Abstract
In a search for Polo-like kinase 1 (Plk1)-interacting proteins using a yeast two-hybrid system, we have identified histone acetyltransferase binding to the origin recognition complex 1 (Hbo1) as a potential Plk1 target. Here, we show that the interaction between Plk1 and Hbo1 is mitosis-specific and that Plk1 phosphorylates Hbo1 on Ser-57 in vitro and in vivo. During mitosis, Cdk1 phosphorylates Hbo1 on Thr-85/88, creating a docking site for Plk1 to be recruited. Significantly, the overexpression of Hbo1 mutated at the Plk1 phosphorylation site (S57A) leads to cell-cycle arrest in the G1/S phase, inhibition of chromatin loading of the minichromosome maintenance (Mcm) complex, and a reduced DNA replication rate. Similarly, Hbo1 depletion results in decreased DNA replication and a failure of Mcm complex binding to chromatin, both of which can be partially rescued by the ectopic expression of WT Hbo1 but not Hbo1-S57A. These results suggest that Plk1 phosphorylation of Hbo1 may be required for prereplicative complex (pre-RC) formation and DNA replication licensing.
Keywords: cell cycle, prereplicative complex, Cdk1, docking site, priming phosphorylation
It has been established that Polo-like kinase 1 (Plk1) plays a critical role in various aspects of mitotic events, such as mitotic entry, centrosome maturation, and mitotic spindle assembly (1). In addition to the N-terminal kinase domain, Plk1 is characterized by the presence of a polo-box domain (PBD) in the noncatalytic C-terminal region (2). The PBD mediates the recruitment of Plk1 to proteins that have been “primed” by phosphorylation at appropriate sites, thus providing an elegant mechanism for controlling Plk1 activity both temporally and spatially (2, 3). Increasing evidence suggests that Plk1 may have additional functions in the regulation of DNA replication. The initiation of DNA replication is tightly regulated in eukaryotic cells to ensure that the genome is precisely duplicated once per cell cycle (4). DNA replication starts with the ordered assembly of a multiprotein complex called the prereplicative complex (pre-RC), whose components are recruited to replication origins in a stepwise manner beginning with the origin recognition complex (Orc). The Orc recruits Cdc6 and Cdt1, which are both required for the subsequent loading of the minichromosome maintenance complex (Mcm). Formation of the pre-RC occurs during late M phase right after sister chromatid segregation and licenses the DNA for replication during S phase (4). A connection between these pre-RC proteins and Plk1 was indicated. The fact that Plk1 is coimmunoprecipitated (co-IPed) with all members (Mcm2–7) of the Mcm complex suggests a new function for Plk1 in coordination of DNA replication and mitotic events (5). Yeast two-hybrid and co-IP were used to reveal interactions between Plk1 and Mcm2, as well as Orc2 (6). Moreover, it was shown that most of the Orc and Mcm subunits are colocalized with Plk1 to centrosomes, and Orc2 is a substrate of Plk1 in vitro (6).
The human histone acetyltransferase binding to Orc1 (Hbo1) protein, a MYST family histone acetyltransferase (HAT), was originally identified through its binding to Orc1 (7). Binding to Mcm2 as well, Hbo1 is required for Orc formation and replication licensing (8) and acts as the major enzyme responsible for histone H4 acetylation (9). In this study, we demonstrate the interaction between Plk1 and Hbo1 and show that Hbo1 is a Plk1 substrate. Depletion of Hbo1 by RNAi results in a reduction of DNA replication and a failure of Mcm protein loading onto chromatin, both of which are partially rescued by ectopic expression of WT Hbo1, but not by a Plk1 unphosphorylatable mutant, suggesting an essential role of Plk1 in pre-RC formation and replication licensing.
Results
Physical Interaction of Plk1 with Hbo1.
