Abstract
A mainstay of ecological theory and practice is that coexisting species use different resources, leading to the local development of biodiversity. However, a problem arises for understanding coexistence of multiple species if they share critical resources too generally. Here, we employ an experimental framework grounded in nutritional physiology to show that closely related, cooccurring and generalist-feeding herbivores (seven grasshopper species in the genus Melanoplus; Orthoptera: Acrididae) eat protein and carbohydrate in different absolute amounts and ratios even if they eat the same plant taxa. The existence of species-specific nutritional niches provides a cryptic mechanism that helps explain how generalist herbivores with broadly overlapping diets might coexist. We also show that performance by grasshoppers allowed to mix their diets and thus regulate their protein–carbohydrate intake matched optimal performance peaks generated from no-choice treatments. These results indicate the active nature of diet selection to achieve balanced diets and provide buffering capacity in the face of variable food quality. Our empirical findings and experimental approach can be extended to generate and test predictions concerning the intensity of biotic interactions between species, the relative abundance of species, yearly fluctuations in population size, and the nature of interactions with natural enemies in tritrophic niche space.
Keywords: biodiversity, competition, resource partitioning, physiological ecology, geometric framework
Ecological niches remain central to explaining community structure (1–7), despite some reservations (8). In general, for within-trophic level interactions, species that differ in their use of resources are more likely to coexist (9–11), even in highly variable environments (9, 10, 12–14). This “resource-partitioning” framework does not obviously apply to coexisting species that use essentially the same resources, including generalist herbivores that eat a diverse and broadly overlapping array of plants (3, 15–18). Alternate models are required to explain these patterns (7). However, because herbivores show differences in the blend of nutrients that maximize growth and fitness (19–21), an opportunity for niche diversification within a resource-partitioning framework exists at the level of macronutrient use rather than discrete plant taxa.
Ecologists search for assembly rules to explain community structure (22), usually as the combined outcome of top-down and bottom-up interactions (6, 7, 23). Although both are important in terrestrial herbivore communities, two observations argue for a critical importance of understanding bottom-up factors. First, interactions among herbivores leading to coexistence can be driven directly by limited food resources (24). Second, plant nutritional quality often mitigates top-down pressures (23, 25), although the converse may also be true. Generally speaking, herbivores are categorized as being specialists or generalists. Specialization is the case for ≈75% of all plant-feeding insects (26), and plant secondary metabolites (PSMs) function as a primary assembly rule for coexistence among specialist plant-feeders because PSMs restrict membership in a specific feeding guild (27, 28). For insect herbivores, specializing on plants that are unpalatable to other species effectively reduces potential interspecific competition. In contrast, PSM assembly rules do not readily predict coexistence of generalist herbivores that are adapted to eat a broad range of plant taxa. Instead, we suggest that community structure for generalist herbivores may be governed in part by assembly rules defined by differences in species-specific nutritional needs. Under this rule, the nutrients within the plants become the currency of importance, and competition or other biotic interactions among coexisting generalist herbivores would be mediated because each species occupies its own unique “nutritional niche.” Here, we define a nutritional niche as the blend and ratio of nutrients that maximize fitness (20, 29).
The extent to which coexisting generalist herbivores might occupy unique nutritional niches can be explored by using a recently developed experimental framework called the “geometric framework” (19–21) (henceforth GF). The GF is a state-space modeling approach that explores how an animal solves the problem of balancing multiple nutritional needs in a multidimensional and variable environment. It treats an animal as living within a multidimensional nutrient space where there are as many axes as there are functionally relevant (fitness-affecting) nutrients. There are >30 required nutrients for most animals, but protein and carbohydrates are among the most important for herbivores because their concentrations in plants are highly variable (depending on plant type, age, and growing conditions) and often limiting (26). The blend of protein and carbohydrate that the herbivore needs to ingest to optimize growth is called an “intake target,” and there is strong evidence that herbivores have evolved a suite of behavioral and physiological mechanisms that enable them to approach this point (19–21). For a generalist herbivore, the intake target can be reached by regulating the amount of an individual food eaten, eating from a range of different foods, or more likely, through a combination of these two mechanisms. A herbivore can reach its intake target by switching back and forth between two foods (represented as trajectories, or “rails” running through protein–carbohydrate “nutritional space”) that alone are nutritionally suboptimal but together are complementary [supporting information (SI) Fig. 3a]. This switching behavior represents a typical situation for most generalist herbivores. If food selection in generalist herbivores is viewed as a problem of collecting and regulating the intake of multiple nutrients, then the GF provides an experimentally accessible framework for explicitly testing whether species-specific nutritional niches and adaptive nutritional physiology can facilitate coexistence in generalist insect herbivore communities (SI Fig. 3 b–d).
