Abstract
For efficient development of an immune response, T lymphocytes require long-lasting calcium influx through calcium release-activated calcium (CRAC) channels and the formation of a stable immunological synapse (IS) with the antigen-presenting cell (APC). Recent RNAi screens have identified Stim and Orai in Drosophila cells, and their corresponding mammalian homologs STIM1 and Orai1 in T cells, as essential for CRAC channel activation. Here, we show that STIM1 and Orai1 are recruited to the immunological synapse between primary human T cells and autologous dendritic cells. Both STIM1 and Orai1 accumulated in the area of contact between either resting or super-antigen (SEB)-pretreated T cells and SEB-pulsed dendritic cells, where they were colocalized with T cell receptor (TCR) and costimulatory molecules. In addition, imaging of intracellular calcium signaling in T cells loaded with EGTA revealed significantly higher Ca2+ concentration near the interface, indicating Ca2+ influx localized at the T cell/dendritic cell contact area. Expression of a dominant-negative Orai1 mutant blocked T cell Ca2+ signaling but did not interfere with the initial accumulation of STIM1, Orai1, and CD3 in the contact zone. In activated T cell blasts, mRNA expression for endogenous STIM1 and all three human homologs of Orai was up-regulated, accompanied by a marked increase in Ca2+ influx through CRAC channels. These results imply a positive feedback loop in which an initial TCR signal favors up-regulation of STIM1 and Orai proteins that would augment Ca2+ signaling during subsequent antigen encounter.
Keywords: calcium signaling, CRAC channel, T lymphocyte
In T lymphocytes, T cell receptor (TCR) engagement leads to depletion of luminal Ca2+ from the endoplasmic reticulum (ER) Ca2+ store followed by activation of calcium release-activated calcium (CRAC) channels (1, 2). The molecular requirements for CRAC channel activity were identified recently by RNA interference screening (3–7). In human T cells, STIM1 senses the ER Ca2+ content, using an EF-hand motif near the N terminus and translocates to the plasma membrane when the Ca2+ store is depleted (1, 8). The Ca2+ channel itself is presumably formed from Orai1 subunits in T cells, because mutation at a conserved glutamate residue between transmembrane segments 1 and 2 in Drosophila Orai or human Orai1 alters the Ca2+ ion selectivity of the channel (6, 9, 10). Importantly, a single point mutation within transmembrane segment 1 of Orai1 in patients with SCID inhibits CRAC channel activity, leading to profound immunosuppression in these patients (5).
After Ca2+ store depletion, STIM1 accumulates in close proximity to the plasma membrane (3, 8, 11–13), which would allow it to interact with CRAC channel subunits or other associated proteins. Indeed, coimmunoprecipitation revealed interactions between Stim and Orai proteins in Drosophila S2 cells (7), and between human STIM1 and Orai1 in HEK293 cells (6). Furthermore, Orai1 accumulates near STIM1 puncta after store depletion, suggesting that both molecules interact to form an elementary unit of store-operated Ca2+ entry (11, 12). Several T cell surface and signaling molecules are recruited to the immunological synapse (IS) at the T cell/antigen-presenting cell (APC) interface (14), thereby optimizing conditions for their interaction to amplify and sustain the signal required for full activation of T cells (15). The present study focused on two questions: (i) whether Orai1 and STIM1 are recruited to the IS during the initial activation phase of T cells in contact with APCs and (ii) whether expression levels of Orai1 and STIM1 correlate with changes in Ca2+ signaling in activated T cells. We also tested the role of CRAC channel-mediated calcium influx in T cell/APC contact formation and in the initial clustering of Orai1 and STIM1 molecules near the interface.
Results
Orai1 and STIM1 Are Recruited to the Immunological Synapse.