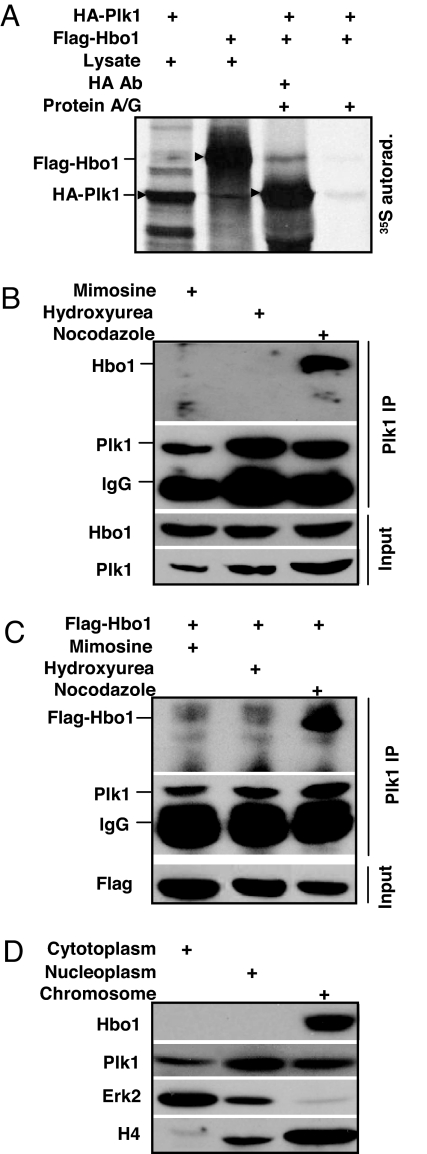
In a search for Plk1-interacting proteins using a yeast two-hybrid system, we have identified Hbo1 as a potential Plk1 target. To determine whether Hbo1 is a bona fide Plk1-interacting partner, we further analyzed the association between Hbo1 and Plk1. Accordingly, HA-Plk1 or Flag-Hbo1 constructs were in vitro translated in a rabbit reticulocyte lysate system in the presence of [35S]methionine. The translation products from the two reactions were mixed together and subjected to HA antibody IP. As shown in Fig. 1A, Hbo1 was co-IPed with HA-Plk1 protein, indicating the association between Plk1 and Hbo1 in vitro. To confirm the interaction between Hbo1 and Plk1 in vivo, HeLa cells were treated with mimosine, hydroxyurea, or nocodazole to arrest cells at G1, S, or M phase, respectively. Total nuclear extracts were prepared and subjected to Plk1 antibody IP. As indicated, endogenous Hbo1 was co-IPed with Plk1 only in nocodazole-treated cells, but not in mimosine- or hydroxyurea-treated cells, suggesting the interaction is mitosis-specific (Fig. 1B). Similarly, overexpressed Flag-Hbo1 also was co-IPed with Plk1 only in mitosis (Fig. 1C). Finally, we tested the subcellular localization of Hbo1 and Plk1. As shown in Fig. 1D, Plk1 can be detected in all three fractions: cytoplasm, nucleoplasm, and chromatin-binding, whereas Hbo1 was only detected in the chromatin-enriched fraction, indicating that Hbo1 binds to Plk1 only in chromatin.
Fig. 1.
Physical interaction between Plk1 and Hbo1. (A) HA-Plk1 or Flag-Hbo1 was translated by using a rabbit reticulocyte system for 30 min at 30°C in the presence of [35S]methionine. Ten percent of the translation products were used as input controls. The remaining products from the two reactions were mixed together, incubated with rotation for 4 h at 4°C, and subjected to HA antibody IP. (B and C) HeLa cells were transfected (C) or not (B) with Flag-Hbo1. At 3 d after transfection, cells were treated with mimosine, hydroxyurea, or nocodazole. Nuclear extracts were prepared, subjected to anti-Plk1 IP, and analyzed by Western blot. (D) Lysates from random growing cells were fractionated into cytoplasmic, soluble nucleoplasmic, and chromatin-enriched fractions and then subjected to Western blot.
Plk1 Phosphorylates Hbo1 in Vitro and in Vivo.
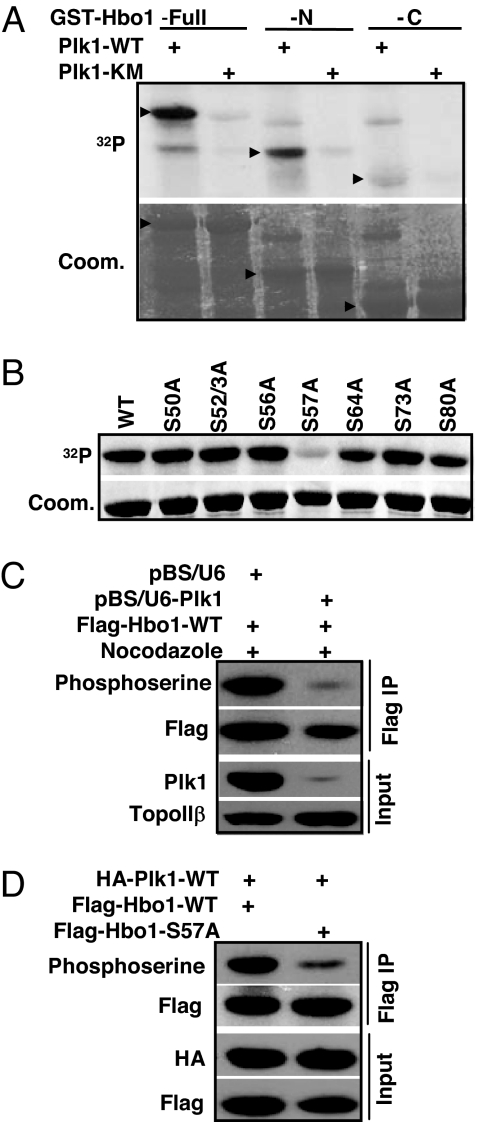
Considering that Hbo1 and Plk1 bind to each other in vivo and in vitro, we asked whether Hbo1 is a substrate of Plk1. Toward this end, purified full-length, N-terminal or C-terminal Hbo1 was incubated with purified Plk1-WT in the presence of [γ-32P]ATP. As shown in Fig. 2A, both full-length and N-terminal fragment of Hbo1 proteins yielded a strong phosphorylation signal. To further narrow down the site, three nonoverlapping Hbo1 fragments (amino acids 1–98, 99–214, and 215–329) were subjected to a kinase reaction, and only the domain containing amino acids 1–98 was a robust substrate for Plk1 (data not shown). Next, every serine in the amino acids 1–98 domain was mutated into alanine in the context of the full-length Hbo1 protein to map the potential phosphorylation site for Plk1. Compared with the phosphorylation level of Hbo1-WT, the phosphorylation level of Hbo1-S57A by Plk1 was significantly reduced, indicating that Ser-57 is the major phosphorylation site for Plk1 in vitro (Fig. 2B).