We apply the GF to seven co-occurring North American grasshoppers within the genus Melanoplus that feed, at the population level (30), on a similar range of host plants, and we test the degree to which these coexisting species occupy unique nutritional niches. We also assess the ability of individuals to regulate nutrient intake to achieve a suitably balanced diet, and we briefly describe the buffering capabilities of diet regulation.
Results
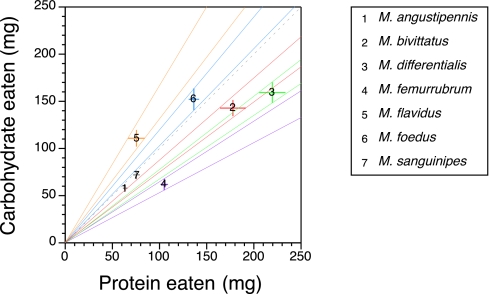
We determined protein–carbohydrate intake targets for each species over their final nymphal stadium by allowing individuals of each species to mix diets between two food dishes that contained nutritionally imbalanced but complementary foods. There were two treatments for each species (Table 1), which allowed us to verify that protein–carbohydrate intake on a treatment was not simply a function of grasshoppers eating equally between the two food dishes (i.e., no discrimination). If grasshoppers actively regulate protein–carbohydrate intake, the amounts of protein and carbohydrate consumed in the two treatments should be similar. Without exception, no significant difference in the amounts of protein and carbohydrate eaten was observed between the two treatments for all species examined (Table 1). Having established that each species was able to regulate its protein–carbohydrate intake, we pooled the protein and carbohydrate consumption values across the two mixed treatments for each species. We then tested the extent to which protein–carbohydrate intake points among the seven species of grasshopper overlapped. Fig. 1 shows a bicoordinate plot of the mean amounts of protein and carbohydrate (± SEM) eaten by each species over the final nymphal stadium, and a comparison of the protein–carbohydrate intake points among the seven species revealed significant differences [multivariate ANOVA (MANOVA): F12,198 = 19.68, P < 0.0001]. Paired comparisons using contrasts were made between species that were “nearest neighbors” in nutritional space (Table 2). All comparisons except one species pair (M. angustipennis and M. sanguinipes) exhibited significant differences in terms of their protein–carbohydrate intake.
Table 1.
Diet pairings for each species plus MANOVA results comparing protein–carbohydrate consumption on the two treatments
| Species | Diet combinations | df | Exact F | Prob > F |
|---|---|---|---|---|
| M. angustipennis | (a) p7:c35 + p35:c7 | 2, 13 | 1.79 | 0.205 |
| (b) p7:c35 + p28:c14 | ||||
| M. bivittatus | (a) p7:c35 + p35:c7 | 2, 14 | 3.71 | 0.051 |
| (b) p7:c35 + p28:c14 | ||||
| M. differentialis | (a) p7:c35 + p35:c7 | 2, 16 | 1.98 | 0.171 |
| (b) p7:c35 + p28:c14 | ||||
| M. femurrubrum | (a) p7:c35 + p35:c7 | 2, 5 | 1.54 | 0.301 |
| (b) p7:c35 + p28:c14 | ||||
| M. flavidus | (a) p7:c35 + p35:c7 | 2, 12 | 0.49 | 0.626 |
| (b) p7:c35 + p28:c14 | ||||
| M. foedus | (a) p14:c28 + p35:c7 | 2, 9 | 0.79 | 0.482 |
| (b) p7:c35 + p28:c14 | ||||
| M. sanguinipes | (a) p7:c35 + p35:c7 | 2, 16 | 0.51 | 0.612 |
| (b) p7:c35 + p28:c14 |
Each species was tested on two treatments, and each treatment contained a pair of food dishes with different diets. The diets differed from one another in their protein (p) and digestible carbohydrate (c) content (e.g., p7:c35 = 7% protein and 35% carbohydrate, expressed on a dry mass basis). If each species is actively regulating protein–carbohydrate intake, no significant difference in protein–carbohydrate consumption should be observed.
Fig. 1.
