Abstract
Resistance (RE) and endurance (EE) exercise stimulate mixed skeletal muscle protein synthesis. The phenotypes induced by RE (myofibrillar protein accretion) and EE (mitochondrial expansion) training must result from differential stimulation of myofibrillar and mitochondrial protein synthesis. We measured the synthetic rates of myofibrillar and mitochondrial proteins and the activation of signalling proteins (Akt–mTOR–p70S6K) at rest and after an acute bout of RE or EE in the untrained state and after 10 weeks of RE or EE training in young healthy men. While untrained, RE stimulated both myofibrillar and mitochondrial protein synthesis, 67% and 69% (P < 0.02), respectively. After training, only myofibrillar protein synthesis increased with RE (36%, P = 0.05). EE stimulated mitochondrial protein synthesis in both the untrained, 154%, and trained, 105% (both P < 0.05), but not myofibrillar protein synthesis. Acute RE and EE increased the phosphorylation of proteins in the Akt–mTOR–p70S6K pathway with comparatively minor differences between two exercise stimuli. Phosphorylation of Akt–mTOR–p70S6K proteins was increased after 10 weeks of RE training but not by EE training. Chronic RE or EE training modifies the protein synthetic response of functional protein fractions, with a shift toward exercise phenotype-specific responses, without an obvious explanatory change in the phosphorylation of regulatory signalling pathway proteins.
Resistance exercise (RE) training results in increases in strength and muscle fibre cross-sectional area (CSA) (Hasten et al. 2000; Balagopal et al. 2001) and, particularly, of myofibrillar protein myosin and actin. Endurance exercise (EE) training is characterized by fatigue resistance due in part to increased oxidative capacity secondary to increased mitochondrial density and thus mitochondrial protein (Morgan et al. 1971; Gollnick et al. 1972; Hoppeler et al. 1973; Fink et al. 1977). Acutely, the rate of mixed muscle protein synthesis (MPS), an average measure of all muscle proteins, is stimulated by resistance (Chesley et al. 1992; Yarasheski et al. 1993; Biolo et al. 1995; MacDougall et al. 1995; Phillips et al. 1997) and endurance (Carraro et al. 1990; Sheffield-Moore et al. 2004) exercise, but the nature of responses of classes or individual proteins within this mixed protein response is unknown. Resistance and endurance exercise must induce protein synthesis in differing fractions of muscle protein, most obviously myofibrillar versus mitochondrial proteins. Chronic repeated performance of resistance exercise induces a more rapid but less long lived rise in mixed muscle protein synthesis after acute resistance exercise than occurs in untrained muscle (MacDougall et al. 1995; Phillips et al. 1997; Phillips et al. 1999, 2002; Rasmussen & Phillips, 2003; Tang et al. 2008). Furthermore, endurance exercise training (Short et al. 2004; Pikosky et al. 2006) as well as resistance training (Yarasheski et al. 1993, 1999; Phillips et al. 2002; Kim et al. 2005) have been shown to increase mixed muscle protein synthesis for up to 2 and 4 days, respectively, after the last exercise session
Feeding (Cuthbertson et al. 2005; Fujita et al. 2007b) and resistance exercise (Coffey et al. 2006; Dreyer et al. 2006; Eliasson et al. 2006; Fujita et al. 2007a) can independently activate translation initiation via multiple signalling proteins (Kimball & Jefferson, 2006), including the protein kinase B (Akt)–mammalian target of rapamycin (mTOR)–70 kDa S6 protein kinase (p70S6K) signal transduction pathway. Several recent investigations have found that the activation of proteins in the Akt–mTOR–p70S6K pathway are enhanced following resistance exercise if amino acids or protein is ingested (Karlsson et al. 2004; Koopman et al. 2007; Dreyer et al. 2008; Drummond et al. 2008) versus resistance exercise alone. These observations corroborate the evidence that provision of amino acids/protein with concurrent resistance exercise synergistically enhances muscle protein synthesis (Biolo et al. 1997; Tipton et al. 1999; Rasmussen et al. 2000; Borsheim et al. 2002, 2004; Miller et al. 2003; Tipton et al. 2004). Therefore, in an effort to contextualize the results of our planned measurements of protein synthesis we also sought to measure phosphorylation of signalling proteins of the Akt–mTOR–p70S6K (Nautilus, Vancouver, USA) pathway shown by others to be responsive to resistance exercise (Karlsson et al. 2004; Coffey et al. 2006; Cuthbertson et al. 2006; Dreyer et al. 2006; Eliasson et al. 2006; Fujita et al. 2007a; Terzis et al. 2007), and endurance exercise (Coffey et al. 2006; Mascher et al. 2007).
Exercise mode-specific responses have been observed in terms of AMP-activated protein kinase (AMPK) activation (Nielsen et al. 2003; Frosig et al. 2004; Coffey et al. 2006; Dreyer et al. 2006; Koopman et al. 2006; Drummond et al. 2008) and inhibition of mTOR in rodents has been speculated to be the underlying reason why resistance exercise is anabolic whereas endurance exercise is not (Atherton et al. 2005). Phosphorylation and enzyme activity of AMPK, which is increased during and briefly after endurance exercise (Nielsen et al. 2003; Frosig et al. 2004; Coffey et al. 2006) as well as resistance exercise (Dreyer et al. 2006; Koopman et al. 2006), has been associated with inhibition of mTOR, p70S6K and 4EBP-1 phosphorylation in rat skeletal muscle (Bolster et al. 2002; Atherton et al. 2005; Thomson et al. 2008); whether a similar mechanism is at play in human muscle is unknown.
Membrane-associated integrins respond to mechanical stimuli by transducing the signal into a cellular response. Focal adhesion kinase (FAK) has been suggested as a possible integrator of load-activated stimuli and integrin signalling in hypertrophying skeletal muscle because stretch has been shown to activate FAK in muscle (Fluck et al. 1999). Moreover, FAK phosphorylation is reduced in unloaded human skeletal muscle (de Boer et al. 2007) suggesting that it is a reasonable candidate for sensing mechanical loads.
We hypothesized that in an untrained state an acute bout of resistance or endurance exercise would elicit a non-exercise-specific rise in the synthetic rate of all protein fractions. As more and more acute bouts are carried out (i.e. comprising a training programme), increasingly exercise-mode and protein-specific protein synthetic responses must occur. Thus, the primary aim of this study was to measure myofibrillar and mitochondrial protein synthesis after an acute bout of either resistance or endurance exercise and repeat this after 10 weeks of resistance or endurance exercise training. The second aim of this study was to examine the effects of bouts of different exercise modes carried out in the fed state, on the activation of signalling proteins regulating protein synthesis (in the Akt–mTOR–p70S6K pathway), to see how these responses were affected by endurance or resistance training. We hypothesized that the phosphorylation of all anabolic signalling molecules would be changed appropriately (increases or decreases of phosphorylation) to promote muscle protein synthesis after acute resistance exercise but possibly suppressed after endurance exercise, at least in the untrained state for the time required for AMP/ATP ratios to normalize. We considered that only endurance exercise would be affected by this mechanism, despite evidence that AMPK is activated with resistance exercise (Dreyer et al. 2006; Koopman et al. 2006; Drummond et al. 2008), since ATP turnover was more than 80-fold greater than that during resistance exercise. After training we expected a different response in as much as training would attenuate the rise in protein synthesis (MacDougall et al. 1995; Phillips et al. 1997, 1999, 2002; Rasmussen & Phillips, 2003; Tang et al. 2008) and that this would be accompanied by a reduced extent of change of the phosphorylation of the regulatory anabolic proteins. We also undertook to measure the responses of molecules likely to regulate activities of members of the Akt–mTOR–p70S6K pathway, namely AMPK and FAK.