Fig. 2.
Mapping of Plk1 phosphorylation sites in Hbo1. (A) Purified GST-Plk1-WT (WT) or −KM (kinase-defective mutant) was incubated with purified GST-Hbo1 (amino acids 1–601), Hbo1-N (amino acids 1–329), or Hbo1-C (amino acids 330–601) for 30 min at 30°C in the presence of [γ-32P]ATP. (B) Purified Plk1 was incubated with various GST-Hbo1 serine-to-alanine mutants. (C) Cells were cotransfected with pBS/U6-Plk1 (or pBS/U6), Flag-Hbo1, and pBabe-puro at a ratio of 6:3:1. Nuclear extracts were prepared and subjected to anti-Flag antibody IP, followed by Western blot analysis with an antiphosphoserine antibody. The same membrane was stripped and probed with an anti-Flag antibody. (D) Cells were cotransfected with HA-Plk1 and Flag-Hbo1-WT (or -S57A) at a ratio of 3:1 and processed as in C.
To test whether Hbo1 could be phosphorylated by Plk1 in vivo, cells were cotransfected with pBS/U6-Plk1 (or pBS/U6), Flag-Hbo1-WT, and pBabe-puro at a ratio of 6:3:1. At 1 d after transfection, puromycin was added to select transfection-positive cells for 2 d. After floating cells were washed away, attached cells were further incubated for 14 h in the presence of nocodazole and harvested. Nuclear extracts were prepared, subjected to anti-Flag IP, and analyzed by immunoblot to detect the phosphorylation of Hbo1 by using a phosphoserine antibody as described (10). The data show that the elimination of endogenous Plk1 attenuated Hbo1 phosphorylation, compared with phosphorylated Hbo1 detected in control cells, suggesting that Plk1 phosphorylates Hbo1 in vivo (Fig. 2C). To determine whether Ser-57 is the in vivo site for Plk1, cells were cotransfected with HA-Plk1-WT and Flag-Hbo1-WT or -S57A at a ratio of 3:1. Hbo1 was IPed with Flag antibody, and the levels of Hbo1 phosphorylation were determined by using the phosphoserine antibody as described above. The S57A mutation significantly reduced Hbo1 phosphorylation, indicating that Ser-57 of Hbo1 is the Plk1 phosphorylation site in vivo (Fig. 2D).
Prior Phosphorylation of Hbo1 by Cdk1 Enhances Recruitment of Plk1 to Hbo1.
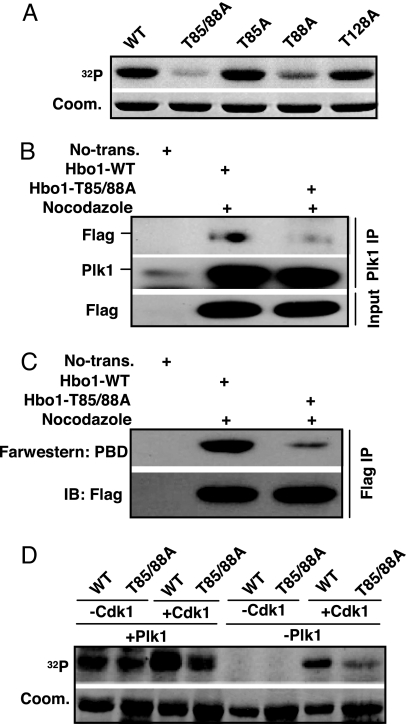
Cdk1-associated phosphorylation has been shown to generate a docking site to recruit Plk1 toward its substrates, such as Cdc25C (2, 11). We asked whether the Hbo1–Plk1 interaction is regulated by a similar mechanism. Accordingly, recombinant Cdk1/cyclin B was incubated with purified Hbo1-WT or various Hbo1 threonine to alanine mutants in the presence of [γ-32P]ATP. We showed that Hbo1 is a robust substrate of Cdk1 and that Thr-85 and Thr-88 are the two sites phosphorylated in vitro (Fig. 3A).
Fig. 3.
Prior phosphorylation of Hbo1 by Cdk1 enhances recruitment of Plk1 to Hbo1. (A) Purified Cdk1/Cyclin B was incubated with various GST-Hbo1 threonine-to-alanine mutants. (B) HeLa cells were transfected with Flag-Hbo1-WT or -T85/88A mutant. At 3 d after transfection, cells were treated with nocodazole for 14 h. Nuclear extracts were subjected to Plk1 antibody IP and analyzed by Western blot. (C) Direct binding of Hbo1 to PBD. After cells were transfected as in B, nuclear extracts were subjected to anti-Flag IP. (Upper) The IP pellets were resolved by SDS/PAGE, transferred to a membrane, and probed with a recombinant GST-Plk1-PBD protein, followed by anti-GST Western blot analysis. (Lower) The same membrane was stripped and reprobed with an anti-Flag antibody to assess the expression level of Hbo1. (D) Purified GST-Hbo1 or -T85/88A proteins were preincubated with or without Cdk1/Cyclin B in the presence of unlabeled ATP. All samples were then incubated with or without Plk1 in the presence of [γ-32P]ATP.
