Abstract
PTEN is a tumor suppressor gene but whether cancer can develop in all PTEN-deficient cells is not known. In T cell-specific PTEN-deficient (tPTEN−/−) mice, which suffer from mature T cell lymphomas, we found that premalignancy, as defined by elevated AKT and senescence pathways, starts in immature T cell precursors and surprisingly not in mature T cells. Premalignancy only starts in 6-week-old mice and becomes much stronger in 9-week-old mice although PTEN is lost since birth. tPTEN−/− immature T cells do not become tumors, and senescence has no role in this model because these cells exist in a novel cell cycle state, expressing proliferating proteins but not proliferating to any significant degree. Instead, the levels of p27kip1, which is lower in tPTEN−/− immature T cells and almost nonexistent in tPTEN−/− mature T cells, correlate with the proliferation capability of these cells. Interestingly, transient reduction of these cancer precursor cells in adult tPTEN−/− mice within a crucial time window significantly delayed lymphomas and mouse lethality. Thus, loss of PTEN alone is not sufficient for cells to become cancerous, therefore other developmental events are necessary for tumor formation.
Keywords: lymphomas, senescence, double positive thymocytes, p27, cyclin A
How premalignancy arises, how the cells respond to it, and what the molecular steps leading to tumors are remain crucial questions in understanding cancer and designing possible future therapies. It has been widely accepted that cancer can be initiated by a combination of gain-of-function mutations of oncogenes and/or loss-of-function mutations of tumor suppressor genes. Whether cells become transformed immediately or other “tumor-initiating” events are still needed for tumor development is not completely known. Recent evidence has demonstrated that leukemia and some solid tumors might contain “cancer stem cells” within the tumors (1, 2). This population is distinct from the cancer cells, exhibit the ability to self-renew, and undergo differentiation to produce cancer cells. However, a more recent study strongly argued against the stem cell model for cancer at least for myc-induced B cell lymphomas and ras-induced T cell lymphomas (3). As the development of B and T cells is a sequential process occurring in different organs, one possible explanation in these cases is that the tumor-initiating cells might be located in other organs rather than the tumor-arising sites.
PTEN is a negative regulator of PI3 kinase pathway (4, 5) and a tumor suppressor gene commonly lost or inactivated in many human cancers. In addition, germ-line PTEN mutations result in the Cowden syndrome, in which patients develop hyperplastic lesions in multiple organs with increased risks of malignant transformation (6, 7). Thus, understanding PTEN biology and how it regulates cell death and cell proliferation during tumor development should yield significant insights into how cancer cells arise. The importance of PTEN in controlling tumor formation was also shown by the phenotype of PTEN heterozygous mice (PTEN+/ −). These mice suffer from multiple types of tumors (8–10) and ≈12% of PTEN+/− mice die between 20 and 50 weeks of age because of massive tumor growth. In animal models where PTEN is lost in both chromosomes in specific tissues, tumors arise early but not immediately. Prostate-specific PTEN conditional-deficient mice suffer from nonlethal high-grade prostatic intraepithelial neoplasia at ≈9 weeks of age (11). p53 seems to be an important fail-safe protein as an inducer of the senescence pathway in this model. Combined p53/PTEN mutations lead to accelerated prostate tumor progression and lethality by 7 months of age (11). Similarly, T cell-specific PTEN conditional mice (lck-cre/PTENfl/fl or tPTEN−/−) suffer from CD4+CD8− T cell lymphomas starting at ≈10 weeks of age (12), and all of them die by 15 weeks of age. Here, we analyzed tumorigenesis of tPTEN−/− mice in detail and found that instead of in lymph nodes and spleen, premalignancy starts in the thymus. Interestingly, significant premalignancy starts in a synchronous fashion in double positive (DP) cells at 9 weeks of age, suggesting that other tumor-initiating events are needed for PTEN-deficient cells to become cancerous. We also found that DP thymocytes exist in a unique state of cell cycle and render senescence program irrelevant in serving as a barrier to cancer. Instead, T cell maturation is an integral part of tumor development. More strikingly, transient administration of dexamethasone into 7.5-week-old tPTEN−/− mice, which reduced the number of DP thymocytes but not mature T cells, led to a significant rescue of lethality and prevented incidence of lymphomas in >50% of the mouse population up to 21 weeks.
Results
Molecular Changes Associated with Premalignancy Appear in DP Thymocytes in a Timed and Synchronous Fashion.