Methods
Subjects
Ten healthy men (mean ±s.e.m.: age, 20.5 ± 0.6 years; mass, 89.4 ± 4.8 kg; height, 179.6 ± 2.2 cm, 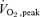 : 43.9 ± 2.1 ml kg−1 min−1) were recruited for the study. Subjects were not actively participating in any weightlifting activities or any programmed endurance activity (< 1 day week−1) for > 8 months prior to the study. Each participant was advised of the purposes of the study and associated risks. Participants were required to complete a health questionnaire and based on responses were deemed healthy. All subjects were non-smokers and were not taking any medication. All subjects gave their written and verbal informed consent prior to participation. The Hamilton Health Sciences Research Ethics Board approved the project, which complies with all standards set by the Declaration of Helsinki on the use of human research subjects.
: 43.9 ± 2.1 ml kg−1 min−1) were recruited for the study. Subjects were not actively participating in any weightlifting activities or any programmed endurance activity (< 1 day week−1) for > 8 months prior to the study. Each participant was advised of the purposes of the study and associated risks. Participants were required to complete a health questionnaire and based on responses were deemed healthy. All subjects were non-smokers and were not taking any medication. All subjects gave their written and verbal informed consent prior to participation. The Hamilton Health Sciences Research Ethics Board approved the project, which complies with all standards set by the Declaration of Helsinki on the use of human research subjects.
Experimental protocol
The subjects underwent two metabolic investigations (described later) separated by 10 week of unilateral leg resistance or endurance exercise training. Participants served as their own controls, one leg being assigned to the resistance exercise and one leg to the endurance exercise condition. The choice of legs for training mode was randomized in a counter-balanced manner. We chose to use a unilateral model of exercise for within-subject comparisons of adaptations, because this had substantial advantage in not having to recruit and train two groups of individuals in two different exercise modes and also being able to directly compare the metabolic responses in the same person (thereby increasing statistical power) and also saving on resources by using only a single isotopic tracer infusion per acute study. Thus, while we acknowledge that our design does not reflect typical training behaviour, it was not designed to do this. Rather we wished to elucidate aspects of the muscle specific changes that underpin changes in muscle phenotype with various forms of exercise training. For these purposes the design was efficient and practical.
Exercise testing
At least 2 weeks before the first infusion trial, subjects reported to the Exercise Metabolism Research Laboratory for familiarization and explanation of all procedures. Afterwards, the baseline physiological characteristics of the legs were measured, in a randomized order. Single leg knee extension strength measurements were determined for both legs over several days of testing before the first and last metabolic investigation. Strength measures included isotonic single repetition maximum (1RM) (Nautilus, Vancouver, WA, USA), isometric 1RM and isokinetic (0.52 rad s−1) 1RM (Biodex-System 3, Biodex Medical Systems, Shirley, NY, USA). Participants completed three 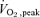 tests on separate days over the 2 week period prior to the first infusion metabolic investigation: a two leg
tests on separate days over the 2 week period prior to the first infusion metabolic investigation: a two leg  test and a single leg
test and a single leg 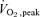 test on each leg.
test on each leg.
To estimate isotonic 1RM for the knee extension, subjects were seated with their hips at 90 deg and their back against a backrest inclined at 30 deg from horizontal. The fulcrum of the machine was aligned with the lateral aspect of the midline of the subject's knee. The leg pad of the machine was positioned ∼5 cm above the subject's ankle and the rotation arm was positioned so that the subject's knee was bent to 90 deg. The subject's ‘settings’ for pin placements for seating in the machine were recorded and kept constant throughout the study. A full repetition was when the subject was able to move the weight through an arc of ∼80 deg (from 90 deg to ∼170 deg). Subjects warmed up with 8–10 repetitions using a light weight. Subjects then performed a single best effort at a weight estimated to be the subject's 1RM based on body weight and height by an experienced trainer. The weight was increased or decreased depending on whether the subject could just manage to perform the task. This estimated 1RM was checked on two subsequent occasions by simply having the subject report to the lab and asking them to once more perform a 1RM at the previously determined weight. For a ‘true’ 1RM to be determined, the coefficient of variation (CV) between two attempts had to be less than 5%. For no subject did it require more than three attempts to determine this 1RM.
Isometric and isokinetic (concentric at an angular velocity of 0.52 rad s−1) knee extensor peak torques were determined using the dynamometer after having a prior familiarization on the dynamometer. The order of testing for each mode was randomized. Subjects had their shoulders strapped to the chair and the chair was adjusted so that the lateral aspect of the midline of the subject's knee lined up with the dynamometer fulcrum. Chair settings were recorded and set to the same settings for subsequent dynamometer testing. The participant's knee was maintained at an angle of 70 deg during three isometric repetitions (5 s) with 90 s of rest between repetitions. For the isokinetic contractions, subjects carried out 10 repetitions throughout the complete 65 deg range of motion. The subjects were given more than 2 min of recovery time between each exercise mode. All subjects were verbally encouraged to voluntarily produce their maximal force and given visual feedback of their force production. The highest peak torque value was considered as the maximal value.
 values for both legs together and separately were determined using an incremental exercise test to exhaustion (usually 7–12 min) on a Lode cycle ergometer (Groningen, Netherlands) with oxygen uptake measured continuously (AEI Technologies, Pittsburgh, PA, USA). Exhaustion was defined at a respiratory exchange ratio > 1.2, a heart rate within 5 beats min−1 of the subject's age-predicted maximal heart rate (for the two leg test), and the inability to maintain 60 r.p.m. on the cycle ergometer at the set workload. The participant's foot was secured to the pedal to enable transmission of force when pushing and pulling the pedals. Prior to training, participants' heart rates during the single leg test were 95 ± 1% of the maximum heart rate achieved in the two leg test. Following training the EE leg achieved 98 ± 3% and the RE leg achieved 95 ± 4% of the two leg maximum heart rate. From the single leg test on the leg assigned to endurance exercise activity, a workload designed to elicit a
values for both legs together and separately were determined using an incremental exercise test to exhaustion (usually 7–12 min) on a Lode cycle ergometer (Groningen, Netherlands) with oxygen uptake measured continuously (AEI Technologies, Pittsburgh, PA, USA). Exhaustion was defined at a respiratory exchange ratio > 1.2, a heart rate within 5 beats min−1 of the subject's age-predicted maximal heart rate (for the two leg test), and the inability to maintain 60 r.p.m. on the cycle ergometer at the set workload. The participant's foot was secured to the pedal to enable transmission of force when pushing and pulling the pedals. Prior to training, participants' heart rates during the single leg test were 95 ± 1% of the maximum heart rate achieved in the two leg test. Following training the EE leg achieved 98 ± 3% and the RE leg achieved 95 ± 4% of the two leg maximum heart rate. From the single leg test on the leg assigned to endurance exercise activity, a workload designed to elicit a 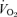 equivalent to 75% of the subject's single leg
equivalent to 75% of the subject's single leg  was selected and confirmed using a 15 min test ride 1 week before the metabolic investigation.
was selected and confirmed using a 15 min test ride 1 week before the metabolic investigation.
