8 and 9 days old. Pups of different treatments were littermates and were returned to the dam after injections. At 10 days of age, pups did not receive any more injections but their intestines (shown as a tube) were each perfused for 4 h with 100 mm fructose. B, average daily increase in BW of pups between 7 and 10 days of age (data are means ±s.e.m., n = 5). RU486 dose-dependently prevented Dex from inhibiting growth. Bars with different letter superscripts are significantly different (P < 0.05). Effects of Dex injection and fructose perfusion on the mRNA abundance of arginase2 (C), GLUT5 (D) and SGLT1 (F), determined by real-time PCR using EF1α as a reference gene. Dex enhanced arginase2 and GLUT5 expression but RU486 clearly blocked this Dex effect. The effects of Dex injection and fructose perfusion on fructose (E) and glucose uptakes (G). Dex allowed fructose to enhance fructose uptake rates, but RU486 blocked the Dex effect. The mRNA abundance and uptake rate in 10-day-old pups injected with vehicle were designated as 100%, in order to normalize abundances and rates in other groups to these values.
Experiment 1B assessed the role of the MR. The two control groups were the same as those in Experiment 1A. Three other groups of five pups each were injected subcutaneously at 7 days of age with spironolactone (S), a specific antagonist of the MR, at 2 (S1x, equivalent to the human dose), 20 (S10x) and 200 (S100x) μg (g BW)−1 using three solutions at 2 μg μl−1 and 20 μg μl−1 and 200 μg μl−1, respectively (a similar volume of 1 μl (g BW)−1 was injected into all pups). Then at days 8 and 9, these pups received S1x plus Dex, S10x plus Dex, or S100x plus Dex.
Experiment 1C evaluated the role of the PXR which has no specific antagonist. Because S and RU486 are also agonists of the PXR, we determined if these drugs were able to induce GLUT5 in the absence of Dex. Four groups of five pups each were injected at 8 and 9 days old with vehicle (5% DMSO in PBS, negative control), Dex alone (positive control), RU10x alone or S10x alone.
Experiment 1D evaluated the role of the progesterone receptor (PR) because Dex and RU486 are also its agonist and antagonist, respectively. Here we used a specific agonist of the PR, norenthindrone (Nor, in 20% ethanol in PBS) which has no GR agonist activity (Schoonen et al. 2000). Three groups of four pups each were injected at 8 and 9 days old with vehicle (20% ethanol in PBS, negative control, 1 μl (g BW)−1), Dex alone (positive control) or norenthindrone alone (at 0.063 μg (g BW)−1, the active dose of norenthindrone for PR activation (Schoonen et al. 2000). The solution of norenthindrone was 0.063 μg μl−1 and the volume injected per pups was 1 μl (g BW)−1.
Experiment 2: Determining whether the effect of Dex on GLUT5 development is direct or requires intermediates
Experiment 2A determined the optimal duration between Dex injection and GLUT5 induction by fructose (see Fig. 7A for a schematic diagram of the design). At 10 days of age, rats (n = 5 rats per group, four groups) were perfused with fructose (100 mm in Krebs Ringer Bicarbonate (KRB)) 0.5, 4, 24 and 48 h after a single subcutaneous injection of Dex (0.1 μg (g BW)−1). Two additional groups (n = 5 rats per group) received daily Dex or vehicle (5% DMSO in PBS) injections 24 and 48 h before fructose perfusion.
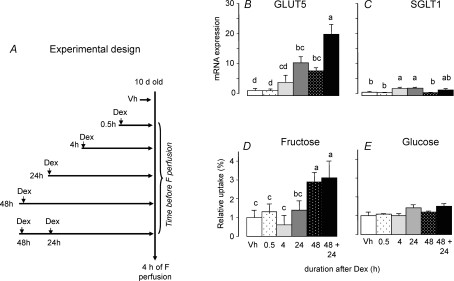
Figure 7.
A, time course of changes in GLUT5 and SGLT1 expression and transport activities as a function of time after injection of Dex to 8–10 days suckling pups prior to an additional 4 h of fructose (F) perfusion. Fructose perfusion was performed 0.5, 4, 24 and 48 h after a single injection of Dex. The negative control group (Vh) was injected with vehicle (5% DMSO solution) 24 h before perfusion and the positive control group received two injections of Dex (0.1 μg (g BW)−1) 48 and then 24 h prior to fructose perfusion. The effects of Dex injection and fructose perfusion on GLUT5 (B) and SGLT1 (C) mRNA levels as measured by real-time PCR using EF1α as a reference gene, and on fructose (D) and glucose (E) uptake rates. The mRNA abundance and uptake rate in 10-day-old pups injected with vehicle were designated as 100%, in order to normalize abundances and rates in other groups to these values. Bars (means ±s.e.m. (n = 5)) with different superscript letters are significantly different (P < 0.05) by one-way ANOVA. The duration required for the effect of a single Dex injection on GLUT5 expression and activity seems to be > 4 h, and optimal at 24–48 h.
Experiment 2B evaluated the existence of a potential intermediate mediating the effect of Dex and GR on the ability of fructose to stimulate GLUT5 in suckling pups < 14 days old (see Fig. 8A for a schematic diagram). Since the effect of Dex is genomic (as suggested by Experiment 2A), we used the transcription inhibitor actinomycin D to prevent GR from inducing the transcription of this intermediate. Two groups (n = 10 per group) of 8-day-old pups were subcutaneously injected with actinomycin D (0.2 μg (g BW)−1) and another two groups were injected with vehicle (for actinomycin D, 5% DMSO in PBS). Three hours later, one subgroup (n = 5 per subgroup) of the actinomycin D group received a single Dex injection (0.1 μg (g BW)−1) and the second subgroup a vehicle injection (for Dex, also 5% DMSO in PBS). Likewise, two subgroups of the vehicle group received either Dex or vehicle. After 48 h when pups were 10 days old, all four subgroups were perfused for 4 h with 100 mm fructose solution.
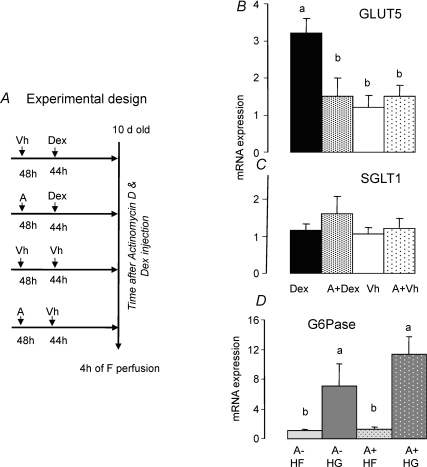
Figure 8. Effect of actinomycin D on GLUT5, SGLT1 and G6Pase mRNA expression in intestine of 10-day-old pups after 4 h of fructose perfusion.