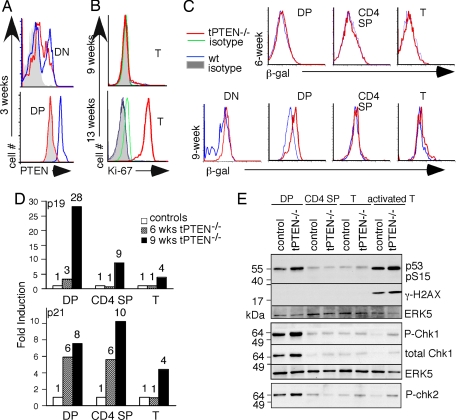
To study how tumors develop in PTEN-deficient cancer cells, we used lck-Cre/PTENfl/fl mice (tPTEN−/−) as a lymphoma mouse model. In these mice, PTEN expression is lost in a T cell lineage-specific fashion because of the expression of the Cre recombinase under the control of the lck proximal promoter, which is active in thymocytes starting from the double negative (DN) stage as early as embryonic day (E) 17 of mouse gestation (13). Intracellular staining with anti-PTEN antibody showed the loss of PTEN in close to 100% of DP thymocytes in all of the mice examined, including 3-week-old mice (Fig. 1A). As noted previously, most of these mice develop CD4+ T cell lymphomas only when they are 10 weeks or older (12). When mice are ≈9 weeks or younger, few tumors develop as measured by the proliferation marker Ki-67 (14) in the spleen and lymph nodes (Fig. 1B). Consistent with published data (15), T cell development and T cell numbers of 9-week-old or younger tPTEN−/− mice were completely normal (data not shown). In contrast, mature T cells from 12 weeks or older tPTEN−/− mice were all Ki-67+. Senescence and DNA damage pathways are activated in premalignant cells and are thought to act as barriers against cancer development (11, 16–20). Senescence can be easily detected by elevated β-gal activity at pH 6.0 [senescence-associated β-gal (SA-β-gal)] and by a dramatic transcriptional increase of several cell cycle inhibitors p19arf, p16ink4a, or p21 (21). We performed quantitative RT-PCR with p19- and p21-specific primers and SA-β-gal staining using C12FDG (22) as the substrate in the presence of chloroquine (23). Whereas 6-week-old thymocytes and T cells from tPTEN−/− mice showed no elevation of SA-β-gal in comparison to their wild-type counterparts, DP thymocytes from 9-week-old mice showed a distinct shift of SA-β-gal (Fig. 1C; n = 6). No changes were detected in other thymocyte populations or peripheral T cells (Fig. 1C). Occasionally, PTEN-deficient CD4 single positive (SP) thymocytes also exhibited an increased staining for SA-β-gal (data not shown), but PTEN-deficient peripheral T cells always showed the same SA-β-gal staining as their wild-type counterparts (n = 6). The same observation was made in examinations of the levels of p19arf and p21. Most of the p19 induction occurred in thymocytes of 9-week- but not 6-week-old tPTEN−/− mice [Fig. 1D and supporting information (SI) Fig. 6]. Although induction of p21 could be found in 6-week-old mice, we concluded that the a robust senescence program does not start until 9 weeks. The level of p16ink4a was undetectable in all T cell populations from either wild-type or PTEN-deficient mice although it was readily seen in mouse fibroblast cells (data not shown).
Fig. 1.
Activation of senescence and DNA damage pathways as markers for premalignancy was detected in DP thymocytes of 9-week-old tPTEN−/− mice. (A) Intracellular staining of PTEN in DN and DP thymocytes of 3-week-old tPTEN−/− mice and their littermate controls. (B) Ki-67 staining of CD4+ splenic T cells of 9- or 13-week-old tPTEN−/− mice or their littermate controls (WT, PTENfl/fl, or /PTENfl//+). (C) The activity of SA-β-gal was measured by flow cytometry in thymocytes and peripheral T cells of 6- or 9-week-old tPTEN−/− mice and littermates (CD4 SP, CD4+CD8− thymocytes; T, peripheral T cells). (D) Quantitative real-time RT-PCR analysis of senescence marker genes in sorted DP thymocytes, CD4 SP thymocytes, and column-purified peripheral T cells (T) of 6- or 9-week-old mice. The graphs represent relative levels of p19 or p21 (normalized by hypoxanthine-guanine phosphoribosyl transferase) in tPTEN−/− samples expressed as fold over the wild-type littermate controls. The numbers above each column denote the corresponding folds of stimulation. (E) Western blot analysis of proteins in the DNA damage pathway using the indicated sorted thymocytes, column-purified peripheral T cells (T) and the ConA/PMA- stimulated mature T cells (activated T). The total level of the MAP kinase ERK5, which does not change during development or with stimulation, is used as a loading control. All of the experiments were done at least three times.