Protein–carbohydrate intake targets (means ± SEM) for each of the seven coexisting species. Each number in the figure corresponds to a particular species, and species that are statistically different from one another have different colored error bars [only species 1 and 7 (M. angustipennis and M. sanguinipes) were statistically similar; for details, see Table 1]. The gray dashed lines represent the two most extreme foods, p7:c35 and p35:c7, and the area between them represents the available nutrient space. The different colored solid lines define the range of nutrient space occupied by a particular species (as determined by the SEM of the intake targets). Overlap in nutrient space is only obvious for species 1 and 7. For a description of how nutrient space was determined for each species, see the Materials and Methods.
Table 2.
Comparison of protein–carbohydrate consumption points for species that were nearest neighbors in nutritional space
| Species comparison | df | Exact F | Prob > F |
|---|---|---|---|
| M. bivittatus (2) vs. M. differentialis (3) | 2, 98 | 5.82 | 0.004 |
| M. bivittatus (2) vs. M. foedus (6) | 2, 98 | 7.46 | 0.001 |
| M. flavidus (5) vs. M. foedus (6) | 2, 98 | 10.09 | <0.001 |
| M. flavidus (5) vs. M. sanguinipes (7) | 2, 98 | 7.52 | 0.004 |
| M. femurrubrum (4) vs. M. sanguinipes (7) | 2, 98 | 3.53 | 0.033 |
| M. angustipennis (1) vs. M. sanguinipes (7) | 2, 98 | 0.88 | 0.417 |
| M. angustipennis (1) vs. M. femurrubrum (4) | 2, 98 | 3.96 | 0.022 |
Protein–carbohydrate consumption points for species that were nearest neighbors in nutritional space were compared by using specified contrast statements after a significant overall species effect (MANOVA: F12,198 = 19.68, P < 0.0001). The number in parentheses after each species refers to the number assigned to that species in Fig. 1. All nearest-neighbor comparisons were significantly different from one another, except for M. angustipennis (1) vs. M. sanguinipes (7).
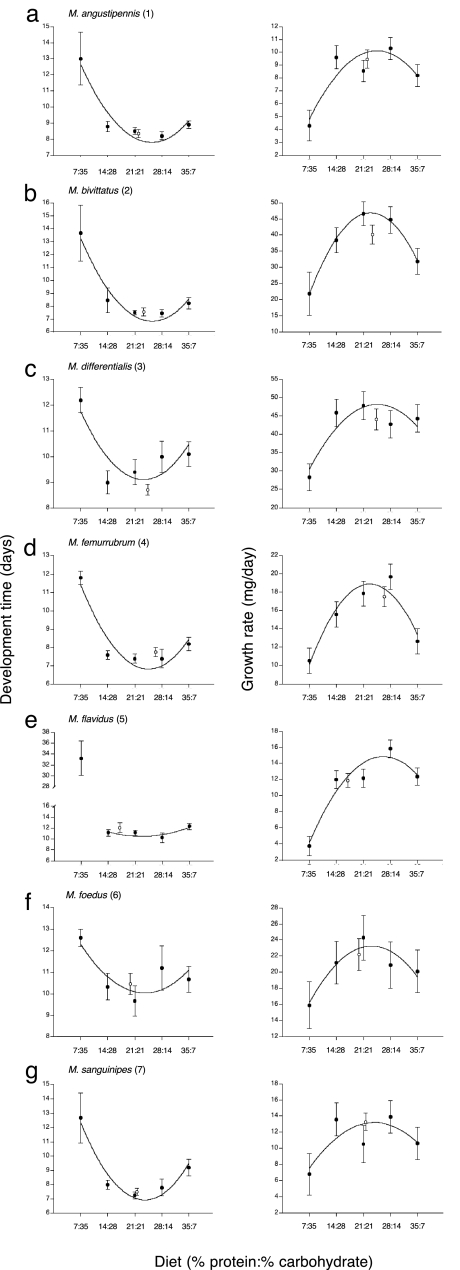
We also compared how insects that were free to mix diets and self-regulate protein–carbohydrate intake performed relative to grasshoppers that were restricted to single diets with different protein:carbohydrate ratios. Our results show, with one possible exception (M. flavidus), that growth rate and development time were near optimal in grasshoppers that were free to mix (Fig. 2).
Fig. 2.