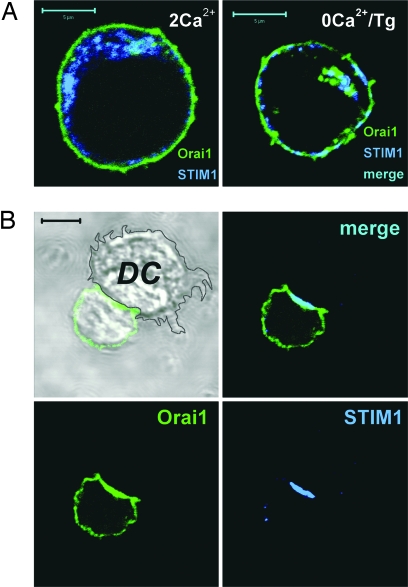
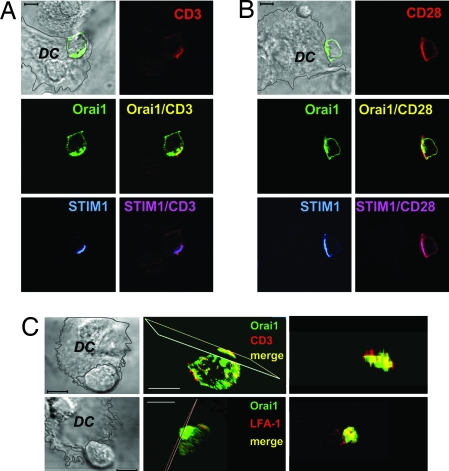
Using primary human T cells or Jurkat T cells cotransfected with GFP-Orai1 and STIM1, we tested whether Orai1 and STIM1 are recruited to the IS upon activation of T cells by contact with superantigen (SEB)-pulsed autologous dendritic cells. Under control conditions without the APC, Orai1 is distributed throughout the cell surface, consistent with plasma membrane localization, whereas the majority of STIM1 was found in an ER pattern of localization [Fig. 1A and supporting information (SI) Fig. 7A]. After treating cells with thapsigargin (Tg), a sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA) inhibitor applied in calcium-free solution to deplete the intracellular Ca2+ store, STIM1 formed puncta and became partially colocalized with Orai1 at the cell surface (Fig. 1A Right). Thus, consistent with the findings in refs. 3, 8, and 11, both STIM1 and Orai1 puncta are found distributed along the entire plasma membrane in store-depleted cells. In contrast, after contact between a T cell and an antigen-pulsed dendritic cell, a pronounced polarization of both STIM1 and Orai1 can be seen (Fig. 1B). Notably, both overexpressed GFP-Orai1 and endogenous Orai1 (SI Fig. 7B) accumulated at high density within the contact zone, in addition to their remaining localization along the rest of the T cell surface. Dendritic cells also demonstrated endogenous Orai1 and low-level STIM1 immunostaining, although without detectable redistribution to the interface (shown for Orai1 in SI Fig. 7B). STIM1 in T cells redistributed more extensively to the T cell/APC interface, with only few puncta found in the contact-free zone. Similar patterns of staining were observed in both resting and SEB-activated T cells cocultured with autologous SEB-pulsed dendritic cells or with Raji B cells as APC (Fig. 1 and SI Figs. 8 and 9). We further showed that Orai1 is colocalized with CD3 and costimulatory CD28 molecules known to be recruited to IS in T cell/APC conjugate pairs (Fig. 2 A and B). Consistent with a multifocal synapse (16, 17), the Orai1 distribution overlapped with both CD3 and lymphocyte function-associated antigen-1 (LFA-1) (Fig. 2C). These results demonstrate that STIM1 and Orai1 are recruited to the IS within the T cell/APC zone of contact.
Fig. 1.
Redistribution of Orai1 and STIM1 in T cells after Ca2+ store depletion with Tg or interaction with SEB-pulsed dendritic cells. (A) Jurkat T cells in control conditions (2 mM Ca2+) and after treatment with Tg in Ca2+-free solution for 15 min. (Scale bars, 5 μm.) (B) Primary human T lymphocyte interacting with SEB-pulsed autologous dendritic cell. Resting primary T cells from peripheral blood were cotransfected with GFP-Orai1 and STIM1, added to dendritic cells, and fixed 30 min after coincubation at 37°C. STIM1 is stained with polyclonal anti-human STIM1 antibody and Cy5-conjugated secondary antibody. (Scale bar, 5 μm.)
Fig. 2.