Muscle metabolic investigation
Subjects participated in two investigations before and after training (see Fig. 1). They were asked to refrain from any strenuous exercise for 2 days beforehand. Therefore, the post-training investigations were carried out at least 2 days after the last training session (9/10 subjects performed the study 4 days post-exercise and only one participant performed the testing 2 days post-exercise due to scheduling). Participants were asked to record what they ate in the afternoon and evening before the first investigation and were asked to consume the same meals again before the second investigation. Subjects were instructed not to consume any food after 20.00 h on the night beforehand and not to consume beverages containing caffeine for 24 h. On each study trial day, subjects consumed a defined formula beverage (2000 kJ; 82 g carbohydrate, 20 g protein and 8 g fat, Boost®, Novartis Nutrition Corporation, Mississauga, ON, Canada) in the morning (05.00 h) after an overnight fast. Two and a half hours later (effectively postabsorptive), subjects reported to the exercise metabolism laboratory. A 20-g polyethylene catheter was then inserted into an antecubital vein from which a baseline blood sample was taken to determine background amino acid enrichment. After the baseline blood sample was drawn, a primed constant infusion of D3-α-ketoisocaproic acid (D3-α-KIC prime: 10 μmol kg−1; infusion rate: 9 μmol kg−1 h−1) was initiated. All isotopes were purchased from Cambridge Isotopes (Andover, MA, USA), dissolved in 0.9% saline, filtered through a 0.2 μm filter and infused using a calibrated syringe pump (KD Scientific, Holliston, MA, USA). The infusion protocol was designed so that steady state was achieved within 1 h in both the intramuscular and plasma pools (Moore et al. 2005; Tang et al. 2008). Immediately after the beginning of the infusion the participant ingested an aliquot of Boost® (75% carbohydrate, 18% protein and 7% fat), which they ingested every 30 min (22 times) for the duration of the metabolic study. The total energy content of all the drinks provided 75% of the daily energy requirements based on the Harris–Benedict equation (Roza & Shizgal, 1984), using a physical activity level of 1.5 (providing 1.1 g of protein per kg body mass). On the basis of the leucine content in protein and the food composition we added sufficient tracer to the drink to maintain expected blood D3-α-KIC labelling of ∼7–8%. After baseline sampling, subjects rested for 1 h, during which time another polyethylene catheter was inserted into the contralateral arm to sample blood for the remainder of the protocol. After 1 h, a blood sample and a percutaneous quadriceps muscle biopsy were obtained using a 5 mm Bergström biopsy needle modified for manual suction under local anaesthesia (1% xylocaine). The muscle was dissected free of any visible fat and connective tissue and was immediately frozen in liquid nitrogen and stored at −80°C prior to analysis. In the untrained situation a single biopsy was taken, but after training, biopsies were taken from each leg. After resting for another 3 h, another blood sample and two more muscle biopsies were taken (one from each leg) via separate incisions. Half the participants were randomized to perform the resistance exercise first and the others performed the endurance first. After 10 week of training, the order of exercise was reversed for each participant so that participants who performed the exercised with resistance exercise first when untrained, underwent endurance exercise first in the untrained situation, and endurance first after training. The acute resistance exercise consisted of five sets of 8–10 repetitions at 80% 1RM of single leg knee extension and the endurance exercise consisted of single leg cycling for 45 min at 75%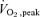 . The same relative intensities for the knee extension and single leg cycling were used in the second trial after training and re-testing of strength and
. The same relative intensities for the knee extension and single leg cycling were used in the second trial after training and re-testing of strength and 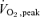 . After the first exercise bout, in whatever mode, a blood sample was taken and then a percutaneous quadriceps muscle biopsy was taken from the leg that had exercised; the second acute bout of exercise in a different mode was then performed followed by blood sampling and a percutaneous muscle biopsy taken from the exercised leg. The participants then rested and blood samples and biopsies were taken 4 h after each respective mode of exercise.
. After the first exercise bout, in whatever mode, a blood sample was taken and then a percutaneous quadriceps muscle biopsy was taken from the leg that had exercised; the second acute bout of exercise in a different mode was then performed followed by blood sampling and a percutaneous muscle biopsy taken from the exercised leg. The participants then rested and blood samples and biopsies were taken 4 h after each respective mode of exercise.
Figure 1.
Infusion trial protocol
Training protocol
After the aforementioned baseline testing was complete, subjects underwent a 10 week training programme in which one leg carried out a resistance-training programme, consisting of knee extension exercise, while the contralateral leg carried out an endurance training programme consisting of one-legged cycling on a cycle ergometer specially designed for this study. The participant's foot was secured to the pedal to enable transmission of force when pushing and pulling the pedals. Subjects alternated training between resistance and endurance training each day so that the first week they performed endurance training three days and resistance training twice. The following week, resistance training was carried out three times and endurance exercise twice.
The training for the resistance-trained leg began with each session consisting of three sets with 10–12 repetitions per set at 80% of their 1RM. On the third week, the volume of training was increased to four sets with 8–10 repetitions per set at 80% 1RM with the last set performed to failure (unable to lift the weight). If the participant could lift more than 10 repetitions on the last set, the training weight was increased on the subsequent session. This mode of adjusting training weight was carried on for the remainder of the protocol. On the fourth and fifth weeks, subjects performed five sets of 8–10 repetitions. At weeks six and seven, the training weight was increased so that participants' goal was to lift eight repetitions for five sets. During weeks 8 to 10, participants completed five sets of six to eight repetitions. After the completion of the training protocol, subjects were tested again to determine their dynamic, isometric and isokinetic strength.
The training for the endurance-trained leg began with exercise for 30 min at 75% of predetermined single leg  for 2 weeks. Participants' heart rates were monitored throughout each training session. As a participant adapted to the training, workload was increased to elicit a heart rate equivalent to the heart rate observe at 75% single leg
for 2 weeks. Participants' heart rates were monitored throughout each training session. As a participant adapted to the training, workload was increased to elicit a heart rate equivalent to the heart rate observe at 75% single leg  in the untrained state. The duration of the exercise was increased in week 3 to 45 min. During the midweek session of weeks 4, 6, 8 and 10, participants completed interval sessions. In brief, participants alternated between two workloads designed to elicit heart rates of ∼140–150 and ∼160–170 for the duration of the training session. During week 5, training workload was adjusted so that participants were training at a heart rate of ∼160. For participants with a below or above average maximal heart rate, workload was adjusted on an individual basis. In week 6, training time was increased to 1 h. After the completion of the training protocol subjects' two leg and single leg
in the untrained state. The duration of the exercise was increased in week 3 to 45 min. During the midweek session of weeks 4, 6, 8 and 10, participants completed interval sessions. In brief, participants alternated between two workloads designed to elicit heart rates of ∼140–150 and ∼160–170 for the duration of the training session. During week 5, training workload was adjusted so that participants were training at a heart rate of ∼160. For participants with a below or above average maximal heart rate, workload was adjusted on an individual basis. In week 6, training time was increased to 1 h. After the completion of the training protocol subjects' two leg and single leg  was tested again on separate days.
was tested again on separate days.