Oncogene-induced senescence is thought to be part of the ATM DNA damage pathway that involves many proteins, including phosphorylation/activation of p53, chk1, chk2, γ-H2AX, and ATM (19, 24). To examine the status of DNA damage pathway during T cell development in tPTEN−/− mice, we performed Western blot analysis with sorted/column-purified T cell populations from 8- to 9-week-old tPTEN−/− mice and their wild-type littermates. We found that p53, chk1, and chk2 were highly phosphorylated in wild-type DP thymocytes, consistent with the stages where V(D)J recombination (and hence RAG1-mediated breaks) occurs (Fig. 1E). Phosphorylation of p53, chk1, and chk2 was negligible in CD4 SP and naïve wild-type T cells. Interestingly, PTEN-deficient DP but not SP thymocytes or mature T cells expressed higher levels of phosphorylated p53, chk1, and chk2 but not γ-H2AX (Fig. 1E). No thymocyte populations expressed γ-H2AX. Thus, DNA damage and senescence pathways overlap to some extent but they are not 100% concordant.
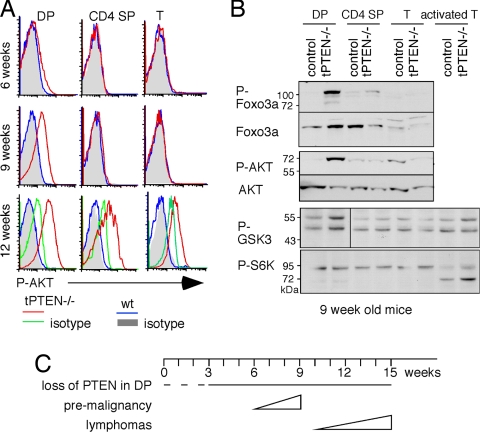
We then examined other molecular changes that might accompany tumorigenesis in PTEN-deficient cells. Intracellular staining with AKT phospho-specific antibody showed an elevated level of activated AKT in DP but not CD4 SP or peripheral T cells of 9-week-old tPTEN−/− mice (Fig. 2A). Phosphorylation of AKT was undetectable in the mice younger than 6 weeks old, whereas a very slight increase was seen in 6- to 8-week-old tPTEN−/− mice (Fig. 2A and data not shown). Consistent with full development of tumors, rampant AKT phosphorylation was seen in all PTEN-deficient T cell populations when mice had reached 12 weeks of age. The AKT downstream protein, Foxo3a, was also heavily phosphorylated in DP but not in CD4 SP or naïve T cells of 9-week-old tPTEN−/− mice (Fig. 2B). Another known AKT downstream target, glycogen synthase kinase 3 (GSK3), was slightly activated but ribosomal S6 kinase (S6K), part of the mTOR pathway, was not differentially phosphorylated in PTEN-deficient DP thymocytes (Fig. 2B). Thus, activation of AKT in DP cells only affect selected downstream pathways. Taken together, these data suggest that although PTEN is lost early, premalignancy does not start until mice are 6 weeks old and becomes very strong by 9 weeks of age. Lymphomas develop later, and all of the mice die by 15 weeks (Fig. 2C).
Fig. 2.
Activation of the AKT pathway was found in DP thymocytes of 9-week-old tPTEN−/− mice. (A) Intracellular staining with AKT phospho-specific antibody showed an elevated level of activated AKT in DP but not CD4 SP or peripheral T cells (T) of 9-week-old tPTEN−/− mice. At 12 weeks of age, rampant AKT phosphorylation was seen in all PTEN-deficient T cell populations. The profiles of isotype controls for 6- and 9-week-old tPTEN−/− mice are the same as the wild-type mice. (B) Activation of AKT downstream genes (FoxO3a, GSK3, and S6K) was measured by Western blot using antibodies specific for each protein or for the corresponding phosphorylated form. All experiments were repeated several times with similar results. (C) A schematic diagram of the timeline to malignancy in tPTEN−/− mice. The dotted line indicates the presumed deletion of PTEN in DP cells.
Wild-Type and PTEN-Deficient DP Thymocytes Exist in a Unique State of Cell Cycle.