Next, we tested whether the phosphorylation state of Cdk1 sites would affect Plk1 binding to Hbo1 in vivo. For that purpose, HeLa cells were transfected with Flag-Hbo1 or Flag-Hbo1-T85/88A constructs and then treated with nocodazole. Nuclear extracts were prepared, subjected to anti-Plk1 IP, and analyzed by Western blot. As shown in Fig. 3B, introduction of the T85/88A mutations strongly reduced the binding affinity between Plk1 and Hbo1, suggesting that Cdk1-associated priming phosphorylation might generate a docking site for Plk1. Furthermore, Far Western blots were used to test the direct binding between Plk1-PBD and Hbo1. After cells were transfected with Flag-Hbo1 and treated with nocodazole, nuclear extracts were prepared and subjected to anti-Flag IP. The IP pellets were resolved by SDS/PAGE and transferred to a membrane, and the membrane was incubated with GST-Plk1-PBD. After extensive washes, the membrane was probed with a GST antibody. As shown in Fig. 3C, alanine substitution of T85/88 dramatically reduced the binding of Plk1-PBD with Hbo1, indicating that Cdk1-associated priming phosphorylation enhances the recruitment of Plk1-PBD to Hbo1.
To determine whether the Cdk1-induced recruitment of Plk1 to Hbo1 converts Hbo1 into an efficient Plk1 substrate, sequential kinase assays were performed (Fig. 3D). For that purpose, recombinant WT and T85/88A Hbo1 proteins were incubated with Cdk1/cyclin B in the presence of unlabeled ATP, followed by incubation with or without Plk1 in the presence of [γ-32P]ATP. Our results showed that the sequential exposure of Hbo1 to Cdk1 and Plk1 resulted in strong phosphorylation of WT Hbo1, but only weak phosphorylation of the T85/88A mutant, suggesting that Cdk1 serves as a priming kinase for Plk1 toward Hbo1.
Overexpression of Hbo1-S57A, but Not Hbo1-WT, Leads to G1/S Phase Arrest, Inhibition of Pre-RC Formation, and a Reduced DNA Replication Rate.
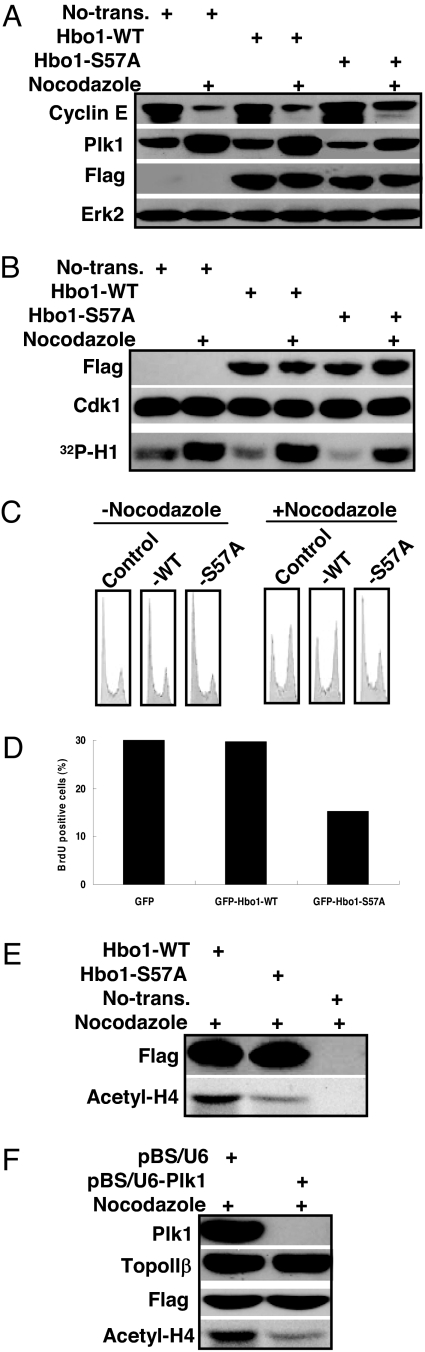
Considering the essential function of Hbo1 in the pre-RC formation and DNA replication licensing, we were interested to test whether these functions of Hbo1 are regulated by Plk1-associated phosphorylation. First, we asked whether overexpression of an Hbo1-S57A mutant would cause cell-cycle arrest. Toward that end, HeLa cells were cotransfected with Hbo1 constructs and pBabe-puro at a ratio of 7:1 and selected with puromycin. After incubation with nocodazole for 10 h, transfection-positive cells were harvested. Lysates were prepared and subjected to Western blot analysis or an anti-Cdk1 IP kinase assay by using histone H1 as a substrate. As shown in Fig. 4 A and B, overexpression of the Hbo1-S57A mutant, but not Hbo1-WT, led to an accumulation of Cyclin E (a G1/S stage marker) and reduced Cdk1 kinase activity in nocodazole-treated cells, suggesting that the overexpression of Hbo1-S57A might cause an arrest in the G1/S phase.