Orai1 and STIM1 are colocalized with T cell receptor molecules at the interface between T cells and dendritic cells. (A) CD3, Orai1, and STIM1 in human T cell pretreated with SEB and transfected with GFP-Orai1 and STIM1 5 min after coincubation with SEB-pulsed autologous dendritic cells at 37°C. (Scale bar, 5 μm.) (B) CD28, GFP-Orai1, and STIM1 in T cells coincubated 30 min with SEB-pulsed dendritic cells. Cells were stained with anti-CD3 or -CD28 antibodies, followed by permeabilization and staining with anti-STIM1 antibody. (C) Images of SEB-primed T cells in contact with SEB-pulsed dendritic cells stained with anti-CD3 (Upper) or anti-LFA-1 antibody (Lower) and projections of the contact regions. T cells were transfected with GFP-Orai1 and STIM1. (Scale bars, 5 μm.)
Relocalization of Orai1 and STIM1 to the Interface Does Not Require Calcium Signaling.
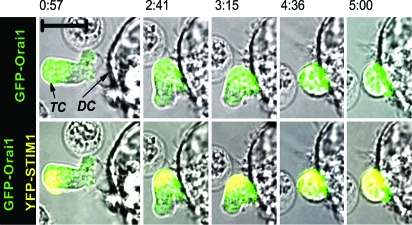
Imaging of living cells confirmed Orai1 and STIM1 accumulation at the immunological synapse and allowed us to examine the kinetics of synaptic recruitment of Orai1 and STIM1. SEB-pretreated human T lymphocytes were cotransfected with GFP-Orai1 and YFP-STIM1 and imaged during migrating and interaction with autologous SEB-pulsed dendritic cells. Before contact with APC, Orai1 in the motile T cells was localized at the cell surface and in the uropod (posterior of the moving polarized lymphocyte), and STIM1 was mostly in the uropod. Contact of the T cell leading edge with an APC induced T cell rounding and establishment of a stable contact with the dendritic cell. Both Orai1 and STIM1 redistributed, accumulating at the T cell/APC interface (Fig. 3 and SI Movie 1) over a 5-min period, during which Ca2+ signaling (data not shown) was initiated.
Fig. 3.
Live-cell imaging of STIM1 and Orai1 relocalization to the APC interface. Human T cell cotransfected with GFP-Orai1 and YFP-STIM1 moving toward and interacting with SEB-pulsed autologous dendritic cell at indicated times (from SI Movie 1). GFP-Orai1 (green, merged with bright-field; Upper) and YFP-STIM1 (yellow, shown together with green GFP-Orai1, merged with bright-field) in T cell (TC) during interaction and establishment of contact with a dendritic cell (DC). Data are representative of seven independent experiments. (Scale bar, 10 μm.)
We further tested whether synaptic recruitment of Orai1, STIM1, and TCR components requires calcium influx through CRAC channels. Point mutation of the conserved glutamate in the loop between S1 and S2 (E106 of human Orai1, E180 in Drosophila Orai) to an alanine or glutamine results in a nonconducting CRAC channel and inhibits native CRAC current, indicating a dominant-negative action that is likely mediated by heteromultimerization with native wild-type Orai or Orai1 subunits (6, 9, 10). Expression of a nonconducting dominant-negative mutant of Orai1 (E106A), identified by fluorescence from the GFP tag, blocked Ca2+ entry in Jurkat and primary human T cells (SI Fig. 9A) but did not disturb initial TCR, STIM1, or Orai1 clustering upon contact with APC. Both Jurkat cells and SEB-pretreated T cells transfected with Orai1 E106A demonstrated interface-associated clustering of mutant Orai1, STIM1, and CD3 after short (10 min) coincubation with SEB-pulsed Raji B lymphocytes (SI Fig. 9 B and C). These results show that Ca2+ influx through Orai1-containing CRAC channels is not critical for the redistribution of Orai1, STIM1, and TCR molecules to the contact zone.
Local Calcium Influx at the T Cell/Dendritic Cell Interface.