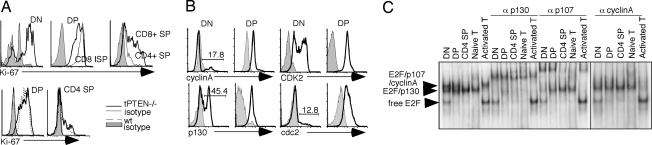
Although PTEN-deficient DP cells show the first signs of tumorigenesis, most of the tPTEN−/− mice develop CD4+CD8− mature T cell lymphomas. To begin understanding why most tPTEN−/− mice do not suffer from DP thymomas and to see whether senescence in DP thymocytes can potentially serve as a barrier to tumor development, we examined the cell cycle status of wild-type and tPTEN−/− DP thymocytes. More than 95% of DP thymocytes from adult mice exhibit 2N DNA content (25) (SI Fig. 6), but their cell cycle state is not completely defined. When mice were injected with one to two pulses of BrdU and thymi were taken 4 h to 11 days later (26), BrdU-labeled DN thymocytes disappeared quickly, consistently with the extensive proliferation of DN compartment. However, DP thymocytes retained BrdU up to 5 days. These published data suggest that DP thymocytes are either proliferating slowly or do not proliferate. Deletion of survivin, a protein required for mitosis, in DP thymocytes had no effect on T cell maturation or apoptosis of DP cells (27), arguing strongly that DP thymocytes are not proliferating. Interestingly, Ki-67, a marker associated with non-G0 cells (14), was highly expressed in 100% of DP thymocytes and was down-regulated as thymocytes matured (Fig. 3A). A fraction of DN thymocytes were Ki-67+, consistent with the fact that some of them were proliferating. In contrast, 100% of CD8 intermediated SP cells, a population before DP cells, and DP cells, expressed Ki-67. Lower levels of Ki-67 were seen in CD4+ CD8−/CD4− CD8+ SP thymocytes (Fig. 3A), ending with Ki-67-negative G0 mature T cells. These data suggest that DP cells are non-G0 cells, and positive selection is accompanied by induction of quiescence. The Ki-67 profiles of 6- to 9-week-old tPTEN−/−mice were indistinguishable from their littermate controls (Fig. 3A), suggesting again that T cell development proceeds normally in these mice. We then analyzed individual cell cycle proteins with Western blot and intracellular staining to see whether any of them might be abnormally changed in tPTEN−/− T cells. Surprisingly, DP thymocytes expressed all of the cell cycle proteins analyzed, including those involved in the G1, S, G2, and M phases. Stimulation with anti-CD3/CD28 either down-regulated or had no effect on the expression levels of these cell cycle proteins (SI Fig. 6). Intracellular staining with antibodies specific for some of these cell cycle proteins showed that these proteins were not expressed in only a subset of cells but in the majority of DP thymocytes (Fig. 3B). No changes in these cell cycle proteins, with the exception of p27kip1, could be seen in tPTEN−/− DP cells (see below and data not shown). We also examined expression of two Rb family members, p130 and p107, and their associations with the E2F transcription factors. Activities of p130 and p107 have been shown to correlate with resting and proliferating cells, respectively (28, 29). DP cells expressed both p130 and p107 that functionally associated with E2F as shown in a gel-shift analysis (Fig. 3C). Unlike activated T cells or DN thymocytes, however, no free E2F could be seen in DP thymocytes, consistent with the notion that DP cells are not proliferating. No significant reproducible changes in expression of p107, p130, or cyclinA were seen in tPTEN−/− DP cells (Fig. 4A). We concluded that DP thymocytes exist in a unique state, expressing many cell cycle proteins but yet do not proliferate to any significant degree. The senescence program found in tPTEN−/− DP cells does not seem to have any effect on the overall cell cycle status of DP cells, including the high level of CDK2 kinase activity.
Fig. 3.
The cell cycle profiles of wild-type and tPTEN−/− thymocytes and mature T cells. (A) (Upper) Intracellular staining of Ki-67 showed a gradient expression level of Ki-67 starting with DN thymocytes. (Lower) The Ki-67 profiles of 9-week-old tPTEN−/− mice are the same as the wild-type littermate controls. (B) Intracellular staining of cell cycle proteins (cyclinA, p130, CDK2, and cdc2) was seen in the majority of DP thymocytes. The staining profiles of DN thymocytes are shown as controls. Gray areas represent the staining profiles of isotype controls. (C) E2F complexes in different T cell populations were characterized by gel-shift analysis. Each of the complexes was identified by the supershifting or blocking of the individual complex with antibodies to p130, p107, or cyclinA.
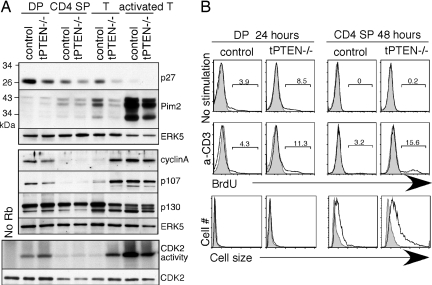
Fig. 4.
Down-regulation of the cell cycle inhibitor p27kip1 correlates with the changes of proliferating proteins. (A) (Top and Middle) Western blot analysis of sorted/column-purified T cell populations (from 9-week-old mice) with antibodies specific for p27kip1, Pim2, cyclinA, p107, p130, and anti-ERK5 for loading control. (Bottom) The CDK2 kinase activities of the indicated T cell populations were measured after immunoprecipitation with anti-CDK2 antibody and incubation with GST-Rb as the substrate in the presence of 32P γ-ATP. As a control, the immunoprecipitated CDK2 was blotted with anti-CDK2 antibodies. (B) (Top and Middle) BrdU staining of untreated and anti-CD3 stimulated thymocytes from 9-week-old tPTEN−/− mice and their littermate controls. The percentages of BrdU+ cells are shown. The gray-shaded peaks are control staining without BrdU. (Bottom) An overlay of side-scatter as an indication of cell size for untreated (gray shading) and anti-CD3 stimulated DP and CD4 SP cells (solid line).