Fig. 4.
The overexpression of Hbo1-S57A leads to G1/S arrest in HeLa cells. (A and B) Cells were cotransfected with Flag-Hbo1 or Flag-Hbo1-S57A and pBabe-puro at a ratio of 7:1 and selected with puromycin. After floating cells were removed, attached cells were further incubated for 10 h in the presence or absence of nocodazole. Lysates were prepared and subjected to Western blot analysis (A) or an anti-Cdk1 IP/kinase assay using histone H1 as a substrate (B). (C) After transfection as in A, cells were incubated for 6 h, treated with nocodazole for 5 h, and harvested for FACS analysis. (D) At 3 d after transfection with GFP-Hbo1 or -S57A, cells were labeled with BrdU for 30 min and stained with BrdU antibody, and the GFP-positive cells were counted. (E and F) Cells were cotransfected with Hbo1 constructs and pBabe-puro at a ratio of 7:1 (E) or with pBS/U6-Plk1, Flag-Hbo1, and pBabe-puro at a ratio of 6:3:1 (F), selected with puromycin, and treated with nocodazole. Nuclear extracts were prepared and subjected to a HAT activity assay.
To further characterize the cell-cycle arrest induced by the Hbo1-S57A mutant, we used FACS to monitor the cell-cycle progression. After cotransfection and selection, puromycin-resistant cells were further incubated for 6 h and then treated for 5 h with nocodazole. As indicated in Fig. 4C, when treated with nocodazole for 5 h, Hbo1-S57A-expressing cells showed an obvious cell-cycle progression delay, compared with that of Hbo1-WT-expressing cells. There was still a lower G2/M peak versus G1 peak in Hbo1-S57A-expressing cells, compared with that of Hbo1-WT-expressing cells (Fig. 4C). Next, we tested whether the overexpression of Hbo1-S57A affects BrdU incorporation. We observed that the overexpression of Hbo1-S57A, but not Hbo1-WT, led to a reduced BrdU incorporation rate, indicating an inhibition of DNA replication in these cells (Fig. 4D).
Considering that Hbo1 is a HAT enzyme responsible for histone H4 acetylation in vivo (9), we asked whether Plk1-associated phosphorylation of Hbo1 affects its histone H4 HAT activity. Accordingly, after cells were transfected with Flag-Hbo1 constructs and treated with nocodazole, nuclear extracts were prepared and subjected to anti-Flag IP, followed by a HAT activity assay using recombinant human histone H4 as the substrate. As shown in Fig. 4E, histone H4 HAT activity of Hbo1-S57A was obviously reduced compared with that of Hbo1-WT, indicating that Plk1-associated phosphorylation of Hbo1 also affects its HAT activity toward histone H4. To further support this notion, Plk1-depleted cells showed lower histone H4 HAT activity (Fig. 4F). To exclude the possibility that S57A mutation might cause some conformational changes in Hbo1 to lead to the decrease of HAT activity, we compared the HAT activity of WT versus phosphomimetic mutation -S57D. Our data showed that S57D retained the HAT activity, compared with that of WT (data not shown).
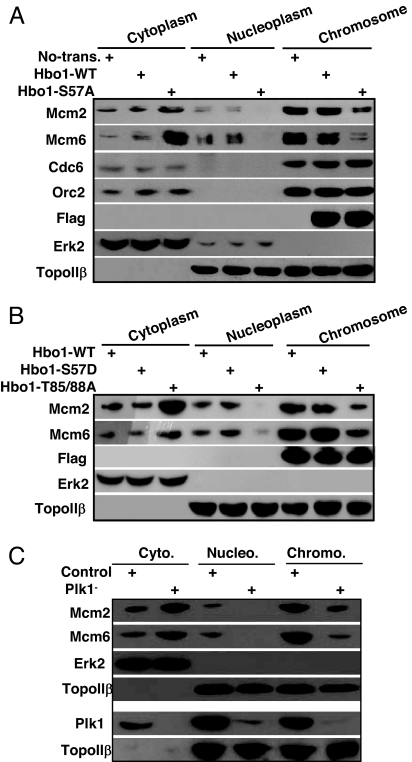
We then examined the effects of overexpression of Hbo1 with different phosphorylation states on the chromatin loading of Mcm proteins. Cells were transfected with Flag-Hbo1 constructs as described above, and cytoplasmic, nucleoplasmic, and chromatin-binding fractions were prepared and subjected to Western blot analysis. In Hbo1-S57A- but not Hbo1-WT- or -S57D-expressing cells, the amounts of Mcm2 and Mcm6 were significantly reduced in the chromosome-binding fraction and recovered instead in the cytoplasmic fraction, indicating that Plk1-associated phosphorylation is required for chromatin loading of Mcm proteins (Fig. 5 A and B). As expected, chromatin binding of Cdc6 and Orc2, two upstream factors of Hbo1 in the formation of pre-RC, was not affected by the phosphorylation of Hbo1. It is worthy to note that the overexpression of Hbo1-T85/T88A double mutant behaved like the S57A mutant on Mcm2/6 chromatin loading (Fig. 5B), further confirming the notion that priming phosphorylation of T85/T88 by Cdk1 has a potent effect on Plk1-mediated phosphorylation toward Hbo1. To directly test the potential involvement of Plk1 in the chromatin loading of Mcm proteins, cells were Plk1-depleted by using vector-based RNAi. Plk1 depletion strongly decreased the binding of Mcm proteins to chromatin, in line with the notion that Plk1-mediated phosphorylation of Hbo1 is involved in the chromatin loading of Mcm proteins (Fig. 5C).