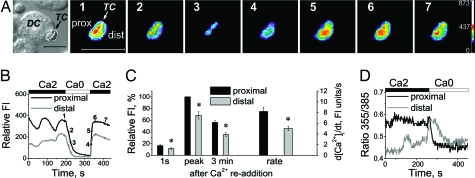
After calcium store depletion by thapsigargin, CRAC channel activity is restricted to areas of STIM1/Orai1 puncta formation (11). Using exogenous Ca2+ buffering to localize the sites of Ca2+ entry in T cells, we tested whether Ca2+ influx induced in T cells upon antigen presentation is enhanced at the IS where STIM1 and Orai1 accumulate. Before coincubation with SEB-pulsed dendritic cells, T lymphocytes were loaded with the Ca2+ indicator fluo-4 along with EGTA, a slow high-affinity Ca2+ buffer. Under these conditions, Ca2+ entering the cell would bind rapidly to the dye, producing a fluorescent signal, and then be captured by EGTA. In the presence of 2 mM extracellular Ca2+, many of those T cells that formed tight contacts with dendritic cells generated Ca2+ oscillations typical for T cells interacting with APCs (Fig. 4 A and B). In addition, an apparent gradient of intracellular Ca2+ with a significantly higher concentration near T cell/dendritic cell interface was observed (Fig. 4A), suggesting that CRAC channels clustered near the interface mediate localized Ca2+ influx in T cells upon contact with APCs. Upon removal of external Ca2+, Ca2+ fell to the same level on both proximal and distal sides of the cell. Subsequent readdition of external Ca2+ reestablished the gradient across the cell, revealing significantly higher Ca2+ influx near the interface compared with other parts of the cell, occurring as soon as 1 s after readdition of Ca2+ (proximal versus distal areas, Fig. 4C). The rate of Ca2+ rise was also significantly higher near the interface compared with the more distal part of the T cell. Furthermore, in similar experiments performed by using ratiometric Ca2+ measurement in Jurkat T cells loaded with fura-2 together with increased EGTA, the Ca2+ level near the interface with APC (proximal) was higher compared with that seen more distally after formation of stable contacts with antigen-presenting Raji B cells (Fig. 4D). Such a difference was never observed in the absence of exogenous intracellular buffering by EGTA (data not shown). Because exogenous Ca2+ chelation was required to reveal the gradients, it is unlikely that the gradients were due to dye compartmentalization. These results show that Ca2+ influx in T cells making contact with APCs preferentially occurs at the interface where STIM1 and Orai1 accumulate.
Fig. 4.
Local Ca2+ influx in the T cell/dendritic cell interface. (A) Intracellular Ca2+ signaling in a representative human T cell (TC) pretreated with SEB and loaded with fluo-4 and EGTA during interaction with an SEB-pulsed autologous dendritic cell (DC). Shown are a T cell interacting with dendritic cell (leftmost panel) and pseudocolor images depicting Ca2+ gradients in the same T cell. (Scale bars, 5 μm.) (B) Relative fluo-4 fluorescence intensity in the proximal and distal region of the same cell. Numbers 1–7 indicate corresponding time points in A. Fluorescence intensity was measured within proximal (near interface) and distal (further from interface) areas of the T cell, as indicated. (C) (Left) Averaged relative [Ca2+]i measured on proximal and distal sides 1s, at the peak of response, and 3 min after readdition of 2 mM Ca2+. (Right) Rate of Ca2+ rise on proximal and distal sides after readdition of 2 mM Ca2+. The data shown are mean ± SE, n = 19 cells; *, P < 0.05. (D) Fura-2 fluorescence ratios in a Jurkat T cell loaded with fura-2 and EGTA interacting with SEB-pulsed Raji B cell. Calcium was measured within proximal and distal areas.
CRAC Channel-Mediated Ca2+ Influx Is Increased in Activated Human T Lymphocytes.