A, actinomycin D (A, 0.2 μg (g BW)−1) was injected in 8-day-old pups 4 h before a single Dex (A + Dex) or vehicle injection (A + Vh) and 2 days before perfusing pups for 4 h with fructose solution. A positive control (Vh + Dex) and negative control group (Vh + Vh) received, respectively, only Dex or vehicle injections before fructose perfusion. The mRNA abundance of GLUT5 (B) and SGLT1 (C) was measured by real-time PCR using EF1α as a reference gene. The mRNA abundance in negative control pups (not injected with Dex and actinomycin D) was designated as 100%, in order to normalize other groups to this value. Bars (means ±s.e.m. (n = 5)) with different superscript letters are significantly different (P < 0.05) by one-way ANOVA. Actinomycin D prevented Dex from allowing fructose to stimulate GLUT5. To ensure that actinomycin D blocked the effect of Dex and not the effect of fructose on GLUT5, we designed another experiment. D, here, actinomycin D (0.2 μg (g BW)−1), was injected in 8-day-old pups 48 h before perfusing pups for 4 h with high (100 mm) fructose (HF) or glucose (HG, negative control) solutions, as the target gene G6Pase can be stimulated by fructose without Dex (Kirchner et al. 2006). The mRNA abundance of G6Pase (measured by real-time PCR using EF1α as a reference gene) in 10 day-old glucose-perfused pups was designated as 100%, in order to normalize other groups to this value. Fructose can stimulate G6Pase 48 h after actinomycin D injection.
Based on results from Experiments 2A and 2B, there may be at least two sequential series of transcription events, that of Dex stimulating the transcription of an intermediate (or intermediates) followed by that intermediate allowing fructose to stimulate the transcription of GLUT5 (see Fig. 9).
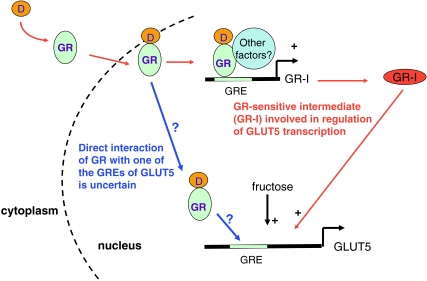
Figure 9. Schematic model of precocious enhancement of GLUT5 expression in suckling pups by Dex.
In suckling pups after Dex (D) injection, Dex binds to the GR in the cytosol and then the Dex–GR complex translocates to the nucleus to stimulate (+) the synthesis of a GR-sensitive intermediate/s (GR-I(s)). This intermediate then allows fructose to stimulate GLUT5 either directly, for example as a cofactor binding to the GLUT5 promoter, or indirectly for example, as a cytoplasmic precursor inducing aggregation of transcription factors required for GLUT5 synthesis. While it seems clear that the effect of Dex on GLUT5 is indirect requiring fructose and at least one intermediate, it is not clear whether GR is again involved (?) in inducing GLUT5 transcription by binding to one or more GR response elements (GRE) identified in the GLUT5 promoter region.
Experiment 2C distinguished between the potential inhibitory effect of actinomycin D on fructose induction of GLUT5 transcription itself from its inhibitory effect on Dex induction of transcription of this unknown intermediate. We showed previously that glucose-6-phosphatase (G6Pase) gene expression increases dramatically in the fructose-perfused intestine of 10-day-old pups even in the absence of Dex (Cui et al. 2004). Two groups of 8-day-old pups (n = 4 rats per group) were subcutaneously injected with actinomycin D (0.2 μg (g BW)−1), and another two with vehicle. About 48 h after Dex injection, actinomycin D as well as vehicle-injected pups were perfused with either 100 mm fructose or glucose.
Intestinal perfusion
Rat intestinal perfusion followed the method previously described (Jiang & Ferraris, 2001). Briefly, rat pups were initially anaesthetized (0.5–0.6 ml (100 g BW)−1, i.p., with 30% urethane) and kept under continuous anaesthesia for 4 h of perfusion. After opening the abdominal cavity 10 cm distal to the stomach, a small incision was made, and a catheter was inserted into the lumen. After the contents were flushed, the small intestine was continuously perfused with fructose solution (100 mm fructose in KRB solution, 37°C) at a rate of 30 ml h−1 at 37°C using a peristaltic pump.
Fructose and glucose uptake measurements
For each pup, the four 1 cm jejunal segments were pre-incubated at 37°C for 5 min in Ringer solution bubbled with 95% O2–5% CO2 as previously described (Karasov & Diamond, 1983; Jiang & Ferraris, 2001). The sleeves were then incubated in 50 mm sugar solutions containing either 10 μCi d-[14C]glucose (NEN, Boston, MA, USA) for 1 min or 10 μCi of d-[14C]fructose for 2 min. We used 20 μCi of l-[3H]glucose to correct for adherent fluid and passive diffusion of glucose or fructose.
Extraction of mRNA and reverse transcription (RT) reaction
Total RNA was isolated from 100 mg of frozen small intestine tissue (−70°C) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed with 5 μg total RNA and 200 units of SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) at 37°C for 1 h as described by the manufacturer.
Real-time PCR
Primers were designed using primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/) (Table 1) and were purchased from Integrated DNA Technologies (IDT, Coralville, IA, USA). Real-time PCR was performed using a Stratagene Mx3000P (Stratagene, La Jolla, CA, USA) as follows: the reactions were performed in 12.5 μl with 300 nm of each primer and 5 μl of a 1: 30 dilution of the RT reaction and the SYBR-Green PCR Master Mix (Stratagene, La Jolla, CA, USA) according to the manufacturer's instructions. The thermal cycling protocol was as follows: 95°C for 10 min, followed by 40 cycles of PCR at 95°C for 30 s, then annealing steps at 59°C (EF1α, Arg2, G6Pase, GLUT5 and SGLT1), 56°C (GR, MR and PXR) or 55°C (MDR1) for 30 s (Table 1) and 72°C for 1 min for extension. After the final cycle, melting curves were monitored from 55°C to 95°C (0.05°C s−1). The relative expression ratios of target genes were calculated as previously described (Douard et al. 2008). In all the experiments, the control group was the 10-day-old pups vehicle-injected and fructose-perfused except for Experiment 2B in which the control group was the 10-day-old pups vehicle-injected and glucose-perfused. The target gene expression was also normalized by a reference gene, α-elongation factor1, EF1α, which we had already validated as having a stable expression level in our experiments (Douard et al. 2008).
Table 1.