Analytic methods
Blood sample analysis
Blood samples were collected into heparinized evacuated containers. Whole blood (100 μl) was added to ice-cold perchloric acid (PCA; 0.6 m, 500 μl), mixed and allowed to sit on ice for 10 min to precipitate all proteins. This mixture was then centrifuged at 20 000 g for 2 min at 4°C. The PCA was neutralized with 250 μl of 1.25 m KHCO3 and the reaction was allowed to proceed on ice for 10 min. Samples were then centrifuged at 4000 g for 2 min at 4°C. The supernatant was stored at −20°C for further analysis of blood amino acid concentrations (Wilkinson et al. 2007) and leucine enrichments. In the fed state there is a rapid equilibration between leucine and α-KIC such that plasma and muscle intracellular enrichments converge (Chinkes et al. 1996). The PCA blood sample was derivatized by adding 50 μl of N-methyl-N-t-butyl-dimethylsilyl-trifluoroacetamide + 1%t-butyl-dimethylchlorosilane (MTBSTFA + 1% TBDMCS, Regis Chemical). 2H3-Leu enrichment was quantified using capillary gas chromatography–electron impact ionization–quadrupole mass spectrometry (GCMS; GC Hewlett Packard 6890, Palo Alto, CA, USA; MSD Agilent 5973, Palo Alto, CA, USA) by monitoring ions at m/z 200 and 203. Plasma was obtained by centrifuging the evacuated tube at 4°C for 10 min at 4000 g. Plasma was stored at −20°C for quantifying insulin and glucose concentrations. Insulin levels were determined using a commercially available radioimmunoassay kit from Diagnostic Products Corp. (Los Angeles, CA, USA). Glucose levels were determined on neutralized blood PCA extracts using a standard enzymatic method (Passoneau & Lowry, 1993).
Muscle biopsy sample analysis
Needle biopsies from the vastus lateralis were obtained under local anaesthesia (1% xylocaine). A 5 mm Bergström biopsy needle modified for manual suction was used to obtain ∼100–200 mg of muscle tissue from each biopsy. Biopsies were obtained from separate incisions from the same leg during each session. The muscle was dissected free of any visible fat and connective tissue and was immediately frozen in liquid nitrogen and stored at −80°C prior to analysis.
One piece of frozen wet muscle (∼10–15 mg) was homogenized using the method described by Henriksson et al. (1986) to a 50 times dilution. The homogenate was subsequently analysed to determine the maximal activity of CS on a spectrophotometer (Ultrospec 3000 pro UV/Vis) using a method described by Carter et al. (2001) and corrected for protein content using a Bradford assay (Passoneau & Lowry, 1993). A second portion of wet muscle (∼10–20 mg) was saved for Western blotting; 10 mg wet muscle was added to 150 μl of homogenizing buffer (1 mm Na3VO4, 50 mm NaF, 40 mmβ-glycerolphosphate, 20 mm sodium pyrphosphate, 0.5% Triton X-100, complete mini protease inhibitor tabs (Roche, Indianapolis, IN, USA) in Tris buffer pH 7.2). After thorough homogenization on ice, homogenates were spun in a 4°C centrifuge at 4500 g for 10 min. The supernatant was removed and stored at –80°C until Western blot analysis. A small portion was saved for Bradford assay for protein (Passoneau & Lowry, 1993).
Aliquots from the homogenates were boiled at 100°C for 5 min in 4 × sample buffer (300 mm Tris-HCl, pH 6.8, 50% glycerol, 8% SDS, 20%β-mercaptoethanol, 0.02% bromophenol blue). Fifty micrograms of sample were added per lane and separated by electrophoresis in running buffer (0.1% SDS, 192 mm glycine, 25 mm Tris base) on 7.5–15% SDS-PAGE gels at 100 V until dye marker had passed through the stacking layer and then at 200 V until the dye marker reached the gel bottom. After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) at 100 V for 2 h at 4°C in transfer buffer (192 mm glycine, 25 mm Tris base, 10% methanol). After transfer, the PVDF membranes were placed in blocking buffer: 5% BSA in Tris-buffered saline (50 mm Tris, 150 mm NaCl) and 0.1% Tween-20 (TBST) for 1 h. Blots were incubated in primary antibody in 5% BSA in TBST overnight at 4°C with constant agitation. The next morning, blots were washed in TBST three times for 5 min and then incubated with secondary antibody (Amersham Biosciences, Little Chalfont, UK) in 5% BSA in TBST for 1 h at room temperature, with continual agitation. Blots were washed in TBST three times for 5 min and then incubated for 5 min with Chemi Glow™ chemiluminescence reagent (Alpha Innotech, San Leandro, CA). Optical density measurements were obtained with a CCD camera, mounted in a Fluorchem SP imaging system (Alpha Innotech). Once the image was captured, densiometric analysis was performed using AlphaEaseFC software (Alpha Innotech). After detection of the phosphorylated antibody, the antibodies were stripped off the PVDF membrane by incubating the blot in stripping buffer (25 mm glycine-HCl, pH 2.0, 1% SDS) for 1 h. We confirmed this stripping buffer was effective by reincubating the blot with secondary antibody then Chemi Glow™ chemiluminescence reagent and determining that no chemiluminescence was observable after incubation with stripping buffer. The blot was then washed in TBST three times for 10 min each, blocked and exposed to the total primary antibody overnight. All data are expressed as the ratio between the phosphorylated protein to the total protein. Primary antibodies were purchased from Cell Signalling (Beverly, MA, USA) as follows: phospho-Akt (Ser473; 1: 1000), total-Akt (1: 1000), phospho-AMPKα (Thr172; 1: 1000), total-AMPKα (1: 1000), phospho-GSK3β (Ser9; 1: 1000), total-GSK3β (1: 1000) phospho-mTOR (Ser2448; 1: 1000), total-mTOR (1: 1000), phospho-S6 ribosomal protein (Ser235/236; 1: 1000), total-S6 ribosomal protein (1: 1000); and from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA): phospho-eIF4E (Ser209; 1: 500), total-eIF4E (1: 500), phospho-FAK (Tyr576/577, 1: 1000), total-FAK (1: 1000), phopho-p70 S6K (Thr389; 1: 1000), total- p70S6K (1: 1000).
The procedure for the isolation of the mitochondria was adapted from Bezaire et al. (2004) and myofibrillar protein from Bohe et al. (2001). Briefly, the remaining portion of muscle was homogenized with a Dounce homogenizer in ice-cold homogenizing buffer (0.1 mm KCl, 50 mm Tris, 5 mm MgCl2 1 mm EDTA, 10 mmβ-glycerophosphate, 50 mm NaF, 1.5% BSA pH 7.5). The homogenate was transferred to a 2 ml Eppendorf tube and spun at 650 g for 10 min at 4°C. The supernatant was transferred to another Eppendorf tube and spun at 10 000 g for 10 min at 4°C to pellet the sarcoplasmic mitochondria (SMs). The supernatant was removed and discarded. The pellet that remained from the original 650 g spin was washed twice with homogenization buffer. A glass pestle was utilized to forcefully homogenize the pellet in ice cold homogenization buffer to liberate intermyofibrillar mitochondria (IMs). The resulting mixture of myofibrils and IMs was spun at 650 g for 10 min at 4°C to pellet out the myofibrils. The supernatant was removed and spun at 10 000 g for 10 min at 4°C to pellet the IMs The myofibrils and SM and IM pellets were washed three times with homogenizing buffer containing no BSA. A BSA-free homogenate was confirmed by electrophoresis. The myofibrils were separated from any collagen by dissolving them in 0.3 m NaCl, removing the supernatant and precipitating them with 1.0 m PCA. All samples were washed once with 95% ethanol and then lyophilized to dryness (Savant, Rockville, MD, USA).