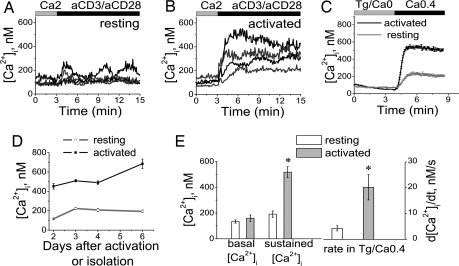
During a second encounter with antigen/APC, TCR engagement induces a stronger Ca2+ signal and a more rapid and profoundly enhanced immune response, compared with that of naïve T cells (18, 19). To activate primary human T cells fully in vitro, we incubated them with plate-bound anti-CD3 in combination with costimulatory anti-CD28 antibody. Under these conditions, T cells up-regulated CD69 expression and proliferated robustly (data not shown). Application of anti-CD3 and -CD28 antibodies consistently induced stronger Ca2+ signaling and higher rates of Ca2+ influx in previously activated T cells compared with resting T cells (Fig. 5). The level of CRAC channel activity evoked by store depletion with Tg was also significantly greater in activated T cells, assessed both by the sustained Ca2+ signal (2.7-fold increase, compared with resting cells) and the maximal rate of Ca2+ rise (dCa2+/dt, 4.7-fold increase; Fig. 5 C and E), consistent with the results in ref. 20. These changes were observed by the second day of activation and lasted for at least 6 days (Fig. 5D). We found no evidence for involvement of voltage-gated Ca2+ channels in either resting or activated human T lymphocytes, in contrast to suggestions that voltage-gated Ca2+ channels mediate or participate in TCR-induced Ca2+ signaling (21). Whole-cell recordings in activated human T cells confirmed functional CRAC channel activity, but not voltage-gated Ca2+ channel activity, under the same conditions that produced voltage-gated Ca2+ current in PC12 cells (SI Fig. 10 A–D). Monitoring of the intracellular Ca2+ concentration ([Ca2+]i) during elevation of external potassium provides a sensitive assay for the type of Ca2+ channel. Again, voltage-gated Ca2+ channel activity was revealed in PC12 cells but not in resting or activated human T cells (SI Fig. 10 E and F). Instead, Tg-dependent Ca2+ influx in T cells was inhibited by high external potassium in a concentration-dependent manner (SI Fig. 10G), consistent with a reduced electrochemical driving force for Ca2+ entry through the CRAC channel. We conclude, therefore, that Ca2+ influx, specifically through CRAC channels, is significantly potentiated after TCR engagement.
Fig. 5.
Ca2+ influx is increased in activated, compared with resting, primary human T cells. (A and B) Individual calcium responses in representative resting (A) and activated (B) human T cells, induced by anti-CD3 and anti-CD28 antibodies. (C) Averaged Tg-dependent influx of [Ca2+]i in 173 resting T cells (three experiments/two donors) and 163 T cells activated with plate-bound antibodies for 3 days (two experiments/two donors). (D) Changes in the amplitude of sustained calcium responses after readdition of Ca2+, measured 2–6 days after activation of cells with plate-bound antibodies or maintaining in control conditions. (E) Averaged values of [Ca2+]i and maximal rate of [Ca2+]i rise in resting cells (454 cells, nine experiments, three donors) and T cells activated with antibodies for 2–6 days (461 cells, eight experiments, three donors), measured initially (basal Ca2+) and 5 min after readdition of Ca2+. The data are shown as mean ± SE (*, P < 0.01). The composition of solutions is given in SI Table 1.
Orai1, -2, and -3 and STIM1 mRNA Expression Is Up-Regulated in Activated T Cells.