Primers chosen for the quantification of target gene transcripts
| Gene (protein) | Accession no. | Direction | Primer sequence (5′→3′) | Probe size (pb) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| Arg2 (Arg2) | NM_019168 | Forward | CGTCTCCCGTCTCCTCCAC | 291 | 59 |
| Reverse | ACCACCTCAGCCAGTTCCTG | ||||
| G6pace (G6pace) | NM_013098 | Forward | GGCTCACTTTCCCCATCAGG | 146 | 59 |
| Reverse | ATCCAAGTGCGAAACCAAACAG | ||||
| EeF1α (EF1 α) | NM_175878 | Forward | CTCCACTTGGTCGTTTTGCTGT | 165 | 59 |
| Reverse | AGACTGGGGTGGCAGGTGTT | ||||
| Slc2a5 (GLUT5) | NM_031741 | Forward | TGCAGAGCAACGATGGAGAAA | 220 | 59 |
| Reverse | ACAGCAGCGTCAGGGTGAAG | ||||
| Abcb1b (MDR1) | NM_012623 | Forward | TGTTGGTCTATGCGTCTTATGC | 246 | 55 |
| Reverse | CTGGTTTGTGTCCCTTGGTT | ||||
| Slc5a1 (SGLT1) | NM_013033 | Forward | GACTGGGTTTGCTTTCCGAGA | 451 | 57 |
| Reverse | TGTTGGGTAGGCGATGTTGG | ||||
| Nr3c1 (GR) | NM_012576 | Forward | TGAAATGGGCAAAGGCGATA | 151 | 56 |
| Reverse | GGAGCAAAGCAGAGCAGGTT | ||||
| Nr3c1 (MR) | M36074 | Forward | TTTGGTGTGTGGAGATGAGG | 319 | 56 |
| Reverse | CGGAGCGATGTATGTGGTC | ||||
| Nr1i2 (PXR) | NM_05298 | Forward | CGACACGGAAACAGGAACC | 261 | 56 |
| Reverse | TGGGGAGAAGAGGGAGATG |
Immunohistochemistry
Rat pup jejunum was fixed in fresh 4% paraformaldehyde in PBS (pH 7.35) overnight at room temperature and then embedded in paraffin. Heat-induced antigen retrieval in a programmed pressure cooker (Retriever, Electron Microscopy Services) was performed on 5 μm sections in 0.01 m sodium citrate buffer (pH 6.0). The sections were rinsed in PBS and blocked in 1% normal goat serum for 1 h at room temperature. The sections were then incubated in rabbit anti-rat GLUT5 (Chemicon International 1: 500 in 1% bovine serum albumin in PBS) for 16 h at 4°C, washed in PBS, blocked in 1% normal mouse serum and incubated for 16 h at 4°C in mouse anti-rat GR (US Biological, 1: 500). Secondary antibodies, goat anti-rabbit IgG labelled with Cy 3 (1: 100) and goat anti-mouse IgG labelled with Cy 2 (1: 100, Chemicon International) were applied in sequence, each for 1 h at room temperature. To examine translocation of GR from cytoplasm to nucleus, immunostaining for GR as described was performed with the addition of propidium iodide staining of nuclei. Immunostaining of phosphorylated (Ser-211) GR was performed according to the same protocol. As controls for non-specific staining, blocked sections were incubated an additional 16 h with normal rabbit or mouse serum at 4°C and stained with the appropriate secondary antibody. Stained sections were mounted in Vectashield (Vector Laboratories) and examined at ×40 with a water immersion lens on a laser-scanning confocal microscope (Biorad, Radiance 2100). All images with the same fluor were obtained with the same settings of the microscope. Non-specific staining with secondary antibodies was consistently negligible.
Statistical analyses
Data are presented as means ±s.e.m. A one-way ANOVA was used to determine the difference of relative mRNA abundance and sugar uptakes among groups with different treatments. If there was a significant difference, Fisher's paired least significant difference (LSD) test was used (STATVIEW, Abacus Concepts). Differences were considered significant at P < 0.05.
Results
Identity of the steroid receptor mediating precocious maturation of GLUT5 regulation
Effect of a GR antagonist on GLUT5 expression levels and fructose uptake rates
To ensure that the nuclear receptors potentially mediating the effect of Dex are found in the small intestine, we showed significant expression levels of GR, MR and PXR in 1-, 10- and 20-day-old pups (data not shown, see Fig. 1S in online Supplemental material). Expression levels between 1- and 10-day-old suckling pups were similar, suggesting that expression levels did not vary among 8-, 9- and 10-day-old pups, the age range used in this group. There were increases in expression in 20-day-old pups.
The weight of vehicle-injected pups increased by 2 g day−1 (Fig. 1B). Dex prevented pups from gaining weight. Pups injected with RU1x gained a modest amount of weight, whereas those injected with 10 × human dose (RU10x) gained as much weight per day as those injected with vehicle alone (P < 0.05). Hence, the inhibitory effect of Dex on growth rate can be reduced by RU486 in a dose-dependent manner. Dex increases the expression of arginase2 (Arg2; Fig. 1C) (P = 0.0094), a gene we used as positive control because its transcription is markedly induced by Dex and mediated by GR (Flynn et al. 1999). This stimulatory effect of Dex on arginase2 expression is blocked by RU486 in a dose-dependent manner. Hence, both the inhibitory and stimulatory effects of Dex are demonstrable in suckling rats, and these effects can be modulated by RU486.
Dex markedly increased (by 9-fold, P < 0.001) GLUT5 mRNA levels in rat small intestine perfused with fructose for 4 h (Fig. 1D). RU486 clearly prevented the Dex-allowed, fructose-induced increase in GLUT5 mRNA expression. In fact, RU10x totally blocked the Dex + fructose-induced increase in GLUT5 expression. In vitro uptake of fructose followed a similar pattern (P < 0.01) (Fig. 1E). In contrast to that of GLUT5, the expression of SGLT1 mRNA was independent (P > 0.58) of Dex- and RU486-injection, suggesting that the effect of Dex and RU486 are specific for GLUT5 (Fig. 1F). Moreover, glucose uptake did not change with either Dex or RU486 treatments (P > 0.17) (Fig. 1G).
Effect of a MR antagonist
Dex-injected pups treated without or with spironolactone at various doses, even at 100 × the human dose, did not grow (Fig. 2A). This suggests that the MR does not mediate the inhibitory effect of Dex on growth. GLUT5 mRNA abundance and fructose uptake rate were low in those injected with vehicle alone and perfused with fructose (Fig. 2B and C). In contrast, GLUT5 expression levels and fructose uptake rates remained high after fructose perfusion in Dex-injected pups treated with or without spironolactone, suggesting that MR blockade did not interfere with the Dex-mediated induction of GLUT5 by fructose. SGLT1 and glucose uptakes were similar in intestines of pups injected with vehicle alone, Dex alone, or Dex with spironolactone, indicating not only that the effect of Dex on GLUT5 is specific, but also that spironolactone in combination with Dex does not compromise glucose uptake in the small intestine (Fig. 2D and E).
Figure 2. Effect of the mineralocorticoid receptor (MR) antagonist spironolactone (S) on the stimulating effect of Dex on fructose-induced GLUT5 expression and activity.
The same design as the one described in Fig. 1A was followed, except that the rats received an injection of spironolactone (S1x: 2 μg g−1; S10x: 20 μg g−1; S100x: 200 μg (g BW)−1) at 7 days of age then a daily injection of Dex + spironolactone (Dex: 0.1 μg (g BW)−1) at 8 and 9 days old. As in Fig. 1, the positive control group (Dex) received vehicle injection at 7 days old and then a daily injection of Dex (0.1 μg (g BW)−1) at 8 and 9 days old. The negative control group received three successive injections of vehicle at 7, 8 and 9 days old. At 10 days of age, all the pups were perfused for 4 h with 100 mm fructose. A, average daily increase in BW of pups between 7 and 10 days of age (bars are means ±s.e.m., n = 5). Bars with different letter superscripts are significantly different (P < 0.05). Rats treated with Dex alone as well as Dex and spironolactone did not grow. The effects of Dex injection and fructose perfusion on the mRNA abundance of GLUT5 (B) and SGLT1 (D) had been determined by real-time PCR using EF1α as a reference gene. The effects of Dex injection and fructose perfusion on fructose (C) and glucose uptakes (E). The mRNA abundance and uptake rate in 10-day-old pups injected with vehicle were designated as 100%, in order to normalize abundances and rates in other groups to these values. Spironolactone did not block the effect of Dex on GLUT5 expression and activity.