We confirmed qualitatively using electron microscopy that the mitochondrial fractions were enriched for mitochondria and that the myofibrillar fraction contained exclusively myofibrils. Furthermore, we measured citrate synthase activity in the mixed muscle homogenate (homogenate prior to differential centrifugation) and each of the mitochondrial fractions. We found that the citrate synthase activities of the mitochondrial fractions were ∼3-fold higher than those of the mixed muscle homogenates.
It was determined the SM and IM protein pellets should be combined in order to quantify 2H3-Leu enrichment. The mitochondria- and myofibrillar-enriched proteins were hydrolysed in 6 m HCl at 100°C for 24 h. Hydrolysates were applied to a cation exchange resin (Dowex AG-50W X8, 100–200 mesh H+ form) and washed with 0.01 m HCl. The amino acids were eluted with 6 m NH4OH, the heptafluorobutyric propyl esters were prepared (Reeds et al. 2006), and 2H3-Leu enrichment in the myofibrillar- and mitochondria-enriched protein fractions was determined using gas chromatography–negative chemical ionization–quadrupole mass spectrometry (GC Hewlett Packard 6890; MSD Agilent 5973) by monitoring ions at m/z 349 and 352. Unfortunately, during processing, the mitochondria-enriched samples from 3 participants were lost, so data represent n = 7. No myofibrillar samples were lost (n = 10).
Calculations
The fractional synthetic rate (FSR) of myofibrillar and mitochondrial proteins was calculated as the rate of tracer incorporation into the appropriate muscle proteins using plasma 2H3-Leu to reflect the precursor pool enrichment, according to the previously published equation (Phillips et al. 1997).
Statistics
Sample size estimates were based on the ability to detect a 25% difference between groups in mixed muscle fractional synthetic rate using α at 0.05 and β at 0.2 (2-sided), with an estimated variance in the measure based on past studies from our lab and from literature values. To protect power we added two subjects to the final calculated sample size estimate. Data were analysed using Statistica (v 6.0, Statsoft, Tulsa, OK, USA) using a repeated measures analysis of variance with planned comparisons. Where a significant F ratio was observed, post hoc analysis using Tukey's test was utilized to identify individual differences. Significance was set at P < 0.05. Data are presented as means ±s.e.m. All relevant comparisons were made for time (within a trial), leg (comparison between EE and RE at each time point within a given trial) and training (comparison of the same leg at the same time point between trials).
Results
Training and functional capacities
Nine out of 10 participants completed 100% of their allocated training sessions. One subject completed 80% of his training sessions, but his results did not differ substantially from those of the other participants so his data are included. All participants maintained stable weight throughout the study (data not shown). There were no significant differences in any strength measurements between legs before training (Table 1). As expected, voluntary 1RM, isokinetic and isometric knee extension strengths increased by 75, 25 and 20%, respectively, after resistance exercise, with all increases being greater or occurring only in the resistance trained leg. We have previously shown that the same programme of resistance training also elicits increases in muscle fibre and whole muscle cross-sectional area with no changes in the contralateral leg (Wilkinson et al. 2006; Tang et al. 2008). Measurements of functional adaptations considered to be characteristic of endurance training are presented in Table 2. There were no significant differences in single leg  between legs before training. Two-leg (i.e. whole-body)
between legs before training. Two-leg (i.e. whole-body)  did not change significantly after 10 weeks of training, but there was a simultaneous 17% increase in the endurance trained single leg
did not change significantly after 10 weeks of training, but there was a simultaneous 17% increase in the endurance trained single leg  which was significant, with no change observed in the resistance exercise leg. Before training, one leg
which was significant, with no change observed in the resistance exercise leg. Before training, one leg  represented 77 ± 2% of two leg
represented 77 ± 2% of two leg  capacity. This capacity increased to 90 ± 2% afterwards (P < 0.05; Table 2) only in the endurance trained leg and did not change after resistance exercise training. Citrate synthase activity, a marker of muscle mitochondrial content (Leek et al. 2001), increased 22 ± 8% (P < 0.05) after endurance training but no change was observed after resistance exercise training.
capacity. This capacity increased to 90 ± 2% afterwards (P < 0.05; Table 2) only in the endurance trained leg and did not change after resistance exercise training. Citrate synthase activity, a marker of muscle mitochondrial content (Leek et al. 2001), increased 22 ± 8% (P < 0.05) after endurance training but no change was observed after resistance exercise training.
Table 1.
Strength measurements
| Endurance exercise | Resistance exercise | |||||
|---|---|---|---|---|---|---|
| Untrained | Trained | % change | Untrained | Trained | % change | |
| 1RM (kg) | 48 ± 3 | 62 ± 3* | 30 ± 6 | 47 ± 3 | 79 ± 3*+ | 75 ± 10 |
| Isometric peak torque (N) | 299 ± 15 | 285 ± 16 | −4 ± 4 | 293 ± 16 | 346 ± 17*+ | 20 ± 7 |
| Isokinetic peak torque (N m) 0.52 rad s−1 | 241 ± 10 | 243 ± 10 | 1 ± 2 | 233 ± 11 | 290 ± 10*+ | 25 ± 4 |
Values are means ±s.e.m.
significantly different from those in the untrained state (same leg);
significantly different from endurance exercise (same training state), P < 0.05.
Table 2.
Measures of endurance capacity
| Endurance exercise | Resistance exercise | |||||
|---|---|---|---|---|---|---|
| Untrained | Trained | % change | Untrained | Trained | % change | |
One leg  (l min−1) (l min−1) |
2.9 ± 0.2 | 3.5 ± 0.2±+ | 17 ± 4 | 3.1 ± 0.2 | 3.1 ± 0.2 | −2 ± 2 |
One leg 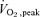 as a percentage as a percentage |
||||||
of two 2 leg 
|
77 ± 2 | 90 ± 2±+ | 19 ± 3 | 81 ± 2 | 81 ± 3 | 0 ± 3 |
| CS activity (mol kg−1 protein h−1) | 9.6 ± 0.3 | 11.7 ± 0.5±+ | 22 ± 8 | 9.6 ± 0.3 | 9.0 ± 0.2 | − 4 ± 5 |
Values are means ±s.e.m.
significantly different from untrained (same leg)
significantly different from resistance exercise (same training state), P < 0.05. Two leg  (l min−1): untrained, 3.9 ± 0.2; trained, 3.8 ± 0.2.
(l min−1): untrained, 3.9 ± 0.2; trained, 3.8 ± 0.2.
Feeding during the protocol resulted in the expected hyperaminoacidaemia, hyperinsulinaemia, and hyperglycaemia (Fig. 2). Isotopic equilibrium was attained in plasma after 1 h of infusion and maintained throughout the tracer infusion (data not shown).
Figure 2.
A, plasma glucose (mm) and insulin (IU ml−1) concentrations. B, whole-blood venous total amino acid concentration (mm). Values are means ±s.e.m.*Significantly different from values in untrained, letters depict a significant effect of time, P < 0.05.