Development time and growth rate for each of the seven coexisting species on treatments with single diets (filled circles) and on the mixed diets (open circles). Development time was measured as the time from the beginning of the final stadium until molting to the adult stage; growth rate was measured as mass gain (in milligrams) in the final stadium divided by development time in the final stadium. In total, there were five single diets that differed in their protein:carbohydrate ratio. The labels on the x axis are the percentage dry mass of protein and carbohydrate, respectively, in each diet. Total macronutrient content for the five single-diet treatments was 42%. For each panel, a best-fit curve is plotted to the data. In the case of M. flavidus developmental time, the curve is only fit through four data points because inclusion of the p7:c35 diet gives an exaggerated optimal diet point. In most instances, development time and growth rate were best on the mixed-diet treatment.
Discussion
This work documents the existence of an explicit nutritional physiological mechanism underlying patterns of food resource partitioning that can facilitate long-term species coexistence of generalist herbivores (2, 9, 10). Specifically, we show that coexisting Melanoplus species partition two key macronutrients, protein and digestible carbohydrates, by differentially regulating their intake (Fig. 1). This partitioning, expressed either in absolute amounts of protein and carbohydrate eaten (their intake targets) or as a protein:carbohydrate ratio (occupying an area, or wedge, of protein–carbohydrate nutritional space), places coexisting species in clearly defined species-specific nutritional niches and may be an effective physiological mechanism to modulate the effects of interspecific competition during critical periods. These regulated species-specific intake targets have functional significance because they correspond to measured performance optima of developmental time and growth rate (Fig. 2). Inspection of performance data also indicates that some buffering capacity exists because relatively large changes in the protein:carbohydrate ratio of the diets result in only small performance penalties. This finding suggests the existence of a mechanism for dealing with often-encountered suboptimal food in a highly variable nutritional environment, although it may come with a cost. It is worth noting that the flatness of our performance curves across the more centrally located diets (e.g., p14:c28, p21:c21, and p28:c14), and to some extent the p35:c7 diet, may reflect the fact that we limited our work to a single developmental stage across a limited number of diets. Recently, Simpson et al. (31) advocated the construction of fitness landscapes over nutrient space, an approach that can reveal a sharp fitness peak at the intake target when a combined performance variable is calculated, for example, growth rate multiplied by percentage survival. In our work, survival was 100% in all but a few diets, so combining growth rate with percentage survival did not provide new insights.
For generalist insect herbivores, the availability of macronutrients such as protein and carbohydrate is highly variable within and among host plants, seasons, and years (32). When limiting, the relative concentrations of macronutrients among alternate food plants in a variable environment can reinforce species-specific niche diversification and determine the outcome of species interactions. For example, current theory of niche differentiation leading to long-term coexistence does not require that resources always be limiting or that species always compete with one another (2, 9). It is expected, however, that coexisting species will diverge in relevant niche space and that resources are sometimes limiting and result in competition, which appears to happen in grasshoppers (33, 34) and other insect herbivores (7). Additional field experiments that alter foliar-N levels directly affect grasshopper performance and species interactions in accordance with ideas presented here (25, 32). Within this framework, our approach provides a starting point for developing and testing specific predictions concerning competition among coexisting species with broadly overlapping diets, the relative abundance of coexisting species, and year-to-year population fluctuations. For example, M. angustipennis and M. sanguinipes share similar protein–carbohydrate intake points and occupy similar positions in nutritional space (their protein:carbohydrate intake ratio is ≈1:1); competition between these two species should be particularly strong. These two species should also exist in similar numbers because they remove similar absolute amounts of protein and carbohydrate from the environment. Additionally, their population sizes relative to the entire community should be relatively stable from year to year because they occupy a central location in protein–carbohydrate nutritional space that makes them less susceptible to yearly permutations in their nutritional environment. In contrast, competition between M. femurrubrum and M. flavidus should be weak because their protein–carbohydrate intake points, and ratios, are very different. However, because the protein–carbohydrate intake targets of these two species are located near the boundaries of nutritional space, yearly shifts in the nutritional environment may cause their populations to fluctuate in a more extreme manner from year to year. Exact predictions of locations of such boundaries in the nutritional landscape based on artificial diets might be tricky, however, because of structural attributes for packaging nutrients in plants and herbivore capabilities in extracting them; some nutrients become less available than others to herbivores (35). However, Jonas (36) has shown for M. bivittatus that the slopes of the boundaries do not change between artificial diets and ground plant material of equal nutritional quality, just the absolute amount of diet eaten, indicating that functional relationships remain intact.