Effect of PXR and PR agonists
PXR and PR have no specific antagonists, hence we determined whether they may be involved in allowing fructose to induce GLUT5 by injecting agonists. Dex, RU486 and spironolactone are all agonists of PXR with affinity values in the 5–20 μm range (Blumberg et al. 1998; Kliewer et al. 1998; Sheppard, 2002), while norenthindrone is a potent agonist of PR (134% relative binding affinities to PR compared to progesterone) (Sitruk-Ware et al. 2007).
Growth rate was normal in vehicle-injected pups, and this rate was matched by pups injected with RU486 and spironolactone at doses 10 × those used in humans (Fig. 3A), hence PXR may not mediate the effect of Dex on growth. The expression level of MDR1 (gene coding for transmembrane ABC-transporter MDR1/P-glycoprotein) increased by 2- to 3-fold (P < 0.01) in RU10x- and S10x-injected pups (Fig. 3B). We used MDR1 as a positive control because its transcription is known to be mediated by PXR (Sheppard, 2002). Surprisingly, pups injected with Dex at 0.1 μg g−1 did not induce an increase in MDR1 mRNA, perhaps because the dose used is much lower than that recommended for humans and therefore insufficient to alter the expression of PXR-mediated genes. Dex induced a marked increase in GLUT5 expression in fructose-perfused pups (Fig. 3C), but RU486 alone or spironolactone alone did not affect GLUT5 mRNA abundance in the intestine of 10-day-old pups perfused with fructose. The data indicate not only that neither of the GR antagonists RU486 and spironolactone has a direct effect on GLUT5, but also that PXR may not be involved in GLUT5 regulation. The expression (Fig. 3D) and activity (not shown) of SGLT1 were also independent of RU10x and S10x alone, suggesting that these GR antagonists and PXR agonists did not affect the uptake of other sugars.
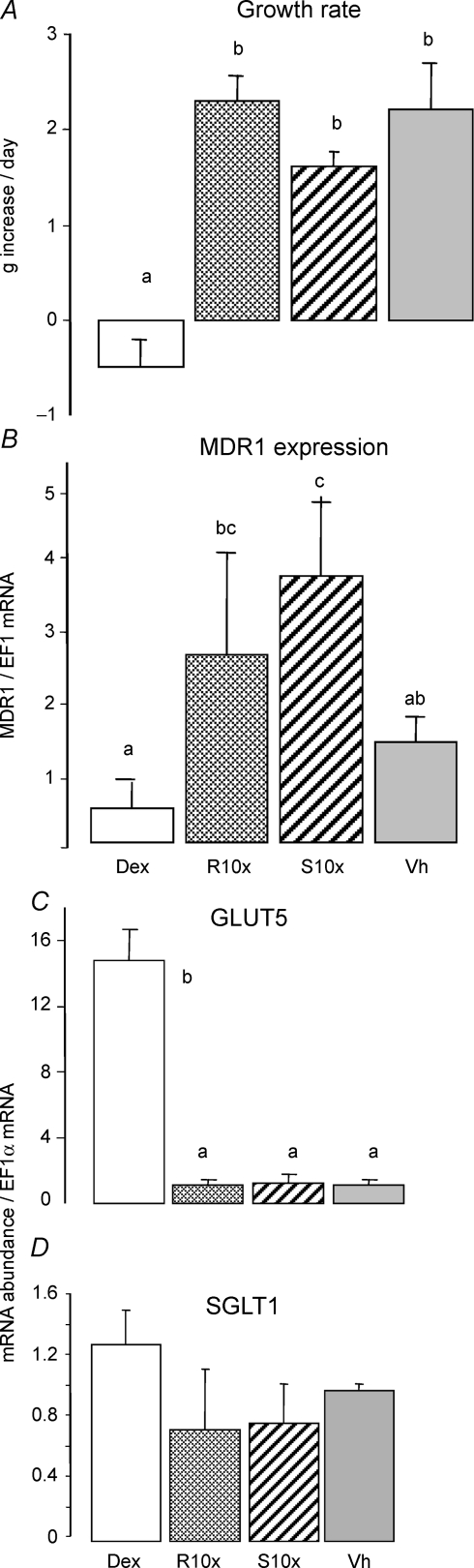
Figure 3. Effect of pregnane-X receptor (PXR) agonists RU486 and spironolactone on GLUT5 expression under fructose perfusion.
The rats received an injection of RU486 (RU10x: 20 μg (g BW)−1), spironolactone (S10x: 20 μg (g BW)−1), or vehicle (Vh) at 7, 8 and 9 days of age. As in Fig. 1, the positive control group (Dex) received vehicle injection at 7 days old and then a daily injection of Dex (0.1 μg (g BW)−1) at 8 and 9 days old. The negative control group received three successive injections of vehicle at 7, 8 and 9 days old. At 10 days of age, all the pups were perfused for 4 h with 100 mm fructose. A, average daily increase in BW of pups between 7 and 10 days of age (bars are means ±s.e.m., n = 5). Bars with different letter superscripts are significantly different (P < 0.05). The mRNA abundance of MDR1 (B), GLUT5 (C) and SGLT1 (D) had been measured by real-time PCR using EF1α as a reference gene. The mRNA abundance and uptake rate in 10-day-old pups injected with vehicle were designated as 100%, in order to normalize abundances and rates in other groups to these values. PXR agonists had no effect on GLUT5 expression.
Norenthindrone had no effect on growth rate, GLUT5 and SGLT1 expression levels (data not shown) (see Supplemental material Fig. 2S, A–C). This experiment excluded the involvement of the PR in fructose-mediated increases in GLUT5 expression. The expression levels of GR, MR and PXR were each independent (P > 0.20) of the various agonists and antagonists used in this study (Fig. 4A–C). Hence, the specific effect of RU486 on Dex-mediated effects, and the absence of effects of spironolactone and norenthindrone were not confounded by alterations in expression levels of the various receptors.
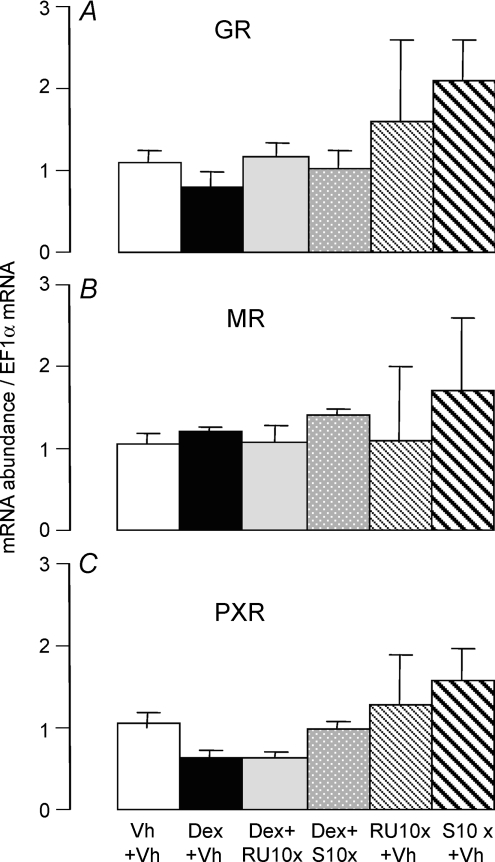
Figure 4. Effects of Dex, RU486, spironolactone (S), Dex + RU486 and Dex + spironolactone on intestinal expression levels of glucocorticoid receptors (GRs), MRs and PXRs in 10-day-old fructose-perfused pups.