Protein synthetic responses
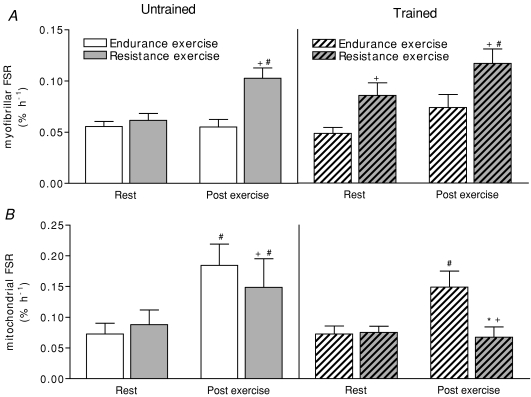
Resting myofibrillar fractional synthetic rate (FSR) was not different between legs prior to training (Fig. 3A). After training, resting myofibrillar FSR was significantly greater in the resistance trained leg than in the endurance trained leg. Prior to training, resistance exercise resulted in a 67% increase in myofibrillar FSR. After 10 weeks of unilateral resistance exercise training, the same relative intensity of acute resistance exercise resulted in a 37% increase above resting myofibrillar FSR. Single leg cycling had no statistically significant effect on myofibrillar FSR in the postexercise period compared to rest prior to (P = 0.96) and after 10 weeks (P = 0.12) of endurance training.
Figure 3.
Myofibrillar (A) and mitochondrial enriched (B) FSR at rest and for the 4 h period after single-leg cycling (endurance) or single leg knee extension exercise (resistance) in the fed state Values are means ±s.e.m. as percentage per hour. *Training effect: significantly different from untrained values with same leg at same time; +leg effect: significantly different from endurance values, same training state and at same time; #time effect: significantly different from values at rest with same leg at same time and from those with same training state, P < 0.05.
Resting mitochondrial FSR was not different between legs before training (Fig. 3B). Both single-leg knee extension resistance exercise and cycling endurance exercise increased in mitochondrial FSR in the untrained state (Fig. 3). However, this postexercise increase in mitochondrial FSR was significantly more pronounced after endurance exercise than after resistance exercise. After training, there were no significant differences between legs or changes due to training at rest. However, the postexercise increase in mitochondrial FSR above resting values was only observed in the endurance trained leg.
Signalling protein phosphorylation
Figure 4 shows representative blots for all signalling proteins measured, in all conditions, in the study. All bands for a particular antibody were obtained from a single Western blot (for details see Methods).
Figure 4.
Examples of analysis of protein Western blots Representative blots for all signalling proteins measured, in all conditions, in this study. All bands for a particular antibody were obtained from a single Western blot.
Akt
In the untrained state trial, Akt phosphorylation was increased by 1.5-fold above rest immediately and 4 h after acute exercise in both the endurance and resistance modes (Fig. 5A). After training in both modes, resting Akt phosphorylation was 1.3-fold greater than in the untrained state. The postexercise phosphorylation response to endurance exercise vanished after endurance training. After resistance exercise training, resistance exercise increased phosphorylation of Akt by 1.5-fold above rest immediately postexercise, but this difference did not persist 4 h later.
Figure 5.
Ratio of phosphorylated to total Akt (Ser473) (A), GSK3β (Ser9) (B), mTOR (Ser2448) (C), p70S6Kinase (Thr389) (D) at rest, immediately after (0 h) and 4 h (4 h) after single-leg knee extension (resistance) or cycling (endurance) in the fed state prior to and after 10 weeks of unilateral resistance or endurance training Values are means ±s.e.m. in arbitrary units. *Training effect: significantly different from untrained values with same leg at same time; +leg effect: significantly different from endurance values, same training state and at same time; time effect: letters depict a significant effect of time (lower case represent untrained and upper case represent trained), P < 0.05.
GSK-3β
GSK-3β (Fig. 5B) phosphorylation increased immediately after and returned to resting phosphorylation values by 4 h after acute exercise in the endurance and resistance modes before (endurance 1.7-, resistance 1.8-fold increases above rest, respectively) and after training (endurance 1.5-, resistance 1.4-fold increases above rest, respectively). After each type of training, resting GSK-3β phosphorylation was 1.3-fold greater than before training.
mTOR
mTOR (Fig. 5C) phosphorylation increased immediately after and returned to resting phosphorylation values by 4 h after acute endurance (untrained 1.7-, trained 1.4-fold increase above rest, respectively) and resistance (untrained 1.6-, trained 1.5-fold increase above rest, respectively) exercise irrespective of training state. The phosphorylation of mTOR was 1.1-fold greater 4 h after acute resistance exercise after training than after endurance exercise.
p70S6K
Acute exercise before training in both modes of exercise increased p70S6K phosphorylation above resting values immediately after exercise (Fig. 5D, endurance 1.9-fold, resistance 1.7-fold). p70S6K phosphorylation remained 1.4-fold elevated above rest 4 h after resistance exercise, whereas after endurance exercise it had returned to baseline. After training acute endurance and resistance exercise caused a similar pattern of immediate postexercise stimulation of p70S6K phosphorylation (endurance 2.2-fold, resistance 2.7-fold), but by 4 h, p70S6K phosphorylation was no different from that at baseline.
rpS6
rpS6 phosphorylation was significantly increased by 1.8-fold 4 h after acute resistance exercise before training (Fig. 6A). Endurance exercise had no effect on rpS6 phosphorylation. After training rpS6 phosphorylation was 50% lower at rest than in the untrained state. Endurance exercise increased rpS6 phosphorylation by 1.6-fold immediately and 4 h after exercise but resistance exercise had no significant effect after training.
Figure 6.
Ratio of phosphorylated to total rpS6 (Ser235/236) (A) and eIF4E (Ser209) (B) at rest, immediately after (0 h) and 4 h after single-leg knee extension (resistance) or cycling (endurance) in the fed state before and after 10 weeks of unilateral resistance or endurance training Values are means ±s.e.m. in arbitrary units. *Training effect: significantly different from untrained values with same leg at same time; +leg effect: significantly different from endurance values, same training state and at same time; time effect: letters depict a significant effect of time (lower case represent untrained and upper case represent trained), P < 0.05.
eIF4E
In the untrained state, eIF4E phosphorylation did not change immediately after acute exercise in either mode (Fig. 6B). However, 4 h after each type of exercise, eIF4E phosphorylation was significantly increased above resting values (endurance 1.5-fold, resistance 1.8-fold). At rest, after resistance training, eIF4E phosphorylation was 1.5-fold greater than in the resting condition in the untrained trial. Increased eIF4E phosphorylation was seen immediately (1.5-fold above trained rest) and persisted until 4 h after both modes of exercise in the TR trial. At 4 h post-exercise, the resistance trained leg had significantly greater (1.8-fold greater than rest) eIF4E phosphorylation than its endurance trained (1.5-fold greater than rest) counterpart.
AMPK
AMPK phosphorylation increased by 6-fold immediately after acute exercise of both modes in both the untrained and trained states, but returned to baseline values by 4 h after exercise (Fig. 7A). There was no effect of mode of exercise or training.
Figure 7.
Ratio of phosphorylated to total AMPKα (Thr172) (A) and FAK (Tyr576/577) (B) at rest, immediately after (0 h) and 4 h after single-leg knee extension (resistance) or cycling (endurance) in the fed state prior to and after 10 week of unilateral resistance or endurance training Values are means ±s.e.m. in arbitrary units. *Training effect: significantly different from untrained with same leg at same time; +leg effect: significantly different from endurance same training state and at same time; time effect: letters depict a significant effect of time (lower case represent untrained and upper case represent trained), P < 0.05.