The extent to which divergence in nutritional requirements by generalist insect herbivores can provide a mechanism for explaining species coexistence and fluctuations in relative abundance is largely unexplored. The GF provides an experimental framework that can inform us about ecological patterns among coexisting insect herbivores to explain patterns of community structure at some appropriate scale. In particular, insights regarding potential competitive interactions determined by bottom-up factors can be deduced. If food is limiting (24), diversification in nutrient space may also help explain the coexistence of a seemingly high diversity of generalist herbivores that share host plants in their diet.
Macronutrient availability is highly variable in time and space, which may contribute to coexistence over the short term, such as through a storage effect (2, 9). Theory concludes that resource partitioning is ultimately required to maintain species coexistence over the long term (9, 10). In this sense, however, we are not claiming that other factors do not contribute or are not important to the coexistence of generalist herbivores (37). Rather, our results provide an important underlying mechanism facilitating explanations based on population fluctuations (2, 38) or top-down effects of natural enemies (6, 7, 37, 39, 40) and extend their predictions. For example, diet selection in herbivores is greatly affected by recent encounters with predators (23, 25, 39), parasitoids (41), and pathogens (42) and often decreases the amount of food eaten and influences which plant species are selected and/or can shift protein–carbohydrate intake targets. In fact, such interactions make it more important to select food closest to optimal protein:carbohydrate ratios whenever possible because fewer nutrients are consumed (25) when foraging time is reduced in the presence of predators. If the highest-quality food is not immediately available in the presence of natural enemies, individual performance and population responses will decline. For example, differences in nutritional niches may alter the population-level responses of prey to generalist predators when available food quality varies if apparent competition is operating (43) by altering the relative susceptibility of each species depending on current conditions. Knowing the parameters of the nutritional niche will make the outcomes of such interactions more predictable (44, 45) as both top-down and bottom-up forces combine in subtle ways to determine the outcomes. Moreover, the observed buffering capacity to performance over a range of protein–carbohydrate foods may further reflect the ability to deal with unpredictable nature of food resources, the impact of nonlethal encounters with predators reducing overall consumption, or both. This physiological buffering may prolong the coexistence of species requiring similar nutrients, facilitating a population-level storage effect (9) and thus promoting long-term coexistence when coupled with resource partitioning. Resource partitioning matters when resources are limited, and we have documented a physiological mechanism that scales within an ecological framework to explain multiple species interactions. Ultimately, the GF may be incorporated as a key mechanism in fractal models of herbivore coexistence (4), further extending its influence. Moreover, the differences in resource needs that we identified for Melanoplus species facilitate coexistence in a variable environment as available nutritional ratios vary over a season and promote equality in fitness across species (2, 38, 46–48) in the context of variable top-down pressures.
Materials and Methods
Insects and Experimental Chambers.
A total of seven grasshopper species (Orthoptera: Acrididae), all within the genus Melanoplus (M. angustipennis, M. bivitattus, M. differentialis, M. femurrubrum, M. flavidus, M. foedus, and M. sanguinipes), were collected as adults in the field at Arapaho Prairie (Arthur Co., Nebraska) and returned to the laboratory and fed a mixture of seedling wheat, wheat germ, and mixed forbs. These species co-occur syntopically and have similar diet breadths, with forbs constituting a large proportion of their diet (49, 50). These seven grasshopper species also share a similar phenology and reproductive output. Eggs from each species were collected, put through a short diapause treatment (≈2 months at 4–6°C), and then allowed to hatch at ambient room temperature. Newly emerged grasshoppers were then reared on seedling wheat, romaine lettuce, and wheat germ until reaching the last nymphal stadium, at which point they were immediately weighed and transferred to small plastic arenas that contained one or two dishes of synthetic food, a small plastic container with water for drinking, and a small aluminum perch for roosting. The last instar is the dominant preadult feeding stage, and the intake target for this developmental stage should be representative of the earlier developmental stages (19). In contrast to nymphs, intake targets are likely more dynamic in adults, particularly females whose nutritional requirements will shift depending on their reproductive status (21). All experiments were conducted in a constant-temperature room at 30–32°C under a 14:10-h light:dark photoregime. Each experimental treatment had between 5 and 10 replicates and approximately equal numbers of males and females.
Experimental Foods.
Dry, granular chemically defined foods were made in a manner similar to those described by Behmer et al. (51). We varied protein and digestible carbohydrates (henceforth, simply carbohydrate) to give the following six combinations of protein (p) and carbohydrate (c) (all values are expressed on a percentage dry mass basis): (i) p7:c35, (ii) p14:c28, (iii) p21:c21, (iv) p28:c14, and (v) p35:c7. All foods had equal amounts of digestible macronutrients (summed protein plus carbohydrate as a proportion of total dry mass = 42%, a typical amount for plant material).