Rats received a daily injection of Dex + RU10x (Dex: 0.1 μg (g BW)−1), Dex + S10x, RU10x alone or S10x alone at 8 and 9 days old. The rats of the positive control group (Dex) received vehicle (Vh) injection at 7 days old and then a daily injection of Dex (0.1 μg (g BW)−1) at 8 and 9 days old. In the negative control group (Vh), the pups received three successive injections of vehicle at 7, 8 and 9 days old. Pups were returned to dams after injection. All the pups were perfused for 4 h at 10 days of age with 100 mm fructose. The mRNA abundance of GR (A), MR (B) and PXR (C) had been measured by real-time PCR using EF1α as a reference gene. The mRNA abundance in 10-day-old fructose-perfused pups injected with vehicle was designated as 100% and abundances in other groups normalized to this value. Bars are means ±s.e.m. (n = 5).
Dex-induced translocation of the GR
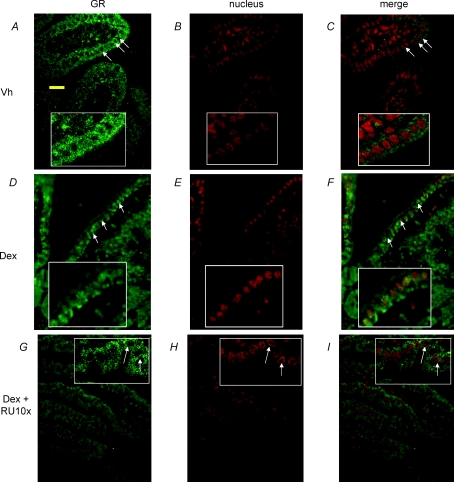
In vehicle-injected pups perfused with fructose, the GR was distributed only in the cytoplasm of intestinal cells (Fig. 5A, B and C). In contrast, in pups injected with Dex before fructose perfusion, GR was observed both in the cytoplasm and in the nuclei of intestinal cells (Fig. 5D, E and F). The merged yellow fluorescence images of GR (green) and nuclei (red) clearly showed that GR was translocated into the nuclei (Fig. 5F). Injecting the GR inhibitor RU486 prior to Dex injection prevented GR translocation (Fig. 5G, H and I). This suggests that translocation of GR may be required for Dex-allowed induction of GLUT5 by fructose. Control slides (not shown) indicate very low levels of non-specific binding.
Figure 5. Localization of the GR in intestinal cells of 10-day-old rats from Experiment 1.
(Fig. 1) A, pups injected with vehicle for 3 days then perfused with fructose at 10 days of age. Cytoplasmic localization of the GR; arrows indicate absence of GR in the central (nuclear) region of cells where nuclei are typically located. Inset: close-up of cells without GR in the central region. Yellow scale bar, 20 μm. B, nuclear staining of intestinal cells with propidium iodide. C, merged image indicating almost complete absence of GR in the nuclei. D, pups injected with Dex at 8 and 9 days of age, then perfused with fructose at 10 days of age. Arrows indicate that the GR is present in most of the cytoplasm, including the central (nuclear) regions. After nuclear staining (E), the merged (F) images now show yellow where the nuclei are, suggesting GR translocation into the nucleus. G, pups injected with RU486 at 7 days of age, then with RU486 + Dex at 8 and 9 days of age, followed by fructose perfusion at 10 days of age. Arrows indicate that RU486 prevented GR from occupying the central region of cells where nuclei are typically located. After nuclear staining (H), the merged (I) image is mostly red where the nuclei are, suggesting absence of GR in the nuclear region. These images are typical of n = 2 pups from each treatment (Fig. 1A).
The immunohistochemical staining of GLUT5 confirms our functional data from Fig. 1E and clearly showed that in the 10-day-old fructose-perfused pups injected with vehicle, when the GR was localized in the cytoplasm only (Fig. 5A), GLUT5 was poorly expressed in the intestinal epithelial cells (Fig. 6A). Little GLUT5 was expressed in the apical membrane, and very little was detectable in the cytoplasm. Once Dex was injected, and then the intestine perfused with fructose (Fig. 6B), GLUT5 proteins became highly expressed in the apical membrane of intestinal epithelial cells where GR proteins were observed not only in the cytoplasm but also in the nuclei (Fig. 5D–F). The injection of GR inhibitor RU486 prior to Dex injection prevented Dex-allowed induction of GLUT5 by fructose (Fig. 6C). This evidence strengthens the link between Dex-allowed, fructose-induced increases in GLUT5 to the GR translocation to the nuclei. Control slides (Fig. 6D) indicate very low levels of non-specific GLUT5 binding.
Figure 6. Localization of GLUT5 protein in intestinal cells of 10-day-old rats from Experiment 1.
(Fig. 1) A, GLUT5 expression in intestinal villi of 10-day-old pups injected with vehicle at 7, 8 and 9 days old and then perfused with fructose at 10 days of age. Yellow scale bar, 20 μm. Low levels of GLUT5 are found in the apical membrane of intestinal villi; almost none can be found in the cytoplasm. B, pups injected with vehicle at 7 days of age followed by Dex at 8 and 9 days old, then fructose perfusion at 10 days of age. There is intense GLUT5 staining in the apical membranes of cells, and cytoplasmic presence of immunoreactive, probably newly synthesized (within the past 4 h following start of fructose perfusion) GLUT5. C, GLUT5 expression in intestinal villi of 10-day-old pups injected with RU486 at 7 days of age, then with RU486 + Dex at 8 and 9 days of age, followed by fructose perfusion at 10 days of age. There is a very low level of GLUT5 staining in the apical membrane of intestinal villi, similar to that observed in A. D, the tissues used for GLUT5 control were incubated with rabbit pre-immune serum followed by secondary antibody as previously described. Because the images were virtually absent at settings used for A–C, gain was increased × 10 to generate an image.
Low levels of staining for phosphorylated GR were present in intestinal epithelial cells of 10 day pups treated with Dex and fructose, or Dex and glucose (not shown). In neither instance was there evidence of nuclear localization of phosphorylated GR.
Duration required for the effect of Dex on GLUT5 expression and activity
Dex via the GR allows fructose to increase GLUT5 in 10-day-old pups, either by directly interacting with the GLUT5 promoter, or indirectly by interacting with the promoter/s of other gene/s that synthesize upstream intermediates and key signalling components mediating the effect of fructose on GLUT5. To distinguish between the two possibilities, we first determined the time course of the Dex-allowed enhancement of GLUT5 by fructose (Fig. 7A), and then used a transcription inhibitor that would block the synthesis of an intermediate without blocking the synthesis of GLUT5 mRNA.
As expected, two daily injections of 0.1 mg kg−1 of Dex 48 h and 24 h before fructose perfusion allowed fructose to dramatically induce GLUT5 (Fig. 7B) but not SGLT1 (Fig. 7C) expression. Likewise, fructose (Fig. 7D) but not glucose (Fig. 7E) uptake increased. This effect of daily Dex injections over 2 days can be replicated by a single dose injected either 24 or 48 h before intestinal fructose perfusion in vivo (Fig. 7B). In contrast, Dex did not induce GLUT5 expression and fructose uptake if injected only 0.5–4 h before fructose perfusion. Hence, Dex has no significant acute, non-genomic effect on GLUT5 and a very modest acute effect on SGLT1 expression as well as activity.