FAK
In the untrained state, immediately after acute exercise of each mode, FAK phosphorylation increased (endurance 2.6-, resistance 2.5-fold) and remained significantly elevated above baseline for 4 h (Fig. 6B, endurance 1.2- and resistance 1.3-fold), but phosphorylation was significantly greater at this time after resistance exercise. After both modes of training resting FAK phosphorylation was significantly greater than before training (endurance 1.4-, resistance 1.5-fold). After both modes of training, FAK phosphorylation was elevated above rest immediately after exercise (endurance 1.8-, resistance 1.7-fold), but returned to baseline at 4 h after exercise.
Discussion
We have characterized the human muscle protein synthetic response and intracellular phosphoprotein signalling alterations (Akt–mTOR–p70S6K) that occur after both endurance and resistance exercise in the fed state and examined how it is altered with exercise training. We observed that in untrained muscle, resistance exercise stimulated both myofibrillar and muscle mitochondrial protein synthesis. We contend that this is the first study to report an increase of human muscle mitochondrial protein synthesis after acute exercise.
After 10 weeks of unilateral resistance training the protein synthetic response was more specific, in that only myofibrillar, and not mitochondrial, protein synthesis increased after an acute bout of resistance exercise. Furthermore, resistance training resulted in a greater resting rate of myofibrillar protein synthesis. Before and after training single-leg endurance exercise, performed at the same relative intensity, stimulated only mitochondrial protein synthesis. Single leg endurance exercise did not acutely stimulate myofibrillar protein synthesis regardless of training state.
In the untrained trial, both forms of exercise increased Akt and mTOR phosphorylation. Only resistance exercise increased rpS6 phosphorylation. Furthermore, increased p70S6K phosphorylation seen immediately after both forms of exercise remained elevated at 4 h after resistance exercise only. Our observation mirror those of previous workers who showed increased Akt and mTOR phosphorylation with no concurrent change in p70S6K or rpS6 phosphorylation after endurance exercise (Coffey et al. 2006; Mascher et al. 2007). A different response was observed after 10 weeks of training; we observed higher resting Akt, GSK3-β, eIF4E and FAK phosphorylation. Similarly, Leger et al. (2006) observed increased Akt, GSK3-β and mTOR phosphorylation at rest after 8 weeks of resistance exercise training. This increased resting phosphorylation status could explain the observed higher resting protein synthesis after resistance training or it may allow for translation initiation to be activated more rapidly after exercise in the trained state. Phosphorylation of several of the key proteins (Akt, p70S6K, GSK3-β) which had remained elevated 4 h after exercise in the untrained state returned to baseline at this stage in the trained state. We propose that exercise training shifts the activation state of key anabolic signalling molecules to a heightened state of ‘responsiveness’ so that they are activated and deactivated more rapidly than in the untrained state. This training enhanced increase in ‘signalling efficiency’ is congruent with a more rapid but much shorter response in the trained versus the untrained state, which we have recently reported (Tang et al. 2008).
Resistance exercise training stimulates mixed MPS at rest and after an acute bout of resistance exercise (Phillips et al. 1999, 2002; Kim et al. 2005). Those studies have also shown that resistance training attenuates the acute postexercise increase of mixed MPS to the same relative exercise intensity (Phillips et al. 1999, 2002; Kim et al. 2005). We did not find an attenuation of myofibrillar protein synthesis after 10 weeks of resistance exercise training. This observation is similar to that reported by Kim et al. (2005). The differing response of mixed and myofibrillar protein synthesis could be due to dampening of the synthetic response of non-myofibrillar proteins while maintaining the synthesis of myofibrillar proteins after training. In support of this we observed that the increase in mitochondrial protein synthesis seen after resistance exercise in the untrained state was absent after 10 weeks of resistance training. This response does not appear to be mediated by changes in the phosphorylation status of known regulatory signalling proteins; however, it is possible that increased specific gene transcripts for myofibrillar proteins remain elevated after resistance training, promoting production of myofibrillar proteins, whereas those for mitochondrial/sarcoplasmic proteins are not. Alternatively, feeding has been shown to have a marked effect on signalling proteins (Karlsson et al. 2004; Dreyer et al. 2008); because our participants were fed throughout the infusion trial and we did not take a biopsy in the fasted state, we may have missed observing a smaller effect of exercise on the phosphorylation of signalling proteins.
Several workers have reported an increase in mixed MPS after endurance or dynamic exercise of various kinds (Carraro et al. 1990; Sheffield-Moore et al. 2004). However, to our knowledge, no other study has examined the relative rates of myofibrillar or mitochondrial protein synthesis after endurance exercise. Miller et al. (2005) used dynamic leg extensor exercise and reported an elevation of myofibrillar and sarcoplasmic (which would include the subsarcolemmal mitochondria that are not adherent to the myofibrils) MPS for 48 h after a 1 h bout. Based on this work (Miller et al. 2005) and the results of others (Carraro et al. 1990; Sheffield-Moore et al. 2004) we proposed that we would observe increases, albeit one that may have been delayed due to AMPK activation (Bolster et al. 2002; Atherton et al. 2005; Thomson et al. 2008), in both mitochondrial and myofibrillar protein synthesis following endurance exercise, although we did not see such a phenomenon. The single leg cycling model we used and the single leg kicking model (Miller et al. 2005) both involve endurance work, since both elicit increases in mitochondrial protein. However, the intensity of muscular work (W per kg active muscle) of the vastus lateralis in the single leg kicking exercise would be greater. This difference in work intensity could explain why our response was confined to mitochondrial proteins, but Miller et al. (2005) reported increases in myofibrillar protein synthesis. Those authors cited the cellular tensegrity hypothesis (Ingber, 2006) as a reason for the robust stimulation of protein synthesis. As a test of this hypothesis we quantified FAK phosphorylation as a reasonable loading responsive protein complex (Gordon et al. 2001; de Boer et al. 2007) but found it to be phosphorylated equally in all conditions, a result which did not illuminate our protein synthetic data. We did, however, observe an elevation of mitochondrial protein synthesis after an acute bout of one-leg cycling before and after training, consistent with the observation of an increased mitochondrial content with endurance training (Morgan et al. 1971; Gollnick et al. 1972; Hoppeler et al. 1973; Fink et al. 1977).
Scheper & Proud (2002) speculate that eIF4E phosphorylation at Ser209 subsequent to formation of the initiation complex may allow for the eIF4F complex to detach from the 5′-cap during elongation, allowing for the rapid recruitment of the next initiation complex or rendering the cap binding factors available for translation of different proteins. Before training, both forms of exercise resulted in an increased eIF4E phosphorylation at Ser209 4 h after exercise. After resistance exercise training, resting eIF4E phosphorylation was elevated. Furthermore, both modes of exercise increased eIF4E phosphorylation immediately and it remained elevated for 4 h. In the resistance trained leg, eIF4E phosphorylation was significantly greater than that observed in the endurance trained leg at 4 h post-exercise. Limited data exist on the activation of eIF4E in skeletal muscle. Williamson et al. (2003) found that in untrained participants, resistance exercise acutely stimulated Mnk1, the upstream kinase of eIF4E, but this was not associated with an increase in eIF4E phosphorylation immediately after exercise. In consonance with the observations made by Williamson et al. (2003), we did not observe an increase in eIF4E phosphorylation immediately after exercise in our untrained participants.