Experimental Protocols.
Two experiments were performed for each species with fifth-instar nymphs. In the first experiment, the protein–carbohydrate intake target for each species was determined by allowing insects to feed between two nutritionally suboptimal but complementary foods. Here, each species received two treatments, which was necessary to verify that intake targets were not simply the result of grasshoppers eating equally from the two available foods. Six of the seven species received a p7:c35 + p28:c14 pairing and a second pairing that consisted of p7:c35 + p35:c7 foods. The seventh species, M. foedus, also received the p7:c35 + p28:c14 pairing, but its second pairing was p14:c28 + p35:c7. We gave M. foedus a slightly different second pairing because it shows a strong preference for forbs over grasses in its diet, whereas the other species tend to be less discriminating in terms of forb/grass preferences (49, 50). In reality, the two diet pairings we use for our seven grasshoppers are not substantially different from one another. However, earlier studies show that tight protein–carbohydrate regulation occurs in the face of subtle or broad pairing differences (20, 21).
The protocol for our first experiment was as follows. Each food was poured into modified Petri dishes [designed to minimize spillage (51)] and then allowed to sit for 24 h so that it could equilibrate to ambient room-humidity levels; individual food dishes were then weighed to the nearest 0.1 mg. After foods were weighed, they were placed inside the arenas, and grasshoppers were allowed to feed for 72 h, after which both food dishes were removed and replaced with fresh, preweighed dishes of food. The food dishes that were removed were then allowed to equilibrate to room-humidity levels for 24 h before being reweighed. This procedure was repeated on days 5, 8, and then every 3 days until the grasshopper either molted or died. Because we knew the amounts eaten from each dish and the protein and carbohydrate content of each food dish, we could calculate precisely the amounts of protein and carbohydrate eaten from each food dish, and by extension, the total amounts of protein and carbohydrate consumed from both foods combined. Mass gain and stadium duration, which both serve as surrogates for fitness, were measured for each individual grasshopper.
We also describe nutrient intake by using the ratio of protein to carbohydrate consumed. More specifically, we identify the area of nutrient space being occupied by a species (see ref. 19), with nutrient space being defined as the area between two lines beginning at the origin and intersecting the upper and lower boundaries of the intake target of that species, with the upper and lower boundaries determined as the combined standard errors for protein and carbohydrate (maximum protein and minimum carbohydrate error bars for upper boundary and minimum protein and maximum carbohydrate error bars for the lower boundary). The importance of using ratios is that it allowed us to correct for size differences among insects. For example, larger grasshoppers typically ingest greater quantities of nutrients than smaller grasshoppers. However, different-sized grasshoppers may be ingesting equal ratios of protein and carbohydrate, and within the context of the GF, this activity places them in the same nutrient space. If nutrient intake were analyzed solely by using absolute amounts consumed, our different-sized grasshoppers would be incorrectly described as occupying unique nutritional niches. A ratio-based approach, via visual inspection of nutrient space, prevents such misinterpretations.
In the second experiment, grasshoppers were given a single food (one of the five experimental foods described previously). The protocols for this experiment are similar to those described above, but here only a single dish of food was presented to each grasshopper. The aim of this experiment was to measure performance (development time and growth rate) for grasshoppers confined to foods with specific protein:carbohydrate ratios.
Data Analysis.
To analyze various aspects of food consumption and insect performance, we used analysis of covariance (ANCOVA) and MANOVA techniques with the statistical packages JMP 5.1.2 and SAS 9.1.3. For ANCOVAs, we used grasshopper initial mass to correct for size differences (on average, females tended to be larger than males). For MANOVA analyses, we used the Pillai test statistic, which is considered the most robust against violations of assumptions (52). When necessary, data were log-transformed to meet the underlying assumptions of these analyses. When significant differences between treatments were detected, pairwise comparisons were made by using contrasts. For MANOVAs, contrasts followed the techniques outlined by Scheiner (52). For all ANCOVAs, tests for heterogeneity of slopes were performed; however, no significant effects were ever observed.
Supplementary Material
ACKNOWLEDGMENTS.
We thank A. Kula, J. Hill, S. Parsons, and Cedar Point Biological Station for help during insect and data collection. We thank D. Tilman for contributing discussion and J. Chase, M. Eubanks, O. Lewis, D. Raubenheimer, G. Rosenthal, and two anonymous reviewers for comments that helped improve the manuscript. This work was supported by National Science Foundation Grant DEB-0456522 (to A.J. and S.T.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711870105/DC1.