PTEN-Deficient CD4+CD8− T Cells Exhibit Propensity to Proliferate.
In contrast to the DP compartment, higher levels of p107 and cyclin A, a slight reduced level of p130, and spontaneous activation of the CDK2 kinase activity were found in mature T cells or SP thymocytes of most 9-week-old tPTEN−/− mice (Fig. 4A and data not shown). Expression of p107 and cyclin A is normally down-regulated during T cell development, whereas p130 expression stays the same as cells develop toward the quiescent state in naive T cells. In this example of 9-week-old tPTEN−/− mice, where no visible tumors were observed, down-regulation of p107, cyclin A, and CDK2 activity in SP thymocytes occurred normally but their mature T cells exhibited abnormal levels of p107, cyclin A, and spontaneous activation of the CDK2 kinase activity (Fig. 4a). Interestingly, the expression levels of the cell cycle inhibitor p27kip1 correlated with these changes. p27 was expressed at a very high level in wild-type DP thymocytes and was significantly reduced in PTEN-deficient DP cells. A gradual decrease of p27 in concordance with T cell maturation was observed in both wild-type and tPTEN−/− mice. PTEN-deficient naive T cells contained virtually no p27. As p27 has been shown to be the downstream gene of Foxo3a (30, 31), the lower levels of p27 can be explained by the lower transcriptional activity of Foxo3a in 9-week-old tPTEN−/− DP cells.
To see whether PTEN-deficient T cells have a tendency to undergo proliferation, we stimulated them with anti-CD3 antibody and measured BrdU incorporation and cell size 24 or 48 h later (by 48 h, most DP thymocytes had died, and no proliferation could be detected in SP at the 24-h time point). Very few DP thymocytes proliferated as expected. Some PTEN-deficient DP cells incorporated BrdU but they didn't increase in cell size (Fig. 4B). In contrast, CD4 SP thymocytes increased in cell size, with more of them in PTEN-deficient cells. tPTEN−/− CD4 SP thymocytes also proliferated more readily than their wild-type counterparts (Fig. 4B). This difference could also be caused by the slight increased capability of tPTEN−/− DP cells that then translates to this enhanced proliferation potential after positive selection. Interestingly, the level of Pim-2, a kinase important for cell size in T cells (32), increased during T cell maturation (Fig. 4A). All of these data combined suggest that molecular changes taking place in the tPTEN−/− DP thymocytes are not sufficient for tumor formation, but T cell maturation that normally down-regulates p27 and up-regulates Pim-2 is another factor that most likely plays a role in development of CD4+CD8− T cell lymphomas.
Transient Reduction of DP Thymocytes Within a 3-Week Window Significantly Halts Lymphoma Development.
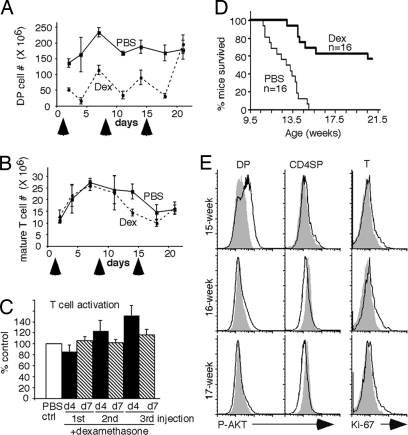
To see whether premalignancy taking place in DP thymocytes is essential for development of CD4+ T lymphomas and hence mouse lethality, we i.p.-injected dexamethasone or PBS to 7.5-week-old tPTEN−/− mice to transiently reduce the number of DP thymocytes (day 1). DP thymocytes but not mature T cells are known to be very sensitive to dexamethasone-induced apoptosis. Dexamethasone has also been shown to have relatively little effect on the ability of mature T cells to proliferate (33). Mice aged 7.5 weeks old have a steady state of T cell development and contain full mature T cell repertoires. We repeated the dexamethasone injection at days 8 and 15. Under this regimen, a significant reduction of DP thymocytes could be seen in these mice (Fig. 5A) but the numbers of splenic or lymph node T cells were not affected (Fig. 5B). The number of DP thymocytes came back 7 days postinjection, presumably because of the clearance of dexamethasone. To confirm that mature T cells from these mice could still undergo proliferation, we stimulated mature T cells taken at different time points after each dexamethasone injection. As shown in Fig. 5c, the abilities of these T cells to undergo proliferation in response to many different stimuli were completely normal. Thus, transient injection of dexamethasone should have a negligible direct effect on any tumor cells (which are mostly CD4+ CD8− mature T cells) that might be arising between 9.5 and 10.5 weeks of age. Indeed, wild-type CD4+CD8− T cells and tPTEN−/− CD4+CD8− tumor cells are similarly resistant to dexamethasone-induced apoptosis in vitro (data not shown). Consistent with the importance of DP thymocytes in lymphoma development, we found that this transient injection of dexamethasone had a major effect on mouse lethality. A significant delay in mouse lethality was observed (Fig. 5D). In the control PBS-injected group, 100% of tPTEN−/− mice (n = 16) had died by 15 weeks of age as expected (12, 34). Forty percent of dexamethasone-treated tPTEN−/− mice died ≈3–4 weeks later, consistent with the idea that reduction of DP thymocytes for 3 weeks led to some delay in lymphoma development. Surprisingly, 56% of dexamethasone-treated tPTEN−/− mice were still alive and healthy when they were 21 weeks of age(Fig. 5D). Some of these mice were 26 weeks or older. Analysis of three dexamethasone-injected tPTEN−/− mice that survived beyond 15 weeks of age showed that none of them exhibited rampant Ki-67 staining in the peripheral T cells, indicating that tumors did not develop. Interestingly, AKT phosphorylation was not even observed in DP thymocytes of two of these mice and was significantly lowered in one of them (Fig. 5E). These data suggest that events that trigger premalignancy in DP thymocytes might only occur transiently during development and thus even a transient reduction of DP thymocytes of adult tPTEN−/− mice within a critical 3-week window could prolong the life of these mice in a significant fashion.