Fig. 5.
Phosphorylation of Hbo1 by Plk1 is essential for pre-RC complex formation. HeLa cells were cotransfected with Flag-Hbo1 (A and B) or pBS/U6-Plk1 (C) and pBabe-puro at a ratio of 7:1 and selected with puromycin. After floating cells were washed away, attached cells were further incubated for 12 h and then harvested. To monitor the Plk1-depletion efficiency, attached cells were treated with nocodazole for 14 h and then harvested (lower portion of C). Lysates were separated into cytoplasmic, soluble nucleoplasmic, and chromatin-enriched fractions and then subjected to Western blot using antibodies indicated at left. The effect of Plk1 depletion on Mcm loading onto chromatin is shown in the upper portion of C.
Hbo1-WT, but Not Hbo1-S57A, Rescues Hbo1-Depletion-Induced Phenotypes in HeLa Cells.
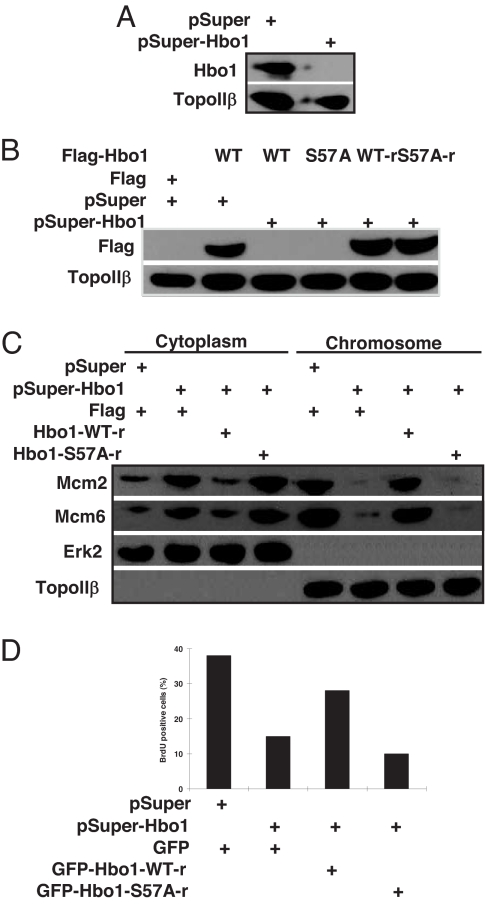
Recently, it was reported that Hbo1 depletion strongly inhibited the chromatin loading of Mcm protein and the DNA replication rate (8). To further address the relevance of Plk1 phosphorylation of Hbo1 in both of these processes, we developed a rescue assay in cells by introducing silent mutations in the RNAi target sequence of the cDNA for Hbo1 expression. First, we tested the depletion efficiency of the RNAi vector pSuper-Hbo1 against endogenous Hbo1 in cells. As shown in Fig. 6A, endogenous Hbo1 was significantly reduced 3 d after transfection, suggesting that pSuper-Hbo1 can efficiently deplete Hbo1 in mammalian cells. Next, cells were cotransfected with pSuper-Hbo1, Flag-Hbo1 (WT or S57A) or RNAi-resistant Flag-Hbo1 (WT-r or S57A-r), and pBabe-puro at a ratio of 6:3:1. As shown in Fig. 6B, overexpressed Flag-Hbo1 was completely depleted, whereas overexpressed Flag-Hbo1-r remained abundant. Under these conditions, we examined whether Hbo1-WT-r could rescue Hbo1-depletion-induced phenotypes such as failure of Mcm protein chromatin loading and inhibition of DNA replication. We showed that Hbo1 depletion strongly reduced Mcm protein loading onto chromatin, and Hbo1-WT-r, but not Hbo1-S57A-r, significantly recovered the chromatin loading of Mcm proteins in Hbo1-depleted cells (Fig. 6C).
Fig. 6.
Hbo1-WT, but not Hbo1-S57A, rescues Hbo1-depletion-induced phenotypes in HeLa cells. (A) Cells were cotransfected with pSuper-Hbo1 and pBabe-puro at a ratio of 7:1 and selected with puromycin. Nuclear extracts were subjected to Western blot analysis. (B) Cells were cotransfected with pSuper-Hbo1, Flag-Hbo1, or RNAi-resistant Flag-Hbo1 and pBabe-puro at a ratio of 6:3:1 and selected as in A. Nuclear extracts were subjected to Western blot analysis. (C) Cells were prepared as in A and harvested. Lysates were separated into cytoplasmic, soluble nucleoplasmic, and chromatin-enriched fractions and then subjected to Western blot. (D) Cells grown on coverslips were cotransfected with pSuper-Hbo1 (or pSuper) and RNAi-resistant GFP-Hbo1-WT (or -S57A) at a ratio of 2:1. At 3 d after transfection, cells were labeled with BrdU for 30 min, and the GFP-positive cells were counted.