References
- 1.Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: Univ Chicago Press; 2003. [Google Scholar]
- 2.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 3.Tilman D. Resource Competition and Community Structure. Princeton: Princeton Univ Press; 1982. [PubMed] [Google Scholar]
- 4.Ritchie ME, Olff H. Spatial scaling laws yield a synthetic theory of biodiversity. Nature. 1999;400:557–560. doi: 10.1038/23010. [DOI] [PubMed] [Google Scholar]
- 5.Chase J, Belovsky GE. Experimental evidence for the included niche. Am Nat. 1994;143:514–527. [Google Scholar]
- 6.Singer MS, Stireman JO. The tri-trophic niche conept and adaptive radiation of phytophagous insects. Ecol Lett. 2005;8:1247–1255. [Google Scholar]
- 7.Kaplan I, Denno RF. Interspecific interactions in phytophagous insects revisited: A quantiative assessment of competition theory. Ecol Lett. 2007;10:977–994. doi: 10.1111/j.1461-0248.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 8.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chesson P. General theory of competitive coexistence in spatially varying environments. Theor Popul Biol. 2000;58:211–237. doi: 10.1006/tpbi.2000.1486. [DOI] [PubMed] [Google Scholar]
- 10.Chesson P, Huntly NJ. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 11.Palmer MW. Variation in species richness: Towards a unification of hypotheses. Folia Geobot Phytotaxon. 1994;29:511–530. [Google Scholar]
- 12.Holt RD. Spatial heterogeneity, indirect interactions, and the coexistence of prey species. Am Nat. 1984;124:377–406. doi: 10.1086/284280. [DOI] [PubMed] [Google Scholar]
- 13.Holt RD. From metapopulation dynamics to community structure: Some consequences of spatial heterogeneity. In: Hanski IA, Gilpin ME, editors. Meta-population Biology: Ecology, Genetics, and Evolution. San Diego: Academic; 1997. pp. 149–164. [Google Scholar]
- 14.Pacala SW, Tilman D. Limiting similarity in mechanistic and spatial models of plant competition in heterogeneous environments. Am Nat. 1994;143:222–257. [Google Scholar]
- 15.Wills C, Condit R, Foster RB, Hubbell SP. Strong density- and diversity-related effects help to maintain tree species diversity in a neotropical forest. Proc Natl Acad Sci USA. 1997;94:1252–1257. doi: 10.1073/pnas.94.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keddy PA. Competition. 2nd Ed. New York: Chapman & Hall; 2001. [Google Scholar]
- 17.Grubb PJ. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol Rev Camb Philos Soc. 1977;52:107–145. [Google Scholar]
- 18.Shmida A, Ellner S. Coexistence of plant species with similar niches. Vegetatio. 1984;58:29–55. [Google Scholar]
- 19.Raubenheimer D, Simpson SJ. Integrating nutrition: A geometrical approach. Entomol Exp Appl. 1999;91:67–82. [Google Scholar]
- 20.Raubenheimer D, Simpson SJ. Organismal stoichiometry: Quantifying nonindependence among food components. Ecology. 2004;85:1203–1216. [Google Scholar]
- 21.Simpson SJ, Raubenheimer D. A multilevel analysis of feeding behaviour: The geometry of nutritional decisions. Philos Trans R Soc London Ser B. 1993;342:381–402. [Google Scholar]
- 22.Weiher E, Keddy PA, editors. Ecological Assembly Rules: Perspectives, Advances, Retreats. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 23.Schmitz OJ. Direct and indirect effects of predation and predation risk in old-field interaction webs. Am Nat. 1998;151:327–342. doi: 10.1086/286122. [DOI] [PubMed] [Google Scholar]
- 24.Denno RF, McClure MS, Ott JR. Interspecific interactions in phytophagous insects: Competition reexamined and resurrected. Annu Rev Entomol. 1995;40:297–331. [Google Scholar]
- 25.Danner BJ, Joern A. Food resource-mediated impact of predation risk on performance in the grasshopper Ageneotettix deorum (Orthoptera). Oecologia. 2003;137:352–359. doi: 10.1007/s00442-003-1362-9. [DOI] [PubMed] [Google Scholar]
- 26.Bernays EA, Chapman RF. Host Plant Selection by Phytophagous Insects. New York: Chapman & Hall; 1994. pp. 7–9. [Google Scholar]
- 27.Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. 2nd Ed. Vol 2. New York: Academic; 1992. [Google Scholar]