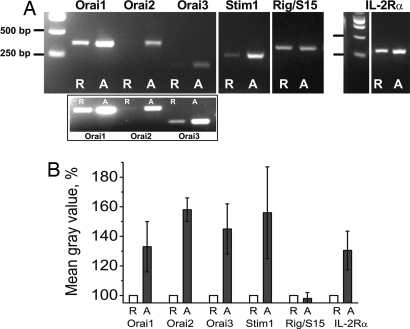
Studies have shown that STIM1 and Orai1 are essential for Ca2+ signaling in T cell lines (4, 5, 22). We confirmed the central role of Orai1 and STIM1 in resting primary T cells; knocking down STIM1 or Orai1 separately with RNAi significantly reduced Ca2+ influx, assessed by Tg-evoked peak Ca2+ levels (data not shown) and the rate of Ca2+ rise, dCa2+/dt (SI Fig. 11A). Global changes in gene expression are characteristic of T cell activation (23). As shown in Fig. 6 A and B, mRNA expression of STIM1 and all three Orai homologs was significantly increased in activated T cells, compared with resting cells. As expected, the expression of IL-2Rα mRNA was also consistently up-regulated in activated cells, serving as a positive control (24), whereas mRNA expression for a housekeeping ribosomal gene, Rig/S15, was not changed. The three Orai homologs, expressed as homomultimers, exhibit distinct pharmacological sensitivity to 2APB (25). Tg-evoked Ca2+ influx in both resting and activated T cells is potentiated by a low concentration (5 μM) of 2APB, and inhibited by a higher 2APB concentration (50 μM) (SI Fig. 11B), consistent with effects of 2APB on Orai1-induced CRAC current, but not channels formed by expression of Orai3 (25). Because Orai1 is a major contributor to store-operated calcium influx in human T lymphocytes, in contrast to Orai2, which has little role (22), or Orai3, which has distinct pharmacological properties (25), we conclude that up-regulation of Orai1 and STIM1 most likely contributes to the enhanced store-operated Ca2+ influx in activated T cells (Fig. 5), although potential contributions of heteromultimers need to be evaluated further.
Fig. 6.
Up-regulation of endogenous human Orai homologs and STIM1 mRNA expression in human T lymphocytes activated with plate-bound antibodies. (A) Orai1, Orai2, Orai3, and STIM1 mRNA expression by RT-PCR analysis in resting (R) and 3-day activated (A) T cells of the same representative donor. (Inset) Orai2 and Orai3 mRNA expression in resting cells revealed by using higher number of cycles (30 cycles compared with 27 cycles in the main image). (B) Relative mRNA expression estimated by measuring the optical density of the bands from activated cells and normalizing to the corresponding bands from resting cells; n = 4 donors (mean ± SE).
Discussion
Our study demonstrates that Orai1 and STIM1—the CRAC channel pore subunit and its activator, respectively—are rapidly recruited to the region of contact between human T lymphocytes and autologous antigen-presenting dendritic cells, resulting in enhanced localized calcium influx in the T cell at the T cell/APC interface. It was shown in solitary T cells that the location of active CRAC channels after store depletion induced by thapsigargin treatment is closely associated with STIM1 puncta in the ER and with colocalized Orai1 in the plasma membrane (11). We also found puncta of colocalized STIM1 and GFP–Orai1 scattered throughout the entire periphery of T cells treated with thapsigargin. Here, we extend these results to the physiological context of human T cells interacting with an antigen-presenting dendritic cell and show that Orai1 and STIM1 colocalize with CD3, CD28 and LFA-1, demonstrating that both Orai1 and STIM1 are recruited to the IS. T cell Ca2+ influx is higher in this region than at the distal pole of the cell, as revealed by [Ca2+]i gradients when Ca2+ buffering was augmented exogenously by EGTA, a slow Ca2+ chelator. These findings are consistent with accumulation of functional CRAC channel components promoting localized Ca2+ influx at the IS.
Before establishment of a stable contact with APC, we found STIM1 predominantly in the trailing uropod, indicating ER localization at the rear of the motile T cell. In a previous study of cytotoxic T cells interacting with their targets in the absence of exogenous buffering, the earliest Ca2+ response was observed at the distal pole of the cell, creating a transient gradient of [Ca2+]i that was highest at the rear end of the cell immediately after contact formation (26). This early Ca2+ gradient may have resulted from IP3-induced Ca2+ release initially at the trailing edge of the cell, leading to STIM1 puncta formation and initiation of CRAC channel activity. Within a few seconds, the global Ca2+ signal was uniform. In our study, we show that CRAC channel components relocalize to and promote Ca2+ entry preferentially at the zone of contact with APC; these results are not in disagreement with the earliest Ca2+ signal occurring at the trailing edge of the cell where STIM1 is initially located. As a consequence of STIM1 relocalization and concentration of Orai1 in the IS, [Ca2+]i may be locally elevated immediately adjacent to the membrane in the IS. However, localized Ca2+ entry produces a uniform global Ca2+ signal in the absence of exogenous Ca2+ buffering.