Effect of actinomycin D on Dex-allowed induction of GLUT5 by fructose
We have previously shown in 20-day-old pups that actinomycin D, if injected 12 h prior to fructose perfusion, can block the direct effect of fructose on GLUT5 transcription (Jiang & Ferraris, 2001). Injecting actinomycin D > 24 h before fructose perfusion had no direct effect on GLUT5 in 20-day-old weaning pups. To evaluate the potential role of de novo mRNA synthesis of an intermediate in the Dex-allowed induction of GLUT5 by fructose, we injected 10-day-old pups with Dex 44 h before fructose perfusion but 4 h after pretreatment with actinomycin D (Fig. 8A). Pretreatment with actinomycin D abolished the increase in GLUT5 expression induced by Dex and fructose (Fig. 8B), suggesting that the transcription of a Dex-dependent intermediate may have been prevented, and the absence of that intermediate in turn prevented fructose from inducing GLUT5. Actinomycin D alone had no effect on steady-state levels of GLUT5 expression in the absence of Dex, suggesting that it did not reduce constitutive, baseline GLUT5 mRNA abundance. Since SGLT1 expression was similar among pups injected with actinomycin D, pups with actinomycin D + Dex, and pups injected with vehicle alone, neither Dex nor actinomycin D perturbed steady-state levels of mRNA expression of SGLT1 transporters (Fig. 8C).
To eliminate the possibility that actinomycin D had a direct effect on GLUT5, i.e. prevented the fructose-induced transcription of GLUT5 instead of preventing the Dex-induced transcription of an intermediate allowing fructose to upregulate GLUT5, we determined the expression of a fructose-responsive gene, G6Pase, 48 h after actinomycin D injection. Since G6Pase responds directly to fructose without Dex injection, only actinomycin D was injected followed by fructose (HF) or glucose (HG) perfusion of the small intestine. Relative to glucose perfusion, fructose induced a 7-fold increase of G6Pase mRNA abundance in the intestine of the 10-day-old pups (Fig. 8D) following earlier results (Cui et al. 2004; Kirchner et al. 2006). The injection of actinomycin D 48 h before the perfusion did not block this fructose-induced increase in G6Pase mRNA levels, indicating that the injection of actinomycin D > 48 h prior to fructose perfusion no longer modifies the direct effect of luminal fructose on intestinal fructose-responsive genes, including GLUT5. Hence, actinomycin D here prevented the Dex-induced synthesis of an intermediate whose absence resulted in the failure of fructose to stimulate GLUT5.
Discussion
This study describes for the first time the role of glucocorticoids and the GR in regulating the development of an intestinal nutrient transport system. Several transport systems are induced only in certain specific stages of mammalian development, such as GLUT5, the apical sodium-dependent bile acid transporter ASBT (Shneider, 1998) and the intestinal type IIb sodium–phosphate cotransporter (Arima et al. 2002; Xu et al. 2002) and their presence or absence can impact nutritional requirements and adaptations. The main finding of this study is that the Dex-mediated precocious development of GLUT5 occurs by indirect genomic mechanisms and acts via GR translocation to the nuclei.
Genomic effect of Dex on GLUT5 transcription may mimic the endogenous corticosterone surge
Corticosterone is the main glucocorticoid in rats and mice, whereas cortisol is predominant in most other mammals. In neonatal rats < 14 days of age, corticosterone plasma concentrations in vivo are < 0.05 ng ml−1; they begin to surge at 14 days of age, peak at around 0.6 ng ml−1 at 20 days of age, and then decrease and reach their adult levels of 0.2 ng ml−1 (Henning, 1987). By introducing exogenous glucocorticoids into 10-day-old pups, we are precociously stimulating a corticosterone surge well before its time of occurrence, thereby triggering a Dex-dependent synthesis of regulatory factors and target genes that otherwise would have been synthesized only after the endogenous corticosterone surge.
Glucocorticoids are known to cause precocious changes in the development of at least 15 different tissues (Ballard & Ballard, 1995). In the intestine of suckling animals, exogenous glucocorticoids enhance maturation of villi and digestive functions as shown by precocious developmental modifications in the activities of sucrase, maltase and lactase (Henning, 1987). The fructose enhancement of GLUT5 expression and activity in the presence of Dex (Douard et al. 2008) gives us a new marker of the maturation of the small intestine and of the intestine's ability to respond to nutrients like fructose normally available only post weaning. However, the mechanism of GLUT5 enhancement by Dex in the 10-day-old pups is different from what was observed previously for sucrase, maltase or other digestive enzymes. Stimulation of GLUT5 expression and transporter activity requires simultaneously the presence of Dex and intestinal luminal exposure to fructose, whereas Dex alone is sufficient for modifying sucrase and lactase expression.
The Dex-allowed, fructose-induced precocious enhancement of GLUT5 is mediated by the GR
The intestinal GR (affinity ∼30 nm) and MR (∼2 nm) are the two high affinity receptors for corticosterone and Dex, while PXR is the rodent steroid/xenobiotic receptor with a lower affinity for Dex and corticosterone (∼30 μm) (Sheppard, 2002). While these steroid receptors are known to be expressed in the small intestine of adult mouse, rat or human (Zhang et al. 1999), here we show for the first time that these receptors are also significantly expressed in the jejunum of suckling and weaning rats, indicating their potential to mediate the effect of Dex in the small intestine of 10-day-old pups.
The effects of Dex on BW of rat (Ma et al. 2003) and on arginase expression in human gastric cancer cells (Wu et al. 1992) are known to be inhibited by RU486 and thus mediated by the GR. It turns out that the Dex-allowed, fructose-mediated increases in levels of GLUT5 mRNA and rates of fructose uptake are also inhibitable by RU486 and follow exactly the same pattern as the effects of Dex and RU486 on arginase, leading to the conclusion that the Dex-mediated fructose induction of GLUT5 expression in suckling rats is mediated by GR. Mediation by the GR seems specific because the MR and PXR are not involved, no other known intestinal steroid receptor binds Dex at high affinity, and SGLT1 is not affected by GR, MR, PXR and PR agonists and antagonists. RU486 and Dex also have modest antagonist and agonist effects, respectively, on the PR, while norenthindrone is a specific agonist of PR and does not bind GR (Ogle & Beyer, 1982). We can, however, conclude that PR does not mediate the effect of Dex on GLUT5 because norenthindrone had no appreciable effect on GLUT5 expression at doses known to activate PR (Schoonen et al. 2000). Moreover, the endogenous glucocorticoid corticosterone has no appreciable binding for the PR (Ogle & Beyer, 1982), which definitely excludes the potential role of PR in the regulation of GLUT5 during neonatal development in vivo.
Since RU486, Dex and spironolactone did not alter the expression levels of the steroid receptors, the inhibition of Dex effects on GLUT5 by RU486 must not have been confounded by changes in the amount of GR, MR or PXR. The RU486-sensitive translocation of the GR to the nucleus in the presence of Dex and fructose affirms the identity of the GR as the mediator of GLUT5 regulation by Dex (Fig. 9). The Ser-211 phosphorylation of GR is known to trigger its translocation into the nucleus. However, we were not able to observe any differences in levels and in location of phosphorylated GR among suckling pups injected with Dex and perfused with fructose, those injected with vehicle and perfused with fructose, and those injected with Dex but perfused with glucose.