Fig. 5.
Injection of dexamethasone specifically reduced the number of DP thymocytes and caused a significant delay of tPTEN−/− mouse lethality. Three doses of dexamethasone or PBS were i.p.-injected starting when mice were 7.5 weeks old. tPTEN−/− mice were used for the survival experiment, and littermate controls were used for the other experiments. Arrows denote the injections. (A and B) The numbers of DP thymocytes and CD4+ mature T cells from spleen were measured at days 2, 4, 7, 11, 14, 18, and 21. Dotted lines denote dexamethasone (Dex)-injected mice, and solid lines represent PBS-injected mice. (C) Four days (d4) or 7 days (d7) after each injection, splenic T cells were isolated and stimulated with anti-CD3, ConA plus PMA, or PMA plus ionomycin for 72 h. The percentages of Ki-67+ T cells were measured. All stimulated samples (n = 6 for each) from dexamethasone-treated mice were grouped together and compared with stimulated samples (set to 100%) from PBS-treated mice. (D) tPTEN−/− mice were injected with dexamethasone (thick line) or PBS (thin line) three times starting when they were 7.5 weeks old. The curve represents the percentages of mice survived over time. (E) Dexamethasone-treated tPTEN−/− mice were analyzed for the presence of Ki-67 and phospho-AKT (dark lines) in different T cell populations by intracellular staining. Gray areas represent staining by isotype controls.
Discussion
Using tPTEN−/− mice as a lymphoma model, we have found a surprising timed appearance of premalignant cells in a precursor organ rather than the place where tumors develop. We also show the important role of normal developmental events in tumorigenesis. Premalignancy, defined by activation of senescence and DNA damage pathways, was found in the thymus but not in spleen or lymph nodes, where lymphomas eventually arise. Although PTEN is presumably lost as early as E17 during gestation because of the activity of the lck proximal promoter, activated AKT and Foxo3a phosphorylation do not appear till 6 weeks of age. It is not clear what events trigger these pathways but it is unlikely caused by random mutations at another gene locus because of the relatively synchronous appearance of these “premalignant” cells. Neither is it likely caused by the accumulated effect of PTEN deficiency, because DP thymocytes live for only 3–4 days (35) and thus DP thymocytes in 6-week-old mice are not the same population from that of 9-week-old mice. Unknown developmental cues that activate the PI3 kinase pathway at ≈8–9 weeks of age are the likely reason for the synchronous molecular changes in tPTEN−/− mice. One theoretical possibility is the advent of puberty and sexual maturation, which occurs ≈6–8 weeks of age for mice. Increased circulating hormones might stimulate tPTEN−/− DP thymocytes that lead to the significant elevated AKT phosphorylation in 9-week-old mice. In assessing the possible role of senescence in tPTEN−/− tumorigenesis, we found that DP thymocytes in both wild-type and tPTEN−/− mice are not proliferating to any significant degree even though they express many cell cycle proteins and exhibit a high level of CDK2 activity. The higher level of p27kip1 is the likely inhibitor that prevents DP cells from entering the S phase. The expression level of p27 is gradually reduced during T cell development in both wild-type and tPTEN−/− mice. The extremely low level of p27 in tPTEN−/− peripheral T cells correlates with higher expression levels of p107 and cyclin A and spontaneous activation of CDk2 kinase activity. Thus, tPTEN−/−/p27−/− mice might suffer from DP thymomas. However, other changes must also contribute to the onset of tumors because p27−/− mice do not have lymphomas (36–38). The increased Foxo3 phosphorylation (and thus presumably decreased Foxo3 protein in the nucleus) might be another crucial event in PTEN tumorigenesis. The Foxo family proteins are transcriptional factors of many key cell cycle proteins and can serve as tumor suppressor genes (39–41), Loss of Foxo1, Foxo3, and Foxo4 led to the development of DP thymomas in 20-week-old mice (39), possibly caused by a complete reduction of p27 levels (39, 40). Interestingly, another important AKT downstream pathway, mTOR (4, 42), is not activated in the DP thymocytes of 9-week-old tPTEN−/− mice. This finding is in contrast to PTEN-deficient hematopoietic stem cells, which exhibit high-level activation of the mTOR kinase pathway (1). Rapamycin, an inhibitor of the TOR pathway, depleted leukemic stem cells and rescued these hematopoietic conditional PTEN-deficient mice from lethality. Thus, the effects of PTEN loss and AKT activation differ between cell types.