Finally, we tested the rescue ability of Hbo1-WT-r on the reduced BrdU incorporation rate in Hbo1-depleted cells. Cells grown on coverslips were cotransfected with pSuper-Hbo1 and RNAi-resistant GFP-Hbo1 (WT-r or S57A-r) at a ratio of 2:1. At 3 d after transfection, cells were labeled with BrdU for 30 min and then stained with a BrdU antibody. Our results showed that the expression of Hbo1-WT-r, but not Hbo1-S57A-r, efficiently rescued the reduced BrdU incorporation rate due to Hbo1 depletion (Fig. 6D). Taken together, these rescue data suggest that phosphorylation of Hbo1 by Plk1 is essential for the chromatin loading of Mcm proteins and DNA replication licensing.
Discussion
In a search for Plk1-interacting proteins using a yeast two-hybrid system, we observed the interaction between Plk1 and Hbo1. Using co-IP and cell-fractionation approaches, we show that Hbo1 binds to Plk1 in vivo. Interestingly, the in vivo binding between Hbo1 and Plk1 is found only in a chromatin-enriched cell fraction during mitosis. A possible explanation for this cell-cycle-specific interaction is that Plk1 serves in the G2/M boundary and reaches a peak during mitosis (12). Furthermore, considering the role of Hbo1 in pre-RC formation and DNA replication, we propose that the interaction between Plk1 and Hbo1 may mainly occur in late mitosis right after sister chromosome segregation.
The sequence context of S57 in Hbo1, the major phosphorylation site for Plk1, is DSSP, which is highly conserved in human, rat, and Xenopus. This sequence also resembles the Plk1 consensus phosphorylation sequence D/E-X-S/T-Φ (X, any amino acid; Φ, a hydrophobic amino acid) (13). Additionally, we provide evidence that Hbo1 could be phosphorylated by Cdk1 at T85 and T88. A single alanine substitution of T88 dramatically reduces the level of Hbo1 phosphorylation, whereas phosphorylation of the double mutant, T85/88A, is further reduced. This indicates that T88 is the major Cdk1 phosphorylation site, but that T85 also is important for phosphorylation by Cdk1. The consensus phosphorylation motif for Cdk1 is S/T-P-X-R/K, in which the Pro at the +1 position is absolutely required, and a basic residue at the +3 position is preferred but not essential for kinase recognition (14). The sequence context of both T85 and T88 (PT85PVT88P) matches this consensus phosphorylation motif, indicating that the phosphorylation of Hbo1 by Cdk1 is specific. Furthermore, alanine substitution of T85, together with T88, significantly reduces the in vivo binding of Hbo1 with Plk1, as well as the in vitro binding between Hbo1 and Plk1-PBD, and affects subsequent phosphorylation by Plk1. Thus far, several physiological substrates that bind to the PBD of Plk1 in a Cdk1 phosphorylation-dependent manner have been identified, such as Cdc25C (2, 11), the peripheral Golgi protein Nir2 (15), and the Plk1-interacting checkpoint “helicase” (16). Here we provide another example that this type of interaction is physiologically relevant by demonstrating that the PBD of Plk1 binds to Hbo1 in a Cdk1 phosphorylation-dependent manner. Finally, the sequence context of T85 and T88 (PT85PVT88P) partially satisfies the optimal sequence motif recognized by the PBD, which is proposed as Ser-[pSer/pThr]-[Pro/X] (2).
The N-terminal domain of Hbo1, which contains a highly conserved serine-rich sequence (amino acids 1–160), serves as the regulatory domain, whereas the C-terminal domain, characterized by a highly conserved C2HC zinc finger and a putative HAT domain, functions as the enzymatic catalytic domain (7, 17). We propose that Plk1 affects the functions of Hbo1 through its phosphorylation of S57 within the N-terminal regulatory domain. It is intriguing to compare the phenotypes induced by the overexpression of WT Hbo1 versus the S57A mutant. We show that the overexpression of Hbo1-S57A induces cell-cycle arrest in the G1 or S phase. In fact, it is more likely that the cell cycle is arrested in the G1 phase because Plk1 levels in Hbo1-S57A-expressing cells are lower than that of Hbo1-WT-expressing cells. This suggests that Plk1-associated phosphorylation of Hbo1 might be essential for G1/S phase transition in cell-cycle progression.
As described above, the major function of Hbo1 is to facilitate the assembly of the pre-RC on replication origins, beginning at late M phase right after sister chromatin segregation and continuing during the G1 phase. Recently, it was reported that the depletion of Hbo1 in mammalian cells or Xenopus eggs led to the failure of Mcm2/6 chromatin loading, although the loadings of geminin, Cdt1, and Cdc6 were not affected (8). In the present study, we provide evidence that the overexpression of Hbo1-S57A, but not Hbo1-WT, prevents the chromatin loading of Mcm2/6, leading to a reduced DNA replication rate. Furthermore, ectopic expression of Hbo1-WT, but not Hbo1-S57A, can significantly rescue these Hbo1-depletion-induced phenotypes. Altogether, our data suggest that Plk1-dependent phosphorylation of Hbo1 plays an essential role in the formation of the pre-RC and DNA replication licensing.