The lack of a known specific target gene for MR made it difficult to prove that MR is not involved in the regulation of GLUT5. However, in COS cells, the affinities of spironolactone and Dex binding to the ligand-binding domain of the MR are 1 nm and 8 nm, respectively (Rogerson et al. 2003, 2004) indicating that at the high dosages used in this study, spironolactone should have blocked the effects of Dex if indeed its effects were mediated by the MR.
Once GR is activated by agonists, it translocates to the nucleus, where GR subunits homodimerize or heterodimerize and act as transcription factors (TFs) (Tsai & O'Malley, 1994). GR-MR heterodimers can be excluded because the effect of Dex on GLUT5 is insensitive to spironolactone. However, the transcriptional regulation of glucocorticoid target genes by GR remains very complex. GR can activate directly or indirectly the transcription of genes carrying the specific glucocorticoid response element (GRE) and modulate, positively or negatively, the activity of its target genes (Jenkins et al. 2001; Kassel & Herrlich, 2007). To examine the potential direct interaction of the GR in GLUT5 promoter, we identified, using the ElDorado system (http://www.genomatix.de), six GR response elements (GREs) in the 0–1250 bp upstream promoter region of GLUT5: −128/−119, −306/−297, −551/−542, −1041/−1032, −1238/−1229, and −1246/−1232. Hence, the direct binding of GR on the promoter area of GLUT5 seems possible. However, the direct interaction of GR with the GLUT5 promoter needs to be confirmed as well as the potential functional GRE(s) identified. Finally, the GR generally also interacts with other TFs: the GR may not bind directly to the DNA, but is recruited to DNA-bound transcription factors in a regulatory complex and then the GR employs protein–protein interactions to exert its effects (Kassel & Herrlich, 2007).
Glucocorticoid-induced transcriptional regulation through the GR involves chromatin remodelling (Trotter & Archer, 2007), and GLUT5 is no exception. Goda and colleagues showed that in IEC6 cells, Dex is able to induce GLUT5 gene expression by controlling acetylation of histones H3 and H4 in the GLUT5 promoter/enhancer region (Takabe et al. 2007). Induction of another sugar-metabolizing gene, sucrase-isomaltase, in the jejunum by a diet rich in carbohydrates is also, in fact, associated with acetylation of histones H3 and H4 (Honma et al. 2007).
GLUT5 regulation by Dex occurs by indirect genomic mechanisms
We clearly showed that fructose-induced increases in GLUT5 mRNA cannot occur within the first 4 h after Dex injection, excluding non-genomic mechanisms known to occur within minutes of glucocorticoid application (Lowenberg et al. 2007) as mediators of Dex effects. Even though regulation of gene expression by steroids could also result from variations in mRNA stability (Schaaf & Cidlowski, 2002; Reddy et al. 2004; Ing, 2005), we can also exclude this process as the mechanism underlying the Dex effect because actinomycin D completely blocked the effect of Dex. Instead, the Dex-allowed, fructose upregulation of GLUT5 most likely requires de novo mRNA synthesis. The mRNA that is synthesized is not GLUT5, and it is not because actinomycin D blocked the fructose-induced increases in GLUT5 mRNA abundance. Since fructose perfusion can enhance G6Pase mRNA abundance 48 h after injection of actinomycin D, this also means that actinomycin D most likely did not prevent fructose from increasing GLUT5 mRNA abundance after 48 h. Rather, our data prove that the direct and genomic effect of fructose on GLUT5 was not altered, and that actinomycin D most likely blocked the synthesis of an intermediate.
Since the induction in GLUT5 mRNA is not noticeable within the first 4 h after Dex, this timing indicates that GLUT5 induction by fructose in the presence of Dex, following the classification of Dean & Sanders for steroid-responsive genes, may be a secondary or delayed primary response (Dean & Sanders, 1996). In 8-day-old mice, primary glucocorticoid response genes, such as Gata4 or Gata6, are induced quickly (1–4 h) after Dex treatment, while the secondary glucocorticoid response genes, such as trehalase, require at least 12 h post injection to be affected and are regulated by the primary response genes (Oesterreicher & Henning, 2004; Agbemafle et al. 2005). Hence, the pattern observed for GLUT5 in neonatal rat small intestine fits the pattern observed for disaccharidases, except GLUT5 requires the luminal presence of fructose as additional signal. Our current model for intestinal development of GLUT5 in suckling rats fits one of two hypotheses (Fig. 9). Either the GR is required for synthesis of both the intermediate and GLUT5, or only of the intermediate. To shed some light on this problem, we are currently identifying by microarray the intestinal genes that respond to Dex 4–12 h after injection, using as a control intestine from pups injected with Dex and actinomycin D.
Physiological and nutritional significance
The results of this study enhance the appreciation of the role of glucocorticoids in regulating the developmental maturation of some digestive and absorptive functions of the intestine, increase our understanding of mechanisms underlying the normal developmental pattern of intestinal fructose absorption, and provide strategies for reprogramming fructose absorption to enhance the nutrition of premature infants. GLUT5 can be used as a general model for exploring the cross-talk between hormonal signals regulating mechanisms of intestinal development, and nutrient signals regulating induction of specific transporters and enzymes as shown in piglets (Petersen et al. 2003). The interaction of GR with fructose is a new finding that opens a wide area of research in which to explore the relationship between glucocorticoids and the major sweetener currently used in human diets, fructose.
Acknowledgments
This work was supported by the NSF Grant Nos. IOS-0722365 and IBN-0235011, as well as NIH Grant No. RDK075617A. We are grateful to Drs Ilene Sugino and Larry Gaspers for help with the micrographs, and Ms Anjali Muduli for help in some experiments.