Atherton et al. (2005) used electrical stimulation in isolated rat muscle to mimic endurance exercise or resistance exercise. AMPK was activated immediately and 3 h after low frequency stimulation mimicking endurance exercise, but was not altered after high-frequency stimulation mimicking resistance exercise. Coffey et al. (2006) found that when trained cyclists and weightlifters performed exercise in their familiar disciplines, no activation of muscle AMPK was observed. However, when they undertook a novel form of exercise (cycling for weightlifters and weightlifting for cyclists) an increase in muscle AMPK phosphorylation was observed immediately after exercise and this returned to baseline within 3 h after exercise. AMPK is also activated following resistance exercise in humans (Dreyer et al. 2006, 2008; Koopman et al. 2006, 2007; Drummond et al. 2008); however, one would suspect that such an elevation would be relatively transient and possibly has less of an effect on mTOR signalling compared to aerobic exercise which has a much greater ATP turnover. Our results are similar in that AMPK phosphorylation was elevated immediately after exercise, but returned to baseline within 4 h post-exercise. In contrast to previous reports (Coffey et al. 2006), we found that 10 weeks of training was insufficient to blunt the exercise stimulation of AMPK activity seen in athletes who had trained in their respective disciplines for years. In rat skeletal muscle, evidence exists that an increase in AMPK phosphorylation is associated with a reduced activation of mTOR, p70S6K and 4EBP-1 (Bolster et al. 2002; Atherton et al. 2005; Thomson et al. 2008). Nevertheless, we observed a simultaneous increase in mTOR and p70S6K phosphorylation above resting values coincident with the increased AMPK phosphorylation. Although unclear, in the current study it is possible that AMPK, mTOR, and p70S6K were concomitantly increased by the feeding-induced hyperaminoacidaemia and hyperinsulinaemia during exercise, and that these factors may override the inhibitory effect that active AMPK has on mTOR (Dreyer et al. 2006; Morrison et al. 2008). Recently, Morrison et al. (2008) reported that 3 h of swimming did not alter the phosphorylation of mTOR, p70S6K, rpS6 or 4EBP-1 compared to rest when rats were fasted. However, when fed carbohydrate and protein, phosphorylation of these signalling proteins increased. While, no measure of AMPK activation was included, this study lends evidence that feeding with exercise may serve to override the inhibition that AMPK has on mTOR (Morrison et al. 2008). Evidence suggests that a similar feeding-induced phenomenon may be occurring in humans (Beelen et al. 2008).
These findings provide evidence that the human skeletal muscle protein synthetic response to different modes of exercise is, as hypothesized, specific for proteins needed for structural and metabolic adaptations to the particular exercise stimulus, and that this specificity of response is altered with training to be more specific. Furthermore, our findings indicate that several factors involved in translation initiation explained little of this differential protein synthetic response. The only notable changes were that resistance exercise resulted in a more prolonged (p70S6K) or later (rpS6 and eIF4E) activation of signalling components than with endurance exercise. It is possible that due to the timing of muscle samples, we missed changes in phosphorylation status of the target phosphoproteins measured, or feeding throughout the protocol may have masked any exercise-specific changes in phosphorylation, or perhaps there are other phosphorylation sites (that were not quantified) or phosphoproteins that are involved in exercise-induced adaptations to muscle protein synthesis. In contrast, we propose that, as opposed to marked changes in signalling protein activation, at least of those proteins that would appear to be most obviously affected, that exercise and training-specific changes in gene transcription may be involved in determining the specific nature of the protein synthetic response that we observed and the changes in phenotype seen with training. What controls these upstream transcriptional changes remains to be answered.
Acknowledgments
This study was sponsored by the National Science and Engineering Research Council of Canada and the Canadian Institutes for Health Research to S.M.P. There were contributions from the UK BBSRC (BB/X510697/1 and BB/C516779/1) and EC EXGENESIS to M.J.R., and K.E.Y. was supported by NIH RR00954, DK20579, DK56341, DK49393, DK74345 and DK59531. Jennifer (Xianghong) Chen provided mass spectrometry analytical support. No authors have any financial or other conflicts of interest to declare.
References
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein co-ingestion stimulates muscle protein synthesis during resistance type exercise. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.00774.2007. in press. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Heigenhauser GJ, Spriet LL. Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E85–E91. doi: 10.1152/ajpendo.00237.2003. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab. 2004;14:255–271. doi: 10.1123/ijsnem.14.3.255. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol Endocrinol Metab. 1990;259:E470–E476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky MA. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001;79:386–392. [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Chinkes D, Klein S, Zhang XJ, Wolfe RR. Infusion of labeled KIC is more accurate than labeled leucine to determine human muscle protein synthesis. Am J Physiol Endocrinol Metab. 1996;270:E67–E71. doi: 10.1152/ajpendo.1996.270.1.E67. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70, S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–E1205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- Fink WJ, Costill DL, Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part II. Muscle fiber composition and enzyme activities. Ann N Y Acad Sci. 1977;301:323–327. doi: 10.1111/j.1749-6632.1977.tb38210.x. [DOI] [PubMed] [Google Scholar]
- Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Cell Physiol. 1999;277:C152–C162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007a;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007b;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert C, 4th, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972;33:312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Fluck M, Booth FW. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174–1183. doi: 10.1152/jappl.2001.90.3.1174. discussion 1165. [DOI] [PubMed] [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Chi MM, Hintz CS, Young DA, Kaiser KK, Salmons S, Lowry OH. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol Cell Physiol. 1986;251:C614–C632. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Luthi P, Claassen H, Weibel ER, Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch. 1973;344:217–232. doi: 10.1007/BF00588462. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568:283–290. doi: 10.1113/jphysiol.2005.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- Koopman R, Pennings B, Zorenc AH, van Loon LJ. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137:1880–1886. doi: 10.1093/jn/137.8.1880. [DOI] [PubMed] [Google Scholar]
- Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290:E1245–E1252. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280:R441–R447. doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3b, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576:923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 2007;191:67–75. doi: 10.1111/j.1748-1716.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Cobb LA, Short FA, Ross R, Gunn DR. Effect of long-term exercise on human muscle mitochondria. In: Pernow B, editor. Muscle Metabolism During Exercise. New York: Plenum; 1971. pp. 87–95. [Google Scholar]
- Morrison PJ, Hara D, Ding Z, Ivy JL. Adding protein to a carbohydrate supplement provided after endurance exercise enhances 4E-BP1 and rpS6 signaling in skeletal muscle. J Appl Physiol. 2008;104:1029–1036. doi: 10.1152/japplphysiol.01173.2007. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Passoneau JA, Lowry OH. Enzymatic Analysis: A Practical Guide. Totawa, NJ, USA: Humana Press; 1993. [Google Scholar]
- Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol. 2002;80:1045–1053. doi: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Pikosky MA, Gaine PC, Martin WF, Grabarz KC, Ferrando AA, Wolfe RR, Rodriguez NR. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr. 2006;136:379–383. doi: 10.1093/jn/136.2.379. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–131. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Reeds DN, Cade WT, Patterson BW, Powderly WG, Klein S, Yarasheski KE. Whole-body proteolysis rate is elevated in HIV-associated insulin resistance. Diabetes. 2006;55:2849–2855. doi: 10.2337/db06-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed-state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R172–R178. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2007;102:145–152. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high–frequency electrically stimulated skeletal muscle contractions. J Appl Physiol. 2008;104:625–632. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36:2073–2081. doi: 10.1249/01.mss.0000147582.99810.c5. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Grant EJ, Correia CE, Phillips SM. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol. 2006;98:546–555. doi: 10.1007/s00421-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am J Physiol Endocrinol Metab. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]