- 28.Becerra JX. The impact of herbivore–plant coevolution on plant community structure. Proc Natl Acad Sci USA. 2007;104:7483–7488. doi: 10.1073/pnas.0608253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clancy KM, King RM. Defining the Western spruce budworm's nutritional niche with response surface methodology. Ecology. 1993;74:442–454. [Google Scholar]
- 30.Fox LR, Morrow PA. Specialization: Species property or local phenomenon? Science. 1981;211:887–893. doi: 10.1126/science.211.4485.887. [DOI] [PubMed] [Google Scholar]
- 31.Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D. Anim Behav. 2004;68:1299–1311. [Google Scholar]
- 32.Joern A, Mole S. The plant stress hypothesis and variable responses by blue grama grass (Bouteloua gracilis) to water, mineral nitrogen, and insect herbivory. J Chem Ecol. 2005;31:2069–2090. doi: 10.1007/s10886-005-6078-3. [DOI] [PubMed] [Google Scholar]
- 33.Chase J. Varying resource abundances and competitive dynamics. Am Nat. 1996;147:649–654. [Google Scholar]
- 34.Chase JM. Differential competitive interactions and the included niche: An experimental analysis with grasshoppers. Oikos. 1996;76:103–112. [Google Scholar]
- 35.Clissold FJ, Sanson GD, Read J. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. J Anim Ecol. 2006;75:1000–1013. doi: 10.1111/j.1365-2656.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- 36.Jonas JL. Manhattan, KS: Kansas State Univ; 2007. Nutrient resources and stoichiometry affect the ecology of above- and belowground invertebrate consumers. p. 159. PhD dissertation. [Google Scholar]
- 37.Chase J, et al. The interaction between predation and competition: A review and synthesis. Ecol Lett. 2002;5:302–315. [Google Scholar]
- 38.Vandermeer JH. Oscillating populations and biodiversity maintenance. BioScience. 2006;56:967–975. [Google Scholar]
- 39.Lima SL. Stress and decision making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Behav. 1998;27:215–290. [Google Scholar]
- 40.Holt RD, Lawton J. The ecological consequences of shared natural enemies. Annu Rev Ecol Sys. 1994;25:495–520. [Google Scholar]
- 41.Bernays EA, Singer MS. Taste alteration and endoparasites. Nature. 2005;436:476. doi: 10.1038/436476a. [DOI] [PubMed] [Google Scholar]
- 42.Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Proc R Soc Lond, Ser B: Biol Sci. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holt RD. Predation, apparent competition, and the structure of prey communities. Theor Popul Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 44.Berdegue M, Trumble JT, Hare JD, Redak RA. Is it enemy free space? The evidence for terrestrial insects and freshwater arthropods. Ecol Entomol. 1996;21:203–217. [Google Scholar]
- 45.Mira A, Bernays EA. Trade-offs in host use by Manduca sexta: Plant characters vs. natural enemies. Oikos. 2002;97:387–397. [Google Scholar]
- 46.Agrawal AA, et al. Filling key gaps in population and community ecology. Front Ecol Environ. 2007;5:145–152. [Google Scholar]
- 47.Agrawal AA, Lau JA, Hamback PA. Community heterogeneity and the evolution of interactions between plants and insect herbivores. Q Rev Biol. 2006;81:349–376. doi: 10.1086/511529. [DOI] [PubMed] [Google Scholar]
- 48.Vandermeer JH, Pascual M. Competitive coexistence through intermediate polyphagy. Ecol Complex. 2006;3:37–43. [Google Scholar]
- 49.Mulkern GB, et al. Food Habits and Preferences of Grassland Grasshoppers of the North Central Great Plains. Fargo, ND: Agricultural Experiment Station, North Dakota State University; 1969. regional publication No. 196. [Google Scholar]
- 50.Joern A. Grasshopper dietary (Orthoptera: Acrididae) from a Nebraska sandhills prairie. Trans Nebr Acad Sci. 1985;8:21–32. [Google Scholar]
- 51.Behmer ST, Raubenheimer D, Simpson SJ. Frequency-dependent food selection in locusts: A geometric analysis of the role of nutrient balancing. Anim Behav. 2001;61:995–1005. [Google Scholar]
- 52.Scheiner SM. MANOVA: Multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. New York: Chapman & Hall; 2001. pp. 99–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.