Similar changes in the relocalization of CRAC channels and potassium channels take place during the first minutes of T cell activation. The two types of T lymphocyte potassium channel, Kv1.3 and KCa3.1, that are indirectly involved in functional Ca2+ signaling by regulating the membrane potential (27) are also recruited to the IS (28–30). Channel function and Ca2+ signaling does not appear important for initial IS formation, because inhibiting either K+ channel with channel blockers (29, 30) or CRAC channel function by expression of the nonconducting dominant-negative Orai1 subunit of the CRAC channel (SI Fig. 9) did not prevent molecular clustering in the contact zone. However, the long-term stability of the IS was shown to be compromised by blocking Ca2+ entry in combination with increasing intracellular Ca2+ buffering (31, 32), although the initial contact formation and redistribution of CD3 to the contact area was not affected (31). Thus, it is possible that the recruitment of potassium and CRAC channels to the IS where receptors, adhesion molecules, and costimulatory molecules also accumulate is important for long-term calcium-dependent regulation of the signaling events triggered upon antigen presentation.
In human T cells after activation, the magnitude of store-operated Ca2+ entry increases ≈10-fold (20); the number of functional voltage-gated K+ channels made up of Kv1.3 subunits increases 3- to 4-fold (33); and the number of functional Ca2+-activated K+ channels made up of KCa3.1 subunits increases 25-fold (34). Thus, all three channel types that are involved directly or indirectly in Ca2+ signaling in T cells are up-regulated upon mitogenic activation after TCR engagement. Our experiments revealed increased expression of Orai1 and STIM1 mRNA in activated T lymphocytes that may contribute to enhanced signaling in activated T cells. Coordinated up-regulation and colocalization of CRAC components (Orai1 pore subunit and activator STIM1) and KCa3.1 channels to the IS may provide positive feedback involving localized Ca2+ entry activating KCa3.1 that would enhance Ca2+ signaling in activated cells. In addition, we found up-regulation of endogenous Orai2 and Orai3 in activated human T cells. Although the stoichiometry of the CRAC channel and the exact mechanism of its activation are still unknown, both Orai1 and STIM1 are undoubtedly crucial for CRAC channel function in human T cells (4, 5, 22, 35). Recent findings (25, 35) suggest that Orai2 and Orai3 can form homomultimeric channel complexes with distinct pharmacological properties and Ca2+ sensitivity. The pharmacological sensitivity to 2-APB of Tg-evoked Ca2+ signaling in activated T cells parallels that of overexpressed Orai1, but not Orai2 or Orai 3, in heterologous cells. However, because Orai1–3 can also heteromultimerize, it remains to be seen what role Orai2 and Orai3 or their heteromultimers play in the regulation of T lymphocyte function. Moreover, T cell proliferation is suppressed in T cells from SCID patients lacking functional CRAC channels due to mutation within the first transmembrane domain of Orai1 (5). One of the implications of Orai1 and STIM1 up-regulation in the early stages of an immune response would be to amplify and ensure CRAC channel-mediated Ca2+ signaling crucial for clonal expansion, differentiation and calcium-dependent regulation of gene expression in T cells. Thus, we propose that positive feedback between the Ca2+ signal and expression of Orai1 and STIM1 would ensure increased response strength to subsequent encounters with antigen.