The timed appearance of premalignant DP thymocytes is crucial for the development of CD4+ T cell lymphomas and cancer treatment in general might benefit from considering not only the organs where tumors develop but also other related organs. Even transient reduction of DP thymocytes by dexamethasone has a profound effect on mouse lethality, which correlates with uncontrolled proliferation of CD4 lymphomas. Indeed, examination of several dexamethasone-treated tPTEN−/− mice showed a complete absence of lymphomas in their spleen and lymph nodes. It is interesting to note that >50% of the mice survived >21 weeks. Some of the dexamethasone-treated mice did not even exhibit increased AKT phosphorylation in their DP thymocyte population, which suggests that events that trigger premalignancy in PTEN-deficient DP thymocytes only happen within a short time window. It is possible that longer dexamethasone treatment might have a more pronounced effect on a larger percentage of tPTEN−/− mice.
Finally, the high-level expression of cell cycle-specific proteins in nonproliferating DP thymocytes might relate to the propensity of these cells to undergo apoptosis. A possible link between cell cycle and apoptotic machineries has been proposed before (43, 44). Expression of cell cycle proteins in DP thymocytes might then be important for T cell development as they render these cells susceptible to negative selection and might also be the basis of DP cells' short life span. Whether this is true and what the molecular cause of the constitutive expression of these cell cycle proteins in DP cells is await further experimentation.
Materials and Methods
Flow Cytometric Analysis.
β-Gal activity was measured as described (23). Briefly, fresh isolated thymocytes and peripheral T cells were cultured and pretreated with 300 μM chloroquine for 2 h to induce lysosomal alkalinization. C12FDG (33 μM) was then added to the pretreatment medium, and the incubation was continued for another 4 h. The β-gal activity was measured after the cell surface staining of CD4 and CD8. Intracellular staining was performed as described (45). Briefly, formaldehyde was added directly to culture medium to a final concentration of 2% and incubated for 10 min at room temperature. The cells were pelleted, resuspended in ice-cold methanol, and incubated for 15–30 min on ice. The cells were then washed three times with staining buffer (0.5% BSA in PBS) and stained with the following antibodies: Ki-67 (BD Biosciences), phospho AKT (Ser-473) and PTEN (Cell Signaling); and p130, cyclinA, CDK2, and cdc2 (Santa Cruz Biotechnology).
Quantitative Real-Time RT-PCR.
RNAs were extracted from sorted DP thymocytes, CD4+CD8− SP thymocytes, and column-purified mature splenic/lymph node T cells. The primer sequences are: p21, forward (5′-GTGTGCCGTTGTCTCTTCGG) and reverse (5′-CTCAGGTAGACCTTGGGCAG); p19arf, forward (5′-GTCGCAGGTTCTTGGTCACT) and reverse (5′-ATCGCACGAACTTCACCAA); p16, forward (GGCACTGAATCTCCGCGA) and reverse (5′-GGGGTACGACCGAAAGAGTT), and HPRT, forward (5′-TGCTCGAGATGTCATGAAGG) and reverse (5′-AATCCAGCAGGTCAGCAAAG).
Western Blotting.
Cell lysates were prepared from sorted DN thymocytes, DP thymocytes, CD4+CD8− SP thymocytes, column-purified mature splenic T cells, or activated T cells [1.5 μg/ml of ConA and 2.5 ng/ml of phorbol 12-myristate 13-acetate (PMA) for 48 h]. Antibodies used were: phospho-p53 (Ser-15), phospho-Chk1 (Ser-345), Chk1, phospho-Chk2 (Thr-387), FoxO3a, phospho-AKT (Ser-473), AKT, phospho-GSK3α/β (Ser-21/9), and phospho-S6K (Thr-389) (Cell Signaling); p107, p130, p27, cyclinA, and Pim2 (Santa Cruz Biotechnology); phospho-histone γ-H2AX (Ser-139) and phospho-FoxO3a (Thr-32), (Upstate Biotechnology); and affinity-purified anti-ERK5 (46).