It has been reported that Hbo1 is the major enzyme responsible for histone H4 acetylation in vivo and that Hbo1 depletion induces a dramatic decrease of histone H4 acetylation in mammalian cells and Xenopus eggs (8, 9). Our data show that the histone H4 HAT activity of Hbo1-S57A is significantly decreased compared with that of Hbo1-WT, suggesting that Plk1 phosphorylation of Hbo1 is involved in the modification of its HAT activity. Interestingly, it was previously shown that the recombinant Hbo1 protein expressed in Escherichia coli had no detectable HAT activity (7). One possible reason that the authors raised is that Hbo1 expressed in E. coli lacked a posttranslational modification such as phosphorylation, resulting in a lack of HAT activity (7). We hypothesize that the regulation of Hbo1 HAT activity by Plk1 phosphorylation might be one of the possible mechanisms by which Hbo1-S57A induces a defect in pre-RC formation.
Overall, we show that Hbo1 is a substrate of Plk1 and that Plk1-dependent phosphorylation of Hbo1 is essential for pre-RC formation and DNA replication licensing. The association of Plk1 with Hbo1 indicates potential functions for Plk1 and the possibility of a link for coordination between DNA replication and mitosis.
Materials and Methods
Plasmid Construction.
Full-length human Hbo1, kindly provided by B. Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY), was amplified by PCR and subcloned into pGEX-KG vector (Amersham), pCMV-Tag-2A (Stratagene), and pEGFP-C1 (Clontech). Human Hbo1 mutants were made by site-directed mutagenesis by using a QuikChange kit from Stratagene according to the manufacturer's instructions. pSuper-Hbo1 RNAi vector was a generous gift of J. Cote (Laval University, Quebec City, QC, Canada) (9). The targeting sequence of Hbo1 was 5′-GAAATGCGCCTTCTTCTGA-3′, corresponding to 392–410 of the coding region relative to the first nucleotide of the start codon. For the RNAi rescue assay, two silent mutations were introduced into the RNAi targeting region of Hbo1. The final mutant, 5′-GAAACGCGCCATCT TCTGA-3′, was created by PCR (mutations italicized).
Supporting Information.
Cell culture, transfections, immunoblotting, kinase assays, and HAT assays are described in supporting information (SI) Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Raymond L. Erikson (Harvard University, Cambridge, MA), in whose laboratory the preliminary experiments were performed, for kindly providing the cell lines and plasmids, and for critical reading of this manuscript; Dr. Jacques Cote for the pSuper-Hbo1 RNAi vector; Dr. Bruce Stillman for cDNA encoding human Hbo1; Dr. Mark Hall for mass spectrometry in preliminary experiments; and Drs. Jiabin Tang and Hongchang Li for helpful discussion. This work was supported by National Cancer Institute Howard Temin Award K01 CA114401 (to X.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712063105/DC1.
References
- 1.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 2.Elia AE, et al. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 3.Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- 4.Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsvetkov L, Stern DF. Interaction of chromatin-associated Plk1 and Mcm7. J Biol Chem. 2005;280:11943–11947. doi: 10.1074/jbc.M413514200. [DOI] [PubMed] [Google Scholar]
- 6.Stuermer A, et al. Mouse pre-replicative complex proteins colocalise and interact with the centrosome. Eur J Cell Biol. 2007;86:37–50. doi: 10.1016/j.ejcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem. 1999;274:23027–23034. doi: 10.1074/jbc.274.33.23027. [DOI] [PubMed] [Google Scholar]
- 8.Iizuka M, Matsui T, Takisawa H, Smith MM. Regulation of replication licensing by acetyltransferase Hbo1. Mol Cell Biol. 2006;26:1098–10108. doi: 10.1128/MCB.26.3.1098-1108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyon Y, et al. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Yuan K, et al. Phospho-regulation of HsCdc14A By Polo-like kinase 1 is essential for mitotic progression. J Biol Chem. 2007;282:27414–27423. doi: 10.1074/jbc.M703555200. [DOI] [PubMed] [Google Scholar]
- 11.Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 12.Golsteyn RM, et al. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 14.Songyang Z, et al. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 15.Litvak V, et al. Mitotic phosphorylation of the peripheral Golgi protein Nir2 by Cdk1 provides a docking mechanism for Plk1 and affects cytokinesis completion. Mol Cell. 2004;14:319–330. doi: 10.1016/s1097-2765(04)00214-x. [DOI] [PubMed] [Google Scholar]
- 16.Baumann C, Korner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. doi: 10.1016/j.cell.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Burke TW, Cook JG, Asano M, Nevins JR. Replication factors MCM2 and ORC1 interact with the histone acetyltransferase HBO1. J Biol Chem. 2001;276:15397–15408. doi: 10.1074/jbc.M011556200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.