Materials and Methods
Jurkat or primary human T cells (resting or previously activated) were incubated together with SEB-pulsed Raji or autologous dendritic cells as APCs on poly-l-lysine-coated dishes, fixed, and stained for localization of STIM1, Orai1, CD3, CD28, and LFA-1, using antibody staining as described in SI Materials and Methods. For live-cell imaging, SEB-pretreated human T cells were cotransfected with GFP-Orai1 (SI Materials and Methods) and YFP-STIM1 [kindly provided by T. Meyer (Stanford University Medical Center, Stanford, CA)] and, 24 h later, added to autologous SEB-pulsed dendritic cells attached to poly-l-lysine-coated glass coverslips. Simultaneous monitoring of GFP- and YFP-positive T cell images were acquired by using an inverted Olympus IX81 microscope equipped with a Olympus PlanApo N 60 × 1.45 N.A. oil objective, a Roper Scientific (Photometrics) Cascade 650 CCD camera, and a T.I.L.L. Photonics Polychrome IV monochromator tuned to independently excite GFP (455 nm), YFP (525 nm), or fura-2 (355 nm and 385 nm). Temperature was maintained at 35–37°C during the entire period of imaging. Ca2+ imaging experiments, using fluo-4 or fura-2, were performed as described in ref. 4. Ca2+ buffering capacity was increased in T cells loaded with fluo-4/AM (4 μM, 30 min) and EGTA/AM (1 μM, 30 min) or with fura-2/AM (2 μM, 30 min) and EGTA/AM (10 μM, 30 min) at room temperature, and images of T cells in contact with APC were acquired under control of Metafluor software. Digital images were acquired for each wavelength, using Metamorph software. Whole-cell recording was performed at room temperature, using an EPC9 amplifier (HEKA Electronik). Intracellular solutions contained 5–10 mM MgCl2 to inhibit the endogenous MIC currents, as described in ref. 36. All solutions are described in SI Table 1.
The remaining methods on cells, transfection, constructs, RNAi, RNA isolation, and RT-PCR can be found in SI Materials and Methods and SI Table 1.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. I. Parker for helpful advice, Dr. L. Forrest for assistance with cell culture, A. Kolski-Andreaco for PC12 cell culture, Dr. Y. Yu for thoughtful discussion, Dr. A. Alcover for the superantigen-specific T cell generation protocol, Drs. Jen Liou and Tobias Meyer for the YFP-STIM1 construct, the Optical Biology Shared Resources at UCI for providing access to confocal microscopy, and the General Research Clinical Center for providing blood samples. This work was supported by National Institutes of Health Grant NS14609 (to M.D.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706122105/DC1.
References
- 1.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 6.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 10.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser CT, Tsien RY. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci USA. 2007;104:3693–3697. doi: 10.1073/pnas.0611713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromley SK, et al. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2:1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 15.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 16.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 17.Brossard C, et al. Multifocal structure of the T cell—dendritic cell synapse. Eur J Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 18.Hess SD, Oortgiesen M, Cahalan MD. Calcium oscillations in human T, natural killer cells depend upon membrane potential and calcium influx. J Immunol. 1993;150:2620–2633. [PubMed] [Google Scholar]
- 19.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: From synapses to fate determination. Nat Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 20.Fomina AF, Fanger CM, Kozak JA, Cahalan MD. Single channel properties and regulated expression of Ca2+ release-activated Ca2+ (CRAC) channels in human T cells. J Cell Biol. 2000;150:1435–1444. doi: 10.1083/jcb.150.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotturi MF, Hunt SV, Jefferies WA. Roles of CRAC and Cav-like channels in T cells: More than one gatekeeper? Trends Pharmacol Sci. 2006;27:360–367. doi: 10.1016/j.tips.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Gwack Y, et al. Biochemical and functional characterization of orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 23.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 24.Hughes-Fulford M, et al. Early immune response and regulation of IL-2 receptor subunits. Cell Signal. 2005;17:1111–1124. doi: 10.1016/j.cellsig.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Lis A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poenie M, Tsien RY, Schmitt-Verhulst AM. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandy KG, et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panyi G, et al. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci USA. 2004;101:1285–1290. doi: 10.1073/pnas.0307421100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beeton C, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolaou SA, Neumeier L, Peng Y, Devor DC, Conforti L. The Ca2+-activated K+ channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am J Physiol Cell Physiol. 2007;292:C1431–9. doi: 10.1152/ajpcell.00376.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3zeta during T cell recognition. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 32.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 33.Lee SC, Sabath DE, Deutsch C, Prystowsky MB. Increased voltage-gated potassium conductance during interleukin 2-stimulated proliferation of a mouse helper T lymphocyte clone. J Cell Biol. 1986;102:1200–1208. doi: 10.1083/jcb.102.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen A, et al. Novel nonpeptide agents potently block the C-type inactivated conformation of Kv1.3 and suppress T cell activation. Mol Pharmacol. 1996;50:1672–1679. [PubMed] [Google Scholar]
- 35.Dehaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 36.Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.