CDK2 Kinase Assay and Gel-Shift Analysis.
CDK2 kinase activity was measured as described (47). Gel-shift analysis was performed as described (28) using the whole-cell extracts. The E2F oligonucleotide used was: 5′-TCATTTAAGTTTCGCGCCCTTTCTCAA-3′.
Dexamethasone Injection Study.
Dexamethasone or PBS i.p. injections were started in 7.5-week-old tPTEN−/− mice (2.5 mg/kg of dexamethasone for male mice and 5 mg/kg of dexamethasone for female mice). Injections were repeated at days 8 and 15. Survival curves were recorded to compare PBS-injected and dexamethasone-injected mice. At the same time, 7.5-week-old littermate mice were injected with the same doses of PBS or dexamethasone. Two, 4, or 7 days after each injection, the number of DP thymocytes and mature T cells was compared between PBS- and dexamethasone-injected mice. Four and 7 days after each injection, splenic T cells were stimulated with 1 μg/ml of anti-CD3, 1.5 μg/ml of ConA, and 2.5 ng/ml of PMA, or 2.5 ng/ml of PMA and 500 ng/ml of ionomycin for 72 h. Ki-67 staining was performed to measure cell proliferation.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Alma Valeros for cell sorting and Mark Schlissel for reading the manuscript. This work was supported by a grant from National Institute of Health (to A.W.) and a postdoctoral fellowship from The Cancer League Inc. (Oakland, CA).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712059105/DC1.
References
- 1.Yilmaz OH, et al. Pten dependence distinguishes hematopoietic stem cells from leukemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Kelly PN, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 4.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 5.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 6.Nelen MR, et al. Germ-line mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 7.Liaw D, et al. Germ-line mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 8.Podsypanina K, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 10.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumor suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki A, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 13.Allen JM, Forbush KA, Perlmutter RM. Functional dissection of the lck proximal promoter. Mol Cell Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Hagenbeek TJ, et al. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 17.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 18.Collado M, et al. Tumor biology: Senescence in premalignant tumors. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 19.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 20.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 21.Campisi J, di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 22.Plovins A, et al. Use of fluorescein-di-beta-d-galactopyranoside (FDG) and C12-FDG as substrates for β-galactosidase detection by flow cytometry in animal, bacterial, and yeast cells. Appl Environ Microbiol. 1994;60:4638–4641. doi: 10.1128/aem.60.12.4638-4641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative aging of human endothelial cells. J Cell Sci. 2000;113:3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 24.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 26.Penit C. In vivo thymocyte maturation: BUdR labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J Immunol. 1986;137:2115–2121. [PubMed] [Google Scholar]
- 27.Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med. 2004;199:69–80. doi: 10.1084/jem.20031588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulligan GJ, Wong J, Jacks T. p130 is dispensable in peripheral T lymphocytes: Evidence for functional compensation by p107 and pRB. Mol Cell Biol. 1998;18:206–220. doi: 10.1128/mcb.18.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura N, et al. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 34.Buckler JL, et al. Cutting edge: T cell requirement for CD28 costimulation is due to negative regulation of TCR signals by PTEN. J Immunol. 2006;177:4262–4266. doi: 10.4049/jimmunol.177.7.4262. [DOI] [PubMed] [Google Scholar]
- 35.Penit C, Vasseur F. Sequential events in thymocyte differentiation and thymus regeneration revealed by a combination of bromodeoxyuridine DNA labeling and antimitotic drug treatment. J Immunol. 1988;140:3315–3323. [PubMed] [Google Scholar]
- 36.Nakayama K, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 37.Kiyokawa H, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 38.Fero ML, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 39.Paik JH, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Myatt SS, Lam EW-F. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 42.Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol. 2007;47:443–467. doi: 10.1146/annurev.pharmtox.47.120505.105359. [DOI] [PubMed] [Google Scholar]
- 43.Abrams JM. Competition and compensation: Coupled to death in development and cancer. Cell. 2002;110:403–406. doi: 10.1016/s0092-8674(02)00904-2. [DOI] [PubMed] [Google Scholar]
- 44.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 45.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
- 46.Kasler H, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osborn SL, Sohn SJ, Winoto A. Constitutive phosphorylation mutation in fadd results in early cell cycle defects. J Biol Chem. 2007;282:22786–22792. doi: 10.1074/jbc.M703163200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.