Supplementary material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.155226/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2008.155226
References
- Agbemafle BM, Oesterreicher TJ, Shaw CA, Henning SJ. Immediate early genes of glucocorticoid action on the developing intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G897–G906. doi: 10.1152/ajpgi.00454.2004. [DOI] [PubMed] [Google Scholar]
- Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Ghishan FK. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-P (i) cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol. 2002;283:G426–G434. doi: 10.1152/ajpgi.00319.2001. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MA, Ye D, Kennedy JC, Al-Sadi R, Shepela C, Ma TY. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2007;292:G590–G598. doi: 10.1152/ajpgi.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol. 2007;292:R862–R867. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- Cui XL, Schlesier AM, Fisher EL, Cerqueira C, Ferraris RP. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1310–G1320. doi: 10.1152/ajpgi.00550.2004. [DOI] [PubMed] [Google Scholar]
- Cui XL, Soteropoulos P, Tolias P, Ferraris RP. Fructose-responsive genes in the small intestine of neonatal rats. Physiol Genomics. 2004;18:206–217. doi: 10.1152/physiolgenomics.00056.2004. [DOI] [PubMed] [Google Scholar]
- Dean DM, Sanders MM. Ten years after: reclassification of steroid-responsive genes. Mol Endocrinol. 1996;10:1489–1495. doi: 10.1210/mend.10.12.8961259. [DOI] [PubMed] [Google Scholar]
- Douard V, Cui XL, Soteropoulos P, Ferraris RP. Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology. 2008;149:409–423. doi: 10.1210/en.2007-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol Gastrointest Liver Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- Flynn NE, Meininger CJ, Kelly K, Ing NH, Morris SM, Jr, Wu G. Glucocorticoids mediate the enhanced expression of intestinal type II arginase and argininosuccinate lyase in postweaning pigs. J Nutr. 1999;129:799–803. doi: 10.1093/jn/129.4.799. [DOI] [PubMed] [Google Scholar]
- Galand G. Brush border membrane sucrase-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp Biochem Physiol B. 1989;94:1–11. doi: 10.1016/0305-0491(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823–832. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromova LV, Grefner NM, Gruzdkov AA, Komissarchik I. [The role of facilitated diffusion in glucose transport across the apical membrane of enterocytes] Ross Fiziol Zh Im I M Sechenova. 2006;92:362–373. [PubMed] [Google Scholar]
- Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- Harvey BJ, Alzamora R, Healy V, Renard C, Doolan CM. Rapid responses to steroid hormones: from frog skin to human colon. A homage to Hans Ussing. Biochim Biophys Acta. 2002;1566:116–128. doi: 10.1016/s0005-2736(02)00589-8. [DOI] [PubMed] [Google Scholar]
- Henning S. Functional development of gastrointestinal tract. In: Johnson LR, editor. Physiology of The Gastrointestinal Tract. 2. New York: Raven Press; 1987. [Google Scholar]
- Honma K, Mochizuki K, Goda T. Carbohydrate/fat ratio in the diet alters histone acetylation on the sucrase-isomaltase gene and its expression in mouse small intestine. Biochem Biophys Res Commun. 2007;357:1124–1129. doi: 10.1016/j.bbrc.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]
- Jenkins BD, Pullen CB, Darimont BD. Novel glucocorticoid receptor coactivator effector mechanisms. Trends Endocrinol Metab. 2001;12:122–126. doi: 10.1016/s1043-2760(00)00357-x. [DOI] [PubMed] [Google Scholar]
- Jiang L, David ES, Espina N, Ferraris RP. GLUT-5 expression in neonatal rats: crypt-villus location and age-dependent regulation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G666–G674. doi: 10.1152/ajpgi.2001.281.3.G666. [DOI] [PubMed] [Google Scholar]
- Jiang L, Ferraris RP. Developmental reprogramming of rat GLUT-5 requires de novo mRNA and protein synthesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G113–G120. doi: 10.1152/ajpgi.2001.280.1.G113. [DOI] [PubMed] [Google Scholar]
- Karasov W, Diamond J. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol B. 1983;152:105–116. [Google Scholar]
- Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects. Mol Cell Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kwon E, Muduli A, Cerqueira C, Cui XL, Ferraris RP. Vanadate but not tungstate prevents the fructose-induced increase in GLUT5 expression and fructose uptake by neonatal rat intestine. J Nutr. 2006;136:2308–2313. doi: 10.1093/jn/136.9.2308. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23:S3–S6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Stahn C, Hommes DW, Buttgereit F. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids. 2007;73:1029–1025. doi: 10.1016/j.steroids.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 2007;22:234–240. doi: 10.1152/physiol.00011.2007. [DOI] [PubMed] [Google Scholar]
- Oesterreicher TJ, Henning SJ. Rapid induction of GATA transcription factors in developing mouse intestine following glucocorticoid administration. Am J Physiol Gastrointest Liver Physiol. 2004;286:G947–G953. doi: 10.1152/ajpgi.00470.2003. [DOI] [PubMed] [Google Scholar]
- Ogle TF, Beyer BK. Steroid-binding specificity of the progesterone receptor from rat placenta. J Steroid Biochem. 1982;16:147–150. doi: 10.1016/0022-4731(82)90160-1. [DOI] [PubMed] [Google Scholar]
- Petersen YM, Hartmann B, Holst JJ, Le Huerou-Luron I, Bjornvad CR, Sangild PT. Introduction of enteral food increases plasma GLP-2 and decreases GLP-2 receptor mRNA abundance during pig development. J Nutr. 2003;133:1781–1786. doi: 10.1093/jn/133.6.1781. [DOI] [PubMed] [Google Scholar]
- Putnam J, Allshouse J, Kantor LS. U.S. per capita food supply trends: more calories, refined carbohydrates, and fats. Food Rev. 2002;25:2–15. [Google Scholar]
- Reddy KV, Bhattacharjee G, Schabbauer G, Hollis A, Kempf K, Tencati M, O'Connell M, Guha M, Mackman N. Dexamethasone enhances LPS induction of tissue factor expression in human monocytic cells by increasing tissue factor mRNA stability. J Leukoc Biol. 2004;76:145–151. doi: 10.1189/jlb.0204068. [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Yao YZ, Smith BJ, Dimopoulos N, Fuller PJ. Determinants of spironolactone binding specificity in the mineralocorticoid receptor. J Mol Endocrinol. 2003;31:573–582. doi: 10.1677/jme.0.0310573. [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Yao Y, Smith BJ, Fuller PJ. Differences in the determinants of eplerenone, spironolactone and aldosterone binding to the mineralocorticoid receptor. Clin Exp Pharmacol Physiol. 2004;31:704–709. doi: 10.1111/j.1440-1681.2004.04079.x. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Schoonen WG, Deckers GH, de Gooijer ME, de Ries R, Kloosterboer HJ. Hormonal properties of norethisterone, 7α-methyl-norethisterone and their derivatives. J Steroid Biochem Mol Biol. 2000;74:213–222. doi: 10.1016/s0960-0760(00)00125-4. [DOI] [PubMed] [Google Scholar]
- Sheppard KE. Nuclear receptors. II. Intestinal corticosteroid receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G742–G746. doi: 10.1152/ajpgi.00531.2001. [DOI] [PubMed] [Google Scholar]
- Shneider BL. A new era in bile acid transport pathophysiology. J Pediatr Gastroenterol Nutr. 1998;26:236–237. doi: 10.1097/00005176-199802000-00025. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R, Bossemeyer R, Bouchard P. Preclinical and clinical properties of trimegestone: a potent and selective progestin. Gynecol Endocrinol. 2007;23:310–319. doi: 10.1080/09513590701267727. [DOI] [PubMed] [Google Scholar]
- Solomon NS, Gartner H, Oesterreicher TJ, Henning SJ. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr Res. 2001;49:782–788. doi: 10.1203/00006450-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Takabe S, Mochizuki K, Goda T. The effects of dephosphorylation signal on glucocorticoid hormone-mediating GLUT5 expression in intestinal cells. FASEB J. 2007;21:839.3. Experimental Biology 2007 Meeting Abstract. [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter KW, Archer TK. Nuclear receptors and chromatin remodeling machinery. Mol Cell Endocrinol. 2007;265–266:162–167. doi: 10.1016/j.mce.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- Wu CW, Wang SR, Chien SL, Yeh TH, Lian SL, Shimizu N, Lui WY, P'Eng FK, Chi CW. Regulation of arginase production by glucocorticoid in three human gastric cancer cell lines. Life Sci. 1992;51:1355–1361. doi: 10.1016/0024-3205(92)90635-3. [DOI] [PubMed] [Google Scholar]
- Xu H, Bai L, Collins JF, Ghishan FK. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)2 vitamin D3. Am J Physiol Cell Physiol. 2002;282:C487–C493. doi: 10.1152/ajpcell.00412.2001. [DOI] [PubMed] [Google Scholar]
- Zhang H, LeCulyse E, Liu L, Hu M, Matoney L, Zhu W, Yan B. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.